Abstract
Purpose
To assess the efficacy of placing a polyethylene glycol (PEG) spacing hydrogel in patients undergoing proton beam radiation therapy for prostate cancer. This study also aims to assess the effect on rectal radiation dose of prostate–rectum separation in various anatomic planes.
Methods and Materials
Seventy-two consecutive prostate cancer patients undergoing conventionally fractionated pencil beam scanning proton radiation therapy with and without hydrogel placement were compared. Magnetic resonance images taken after hydrogel placement measured prostate–rectum separation and were correlated to rectal dosing and rectal toxicity. Univariate analysis of clinical variables and radiation dosing was conducted using nonparametric Wilcoxon rank-sum test with continuity correction between groups (hydrogel spacer vs controls). Spearman's rank correlation coefficient assessed relationships between the various anatomic dimensions of perirectal space and rectal radiation dosing.
Results
Fifty-one patients had hydrogel placement before therapy and 21 did not. There was a 42.2% reduction in rectal dosing (mL3 rectum) in hydrogel patients (P < .001). Increasing midline sagittal lift resulted in a greater mitigation of total rectal dose (P = .031). The degree of prostate surface area coverage on coronal plane did not correlate with further reductions in rectal radiation dose (P = .673). Patients who had PEG hydrogels placed reported more rectal side effects during treatment compared with those patients who did not (35.3% vs 9.5%, P = .061). At median 9.5-month follow-up, there was no difference in reporting of grade ≤2 rectal toxicity between the 2 groups (7.7% vs 7.1%, P = .145).
Conclusions
Polyethylene glycol hydrogel placement before pencil proton beam radiation therapy for prostate cancer reduced rectal radiation dose. The most important factor reducing total rectal dose was the degree of sagittal midline separation created by the PEG hydrogel. This is the largest study with the longest follow-up to investigate hydrogel placement in the proton beam radiation setting.
Introduction
Polyethylene glycol (PEG) hydrogel (SpaceOAR, Augmenix, Bedford, MA) is a slowly resorbing hydrogel injected into Denonvillier's space before external beam radiation (EBRT) for prostate cancer to limit radiation exposure to the rectum. The gel undergoes hydrolysis and dissolves by 6 to 12 months1,2 and was approved by the US Food and Drug Administration (FDA) in 2015.3 Most large series published to date focus on patients undergoing conventional photon beam EBRT2 where utilization of this adjunct has become commonplace.
Displacement of the rectal wall from the prostate by approximately 1 cm allows for a reduction of rectal radiation dose up to 60.6%.1 Recognition of this fact has led to deliberate (saline hydrodissection) efforts to create the space between the prostate and rectum with the hydrogel.
Proton beam radiation therapy (PBRT) is available as an option for men with prostate cancer who choose definitive radiation treatment.4 Proton beams release energy at the Bragg peak, resulting in the benefit of limiting the dose to normal tissue with potential improvement in side effects.4 The rectum has been identified as the dose-limiting collateral structure for traditional photon beam prostate radiation therapy.5 In a randomized trial comparing conventional dose PBRT and high-dose PBRT, Zietman et al reported acute grade 2 or higher gastrointestinal (GI) toxicity as 45% and 64%, respectively.6 Currently, the Prostate Advanced Radiation Technologies Investigating Quality of Life (PARTIQoL) trial (NCT01617161) is ongoing to compare proton therapy to intensity-modulated radiation therapy (IMRT) for low or intermediate risk prostate cancer, with change in bowel function as the primary endpoint. No randomized trials have compared the efficacy and toxicity of PBRT to photon EBRT. Given the proposed advantage of PBRT, the additional benefit of perirectal spacing hydrogel has been questioned. Analysis of Medicare data has previously demonstrated comparatively increased rates of rectal toxicity in PBRT for prostate cancer compared with IMRT during its early adoption,7,8 and hence there is justification for the use of a perirectal spacing agent in this setting.
This is the largest series with the longest follow-up comparing patients with prostate cancer undergoing pencil scanning PBRT with and without placement of PEG hydrogel. The purpose was to determine the effect of the PEG hydrogel on rectal proton beam radiation exposure and relationship between exposure and the degree of prostate–rectum separation. Additional aims were to determine the subsequent effect of PEG hydrogel placement on reducing rectal toxicities from proton beam radiation at early patient follow-up.
Materials and Methods
After institutional review board approval, a retrospective review of patients who underwent conventionally fractionated pencil beam scanning proton EBRT with and without PEG hydrogel placement at Mayo Clinic Arizona was performed.
