Abstract
Purpose
To inform development of procedures for using tumor-treating field arrays (TTFields) during glioblastoma radiation therapy by determining whether the placement and repositioning of arrays affects target volume coverage and cranial skin dose.
Methods and Materials
Radiation plans from 10 consecutive patients treated for glioblastoma were copied to a cranial phantom and reoptimized for phantom anatomy. Dose distributions were then recalculated on 3 additional computed tomographic scans of the phantom with the TTFields electrode arrays placed over distinct locations on the phantom scalp to compare planning target volume (PTV) coverage and skin dose with and without TTFields in place in varying positions. Percent depth dose curves were also measured for radiation beams passing through the electrodes and compared with commonly used bolus material.
Results
The presence of TTFields arrays decreased PTV V97% and D97% by as much as 1.7% and 2.7%, respectively, for a single array position, but this decrease was mitigated by array repositioning. On averaging the 3 array positions, there was no statistically significant difference in any dosimetric parameter of PTV coverage (V95-97%, D95-97%) across all cases compared with no array. Mean increases in skin D1cc and D20cc of 3.1% were calculated for the cohort. Surface dose for TTFields electrodes was less than that with a 5-mm superflab bolus.
Conclusions
Our work demonstrates that placement of TTFields arrays does not significantly affect target volume coverage. We show that repositioning of TTFields arrays, as is required in clinical use, further minimizes any dosimetric changes and eliminates the need for replanning when arrays are moved. A slight, expected bolus effect is observed, but the calculated increases in skin dose are not clinically significant. These data support the development of clinical trials to assess the safety and efficacy of combining concurrent chemoradiotherapy with TTFields therapy for glioblastoma.
Introduction
Glioblastoma (GBM) is the most common primary brain malignancy in adults.1 Standard-of-care treatment since the mid-2000s has comprised maximal safe resection followed by radiation therapy with concurrent and adjuvant temozolomide, but the majority of patients will still die within 2 years of diagnosis.2 One of the few modifications to this chemoradiotherapy regimen that has shown clinical benefit in GBM has been the introduction of tumor-treating fields (TTFields). TTFields are a noninvasive local therapy in which transducer arrays are affixed to the scalp and deliver focal, low-intensity alternating electric fields to tumor cells.3, 4 The EF-14 phase 3 clinical trial conducted by Stupp et al showed a significant improvement in both progression-free (6.7 vs 4.0 mo), overall (20.9 vs 16.0 mo), and 5-year survival (13% vs 5%) with the addition of TTFields to adjuvant temozolomide in patients with newly diagnosed GBM after chemoradiotherapy.5, 6
The antitumor mechanism of TTFields occurs through inhibition of mitotic spindle assembly, inducing mitotic arrest or apoptosis.7, 8 Preclinical evidence has shown synergism between the antineoplastic effects of TTFields and taxanes9; tumor cells exposed to TTFields may also show a heightened sensitivity to radiation therapy (RT) owing to delayed DNA repair and accumulation within the G2/M-phase of the cell cycle.10, 11, 12, 13 Given this potential for synergism between TTFields and RT, earlier integration of TTFields into the typical treatment paradigm for newly diagnosed GBM, during concurrent chemoradiotherapy, may theoretically induce a more robust antitumor response compared with use in the setting of adjuvant temozolomide. Because reirradiation of progressive disease after definitive treatment is also considered the standard of care, the combination of RT and TTFields also remains to be explored in the setting of recurrent GBM.
Investigation into clinical synergism between RT and TTFields has thus far been limited by practical considerations. Treatment with TTFields necessitates the placement of multiple metal-ceramic electrode arrays directly onto the scalp, within the radiation field.14 For a typical intensity modulated radiation therapy or volumetric modulated arc therapy radiation plan for GBM, it is not well defined as to whether the presence of these high-density scalp arrays will influence RT dose distribution, create imaging artifact on cone beam computed tomography (CT) that affects daily patient set-up, or increase superficial skin dosage owing to bolus effect. Prior phantom studies have demonstrated that dosimetric changes with TTFields are small and likely to be manageable but have not accounted for the shifting scalp positions of the TTFields arrays ie, necessary to avoid skin irritation (10-14 replacements during a 6-week course of RT).15, 16
To more completely understand how radiation dosimetry is affected by shifting array positions during RT, we quantified the dosimetric effect of placement and repositioning of TTFields arrays using an anthropomorphic phantom. We hypothesized that any decreases in target volume coverage or increases in dose to skin would be mitigated by dose averaging that occurs with repositioning of the arrays. We further aimed to establish whether this dose averaging would make the combination of RT and TTFields feasible for GBMs of varied size, location, and depth.
