Abstract
Purpose
To evaluate whether response assessment of newly diagnosed glioblastoma at 3 months using 11C-methionine-positron emission tomography (MET-PET) is better associated with patient outcome compared with baseline MET-PET or anatomic magnetic resonance imaging alone.
Methods and Materials
Patients included were participants in a phase I/II trial of dose-escalated chemoradiation based on anatomic magnetic resonance imaging. Automated segmentation of metabolic tumor volume (MTV) was performed at a threshold of 1.5 times mean cerebellar uptake. Progression-free (PFS) and overall survival were estimated with the Kaplan-Meier method and compared with log-rank tests. Multivariate analysis for PFS and overall survival was performed using Cox proportional hazards, and spatial overlap between imaging and recurrence volumes were analyzed.
Results
Among 37 patients, 15 had gross total resection, of whom 10 (67%) had residual MTV, 16 subtotal resection, and 6 biopsy alone. Median radiation therapy dose was 75 Gy (range, 66-81). Median baseline T1 Gd-enhanced tumor volume (GTV-Gd) was 38.0 cm3 (range, 8.0-81.5). Median pre-CRT MTV was 4.9 cm3 (range, 0-43.8). Among 25 patients with 3-month MET-PET, MTV was only 2.4 cm3 (range, 0.004-18.0) in patients with uptake. Patients with MTV = 0 cm3 at 3 months had superior PFS (18.2 vs 10.1 months, P = .03). On multivariate analysis, larger 3-month MTV (hazard ratio [HR] 2.4, 95% confidence interval [CI], 1.4-4.3, P = .03), persistent MET-PET subvolume (overlap of pre-CRT and 3 month MTV; HR 2.0, 95% CI, 1.2-3.4, P = .06), and increase in MTV (HR 1.8, 95% CI, 1.1-3.1, P = .09) were the only imaging factors significant for worse PFS. GTV-Gd at recurrence encompassed 97% of the persistent MET-PET subvolume (interquartile range 72%-100%), versus 71% (interquartile range 39%-93%) of baseline MTV, 54% of baseline GTV-Gd (18%-87%), and 78% of 3-month MTV (47%-95%).
Conclusions
The majority of patients with apparent gross total resection of glioblastoma have measurable postoperative MTV. Total and persisting MTV 3 months post-CRT were significant predictors of PFS, and persistent MET-PET subvolume was the strongest predictor for localizing tumor recurrence.
Introduction
Glioblastoma (GBM) is traditionally treated by maximal safe resection followed by chemoradiation (CRT); however, overall survival remains poor despite decades of research.1, 2 Radiation therapy is a backbone of treatment because these tumors are always incompletely surgically removed. Typically, radiation planning relies on T1-weighted gadolinium enhanced (T1-Gd) and T2/fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) to define tumor treatment volume.3, 4 However, the T1-Gd volume does not fully encompass the tumor volume as some infiltrative portions of tumor are nonenhancing.5 This has led to the investigation of biologically based imaging techniques, such as advanced MRI and radiolabeled positron emission tomography (PET), which have been shown to identify areas of tumor not identified with traditional MRI.6, 7, 8, 9, 10, 11, 12, 13
Glucose-labeled radiotracers like 2-deoxy-2-(18F)fluoro-D-glucose are less useful in gliomas owing to insufficient distinction between tumor and the background cortex and the inability to distinguish from benign sources of inflammation.14, 15 In contrast, amino acid tracers have superior ability to diagnose, grade, and delineate extent of glioma; guide surgical and radiation treatment planning; and assess response.14 In particular (11C-methionine)-positron emission tomography (MET-PET), the most well-studied amino acid radiotracer in malignant brain tumors, has been found to have a high detection rate with improved tumor delineation based on histopathologic confirmation.16 MET-PET uptake is increased in GBM owing to increased expression of L-amino acid transporter in tumor cells compared with normal brain tissue and increased metabolism of methionine which results in higher avidity for 11C-methionine in tumor.17 In contrast, T1-gadolinium enhanced MRI, the standard imaging for GBM diagnosis and therapeutic intervention, relies upon nonspecific increased permeability of the blood-brain barrier, which may be present in the absence of tumor or may be absent in regions of infiltrative disease.18, 19, 20 Both standardized uptake value metrics (the ratio of intensity in tumor compared with normal brain tissue) as well as metabolic tumor volume (MTV) may be quantitated from amino acid PET scans including 11C-MET PET. Direct comparison between MTV and standardized uptake value suggests that the MTV may be more prognostic for overall survival because it better characterizes the whole tumor volume rather than the evaluation of a single voxel with greater intensity.21, 22
Recognizing the prognostic limitations of anatomic MRI and the previously identified value of MTV, we sought to evaluate the prognostic value of MTV identified by MET-PET before and after chemoradiation, and whether 3-month response assessment was better associated with patient outcome compared with baseline or anatomic MRI alone. Additionally, spatial correlation between MTV and persistent MTV with recurrent tumor volume was explored to determine whether biologically significant tumor subvolumes could be identified that may serve as potential radiation therapy targets in future studies.
