Abstract
In the common pest cockroach, Periplaneta americana, behavioural responses to the sex and aggregation pheromones change in an age-dependent manner. Nymphs are attracted by the aggregation pheromone periplanolide-E (PLD-E) but not by the sex pheromone periplanone-B (PB) in faeces. Adults display prominent behaviours to PB but not to PLD-E. Despite the significant behavioural differences depending on postembryonic developmental stages, peripheral codings of the sex and aggregation pheromones have not been studied in the nymph of any insects as far as we know. In this study, we morphologically and electrophysiologically identified antennal sensilla that respond to PB and PLD-E in nymphal cockroaches. Although nymphs lacked the sex pheromone-responsive single-walled B (sw-B) sensilla identified in adult males, we found PB-responsive sensory neurons (PB-SNs) within newly identified sw-A2 sensilla, which exhibit different shapes but have the same olfactory pores as sw-B sensilla. Interestingly, PLD-E-responsive sensory neurons (PLD-E-SNs) were also identified in the same sensillar type, but PB and PLD-E were independently detected by different SNs. Both PB-SNs and PLD-E-SNs showed high sensitivity to their respective pheromones. The hemimetabolous insect nymph has an ability to detect these pheromones, suggesting that behaviours elicited by pheromones might be established in brain centres depending on postembryonic development.
Subject terms: Olfactory system, Peripheral nervous system, Sensory processing, Sexual behaviour, Animal physiology
Introduction
Pheromones are chemical agents that effectively trigger species-specific behaviours to conspecifics, such as aggregation, sexual and social behaviors1. As cockroaches are one of the major pests in urban environments, effective control of their behaviour using pheromones has been extensively investigated with regarding to ecological, pharmacological and physiological aspects. Thus, pheromones have been identified in several species of cockroaches2–5. In the American cockroach, Periplaneta americana, two sex pheromones have been isolated, the subcomponent periplanone-A (PA) and the main component periplanone-B (PB)6–8. Adult male cockroaches are strongly attracted by the sex pheromones emitted by adult females, which elicit sexual behaviours9, but nymphal cockroaches do not exhibit any behavioural responses to the sex pheromones10,11. In the cockroach, adults and nymphs at various developmental stages rest in a group in nature environment12. Especially, nymphs are strongly attracted by their faeces13. Recently, six attractive compounds, termed periplanolide-A to -F (PLD-A to -F), were identified from dried faeces of P. americana and were successfully synthesised14. Behavioural experiments showed that each PLD compound strongly and weakly attracts early instar nymphs and adults, respectively14. Thus, behaviours in response to the sex and aggregation pheromones are significantly different depending on the postembryonic developmental stages of the cockroach. However, it is still unknown how nymphal cockroaches detect and process these pheromones.
In P. americana, neural processing of sex pheromones has been well studied on levels ranging from peripheral to higher brain centres in adult males. Odourants including pheromones are generally detected by olfactory sensory neurons (OSNs) in antennal olfactory sensilla. In the adult, antennal olfactory sensilla are classified into three morphological types: perforated basiconic, grooved basiconic and trichoid sensilla15. Among them, perforated basiconic sensilla are further classified on the basis of shaft length into single walled-A (sw-A) sensilla (Fig. 1; 8–12 µm in length) and sw-B sensilla (Fig. 1; 18–28 µm in length)15–18. Sw-A and sw-B sensilla contain two and four OSNs, respectively15–18. Both of the OSNs in sw-A sensilla detect general odours, but single sw-B sensilla contain PA- and PB-responsive sensory neurons (PA-SNs and PB-SNs) in addition to two OSNs that detect general odours19–22. PA-SNs and PB-SNs extend their axons into A- and B-glomeruli in the antennal lobe (AL), respectively. Because of the greater number of sw-B sensilla on the male antenna, the A- and B-glomeruli are enlarged in males compared with the homologous glomeruli in females23. In the A- and B-glomeruli, PA- and PB-SNs synapse onto PA- and PB-responsive projection neurons (PA-PNs and PB-PNs)24–26.
Figure 1.
Classification of antennal olfactory sensilla in the nymphal and adult male cockroaches. (A) Sensillar distribution on a flagellomere in the distal part of a fourth instar male antenna. A SEM image of the 43rd flagellomere (left) and its schematic drawing (right) denote the sensillar distribution. (B) Sensillar distribution on a flagellomere in the distal part of an adult male antenna. A SEM image of the 94th flagellomere (left) and its schematic drawing (right) denote the sensillar distribution. Adult male antenna equips a large number of sex pheromone-responsive sw-B sensilla (blue triangles). cha: chaetic sensillum. (C) Nymphal olfactory sensilla. The antenna contains perforated basiconic (p-bas), grooved basiconic (g-bas), and trichoid (tri) sensilla. Based on the shapes of olfactory pores, p-bas sensilla further divided into sw-A1 and sw-A2 sensilla in nymphs (insets in C). (D) New classification of adult olfactory sensilla. Based on differences of sensillar lengths and olfactory pore shapes, p-bas sensilla further divided into sw-A1, sw-A2 and sw-B sensilla in adult males (insets in D). In both postembryonic developmental stages, sw-A1 sensilla equip with slit-like olfactory pores, whereas sw-A2 and sw-B sensilla equip with circular pores.