Methods and Materials
Treatment-planning system analysis
A kilovoltage (kV) CT scan was obtained of an anthropomorphic cranial phantom (Accuray, Sunnyvale, CA) for radiation planning. Additional CT scans were obtained of the phantom wearing an Optune TTFields array (NovoCure, St. Helier, Jersey, England) in 3 distinct, partially overlapping positions on the scalp and immobilized by a thermoplastic mask to simulate routine repositioning during treatment (Fig 1a). Each TTFields array consisted of a 3 × 3 grid of metal-ceramic composite electrodes within an adhesive patch that can be attached to the scalp. Each electrode is a disc of diameter 20 mm and thickness 2.5 mm, with a material density of 5 g/mL. In standard clinical use for GBM and in this study, 4 TTFields arrays are affixed to the scalp (2 temporoparietal, one occipital, and one frontal).
Figure 1.
(a) The tumor-treating fields (TTFields) array electrodes are visible on the phantom scalp and were immobilized using a thermoplastic mask as in typical patient simulation and set-up. (b) Representative sagittal and coronal views of the anthropomorphic phantom imaged by kVCT, with metal artifact from the TTFields electrodes. (c) The anthropomorphic phantom imaged by Tomotherapy axial megavoltage computed tomography, with the TTFields arrays in place and showing no metal artifact. Each panel reflects a unique array position on the scalp.
Scans with the TTFields arrays in place were performed using the megavoltage (MV) CT of a Tomotherapy unit (Accuray, Sunnyvale, CA) to minimize metal artifact that was observed on kVCT (Fig 1b-c). The no-array phantom was chosen for planning purposes as patients in actual clinical practice would not have the TTFields array in place at the time of simulation.
Radiation beams, target volumes, and organs at risk from 10 consecutive patients previously treated at our institution for newly diagnosed GBM were then copied to the planning kVCT and the contours were minimally adjusted by a radiation oncologist specializing in neuro-oncology to account for differences in phantom anatomy. Contour adjustment consisted of removal of areas of the clinical target volume that overlapped with the bone of the cranial phantom before isometric expansion to the planning target volume (PTV). The patient and treatment characteristics for each case are summarized in Table 1. A 5-mm skin contour was created to quantify superficial hot spots secondary to bolus effect.
Table 1.
Patient, tumor, and planning characteristics for each of the 10 cases
| Case | Sex | GBM location | PTV volume (mL) | Prescription isodose (%) | Planning technique |
|---|---|---|---|---|---|
| 1 | Male | Right frontal | 372.7 | 96 | 2-arc VMAT |
| 2 | Male | Right parietal | 225.1 | 98 | 1-arc VMAT |
| 3 | Female | Left frontal | 128.1 | 97.5 | 1-arc VMAT |
| 4 | Male | Left occipital | 440.7 | 96.5 | 5-beam IMRT |
| 5 | Male | Right temporal | 644.1 | 97.5 | 5-beam IMRT |
| 6 | Male | Right temporal | 206.4 | 99 | 2-arc VMAT |
| 7 | Female | Left temporal | 179.8 | 96.5 | 2-arc VMAT |
| 8 | Male | Left temporal | 167.7 | 97.5 | 7-beam IMRT |
| 9 | Male | Right frontal | 374.6 | 98 | 5-beam IMRT |
| 10 | Female | Right parietal | 274.7 | 98.5 | 2-arc VMAT |
Abbreviations: GBM = glioblastoma; IMRT = intensity modulated radiation therapy; PTV = planning target volume; VMAT = volumetric modulated arc therapy.
