Abstract
Escherichia coli is an important pathogenic indicator in drinking water. Viable but non-culturable (VBNC) E. coli induced by low level chlorination was found to have higher antibiotic tolerance. The emerging of VBNC bacteria in drinking water systems is posing challenges to the control of bio-safety. It is necessary to study the underlying mechanisms of VBNC state E. coli under actual residual chlorine condition of drinking water pipe network. In this study, we investigated the changes of morphology and gene expressions that might present such state. The results indicated that the size of VBNC E. coli was not remarkably changed or recovered culturability under favorable environmental conditions. Results from transcriptomic analysis revealed that the regulated genes related to fimbrial-like adhesin protein, putative periplasmic pilin chaperone, regulators of the transcriptional regulation, antibiotic resistance genes and stress-induced genes, rendering VBNC cells more tolerant to adverse environmental conditions. In total of 16 genes were significantly up-regulated under the VBNC state, including three genes encoding toxic protein (ygeG, ibsD, shoB), indicating that VBNC E. coil was still a threat to human. The work is of great relevance in the context of better understanding this poorly understood physiological state.
Subject terms: Microbiology, Environmental sciences
Introduction
Bacteria are prime targets to remove from drinking water since waterborne pathogenic microorganisms can cause epidemic diseases. In many countries, chlorine at levels of 0.05–0.5 mg L−1 is generally applied to repress bacterial regrowth in drinking water distribution systems (DWDS)1,2 so that the number of bacteria in tap water can meet the water quality standards. However, several reports have mentioned that some waterborne pathogens could be induced into a viable but nonculturable (VBNC) state by chlorine or chloramine disinfectants3–10. VBNC bacteria cannot be detected by the conventional plate counting method which leads to an underestimation of the potential microbiological risks. Thus, the pathogenic bacteria in the VBNC state may pose a threat to public health.
It has been reported that VBNC bacteria exhibit an extraordinary tolerance to adverse conditions. Escherichia coli in the VBNC state persisted in the presence of certain antibiotics, e.g., 200 μg mL−1 ampicillin (Amp) and 5 μg mL−1 ofloxacin (Ofx) (approximately 128 MIC and 64 MIC, respectively)11. In addition, Oliver et al. reported that Vibrio vulnificus could enter the VBNC state, protecting cells against a variety of potentially lethal environmental challenges (heat, oxidative stress, osmotic stress, pH fluctuations, ethanol, antibiotics and heavy metals)12. In our previous study, it was found that low doses of chlorine (0.5 mg L−1) disinfection effectively reduced the number of culturable E. coli and induced a VBNC state, after which the metabolic activity of the bacteria was reduced and persistence of E. coli in the presence of 9 typical antibiotics was enhanced13. Moreover, Moreno et al. also reported that low-level of residual chlorine in DWDS was capable of inducing VBNC state antibiotic-tolerant bacteria, which could be considered as an undetectable risk8. Chlorination, a vital step in controlling the biosafety of drinking water, was directly related to public and environmental health. Thus, it is necessary to thoroughly investigate the VBNC state induced by low concentrations of chlorine.
In addition to the compromise of physical structure, chlorine has also been reported to impact the metabolic processes, efflux pumps, and pathogenicity of bacteria by regulating gene expression. Therefore, it is important to comprehensively investigate the antibiotic tolerance of VBNC bacteria at the morphological and genetic levels. Generally, quantitative reverse transcription PCR (RT-qPCR) is applied to quantitatively determine the expression levels of known target genes in viable cells. It was reported that human pathogenic Vibrio spp. and Pseudomonas aeruginosa in the VBNC state could continuously express known toxin and virulence genes14,15. To survive under unfavorable conditions, major metabolic pathways (e.g., central carbohydrate metabolic pathways) could be altered to obtain energy and reduce the metabolic rate under the VBNC state16. Some studies focused on the gene expression of VBNC bacteria to explore their regulatory mechanisms17–19.
The regulation of gene expression involves complex and specific biological processes composed of a series of functionally related molecules20. Conventional investigation through individual genes is inadequate for discovering the complete regulation and cross talk of these genes. Nowadays, the high-throughput transcriptome sequencing (RNA-seq) technique has been applied in constructing frameworks of gene expression which has markedly accelerated the capacity of analyses21. Several comparative transcriptome analyses focused on some important environmental bacteria, including Pseudomonas syringae (Fmicb)18, Rhodococcus biphenylivorans19 and Vibrio splendidus22. These studies highlighted a more comprehensive and data-intensive approach for better understanding of the mechanisms of bacterial stress responses. In DWDS, the inducer is chlorine, a chemical oxidant that may endow many different characteristics to the VBNC bacteria compared with physical inducers such as low temperature. Accordingly, it is worthwhile to analyze the adaptive responses of E. coli in the VBNC state induced by low-level chlorine via transcriptomic analysis.
In this study, as a continuation of the previous work, E. coli was selected as the test which is the indicator for pathogenic bacteria in the drinking water quality standards all over the world23,24. The bacteria were exposed to chlorine at the level comparable to residual chlorine in a DWDS to induce the VBNC state. The gene expression variation of the VBNC bacteria was assessed via transcriptome approaches. Then, the morphology was investigated by scanning electron microscope (SEM) and flow cytometry (FCM). It was expected that the special regulation behavior at low level chlorination circumstances could be revealed and deeper understanding of VBNC cells could be acquired.
Results and Discussion
Resuscitation of VBNC state
Chlorination is vital for controlling bacterial regrowth in DWDS. It usually takes several hours to days to transport water from a treatment plant to household taps. Such residence time is sufficient for the induction and resuscitation of bacteria into/from the VBNC state. To induce a VBNC state, we chose chlorine at 0.5 mg L−1, the concentration generally applied in DWDS1,2. In our previous study, it demonstrated that 105–6 cells mL−1 of bacteria entered the VBNC state at 0.5 mg L−1 residual chlorine in 6 h (Fig. S1 red column). Our result is consistent with Lee et al.; although no bacteria were observed in the chlorinated influent of the model system by heterotrophic plate count (HPC) method, a direct viable count (DVC) still presented observed growth in the range between 3- and 4-log cells mL−1. Significantly high numbers of HPC and DVC were found both in the biofilm and effluent of the model system25.