Although the antennae of nymphal cockroaches lack sw-B sensilla17,27,28, previous studies suggested that nymphal cockroaches sense sex pheromones as follows. First, the A- and B-glomeruli are formed during early development, and the volumes of these glomeruli gradually increase at every moult concomitantly with the elongation of the antennae28–30. Second, the nymphal antennae exhibit weak but reliable electroantennogram responses to sex pheromones31–33. Third, later instar nymphs have PB-PNs that have dendrites in the nymphal B-glomerulus34. Finally, a large majority of nymphal sw-A sensilla are transformed into sw-B sensilla at the final moult, and some OSNs in the nymphal sw-A sensilla extend their axon terminals into the A- and B-glomeruli28. Therefore, nymphal sw-A sensilla may be able to detect sex pheromones. However, the physiological properties of nymphal OSNs are still unclear. Furthermore, neural processing of aggregation pheromones has not been clarified in either nymphal or adult cockroaches.
In this study, morphological and electrophysiological analyses of nymphal antennal sensory systems revealed that male and female nymphal cockroaches can sense both sex and aggregation pheromones via OSNs in specific sensilla with high sensitivities. It is unclear the function of sex pheromone detection ability of nymphal stages because of lack of behavioural bioassay. However, comparisons of pheromone processing between nymphs and adults will provide valuable insights for understanding the neural mechanisms of behaviours elicited by pheromones in insects.
Results
Olfactory sensilla of nymphal cockroaches
To characterise the morphological features of antennal olfactory sensilla in nymphal cockroaches, we observed flagella of the distal parts of antennae in fourth instar males (N = 5) and females (N = 5) with a field emission scanning electron microscope (SEM) and compared with those of the adult male antennae (N = 5) (Fig. 1). In the cockroach, the antenna is composed of the scape, pedicel, and flagellum, and the flagellum of the fourth instars and adult males consist of approximately 70 and 140 flagellomeres, respectively27. On the flagellum of nymphal antennae, antennal sensilla were sparsely distributed compared with those of adult males (Fig. 1A,B)17,28. We distinctively observed perforated basiconic, grooved basiconic, and trichoid sensilla on nymphal antennae (Fig. 1A,C), which have been identified as olfactory sensilla in adult antennae (Fig. 1B,D)15,17. Based on the differences of sensillar lengths, adult males have two types of perforated basiconic sensilla, sw-A (8–12 µm in length) and sw-B sensilla (18–28 µm in length) (Fig. 1D)17. Although nymphal antennae lacked the sw-B sensilla, as reported previously (Fig. 1A)17,27,28, we found that nymphal sw-A sensilla could be classified into sw-A1 and sw-A2 sensilla on the basis of olfactory pore shapes (Fig. 1C). The sw-A1 sensilla had larger basal diameters (~3 µm) and were equipped with slit-like olfactory pores on their cuticular apparatus, whereas the sw-A2 sensilla had smaller basal diameters (~2 µm) and were equipped with circular olfactory pores (Fig. 1C). A few sw-A1 sensilla were present in the distal region of each nymphal flagellomere, whereas many sw-A2 sensilla were distributed throughout the flagellomere (Fig. 1A). No sex differences were observed in the sensillar distribution pattern in nymphs. We also observed shapes of olfactory pores of adult sw-A and sw-B sensilla. We found that the morphological criteria of sw-A1 and sw-A2 sensilla in nymphs were also applicable in adult sw-A sensilla (Fig. 1D). Interestingly, all observed sw-B sensilla equipped with circular pores as same as the sw-A2 sensilla (Fig. 1D). In adults, sw-A1 sensilla selectively distributed in the distal region of each flagellomere, suggesting loci of sw-A1 sensilla were maintained during postembryonic development (Fig. 1A,B). In adult males, both sw-A2 and sw-B sensilla were sparsely and densely distributed throughout a given flagellomere, respectively (Fig. 1B).
