Abstract
Purpose
Utilization of stereotactic radiosurgery (SRS) for brain metastases (BM) has increased, prompting reassessment of whole brain radiation therapy (WBRT). A pattern of care analysis of SRS and WBRT dose-fractionations was performed in patients presenting with BM at the time of cancer diagnosis.
Methods and Materials
Adults with BM at cancer diagnosis between 2010 to 2015 and no prior malignancy were identified in the National Cancer Database. SRS was defined using published thresholds. Short (ShWBRT), standard (StWBRT), and extended (ExWBRT) dose-fractionations were defined as 4 to 9, 10 to 15, and >15 fractions. Radioresistant histology was defined as melanoma, renal cell carcinoma, sarcoma or spindle cell, or gastrointestinal primary.
Results
Of 4,087,967 adults with their first lifetime cancer, 90,388 (2.2%) had BM at initial diagnosis. Of these, 11,486 (12.7%) received SRS and 24,262 (26.8%) WBRT as first-course radiation therapy. The proportion of annual WBRT use decreased from 27.8% to 23.5% of newly diagnosed patients, and SRS increased from 8.7% to 17.9%. Common dose-fractionations were 30 Gy in 10 fractions (56.8%) for WBRT and 20 Gy in 1 fraction (13.0%) for SRS. On multivariate analysis, factors significantly associated with SRS versus WBRT included later year of diagnosis (2015 vs 2010, adjusted odds ratio [aOR] = 2.4), radioresistance (aOR = 2.0), academic facility (aOR = 1.9), highest income quartile (aOR = 1.6), chemotherapy administration (aOR = 1.4), and longer travel distance (>15 vs < 5 miles, aOR = 1.4). Linear regression revealed significant ExWBRT reductions (–22.4%/y, R2 = 0.97, P < .001) and no significant change for ShWBRT or StWBRT. Patients were significantly more likely to receive ShWBRT than StWBRT if not treated with chemotherapy (aOR = 3.5).
Conclusions
Utilization of WBRT, particularly ExWBRT, decreased while SRS utilization doubled as the first radiation therapy course in patients with BM at diagnosis. Patients with radioresistant histologies were more likely to receive SRS. Those not receiving chemotherapy, potentially owing to poor performance status, were less likely to receive SRS and more likely to receive ShWBRT.
Summary.
This analysis of the National Cancer Database examined utilization of whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS) for patients with brain metastases at initial cancer diagnosis. From 2010-2015, the findings demonstrate a declining overall use of WBRT and concomitant increase in SRS, particular among subjects in high income areas or with insurance other than Medicaid. The most commonly used dose-fractionations were 30 Gy in 10 fractions for WBRT and 20 Gy in 1 fraction for SRS.
Introduction
Brain metastases (BM) are the most common intracranial tumor, affecting up to 40% of all patients with cancer.1 Identifying the optimal treatment for BM in an individual patient involves considerations of life expectancy, quality of life, treatment logistics, and potential neurocognitive effects both from treatment and uncontrolled intracranial disease.2 Treating brain metastases with whole brain radiation therapy (WBRT) and steroids has been widely used since the 1950s to 1960s,3, 4 but comes at the cost of significant morbidity. To limit cognitive dysfunction and improve WBRT's therapeutic index, treatment with memantine, hippocampal avoidance, and longer fractionation schedules with smaller doses (≤2.0 Gy per fraction) have been investigated.5, 6, 7 Despite this, neurocognitive toxicity remains a significant issue with WBRT.
Stereotactic radiosurgery (SRS) is an alternative to WBRT for the treatment of limited BM. When a limited number of lesions are present, typically 1 to 3, survival is equivalent and there is less cognitive deterioration for patients initially treated with SRS alone versus SRS and WBRT.8, 9, 10, 11 Additionally, SRS feasibility and efficacy is being increasingly demonstrated in patients with larger disease burden.12 As a result, retrospective analyses of radiation utilization trends in the treatment of brain metastases have shown increasing SRS use, particularly among radioresistant histologies like melanoma.[13, 14] The proportion of patients receiving SRS significantly increased from 7% in 2004 to 37% in 2014 for BM from non-small cell lung cancer (NSCLC),13 and from 9.8% to 25.6% in a combined cohort of non-small cell lung cancer, breast cancer, colorectal cancer, and melanoma.14 Disparities in increasing SRS utilization have been associated with treatment facility characteristics, patient insurance status, and regional income.13, 14 For example, in a study of 7 Canadian cancer centers, the availability of on-site SRS was found to be more influential than clinical eligibility in the provision of SRS for BM treatment, suggesting patients in resource-poor areas may not receive optimal radiation therapy.15
This study's purpose was to analyze patterns-of-care for multiple WBRT dose-fractionations and SRS in the treatment of patients with BM at the time of cancer diagnosis. Although prior studies have used the National Cancer Database (NCDB) to investigate BM radiation therapy trends, this is the first study limited to patients with BM at initial cancer diagnosis, using a NCDB variable introduced in 2010. Additionally, this study is inclusive of BM originating from primary cancers of multiple organ systems to a greater extent than past work. Finally, we apply a more expansive definition of SRS while conducting subanalyses of WBRT dose-fractionations.
Methods and Materials
Data source and cohort selection
A retrospective cohort analysis was conducted using the NCDB, a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB is an oncology outcomes database compiled from >1500 cancer programs in the United States capturing approximately 70% of newly diagnosed cases. This research was determined exempt after initial review by our institution's institutional review board. Inclusion criteria included adult patients with no prior malignancy who had BM at time of new cancer diagnosis from 2010 to 2015 and who had a primary tumor site of lung, breast, skin (excluding basal and squamous cell carcinoma), urinary, gastrointestinal, female genital, or head and neck.
Receipt of SRS or WBRT as first course of radiation therapy was defined as follows. A SRS cohort was created by identifying patients coded as treated with a radiosurgery modality (stereotactic radiosurgery, GammaKnife, or LINAC radiosurgery), a single fraction of 12 to 24 Gy, 2 fractions of 18 to 30 Gy, 3 or 4 fractions of 21 to 36 Gy, or 5 fractions of 25 to 40 Gy to the brain.13, 16, 17, 18, 19, 20, 21, 22, 23, 24 The WBRT cohort was created by excluding patients if they were included in the SRS cohort; received partial brain irradiation, a regional radiation dose <20 or >50 Gy, <4 or >44 fractions, <1.6 or >6.0 Gy/fraction, a biologically effective dose (BED) of <60 or >100 Gy2; or completed radiation therapy in >60 days. Regional dose limits were selected based on prior efficacy data of low- versus high-dose treatment.25 The upper limit of total fractions was selected based on a randomized trial of accelerated hyperfractionation.26 It should be noted that the NCDB only reports the total number of fractions administered (ie, regional plus boost). Thresholds for dose-per-fraction were set based on accelerated hyperfractionation for the lower limit26, 27 and clinical trials of palliative treatment schedules for the upper limit.28, 29 The thresholds of BED 60 and 100 Gy2 using an α/β of 2 were chosen based on 20 Gy in 5 fractions (ie, the lowest BED among the most common dose-fractionations used in clinical trials)30, 31 and a conservative estimate of the lower threshold for spinal-cord tolerance,32 respectively.
