Abstract
Purpose
Multiple studies have reported favorable outcomes for stereotactic radiosurgery (SRS) in the treatment of limited brain metastases. An obstacle of SRS in the management of numerous metastases is the longer treatment time using traditional radiosurgery. Single-isocenter multitarget (SIMT) SRS is a novel technique that permits rapid therapy delivery to multiple metastases. There is a lack of clinical evidence regarding its efficacy and safety. We report the outcomes of patients treated with this technique.
Methods and Materials
We reviewed the records of patients with intact or resected brain metastases treated with SRS in 1 to 5 fractions using SIMT technique at our institution, with at least 1 available follow-up brain magnetic resonance imaging. Survival, disease control, and toxicity were evaluated using Cox regression, logistic regression, and Kaplan-Meier analysis.
Results
We identified 173 patients with 1014 brain metastases. Median follow up was 12.7 months. Median beam-on time was 4.1 minutes. The median dose to the brain was 219.4 cGy. Median overall survival and freedom from intracranial progression were 13.2 and 6.3 months, respectively. Overall survival did not differ between patients treated with greater than or less than 4 lesions (hazard ratio, 1.03; 95% confidence interval 0.66-1.61; P = .91). Actuarial 1- and 2-year local control were 99.0% and 95.1%, respectively. Rates of grade 2 and grade 3 or higher radionecrosis were 1.4% and 0.9%, respectively.
Conclusions
SIMT radiosurgery delivered in 1 to 5 fractions offers excellent local control and acceptable toxicity in the treatment of multiple intact and postoperative brain metastases. This technique should be evaluated prospectively.
Introduction
Brain metastases are the most common intracranial malignancy and occur in roughly 20% to 40% of patients with cancer.1, 2 Although surgery is typically reserved for solitary or symptomatic metastases, historically, whole brain radiation therapy (WBRT) has been the treatment of choice for multiple intracranial metastases. Stereotactic radiosurgery (SRS), a radiotherapeutic technique that employs high dose and target conformity, has been increasingly used as an alternative to WBRT as a means of achieving intracranial disease control while offering superior normal tissue sparing with less cognitive deterioration.3
Although multiple phase III trials have evaluated SRS in the treatment of 1 to 4 brain metastases,4, 5, 6, 7 there are limited data regarding the role of SRS for a larger number of metastases,8 in part because of the prohibitively long treatment times associated with an increasing number of lesions, each requiring additional shots or separate isocenters for gamma knife–based radiosurgery or linear-accelerator based radiosurgery, respectively.9, 10 Volumetric modulated arc therapy (VMAT) is a novel, intensity modulated radiation therapy–based plan optimization platform that permits treatment of multiple lesions using a single isocenter through modulation of gantry rotation, collimation, and dose rate.11 Although several studies have reported the dosimetric feasibility of this technique,12, 13, 14 there are little clinical data to support its safety and efficacy.15, 16 In this report, we present the results of our institutional experience using single-isocenter, multitarget (SIMT) SRS technique in the treatment of intact and postoperative brain metastases using 1 to 5 fractions.
Methods and Materials
We reviewed the records of patients with intact or resected brain metastases treated with SRS in 1 to 5 fractions using SIMT technique at our institution between 2015 and 2018, with at least 1 available follow-up brain magnetic resonance imaging (MRI) scan. This study was approved by our institutional review board (no. 2013C0145). All patients underwent computed tomography (CT) simulation in the supine position using a Qfix Encompass thermoplastic mask (QFix Inc, Avondale, PA). The CT scan with 1.25-mm slice thickness was fused with a thin-cut, gadolinium-enhanced axial T1-weighted MRI scan. For intact metastases, the planning target volume (PTV) was generated using a 2- to 3-mm expansion of the gross tumor volume, which included contrast-enhancing lesions on the T1 MRI. For postoperative brain metastases, the PTV was created from a 2- to 3-mm expansion of the clinical target volume, which included the postoperative surgical bed and any associated contrast enhancement. Typically, 2-mm expansions were used for targets placed at isocenter and 3-mm expansions were used for all other lesions to account for the added possibility of rotational error with distance from isocenter. Brain, brainstem, optic pathway, and spinal cord volumes were contoured, with additional organs at risk contoured per the discretion of the radiation oncologist, depending on tumor location.