Responses of nymphal sensilla to sex and aggregation pheromones
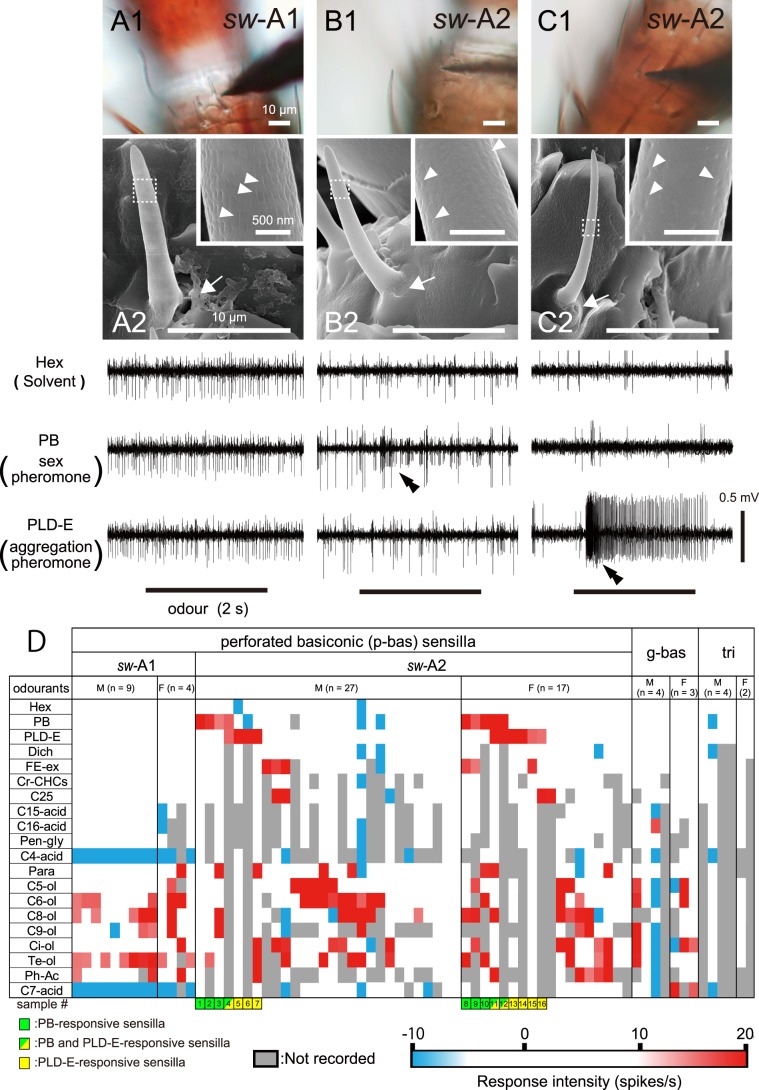
To identify the olfactory sensilla that detect sex and aggregation pheromones, we recorded responses from a total of 213 olfactory sensilla of fourth instar nymphs of both sexes using the single sensillum recording (SSR) method. We successfully recorded olfactory responses from 57 perforated basiconic, seven grooved basiconic, and six trichoid sensilla on male and female nymphal antennae. In each SSR, several different shapes of spikes that were discharged from different OSNs in a single sensillum were concurrently recorded (Fig. 2A–C). Based on these spike shapes, we identified two, three, and two OSNs in single perforated basiconic, grooved basiconic, and trichoid sensilla, respectively. To classify recorded perforated basiconic sensilla into sw-A1 and sw-A2 sensilla, recorded sensilla were further observed in detail with SEM (Fig. 2A2–C2). In the result, we successfully recorded olfactory responses of 13 sw-A1 and 44 sw-A2 sensilla in this study. In both sw-A1 and sw-A2 sensilla, large- and small-amplitude spikes were spontaneously discharged (Fig. 2A–C). The averaged spontaneous firing rate of 13 sw-A1 sensilla (13.1 Hz) was higher than that of 44 sw-A2 sensilla (9.6 Hz). As shown in Fig. 2B, PB activated the small-amplitude spike OSN but not the large-amplitude spike OSN in a sw-A2 sensillum. Thus, each sw-A sensillum was innervated by paired OSNs with different response profiles. Although the spike amplitudes were distinguishable in several traces, the spikes were not easily distinguishable in many recorded sensilla. Therefore, we first characterised recorded sensilla in terms of the total number of spikes per sensillum and then calculated the increase in spike frequency from the spontaneous level (Fig. 2D).
Figure 2.
Olfactory responses of single sensilla on the nymphal antennae. (A–C) Typical responses and morphological features of sw-A1 (A), PB-responsive sw-A2 (B), and PLD-E-responsive sw-A2 sensilla (C). Because we could not identify sensillar types of sw-A sensilla under the light microscope (A1–C1), recorded sensilla were observed using SEM in detail (A2–C2). SEM observation reveals olfactory pores of recorded sensilla (arrowheads in insets of A2–C2). Arrows in A2–C2 indicate the insertion sites of electrodes. Sex and aggregation pheromones elicited small-amplitude and large-amplitude spikes in single sw-A2 sensilla, respectively (double arrowheads in B,C). (D) Response intensities of single sensilla to tested odourants. Response intensity to a given odourant is colour-coded according to the “R − R0” value (see Material and Methods). Excitatory and inhibitory responses are shown as warm and cold colours, respectively. 16 sw-A2 sensilla which exhibited excitatory responses to PB and/or PLD-E are numbered (sample numbers below the heatmap). M: male, F: female. g-bas: grooved basiconic sensilla. tri: trichoid sensilla.
In SSRs, we initially attempted to record the responses to 2 ng of PB, 2 ng of PLD-E and faecal extract, which contains the equivalent to 2 ng of PLD-E (Table 1)14. These pheromones elicited a strong excitatory response from some sw-A2 sensilla but did not elicit any response from other morphological types of sensilla (Fig. 2B–D). Thereafter, we increased the number of test odourants included six reported attractants of nymphal cockroaches5,35–37 and eight chemically diverse odourants that are frequently used for classification of OSN types (Table 1)20,22,38. Each of the general odours largely activated the sw-A1, sw-A2 and grooved basiconic sensilla (Fig. 2D). Most of the sw-A1 sensilla exhibited strong inhibitory responses to C4- and C7-acids, which are known to elicit excitatory responses from OSNs in the grooved basiconic sensilla of adults20,22. Among the six attractive odourants, pentacosane (C25) selectively activated a small subset of sw-A2 sensilla. Because pheromones and attractive odours were selectively detected by sw-A2 sensilla, we focused on the physiological properties of this type of sensillum in subsequent studies.