Dose distributions for each case were reconstructed using an adaptive dose convolution algorithm in the Pinnacle v9.10 treatment-planning system (Philips Healthcare, Amsterdam, Netherlands) and reoptimized using accepted target coverage and normal tissue constraints for GBM. The megavoltage CT scans of the phantom with the array in each of the 3 positions were then fused to the planning kVCT and the densities of autocontours of each ceramic disc of the array were overridden to the known density value of 5 g/mL. Radiation dose distributions were recalculated on the array scans to assess the effect of the electrodes on dosimetric parameters.
We analyzed PTV coverage (V97%, V96%, V95%, D97%, D96%, D95%) and volumetric RT dose to the 5-mm skin contour. Statistical significance was assessed using the Wilcoxon signed-rank test. Institutional review board approval was obtained for this study.
Physical measurements
To assess the surface dose effects of the TTFields array, a 2.5-in square piece of EBT 3 GAF chromic film (ISP Corp., Wayne, NJ) was oriented vertically in a rectangular phantom and irradiated with a 10 × 10 cm 6 MV linear accelerator beam. Measurements were obtained using a single electrode of the Optune TTFields array aligned to the central axis of the beam, a piece of 5-mm thick superflab bolus in the beam, and a sheet of brass bolus in the beam, as well as without any type of bolus for calibration purposes.
Results
Planning target volume coverage
On treatment-planning system analysis, glioblastoma PTV coverage was not compromised by the presence of TTFields arrays on the phantom scalp (Tables 2 and 3). The mean of the 3 individual TTFields array positions are given for each dosimetric parameter for the 10 cases analyzed and the mean and standard deviation of these values. The mean decrease of the percentage of the PTV receiving 97% of the prescription dose (V97%) was 0.3% ± 0.6%, with no mean decrease observed for the V95%. The mean decrease in the minimum radiation dose covering 97% of the PTV (D97%) was 0.8% ± 1.0% (48 cGy ± 60 cGy for a standard 60 Gy treatment), with similar values observed for D96% and D95%. The differences in PTV coverage with and without the TTFields arrays in place did not reach the P = .05 level of significance for any of the dosimetric parameters, as assessed by the Wilcoxon signed-rank test.
Table 2.
Mean percent change in dosimetric parameters of planning target volume coverage for the 3 TTFields array positions relative to coverage without the array in place
| Case | V97% | V96% | V95% | D97% | D96% | D95% |
|---|---|---|---|---|---|---|
| 1 | −0.9% | −0.6% | −0.4% | −1.5% | −1.4% | −1.3% |
| 2 | −0.1% | −0.1% | 0.0% | −0.6% | −0.6% | −0.6% |
| 3 | 0.0% | 0.0% | 0.0% | −0.9% | −0.9% | −0.9% |
| 4 | 0.3% | 0.2% | 0.2% | 0.0% | −0.1% | −0.1% |
| 5 | −1.7% | −0.4% | −0.1% | −2.7% | −2.7% | −2.7% |
| 6 | 0.1% | 0.0% | 0.0% | 0.3% | 0.4% | 0.4% |
| 7 | −0.6% | −0.2% | −0.1% | −1.3% | −1.3% | −1.3% |
| 8 | −0.2% | 0.0% | 0.0% | −1.0% | −1.0% | −1.0% |
| 9 | 0.3% | 0.2% | 0.1% | 0.7% | 0.6% | 0.5% |
| 10 | −0.3% | −0.1% | 0.0% | −1.3% | −1.3% | −1.2% |
| Mean | −0.3% | −0.1% | 0.0% | −0.8% | −0.8% | −0.8% |
| SD | 0.6% | 0.3% | 0.1% | 1.0% | 1.0% | 0.9% |
Abbreviation: TTFields = tumor-treating fields.
Table 3.
Percent change in dosimetric parameters for individual array positions for case 5, which demonstrated the greatest absolute difference in coverage with and without TTFields arrays
| Array position for case 5 | V97% | V96% | V95% | D97% | D96% | D95% |
|---|---|---|---|---|---|---|
| 1 | −2.1% | −0.4% | −0.1% | −3.0% | −3.0% | −3.0% |
| 2 | −0.7% | −0.1% | 0.0% | −2.2% | −2.1% | −2.0% |
| 3 | −2.3% | −0.7% | −0.2% | −3.0% | −3.0% | −2.9% |
| Mean | −1.7% | −0.4% | −0.1% | −2.7% | −2.7% | −2.7% |
Abbreviations: SD = standard deviation; TTFields = tumor-treating fields.