The decision to treat with a single isocenter was made on a case-by-case basis by the treating physician and dosimetrist. Typically, lesions were treated with a single isocenter if they were no more than 4 cm apart, although the size of respective targets was also taken into consideration (eg, lesions with a large range of sizes were typically excluded from single isocenter treatment because the distinct multileaf collimator arrangements required to optimally constrict the dose for each lesion was better suited by multiple isocenters). Volumes were treated with either 18 to 24 Gy in a single fraction, 21 to 27 Gy in 3 fractions, or 25 to 30 Gy in 5 fractions. VMAT treatment plans were created using the Varian Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA) and were normalized such that a minimum of 95% of the PTV volume received 100% of the prescribed dose. Treatments were planned using 3 to 5 noncoplanar arcs using 6 MV beams with or without a flattening filter. For a relatively close group of lesions, 3 to 4 arcs using angles customized per patient were used. Otherwise, a standard 5-arc technique was used with 2 full coplanar 360° arcs and 3 half vertex arcs equidistant every 45° using couch kicks. All plans were generated using the RapidArc Progressive Resolution Optimization algorithm (version 11.0 or 13.0) in Varian Eclipse. Mid- and low-dose “arcing” between lesions was reduced using individualized planning rings to constrict 100%, 50%, and 20% isodose lines. Per departmental guidelines, dose constraints followed Task Group 101 recommendations for normal tissue constraints.17 Additional constraints included Radiation Therapy Oncology Group Conformity Index <1.05. The acceptable volume of brain receiving 12 Gy and gradient index were determined on a case-by-case basis by the treating physician.
Patients were treated using a Varian Edge linear accelerator with RapidArc technology on a robotic couch with 6 dof, with daily kV cone beam CT image guidance. In addition to daily cone beam CT alignment, a surface guided radiation therapy technology, Optical Surface Monitoring System (Varian Medical Systems), was implemented to confirm patient positioning during treatment for all fractions. Patients were followed every 2 to 3 months with a brain MRI and additionally as needed for new or worsening neurologic symptoms. All statistical analyses were performed using R.18 Overall survival was defined as the time from treatment completion to death from any cause. Local recurrence was defined as any progression of unresected brain metastases or recurrence within a postoperatively treated surgical bed that subsequently led to either retreatment with SRS or to surgical resection with resultant pathologic testing indicating predominantly viable malignant cells. This definition was favored over Response Assessment in Neuro-Oncology criteria because of the high rate of transient growth after stereotactic radiosurgery.19, 20 Radionecrosis was defined as either histologic evidence of necrosis or any new enhancement or progression of preexisting enhancement of treated lesions on MRI that stabilized on consecutive MRI scans and did not require local salvage therapy. Radionecrosis was graded according to Common Terminology Criteria for Adverse Events version 5.0 grading for central nervous system necrosis. Distant recurrence was defined as the presence of any new contrast-enhancing lesion within the brain parenchyma that either was ≥5 mm with growth on 2 consecutive follow-up brain MRIs or prompted radiotherapeutic or surgical intervention. Intracranial progression was defined as local recurrence, distant brain recurrence, or development of leptomeningeal disease. Survival, intracranial control, and local control endpoints were analyzed on a per-patient, per-treatment, and per-lesion basis, respectively. Crude and actuarial radionecrosis were evaluated on a per-treatment and per-lesion basis, respectively. Endpoints were evaluated using Cox univariate and multivariate regression, logistic regression, and Kaplan-Meier analysis.