Table 1.
Pheromones and odourants used in this study.
| Odourants | Abbreviations | Purity (%) | Solvent | Concentration | Ref. |
|---|---|---|---|---|---|
| Solvent | |||||
| n-hexane | Hex | ≥99% | |||
| dichloromethane | Dich | ≥99% | |||
| n-paraffin-oil | Para | ||||
| Sex pheromone | |||||
| periplanone-B | PB | Hex | 2 ng/µl | 50 | |
| Aggregation pheromone | |||||
| periplanolide-E | PLD-E | Hex | 2 ng/µl | 14 | |
| faecal extract | FE-ex | Dich | 16.6 µl | 13 | |
| Aggregation-inducing substances | |||||
| crude extract of CHCs | Cr-CHCs | Dich | 10 µl | 35,36 | |
| pentacosane | C25 | ≥97% | Dich | 20 µg/µl | 35,36 |
| pentadecanoic acid | C15-acid | ≥99% | Dich | 10 µg/µl | 5 |
| hexadecanoic acid | C16-acid | ≥99% | Dich | 10 µg/µl | 5 |
| pentaethylene glycol | Pen-gly | ≥90% | Dich | 10 µg/µl | 5 |
| butyric acid | C4-acid | ≥98% | Para | 10 mM | 37 |
| General odours | |||||
| n-pentanol | C5-ol | ≥98% | Para | 10 mM | 20,22 |
| n-hexanol | C6-ol | ≥97% | Para | 10 mM | 20,22 |
| n-octanol | C8-ol | ≥98% | Para | 10 mM | 20,22 |
| n-nonanol | C9-ol | ≥99% | Para | 10 mM | 20,22 |
| cineol | Ci-ol | ≥85% | Para | 10 mM | 20,22 |
| α-terpineol | Te-ol | ≥80% | Para | 10 mM | 20,22 |
| phenyl acetate | Ph-Ac | ≥98% | Para | 10 mM | 20,22 |
| heptanoic acid | C7-acid | ≥98% | Para | 10 mM | 20,22 |
Among 44 recorded sw-A2 sensilla, 16 sw-A2 sensilla exhibited phasic-tonic responses to PB and/or PLD-E (numbered sensilla in Fig. 2D), and another 28 sensilla did not respond to these pheromones (Fig. 2D). That is, nymphal antennae contained both pheromone-responsive sw-A2 and pheromone-unresponsive sw-A2 sensilla. The pheromone-responsive sw-A2 sensilla were narrowly tuned to the pheromones and a few general odours, whereas the pheromone-unresponsive sw-A2 sensilla broadly responded to many general odours. The response spectra of pheromone-unresponsive sw-A2 sensilla differed from those of sw-A1 sensilla. For example, C4- and C7-acids generally elicited inhibitory responses in sw-A1 sensilla, but these acids did not elicit any responses in sw-A2 sensilla. These results indicate that OSNs in sw-A1 and sw-A2 sensilla express distinct repertories of olfactory receptors. Both PB- and PLD-E-responsive sw-A2 sensilla were commonly present in both male and female nymphs (Fig. 2D). Therefore, nymphal cockroaches can sense sex pheromones irrespective of their sexes.
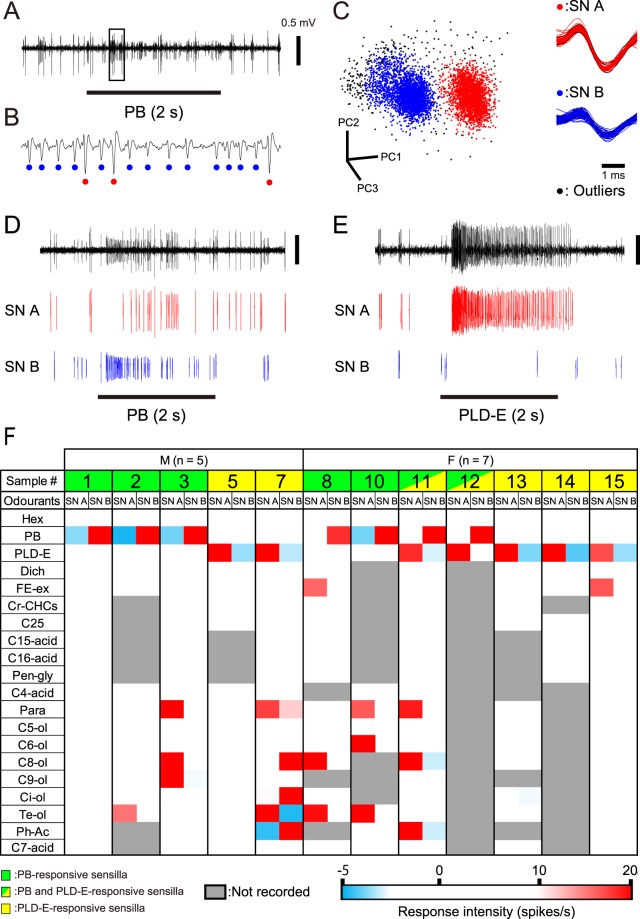
Among pheromone-responsive sw-A2 sensilla, three sensilla exhibited excitatory responses to both sex and aggregation pheromones (#4, #11 and #12 indicated in Fig. 2D). This result raises a question of whether a specific OSN that can process both sex and aggregation pheromones exists in sw-A2 sensilla. Therefore, we sorted spikes using specific electrophysiological data of pheromone-responsive sw-A2 sensilla, and revealed the response properties of each of the two OSNs in these sensilla (Fig. 3).