VBNC microorganisms can be resuscitated under certain conditions. In this study, the resuscitation of VBNC E. coli (initial concentration of approximately 104 cells mL−1) was verified. Resuscitation experiments were performed with VBNC cells using liquid media. As shown in Fig. 1, the results of multiple experiments indicated that within the first 20 hours of culture, there was no colony formation on the plate. However, 1 hour later, the bacteria showed an “outbreak” increase of 3 orders of magnitude (from 0 to 1.4 × 103 CFUs mL−1). The same initial concentration of culturable E. coli was used to draw the growth curve as a control. We found that the growth curve of VBNC cells was significantly different from that of the conventional growth curve, suggesting the occurrence of resuscitation (Fig. S2). The theoretical growth rate of the cells in LB broth medium would be approximately 6 min (approximately 10 generations for one hour), which demonstrated that resuscitation occurred at a rate much faster than the typical doubling time of E. coli (which is approximately 30 min). In our results, approximately one in twelve (1/12) tubes displayed this recovery.
Figure 1.
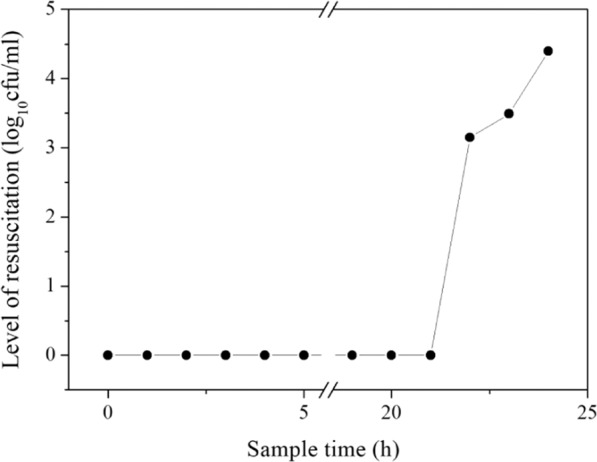
Resuscitation of the VBNC cells in LB broth.
This recovery was not high for the absolute percentage of bacteria. However, the risk of resuscitation will be even more serious considering the large water supply in large modern cities. Currently in China, many cities have a water supply capacity of over 1 million tons/day. The probability of VBNC cells is approximately 101 to 103 cells/mL in real drinking water. This means that residents in these cities will be exposed to up to 1013–15 VBNC (101–3 cells/mL = 1013–15 cells/million tons) cells per day, which might resuscitate under the appropriate conditions.
Characterization of VBNC E. coil
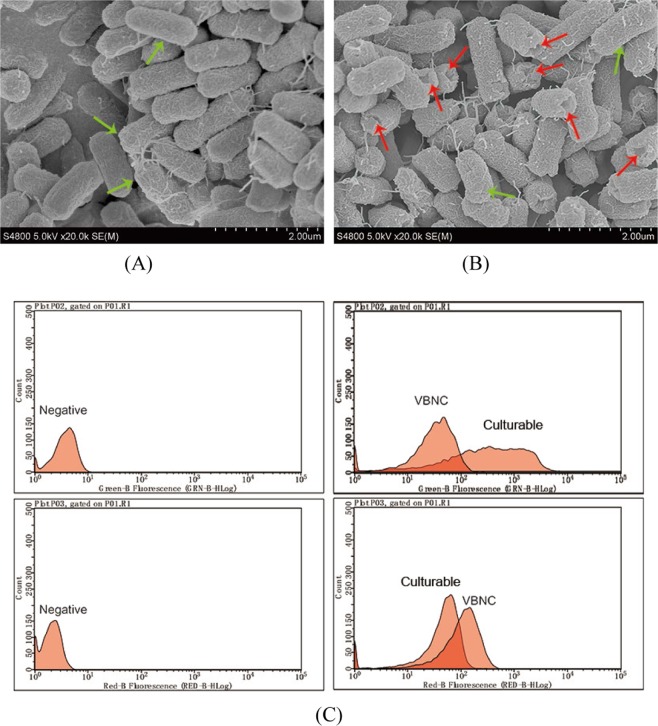
VBNC bacteria may present morphological changes such as cellular shrinkage and size reduction due to physical environmental stresses (e.g., low temperature)26. Unlike the VBNC state induced by cold temperature, the bacteria sterilized by low chlorine concentrations showed no changes in cell morphology. However, we observed a number of damages on the cell membrane (walls) of the chlorinated bacteria (red arrows), as shown Fig. 2(B), whereas we observed nearly intact cell membranes (walls) of the culturable cells (Fig. 2(A)). Jang et al. confirmed that bleach inhibits the biosynthesis of outer cell wall mycolic acids and induces oxidative damage in bactreria27. In the presence of 0.5 mg L−1 chlorine, some bacteria were relatively intact (marked by the green arrow in Fig. 2(B)), the cell membrane was not completely destroyed, and the bacteria in the VBNC state belonged to the group with intact membranes.
Figure 2.
Morphological characteristics of E. coli under a scanning electron microscopy (Magnification, 20,000×) and a flow cytometry. SEM (A) culturable cells, (B) chlorinated cells, (the damaged bacteria with red arrow, green arrow labels the relative integrated cell membrane.) FCM (C) Peak diagram for red/green fluorescence analysis. (negative: undyed culturable cells/chlorinated cells).
The results of FCM corroborated the conclusion that some cells were SYTO 9 positive (Fig. 2(C)). FCM analysis showed that the green fluorescence intensity of culturable and chlorinated cells (including VBNC cells) was greater than that of the negative control bacteria and the green fluorescence intensity of the VBNC cells was clearly weaker than that of the culturable cells. Furthermore, as shown in Fig. 2(C), the mean intensity of green fluorescence from the VBNC cells showed a trend of shifting to the left, and the red fluorescence intensity showed a reverse trend, revealing that the cell permeability of the VBNC bacteria increased. Moreover, we quantified the changes in cell membrane permeability under the VBNC state. Through the definition of the gate, 98.44% VBNC E. coli had enhanced permeability in the presence of selected chlorine concentrations (data not shown). These results were in accordance with the conclusions reached by Virto et al.28 and Tatsumi et al.29 who suggested that approximately a dose of 1 mg L−1 chlorine was linked to an increase in the membrane permeability of four types of bacteria including E. coli and neutrophils.