Patients were positioned in dorsal lithotomy under general anesthesia and 4 carbon fiducial markers were inserted into the prostate. A 15-cm, 18-gauge bevel needle was used to hydro-dissect the potential Denonvillier's space with minimal normal saline (<2-3 mL) to confirm good needle position before the hydrogel was injected transperineally. A brachytherapy step device was used to hold the rectal ultrasound probe (BK 3000, BK Ultrasound, Peabody MA), and a side-firing biplanar transrectal ultrasound provided real-time visualization ensuring accuracy of hydrogel placement in axial and sagittal planes. A minimum of 1 vial of PEG hydrogel (10 mL) was injected in each patient per manufacturer recommendations.
PBRT was delivered with 2 lateral fields and with 67.5 to 79 Gy in 25 to 44 fractionations depending on the clinical situation. All patients had an endorectal balloon filled with 100 mL of water for each treatment cycle to limit natural rectal displacement.
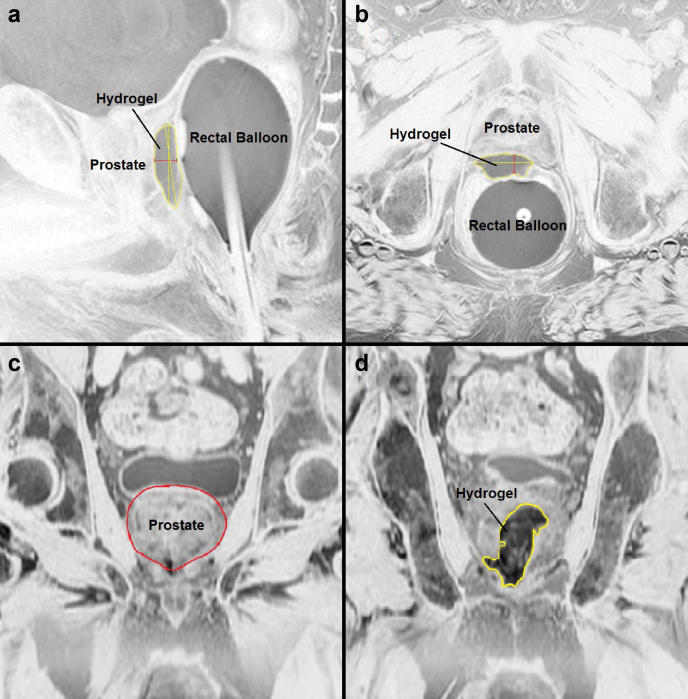
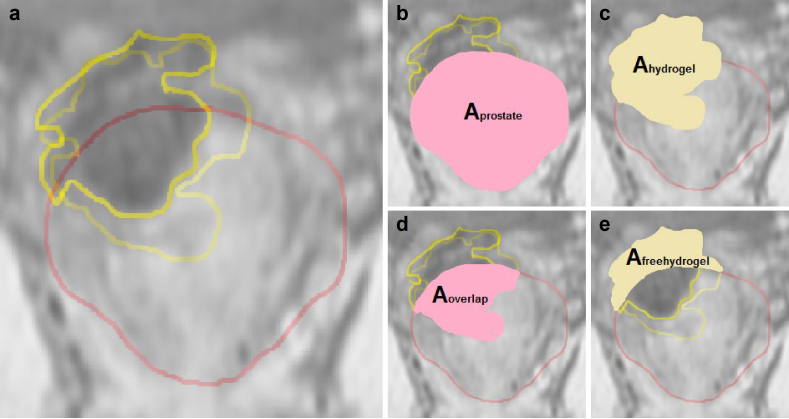
All patients had a post hydrogel MRI on a 3T platform without an endorectal coil within 7 days to confirm location of the PEG hydrogel and fiducial markers. The following measurements were obtained: (1) Hydrogel thickness: this was measured at the midline of prostate gland on both sagittal and axial images (Fig 1a-b). (2) Maximal surface area of the prostate (AProstate): This was obtained using a reference plane that was 3.51 mm (3 1.17 mm slices on MRI) anterior to the PEG hydrogel (Fig 1c). (3) Maximal contact surface area of the PEG hydrogel (AHydrogel): This was an average of the 2 largest surface area measurements of the PEG hydrogel from coronal images. (4) The difference between points 2 and 3 above and the percentage of prostate coverage were calculated (AOverlap; Fig 2d). Coronal cross-sectional images of the hydrogel and the prostate were superimposed to determine coverage of the prostate using Adobe Photoshop (Adobe Systems, San Jose, CA; Fig 2). Measurements were verified by a single radiologist with expertise in prostate MRI and imaging processing techniques.
Figure 1.