Short (ShWBRT), standard (StWBRT), and extended (ExWBRT) fractionation groups of WBRT courses were defined as 4 to 9, 10 to 15, and >15 fractions, respectively. Additional variables of interest included age, sex, race, chemotherapy administration, radioresistant histology, Charlson-Deyo comorbidity index, insurance status, 2012 median income at the ZIP code of residence, great circle distance to care (miles between the hospital and patient's ZIP code), and treatment facility type and location. Radioresistant histology was defined as melanoma, renal cell carcinoma, sarcoma or spindle cell, or gastrointestinal primary.
Statistical methods
Statistical analyses were performed in R version 3.5.1 (R Foundation, Vienna, Austria),33 graphics made with the ggplot234 and survminer packages,35 and adjusted odds ratios (aOR) calculated with the odds ratio package.36 Categorical characteristics were compared using the Pearson χ2 test with Yates’ continuity correction or Fisher exact test when expected values were low. Continuous variables were compared using Kruskal-Wallis tests. These characteristics were compared between SRS and WBRT and between WBRT fractionation groups. Yearly trends were analyzed with a linear regression, using year of diagnosis as a continuous variable. The aOR for comparing factors influencing treatment modality were estimated from multivariate logistic regression. Variables with P values < .05 on individual comparison were included in the multivariate model. When comparing WBRT dose-fractionations, age at diagnosis was included as a continuous variable, but violated linearity assumptions and so was adjusted using the restricted cubic spline approach.37
Based on time from diagnosis to last contact, median follow-up time was calculated by the reverse Kaplan-Meier method, whereas median, 6-month, and 36-month overall survival (OS) were calculated using Kaplan-Meier estimation. Multivariate Cox proportional hazard results are not reported because treatment variables (WBRT, SRS, and chemotherapy use) violated the proportional hazard assumption. Assumption violations remained when conducting a landmark analysis, using a robust-variance estimator, and excluding WBRT dose-fractionation groups that most severely violated assumptions.
Results
Cohort derivation and dose-fractionations
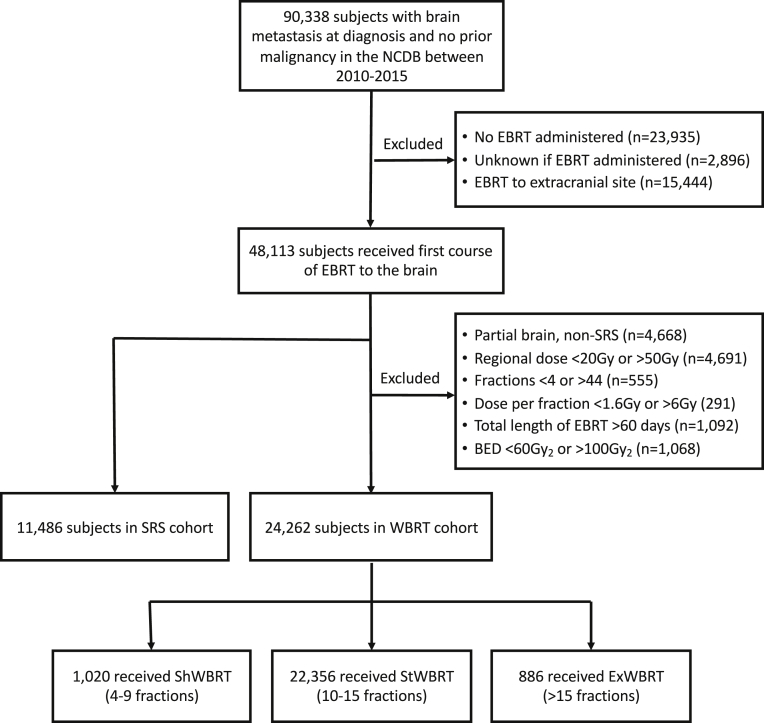
Between 2010 and 2015, 90,388 (2.2%) of 4,087,967 adults with their first lifetime cancer had BM at the time of diagnosis (Fig 1). Among these 90,388, 23,935 did not receive brain radiation therapy for reasons including: not part of treatment course (81.1%), contraindicated owing to risk factors (4.2%), and refused (10.7%). Overall, 11,486 (12.7%) met inclusion criteria for the SRS and 24,262 (26.8%) for the WBRT cohort (Fig 1).
Figure 1.
Cohort derivation. Abbreviations: BED = biologically effective dose; EBRT = external-beam radiation therapy; ExWBRT = extended-course WBRT; ShWBRT = short-course; SRS, stereotactic radiosurgery; WBRT = whole brain radiation therapy; StWBRT = standard-course WBRT.
Elapsed time from diagnosis to radiation therapy was significantly longer for SRS than WBRT (median 17 vs 8 days; Table 1). The most common WBRT dose-fractionations were 30 Gy in 10 fractions (56.8%), 37.5 Gy in 15 fractions (15.5%), and 35 Gy in 14 fractions (11.0%). StWBRT accounted for 92.1% of WBRT dose-fractionations in the cohort, followed by ShWBRT (4.2%) and ExWBRT (3.7%; Table 1). The most common SRS dose-fractionations (Table E1, available online at https://doi.org/10.1016/j.adro.2019.07.012) were 12 to 24 Gy in 1 fraction (n = 5,458, 47.5%), particularly 20 Gy in 1 fraction (13.0%), 18 Gy in 1 fraction (11.3%), and 24 Gy in 1 fraction (6.4%). The most common SRS dose-fractionations with >1 fraction were 24 Gy in 3 fractions (3.4%), 30 Gy in 5 fractions (3.3%), and 25 Gy in 5 fractions (3.1%). Included in the SRS cohort were 374 patients receiving >10 fractions and reported as receiving a radiosurgery modality, potentially reflecting combination WBRT and SRS.
Table 1.