Figure 3.
Olfactory responses of OSNs in single pheromone-responsive sw-A2 sensilla. (A) The response of a sw-A2 sensillum to PB. (B) Expanded electrophysiological trace shown in A. The trace shows small-amplitude spikes (blue dots) and large-amplitude spikes (red dots). (C) Identification of two OSNs in single sw-A2 sensilla. Each of spikes is plotted based on the first three principal components (PC1-PC3) obtained from the principal component analysis using the spike amplitudes and durations (left). Cluster analysis identified two groups of spikes (red and blue dots). Outliers of the cluster analysis are shown as black dots in the 3D-space and removed from the subsequent analyses. The spike waveforms of red and blue dots are superimposed (right), and large-amplitude spikes of SN A and small-amplitude spikes of SN B are denoted by red and blue colours, respectively. (D,E) Segregated activities of SN A and SN B in PB-responsive (D) and PLD-E-responsive sw-A2 sensilla (E). (F) Olfactory responses of SN A and SN B to the tested odourants. We selected 12 pheromone-responsive sw-A2 sensilla from our SSRs (sample numbers in F and Fig. 2D) and sorted spikes of SN A and SN B. M: male, F: female.
Response properties of PB- and PLD-E-responsive SNs in sw-A2 sensilla
Small- and large-amplitude spikes from different OSNs were identical in each SSR from single sw-A2 sensilla of fourth instar nymphs (Figs. 2 and 3). We sorted these spikes using spike sorting software based on the spike amplitudes and durations (Fig. 3A–E) and successfully sorted into the two types of spikes from 12 pheromone-responsive sw-A2 sensilla (Fig. 3F). We termed large-spike and small-spike OSNs as “SN A” and “SN B”, respectively. PB exclusively activated SN B in PB-responsive sw-A2 sensilla, whereas PLD-E exclusively activated SN A in PLD-E-responsive sensilla (Fig. 3D,E). In the sw-A2 sensilla which responded to both pheromones, PLD-E-responsive SN A and PB-responsive SN B were co-localised (#11 and #12 in Fig. 3F). Thus, PB and PLD-E were independently detected by different OSNs, and we termed these as PB-SNs and PLD-E-SNs. None of PB-SNs responded to other tested odours (Fig. 3F). In contrast to the PB-SNs, PLD-E-SNs showed diverse response spectra; some PLD-E-SNs exhibited excitatory responses not only to PLD-E but also to general odours (#7 and #15 SN A in Fig. 3F).
We observed that an OSN which paired with pheromone-responsive OSN in single sw-A2 sensilla was activated by general odours (Fig. 3F). In each sw-A2 sensillum, both SN A and SN B exhibited spontaneous spike activity and tended to be reciprocally inhibited by effective odours; the spike activity of an OSN was decreased during the period that the other OSN exhibited a high spike frequency (Figs. 3F and 4).
Figure 4.
Temporal activity patterns of SN A and SN B that respond to sex and aggregation pheromones. (A,B) Temporal activity patterns of SN A and SN B to a given concentration of PB (A) and PLD-E (B). Olfactory responses (left panels) are obtained from a PB-responsive sw-A2 sensillum (A: #11 in Figs. 2D and 3F) and a PLD-E-responsive sw-A2 sensillum (B: #13 in Figs. 2D and 3F). Responses of SN A and SN B are displayed as peri-stimulus time histograms with 100 ms bins (right panels).
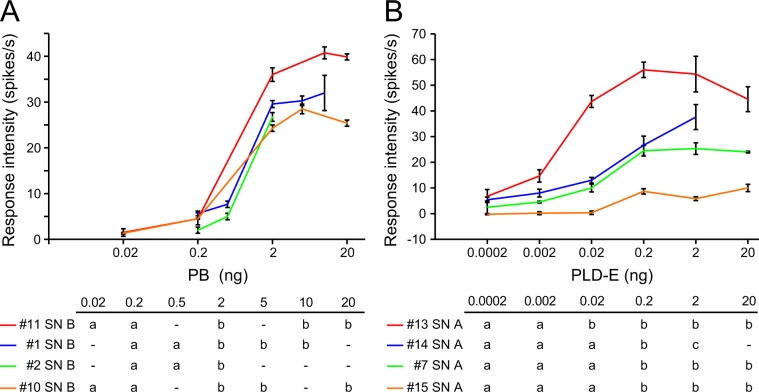
Finally, we examined the dose-response relationships of PB-SN responses to PB and PLD-E-SN responses to PLD-E. In both PB-SNs and PLD-E-SNs, temporal response patterns to pheromones were changed from phasic to phasic-tonic according to the increase of concentrations (Fig. 4). The tonic phases elicited by high pheromone concentration lasted long beyond the stimulus periods (arrowhead in Fig. 4A,B). We evaluated the increase in spike frequency during the 1-sec period after stimulus onset (Fig. 5). All responses of PB-SNs typically showed sigmoidal dose-response curves and began to increase monotonically at 0.2 ng of PB and nearly reached a plateau at 2 ng (Fig. 5A). The curves indicated that the threshold concentration of PB lies between these concentrations (one-way ANOVA and post-hoc Tukey’s test, different alphabet letters; P < 0.05 in Fig. 5A). All recorded PB-SNs exhibited similar dose-response curves, but the dose-response curves to PLD-E varied across four PLD-E-SNs (Fig. 5B). The responses of the most sensitive PLD-E-SN began to increase when 2 pg of PLD-E was presented and reached a plateau at 20 pg (#13 SN A in Fig. 5B; one-way ANOVA and post-hoc Tukey’s test). Although the sensitivity of recorded PLD-E-SNs was various, as shown in Fig. 5B, threshold concentration of all tested PLD-E-SNs to PLD-E appeared to be higher than that of PB-SNs to PB. Interestingly, the maximal spike frequency widely varied from 15 to 55 Hz in PLD-E-SNs. In addition, the PLD-E-SN responded only to PLD-E (#13 SN A in Figs. 3F and 5B) exhibited higher sensitivity than those responded both PLD-E and general odours (#7 SN A in Figs. 3F and 5B). These results showed that there are several types of PLD-E-SNs that have different physiological properties in the nymphal antenna.
