Abstract
Purpose
Superficial dose is an important parameter in breast cancer radiation therapy. When treated with conventional linacs, bolus is commonly applied to improve target coverage near the surface while also managing the risk of severe skin reactions and negative cosmesis. With the introduction of modern linacs with 6X flattening filter free (FFF) photon beams, the effect on superficial dose and the need for bolus must be evaluated.
Methods and Materials
In vivo measurements of superficial dose were made with optically stimulated luminescence dosimeters on 11 breast cancer patients treated with the Halcyon 6X FFF linac (Varian Medical Systems, Palo Alto, CA). Additionally, measurements were made with the Halycon 6X FFF beam and a 6X beam with flattening filter (FF) delivered to an anthropomorphic phantom. A planning study was carried out in which 14 patients treated on the Halcyon were replanned with a conventional linac to determine the difference in superficial dose predicted by the treatment planning system. Measures were taken to increase the accuracy of the treatment planning system superficial dose.
Results
The use of the Halcyon 6X FFF beam led to higher superficial dose compared with 6X FF beams. The in vivo measurements show an average superficial dose of 83.8% ± 0.6%, which is an increase of approximately 10% compared with published measurements for a 6X FF linac. Comparison of superficial dose for 6X FF and 6X FFF beams in the phantom measurements show an increase from 70% ± 1.3% to 84% ± 1.3%, which is consistent with the in vivo measurements. The planning comparison shows an increase in V70%Rx from 62% ± 4.4% to 81% ± 2.2% for the superficial breast tissue for the Halcyon 6X FFF beam compared with a standard C-arm linac with FF.
Conclusions
The use of the Halcyon 6X FFF beam was associated with higher superficial dose which may obviate the use of bolus.
Introduction
A common strategy in treating breast cancer is a combined approach, which involves either mastectomy or breast-conserving surgery followed by adjuvant radiation therapy.1 Adjuvant radiation is more common after breast-conserving surgery; however, post mastectomy radiation therapy (PMRT) can also be recommended for patients with advanced stage II or III disease.2 Chest wall recurrences after mastectomy often involve the scar, subcutaneous tissue, or the dermis itself.1 It is common in post mastectomy radiation therapy to use a layer of water equivalent bolus material, typically either 0.5 cm daily or 1 cm every other day, to increase the dose to superficial tissue while also managing the risk of excessive skin toxicity.3
The Halcyon (Varian Medical Systems, Palo Alto, CA) is a novel medical accelerator that features a straight-through linac with a single 6 MV flattening filter free (FFF) beam in an enclosed bore geometry. The removal of the flattening filter increases the dose rate and reduces the scatter and leakage radiation, which in turn reduces the shielding requirements, lowering cost. The enclosed bore geometry allows the gantry to rotate 4 times faster than a C-arm linac without risk of collision with the patient. This, combined with the higher dose rate of the FFF beam, reduces treatment time and consequently increases the patient throughput.4, 5
A consequence of the removal of the flattening filter for Varian linacs is that the mean energy of the beam is lower than a flattened beam of the same nominal energy. The disproportionate attenuation of low energy photons leads to beam hardening in linacs with flattening filters. This hardening effect is reduced in FFF beams because there is significantly less material in the beam path, leading to higher superficial dose.6 Also, Monte Carlo (MC) studies have shown that the fluence of contamination electrons is greater in FFF beams, which contributes further to the rise in superficial dose.7, 8 Published studies have demonstrated an increase in mean energy at the surface and superficial dose for standard C-arm linacs in FFF mode in both MC simulations7, 8 and commissioning beam data.5, 9 Another important consequence of the Halcyon design that may affect superficial dose is the presence of the bore cover in the beam path, which may act like a beam spoiler increasing the superficial dose to the patient.
Given these factors, the superficial dose must be assessed in breast cancer patients treated with Halcyon to determine the appropriate treatment techniques for the management of skin coverage and toxicity, in particular the need for bolus.