Demographic, clinical, and treatment characteristics
| Factor | SRS, n = 11,486 | WBRT, n = 24,262 | P value | ShWBRT, n = 1020 | StWBRT, n = 22,356 | ExWBRT, n = 886 | P value |
|---|---|---|---|---|---|---|---|
| Age at Dx | .810 | <.001 | |||||
| Median (IQR) | 63 (56-71) | 63 (56-70) | 65 (57-73) | 63 (56-70) | 62 (55-69) | ||
| Min, Max | 18, 90 | 18, 90 | 30, 90 | 18, 90 | 27, 87 | ||
| Sex, n (%) | .578 | .425 | |||||
| Male | 5925 (51.6) | 12,445 (51.3) | 543 (53.2) | 11,453 (51.2) | 449 (50.7) | ||
| Female | 5561 (48.4) | 11,817 (48.7) | 477 (46.8) | 10,903 (48.8) | 437 (49.3) | ||
| Race, n (%) | .001 | .219 | |||||
| Non-Hispanic white | 9324 (81.2) | 19,815 (81.7) | 811 (79.5) | 18,285 (81.8) | 719 (81.1) | ||
| Non-Hispanic black | 1167 (10.2) | 2816 (11.6) | 133 (13.0) | 2564 (11.5) | 119 (13.4) | ||
| Hispanic | 408 (3.6) | 755 (3.1) | 32 (3.1) | 695 (3.1) | 28 (3.2) | ||
| Other | 22 (0.2) | 48 (0.2) | 4 (0.4) | 42 (0.2) | 2 (0.2) | ||
| Unknown | 565 (4.9) | 828 (3.4) | 40 (3.9) | 770 (3.4) | 18 (2.0) | ||
| Charlson-Deyo comorbidity index, n (%) | <.001 | .042 | |||||
| 0 | 7999 (69.6) | 15,627 (64.4) | 616 (60.4) | 14,444 (64.6) | 567 (64.0) | ||
| 1 | 2460 (21.4) | 6029 (24.8) | 281 (27.5) | 5523 (24.7) | 225 (25.4) | ||
| 2 | 713 (6.2) | 1817 (7.5) | 75 (7.4) | 1675 (7.5) | 67 (7.6) | ||
| ≥3 | 314 (2.7) | 789 (3.3) | 48 (4.7) | 714 (3.2) | 27 (3.0) | ||
| Insurance, n (%) | <.001 | <.001 | |||||
| Medicaid | 1133 (9.9) | 2925 (12.1) | 149 (14.6) | 2654 (11.9) | 122 (13.8) | ||
| Medicare | 4842 (42.2) | 10,566 (43.5) | 507 (49.7) | 9703 (43.4) | 356 (40.2) | ||
| Private | 4780 (41.6) | 8368 (34.5) | 255 (25.0) | 7777 (34.8) | 336 (37.9) | ||
| Other government | 221 (1.9) | 486 (2.0) | 23 (2.3) | 452 (2.0) | 11 (1.2) | ||
| Uninsured | 372 (3.2) | 1555 (6.4) | 70 (6.9) | 1438 (6.4) | 47 (5.3) | ||
| 2012 income, n (%) | <.001 | <.001 | |||||
| Lowest quartile | 1953 (17.0) | 5116 (21.0) | 253 (24.8) | 4631 (20.7) | 232 (26.2) | ||
| Second quartile | 2625 (22.9) | 6270 (25.8) | 261 (25.6) | 5774 (25.8) | 235 (26.5) | ||
| Third quartile | 3063 (26.7) | 6614 (27.3) | 281 (27.5) | 6131 (27.4) | 202 (22.8) | ||
| Highest quartile | 3776 (32.9) | 6080 (25.1) | 215 (21.1) | 5654 (25.3) | 211 (23.8) | ||
| Facility type, n (%) | <.001 | <.001 | |||||
| Academic or research | 5470 (47.6) | 7779 (32.1) | 403 (39.5) | 7215 (32.3) | 161 (18.2) | ||
| CCP or integrated network | 1926 (16.8) | 5205 (21.5) | 208 (20.4) | 4838 (21.6) | 159 (17.9) | ||
| Comprehensive CCP | 3864 (33.6) | 11,009 (45.4) | 401 (39.3) | 10,056 (45.0) | 552 (62.3) | ||
| Distance, n (%) | <.001 | .132 | |||||
| ≤5 miles | 2700 (23.5) | 7264 (29.9) | 291 (28.5) | 6710 (30.0) | 263 (29.7) | ||
| >5 and ≤ 15 miles | 3597 (31.3) | 8016 (33.0) | 323 (31.7) | 7422 (33.2) | 271 (30.6) | ||
| >15 miles | 5109 (44.4) | 8805 (36.3) | 396 (38.8) | 8063 (36.1) | 346 (39.1) | ||
| Primary tumor, n (%) | <.001 | .001 | |||||
| Breast | 333 (2.9) | 836 (3.4) | 34 (3.3) | 763 (3.4) | 42 (4.7) | ||
| Female genital | 66 (0.6) | 125 (0.5) | 12 (1.2) | 112 (0.5) | 2 (0.2) | ||
| Gastrointestinal | 563 (4.9) | 853 (3.5) | 60 (5.9) | 765 (3.4) | 31 (3.5) | ||
| Head and neck | 23 (0.2) | 33 (0.1) | 4 (0.4) | 28 (0.1) | 2 (0.2) | ||
| Lung | 9048 (78.8) | 21,163 (87.2) | 913 (89.5) | 19,545 (87.4) | 755 (85.2) | ||
| Skin | 744 (6.5) | 705 (2.9) | 29 (2.8) | 649 (2.9) | 30 (3.4) | ||
| Urinary | 709 (6.2) | 547 (2.3) | 35 (3.4) | 494 (2.2) | 24 (2.7) | ||
| Radioresistance, n (%) | <.001 | .022 | |||||
| Yes | 1655 (14.4) | 1911 (7.9) | 100 (9.8) | 1734 (7.8) | 77 (8.7) | ||
| No | 9728 (84.7) | 22,121 (91.2) | 889 (87.2) | 20,431 (91.4) | 801 (90.4) | ||
| Chemotherapy, n (%) | <.001 | <.001 | |||||
| Yes | 8059 (70.2) | 15,416 (63.5) | 334 (32.7) | 14,463 (64.6) | 619 (69.9) | ||
| No | 3123 (27.2) | 8302 (34.2) | 657 (64.4) | 7385 (33.0) | 260 (29.3) | ||
| RT total dose, Gy | <.001 | <.001 | |||||
| Median (IQR) | 24 (20-36) | 30 (30-35) | 24 (20-27) | 30 (30-35) | 40 (36-40) | ||
| Fractions | <.001 | <.001 | |||||
| Median (IQR) | 1 (1-3) | 10 (10-14) | 8 (5-9) | 10 (10-14) | 20 (18-20) | ||
| BED, Gy2 | <.001 | <.001 | |||||
| Median (IQR) | 220 (128-312) | 75 (75-79) | 60 (60-68) | 75 (75-79) | 80 (72-81) | ||
| Time from Dx to RT, days | <.001 | .020 | |||||
| Median (IQR) | 17 (5-31) | 8 (4-20) | 8 (4-19) | 9 (4-20) | 7.5 (3-19) | ||
| Reverse Kaplan-Meier follow-up time, mo | .030 | <.001 | |||||
| Median (95% CI) | 37.6 (36.5-38.8) | 41.7 (40.5-42.9) | 31.6 (28.3-45.4) | 41.2 (40.0-42.4) | 54.4 (51.6-61.3) |
Abbreviations: BED = biologically effective dose; CI = confidence interval; CCP = Community Cancer Program; Dx = diagnosis; ExWBRT = extended-course WBRT; Gy = gray; IQR = interquartile range; RT = radiation therapy; ShWBRT = short-course WBRT; SRS = stereotactic radiosurgery; StWBRT = standard-course WBRT; WBRT = whole brain radiation therapy.