Magnetic resonance images demonstrating measurements of polyethylene glycol hydrogel taken. (a) Sagittal thickness of hydrogel in midline of prostate. The red line represents thickness measurement in cm. (b) Axial thickness of hydrogel in midline of prostate. The red line represents thickness measurement in centimeters. (c) Coronal measurement of prostate surface area. The measurement taken 3.51 mm anterior to hydrogel and red line represents area used for surface area estimation. (d) Coronal measurement of polyethylene glycol Hydrogel maximal surface area. The yellow line represents area used for surface area estimation.
Figure 2.
Comparison of Hydrogel placement compared to area under the curve (AUC) rectal radiation dosage (cc3.Gy) with Spearman rank correlation calculation (rho). (a) Sagittal thickness of hydrogel in midline (cm) versus AUC (P = .031). (b) Axial thickness of hydrogel in midline (cm) versus AUC (P = .222). (c) Percentage of prostate coverage or overlap (Aoverlap) in the coronal plane versus AUC (P = .673).
The effect of PEG hydrogel placement on rectal radiation exposure was calculated using area under the curve (AUC) for the histogram data of each patient to determine the overall rectal dose based on each patient's individual dosimetry histogram data (V40 Gy, V50 Gy, V60 Gy, V65 Gy, V70 Gy, and V75 Gy; V indicates the volume of rectum receiving specific radiation dose, eg, V40 Gy is the volume of rectum receiving 40 Gy of radiation). Although previous literature focused on the volume of rectum exposed to the highest recorded radiation dose,2 the AUC calculation allowed us to estimate overall dosage received by the rectum (Figure E1; available online at https://doi.org/10.1016/j.adro.2019.08.007). Rectal toxicity was graded prospectively by either the clinic nurse or physician at the time of patient follow-up. The follow-up protocol was not standardized and was based on the National Comprehensive Cancer Network guidelines. Toxicities were graded prospectively by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.9
Median and interquartile range values described patient demographic and clinical characteristics. Maximum grade toxicity was compared between groups by use of χ2 or Fisher exact test. Univariate analysis of clinical variables and radiation dosing (ie, AUC) was conducted using nonparametric Wilcoxon rank-sum test with continuity correction between groups (hydrogel spacer versus controls). Among hydrogel spacer patients, Spearman's rank correlation coefficient was used to assess relationships between the various dimension of perirectal space and rectal radiation dosing. R version 3.4.2 (R Software, Open Source) was used for analysis. P values < .05 were considered statistically significant.
Results
Seventy-two consecutive patients with prostate cancer treated with pencil scanning PBRT from January 2016 to August 2017. Fifty-one patients received PEG hydrogel before therapy compared with 21 patients who did not. Table 1 details baseline patient characteristics. There were no differences between the 2 groups at median follow-up of 9.5 (8.6-11.5) months after treatment.
Table 1.
Baseline patient characteristics
| PEG hydrogel placement (n = 51) | Controls (n = 21) | P Value | |
|---|---|---|---|
| Median age (IQR) | 73.9 (70.0-78.0) | 74.9 (73.0-78.05) | .291 |
| Median BMI (IQR) | 26.7 (4.8-30.1) | 26.3 (24.9-30.0) | .78 |
| Median PSA (ng/mL) (IQR) | 6.9 (4.5-10.2) | 9.7(4.8-12.4) | .222 |
| Patient on ADT | 26 (n = 40, 65%) | 19 (n = 10, 95%) | .027 |
| Gleason score | .361 | ||
| 3 + 3 | 10 (19.6%) | 1 (4.8%) | |
| 3 + 4 | 15 (29.4%) | 4 (19.0%) | |
| 4 + 3 | 15 (29.4%) | 8 (38.1%) | |
| 4 + 4 | 7 (13.7%) | 6 (28.6%) | |
| 4 + 5 | 2 (3.9%) | 2 (9.5%) | |
| 5 + 4 | 1 (2.0%) | 0 | |
| 5 + 5 | 1 (2.0%) | 0 | |
| Clinical stage | .65 | ||
| T1 (T1a, T1b, T2c) | 22 (43.1%) | 8 (38.1%) | |
| T2 | 24 (47.1%) | 12 (57.1%) | |
| T3 (T3a, T3b) | 5 (9.8%) | 1 (4.8%) | |
| Median radiation dose delivered Gy (IQR) | 79.2 (79.2-79.2) | 79.2 (79.2-79.2) | .621 |
| Median no. of fractions delivered | 44.0 (44.0-44.0) | 44.0 (44.0-44.0) | .786 |
Abbreviations: ADT = androgen deprivation therapy; BMI = body mass idex; IQR = interquartile ratio; PEG = polyethylene glycol; PSA = prostate-specific antigen.