Global transcriptional profiles of E. coli in VBNC state and culturable state
Volcano Plot and venn diagram of VBNC versus culturable cells
Recently, several studies have focused on the induction conditions and properties of VBNC cells30–33, but few studies have investigated VBNC cells produced in municipal pipe networks. Their researches confirmed that VBNC cells existed in municipal pipe networks exhibited strong viability, and they could resuscitate6,25. Undoubtedly, more researches are needed to explore bacteria in a VBNC state, which may yield insight into strategies for the prevention and control of VBNC bacteria.
cDNA libraries derived from VBNC and culturable E. coli have been constructed. The libraries of VBNC cells and culturable cells had 27.97 million and 29.82 million reads respectively, and these reads were aligned with the E. coli K12 genome sequence (NCBI GenBank accession No. NC_000913). After incubation with a low concentration of chlorine, the expression of 362 genes of E. coli was found to be changed significantly (expressed in the log2 (FoldChange) with base 2, hereinafter indicated as |log2 (FoldChange)|) (|log2 (FoldChange)| >1 and p < 0.05) (Fig. S3(A)), of which 203 genes were upregulated (red plots) and 159 genes were downregulated (green plots). In particular, 3 downregulated and 22 upregulated genes with |log2 (FoldChange)| >5 indicated that these genes had extremely significant changes after exposure to chlorine and needed special attention. Detailed information of the 362 genes with significant differences was provided in supporting information Table S3. These changes were categorized as follows: (1) biochemical process such as transport, establishment localization, localization, oxidation-reduction process, biological process; and (2) molecular function such as transporter activity, ATPase activity, GTPase activity, guanyl ribonucleotide binding, guanyl nucleotide binding, etc.
In the Venn diagram, the total expressed genes were plotted with two comparisons of transcriptional profiles (Fig. S3(B)). Established on the standard of gene expression with FPKM >1, the sum of all the numbers (3900) in orange circle represented the expression level of genes in culturable cells, and the sum of all the numbers (3955) in green circle represented the expression level of genes in VBNC cells, suggesting that 3880 genes were in common between the two groups (overlapping yellow circle). Obviously, compared with the culturable state, the VBNC cells had some new genes that were activated or silenced to survive under environmental stresses.
Gene ontology (GO) and KEGG analysis
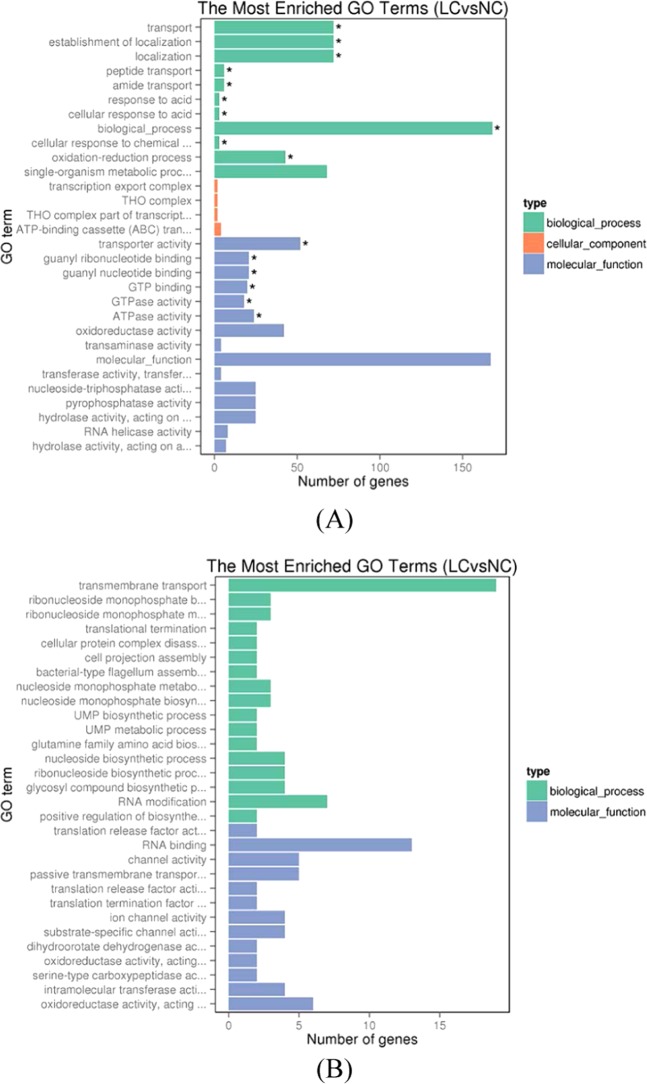
To ascribe gene functions to differentially expression genes (DEGs), a total of 3194 DEGs were assigned to 3 parts: biological process, molecular function and cellular component. To determine the Gene Ontology (GO) function of the up- or downregulated genes, GO enrichment analysis was performed on the 2048 upregulated and 1777 downregulated genes. The 30 most enriched GO terms are presented in Fig. 3. For the enriched GO terms of the 2048 upregulated genes, there were 1476, 467, 105 significantly enriched GO terms that corresponded to biological process, molecular function, and cellular component processes, respectively. It is worth mentioning that biological processes such as transportation, localization, and response to acid were significantly enriched. Furthermore, transporter activity and ATPase activity of the molecular component were also remarkedly enriched (P < 0.05), suggesting that the transport of some substance could be enhanced to excrete harmful substances in the VBNC state. The evidence indicated that VBNC cells were not just in a state of dormancy and still demonstrated highly expressed energy metabolism. The cellular component was relatively unchanged before or after treatment with a low concentration of chlorine, compared with the other 2 GO terms.
Figure 3.
(A) Histogram of GO enrichment of up-regulated genes. (B) Histogram of GO enrichment of down-regulated genes. The significant enriched GO terms were presented with P < 0.05.
Additionally, the genes were mapped to the KEGG pathway. Among the mapped genes, the upregulated genes in the VBNC cells were mainly enriched in two pathways: ABC transporters and microbial metabolism in diverse environments. The enriched genes had lower Q values, indicating significant enrichment (Fig. 4). In Fig. 3(B), the GO enrichment of downregulated genes in the VBNC cells was presented by 2 groups: biological process and molecular function. Similar to GO terms identified for the upregulated genes, the cellular component category was unchanged before or after the low chlorine treatment. The top 2 enriched pathways were transmembrane transporter and RNA binding while no enrichment pathways were observed in downregulated GO.
Figure 4.