Yearly trends and demographic characteristics
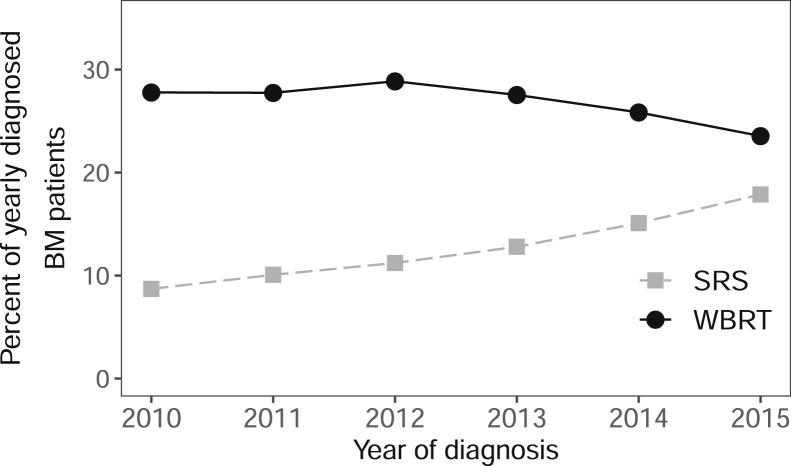
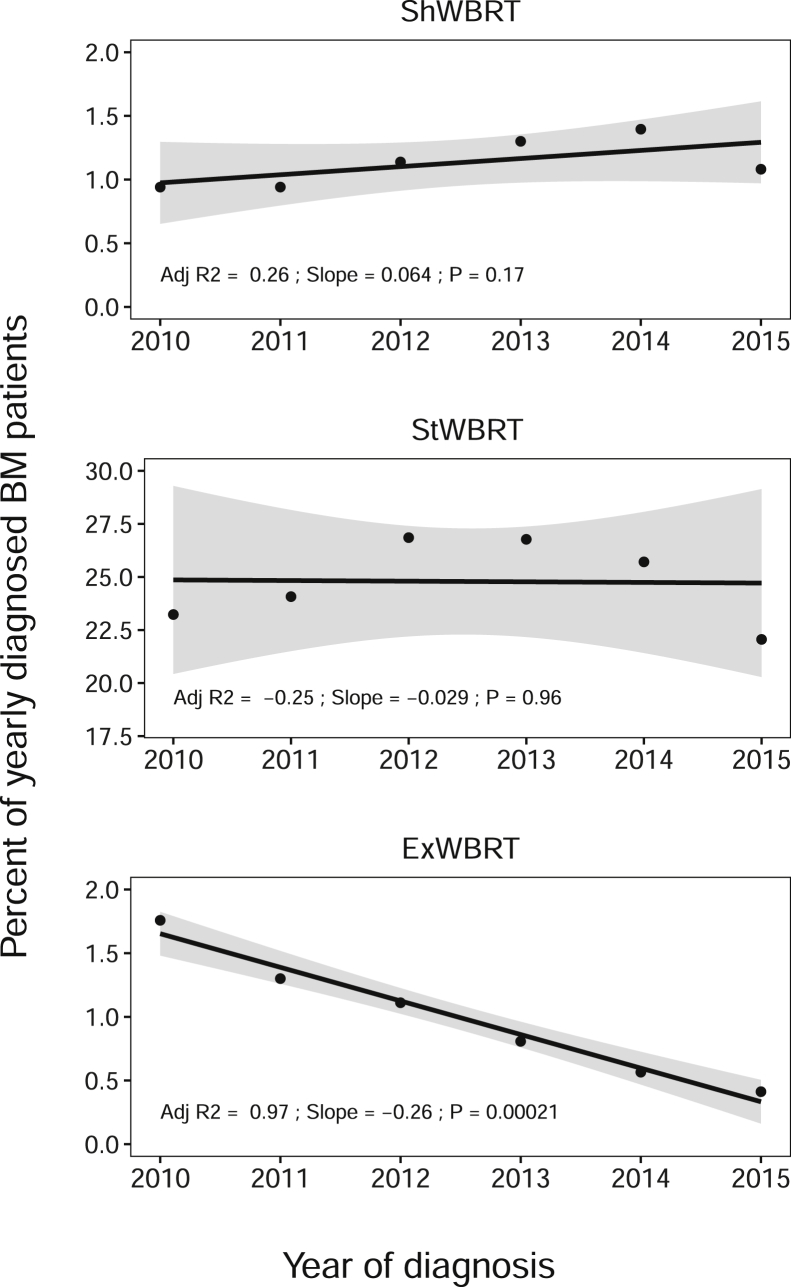
From 2010 to 2015, the use of SRS increased from 8.7% to 17.9% of annually diagnosed patients, whereas WBRT decreased from 27.8% to 23.5% (Fig 2). Compared with 2010, the probability of treatment with SRS increased significantly each year, with maximum probability in 2015 (aOR = 2.4; 95% confidence interval [CI], 2.2-2.6; Table 2). There was no significant annual trend in overall ShWBRT or StWBRT use (Fig 3); however, there was a 22.4% annual decrease in ExWBRT use (–0.26% of newly diagnosed BM patients per year), resulting in a 76.5% absolute reduction. Compared with 2010, the probability of treatment with ExWBRT compared with StWBRT decreased significantly each year, with lowest probability in 2015 (aOR = 0.3; 95% CI, 0.2-0.4; Table 2).
Figure 2.
Yearly trends of whole brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) for patients with their first lifetime malignancy and brain metastasis (BM) at diagnosis. The y-axis represents the percentage of patients diagnosed with BM in a year (2010, n = 14,613; 2011, n = 14,529; 2012, n = 14,665; 2013, n = 15,347; 2014, n = 15,727; 2015, n = 15,507) treated with the modality.
Table 2.