Methods
Study patients and data collection
We performed an institutional review board–approved retrospective analysis of patients with newly diagnosed histologically confirmed supratentorial World Health Organization grade IV gliomas who were treated on a phase I/II clinical trial evaluating dose-escalated radiation therapy (RT) based on anatomic MRI alone from 2003 to 2007.23 After surgery but before radiation treatment, baseline anatomic MRI, including T1-Gd and T2/FLAIR images and MET-PET scans were obtained. A subset of patients underwent MET-PET imaging 3 months postchemoradiation. Clinical and pathologic information was obtained from the medical record. The extent of resection was defined based on the postoperative imaging and multidisciplinary assessment.
Radiation treatment
All patients were treated on a prospective dose escalation trial using standard anatomic MRI that has previously been published.23 Residual T1-weighted gadolinium enhanced tumor or resection cavity (after gross total resection) was defined on the T1-Gd MRI (GTV-Gd). Clinical target volume was defined as GTV-Gd expanded by 1.5 cm delimited by anatomic boundaries. Two planning target volumes (PTV) were defined: PTV1 was clinical target volume plus 0.5 cm which was planned to 60 Gy in 30 fractions. PTV2 was GTV-Gd plus 0.5 cm planned using simultaneous integrated boost to an assigned dose level (66-81 Gy in 30 fractions) via forward- or inverse-planned intensity modulated radiation therapy. Per protocol, at least 99.5% of the PTV was encompassed by the 95% prescription isodose surface (IDS).
MET-PET imaging
Baseline and 3-month follow-up MET-PET scans were obtained on a Siemens ECAT EXACT HR plus whole-body tomography with axial resolution 4.1 mm FWHM in the center of the field of view.24 Owing to the short half-life of 11C-methionine, a cyclotron was required on site. A 6-minute long (approximately 1.5 million counts) transmission scan was obtained using 3 Ge-68 rod sources before intravenous injections of approximately 740 MBq of MET. Ten to 30 minutes later emissions scan image data was obtained in 3-dimensional mode, which was summed into a single frame and analyzed. An all-pass filter reconstructed iteratively emission data corrected for attenuation, scatter, and random coincidences with 4 iterations and 16 subsets (128 × 128 pixel matrix).
Image registration
The pre-RT treatment planning (computed tomography) CT, FLAIR MRI, and MET-PET images were coregistered with the T1-Gd MRI using an in-house functional imaging analysis tool (imFIAT).25 The 95% IDS was generated from the planned composite dose distribution and aligned with the T1-Gd MRI through coregistration with CT. The T1-Gd recurrence MRI was coregistered to the baseline T1-Gd MRI. All image registrations were done using rigid body transformation and mutual information. Nonrigid transformation was necessary for one patient owing to cavity change over time. Image registration was confirmed by visual inspection, split-screen, scrolling slice by slice, viewing in different planes, and by comparing contour overlays.
Metabolic tumor volume delineation
For MET-PET scans at baseline and 3 months, automated segmentation of MTV was performed using imFIAT and a threshold of 1.5 times mean cerebellar uptake as previously described.13 Uptake in normal tissue (ie, lacrimal and pituitary glands) was manually excluded to create the MTV.26 Persistent MTV at 3 months was defined as the subvolume of overlap between the 3-month MTV and the pre-CRT (baseline) MTV.
Follow-up and patterns of failure analysis
Progression was typically defined by multidisciplinary determination as radiographic evidence of Gd-enhanced progression and clinical findings. The pattern of failure tumor volume (TVPOF) was defined based on the T1-Gd MRI obtained at time of progression in all but 2 cases who underwent a contrast CT instead of MRI. One of these patients had analyzable imaging data and was included in the pattern of failure analysis, whereas the other had imaging data that was unable to be used due to errors in the image file.
Statistical analysis
Progression-free survival (PFS) was defined as the interval from diagnosis to progression or death. Overall survival was defined as the interval from diagnosis to death of any cause or date of last follow-up for alive patients, calculated using Kaplan-Meier methods. Comparison of PFS and OS outcomes for patients with and without detectable MTV at 3 months was performed using log-rank test. Univariate and multivariate Cox proportional hazard models were used to identify factors associated with PFS and OS. Clinical factors included were age (continuous), sex, Karnofsky Performance Status (KPS; ≥90 vs < 90), extent of surgery (gross total resection [GTR] vs subtotal resection [STR] vs biopsy), O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status, and radiation dose (continuous). Imaging variables tested included GTV-Gd, GTV-FLAIR, MTV at baseline, and MTV at 3 months. Imaging subvolumes included persistent MTV at 3 months, MTV inside and outside the 95% IDS, and MTV outside GTV-FLAIR. Progression related features were included as time dependent covariates in the OS models. In the multivariate analysis, clinical covariates that were significant in the univariate analysis were included (KPS, age, and extent of resection) and one imaging covariate was added at a time in a step-wise fashion to create 11 multivariate models with 4 covariates each. For multiple testing of imaging covariates, the Benjamini-Hochberg method was used for false discovery rate (FDR) control with the FDR control rate set to 10%.27 For continuous covariates, the reported hazard ratio (HR) was per 1 standard deviation increase to allow for comparison of the magnitude of HRs between different imaging metrics.