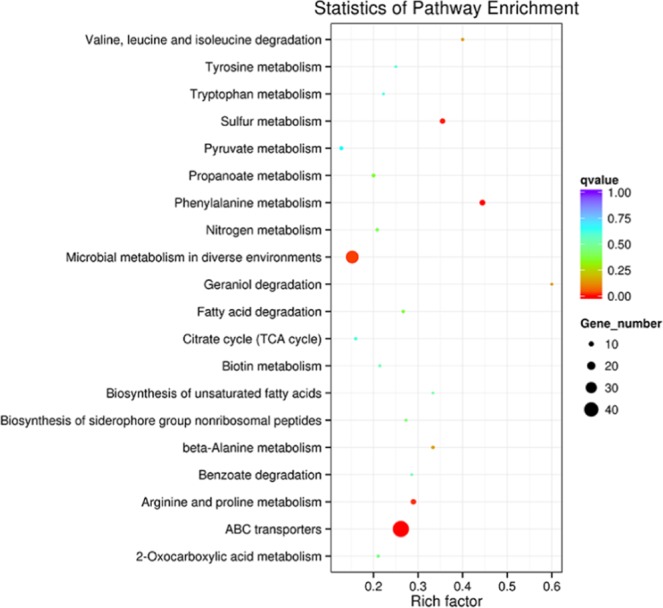
KEGG scatter plot of 20 extremely enriched DEGs. The abscissa represents Rich factor; the ordinate represents name of pathway; the plot size represents the expression number of DEGs in pathways; the color of plot represents corresponding Q value.
Functional analysis of the up/down-regulated genes in low chlorine-induced VBNC E. coli
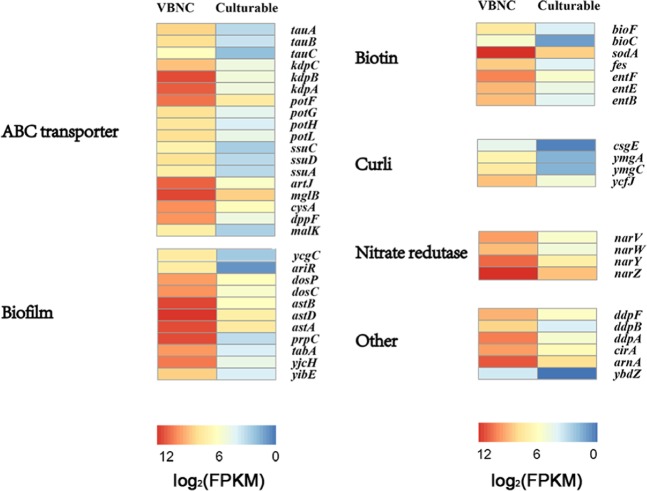
A VBNC state is a state of dormancy. In the VBNC state, many genes are downregulated to reduce energy consumption4. However, VBNC cells still have high level of gene expression, and the behavior of these genes has a greater contribution to the survival mechanisms and resuscitation of VBNC cells. The present study focused on highly expressed genes. Figure 5 revealed that highly expressed genes of VBNC E. coli were related to 6 functional groups, including ABC transporter, biotin, curli, biofilm, nitrate reductase and others. ABC transporters, including ATP-binding components of amino acid ABC transporters, ATP-dependent high affinity P-Type ATPase metal ion transporters, saccharides ABC transportors, etc., were upregulated from 3.15 to 6.39 log2 (FoldChange), suggesting that some genes could be highly expressed in VBNC bacteria to survive34,35. With respect to the expression of other genes, some genes involved in biotin synthesis could encode proteins that produce some growth factors, increasing the survivability of bacteria. Enterobactin (also known as enterochelin) is a high-affinity siderophore that acquires iron for microbial systems. Pathogenic bacteria can steal iron from other living organisms using this mechanism, even though the concentration of iron is kept extremely low due to the toxicity of free iron34,36,37. The existence of such levels of biotin not only increases the pathogenicity of pathogenic bacteria (because of the involvement of metal ions in oxygen transport and gall storage, electron transfer, DNA synthesis and other physiological processes), but also sequesters the iron resources of other bacteria. Curli is composed of two subunits: CsgA and CsgB. CsgE prohibits the self-assembly of CsgA into amyloid fibers. CsgE acts directly on the secreted substrate CsgA to prevent premature subunit assembly38. The expression levels of the csgE gene were significantly higher in bacteria in the VBNC state (4.18 log2 (FoldChange), P < 0.05) compared to the control bacteria, and the formation of pili was reduced, leading to the reduced motility of bacteria39. The cells that cannot swim could reduce energy consumption and are more suitable for dormancy. Additionally, biofilm formation would be regulated through the high expression of yjcH (the gene yjcG, is co-transcribed with the gene acs, which encodes an acetate permease in E. coli) and yibE (YibE is involved in extracellular polysaccharide production and is a threonine-rich protein). Overexpression of the yjbEFGH operon alters colony morphology, leading to the production of novel extracellular polysaccharide genes that promote the production of extracellular polymer substances and enhance the mechanical strength of biofilms40. Due to the bacterial reduction of nitrate and generation of nitrite with high expression of the nar V, W, Y, Z genes, more amino acids and other nitrogen compounds would be formed in cells, enhancing the strength of bacterial cell walls41,42. In other words, these enhanced processes would increase the persistence and survivability of the cells. Furthermore, the VBNC cells showed partial resistance, such as the expression of resistant genes (ddp A, B, F) that are homologues of vancomycin resistance genes43. The polymyxin b resistance protein (ArnA)44, TA systems and the porin protein (CirA) increase the transcription of ybdZ.
Figure 5.
The FPKM hierarchical cluster analysis of differentially expressed genes of VBNC versus culturable cells. Red: high-expressed genes; blue: low-expressed genes. Changes in color from red to blue indicates that the difference values of log2 (FPKM) from large to small.
Dormant bacteria improve their prospects of survival by reducing metabolic activity4. Thus, the downregulation of gene expression in dormant bacteria seems to be a common conclusion. In this study, we also listed genes that were extremely downregulated (log2 (FoldChange) < −4) (Table S1). Eleven downregulated genes mainly encode hydrogenase 1 (HYD 1) (hyaA, B, C, D, E, F) and cold shock protein (cspA, B). Hydrogen metabolism in E. coli is tightly regulated by anaerobic conditions of growth produced. The genes hyaA, B, C, D, E, and F were downregulated, which could decrease the synthesis of active HYD1, and reduce energy metabolisms1. CspA and CspB have been shown to be cold shock-inducible in E. coli. The low expression levels of CspA and B in VBNC cells suggested that bacteria were reduced in response to low temperature stresss2. Other downregulated genes (yjiG, yhiD, appC) involved in ATP-dependent transport reduced the related biological process energy consumptions3, 4. The results of downregulation gene expression were consistent with previous studies.