Results
Patient cohort
We identified 173 patients with a total of 1014 intact (n = 949) or resected (n = 65) brain metastases, treated in 208 separate SIMT courses at our institution between 2015 and 2018 (Table 1). Median follow-up for living patients was 12.7 months (range, 1.5-43.0 months) and for all patients was 7.0 months (range, 0.7-43 months). All treatments used VMAT technique with noncoplanar arcs, with the exception of a single course that used noncoplanar dynamic conformal arc therapy. Treatments were delivered using a median of 5 arcs (range, 2-7), in a median of 4.1 minutes of beam-on time per fraction (range, 1.0-15.8 minutes). The median number of lesions treated per course was 3 (range, 2-30). Nearly one-half of treatments (n = 101; 48.6%) were to ≥4 lesions and 21 treatments (10.1%) targeted 10 or more lesions. The median volume of intact lesions was 0.11 cm3 (range, 0.001-41.1 cm3), corresponding to an equivalent spherical diameter of 0.6 cm (range, 0.04-4.28 cm). The median volume of postoperative cavities was 10.4 cm3 (range, 2.6-70.2 cm3), corresponding to an equivalent spherical diameter of 2.7 cm (range, 1.7-5.1 cm). The majority of treatments were the first SRS course received by the patient (n = 127; 61.1%). For the remaining treatments, the median number of prior SRS courses received was 1 (range, 1-5), and the median number of lesions previously treated with SRS was 4 (range, 1-30). A total of 37 of the 208 (17.8%) SIMT courses were performed as a salvage therapy in patients who had received prior WBRT. In 27 treatments (13.0%), there was simultaneous treatment to another intracranial target using a separate isocenter.
Table 1.
Patient and treatment characteristics of cohort
| All patients (N = 173) | |
|---|---|
| Age | |
| Median (IQR) | 61 (52-68) |
| Sex | |
| Male | 77 (44.5%) |
| Female | 96 (55.5%) |
| Race | |
| White | 153 (88.4%) |
| Black | 15 (8.7%) |
| Other | 5 (2.9%) |
| KPS | |
| Median (IQR) | 80 (70-90) |
| GPA | 2 (1.5-2.5) |
| Median (IQR) | |
| Primary tumor site | |
| Lung | 77 (44.5%) |
| Breast | 33 (19.1%) |
| Genitourinary | 9 (5.2%) |
| Melanoma | 24 (13.9%) |
| Head and neck | 3 (1.7%) |
| Gynecologic | 6 (3.5%) |
| Gastrointestinal | 10 (5.8%) |
| Sarcoma | 3 (1.7%) |
| Other | 8 (4.6%) |
| Total no. of SRS courses | |
| Median (range) | 1 (1-9) |
| Total no. of brain metastases treated with SRS | |
| Median (range) | 5 (2-58) |
| All SIMT SRS courses (N = 208) | |
|---|---|
| Dose and fractionation | |
| 18-24 Gy × 1 fraction | 93 (44.7%) |
| 7-9 Gy × 3 fractions | 82 (39.4%) |
| 5-6 Gy × 5 fractions | 33 (15.9%) |
| Beam energy | |
| 6 MV | 109 (52.4%) |
| 6 MV-FFF | 91 (43.8%) |
| 10 MV-FFF | 8 (3.8%) |
| No. of target lesions | |
| Median (range) | 3 (2-30) |
| Prior WBRT | |
| No | 171 (82.2%) |
| Yes | 37 (17.8%) |
Abbreviations: FFF = flattening filter-free; GPA = graded prognostic assessment; IQR = interquartile range; KPS = Karnofsky performance status; SIMT = single-isocenter multitarget; SRS = sterotactic radiosurgery; WBRT = whole brain radiation therapy.