Forty-seven (92.2%) patients had 1 hydrogel vial (10 mL) injected, and 4 (7.8%) patients had 2 vials injected. Mean volume of hydrogel injected was 10.75 ± 2.67 mL. All patients had concurrent placement of 4 carbon fiducial markers. No patients experienced any immediate complications.
Median midline separation produced by PEG hydrogel was 10.5 mm (9.4-12.1) and 10.1 mm (8.6-11.42) on sagittal and axial planes, respectively. Only 32 patients had the necessary coronal images to calculate coverage of prostate by the PEG hydrogel in the coronal plane. Median percentage of prostate covered by the PEG hydrogel in coronal plane (Aoverlap) was 49.0% (39.9-57.7).
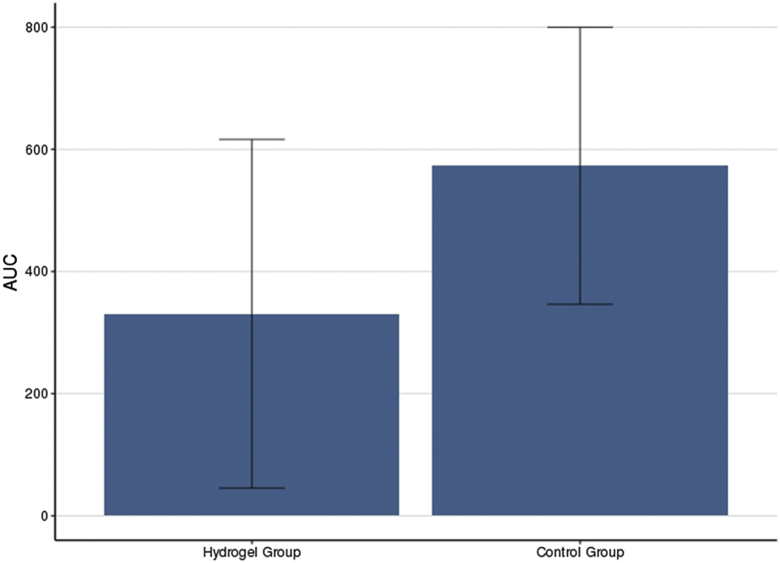
Patients who had PEG hydrogel placement had reduced radiation dose per volume of rectum at all histogram dosimetry levels compared with those with no PEG hydrogel (P < .001; Figure E2; available online at https://doi.org/10.1016/j.adro.2019.08.007). Volume of rectum exposed was reduced by 42.2% and 41.8% for V70 Gy and V75 Gy, respectively. AUC analysis also demonstrated a 42.2% relative reduction in overall rectal dose delivery in hydrogel patients (P < .001; Fig 3).
Figure 3.
Area under curve (AUC) calculation of overall rectal radiation dose (cc3.Gy) in patients with and without (control) Hydrogel placement. Median Hydrogel AUC was 330.90 (IQR, 136.5-421.9). Median control group AUC was 572.97 (IQR, 494.3-721.0). There was a 42.2% relative reduction in the Hydrogel group. Wilcoxon rank sum test with continuity correction was used (P < .001).
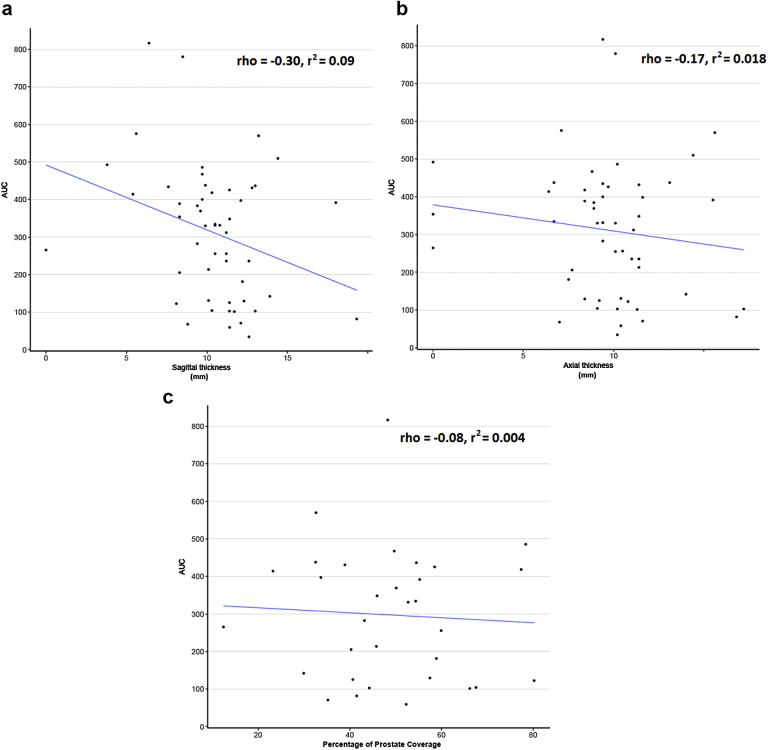
When overall rectal dosimetry based on AUC calculation was compared with prostate–rectum separation measurements, greater mitigation of rectal dosing was seen with increased midline prostate–rectum separation on sagittal MRI (P = .031). Increased coverage of the prostate by PEG hydrogel in the coronal plane was not associated with reduced rectal radiation dose based on our measurements (Fig 4).