Multivariate logistic regression adjusted odds ratios (aOR) for treatment of newly diagnosed brain metastasis with stereotactic radiosurgery (SRS) versus whole brain radiation therapy (WBRT), short course WBRT (ShWBRT) versus standard WBRT (StWBRT), and extended WBRT (ExWBRT) versus StWBRT
| SRS vs WBRT n = 10,648 and n = 22,706 |
ShWBRT vs StWBRT n = 933 and n = 20,951 |
ExWBRT vs StWBRT n = 837 and n = 20,951 |
||||
|---|---|---|---|---|---|---|
| Variable | aOR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value |
| Year of diagnosis | ||||||
| 2010 | Reference | Reference | Reference | |||
| 2011 | 1.1 (1.0-1.2) | .013 | 1.0 (0.8-1.3) | .876 | 0.7 (0.6-0.9) | .003 |
| 2012 | 1.3 (1.1-1.4) | <.001 | 1.0 (0.8-1.3) | .707 | 0.6 (0.5-0.7) | <.001 |
| 2013 | 1.5 (1.4-1.6) | <.001 | 1.2 (0.9-1.5) | .159 | 0.4 (0.3-0.6) | <.001 |
| 2014 | 1.8 (1.7-2.0) | <.001 | 1.4 (1.1-1.7) | .010 | 0.4 (0.3-0.5) | <.001 |
| 2015 | 2.4 (2.2-2.6) | <.001 | 1.1 (0.9-1.4) | .312 | 0.3 (0.2-0.4) | <.001 |
| Charlson-Deyo comorbidity index | ||||||
| 0 | Reference | Reference | Reference | |||
| 1 | 0.9 (0.8-0.9) | <.001 | 1.2 (1.0-1.4) | .052 | 1.0 (0.9-1.2) | .633 |
| 2 | 0.9 (0.8-0.9) | .003 | 1.0 (0.7-1.2) | .756 | 1.0 (0.8-1.3) | .867 |
| ≥3 | 0.9 (0.8-1.1) | .292 | 1.3 (0.9-1.7) | .125 | 1.0 (0.7-1.5) | .902 |
| Income quartile | ||||||
| Lowest | Reference | Reference | Reference | |||
| Second | 1.1 (1.0-1.2) | .015 | 0.9 (0.7-1.1) | .178 | 0.8 (0.6-0.9) | .008 |
| Third | 1.2 (1.1-1.3) | <.001 | 0.9 (0.7-1.1) | .251 | 0.6 (0.5-0.8) | <.001 |
| Highest | 1.6 (1.4-1.7) | <.001 | 0.8 (0.6-0.9) | .010 | 0.7 (0.6-0.9) | <.001 |
| Radioresistance | ||||||
| Yes | 2.0 (1.9-2.2) | <.001 | 1.2 (1.0-1.5) | .111 | 1.1 (0.9-1.4) | .375 |
| No | Reference | Reference | Reference | |||
| Chemotherapy | ||||||
| Yes | 1.4 (1.4-1.5) | <.001 | Reference | 1.2 (1.0-1.4) | .026 | |
| No | Reference | 3.5 (3.1-4.1) | <.001 | Reference | .026 | |
| Facility type | ||||||
| Academic | 1.9 (1.8-2.0) | <.001 | 1.4 (1.2-1.6) | <.001 | 0.4 (0.3-0.5) | <.001 |
| CCP or IN | 1.1 (1.0-1.2) | .006 | 1.1 (0.9-1.3) | .588 | 0.6 (0.5-0.7) | <.001 |
| Comprehensive CCP |
Reference | Reference | Reference | |||
| Insurance | ||||||
| Medicaid | Reference | Reference | Reference | |||
| Medicare | 1.3 (1.2-1.4) | <.001 | 0.8 (0.6-1.0) | .033 | 0.8 (0.6-1.1) | .162 |
| Private | 1.4 (1.3-1.5) | <.001 | 0.7 (0.6-0.9) | .003 | 0.9 (0.7-1.1) | .270 |
| Other government | 1.2 (1.0-1.5) | .037 | 0.8 (0.5-1.2) | .287 | 0.6 (0.3-1.1) | .124 |
| Uninsured | 0.7 (0.6-0.8) | <.001 | 1.0 (0.7-1.3) | .743 | 0.7 (0.5-0.9) | .019 |
| Race and ethnicity | ||||||
| Black, non-Hispanic | Reference | Not in model | Not in model | |||
| White, non-Hispanic | 0.9 (0.8-1.0) | .051 | ||||
| Hispanic | 1.2 (1.0-1.4) | .048 | ||||
| Other, non-Hispanic | 0.9 (0.5-1.6) | .706 | ||||
| Distance to care | Not in model | Not in model | ||||
| <5 miles | Reference | |||||
| 5-15 miles | 1.1 (1.0-1.1) | .085 | ||||
| >15 miles | 1.4 (1.3-1.5) | <.001 | ||||
Abbreviations: CCP = Community Cancer Program; CI = confidence interval; IN = integrated network.
Sample size in this analysis was limited by the number of patients having complete data for the modeled variables. Age at diagnosis was adjusted using the restricted cubic spline approach and included as a continuous variable in the ShWBRT and ExWBRt models to control for the nonlinear influence of this variable.
Figure 3.
Yearly trends of whole brain radiation therapy (WBRT) dose-fractionations for patients with their first lifetime malignancy and brain metastasis (BM) at diagnosis. The y-axis represents the percentage of patients diagnosed with BM in a year (2010, n = 14,613; 2011, n = 14,529; 2012, n = 14,665; 2013, n = 15,347; 2014, n = 15,727; 2015, n = 15,507) treated with the modality. Linear regression model fits are plotted with 95% confidence intervals.
There were significant differences in WBRT dose-fractionations by age with the ShWBRT cohort having the oldest median age (Table 1). There was no significant difference in WBRT dose-fractionation by race or distance to care. In multivariable analysis, compared with StWBRT, patients from high-income ZIP codes had a lower likelihood of ShWBRT (highest vs lowest quartile: aOR = 0.8; 95% CI, 0.6-0.9) and ExWBRT treatment (highest vs lowest quartile: aOR = 0.7; 95% CI, 0.6-0.9; Table 2). Compared with Medicaid, there was a significantly lower likelihood of ShWBRT in patients with private insurance (aOR = 0.7; 95% CI, 0.6-0.9), and uninsured patients had a lower likelihood of receiving ExWBRT (aOR = 0.7; 95% CI, 0.5-0.9; Table 2). Compared with StWBRT, academic facilities had a significantly higher use of ShWBRT than comprehensive CCPs (aOR = 1.4; 95% CI, 1.2-1.6), whereas academic (aOR = 0.4; 95% CI, 0.3-0.5) and integrated networks and CCPs (aOR = 0.6; 95% CI, 0.5-0.7) had significantly lower ExWBRT use than comprehensive CCPs (Table 2).