Figure 5.
Dose-response curves of PB-SNs (A) and PLD-E-SNs (B). The averaged “R−R0” values of four PB-SNs (A) and four PLD-E-SNs (B) to a given concentration of pheromones are plotted with the standard error (vertical bars). Response spectra of four PB-SNs and four PLD-E-SNs are denoted in Fig. 3F (see sample numbers). In each of recordings, response intensities to different concentrations of pheromones are compared (one-way ANOVA and post-hoc Tukey’s test), and the same alphabet letters represent no statistical significant differences (P > 0.05).
Discussion
We electrophysiologically examined pheromone and odour detection in nymphal cockroaches and found that sex and aggregation pheromones are independently detected by distinct OSNs in the newly identified sw-A2 sensilla in nymphs. As far as we know, this is the first report on the cellular mechanisms of pheromone detection in nymphs of hemimetabolous insects. Sex and aggregation pheromone-responsive SNs exhibited high specificities and high sensitivities to their respective pheromones. Because the nymphal cockroaches lack sw-B sensilla, which have been confirmed to respond to sex pheromones in adults, and exhibits no behavioural responses to sex pheromones, it is surprising that nymphs possess high sensitive sex pheromone-responsive SNs. The behaviours elicited by sex and aggregation pheromones differ depending on the postembryonic developmental stages of the cockroach. Thus, the postembryonic developments of neural olfactory circuits and of their physiological properties are important factors for understanding expression of pheromone behaviours in hemimetabolous insects.
In this study, we classified nymphal and adult sw-A sensilla into sw-A1 and sw-A2 sensilla based on the external appearance of olfactory pores (Fig. 1). Different sizes and spatial patterns of olfactory pores in a given morphological type of olfactory sensilla have not been reported in other insects. In fruit flies, the formation of olfactory pores is controlled by a specific gene for cuticle secretion in single-walled olfactory sensilla39. Thus, olfactory pores of the cockroach might also be formed by specific gene control. Furthermore, sw-A1 and sw-A2 sensilla exhibited different olfactory response spectra, suggesting that OSNs in the two sensilla types might express different repertories of olfactory receptors. Therefore, sw-A1 and sw-A2 sensilla appear to have separate origins similar to the single-walled and double-walled olfactory sensilla, which exhibit different pore structures40.
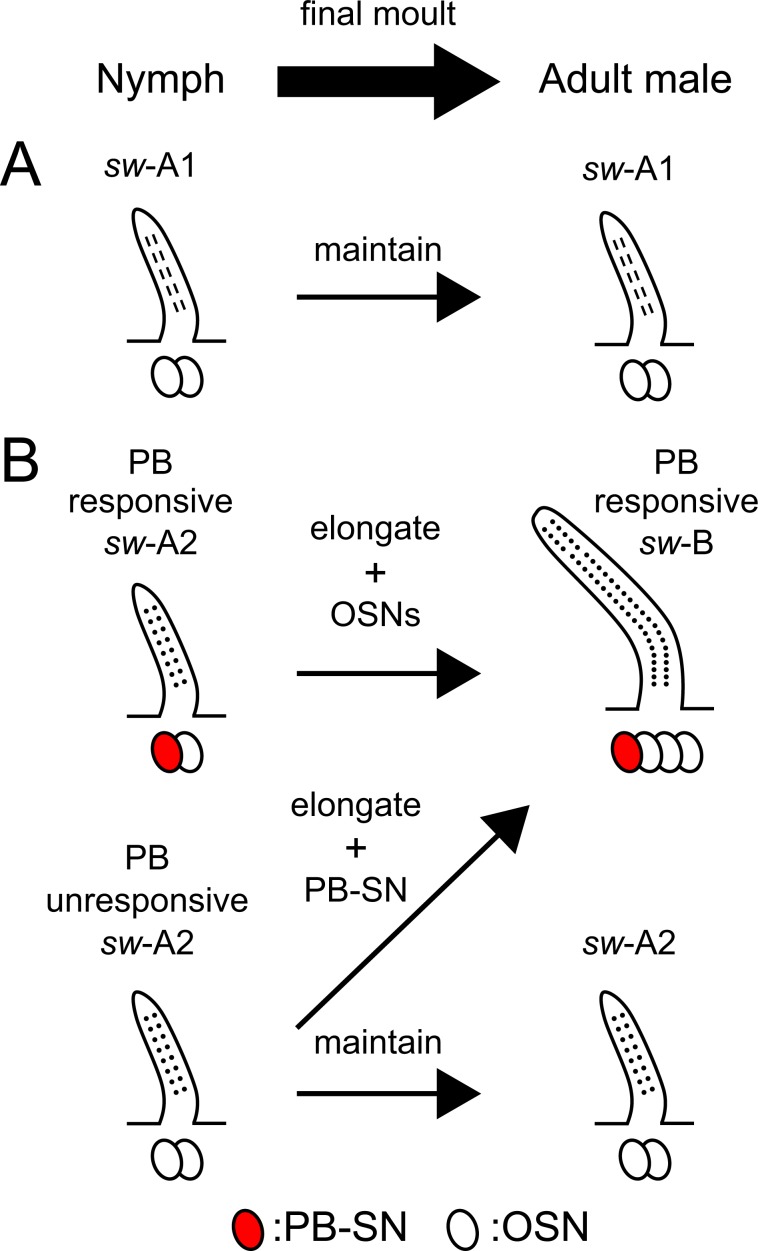
Taken together with previous and current results, we hypothesized postembryonic developments of sw-A and sw-B sensilla in the cockroach (Fig. 6). In the male cockroach, at the final moult, a large majority of the nymphal sw-A sensilla transform into sw-B sensilla, and the remaining sw-A sensilla retain their morphology17,28. In this study, we revealed that adult sw-A sensilla also classified into sw-A1 and sw-A2 sensilla based on differences of olfactory pore shapes. The nymphal and adult sw-A1 sensilla share common morphological features, such as sensillar sizes, olfactory pores (Fig. 1C,D), number of OSNs17 and distribution patterns (Fig. 1A). In addition, OSNs in nymphal sw-A1 sensilla and a subset of adult sw-A sensilla have similar response spectra to general odours (Fig. 