Figure 4.
Comparison of Hydrogel placement with area under the curve (AUC) rectal radiation dosage (cc3.Gy) with Spearman rank correlation calculation (rho). (a) Sagittal thickness of hydrogel in midline (cm) versus AUC (P = .031). (b) Axial thickness of hydrogel in midline (cm) versus AUC (P = .222. (c) Percentage of prostate coverage/overlap (AOverlap) in coronal plane versus AUC (P = .673).
Patients with hydrogel placement reported more toxicity during treatment compared with controls. At least one grade 1 acute rectal toxicity was experienced in 35.3% of hydrogel patients compared with 9.5% of controls (P = .061) during the course of therapy.
With longer follow-up (median 10.3 vs 8.7 months, hydrogel vs control), the overall reporting of rectal toxicity was not different between the 2 groups (Table 2). There was no association between rectal toxicity at any point and PEG hydrogel measurements.
Table 2.
Reported rates of rectal toxicity during treatment and then at subsequent follow-up (at completion of radiation therapy) in PEG Hydrogel versus control group
| Hydrogel group | Control group | P Value | |
|---|---|---|---|
| During treatment∗ | |||
| No. of patients | 51 | 21 | .061 |
| No toxicity | 32 (62.7%) | 19 (90.5%) | |
| Grade 1 rectal toxicity | 18 (35.3%) | 2 (9.5%) | |
| Grade 2 rectal toxicity | 1 (2%) | 0 | |
| Subsequent to treatment† | |||
| Median follow-up time (IQR) (mo) | 10.3 (9.02-11.7) | 8.7 (7.5-9) | .004 |
| No. of patients | 39 | 14 | .145 |
| No toxicity | 36 (92.3%) | 13 (92.9%) | |
| Grade 1 rectal toxicity | 3 (7.7%) | 0 (0%) | |
| Grade 2 rectal toxicity | 0 (0%) | 1 (7.1%) |
Abbreviations: IQR = interquartile ratio; PEG = polyethylene glycol.
No patients experienced grade ≥3 rectal toxicity.
No patients experienced grade ≥2 rectal toxicity.
Discussion
The success of PEG hydrogel spacers in significantly reducing short- to medium-term rectal toxicity has been well documented in the photon EBRT setting.2 In this pivotal trial that led to the approval of hydrogel by the FDA, no rectal balloon was used. Placement of hydrogel is well tolerated, improves GI quality of life scores at 5 years, and has been shown to be cost effective.10,11 PBRT alone has previously demonstrated low rates of rectal toxicity even without the use of a spacer.12 The added benefit of placing a PEG hydrogel spacer in this setting has not been well investigated and can be questioned given the theorized reduction in collateral radiation from proton beam radiation. Only a single small series of 12 patients has previously demonstrated a relative reduction in V70 Gy rectal radiation dose of 63% and 70% based on 2 separate proton radiation plans using an alternative PEG Hydrogel (Duraseal, Covidien, Mansfield, MA). We report the largest study to directly compare PBRT patients treated with and without PEG hydrogel placement.