There was a significant difference in race between SRS and WBRT cohorts, even when unknown race was excluded. A higher likelihood of SRS in Hispanic (aOR = 1.2; 95% CI, 1.0-1.4) than non-Hispanic black or white patients characterized this racial difference (Table 2). There was no significant difference in SRS versus WBRT between non-Hispanic black and white patients. Greater distance (>15 miles) from a patient's ZIP code of residence to the treatment facility significantly related to higher SRS use (aOR = 1.4; 95% CI, 1.3-1.5; Table 2). Patients from ZIP codes in the highest income quartiles had significantly higher incidence of treatment with SRS than WBRT (highest vs lowest quartile: aOR = 1.6; 95% CI. 1.4-1.7; Table 2). Compared with Medicaid, patients with private insurance had a significantly higher (aOR = 1.4; 95% CI, 1.3-1.5), and uninsured patients had a significantly lower (aOR = 0.7; 95% CI, 0.6-0.8), likelihood of SRS. Compared with comprehensive CCPs, there was a significantly higher likelihood of SRS at academic facilities (aOR = 1.9; 95% CI, 1.8-2.0) and CCPs and integrated networks (aOR = 1.1; 95% CI, 1.0-1.2; Table 2).
Clinical characteristics and survival
Nonzero Charlson-Deyo indices were significantly associated with a lower likelihood of SRS (Table 2). There was a significant difference in primary tumor site between WBRT and SRS cohorts (Table 1). Lung primaries accounted for the majority of patients, but a significantly smaller proportion of the SRS (78.8%) than WBRT (87.2%) cohort. Urinary, skin, and gastrointestinal tumors constituted a significantly larger proportion of the SRS than WBRT cohort. These differences extend to histology and presumed radioresistance, with a higher proportion of patients with a radioresistant histology in the SRS (14.4%) than WBRT (7.9%) cohort (Table 1). Radioresistance was associated with a significantly higher likelihood of SRS (aOR = 2.0; 95% CI, 1.9-2.2; Table 2). Chemotherapy was part of treatment for the majority of patients (65.7%) and related to significantly higher receipt of SRS (aOR = 1.4; 95% CI, 1.4-1.5) or ExWBRT (aOR = 1.2; 95% CI, 1.0-1.4), whereas those not receiving chemotherapy had significantly higher receipt of ShWBRT (aOR = 3.5; 95% CI, 3.1-4.1; Table 2). Median OS (SRS = 11.7; 95% CI, 11.3-12.0; WBRT = 5.7 months; 95% CI, 5.6-5.9), 6-month OS (SRS = 72.2%; 95% CI, 71.2-73.0; WBRT = 48.5%; 95% CI, 47.8-49.2), and 36-month OS (SRS = 18.9%; 95% CI, 18.0-19.8; WBRT = 6.4%; 95% CI, 6.0-6.8; Table 3) differed between SRS and WBRT cohorts. Median OS by WBRT dose-fractionation was 1.9 months (95% CI, 1.8-2.2) for ShWBRT, 5.9 months (95% CI, 5.8-6.0) for StWBRT, and 8.3 months (95% CI, 7.4-9.3) for ExWBRT.
Table 3.
Kaplan-Meier overall survival analysis for patients with brain metastases at diagnosis treated with whole brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS)
| Median survival, months (95% CI) | 6-mo survival, % (95% CI) | 36-mo survival, % (95% CI) | |
|---|---|---|---|
| SRS (n = 8715) | 11.7 (11.3-12.0) | 72.2 (71.2-73.0) | 18.9 (18.0-19.8) |
| WBRT (n = 20,611) | 5.7 (5.6-5.9) | 48.5 (47.8-49.2) | 6.4 (6.0-6.8) |
| ShWBRT (n = 854) | 1.9 (1.8-2.2) | 16.9 (14.5-19.7) | 2.7 (1.7-4.3) |
| StWBRT (n = 18,935) | 5.9 (5.8-6.0) | 49.4 (48.7-50.1) | 6.4 (6.1-6.9) |
| ExWBRT (n = 822) | 8.3 (7.4-9.3) | 59.8 (56.5-63.2) | 9.3 (7.5-11.7) |
Abbreviations: CI = confidence interval; ExWBRT = extended dose-fractionations; ShWBRT = short dose-fractionations; SRS = stereotactic radiosurgery; StWBRT = standard dose-fractionations.
Discussion
This study identified a 2.2% incidence of BM at the time of cancer diagnosis. Among these patients, there was a decline in WBRT and concomitant doubling of SRS utilization as the first course of radiation therapy from 2010 to 2015. The most common WBRT dose-fractionation was 30 Gy in 10 fractions, which was used in more than half of the WBRT cohort, whereas SRS was most commonly given in 1 fraction of 12 to 24 Gy. Contrary to prior work finding that black and Hispanic patients were less likely to receive SRS, this study found Hispanic patients were more likely to receive SRS, and that no significant difference in SRS between non-Hispanic white and black patients existed. Unlike prior NCDB studies of BM radiation therapy trends, this work detailed WBRT dose-fractionation trends, showing that ExWBRT decreased significantly during the study period. Owing to the selection bias inherent in the relationship between expected outcome and selection of treatment modality, the study did not define an effect of dose-fractionation on survival.
Nonzero Charlson-Deyo indices and a lack of chemotherapy were associated with worse survival as indicated by higher use of WBRT. Patients not receiving chemotherapy also had a 3.5-fold higher odds ratio of ShWBRT, the dose-fractionation cohort with the shortest survival. The associations between these NCDB variables and the cohorts with the lowest survival suggests these variables may serve as surrogates of poor baseline performance status or prognosis.
Although the reasons for decreased WBRT utilization cannot be definitively determined, multiple events occurred during the study period that likely influenced WBRT trends. For example, ASTRO's September 2014 Choosing Wisely recommendations included not routinely adding WBRT to SRS in cases of limited BM.38 An interim report for the Quality of Life after Treatment for Brain Metastases (QUARTZ) trial was released in 2013 and reported no significant benefit of adding WBRT to optimal supportive care.39 Diagnostic advances during the study period, such as identifying biomarkers of radioresistance, may have contributed to excluding patients with poor performance status from WBRT treatment.40, 41, 42 However, a decline in ShWBRT, the treatment associated with the worst performance status, was not seen. The overall decline in WBRT use has likely continued since the end of the study period owing to the influence of the QUARTZ trial, which demonstrated no significant improvement in overall survival or overall quality of life for WBRT in patients with poor prognosis.31
Increasing SRS use may have been driven by an expansion of clinical indications and due to facility-related factors. Indications for SRS have expanded owing to studies showing the efficacy of SRS for multiple BM lesions,8, 9, 10, 12 enhanced survival for SRS in treating BM from certain cancers,11 and a lack of benefit when adding WBRT to SRS.43 Academic centers, which are more likely to have on-site SRS services, have previously been shown to drive increased SRS usage.14 Academic centers are nearly 2-fold more likely to use SRS than WBRT, and more than 2-fold more likely to use StWBRT than ExWBRT. The relatively low use of ExWBRT and high use of SRS suggests a shift to SRS to avoid late toxicities in those expected to have the longest life expectancy. Prior studies demonstrated that the availability of on-site SRS is the most predictive factor of SRS use,15 and 50% of facilities in 2014 did not report treatment with brain SRS.14 We speculate that because more facilities have adopted SRS since the end of this study period, the trend of increasing SRS and decreasing ExWBRT has continued.