Effect of array repositioning
During routine clinical use, the scalp position of TTFields arrays are changed every 3 to 5 days to avoid potential skin rash. We hypothesized that radiation dosimetric perturbation would be decreased for each of the 10 GBM plans when averaged across the 3 array positions, compared with the maximal absolute change (increase or decrease) for any single array. For both PTV V97% and D97% (Fig 2a-b), mean percent change in each parameter over 3 array positions was significantly decreased from maximal percent change for a single array position (P < .05). Across the 10 plans, mean change in PTV V97% and D97% were 0.31% and 0.80%, respectively, for the 3 TTFields arrays and 0.54% and 1.1%, respectively, for the maximal single array change (P < .05).
Figure 2.
Paired comparison of maximal absolute change for an individual array position (blue) and mean change over all 3 array positions (red) for each patient case for (a) planning target volume V97% and (b) planning target volume D97%. Differences were significant at the P = .05 level for both metrics using the Wilcoxon signed-rank test.
Skin dose
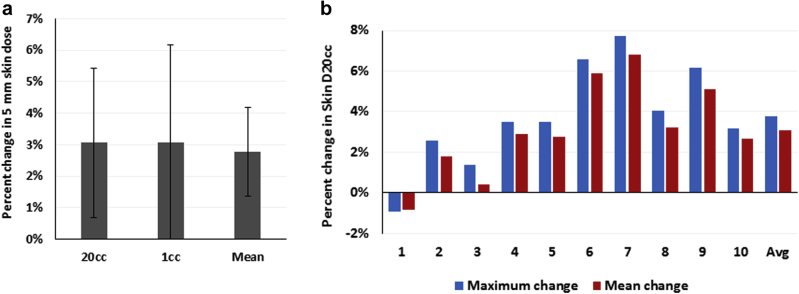
The cranial skin was modeled using a 5 mm wide contour from the most external surface visible on CT of the phantom. The radiation dose to at least 20 mL and 1 mL volumes of the skin contour (D20cc and D1cc) and the mean skin dose were increased by 3.1% ± 2.4%, 3.1% ± 3.1%, and 2.8% ± 1.4%, respectively, in the patient plans with TTFields arrays relative to those without the device (Fig 3a; P = .009, .012, and 0.006, respectively). Similar to PTV coverage, the mean increase in calculated skin D20cc was significantly decreased over the average of the 3 TTFields array positions relative to the maximum increase (Fig 3b; P < .05).
Figure 3.
(a) Mean change in skin dosimetry with the addition of tumor-treating fields electrodes. Error bars represent standard deviation. (b) Paired comparison of maximal absolute change for an individual array position (blue) and mean change over all 3 array positions (red) for each patient case for skin D20cc. The difference was significant at the P = .05 level using the Wilcoxon signed-rank test.
Surface dose measurement
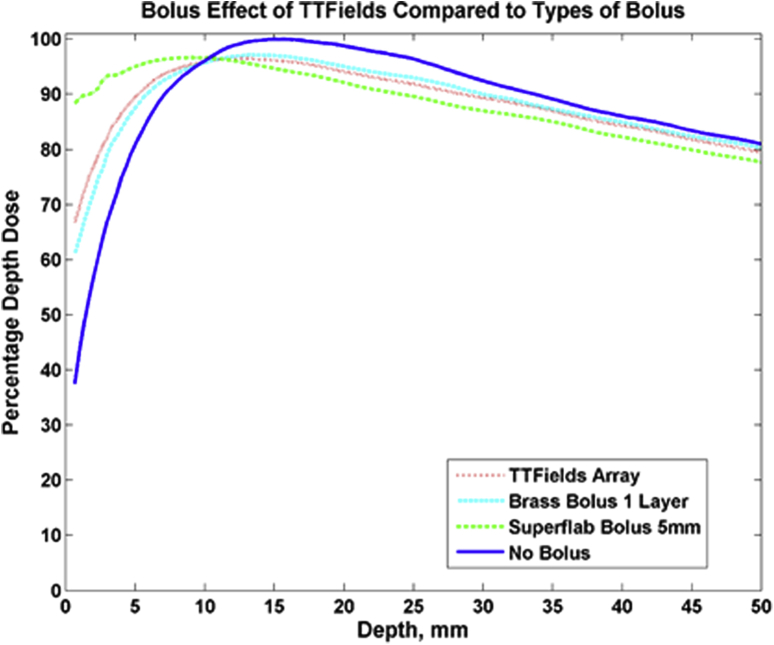
Increased skin toxicity owing to shifting of the radiation dose distribution toward the surface is a concern for any material that may produce bolus effect. Using radiochromic film to measure surface dose, we observed that the bolus effect of the ceramic TTFields array electrodes is comparable to that of single-layer brass bolus and less than that of 5-mm thick superflab bolus (Fig 4). Superflab bolus resulted in approximately 90% of maximum dose deposited at the surface compared with approximately 70% of maximum dose deposited at the surface for TTFields electrodes. The 90% dose for TTFields remained at a depth of 5 mm, compared with 8 mm with no bolus. From the entire range from the surface to the depth of maximum dose, the no bolus percent depth dose curve as measured with film in solid water matched our commissioned beam data to within 1.2 mm.