Our study demonstrated a 42.2% relative reduction of rectal exposure in PBRT patients with the use of the hydrogel spacer during treatment. Each rectal dosimetry level demonstrated significantly less radiation exposure. This is consistent with previously published results in the photon EBRT population, which have demonstrated a relative rectal dose reduction of 25% to 59% in patients who had PEG Hydrogel placed before therapy.13, 14, 15
A median prostate–rectum separation at midline of 10.5 mm on sagittal imaging was achieved in the hydrogel group and was positively correlated with dose reduction. A statistically significant association was not seen between dose reduction and length of separation on axial images. Pinkawa et al have previously demonstrated that there was a learning curve required to obtain up to 15 mm of separation from of the prostate to the anterior rectal wall.16 Preclinical studies have shown that no further reduction in V70 Gy occurred with separation >15 mm after 20 mL of hydrogel injection.17 Furthermore, these results are consistent with data in the photon EBRT population demonstrating separation of 10 to 13 mm is most effective at reducing rectal exposure.1, 2 Cadaver and small clinical series of treatment planning with PBRT did demonstrate that 7 to 9 mm of prostate–rectum separation can result in reduced rectal radiation dose.18,19
Coronal coverage maps demonstrated that a median of 49.0% of the contact surface area of the prostate was covered by the PEG hydrogel. Often, <50% of the contact surface of the prostate was overlapped by the PEG hydrogel in the coronal plane due to displacement of the hydrogel away from the midline. This suggests that the hydrodissection of the potential space of Denonvillier's may not be predictable or that hydrogel placement is more unpredictable than wanted. It was anticipated that the degree of prostate covered by the hydrogel on coronal imaging would correlate with a more uniform separation of the prostate from the rectum and greater rectal dose reduction; our study did not demonstrate this association. This questions the necessity of ensuring even distribution of hydrogel in the perirectal space rather than focusing on the goal of adequate lift of the prostate at midline.
No relationship was found between the thickness of prostate–rectum separation at midline on imaging and rectal toxicity reported either during treatment or on follow-up. Although increasing thickness of separation may improve dosimetry scores, its clinical effect remains uncertain. Mariados et al demonstrated that hydrogel absorption began at 3 months and completely resolved by 12 months.2 It is difficult to assess the clinical effect of initial separation given the variable rates of absorption occurring between patients owing to differences in renal clearance and proteolytic resistance.20
During treatment, greater rectal toxicity was reported in patients with PEG hydrogel placement than without. It must be acknowledged that this is a near-significant approximate 4-fold increase of in-treatment rectal toxicity in those patients who received PEG hydrogel. None of these patients needed intervention for these side effects and all toxicities were grade 1 and did not require any intervention. This increased propensity for hydrogel patients to experience rectal side effects may be due to interaction between the rectal balloon and PEG hydrogel in place concurrently with each radiation treatment (Fig 1a-b demonstrating proximity of the 2 structures). Rectal balloons have been used in PBRT to reduce variability in prostate position given the increased sensitivity of PBRT to target motion owing to steep dose depletion beyond the Bragg peak.4 We have used an endorectal balloon for PBRT patients as a method of reducing intrafraction movement, extrapolating from previous studies of IMRT for prostate cancer.21,22 Small studies have described patients complaining about local side effects from PEG hydrogel placement. A recent retrospective review of 125 patients undergoing photon EBRT revealed an increased rate of hemorrhoids in patients who had hydrogel placement.23 Another study described transient increase in rectal discomfort in 4 of 11 patients who received PEG hydrogel before photon EBRT. These side effects resolved within 12 weeks of hydrogel placement.24 Interaction of the PEG hydrogel and rectal balloon on the anterior rectal wall may represent a side effect of the hydrogel as opposed to true radiation toxicity. It is also possible that Common Terminology Criteria for Adverse Events, version 4 is not sensitive enough to differentiate rectal discomfort secondary to the simultaneous use of the hydrogel and rectal balloon, and the discomfort was graded as proctitis at the weekly management visit during the treatment course. The concurrent placement of a PEG hydrogel and endorectal balloon requires more investigation of its effect on intrafraction prostate movement.
Fifty-three patients (73.6%) had completed follow-up at a median time of 9.5 (8.6-11.5) months. Of these, only 7.5% experienced grade ≤2 rectal toxicity. There was no difference in rectal toxicity between the 2 groups at early follow-up. The median follow-up time for the hydrogel group was 1.6 months longer than the control group. A possible explanation is that the physical discomfort caused by the hydrogel and rectal balloon resolved after resorption of the hydrogel in 3 to 6 months. Large series of PBRT patients without hydrogel has variable reported rectal toxicity rates ranging from 0% to 64%.6,25 The pivotal randomized controlled trial that led to FDA approval of PEG hydrogel in photon EBRT demonstrated no difference in acute toxicity rates (<3 months after therapy) with 23% and 28% in hydrogel and control groups, respectively. However, at late follow-up (>3 months), there was a significant reduction in rectal toxicity (grade ≤2) in those who had hydrogel placement of 7% versus 2%.2 This study demonstrates no difference in rectal toxicity rates on longer follow-up (>3 months). This was recently updated with 3-year follow-up and it was demonstrated that patients with PEG hydrogel experienced statistically significantly less rectal toxicity compared with controls. They also demonstrated that improved bowel quality of life scores (Expanded Prostate Cancer Index Composite) were maintained at 3 years in patients who had hydrogel placement. The authors determined that the number needed to treat to spare grade ≥1 and ≥ 2 toxicity at 3 years were 1.3 and 16.7, respectively, which raises the clinical significance of these improvements. Our study requires longer term follow-up to determine the effect of the decrease in rectal dose secondary to PEG hydrogel placement on reporting of rectal toxicity in PBRT.26 Although this study also investigated the effect of PEG hydrogel on urinary toxicity, this was not an endpoint of our study.