Survival and disease control
Figure 1A shows the Kaplan-Meier curve for overall survival of the entire cohort of patients. Median, 1-year, and 2-year overall survival were 13.2 months (95% confidence interval [CI], 8.5-18.7 months), 53.5% (95% CI, 45.9%-62.3%), and 34.2% (95% CI, 26.1%-44.8%), respectively. In the patients who died, the rate of neurologic death was 16.5%. On univariate analysis, overall mortality was associated with age (hazard ratio [HR], 1.02; 95% CI 1.00-1.0; P = .019), low graded prognostic assessment (HR, 1.45; 95% CI, 1.12-1.85; P = .003), head and neck histology (HR, 5.02; 95% CI, 1.51-16.71; P = .009), and gastrointestinal histology. There was a trend toward association of overall mortality with male sex (HR, 1.47; 95% CI, 0.97-2.22; P = .069), whereas there was no association with total target volume (P = .43), number of target lesions (P = .81), or postoperative SRS (P = .37). Overall survival did not differ between patients treated to 4 or fewer lesions versus greater than 4 (HR = 1.03 l 95% CI 0.66-1.61; P = .91). On multivariate analysis, low graded prognostic assessment, head and neck histology, and gastrointestinal histology maintained statistically significant associations with overall mortality (Table E1; available online at https://doi.org/10.1016/j.adro.2019.08.013.)
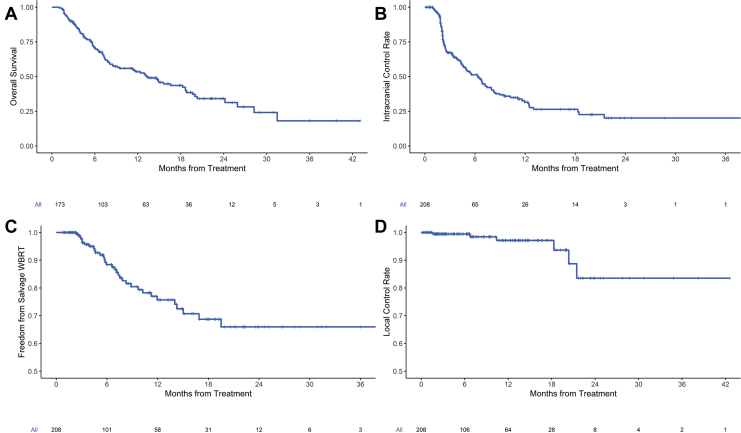
Figure 1.
Kaplan-Meier curves for (A) overall survival for all patients, (B) intracranial control for all treatments, (C) freedom from salvage whole brain radiation therapy (WBRT) for all treatments, and (D) local control for all lesions.
Figure 1B shows Kaplan-Meier curves for intracranial control for all SIMT SRS courses. Median freedom from intracranial progression was 6.3 months (95% CI, 4.6-8.0 months), and 1-year and 2-year freedom from intracranial progression were 31.4% and 20.1%, respectively. One- and 2-year freedom from salvage WBRT were 75.7% and 66.0%, respectively (Fig 1C). Additionally, 1- and 2-year freedom from leptomeningeal disease were 89.4% and 87.4%, respectively (Fig E1; available online at https://doi.org/10.1016/j.adro.2019.08.013). The crude rate of posttreatment leptomeningeal disease was 7.2%. In the patients who developed leptomeningeal disease, the median time to leptomeningeal disease was 5.3 months.
Of the 1014 treated lesions, there were only 7 local recurrences, 2 of which were in the same treatment course, yielding a crude per-lesion local recurrence rate of 0.69% and crude per-treatment local recurrence rate of 2.9%. Actuarial 1-year and 2-year local recurrence rates were 99.0% and 95.1%, respectively, for all lesions (Fig 1D). Table E2 (available online at https://doi.org/10.1016/j.adro.2019.08.013) shows the characteristics of the treatments that had local failure of a lesion. The median time to local failure was 10.4 months (range, 8.6-19.3 months). Salvage surgery was performed for 3 recurrences, and the remainder were treated with salvage SRS. On logistic regression, there was an association of local recurrence with total gross tumor/cavity volume of all treated lesions (odds ratio [OR], 1.04; 95% CI, 1.01-1.06; P = .002), however there was no association of local recurrence with individual gross tumor volume/cavity volume (OR = 1.00; 95% CI 0.78-1.08; P = .994), dose per fraction (OR, 0.89; 95% CI 0.70-1.02; P = .18), or postoperative SRS (OR, 2.46; 95% CI, 0.13-14.67; P = .41).