Results
Baseline patient and imaging characteristics
The 37 patients who participated on a phase I/II dose escalation study were included in this analysis. The median age was 53 years (range, 20-75), and 49% of patients were male.23 Fifteen had GTR, 16 STR, and 6 biopsy alone (Table 1). Median time from surgery until baseline pre-RT MRI and MET-PET was 20 days (range, 1-43) and 21 days (range, 6-40), respectively. Median prescribed RT dose was 75 Gy (range, 66-81). All patients received concurrent temozolomide (TMZ) and 32 patients (86%) received adjuvant TMZ; 5 did not receive adjuvant TMZ owing to clinical deterioration or death. Among the subset of patients in whom MGMT methylation status was available, 6 of 10 had MGMT promoter methylation.
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic (n = 37) | No. (percent) |
|---|---|
| Age (y) | |
| Mean | 53 |
| Standard deviation | 14.80 |
| Sex | |
| Female | 19 (51) |
| Male | 18 (49) |
| KPS | |
| ≥90 | 32 (87) |
| 80 | 3 (8) |
| 70 | 2 (5) |
| MGMT∗ | |
| Methylated | 6 (16) |
| Unmethylated | 4 (11) |
| Unknown | 27 (73) |
| Received adjuvant TMZ | 32 (86) |
| Surgery type | |
| Biopsy | 6 (16) |
| Subtotal resection | 16 (43) |
| Gross total resection | 15 (41) |
| Dose (Gy) | |
| Median | 75 |
| Range | 66-81 |
Abbreviations: KPS = Karnofsky Performance Status; MGMT = O6-methylguanine-DNA methyltransferase; TMZ, temozolomide.
Ten patients with available data.
The median postoperative GTV-Gd was 38.0 cm3 (range, 8.0-81.5) and the median GTV-FLAIR was 73.2 cm3 (range, 9.7-206.2; Table 2). In comparison, the median postoperative MTV was 4.9 cm3 (range, 0-43.8). Ten of the 15 (67%) patients with apparent GTR had residual MTV. The median enhancing component of the MTV was 4.2 cm3 (range, 0-32.8) with a median of 77% of the MTV demonstrating enhancement (range, 2%-100%). The median nonenhancing component was 1.2 cm3 (range, 0-24.5), and the median MTV outside of GTV-FLAIR was 0.2 cm3 (range, 0-4.7); 8 of 37 had ≥1 cm3 of MTV outside of GTV-FLAIR. Therefore, 3 patients had ≥1 cm3 MTV extending outside of the 95% IDS, because GTV-FLAIR was not specifically targeted.
Table 2.
Characteristics of anatomic and metabolic imaging volumes
| Imaging variable (cm3) | Median (range) |
|---|---|
| Preradiation therapy | |
| MTV | 4.9 (0-43.8) |
| GTV-Gd | 38.0 (8.0-81.5) |
| GTV-FLAIR | 73.2 (9.7-206.2) |
| Enhancing MTV | 4.2 (0-32.8) |
| Nonenhancing MTV | 1.2 (0-24.5) |
| MTV outside 95% IDS | 0 (0-3.6) |
| 3 mo | |
| MTV at 3 mo | 0.1 (0-18.0) |
| Change in MTV from baseline | –2.7 (–41.4-0.1) |
| Persistent MTV subvolume | 1.5 (0.0-10.9) |
Abbreviations: IDS = isodose surface; GTV-FLAIR = volume of fluid-attenuated inversion recovery intensity; GTV-Gd = gadolinium-enhanced gross tumor volume; MTV = metabolic tumor volume.
Changes in MTV 3 months post chemoradiation
Twenty-five of the 37 patients underwent MET-PET imaging approximately 3 months from completion of chemoradiation. In contrast to the baseline MTV (4.9 cm3), the median MTV at 3 months among patients with uptake was only 2.4 cm3 (range, 0.004-18.0), and 9 of 25 patients had no uptake at 3 months. Only one patient had an increase in MTV and experienced early failure <6 months posttreatment, whereas the remaining patients had a reduction in absolute volume in MTV. Six of 22 patients (27%) with uptake pre-CRT had no residual MTV at 3 months (Fig 1). The median reduction in MTV from baseline was 2.7 cm3 (range, –0.1 to 41.4). At 3 months, the median persistent MET-PET subvolume (overlap between the baseline and 3-month MET-PET) was 1.5 cm3 (range, 0-10.9), and 9 of 25 patients had ≥1 cm3 of overlap.