Extremely high expression genes of VBNC E. coli
For further analysis, we selected genes that were expressed at extremely high levels (log2 (FoldChange) >5) (Table 1). There were 16 upregulated genes, including 3 encoding toxic proteins (ygeG, ibsD, shoB), 5 encoding stress-induced proteins (spy, fade, narV, yeaG, ycgB), 3 encoding regulators (ebgC, ygcW, yaiV) and 3 encoding efflux systems (cusF, cusC, garP) (Table 1). These genes could be classified into two groups. The genes of the first group were related to the transportation of substances (e.g., efflux pump-encoding genes and transcripts), which helped to lower the level of toxic or harmful substances in cells. The type III secretion system (T3SS) in several gram-negative bacteria, which contains the ygeG gene, an important virulence factor that subverts host cellular processes45. As a result, such “dormancy” cells still carry risks that threaten human health. The second group was involved in gene regulation (including transcriptional regulatory factors, stress-induced protein-encoding genes and virulence genes) which could be useful for regulating the metabolic levels. RpoS is the master regulator of the general stress response in E. coli. Rapidly growing cells contain little, if any, RpoS. In contrast, in order to response to many different stress conditions, cellular RpoS levels rapidly increased up to 20-fold. This regulation occurred at the levels of transcription and translation, and RpoS proteolysis occurred in various stress conditions (starvation, hyperosmotic shift, or pH downshift) that differentially affected these levels of control46,47. RpoS is a typical upregulated gene47 which could lead to the inhibition of bacterial division and increase the survivability under high-level expression. In particular, the metal efflux system genes (cusC and F)48,49 and the multidrug efflux protein gene (garP)30 were extremely upregulated, which could increase metal- and antibiotic persistence.
Table 1.
List of log2 (FoldChange) >5 genes in VBNC cells.
| Gene ID | Gene symbol | Gene description | Gene function | Conferences |
|---|---|---|---|---|
| b2851 | ygeG | SycD-like chaperone family TPR-repeat-containing protein | toxic-protein | 45 |
| b4664 | ibsD | toxic membrane protein | toxic-protein | |
| b4687 | shoB | toxic membrane protein | toxic-protein | |
| b2975 | glcA | glycolate transporter | transporter | 57,58 |
| b3599 | mtlA | mannitol-specific PTS enzyme: IIA, IIB and IIC components | transporter | |
| b1743 | spy | periplasmic ATP-independent protein refolding chaperone, stress-induced | stress-induced protein | 59 |
| b0221 | fadE | acyl coenzyme A dehydrogenase | stress-induced protein | 60 |
| b1465 | narV | nitrate reductase 2 (NRZ), gamma subunit | rpos | 41 |
| b1783 | yeaG | protein kinase, endogenous substrate unidentified; autokinase | rpos | 61,62 |
| b1188 | ycgB | SpoVR family stationary phase protein | rpos | |
| b3077 | ebgC | volved beta-D-galactosidase, beta subunit; cupin superfamily | regulator | 63 |
| b2774 | ygcW | putative SDR family oxidoreductase | regulator | 64 |
| b0375 | yaiV | putative transcriptional regulator | regulator | 65 |
| b0573 | cusF | periplasmic copper- and silver-binding protein | efflux | 48 |
| b0572 | cusC | copper/silver efflux system, outer membrane component | efflux | 49 |
| b3127 | garP | putative (D)-galactarate transporter | efflux | 30 |
Conclusion
From the morphology and based on transcriptomic analysis, we investigated the regulation of gene expression of VBNC cells induced by a low chlorine level. E. coli cells could be induced into the VBNC state if water was treated with low concentrations (0.5 mg L−1) of chlorine and VBNC E. coli had the potential to resuscitate. Results of the SEM showed that: the size of VBNC bacteria was not markedly changed, and FCM examination showed that: VBNC cells’ permeability was enhanced. Transcriptomic analysis suggested that the enhancement of the efflux system and stress-induced function contributed to the strong environmental tolerance of the VBNC cells and VBNC E. coli was still a threat to human health.
Methods
Preparation of strain
E. coli CMCC4410315 was cultured with Luria-Bertani (LB) broth (Haibo, China) at 37 °C, 180 rpm until logarithmic phase (12 h). Cells were harvested (5000 rpm, 10 min) and re-suspended in sterilized buffered saline and then diluted to a final concentration of 107–108 CFU mL−1. The cell number was counted by plate counting and disposable counting chambers (CELL.VU, USA). The bacteria were divided into two parts, one of which was used to induce the VBNC state, and the other for control. The control was culturable E. coli with the same treatment except chlorination.
Induction of VBNC E. coli
An initial disinfection concentration of 0.5 mg L−1 was made by addition of disinfectant stock solution. To maintain stable chlorine level, chlorine was added every 2 hours. The concentration of free chlorine was determined using the DPD method (Hach, USA). All cells were incubated at 25 °C, 180 rpm and sampled at 0 min, 15 min, 30 min, 1 h, 3 h, 6 h, 9 h, 12 h, 18 h and 24 h. Before sampling, 100 μL of 20% sodium thiosulfate (1:1 v/v in sterilized buffered saline) was added to inactivate residual chlorine.
In this study, culturable cells were identified by plate counting on NA agar. The number of viable cells was counted by flow cytometry. The number of VBNC cells was calculated as the difference between the numbers of viable and culturable cells. This is a classic characterization method that could count numbers of VBNC cells4. When cells were exposed to chlorine for 6 hours or longer, the numbers of VBNC and viable cells were equal because no colonies were formed on the plates. Cells were considered to have entered VBNC state (105–6 cells mL−1) and have been ready for the next preparation step in this study (Fig. S1 red column). The VBNC preparations were enriched about 50 times (final concentration of 107–8 cells mL−1) by centrifuging at 7800 rpm, 4 °C, 20 minutes and harvested for RNA extraction.
Resuscitation of VBNC cells
Resuscitation experiments were conducted in LB broth medium with the prepared VBNC state E. coli. In each experiment, 4 mL of prepared VBNC state bacteria were separately dispensed into 50 mL centrifuge tubes. Each tube contained 36 mL sterilized LB broth medium and 4 mL bacterial culture, the ratio of VBNC E. coli to LB broth was 1:9. The initial concentration of VBNC cells was about 104 cells mL−1. All of these tubes were cultured at 37 °C, 180 rpm. 1 mL of sample was collected hourly and coated on nutrient agar (NA) (Haibo, China) plate, and the resuscitation was evaluated by plate counting.