Toxicity
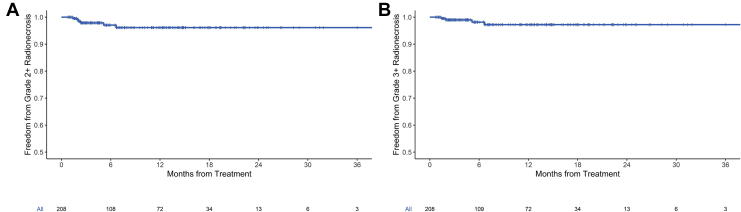
The median minimum dose to the brain was 23.9 cGy (range, 0.7-449.6 cGy) and mean dose to the brain minus PTV was 219.4 cGy (range, 46.4-1050.9 cGy). The mean dose to the brain minus PTV for 1-, 3-, and 5-fraction regimens was 147.4 cGy (range, 46.4-446.4 cGy), 294.6 cGy (84.8-878.9 cGy), and 329 cGy (112.3-1050.0 cGy), respectively (Kruskal-Wallis P < .001). Actuarial 1-year freedom from grade 2 + radionecrosis and freedom from grade 3 + radionecrosis were 96.1% and 97.2%, respectively, for the 208 treatment courses (Fig 2).
Figure 2.
Kaplan-Meier curves for (A) freedom from grade 2 + radionecrosis and (B) freedom from grade 3 + radionecrosis for all treatments.
Grade 2 or higher (grade 2+) and grade 3 or higher (grade 3+) radionecrosis occurred in 14 (crude rate 1.4%) and 9 (crude rate 0.9%) of the 1014 treated lesions, respectively. Prior WBRT was significantly associated with development of lesional grade 2+ (OR, 1.04; 95% CI, 1.02-1.06; P < .001) and grade 3 + radionecrosis (OR, 1.03; 95% CI, 1.01-1.05; P < .001).
Discussion
To our knowledge, this is the first study of clinical outcomes in patients treated with single-fraction and multifraction, single-isocenter, multitarget stereotactic radiosurgery to intact and postoperative brain metastases. We found that this technique is associated with high local control and acceptable rates of radionecrosis. Additionally, the patients in this cohort had favorable overall survival, with relatively low rates of salvage WBRT and leptomeningeal disease progression.
Multiple studies have found that focal SRS offers comparable local control with less neurocognitive deterioration compared with WBRT, particularly with a limited number of brain metastases.3, 5, 6, 7, 21, 22 This has led to the adoption of SRS as the preferred modality in patients with 1 to 4 brain metastases, and otherwise controlled systemic disease, in national consensus guidelines.23 Although select studies have prospectively evaluated the efficacy of SRS in the management of greater than 3 metastases,8 long treatment times associated with traditional SRS technique have impeded the evaluation and application of SRS in this setting.