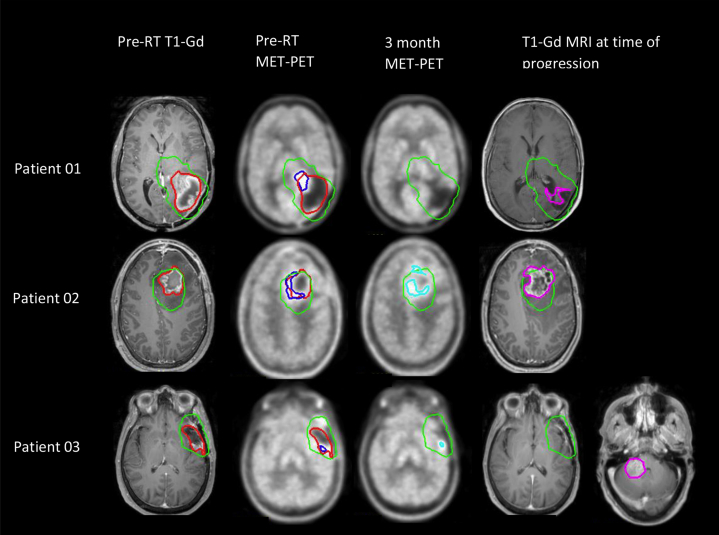
Figure 1.
Anatomic and metabolic tumor volumes (MTV) before and after chemoradiation in relation to recurrence. Patient 01 had a subtotal resection with residual MTV extending anteromedially beyond the surgical cavity, with a complete metabolic response 3 months post chemoradiation. He experienced delayed progressed at 19 months. Patient 02 underwent subtotal resection and demonstrated residual MTV along the medial cavity that persisted 3 months post-chemoradiation, with subsequent early recurrence at 9 months. Patient 03 underwent gross total resection, yet had residual MTV that reduced in volume at 3 months. He experienced distant recurrence remote from the original resection bed and MTV at 18 months. Red = enhancing gross tumor volume and surgical cavity (GTV-Gd); blue = MTV pre-CRT; cyan = MTV at 3 months; green = 95% isodose surface; purple = enhancing tumor recurrence volume.
Survival outcomes
Among all patients, median PFS was 9.5 months (95% CI, 7.2-17.9) and median OS was 20.4 months (95% CI, 15.6-36.8). The subset of patients with MTV = 0 cm3 at 3 months had superior PFS: 18.2 (95% CI, 9.5-NA) versus 10.1 months (95% CI, 6.8-17.9; P = .03, Fig 2). Results of the univariate analysis for PFS and OS are shown in Table 3. On multivariate analysis (Table 4), worse KPS (HR 6.0, 95% CI 1.5-23.8, P = .01) and biopsy compared with GTR (HR 5.1, 95% CI 1.7-15.3, P < .01) were associated with worse PFS. The only imaging factors significantly associated with worse PFS were larger 3-month MTV (HR 2.4, 95% CI, 1.4-4.3, P < .01, P value after FDR 0.03), persistent MET-PET subvolume (HR 2.0, 95% CI, 1.2-3.4, P = .01, P value after FDR 0.06), and an increase in MTV from baseline (HR 1.8, 95% CI, 1.1-3.1, P = .02, P value after FDR 0.09). For OS, only KPS (HR 15.6, 95% CI, 4.0-62.5, P < .01), age (HR 1.5, 95% CI, 1.0-2.2 P = .05), and biopsy versus GTR (HR 3.3, 95% CI, 1.2-9.3, P = .03) were significant (Table 4).
Figure 2.
Progression free (PFS) and overall survival (OS) Kaplan-Meier curves of patients with. and without metabolic tumor volume (MTV) at 3 months demonstrating significantly improved PFS and a trend toward improved OS in patients without MTV at 3 months post chemoradiation.
Table 3.