Methods and Materials
In vivo measurements
Superficial dose measurements were carried out on 11 breast cancer patients treated on Halcyon at the University of Pennsylvania Perelman Center for Advanced Medicine between March and November 2018. The prescription doses ranged from 4005 to 5040 cGy to the whole breast with a local boost of 1000 cGy to the surgical bed in some cases. Measurements were made at 9 locations across the surface of the breast for each patient using Landauer Nanodot optically stimulated luminescence detectors (OSLDs). Figure 1a shows an example of the OSLD placement for a patient in the study. Two OSLDs were placed at each location, each OSLD was read 5 times, and the average of all 10 readings was used to estimate the superficial dose in that region. Table 1 shows a summary of the characteristics of the patients included in the in vivo measurement study.
Figure 1.
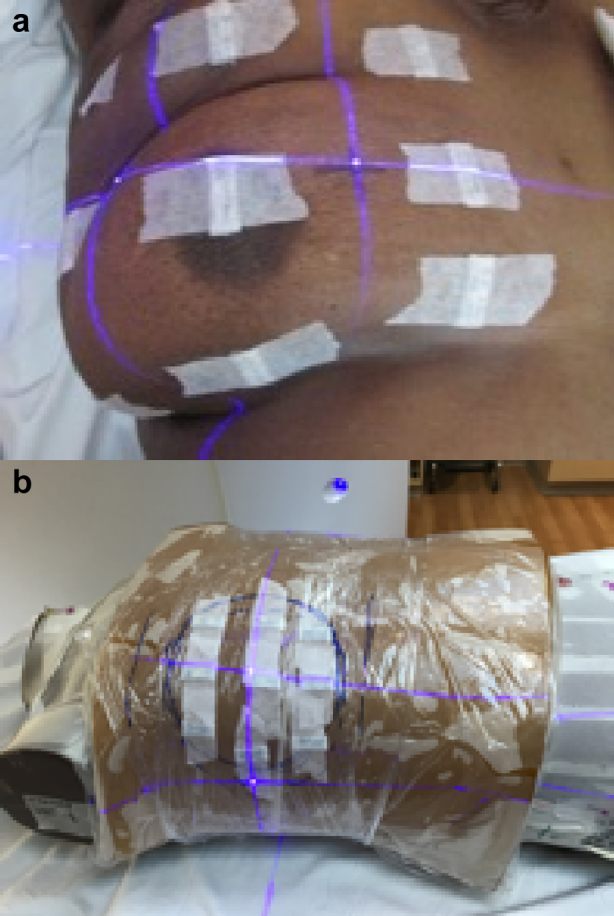
(a) In vivo setup: image of patient taken before treatment showing array of optically stimulated luminescence detectors. (b) Phantom setup: Rando phantom setup showing the optically stimulated luminescence detector placement for the phantom measurements.
Table 1.
Patient characteristics: summary of the patient characteristics for the in vivo and planning studies
| Patient characteristics | In vivo (11 pts) | Planning (14 pts) |
|---|---|---|
| Laterality | ||
| Right | 8 (72.7%) | 8 (57.1%) |
| Left | 3 (27.3%) | 6 (42.8%) |
| Position | ||
| Supine | 9 (81.8%) | 12 (85.7%) |
| Prone | 2 (18.2%) | 2 (14.3%) |
| Target | ||
| Whole breast | 6 (54.6%) | 12 (85.7%) |
| Whole breast + LNs | 5 (45.4%) | 2 (14.3%) |
| Surgery | ||
| Intact breast | 7 (63.6%) | 12 (85.7%) |
| Mastectomy without reconstruction | 3 (27.3%) | 2 (14.3%) |
| Mastectomy without reconstruction | 1 (9.1%) | 0 (0.0%) |
Landauer nanodots are composed of carbon doped aluminum oxide (Al2O3:C) disks, 7 mm in diameter and 0.2 mm thick enclosed in a 1 cm × 1 cm × 2 mm light tight plastic casing of 0.04 g/cm2 intrinsic buildup.10 The OSLD batch was calibrated following the vendor recommended procedure.11 The calibration was then validated to an accuracy of ± 1.5%, which is within the accuracy of ≤5% claimed by the vendor.