2D)20,22. It suggests that sw-A1 sensilla are morphologically and physiologically maintained during postembryonic developments (Fig. 6A). Interestingly, both adult sw-B and sw-A2 sensilla exhibited circular olfactory pores which identified in nymphal sw-A2 sensilla. SSR experiments revealed that nymphal sw-A2 sensilla had two OSNs and classified into PB-responsive and PB-unresponsive types. Whereas, in adult males, sw-B sensilla are always PB-responsive19 and each sw-B sensillum contains four OSNs including a PB-SN15,17,22. Because the morphogenesis from sw-A to sw-B sensilla is often accompanied by an increasing number of OSNs28, nymphal sw-A2 sensilla, especially PB-responsive sw-A2 sensilla, must transform into the adult sw-B sensilla (Fig. 6B). Therefore, PB-SNs in the nymphal sw-A2 sensilla might be inherited by the adult sw-B sensilla. Adult sw-A2 sensilla must be originated from PB-unresponsive sw-A2 sensilla (Fig. 6B). But, we can not deny the possibility that PB-unresponsive sw-A2 sensilla transform into the sw-B sensilla by acquiring the PB-SNs during the postembryonic development (Fig. 6B). It needs more detailed observations of sensillar morphogenesis during the postembryonic development.
Figure 6.
Leading models of the postembryonic developments of olfactory sensilla. (A) Schematic drawing of the postembryonic development of sw-A1 sensilla. Anatomical and physiological features indicate that nymphal sw-A1 sensilla are maintained during the postembryonic development of the cockroach. (B) Schematic drawing of the postembryonic development of sex pheromone-responsive sensilla. In this study, we obtained following results. (1) Both nymphal sw-A2 and adult sw-B sensilla have common shapes of olfactory pores. (2) All recorded nymphal sw-A2 sensilla have two OSNs, and there are PB-responsive and PB-unresponsive sw-A2 sensilla in nymphs. It has been known that each sw-B sensillum has one PB-SN and three other OSNs17,19. Furthermore, a large majority of nymphal sw-A sensilla transform to the sw-B sensilla at the final moult, and the morphogenesis is accompanied the increase of the number of OSNs28. Taken together current and previous results, we hypothesized developmental schemes of the sex pheromone-responsive sensilla.
The length of sw-B sensillum in the adult is about two-fold longer than that of a nymphal sw-A2 sensillum17,28. In accordance with the sensillar morphogenesis at the final moult, dendrites of PB-SNs expand17, which might result in a greater number of PB receptors on the dendrite and greater number of olfactory pores on cuticular apparatus. Therefore, PB-SNs in sw-B sensilla are hypothesised to exhibit higher sensitivity to sex pheromones than those in nymphal sw-A2 sensilla17,28. The response thresholds of PB-SNs in nymphal sw-A2 sensilla range between 0.2 and 2 ng of PB, and those in adult sw-B sensilla have been reported to range between 0.1 ng and 1 ng of PB19. Thus, the PB sensitivity of PB-SNs is not significantly different between nymphs and adults. However, other physiological features of PB-SNs significantly differ between nymphs and adults; PB-SNs in sw-B sensilla frequently adapt to higher concentrations of PB19, but this does not occur in nymphal sw-A2 sensilla. Further electrophysiological experiments are needed to determine the functional effects of morphogenesis from sw-A2 to sw-B sensilla.