Univariate Cox proportional hazards model of progression free survival and overall survival
| OS |
PFS |
|||||
|---|---|---|---|---|---|---|
| Covariates∗.† | HR | 95% CI | P value‡ | HR | 95% CI | P value‡ |
| Age | 1.5 | 1.0-2.1 | .04 | 1.0 | 0.7-1.5 | .79 |
| Male sex (vs female) | 1.2 | 0.6-2.4 | .60 | 1.6 | 0.8-3.1 | .20 |
| KPS (vs KPS ≥90) | 14.5 | 3.8-55.6 | .000 | 0.2 | 0.1-0.8 | .03 |
| STR (vs GTR) | 0.9 | 0.4-1.9 | .74 | 1.4 | 0.7-3.0 | .34 |
| Biopsy (vs GTR) | 2.1 | 0.8-5.5 | .14 | 4.3 | 1.5-12.5 | .01 |
| RT dose | 0.9 | 0.6-1.2 | .44 | 0.9 | 0.6-1.3 | .70 |
| MGMT methylated (vs unmethylated) | 0.2 | 0.0-1.2 | .07 | 0.3 | 0.1-1.3 | .11 |
| Gd-enhancing GTV | 1.1 | 0.8-1.6 | .95 | 1.4 | 0.9-2.0 | .17 |
| FLAIR-GTV | 1.1 | 0.8-1.5 | .95 | 1.4 | 1.0-2.0 | .09 |
| Baseline MTV | 1.3 | 0.9-1.8 | .90 | 1.6 | 1.2-2.3 | .02 |
| Baseline enhancing MTV | 1.2 | 0.9-1.8 | .90 | 1.6 | 1.1-2.2 | .03 |
| Baseline nonenhancing MTV | 1.0 | 0.8-1.4 | .95 | 1.2 | 0.8-1.6 | .42 |
| Baseline MTV outside FLAIR | 1.2 | 0.8-1.8 | .93 | 1.1 | 0.8-1.6 | .57 |
| Baseline MTV outside 95% IDS | 1.0 | 0.7-1.3 | .95 | 1.4 | 1.0-1.9 | .10 |
| Baseline MTV inside 95% IDS | 1.3 | 0.9-1.8 | .90 | 1.6 | 1.2-2.2 | .02 |
| 3-month MTV | 1.0 | 0.6-1.5 | .95 | 1.8 | 1.1-2.7 | .03 |
| Persistent MTV subvolume at 3 months | 1.0 | 0.6-1.6 | .95 | 1.6 | 1.1-2.6 | .07 |
| Change in MTV from baseline | 1.0 | 0.7-1.6 | .95 | 1.3 | 0.9-2.0 | .20 |
Abbreviations: CI = confidence interval; FLAIR = fluid attenuated inversion recovery; Gd = gadolinium; GTR = gross total resection; GTV = gross tumor volume; HR = hazard ratio; IDS = isodose surface; KPS = Karnofsky Performance Status; MGMT = O6-methylguanine-DNA methyltransferase; MTV = metabolic tumor volume; OS = overall survival; RT = radiation therapy; STR = subtotal resection.
For continuous covariates, HR is per 1 SD.
HR for imaging covariates are per 10 cm3.
For multiple testing, Benjamini-Hochberg method was used for false discovery rate control with the false discovery rate control rate set to 10%.
Table 4.
Multivariate Cox proportional hazards model of progression free survival and overall survival
| OS∗ |
PFS∗ |
|||||
|---|---|---|---|---|---|---|
| Covariates† | HR | 95% CI | P value‡ | HR | 95% CI | P value‡ |
| KPS (vs KPS ≥90) | 15.6 | 4.0-62.5 | .000 | 6.0 | 1.5-23.8 | .01 |
| Age | 1.5 | 1.0-2.2 | .05 | 1.0 | 0.7-1.4 | .88 |
| STR (vs GTR) | 1.2 | 0.5-2.6 | .73 | 1.6 | 0.7-3.4 | .26 |
| Biopsy (vs GTR) | 3.3 | 1.2-9.3 | .03 | 5.1 | 1.7-15.3 | .004 |
| Gd-enhancing GTV | 1.3 | 0.8-2.0 | .91 | 1.2 | 0.8-2.1 | .45 |
| FLAIR-GTV | 1.0 | 0.7-1.5 | .98 | 1.3 | 0.9-2.0 | .28 |
| MTV pre-CRT | 1.2 | 0.7-2.2 | .91 | 1.6 | 0.8-3.0 | .28 |
| Baseline enhancing MTV | 1.4 | 0.8-2.5 | .91 | 1.4 | 0.8-2.5 | .29 |
| Baseline nonenhancing MTV | 0.9 | 0.6-1.4 | .91 | 1.0 | 0.7-1.5 | .98 |
| Baseline MTV outside FLAIR | 1.1 | 0.6-1.8 | .95 | 0.7 | 0.4-1.2 | .28 |
| Baseline MTV outside 95% IDS | 1.0 | 0.7-1.4 | .95 | 1.4 | 0.9-2.0 | .26 |
| Baseline MTV inside 95% IDS | 1.2 | 0.7-2.3 | .91 | 1.5 | 0.8-2.9 | .29 |
| MTV 3 month | 1.3 | 0.8-2.4 | .91 | 2.4 | 1.4-4.3 | .03 |
| Persistent MET-PET subvolume | 1.3 | 0.7-2.4 | .91 | 2.0 | 1.2-3.4 | .06 |
| Change in MTV from baseline | 1.1 | 0.7-1.7 | .95 | 1.8 | 1.1-3.1 | .09 |
Abbreviations: CI = confidence interval; FLAIR = Fluid attenuated inversion recovery; Gd = gadolinium; GTR = gross total resection; GTV = gross tumor volume; HR = hazard ratio; IDS = isodose surface; KPS = Karnofsky Performance Status; MGMT = O6-methylguanine-DNA methyltransferase; MTV = metabolic tumor volume; OS = overall survival; RT = radiation therapy; STR = subtotal resection.
11 models included with 4 covariates per model (age, KPS, extent of resection and imaging variable).
For continuous covariates, HR is per 1 SD.
For multiple testing, Benjamini-Hochberg method was used for false discovery rate (FDR) control with the FDR control rate set to 10%.