There were several limitations in this study. The measured height of prostate–rectum separation at a single midline point in the sagittal and axial planes was arbitrarily determined. Although this allowed us to measure the length of separation in a standardized fashion, measurements at other points of reference may have resulted in different correlations between length of separation and rectal radiation dose. The study was also limited by its relatively small number of patients and may be underpowered to detect differences between the groups. Additionally, because both PEG hydrogel and pencil beam scanning PBRT are relatively new therapies for prostate cancer treatment, the patients in our study have a relatively short follow-up. Additional follow-up is required to compare the effectiveness of PBRT with hydrogel spacer to photon EBRT with hydrogel spacer in reducing long term rectal toxicity. Finally, the retrospective nature of our review resulted in inconsistencies of data reporting, particularly pertaining to toxicity scores.
Conclusions
This is the largest study to investigate the effect of PEG hydrogel placement in patients undergoing proton beam radiation therapy for prostate cancer. PEG hydrogel placement before pencil beam scanning PBRT reduces overall rectal dose by 42.2%. Increased midline separation in the sagittal plane correlates with reduced rectal dosing. Placement of the hydrogel in Denonvillier's space may not be predictable given the relatively low overlap between hydrogel and prostate on coronal imaging; however, this does not affect the efficacy of the PEG hydrogel. Concurrent presence of the PEG hydrogel and rectal balloon during PBRT may result in increased reporting of rectal toxicity during treatment, which may represent a shortfall of the reporting system to differentiate rectal discomfort from true radiation toxicity. There was no difference between the rates of rectal toxicity reported between patients with and without PEG hydrogel at 9.5 months after completion of PBRT. Additional follow-up will allow the determination if placement of PEG hydrogel in the PBRT setting results in reduction in long-term rectal toxicity, as well as ensuring there is no deterioration in the treatment effect of PBRT.
Footnotes
Sources of support: A.N. received a scholarship from the Australasian Urologic Foundation.
Disclosures: The authors have no conflicts of interest to disclose.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2019.08.007.
Supplementary data
Area under curve calculation of overall rectal radiation dose (cc3.Gy) in patients with and without (control) hydrogel placement. Median hydrogel area under the curve 330.90 (136.5-421.9). Median (interquartile range) control group area under the curve 572.97 (494.3-721.0). 42.2% relative reduction in hydrogel group. Wilcoxon rank-sum test with continuity correction P < .001.
Coronal magnetic resonance imaging sections through prostate (a) Yellow = 2 hydrogel surface area outlined; red = prostate surface area. (b) Surface area of the prostate in coronal section 3.51 mm from anterior surface of hydrogel. (c) Combined surface area of 2 hydrogel surface area measurements. (d) Surface area of hydrogel overlapping prostate. (e) Surface area of free hydrogel not covering prostate.
References
- 1.Hatiboglu G., Pinkawa M., Vallee J.P., Hadaschik B., Hohenfellner M. Application technique: Placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012;110:E647–E652. doi: 10.1111/j.1464-410X.2012.11373.x. [DOI] [PubMed] [Google Scholar]
- 2.Mariados N., Sylvester J., Shah D. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Hedrick S.G., Fagundes M., Case S. Validation of rectal sparing throughout the course of proton therapy treatment in prostate cancer patients treated with SpaceOAR. J Appl Clin Med Phys. 2017;18:82–89. doi: 10.1002/acm2.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wisenbaugh E.S., Andrews P.E., Ferrigni R.G. Proton beam therapy for localized prostate cancer 101: Basics, controversies, and facts. Rev Urol. 2014;16:67–75. [PMC free article] [PubMed] [Google Scholar]
- 5.Kuban D.A., Tucker S.L., Dong L. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 6.Zietman A.L., Bae K., Slater J.D. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheets N.C., Goldin G.H., Meyer A.M. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S., Shen S., Moore D.F. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60:908–916. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute, National Institutes of Health, US Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. 2010. http://evsncinihgov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_5×7pdf Available at.