The treatment plans for all patients were generated using the Eclipse treatment planning system version 15.5-6 with the AAA dose calculation algorithm and Halcyon version 1.0 and version 2.0. Part way through the study the Halcyon was upgraded from version 1.0 to version 2.0 to include kV cone beam CT (CBCT) imaging capability. To support this upgrade, Eclipse was also upgraded from version 15.1 to version 15.6. The first 5 patients were treated on Halcyon version 1 with MV CBCT. The remaining 6 patients were treated on version 2 with kV CBCT. A CBCT was taken for all patients at every fraction to ensure proper alignment. This is necessary because the Halcyon has no light field or optical distance indicator. The OSLDs were placed before the CBCT so the raw measurements include the combined dose from the imaging and treatment fields. However, phantom and in vivo measurements of the CBCT dose on Halcyon indicate that it is a negligible contribution to the total dose for both kV and MV CBCT. Nevertheless, the CBCT dose was subtracted from the raw measurements to consider the dose from the treatment fields only because imaging practice may vary between institutions.
The treatment plans for all but one of the patients for which measurements were taken were generated using the electronic tissue compensation12 technique in which MLCs are swept across the field to produce a homogeneous dose in the target. The remaining patient was treated with the standard field-in-field technique.
Phantom measurements
To study the difference in superficial dose in a controlled setting, phantom measurements were made with OSLDs in the same configuration as described for the patient measurements. 6X FF and FFF tangential fields were delivered, with TrueBeam (Varian Medical Systems, Palo Alto, CA) and Halcyon linacs, respectively, to an anthropomorphic Rando phantom (Imaging Solutions, Brisbane, Australia) with a 2-cm layer of bolus used to simulate breast tissue attached to the right anterior chest of the phantom. The superficial dose for the FF fields on TrueBeam was measured with and without a 1-cm bolus placed on top of the OSLDs. The measurements were used to estimate the superficial dose for Halcyon and a C-arm FF linac with and without bolus and with bolus applied on alternate days which is a common clinical practice. The phantom setup with the bolus and OSLDs placed is shown in Fig 1b. These measurements allow for a direct comparison between 6X FF and FFF fields measured with the same detector system and setup.
For each set of phantom measurements, a CBCT was done before delivering the treatment fields to ensure that all OSLDs were well within the treatment fields. An extra OSLD pair was placed in the center of the breast for the CBCT only to estimate its contribution to the total dose.
Treatment plans were generated on the phantom with the Eclipse TPS version 15.5-6 for Halcyon and TrueBeam with opposed tangent fields and a prescription of 4256 cGy in 266 cGy fractions. An additional plan was optimized for TrueBeam with a 1-cm bolus added in planning. A volume was drawn to simulate a typical breast target and the plans were optimized to achieve approximately the same target coverage.
Treatment planning system calculated dose comparison
To complement the patient and phantom measurements, a planning study was carried out to make a direct comparison of the superficial dose calculated in the treatment planning system (TPS). This comparison removes complicating factors that may influence the superficial dose such as variations in patient anatomy and OSLD placement. However, it is subject to other limitations, namely the accuracy of the TPS dose calculation algorithm near the patient surface. Measures were taken to reduce this uncertainty to allow for a meaningful comparison of superficial dose.13
Fourteen breast patients planned and treated on the Halcyon were retrospectively replanned with either a Clinac (Varian Medical Systems, Palo Alto, CA) or TrueBeam 6 MV FF beam model. These are collectively referred to in the following as C-arm FF linac. The plans were renormalized to achieve approximately the same target coverage and were required to meet all clinical constraints. The study includes a wide range of clinical and treatment characteristics, such as laterality, treatment position, and planning techniques, which are summarized in Table 1. Of the 14 patients in the study, 11 were planned on Halycon with Varian's implementation of the electronic tissue compensation technique known as the irregular surface compensator, 2 were planned using the field-in-field (FiF) technique with dynamic beam flattening and one with the FiF technique with the native FFF beam. All the comparative C-arm FF linac plans were planned with the FiF technique and reviewed by a physician and deemed clinically acceptable.14
For all patients treated in the supine position, 2 treatment plans were generated with and without a 1 cm bolus and an equally weighted plan sum of the 2 was generated to represent the common clinical practice of applying bolus on alternate days throughout the course of treatment. The superficial dose was then compared between the Halcyon plans and the standard C-arm FF linac plans.