This study highlights demographic trends related to income level and insurance status. SRS was more likely to be used for patients from wealthier areas or who were insured. A previous NCDB study found a growing disparity in SRS use for patients from less wealthy areas, less educated areas, or who had Medicaid or no insurance.14 Although that study found that black and Hispanic patients were less likely to receive SRS, we found higher likelihood of SRS for Hispanic, and no difference between non-Hispanic white and black patients. For WBRT dose-fractionations, ShWBRT was more likely to be used than StWBRT in patients with Medicaid or no insurance and less likely to be used for those from the wealthiest areas. Previously, similar findings have been attributed to poor insurance status relating to diagnostic delays that result in a worse prognosis at time of diagnosis, or owing to late presentation to medical care among those of lower socioeconomic status.44 Alternatively, sociodemographic factors may relate to differential adherence to National Comprehensive Cancer Network radiation therapy guidelines, which has been previously suggested.45
Given the retrospective nature of this analysis, we could not define the casual relationship between radiation therapy modality and patient survival, and it is likely that treatment with SRS or WBRT, as well as WBRT dose-fractionation, was selected based on patient characteristics that were responsible for the underlying differences in survival. Analysis was limited by a lack of data on performance status as well as the number, location, and size of BM, all of which can influence treatment modality selection. Thus, although this study confirmed increasing use of SRS for BM, it does not definitively differentiate patient characteristics used to select treatment modality. Furthermore, the NCDB only reports the total number of fractions administered (ie, regional plus boost if applicable), making accurate determination of radiation therapy dose-fractionation difficult. Additional limitations include a lack of data on treatment-related morbidity and additional radiation therapy beyond the first course of treatment. Finally, this study only included patients with BM at the time of initial cancer diagnosis, the majority from lung cancer, meaning the results may not be reflective of patients presenting with BM at later stages of disease.
Conclusions
Although WBRT for BM as the first course of radiation therapy is declining and SRS is increasing in the United States, WBRT was still used more than SRS in the period analyzed. Furthermore, the increasing application of SRS is not occurring solely at the expensive of WBRT because the population treated by either SRS or WBRT increased from 36.5% of newly diagnosed BM patients in 2010 to 41.4% in 2015. This analysis demonstrated SRS is likely being increasingly used to deliver a higher BED for overcoming radioresistance. We speculate the decline in ExWBRT is related to a shift to SRS to avoid late toxicities in those expected to have the best outcomes. A difference in radiation therapy modality by insurance status requires additional attention because it is unclear whether this is driven by differences in prognosis at diagnosis, selection bias owing to socioeconomic factors such as reimbursement, or factors not identified in the NCDB.
Footnotes
Sources of support: Dr Barbour is supported by the Stead Scholarship Program of the Duke University Department of Medicine. Dr Suneja is supported by grants K08CA228631 and P30AI064518 from the USNational Institutes of Health. Dr Floyd is supported by Burroughs Wellcome Fund Career Award for Medical Scientists.
Disclosures: Dr Kirkpatrick reports grants from Varian Medical System and personal fees from ASTRO. Disclaimer: The data are derived from a de-identified National Cancer Database file. The American College of Surgeons and Commission on Cancer have not verified and are not responsible for the analytical or statistical methodology used or the conclusions drawn by the investigators.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2019.07.012.
Supplementary data
References
- 1.Nussbaum E.S., Djalilian H.R., Cho K.H., Hall W.A. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–1788. [PubMed] [Google Scholar]
- 2.Chen R.C., Punglia R.S., Sher D.J. Stereotactic radiosurgery (SRS) vs. whole brain radiation therapy (WBRT) vs. combined treatment (SRS & WBRT) for brain metastases: A decision analysis. Int J Radiat Oncol Biol Phys. 2009;75:S89. [Google Scholar]
- 3.Chao J.H., Phillips R., Nickson J.J. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682–689. doi: 10.1002/1097-0142(195407)7:4<682::aid-cncr2820070409>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Order S.E., Hellman S., Von Essen C.F., Kligerman M.M. Improvement in quality of survival following whole-brain irradiation for brain metastasis. Radiology. 1968;91:149–153. doi: 10.1148/91.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Brown P.D., Pugh S., Laack N.N., Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncology. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sneed P.K., Larson D.A., Wara W.M. Radiotherapy for cerebral metastases. Neurosurg Clin N Am. 1996;7:505–515. [PubMed] [Google Scholar]
- 7.Graham P.H., Bucci J., Browne L. Randomized comparison of whole brain radiotherapy, 20 Gy in four daily fractions versus 40 Gy in 20 twice-daily fractions, for brain metastases. Int J Radiat Oncol Biol Phys. 2010;77:648–654. doi: 10.1016/j.ijrobp.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Brown P.D., Ballman K.V., Cerhan J.H. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama H., Shirato H., Tago M. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 11.Robin T.P., Jones B.L., Amini A. Radiosurgery alone is associated with favorable outcomes for brain metastases from small-cell lung cancer. Lung Cancer. 2018;120:88–90. doi: 10.1016/j.lungcan.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M., Serizawa T., Higuchi Y. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 13.Modh A., Burmeister C., Elshaikh M.A. Radiation utilization trends in the treatment of brain metastases from non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99:E94. [Google Scholar]
- 14.Kann B.H., Park H.S., Johnson S.B., Chiang V.L., Yu J.B. Radiosurgery for brain metastases: Changing practice patterns and disparities in the United States. J Natl Compr Canc Netw. 2017;15:1494–1502. doi: 10.6004/jnccn.2017.7003. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson D.C., Charpentier A.M., Cigsar C. A multi-institutional study of factors influencing the use of stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013;85:335–340. doi: 10.1016/j.ijrobp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Fahrig A., Ganslandt O., Lambrecht U. Hypofractionated stereotactic radiotherapy for brain metastases—results from three different dose concepts. Strahlenther Onkol. 2007;183:625–630. doi: 10.1007/s00066-007-1714-1. [DOI] [PubMed] [Google Scholar]