Figure 4.
Measured percentage depth dose curves comparing the surface dose with tumor-treating fields arrays to brass bolus, 5-mm superflab bolus, and no bolus. The bolus effect observed with tumor-treating fields was similar to brass bolus and less than that of superflab bolus.
Discussion
Tumor-treating fields are a relatively novel component of multimodality therapy for glioblastoma, which typically includes neurosurgery, radiation therapy, and alkylating-agent chemotherapy. The utility of TTFields therapy after chemoradiotherapy for GBM has been established by a multinational phase-3 clinical trial demonstrating significant increases in both overall and progression-free survival.6 TTFields currently hold Food and Drug Administration approval and are in the National Comprehensive Cancer Network guidelines for newly diagnosed GBM in the adjuvant setting, but little work yet exists to assess their safety and efficacy when combined with chemoRT, in part owing to practical uncertainties regarding RT delivery through the scalp electrodes. Concurrent chemoRT and TTFields are being evaluated in the NCT03232424 trial with daily removal and replacement of transducer arrays for each RT fraction, although this is logistically challenging and introduces the possibility of mechanical scalp injury from frequent array changes.
To assess the feasibility of arrays remaining on the scalp during treatment, we conducted a phantom study of the alterations to RT dosimetry produced by TTFields arrays on GBM plans from actual patients. In this work, we demonstrate that there is no significant decrease in well-established parameters of planning target volume coverage in the presence of TTFields arrays over our cohort. More importantly, we show that even in cases with a higher than average decrease in PTV coverage, this effect is mitigated by repositioning of the TTFields arrays on the scalp, suggesting that concurrent therapy may be feasible even for tumors in which a single array position creates undesirable dosimetric changes. Finally, we quantify the bolus effect of the high-density TTFields electrodes using physical measurements of percent depth dose and show that calculated increases in skin dose are small.
Case 5, the GBM plan demonstrating the largest decrease in PTV V97% and D97% for both any individual array position and the average of the 3 array positions, had a PTV measuring 644.1 mL, over twice as large as the mean PTV of 301.4 mL for our patient cohort. This represented nearly hemispheric right-sided tumor involvement and suggests that RT dose perturbation in the presence of TTFields arrays may be more apparent for large radiation fields. We hypothesize that this may occur because a greater amount of the radiation beams pass through the electrodes. Conversely, the 5 plans with PTVs less than the median volume accounted for the 4 lowest absolute changes in the V97%. Tumor location and planning technique (intensity modulated radiation therapy or volumetric modulated arc therapy) did not correlate with decreased PTV coverage in our cohort.
Average human cranial skin thickness is 3 to 4.5 mm17; for some patients the deep subcutaneous tissue may therefore be in the region of the measured 90% depth dose for TTFields electrodes in our analysis, raising the potential for increased toxicity. We have shown, however, that skin hot-spots are mitigated by as few as 3 distinct placements of the TTFields array. The actual number of such repositionings during a standard 6-week course of glioblastoma radiation therapy is likely to be ≥10. Because each array placement will thus cover a given area of scalp for a smaller percentage of the total RT dose, we hypothesize that this decrease in local hot spots is likely to be even more apparent in clinical practice. Nevertheless, given that the TTFields device may cause skin irritation even in the absence of RT, we strongly recommend considering scalp dose in the initial planning algorithm and procedures for standard replacement of the applicator to minimize the risk of toxicity.