A well-known limitation of TPS dose calculation algorithms, including the Eclipse AAA algorithm implemented in this study, is the underestimation of dose in the superficial region. A study comparing AAA to MC simulations and phantom measurements shows that this underestimation can be reduced from 14% to 4% without affecting the dose to underlying structures by extending the body contour by 2 cm into the air surrounding the patient.13 This recommendation was implemented in the present study to improve the accuracy of the superficial dose comparison.
A superficial tissue structure was defined as a 2-mm inner margin on the patient contour defined with a threshold CT number of –800 HU. This is the smallest thickness at which the TPS can generate a continuous structure owing to the limited segmentation resolution. A superficial breast tissue structure was then taken as the overlap of the superficial tissue structure and a 2-cm expansion on the breast target structure. Each structure was defined as a high-resolution contour in the planning system to capture the superficial tissue as accurately as possible.
All data were analyzed using MatLab R2017a Statistics toolbox (The MathWorks Inc, Natick, MA). Statistical significance was assessed at the 95% confidence level using a 2-sided Student t test comparing 2 unpaired distributions with unequal variance, also known as a Welch t test.
Results
Figure 2 shows a histogram of all Halcyon in vivo and phantom surface dose measurements. There are a total of 228 measurements with a mean value of 83.8% ± 0.6% of the prescription dose. The error is calculated as the standard error of the mean = σ/√n. The histogram is fit to a Gaussian, yielding a fit mean and standard deviation of 83.9% and 8.3%, respectively with a χ2d value of 2.2. The mean of the Halcyon in vivo and phantom measurements considered separately are 83.8 ± 0.6% and 84 ± 1.3%, respectively.
Figure 2.
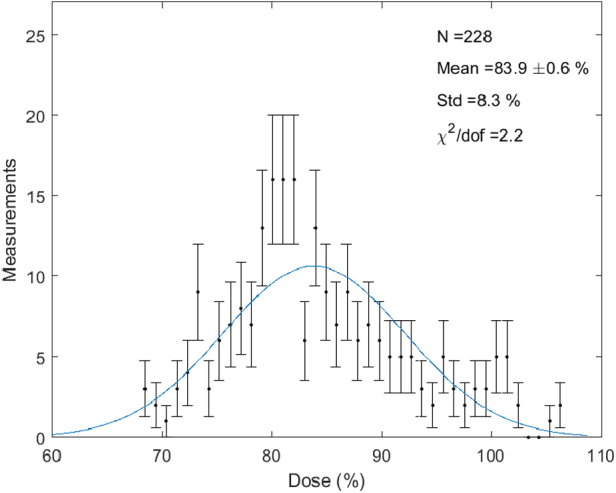
Superficial dose histogram: histogram of all superficial dose measurements for the 11 breast cancer patients and the Rando phantom treated on Halcyon.
The distribution of measurements for each individual patient, including the mean and the spread of the data are shown in Figure 3. The plot also shows the distribution of phantom measurements treated with breast tangents on the Halcyon, the TrueBeam with and without bolus and with bolus applied on alternate days. The average superficial dose measured on the Rando phantom irradiated with the TrueBeam breast plan is 70% ± 1.3% without bolus, 104% ± 2% with bolus, and 87% ± 1.3% with bolus applied on alternate days. The superficial dose for the Halcyon phantom measurement is 84% ± 1.3% which demonstrates an increase of approximately 14% with respect to TrueBeam. This is consistent within measurement error with the difference seen in the in vivo study.
Figure 3.