- 17.Minniti G., D'Angelillo R.M., Scaringi C. Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol. 2014;117:295–301. doi: 10.1007/s11060-014-1388-3. [DOI] [PubMed] [Google Scholar]
- 18.Marcrom S.R., McDonald A.M., Thompson J.W. Fractionated stereotactic radiation therapy for intact brain metastases. Adv Radiat Oncol. 2017;2:564–571. doi: 10.1016/j.adro.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton B.R., Gebhardt B., Prabhu R. Hypofractionated radiosurgery for intact or resected brain metastases: Defining the optimal dose and fractionation. Radiat Oncol. 2013;8:135. doi: 10.1186/1748-717X-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler J.R., Jr., Gibbs I.C., Puataweepong P., Chang S.D. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2006;59:244–254. doi: 10.1227/01.NEU.0000223512.09115.3E. discussion 244-254. [DOI] [PubMed] [Google Scholar]
- 21.Mehta V.K., Lee Q.T., Chang S.D., Cherney S., Adler J.R., Jr. Image guided stereotactic radiosurgery for lesions in proximity to the anterior visual pathways: A preliminary report. Technol Cancer Res Treat. 2002;1:173–180. doi: 10.1177/153303460200100302. [DOI] [PubMed] [Google Scholar]
- 22.Milano M.T., Usuki K.Y., Walter K.A., Clark D., Schell M.C. Stereotactic radiosurgery and hypofractionated stereotactic radiotherapy: Normal tissue dose constraints of the central nervous system. Cancer Treat Rev. 2011;37:567–578. doi: 10.1016/j.ctrv.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Kirkpatrick J.P., Soltys S.G., Lo S.S. The radiosurgery fractionation quandary: Single fraction or hypofractionation? Neuro Oncol. 2017;19(Suppl 2):ii38–ii49. doi: 10.1093/neuonc/now301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lischalk J.W., Oermann E., Collins S.P. Five-fraction stereotactic radiosurgery (SRS) for single inoperable high-risk non-small cell lung cancer (NSCLC) brain metastases. Radiat Oncol. 2015;10:216. doi: 10.1186/s13014-015-0525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtz J.M., Gelber R., Brady L.W., Carella R.J., Cooper J.S. The palliation of brain metastases in a favorable patient population: A randomized clinical trial by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 1981;7:891–895. doi: 10.1016/0360-3016(81)90005-5. [DOI] [PubMed] [Google Scholar]
- 26.Epstein B.E., Scott C.B., Sause W.T. Improved survival duration in patients with unresected solitary brain metastasis using accelerated hyperfractionated radiation-therapy at total doses of 54.4 Gray and greater: Results of radiation-therapy oncology group 85-28. Cancer. 1993;71:1362–1367. doi: 10.1002/1097-0142(19930215)71:4<1362::aid-cncr2820710431>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Murray K.J., Scott C., Greenberg H.M. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: A report of the radiation therapy oncology group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39:574. doi: 10.1016/s0360-3016(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 28.Haie-Meder C., Pellae-Cosset B., Laplanche A. Results of a randomized clinical trial comparing two radiation schedules in the palliative treatment of brain metastases. Radiother Oncol. 1993;26:111–116. doi: 10.1016/0167-8140(93)90091-l. [DOI] [PubMed] [Google Scholar]
- 29.Komarnicky L.T., Phillips T.L., Martz K. A randomized phase-III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916) Int J Radiat Oncol Biol Phys. 1991;20:53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 30.Slotman B., Faivre-Finn C., Kramer G. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 31.Mulvenna P., Nankivell M., Barton R. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieder C., Grosu A.L., Andratschke N.H., Molls M. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys. 2006;66:1446–1449. doi: 10.1016/j.ijrobp.2006.07.1383. [DOI] [PubMed] [Google Scholar]
- 33.Team R.C.R. R Foundation for Statistical Computing; Vienna, Austria: 2018. A language and environment for statistical computing. [Google Scholar]
- 34.Wickham H. Springer-Verlagl; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 35.Kassambara A, Kosinski M. Survminer: Drawing Survival Curves using ‘ggplot2’. 2018.
- 36.Schratz P. R Package ‘Oddsratio’: Odds Ratio Calculation for GAM(M)s & GLM(M)s. 2017.
- 37.Groenwold R.H., Klungel O.H., Altman D.G. Adjustment for continuous confounders: An example of how to prevent residual confounding. CMAJ. 2013;185:401–406. doi: 10.1503/cmaj.120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn C., Kavanagh B., Bhatnagar A. American Society for Radiation Oncology: Ten things physicians and patients should question. Pract Radiat Oncol. 2014;4:349–355. doi: 10.1016/j.prro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Langley R.E., Stephens R.J., Nankivell M. Interim data from the Medical Research Council QUARTZ Trial: Does whole brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin Oncol (R Coll Radiol) 2013;25:e23–30. doi: 10.1016/j.clon.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Sais E., Menéndezn J.A., Bosch-Barrera J. The practice-changing QUARTZ trial: Is there any role for whole brain radiotherapy in patients with non-small cell lung cancer and brain metastases? Translational Cancer Research. 2017;6(Suppl 1):S201–S204. [Google Scholar]
- 41.Ponz-Sarvisé M., Nguewa P.A., Pajares M.J. Inhibitor of differentiation-1 as a novel prognostic factor in NSCLC patients with adenocarcinoma histology and its potential contribution to therapy resistance. Clin Cancer Res. 2011;17:4155–4166. doi: 10.1158/1078-0432.CCR-10-3381. [DOI] [PubMed] [Google Scholar]
- 42.Castañon E., Bosch-Barrera J., López I. Id1 and Id3 co-expression correlates with clinical outcome in stage III-N2 non-small cell lung cancer patients treated with definitive chemoradiotherapy. J Transl Med. 2013;11:13. doi: 10.1186/1479-5876-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Churilla T.M., Ballman K.V., Brown P.D. Stereotactic radiosurgery with or without whole-brain radiation therapy for limited brain metastases: A secondary analysis of the North Central Cancer Treatment Group N0574 (Alliance) randomized controlled trial. Int J Radiat Oncol Biol Phys. 2017;99:1173–1178. doi: 10.1016/j.ijrobp.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin S., Ulrich C., Munsell M. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12:816–824. doi: 10.1634/theoncologist.12-7-816. [DOI] [PubMed] [Google Scholar]
- 45.Graboyes E.M., Garrett-Mayer E., Sharma A.K., Lentsch E.J., Day T.A. Adherence to National Comprehensive Cancer Network guidelines for time to initiation of postoperative radiation therapy for patients with head and neck cancer. Cancer. 2017;123:2651–2660. doi: 10.1002/cncr.30651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.