Our analysis does have several limitations. Our sample size is relatively small, although still encompasses a variety of tumor sizes and locations despite representing an unbiased, consecutive series of patients treated for GBM. Furthermore, although the collapsed cone convolution algorithm used for dose calculation in the Pinnacle treatment-planning system is generally able to accurately calculate dose at interfaces of differing density (eg, tissue-bone or, in our study, tissue-electrode), a Monte Carlo algorithm could potentially provide greater accuracy in calculating dose distribution.18 We attempted to reduce any dosimetric uncertainty in the study by contouring the individual array electrodes and overriding the density derived from CT imaging to the known density of 5 g/mL. Pinnacle's dose engine will treat this density as if it were titanium for attenuation of the primary radiation beam.
Conclusions
This work demonstrates that target volume dosimetry for glioblastoma radiation therapy is not significantly affected by the presence or repositioning of tumor-treating fields electrode arrays. An increased dose to skin due to bolus effect is observed ie, unlikely to be clinically relevant but emphasizes the need for close monitoring in patients undergoing concurrent therapy. We are initiating a clinical trial to assess the safety and logistics of the combination of tumor-treating fields and chemoradiotherapy in patients with newly diagnosed glioblastoma.
Footnotes
Sources of support: This work was partially supported by a grant from NovoCure, Inc.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Ostrom Q.T., Bauchet L., Davis F.G. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omuro A., DeAngelis L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.Pless M., Weinberg U. Tumor treating fields: Concept, evidence and future. Expert Opin Investig Drugs. 2011;20:1099–1106. doi: 10.1517/13543784.2011.583236. [DOI] [PubMed] [Google Scholar]
- 4.Swanson K.D., Lok E., Wong E.T. An overview of alternating electric fields therapy (NovoTTF therapy) for the treatment of malignant glioma. Curr Neurol Neurosci Rep. 2016;16:8. doi: 10.1007/s11910-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R., Taillibert S., Kanner A.A. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R., Taillibert S., Kanner A.A. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirson E.D., Dbaly V., Tovarys F. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giladi M., Schneiderman R.S., Voloshin T. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5:18046. doi: 10.1038/srep18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneiderman R.S., Shmueli E., Kirson E.D. TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 2010;10:229. doi: 10.1186/1471-2407-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giladi M., Munster M., Scheiderman R.S. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12:206. doi: 10.1186/s13014-017-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirson E.D., Gurvich Z., Schneiderman R.S. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karanam N.K., Srinivasan K., Ding L. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 2017;8:e2711. doi: 10.1038/cddis.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim E.H., Kim Y.H., Song H.S. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. 2016;7:62267–62279. doi: 10.18632/oncotarget.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hottinger A.F., Pacheco P., Stupp R. Tumor treating fields: A novel treatment modality and its use in brain tumors. Neuro Oncol. 2016:1338–1349. doi: 10.1093/neuonc/now182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straube C., Oechsner M., Kampfer S. Dosimetric impact of tumor treating field (TTField) transducer arrays onto treatment plans for glioblastomas—a planning study. Radiat Oncol. 2018;13:31. doi: 10.1186/s13014-018-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T., Shukla G., Peng C., Lockamy V., Liu H., Shi W. Dosimetric impact of a tumor treating fields device for glioblastoma patients undergoing simultaneous radiation therapy. Front Oncol. 2018;8:51. doi: 10.3389/fonc.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oltulu P., Ince B., Kokbudak N. Measurement of epidermis, dermis, and total skin thicknesses from six different body regions with a new ethical histometric technique. Turk J Plast Surg. 2018;26:56–61. [Google Scholar]
- 18.Chopra K.L., Leo P., Kabat C. Evaluation of dose calculation accuracy of treatment planning systems in the presence of tissue heterogeneities. Ther Radiol Oncol. 2018;2:28. [Google Scholar]