Pattern of failure analysis
Thirty-four of 37 patients had documented progression of disease. The median TVPOF was 28.3 cm3 (range, 1.1-109.6). Across patients, the median GTV-Gd at recurrence that encompassed the persistent MET-PET subvolume was 97% (interquartile range 72%-100%), compared with 71% (interquartile range 39%-93%) of the baseline MTV, 54% of the baseline GTV-Gd (18%-87%), and 78% of the 3-month MTV (47%-95%).
Discussion
In our study we found that despite apparent gross total resection, the majority of patients had residual MTV detected by MET-PET before chemoradiation. However, neither MTV nor GTV-Gd before chemoradiation were associated with PFS or OS when accounting for 3-month metabolic response. In contrast, the presence of MTV 3 months post-CRT was significantly associated with worse PFS and demonstrated a spatial relationship to eventual progression. These findings suggest that 3-month metabolic response assessment may be used as a biomarker for prognostication and early treatment decision making and may signify regions of treatment resistance that eventually repopulate tumor recurrence. If these treatment-resistant subvolumes could be identified before treatment, they may serve as potential targets for intensified local therapy with surgery or radiation.
Response assessment after treatment of GBM standardly relies upon anatomic MRI.28 However, the usefulness of anatomic MRI several months after CRT is known to be limited owing to difficulty in distinguishing between tumor and other processes that disrupt the blood-brain barrier such as medication effects, variation in imaging technique, treatment-related inflammation, seizures, and postsurgical changes.29 As a result, advanced MRI and PET imaging have been investigated as techniques for response assessment.14 One study found that a decrease in O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET PET) maximum and mean tumor-to-brain ratio from pre-CRT to 7 to 10 days after completion of CRT was associated with improved PFS and OS.30 In the same study, reduction of 18F-FET uptake at 6 to 8 weeks after completion of CRT was associated with improved PFS and trended toward improved OS. Post-CRT GTV-Gd based on anatomic T1-Gd MRI was not associated with PFS or OS in this study. Response assessment using amino acid tracers has also been studied after treatment with antiangiogenic therapies (bevacizumab) and alkylating chemotherapy.31, 32, 33, 34, 35 Decreased uptake of 3,4-dihydroxy-6-[(18)F]-fluoro-l-phenylalanine ((18)F-FDOPA), 18F-FET, and MET-PET after chemotherapy in patients with recurrent GBM have been shown to be associated with improved survival.
Response assessment early during CRT using advanced MRI techniques, such as parametric response maps based on quantitative markers like relative cerebral blood volume and apparent diffusion coefficient, in addition to reduction in fractional high-cerebral blood volume tumor volume have been associated with overall survival.36, 37, 38, 39, 40 The relationship between these advanced MRI techniques and various types of amino acid PET imaging has not been well-characterized, and studies are needed to evaluate whether these advanced imaging modalities are complementary in their characterization of tumor biology and prognosis, and whether the combination of multiple imaging approaches may more optimally characterize tumor heterogeneity and potentially guide treatment.41
In our study, neither baseline MTV nor baseline GTV-Gd was significant for PFS or OS. A previous study looking at pretreatment MET-PET scans found MTV was associated with OS on multivariate analysis and GTV-Gd was not.42 Another study of 18 patients with newly diagnosed GBM found preoperative MTV to be the only significant factor associated with PFS on multivariate analysis.43 No postoperative MTV was obtained to verify extent of resection. Although we did not find baseline MTV to be significantly associated with PFS, our imaging timepoints included postoperative pre-CRT MET-PET and post-CRT imaging, which provides a true metabolic response assessment of the postoperative residual MTV after CRT.
A prior study demonstrated that MTV outside of the 95% IDS was associated with noncentral tumor recurrence.13 In our present study, we found that baseline MTV was not prognostic for PFS when accounting for 3-month metabolic response, but did find a spatial association between eventual tumor recurrence and MTV persisting from baseline to 3 months post-CRT. Taken together, these results suggest that successful identification of these treatment-resistant subregions before CRT may enable a dose-intensified approach that could alter patterns of failure and prolong time to progression.44 Although the number of patients with persistent MTV was small, persistent MTV was more likely to be encompassed in the TVPOF than any other imaging subvolume. Additionally, MTV at baseline and at 3 months was more likely to be encompassed by TVPOF than the GTV-Gd, suggesting MTV can help identify the volumes of tumor most likely progress. However, the spatial analysis is limited because the MTV volumes were much smaller than the GTV-Gd, which would make it more likely to be encompassed by the TVPOF. Moreover, the comparison of MTV with TVPOF is limited as it presumes TVPOF defined on the T1-Gd MRI accurately describes the recurrence volume. T1-Gd MR imaging is the standard modality to assess tumor response and disease status for GBM, but is nonspecific and may not fully depict tumor extent posttreatment, or readily distinguish between treatment effect and tumor progression.28 The spatial analysis is hypothesis generating as it seems to suggest a relationship between the MTV and eventual recurrence. This persistent MTV identifies a distinct tumor subvolume potentially at highest risk of recurrence compared with the remainder of the MTV or anatomic imaging defined tumor volumes. An aim of future studies would be to identify this treatment resistant tumor subvolume before surgery or radiation. One possible approach could be to correlate MTV with advanced MRI techniques like perfusion and high b-value diffusion weighted imaging. This is one of the goals of an ongoing phase II clinical trial at our institution.45
We did not observe a relationship between metabolic imaging at baseline or 3 months and overall survival. This could be due to limited patient numbers or collinearity with extent of resection. Another limitation of our study is a lack of molecular markers like MGMT promoter methylation and IDH mutation status due to the era of treatment. It would be interesting to note if MGMT methylation was associated with MTV response as it has been associated with change in standardized uptake.46
Conclusions
The majority of patients with apparent GTR of newly diagnosed GBM have measurable postoperative MTV, and total and persisting MTV volumes 3 months post-CRT were the only imaging predictors of PFS in our study. In particular, the persistent MET-PET subvolume is the strongest predictor for localizing tumor recurrence, and additional advanced imaging strategies should be investigated to identify whether these highest-risk tumor subregions can be identified before and during treatment for adaptive radiation therapy strategies and prognostication during and after CRT.