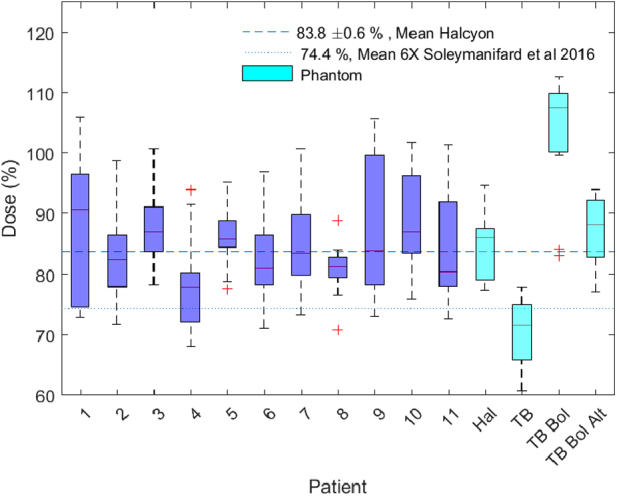
Superficial dose versus patient: in vivo superficial dose measurements made with optically stimulated luminescence detectors for the 11 patients in the study and on the Rando phantom treated with tangential fields on the Halcyon, TrueBeam with and without bolus and with bolus applied on alternate days. The boxes represent the range of data from the 25% to the 75% percentile and whiskers are drawn to the furthest observations not considered outliers (outliers defined as >1.5 × range).
The OSLDs were placed before imaging and therefore included the dose from the CBCT. This represents a very small contribution to the overall dose. The dose from CBCT alone was estimated with an OSLD measurement of the CBCT field and found to be 0.8 ± 0.1 cGy for the MV CBCT and 0.27 ± 0.01 cGy for the kV CBCT, which is <0.5% of the prescription for all patients. For the kV CBCT dose the OSLD response was scaled down by a factor of 3.5 to account for the known over response of Al2O3:C OSLDs to kilovoltage x-rays.10, 15 The CBCT dose was then subtracted from all measurements to consider the dose from the treatment fields only.
Figure 4 show the results of the TPS superficial dose comparison. Figure 4a shows a comparison of the average dose volume histogram (DVH) for the superficial breast tissue for the 4 scenarios considered: C-arm FF linac, Halcyon and C-arm FF linac with and without bolus and with bolus applied on alternate days. Figure 4b shows the distributions of 3 dose volume parameters (Dmax, Dmean, and V70%Rx) for the superficial breast tissue in these 4 scenarios.
Figure 4.
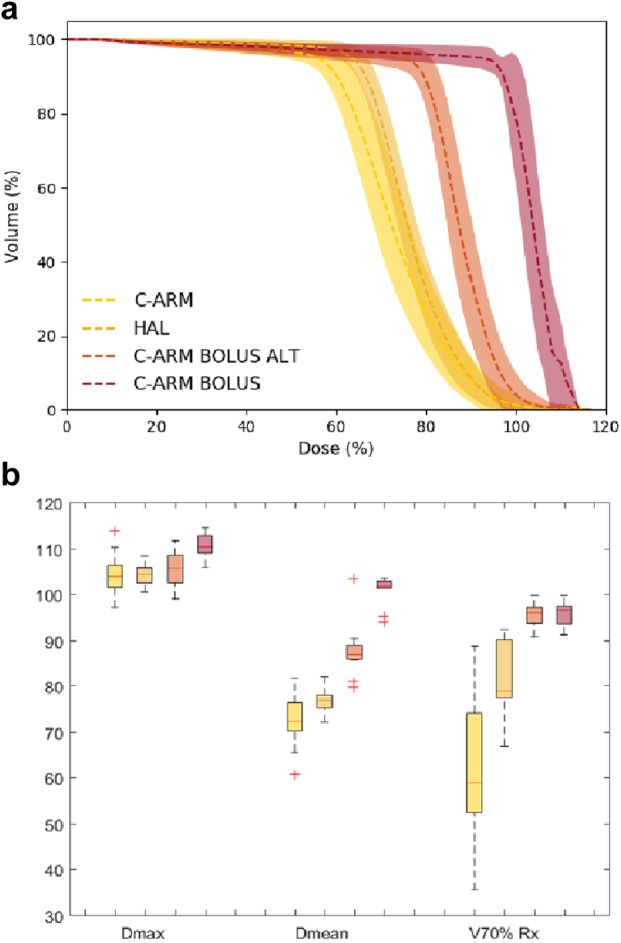
(a) Breast skin DVH comparison: comparison of the breast skin DVH for the C-arm linac (yellow), Halcyon (dark yellow), C-arm linac with bolus applied every other day (orange), and C-arm linac with bolus (red). The dashed curve and bands represent the average and 1 sigma variation of the DVHs across all 14 patients in the planning study. (b) Breast skin dosimetry: comparison of Dmax, Dmean, and V70%Rx of the breast skin structure calculated in eclipse for the same 4 scenarios. DVH = dose volume histogram.