Analysis of viability and culturability
Cell viability was analyzed by flow cytometer (FCM) (Millipore Guava EasyCyte, USA). SYBR Green was used for total cells counting (Thermo Fisher Scientific, USA)50, whereas 5-cyano-2, 3-ditolyl tetrazolium (CTC) was used for viable cells counting (Thermo Fisher, USA)51. SYBR Green (The final concentration of 10,000 times dilution) was added into 0.2 mL suspensions in 1.5 mL tubes (20 min, room temperature). CTC was added to the same system at a final concentration of 2 mM (37 °C, 3 h). Samples were analyzed by FCM (Millipore Guava EasyCyte, USA) within 2 h after resuspension, and Forward scatter (FSC), side scatter (SSC), fluorescence signals SYBR Green and CTC using 525 nm and 620 nm band pass filters were measured in logarithmic amplification mode. 5,000 cells for each sample were collected. Dyeing operations were all conducted in the dark.
For culturability analysis, each sample was incubated on NA at 28 °C for 24 h according to the national standards1. All these experiments were performed in triplicate. Cells were considered to be in VBNC state when none of the colonies was demonstrated on a NA plate (1 mL of sample was coated).
RNA extraction and qualification
Previously prepared control (4.1) and VBNC (4.2) bacteria solutions (107–8 cells mL−1) were centrifuged at 12,000 × g for 1 min and re-suspended in sterilized buffered saline. The control in this paper was culturable E. coli with the same treatment except chlorination. RNA was extracted using EasyPure RNA Kit (Transgen Biotech, China). RNA purity was examined using the micro-spectrophotometer Nano-100 (Aosheng, China). Qubit (Life Technologies, USA) was used to measure the RNA concentration. The experiments were repeated three times under the same conditions. The RNA which was extracted from biological duplication was used for RNA sequencing. In addition, Pearson correlation between samples was checked to ensure accuracy of the results of RNA-seq.
Transcriptome sequencing
Library preparation and sequencing
Transcriptome sequencing was performed by the Novogene company (Novogene, China). Sequencing libraries were built using NEBNext® Ultra™ Directional RNA Library Prep Kit (NEB, USA) following the manufacturer’s instructions. Briefly, the mRNA was fragmented and purified with poly-T oligo-attached magnetic beads (Beckman Coulter, USA). Then, first strand cDNA and second strand cDNA were synthesized. PCR was performed of universal PCR primers and Index (x) Primer. Finally, the Library preparations were sequenced on an Illumina Hiseq 2000 platform.
Gene expression level analysis
FPKM (Expected number of Fragments Per Kilobase of transcript sequence per Millions base pairs sequenced) method52,53 was used to analyze the gene expression variations between culturable and VBNC cells. The reads numbers mapped to each gene was counted by HTSeq v0.6.1.
Differential expression analysis
DESeq R package was used to analyze differential expression of culturbale and VBNC state. After multiple testing correction, the adjusted P-value less than 0.05 by DESeq was selected as a threshold of differential expression54.
Gene Ontology (GO) enrichment analysis
GOseq R package was used to GO enrichment analysis of differential expressed genes. GO terms with adjusted P-value < 0.05 were assigned as significantly enriched by differential expressed genes55.
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
KEGG was used to understand functions of the biological system from molecular-level information. In this study, KOBAS software was adopted to analyze the statistical enrichment of differential expression genes in KEGG pathways56.
Quantitative real-time PCR (qRT-PCR) validation
Five typical genes were chosen for the expression levels via qRT-PCR to confirm the transcriptome results. The information of primers was listed in Table S2. Samples were run on Applied Biosystems Q6 instrument (Life Technology, Singapore). The reaction was performed in triplicate at 30 s denaturation at 94 °C, with 40 cycles of 5 s annealing at 94 °C and extension step at 60 °C for 40 s. The comparative threshold cycle method was used to calculate gene expression levels as described previously (Fig. S4).
Morphology
Morphological characteristics of culturable cells and VBNC cells were investigated using the LIVE/DEAD BacLight bacterial viability kit (Invitrogen, Inc. L7012, USA)9,25. Briefly, SYTO 9 and propidium iodide (PI) were added in 1.5 mL tubes at a final concentration of 0.5 μM and 10 μM, respectively (20 min, room temperature). Samples were analyzed by FCM (the same operations as method 4.4), and the fluorescence was recorded for SYTO 9 and PI using 480/525 nm and 488/617 nm, respectively. The morphological structures were observed by SEM (Hitachi S4800, Japan) at the 20,000 × magnification.
Supplementary information
Characterization and potential mechanisms of highly antibiotic tolerant VBNC Escherichia coli induced by low level chlorination.
Acknowledgements
This research was supported by the National Key Research and Development Program of China-International collaborative project from Ministry of Science and Technology (Grant No. 2017YFE0107300), Natural Science Foundation of China (41861144023, 51678551, 51708534).
Author contributions
Xin Yu conceived the experiment(s), Chengsong Ye and Huirong Lin conducted the experiment(s), Chengsong Ye, Menglu zhang and Sheng Chen analysed the results. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chengsong Ye and Huirong Lin.
Supplementary information
is available for this paper at 10.1038/s41598-020-58106-3.
References
- 1.GB Standardization Administration of China. The People’s Republic of China drinking water sanitary standard. Vol. GB 5749–2006 (China, 2006).