- 10.Pinkawa M., Berneking V., Schlenter M., Krenkel B., Eble M.J. Quality of life after radiation therapy for prostate cancer with a hydrogel spacer: 5-year results. Int J Radiat Oncol Biol Phys. 2017;99:374–377. doi: 10.1016/j.ijrobp.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson R.C., Sundaram V., Folkert M., Lotan Y. Decision analysis model evaluating the cost of a temporary hydrogel rectal spacer before prostate radiation therapy to reduce the incidence of rectal complications. Urol Oncol. 2016;34:291 e19–291 e26. doi: 10.1016/j.urolonc.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Slater J.D., Rossi C.J., Jr., Yonemoto L.T. Proton therapy for prostate cancer: The initial loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–352. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Pinkawa M., Corral N.E., Caffaro M. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2011;100:436–441. doi: 10.1016/j.radonc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Song D.Y., Herfarth K.K., Uhl M. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: Analysis of dosimetric outcomes. Int J Radiat Oncol Biol Phys. 2013;87:81–87. doi: 10.1016/j.ijrobp.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanneste B.G., Pijls-Johannesma M., Van De Voorde L. Spacers in radiotherapy treatment of prostate cancer: Is reduction of toxicity cost-effective? Radiother Oncol. 2015;114:276–281. doi: 10.1016/j.radonc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Pinkawa M., Klotz J., Djukic V. Learning curve in the application of a hydrogel spacer to protect the rectal wall during radiotherapy of localized prostate cancer. Urology. 2013;82:963–968. doi: 10.1016/j.urology.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Susil R.C., McNutt T.R., DeWeese T.L., Song D. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1251–1258. doi: 10.1016/j.ijrobp.2009.07.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christodouleas J.P., Tang S., Susil R.C. The effect of anterior proton beams in the setting of a prostate-rectum spacer. Med Dosim. 2013;38:315–319. doi: 10.1016/j.meddos.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung H., Polf J., Badiyan S., Biagioli M. Rectal dose to prostate cancer patients treated with proton therapy with or without rectal spacer. J Appl Clin Med Phys. 2017;18:32–39. doi: 10.1002/acm2.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guichard M.J.L.T., Van bever R. PEGylation, an approach for improving the pulmonary delivery of biopharmaceuticals. Curr Opin Colloid Interface Sci. 2017;31:43–50. [Google Scholar]
- 21.Both S., Wang K.K., Plastaras J.P. Real-time study of prostate intrafraction motion during external beam radiotherapy with daily endorectal balloon. Int J Radiat Oncol Biol Phys. 2011;81:1302–1309. doi: 10.1016/j.ijrobp.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 22.Smeenk R.J., Louwe R.J., Langen K.M. An endorectal balloon reduces intrafraction prostate motion during radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:661–669. doi: 10.1016/j.ijrobp.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Te Velde B.L., Westhuyzen J., Awad N., Wood M., Shakespeare T.P. Can a peri-rectal hydrogel spaceOAR programme for prostate cancer intensity-modulated radiotherapy be successfully implemented in a regional setting? J Med Imaging Radiat Oncol. 2017;61:528–533. doi: 10.1111/1754-9485.12580. [DOI] [PubMed] [Google Scholar]
- 24.Eckert F., Alloussi S., Paulsen F. Prospective evaluation of a hydrogel spacer for rectal separation in dose-escalated intensity-modulated radiotherapy for clinically localized prostate cancer. BMC Cancer. 2013;13:27. doi: 10.1186/1471-2407-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arimura T., Yoshiura T., Matsukawa K., Kondo N., Kitano I., Ogino T. Proton beam therapy alone for intermediate- or high-risk prostate cancer: An institutional prospective cohort study. Cancers (Basel) 2018;10 doi: 10.3390/cancers10040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamstra D.A., Mariados N., Sylvester J. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III Trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Area under curve calculation of overall rectal radiation dose (cc3.Gy) in patients with and without (control) hydrogel placement. Median hydrogel area under the curve 330.90 (136.5-421.9). Median (interquartile range) control group area under the curve 572.97 (494.3-721.0). 42.2% relative reduction in hydrogel group. Wilcoxon rank-sum test with continuity correction P < .001.
Coronal magnetic resonance imaging sections through prostate (a) Yellow = 2 hydrogel surface area outlined; red = prostate surface area. (b) Surface area of the prostate in coronal section 3.51 mm from anterior surface of hydrogel. (c) Combined surface area of 2 hydrogel surface area measurements. (d) Surface area of hydrogel overlapping prostate. (e) Surface area of free hydrogel not covering prostate.