The results of the TPS dosimetry study indicate that the AAA dose calculation algorithm also shows an increase in superficial dose for Halcyon compared with a standard C-arm FF linac. However, the superficial dose is still less than that for a C-arm FF linac with bolus applied every other day. This is consistent with the in vivo and phantom measurements. The mean dose to the superficial breast tissue for Halcyon and C-arm FF linac is 76.7% ± 0.8% and 73% ± 1.6% respectively (P = .017). The average value of V70%Rx is 81% ± 2.2% for Halcyon and 62% ± 4.4% for the C-arm FF linac (P < .01). This increase is clearly visualized in the average DVH comparison plot in Figure 4b. No significant dosimetric differences were observed for other organs at risk other than a moderate increase in mean heart dose for Halcyon due to the daily CBCT. Published studies have demonstrated that treatment plans generated for breast cancer with flattened and FFF beams are dosimetrically comparable for structures other than superficial tissue.16
Discussion
The in vivo and phantom measurements demonstrate that the average surface dose in breast cancer treatment with tangential fields is significantly higher on the Halcyon than on a conventional C-arm 6 MV linac with flattening filter. Similar in vivo measurements made by Soleymanifard et al, with thermo-luminescent dosimeters on a C-arm linac with 6 MV beams and flattening filter yield an average value of 74.4% ± 4.5%.17 The surface dose measured on Halcyon in our study is approximately 10% higher (P = .046).
To rule out the possibility that the observed difference in superficial dose is caused by the difference in effective buildup of the dosimeters, additional phantom measurements were carried out on a TrueBeam with 6X FF tangential beams. As shown in Fig 4, these measurements are consistent with those presented by Soleymanifard demonstrating that the difference is in fact due to the linac rather than the dosimeters. Also of note, the Halcyon phantom measurement is comparable to that on the TrueBeam with bolus applied alternately with a difference of only 2.6% (P = .028).
Given the geometry of tangential fields and the irregular shape of the breast contour the superficial doses presented here represent an average over a range of angles of incidence. To provide a reference to zero angle of incidence measurements were carried out on the Halcyon and TrueBeam of an en face 10 × 10 cm2 beam at 100 SSD in solid water with a PTW TN34045 Markus plan parallel chamber (PTW-Freiburg, Freiburg, Germany). The measurements yield superficial doses of 26.8% at the surface and 57.9% at 1-mm depth for Halcyon and 19.7% at the surface and 47.4% at 1-mm depth for TrueBeam. Thus, for an en face field the superficial dose for Halcyon is approximately 8% to 11% higher than TrueBeam, which is consistent with the increase seen for the breast fields.
It was observed that the relative increase in superficial dose predicted by AAA for Halcyon versus C-arm FF linac was smaller compared with that observed in the in vivo and phantom measurements. This is most likely a reflection of the limited accuracy of the surface dose modeling in the current AAA algorithm (version 15.6.03). Caution should be taken when evaluating superficial dose for Halcyon plans as it might underestimate the actual dose received by superficial structures.