Several studies have found that VMAT is associated with high target conformity and relatively rapid treatment time compared with 3-dimensional conformal and intensity modulated radiation therapy in the treatment of prostate and cervical malignancies and benign intracranial tumors.24, 25, 26, 27, 28, 29 Compared with traditional gamma knife SRS or multi-isocenter linear accelerator-based SRS, which typically require 20 minutes per lesion, VMAT-based radiosurgery using 7 arcs can deliver focal treatment to numerous brain metastases in 20 minutes.14, 30
This potential advantage in treatment efficiency has prompted dosimetric feasibility studies finding target conformity comparable to multi-isocentric treatment.14, 31 Nevertheless, dose conformity and other dose parameters do not necessarily correlate with clinical toxicity,32 and clinically meaningful endpoints should be evaluated before the large-scale adoption of any technique. Two prior studies, one with 15 patients16 and the other with a subset of 55 patients15 treated with single-isocenter SRS, suggested outcomes comparable to conventional radiosurgery. However, these studies had small numbers of patients and lacked data on postoperative and multiple-fraction treatment, respectively. These exclusions limit the generalizability of these studies, particularly as the benefits of surgical resection and fractionation for larger tumors are becoming increasingly realized.20, 33
The hesitancy to employ VMAT-based SRS planning largely stems from theoretical concerns that such planning may be associated with diminished dose conformity and robustness versus rotational errors,12 in addition to reduced dose heterogeneity in the tumor, which is thought to be beneficial for local control.34 In our study, patients had overall survival and local control comparable to modern-day studies, in addition to relatively low rates of salvage WBRT and leptomeningeal disease progression.8, 22, 35 Although a significant proportion of our cohort received either prior SRS or WBRT, the crude rate of symptomatic radionecrosis remained considerably less than 10%, commensurate with the toxicity noted in other radiosurgical reports.36, 37, 38 Thus, the abbreviated treatment time associated with SIMT SRS does not appear to come at the expense of meaningful clinical outcomes, notably under conditions of robust image and surface guidance that ensure reproducibility of patient setup. Nevertheless, these results come with the caveats inherent to single-institution, retrospective studies, and this technique should be studied prospectively. Additionally, we acknowledge that a detailed analysis of dosimetry and plan quality indices, correlating with the clinical outcomes in this study, would be further beneficial in mitigating the concerns regarding the theoretical dosimetric limitations of VMAT-based planning.
In conclusion, single-isocenter multitarget stereotactic radiosurgery technique, in 1 to 5 fractions, offers high local control with acceptable rates of radionecrosis in the management of multiple intact or postoperative brain metastases. Thus, this technique likely offers a more convenient alternative to gamma knife or multi-isocentric stereotactic radiosurgery and merits comparison with WBRT in the management of numerous (>4) brain metastases. These findings warrant evaluation of SIMT SRS in a prospective cohort such as the Canadian Cancer Trials Group Ce.7 (NCT03550391) randomized trial.
Footnotes
Sources of Support: This work had no specific funding.
Disclosures: Dr Palmer declares grant funding from Varian Medical Systems. Dr Brown reports personal fees from UpToDate (contributor) outside the submitted work.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2019.08.013.
Supplementary data
References
- 1.Patchell R.A. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 2.Bradley K.A., Mehta M.P. Management of brain metastases. Semin Oncol. 2004;31:693–701. doi: 10.1053/j.seminoncol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama H., Shirato H., Tago M. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 6.Chang E.L., Wefel J.S., Hess K.R. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 7.Kocher M., Soffietti R., Abacioglu U. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2010;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto M., Serizawa T., Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 9.Johnson P.B., Monterroso M.I., Yang F. Optimization of the prescription isodose line for Gamma Knife radiosurgery using the shot within shot technique. Radiat Oncol. 2017;12:187. doi: 10.1186/s13014-017-0919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L., Nichol A., Hossain S. Variable dose interplay effects across radiosurgical apparatus in treating multiple brain metastases. Int J CARS. 2014;9:1079–1086. doi: 10.1007/s11548-014-1001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 12.Roper J., Chanyavanich V., Betzel G. Single-isocenter multiple-target stereotactic radiosurgery: Risk of compromised coverage. Int J Radiat Oncol Biol Phys. 2015;93:540–546. doi: 10.1016/j.ijrobp.2015.07.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzell G.A. The spatial accuracy of two frameless, linear accelerator-based systems for single-isocenter, multitarget cranial radiosurgery. J Appl Clin Med Phys. 2017;18:37–43. doi: 10.1002/acm2.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark G.M., Popple R.A., Young P.E. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys. 2010;76:296–302. doi: 10.1016/j.ijrobp.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Limon D., McSherry F., Herndon J. Single fraction stereotactic radiosurgery for multiple brain metastases. Adv Radiat Oncol. 2017;2:555–563. doi: 10.1016/j.adro.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau S.K.M., Zakeri K., Zhao X. Single-isocenter frameless volumetric modulated arc radiosurgery for multiple intracranial metastases. Neurosurgery. 2015;77:233–240. doi: 10.1227/NEU.0000000000000763. discussion 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/. Accessed July 2018.