Footnotes
Sources of support: This study was funded in part by the National Institutes of Health P01 CA059827.
Disclosures: No authors have pertinent conflicts of interest to disclose.
References
- 1.Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R., Taillibert S., Kanner A. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang E.L., Akyurek S., Avalos T. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68:144–150. doi: 10.1016/j.ijrobp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald D.R., Cascino T.L., Schold S.C., Cairncross J.G. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 5.Autry A., Phillips J.J., Maleschlijski S. Characterization of metabolic, diffusion, and perfusion properties in gbm: Contrast-enhancing versus non-enhancing tumor. Transl Oncol. 2017;10:895–903. doi: 10.1016/j.tranon.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosu A.L., Weber W.A., Riedel E. L-(methyl-11c) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:64–74. doi: 10.1016/j.ijrobp.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 7.Pirotte B., Goldman S., Dewitte O. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: A report of 103 consecutive procedures. J Neurosurg. 2006;104:238–253. doi: 10.3171/jns.2006.104.2.238. [DOI] [PubMed] [Google Scholar]
- 8.Heiss W.D. Pet in gliomas. Overview of current studies. Nuklearmedizin. 2014;53:163–171. doi: 10.3413/Nukmed-0662-14-04. [DOI] [PubMed] [Google Scholar]
- 9.Pirotte B.J., Levivier M., Goldman S. Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: A survival analysis in 66 consecutive patients. Neurosurgery. 2009;64:471–481. doi: 10.1227/01.NEU.0000338949.94496.85. [DOI] [PubMed] [Google Scholar]
- 10.Barajas R.F., Jr., Phillips J.J., Parvataneni R. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro Oncol. 2012;14:942–954. doi: 10.1093/neuonc/nos128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Costanzo A.1, Scarabino T., Trojsi F. Multiparametric 3t MR approach to the assessment of cerebral gliomas: Tumor extent and malignancy. Neuroradiology. 2006;48:622–631. doi: 10.1007/s00234-006-0102-3. [DOI] [PubMed] [Google Scholar]
- 12.McKnight T.R., von dem Bussche MH., Vigneron DB. Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg. 2002;97:794–802. doi: 10.3171/jns.2002.97.4.0794. [DOI] [PubMed] [Google Scholar]
- 13.Lee IH., Piert M., Gomez-Hassan D. Association of 11c-methionine pet uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:479–485. doi: 10.1016/j.ijrobp.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert N.L., Weller M., Suchorska B. Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18:1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omuro A.M., Leite C.C., Mokhtari K. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006;5:937–948. doi: 10.1016/S1474-4422(06)70597-X. [DOI] [PubMed] [Google Scholar]
- 16.Glaudemans A.W., Enting R.H., Heesters M.A. Value of 11c-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–635. doi: 10.1007/s00259-012-2295-5. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom M., Lundqvist H., Ericson K. Comparison of the accumulation kinetics of l-(methyl-11c)-methionine and d-(methyl-11c)-methionine in brain tumors studied with positron emission tomography. Acta Radiol. 1987;28:225–229. [PubMed] [Google Scholar]
- 18.Langen K.J., Muhlensiepen H., Holschbach M. Transport mechanisms of 3-[123i]iodo-alpha-methyl-l-tyrosine in a human glioma cell line: Comparison with [3h]methyl]-l-methionine. J Nucl Med. 2000;41:1250–1255. [PubMed] [Google Scholar]
- 19.Okubo S., Zhen H.N., Kawai N. Correlation of l-methyl-11c-methionine (met) uptake with l-type amino acid transporter 1 in human gliomas. J Neurooncol. 2010;99:217–225. doi: 10.1007/s11060-010-0117-9. [DOI] [PubMed] [Google Scholar]
- 20.Upadhyay N., Waldman A.D. Conventional MRI evaluation of gliomas. Br J Radiol. 2011;84 Spec:S107–111. doi: 10.1259/bjr/65711810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.I., Kim Y., Lee J.Y. Prognostic value of the metabolic and volumetric parameters of (11)c-methionine positron-emission tomography for gliomas: A systematic review and meta-analysis. Am J Neuroradiol. 2018;39:1629–1634. doi: 10.3174/ajnr.A5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boellaard R., Nc K., Os H. Effects of noise, image resolution, and roi definition on the accuracy of standard uptake values: A simulation study. J Nucl Med. 2004;45:1519–1527. [PubMed] [Google Scholar]
- 23.Tsien CI., Brown D., Normolle D. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18:273–279. doi: 10.1158/1078-0432.CCR-11-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brix G., Zaers J., Adam L.E. Performance evaluation of a whole-body PET scanner using the nema protocol. National Electrical Manufacturers Association. J Nucl Med. 1997;38:1614–1623. [PubMed] [Google Scholar]
- 25.Cao Y. We-d-t-6c-03: Development of image software tools for radiation therapy assessment. Medical Physics 2005;32:2136.