The extent to which the observed increase in superficial dose was influenced by the spoiler effect of the bore cover was tested by making a series of measurements with and without the bore cover. Percent depth dose curves were measured with a parallel plate chamber in water for a 10 × 10 field at 80 cm and 100 cm SSD. Figure 5 shows the difference in these measurements in the superficial region. It can be observed that the difference with and without the bore cover is minimal (<1%), suggesting that it is not the main contributor to the higher superficial dose observed for Halcyon 6 FFF beams. This implies that the increase is caused primarily by the lower average energy of the beam and the higher fluence of contamination electrons in the absence of the flattening filter.
Figure 5.
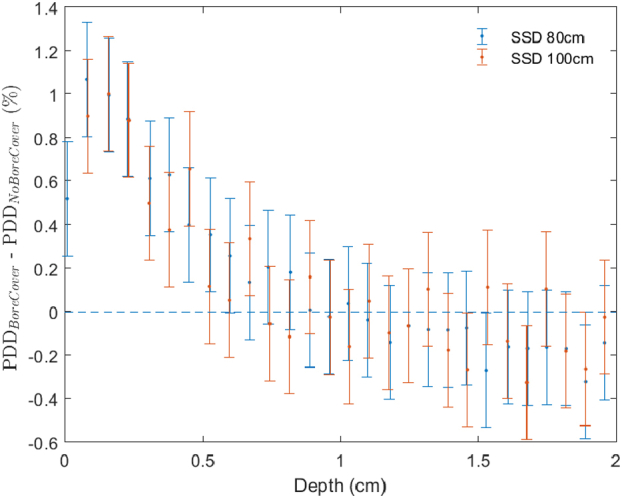
Effect of bore cover: difference in percent depth dose (PDD) in the superficial region with and without the bore cover in place for Halcyon 6FFF at 80 cm and 100 cm SSD measured with a parallel plate chamber in water. The error was estimated as the root mean square of the data in the flat region of the curve (not shown) for depth >2 cm.
Conclusions
The combination of in vivo and phantom measurements establishes that there is a significant increase (10%-15%) in superficial dose for whole breast irradiation with Halcyon compared with a standard 6X linac with flattening filter. The superficial dose with Halcyon is approximately equal to that achieved with a standard 6X linac by applying a 1-cm bolus for half of the treatment fractions. Comparison of the dose calculated with the Eclipse TPS also shows an increase; however, it does not reflect the full magnitude of the increase observed in the in vivo measurements. The results of the study suggest that it may be possible to cover the full breast or chest wall after mastectomy with sufficient dose to superficial and subcutaneous tissues without applying bolus. The traditional strategy of applying bolus every other day should be carefully re-evaluated to reduce the chance of overdosing superficial tissue for patients treated on Halcyon.
Footnotes
Sources of support: No funding was received for this work.
Disclosures: J.M.M. reports grants and personal fees from Varian Medical Systems during the conduct of the study; personal fees from IBA, personal fees from NCC Singapore Proton Advisory Board, personal fees from Global Advisory Board, outside the submitted work; G.D.H. reports grants from Varian Medical Systems during the conduct of the study; grants from ViewRay, Inc, outside the submitted work; L.D. reports grants and personal fees from Varian Medical Systems outside the submitted work; C.K. reports honoraria for speaking engagements from Varian Medical Systems during the conduct of the study outside the submitted work; S.M. reports grants, personal fees and nonfinancial support from Varian Medical outside the submitted work; B.C. reports grants from Varian Medical System outside the submitted work.
References
- 1.National Comprehensive Cancer Network Guidelines For Breast Cancer. 3.2018:BINV-2. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed February 1, 2018.