- 19.Ruzevick J., Kleinberg L., Rigamonti D. Imaging changes following stereotactic radiosurgery for metastatic intracranial tumors: Differentiating pseudoprogression from tumor progression and its effect on clinical practice. Neurosurg Rev. 2014;37:193–201. doi: 10.1007/s10143-013-0504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhu R.S., Press R.H., Patel K.R. Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: A multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99:459–467. doi: 10.1016/j.ijrobp.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Tsao M., Xu W., Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 22.Brown P.D., Ballman K.V., Cerhan J.H. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network Central nervous system cancers (version 2.2018) Available at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- 24.Palma D., Vollans E., James K. Volumetric modulated arc therapy for delivery of prostate radiotherapy: Comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 25.Cozzi L., Dinshaw K.A., Shrivastava S.K. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 2008;89:180–191. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Fogliata A., Clivio A., Nicolini G. Intensity modulation with photons for benign intracranial tumours: A planning comparison of volumetric single arc, helical arc and fixed gantry techniques. Radiother Oncol. 2008;89:254–262. doi: 10.1016/j.radonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P., Happersett L., Hunt M. Volumetric modulated arc therapy: Planning and evaluation for prostate cancer cases. Int J Radiat Oncol Biol Phys. 2010;76:1456–1462. doi: 10.1016/j.ijrobp.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Yoo S., Wu Q.J., Lee W.R. Radiotherapy treatment plans with RapidArc for prostate cancer involving seminal vesicles and lymph nodes. Int J Radiat Oncol Biol Phys. 2010;76:935–942. doi: 10.1016/j.ijrobp.2009.07.1677. [DOI] [PubMed] [Google Scholar]
- 29.Wolff D., Stieler F., Welzel G. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93:226–233. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Roa D.E., Schiffner D.C., Zhang J. The use of RapidArc volumetric-modulated arc therapy to deliver stereotactic radiosurgery and stereotactic body radiotherapy to intracranial and extracranial targets. Med Dosim. 2012;37:257–264. doi: 10.1016/j.meddos.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Wu Q., Snyder K.C., Liu C. Optimization of treatment geometry to reduce normal brain dose in radiosurgery of multiple brain metastases with single-isocenter volumetric modulated arc therapy. Sci Rep. 2016;6:34511. doi: 10.1038/srep34511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura J.L., Verhey L.J., Smith V. Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys. 2001;51:1313–1319. doi: 10.1016/s0360-3016(01)01757-6. [DOI] [PubMed] [Google Scholar]
- 33.Lehrer E.J., Peterson J.L., Zaorsky N.G. Single versus multifraction stereotactic radiosurgery for large brain metastases: An international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys. 2019;103:618–630. doi: 10.1016/j.ijrobp.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Lucia F., Key S., Dissaux G. Inhomogeneous tumor dose distribution provides better local control than homogeneous distribution in stereotactic radiotherapy for brain metastases. Radiother Oncol. 2019;130:132–138. doi: 10.1016/j.radonc.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Mahajan A., Ahmed S., McAleer M.F. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 37.Blonigen B.J., Steinmetz R.D., Levin L. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.M., Miller J.A., Kotecha R. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol. 2017;133:357–368. doi: 10.1007/s11060-017-2442-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.