- 2.Lewis AR, Robert MC, Walter MG. Modeling Chlorine Residuals in Drinking‐Water Distribution Systems. J. Environ. Eng. 1994;120:803–820. doi: 10.1061/(ASCE)0733-9372(1994)120:4(803). [DOI] [Google Scholar]
- 3.Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicol. 2004;198:229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver JD. The Viable but Nonculturable State in Bacteria. J. Microbiol. 2005;43:93–100. [PubMed] [Google Scholar]
- 5.Byrd JJ, Xu HS, Colwell RR. Viable but nonculturable bacteria in drinking water. Appl. Environ. Microbiol. 1991;57:875–878. doi: 10.1128/AEM.57.3.875-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams BL, Bates TC, Oliver JD. Survival of Helicobacter pylori in a Natural Freshwater Environment. Appl. Environ. Microbio. 2003;69:7462–7466. doi: 10.1128/aem.69.12.7462-7466.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Wang C, Tyrrell G, Hrudey SE, Li XF. Induction of Escherichia coli O157:H7 into the viable but non-culturable state by chloraminated water and river water, and subsequent resuscitation. Environ. Microbiol. Rep. 2009;1:155–161. doi: 10.1111/j.1758-2229.2009.00024.x. [DOI] [PubMed] [Google Scholar]
- 8.Moreno Y, et al. Survival and viability of Helicobacter pylori after inoculation into chlorinated drinking water. Water Res. 2007;41:3490–3496. doi: 10.1016/j.watres.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Dinu LD, Bach S. Induction of viable but nonculturable Escherichia coli O157:H7 in the phyllosphere of lettuce: a food safety risk factor. Appl. Environ. Microbiol. 2011;77:8295–8302. doi: 10.1128/AEM.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lleo MdM, Bonato B, Signoretto C, Canepari P. Vancomycin Resistance Is Maintained in Enterococci in the Viable but Nonculturable State and after Division Is Resumed. Antimicrob. Agents Chemother. 2003;47:1154–1156. doi: 10.1128/aac.47.3.1154-1156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orman MA, Brynildsen MP. Establishment of a method to rapidly assay bacterial persister metabolism. Antimicrob. Agents Chemother. 2013;57:4398–4409. doi: 10.1128/AAC.00372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowakowska J, Oliver JD. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiol. Ecol. 2013;84:213–222. doi: 10.1111/1574-6941.12052. [DOI] [PubMed] [Google Scholar]
- 13.Lin H, Ye C, Chen S, Zhang S, Yu X. Viable but non-culturable E. coli induced by low level chlorination have higher persistence to antibiotics than their culturable counterparts. Environ. Pollut. 2017;230:242–249. doi: 10.1016/j.envpol.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 14.Vora GJ, et al. Microarray-based detection of genetic heterogeneity, antimicrobial resistance, and the viable but nonculturable state in human pathogenic Vibrio spp. P. Natl. Acad. Sci. USA. 2005;102:19109–19114. doi: 10.1073/pnas.0505033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Ye C, Lin H, Lv L, Yu X. UV disinfection induces a VBNC state in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 2015;49:1721–1728. doi: 10.1021/es505211e. [DOI] [PubMed] [Google Scholar]
- 16.Heim S, Del Mar Lleo M, Bonato B, Guzman CA, Canepari P. The Viable but Nonculturable State and Starvation Are Different Stress Responses of Enterococcus faecalis, as Determined by Proteome Analysis. J. Bacteriol. 2002;184:6739–6745. doi: 10.1128/jb.184.23.6739-6745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asakura H, et al. Gene expression profile of Vibrio cholerae in the cold stress-induced viable but non-culturable state. Environ. Microbiol. 2007;9:869–879. doi: 10.1111/j.1462-2920.2006.01206.x. [DOI] [PubMed] [Google Scholar]
- 18.Postnikova OA, Shao J, Mock NM, Baker CJ, Nemchinov LG. Gene Expression Profiling in Viable but Nonculturable (VBNC) Cells of Pseudomonas syringae pv. syringae. Front. Microbiol. 2015;6:1419. doi: 10.3389/fmicb.2015.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su X, et al. Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci. Rep. 2015;5:18590. doi: 10.1038/srep18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crapoulet N, Barbry P, Raoult D, Renesto P. Global transcriptome analysis of Tropheryma whipplei in response to temperature stresses. J. Bacteriol. 2006;188:5228–5239. doi: 10.1128/JB.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorek R, Cossart P. Prokaryotic transcriptomics: a new view on regulation, physiology and pathogenicity. Nat. Rev. Genet. 2010;11:9–16. doi: 10.1038/nrg2695. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, et al. Comparative Transcriptome Analysis of Vibrio splendidus JZ6 Reveals the Mechanism of Its Pathogenicity at Low Temperatures. Appl. Environ. Microbiol. 2016;82:2050–2061. doi: 10.1128/AEM.03486-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton. GA, Gunnison D, Lanza GR. Survival of Pathogenic Bacteria in Various Freshwater Sediments. Appl. Environ. Microbio. 1987;53:633–638. doi: 10.1128/AEM.53.4.633-638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edberg SC, Rice EW, Karlin RJ, Allen MJ. Escherichia coli the best biological drinking water indicator for public health protection. J. App. Microbio. 2000;88:106S–116S. doi: 10.1111/j.1365-2672.2000.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee DG, Seong JP, Sang-Jong K. Influence of pipe materials and VBNC cells on culturable bacteria in a chlorinated drinking water model system. J. microbio. biotechnol. 2007;17:1558–1562. [PubMed] [Google Scholar]
- 26.Xu, H. S. et al. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol., 313–323 (1982). [DOI] [PubMed]
- 27.Jang HJ, Nde C, Toghrol F, Bentley WE. Global transcriptome analysis of the Mycobacterium bovis BCG response to sodium hypochlorite. Appl. Microbiol. Biotechnol. 2009;85:127–140. doi: 10.1007/s00253-009-2208-0. [DOI] [PubMed] [Google Scholar]
- 28.Virto R, Manas P, Alvarez I, Condon S, Raso J. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl. Environ. Microbiol. 2005;71:5022–5028. doi: 10.1128/AEM.71.9.5022-5028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatsumi T, Fliss H. Hypochlorous acid and chloramines increase endothelial permeability: possible involvement of cellular zinc. Am. J. Physiol. Heart Circ. Physiol. 1994;267:1597–1607. doi: 10.1152/ajpheart.1994.267.4.H1597. [DOI] [PubMed] [Google Scholar]
- 30.Duo M, Hou S, Ren D. Identifying Escherichia coli genes involved in intrinsic multidrug resistance. Appl. Microbiol. Biotechnol. 2008;81:731–741. doi: 10.1007/s00253-008-1709-6. [DOI] [PubMed] [Google Scholar]