- 2.Bellavance E.C., Kesmodel S.B. Decision-making in the surgical treatment of breast cancer: Factors influencing women’s choices for mastectomy and breast conserving surgery. Front Oncol. 2016;6:1–7. doi: 10.3389/fonc.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibollahi F., Mayles H.M., Mayles W.P. Assessment of skin dose and its relation to cosmesis in the conservative treatment of early breast cancer. Int J Radiat Oncol Biol Phys. 1988;14:291–296. doi: 10.1016/0360-3016(88)90435-x. [DOI] [PubMed] [Google Scholar]
- 4.Michiels S., Poels K., Crijns W. Volumetric modulated arc therapy of head-and-neck cancer on a fast-rotating o-ring linac: Plan quality and delivery time comparison with a c-arm linac. Radiother Oncol. 2018;128:479–484. doi: 10.1016/j.radonc.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Cashmore J. The characterization of unflattened photon beams from a 6 MV linear accelerator. Phys Med Biol. 2008;53:1933–1946. doi: 10.1088/0031-9155/53/7/009. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Y., Kry S.F., Popple R. Flattening filter-free accelerators: A report from the AAPM Therapy Emerging Technology Assessment Work Group. Med Phys. 2015;16 doi: 10.1120/jacmp.v16i3.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammed M., Chakir E., Boukhal H., Mroan S., El Bardouni T. Evaluation of the dosimetric characteristics of 6 MV flattened and unflattened photon beam. J King Saud Univ Sci. 2017;29:371–379. [Google Scholar]
- 8.Mesbahi A. Dosimetric characteristics of unflattened 6 mv photon beams of a clinical linear accelerator: A Monte Carlo study. Appl Radiat Isot. 2007;65:1029–1036. doi: 10.1016/j.apradiso.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Vassiliev O.N., Titt U., Pönisch F., Kry S.F., Mohan R.G.M. Dosimetric properties of photon beams from a flattening filter free clinical accelerator. Phys Med Biol. 2006;51:1907–1917. doi: 10.1088/0031-9155/51/7/019. [DOI] [PubMed] [Google Scholar]
- 10.Jursinic P.A. Characterization of optically stimulated luminescent dosimeters, oslds, for clinical dosimetric measurements. Med Phys. 2007;34:4594–4604. doi: 10.1118/1.2804555. [DOI] [PubMed] [Google Scholar]
- 11.Yahnke C.J. Calibrating the microStar. Landauer inLight Systems. 2009 https://pdfs.semanticscholar.org/d29d/e4a28e2e10314d20b92fc4ac75e88a3a1125.pdf Available from. [Google Scholar]
- 12.Kennedy C., Freedman G., Taunk N. Whole Breast irradiation with HalcyonTM 2.0: Workflow and efficiency of field-in-field treatment with dynamic beam flattening technique and kV cone beam computed tomography. Cureus. 2018;10:1–12. doi: 10.7759/cureus.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Cmelak A.J., Ding G.X. A simple technique to improve calculated skin dose accuracy in a commercial treatment planning system. J Appl Clin Med Phys. 2018;19:191–197. doi: 10.1002/acm2.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttmann D, Gabriel P, Kennedy C. Comparison of acute toxicities between contemporary forward-planned 3D conformal radiotherapy and inverse-planned intensity-modulated radiotherapy for whole breast radiation. Breast J. 2018;24:128–132. doi: 10.1111/tbj.12857. [DOI] [PubMed] [Google Scholar]
- 15.Scarboro S.B., Kry S.F. Characteristics of energy response of Al2O3:C optically stimulated luminescent dosimeters (OSLDs) using cavity theory. Radiat Prot Dosim. 2013;153:23–31. doi: 10.1093/rpd/ncs086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spruijt K.H., Dahele M., Cuijpers J.P. Flattening filter free vs flattened beams for breast irradiation. Int J Radiat Oncol Biol Phys. 2013;85:506–5013. doi: 10.1016/j.ijrobp.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Soleymanifard S., Aledavood S.A., Noghreiyan A.V., Ghorbani M., Jamali F., Davenport D. In vivo skin dose measurement in breast conformal radiotherapy. Wspolczesna Onkol. 2016;20:137–140. doi: 10.5114/wo.2015.54396. [DOI] [PMC free article] [PubMed] [Google Scholar]