- 26.Torii K., Tsuyuguchi N., Kawabe J. Correlation of amino-acid uptake using methionine pet and histological classifications in various gliomas. Ann Nucl Med. 2005;19:677–683. doi: 10.1007/BF02985116. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 28.Wen P.Y., Macdonald DR., Reardon DA. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 29.Vogelbaum MA., Wen PY. End point assessment in gliomas: Novel treatments limit usefulness of classical MacDonald's criteria. J Clin Oncol. 2009;27:2905–2908. doi: 10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galldiks N.1, Langen K.J., Holy R. Assessment of treatment response in patients with glioblastoma using o-(2-18f-fluoroethyl)-l-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53:1048–1057. doi: 10.2967/jnumed.111.098590. [DOI] [PubMed] [Google Scholar]
- 31.Galldiks N., Kracht L.W., Burghaus L. Use of 11c-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur J Nucl Med Mol Imaging. 2006;33:516–524. doi: 10.1007/s00259-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 32.Beppu T., Terasaki K., Sasaki T. MRI and 11c-methyl-l-methionine PET differentiate bevacizumab true responders after initiating therapy for recurrent glioblastoma. Clin Nucl Med. 2016;41:852–857. doi: 10.1097/RLU.0000000000001377. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzenberg J., Czernin J., Cloughesy T.F. Treatment response evaluation using 18f-fdopa PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20:3550–3559. doi: 10.1158/1078-0432.CCR-13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutterer M., Nowosielski M., Putzer D. O-(2-18f-fluoroethyl)-l-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52:856–864. doi: 10.2967/jnumed.110.086645. [DOI] [PubMed] [Google Scholar]
- 35.Galldiks N., Rapp M., Stoffels G. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18f]fluoroethyl-l-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40:22–33. doi: 10.1007/s00259-012-2251-4. [DOI] [PubMed] [Google Scholar]
- 36.Moffat B.A., Chenevert T.L., Lawrence T.S. Functional diffusion map: A noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA. 2005;102:5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsien C., Galbán C.J., Chenevert T.L. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol. 2010;28:2293–2299. doi: 10.1200/JCO.2009.25.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galbán C.J., Chenevert T.L., Meyer C.R. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15:572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y., Tsien CI., Nagesh V. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected] Int J Radiat Oncol Biol Phys. 2006;64:876–885. doi: 10.1016/j.ijrobp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Galban C.J., Lemasson B., Hoff B.A. Development of a multiparametric voxel-based magnetic resonance imaging biomarker for early cancer therapeutic response assessment. Tomography. 2015;1:44–52. doi: 10.18383/j.tom.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohmann P., Werner J., Shah N.J. Combined amino acid positron emission tomography and advanced magnetic resonance imaging in glioma patients. Cancers. 2019;11:153. doi: 10.3390/cancers11020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galldiks N., Dunkl V., Kracht L.W. Volumetry of [11c]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol Imaging. 2012;11:516–527. [PubMed] [Google Scholar]
- 43.Yoo M.Y., Paeng J.C., Cheon G.J. Prognostic value of metabolic tumor volume on (11)c-methionine pet in predicting progression-free survival in high-grade glioma. Nucl Med Mol Imaging. 2015;49:291–297. doi: 10.1007/s13139-015-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MM., Speers C., Li P. Dose-intensified chemoradiation is associated with altered patterns of failure and favorable survival in patients with newly diagnosed glioblastoma. J Neuro Oncol. 2019;143:313–319. doi: 10.1007/s11060-019-03166-3. [DOI] [PubMed] [Google Scholar]
- 45.Kim MM., Parmar HA., Aryal MP. Developing a pipeline for multiparametric MRI-guided radiation therapy: Initial results from a phase II clinical trial in newly diagnosed glioblastoma. Tomography. 2019;5:118–126. doi: 10.18383/j.tom.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Rapalino O., Heidari P. C11 methionine PET (MET-PET) imaging of glioblastoma for detecting postoperative residual disease and response to chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2018;102:1024–1028. doi: 10.1016/j.ijrobp.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]