- 31.Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 2010;34:415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 32.Ayrapetyan M, Williams TC, Oliver JD. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 2015;23:7–13. doi: 10.1016/j.tim.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014;5:258. doi: 10.3389/fmicb.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymond KN, Dertz EA, Kim SS. Enterobactin an archetype for microbial iron transport. P. Natl. A. Sci. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niederhoffer EC, Naranjo CM, Bradley KL, Fee JA. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J. Bacterial. 1990;172:1930–1938. doi: 10.1128/JB.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosa JH, Walsh CT. Genetics and Assembly Line Enzymology of Siderophore Biosynthesis in Bacteria. Microbio. Molecular. Biol. Rev. 2002;66:223–249. doi: 10.1128/mmbr.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Path. 2010;6:e1000949. doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nenninger AA, et al. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Mol. Microbiol. 2011;81:486–499. doi: 10.1111/j.1365-2958.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, et al. Structure and function of the Escherichia coli protein YmgB: a protein critical for biofilm formation and acid-resistance. J. Mol. Biol. 2007;373:11–26. doi: 10.1016/j.jmb.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gimenez R, Nunez MF, Badia J, Aguilar J, Baldoma L. The Gene yjcG, Cotranscribed with the Gene acs, Encodes an Acetate Permease in Escherichia coli. J. Bacteriol. 2003;185:6448–6455. doi: 10.1128/jb.185.21.6448-6455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Showe MK, DeMoss JA. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J. Bacterial. 1968;95:1305–1313. doi: 10.1128/JB.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang L, Wei LIC, Audia JP, Morton RA, Schellhorn HE. Expression of the Escherichia coli NRZ nitrate reductase is highly growth phase dependent and is controlled by RpoS, the alternative vegetative sigma factor. Mol. Microbiol. 1999;34:756–766. doi: 10.1046/j.1365-2958.1999.01637.x. [DOI] [PubMed] [Google Scholar]
- 43.Lessard IA, et al. Homologs of the vancomycin resistance D-Ala-D-Ala dipeptidase VanX in Streptomyces toyocaensis, Escherichia coli and Synechocystis: attributes of catalytic efficiency, stereoselectivity and regulation with implications for function. Chem. Biol. 1998;5:489–504. doi: 10.1016/S1074-5521(98)90005-9. [DOI] [PubMed] [Google Scholar]
- 44.Gatzeva-Topalova PZ, May AP, Sousa MC. Crystal structure and mechanism of the Escherichia coli ArnA (PmrI) transformylase domain. An enzyme for lipid A modification with 4-amino-4-deoxy-L-arabinose and polymyxin resistance. Biochem. 2005;44:5328–5338. doi: 10.1021/bi047384g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ideses D, et al. A degenerate type III secretion system from septicemic Escherichia coli contributes to pathogenesis. J. Bacteriol. 2005;187:8164–8171. doi: 10.1128/JB.187.23.8164-8171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mihaela P, Regine HA. The cellular level of the recognition factor RssB is rate limiting for σs proteolysis implications for RssB regulation and signal transduction in σs turnover in Escherichia coli. Mol. Microbio. 2002;45:1701–1713. doi: 10.1046/j.1365-2958.2002.03123.x. [DOI] [PubMed] [Google Scholar]
- 47.Mika F, Hengge R. A two-component phosphotransfer network involving ArcB, ArcA, and RssB coordinates synthesis and proteolysis of sigmaS (RpoS) in E. coli. Genes Dev. 2005;19:2770–2781. doi: 10.1101/gad.353705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung DK, Lau WY, Chan WT, Yan A. Copper efflux is induced during anaerobic amino acid limitation in Escherichia coli to protect iron-sulfur cluster enzymes and biogenesis. J. Bacteriol. 2013;195:4556–4568. doi: 10.1128/JB.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padilla-Benavides T, George Thompson AM, McEvoy MM, Arguello JM. Mechanism of ATPase-mediated Cu+ export and delivery to periplasmic chaperones: the interaction of Escherichia coli CopA and CusF. J. Biol. Chem. 2014;289:20492–20501. doi: 10.1074/jbc.M114.577668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez GG, Phipps D, Ishiguro K, Ridgway HF. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Micro. 1992;58:1801–1808. doi: 10.1128/AEM.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y, Yang T, Li D, He M. Rapid detection of viable bacteria by integrated CTC (5-Cyano-2, 3-ditoyl tetrazolium chloride) dying and flow cytometry assay (CTC-FCM) Acta. Sci. Circumst. 2013;33:2511–2515. [Google Scholar]
- 52.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anders, S. & Huber, W. Differential expression of RNA-Seq data at the gene level–the DESeq package. Eur. Mol. Biol. Lab. 1–24 (2012).
- 55.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanehisa M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prakash S, Cooper G, Singhi S, Saier MH. The ion transporter superfamily. Biochim. Biophys. Acta. 2003;1618:79–92. doi: 10.1016/j.bbamem.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Kostner M, Schmidt B, Bertram R, Hillen W. Generating tetracycline-inducible auxotrophy in Escherichia coli and Salmonella enterica serovar Typhimurium by using an insertion element and a hyperactive transposase. Appl. Environ. Microbiol. 2006;72:4717–4725. doi: 10.1128/AEM.00492-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto K, Ogasawara H, Ishihama A. Involvement of multiple transcription factors for metal-induced spy gene expression in Escherichia coli. J. Biotechnol. 2008;133:196–200. doi: 10.1016/j.jbiotec.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Michael PS, et al. The medium-/long-chain fatty acy l-CoA dehydrogenase (fadF) gene of Salmonella typhimurium is a phase 1 starvation-stress response (SSR) locus. Microbio. 1999;145:15–31. doi: 10.1099/13500872-145-1-15. [DOI] [PubMed] [Google Scholar]
- 61.Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R. Genome-wide analysis of the general stress response network in Escherichia coli: sigmaS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005;187:1591–1603. doi: 10.1128/JB.187.5.1591-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tagourti J, Landoulsi A, Richarme G. Cloning, expression, purification and characterization of the stress kinase YeaG from Escherichia coli. Protein Expr. Purif. 2008;59:79–85. doi: 10.1016/j.pep.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Hall BG. Evolutionary potential of the ebgA gene. Mol. Bio. Evol. 1995;12:514–517. doi: 10.1093/oxfordjournals.molbev.a040225. [DOI] [PubMed] [Google Scholar]
- 64.Monterrubio R, Baldoma L, Obradors N, Aguilar J, Badia J. A Common Regulator for the Operons Encoding the Enzymes Involved in D-Galactarate, D-Glucarate, and D-Glycerate Utilization in Escherichia coli. J. Bacteriol. 2000;182:2672–2674. doi: 10.1128/JB.182.9.2672-2674.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazar V, et al. Bacterial evolution of antibiotic hypersensitivity. Mol. Syst. Biol. 2013;9:700. doi: 10.1038/msb.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization and potential mechanisms of highly antibiotic tolerant VBNC Escherichia coli induced by low level chlorination.