Although both nymphal and adult cockroaches sense sex pheromones, the expression of sexual behaviours induced by sex pheromones is restricted to adults10,11. Sexual behaviours may be driven by physiological changes in pheromone-responsive neurons during postembryonic development because the major pheromone processing pathway from peripheral to higher brain centres is established during early development in the insect29,30,41. At the peripheral level, numbers of olfactory sensilla and sensory afferents including PB-SNs in the adult male antenna are more than 10-fold greater than those in the fourth instar antenna17,27. Reflecting the number of PB-SNs, the size of B-glomerulus in adult males is also approximately 250-fold greater than that of fourth instars30. Therefore, projection neurons innervating the B-glomerulus (B-PNs) in adult males may exhibit higher sensitivity to PB than that in fourth instar nymphs. In fact, in adult females which have a small number of sw-B sensilla and a small sized B-glomerulus that is equivalent in size to that of mid-instar nymphs42, B-PNs exhibited the weaker sensitivity to PB compared with those in adult males43. It suggests that female and nymphal B-PNs require higher concentrations of PB to generate responses equivalent to those of males. The functional significance of sex pheromones in nymphal cockroaches is still unknown, but nymphal cockroaches sufficiently detect and process higher concentrations of PB.
In this study, we identified olfactory sensilla and OSNs in nymphal cockroaches that detect the aggregation pheromone PLD-E. Aggregation pheromones have been behaviourally identified in many insect species1, but the neural processing mechanisms of the pheromone are still unknown. On the basis of the dose-response curves and response spectra of PLD-E-SNs, we can speculate that there are several types of PLD-E-SNs which express different olfactory receptors. Among them, PLD-E-SNs that are selectively tuned to PLD-E exhibited high sensitivity to this pheromone. Their low threshold for PLD-E is consistent with the behavioural threshold as shown in the olfactometer assay using the early instar cockroaches14.
There are 205 glomeruli in the antennal lobe of P. americana regardless of sexes and instar stages, and these loci are maintained throughout all instar stages, although their sizes are larger in the later instar nymphs23,30,42. As PB is processed by B-glomerulus in the later instar nymphs, adult males and females24,34,43, PB would be also processed in the B-glomerulus in the earlier instar nymphs. In contrast, glomerulus processing PLD-E in both adults and nymphs has not been identified. In adult AL, 205 glomeruli are clearly organized into the anterodorsal and the posteroventral groups on the basis of differences in their locations, morphologies, functions and developments23,38,41,44. Except for PB-SNs and PA-SNs, OSNs in sw-A and sw-B sensilla exclusivelly terminate in ordinary glomeruli in the anterodorsal group in adults44. If this feature is present in nymphs, PLD-E-SNs in sw-A2 sensilla might also project to given glomeruli of the anterodorsal group. Interestingly, in first to fourth instar nymphs of the cockroach, the nymphal B-glomerulus is smaller than other ordinary glomeruli in the anterodorsal group30. As glomeruli receiving many OSNs generally tend to be large in size28,30, the earlier instar nymphs may have a larger number of PLD-E-SNs than PB-SNs. In addition, PLD-E-SNs have a 100 times higher sensitivity than PB-SNs to their effective pheromones in fourth instar nymphs. Taking these results together, the nymphal olfactory system appears to be tuned to detection of the aggregation pheromone instead of the sex pheromone. This finding is correlated with the behaviour of nymphal cockroaches, especially early instars, in that they are strongly attracted to aggregation pheromones but not to sex pheromones14.
Both PB- and PLD-E-SNs tended to inhibit during the activated period of the other OSN in a given sensillum (Figs. 3F and 4). This inhibition among co-localised OSNs affects the detection of pheromones and general odours. In the Japanese beetle Popillia japonica, two OSNs in a sensillum, which detect a conspecific pheromone and heterospecific kairomone, inhibit each other and increase the perceived contrast between these two odours45. In fruits flies and mosquitoes, inhibition of co-localised OSNs in single olfactory sensillum is mediated by non-synaptic interaction and improves detection of the given odour stimulus46,47. Because both sex and aggregation pheromones are contained in the faeces, the cockroach receives these pheromones and many other odourants at the same time in the natural condition. Therefore, the strong activity elicited by pheromones might inhibit the activity of co-localized OSNs. Cockroaches tune peripheral system that can detect behaviourally salient pheromones from complex and cluttered odour environments.
Materials and Methods
Insects
Fourth instar males and females, and adult males of P. americana with intact antennae were used in this study. P. americana develops to the adult stage via 11 moults27,28,30. The cockroaches were obtained from laboratory colonies maintained at 28 °C under a 12:12 light-dark cycle at Fukuoka University. Since the aggregation pheromone PLD-E effectively attracts earlier instar cockroaches14, fourth instar nymphs were used for the electrophysiological study because of the technical limitations of SSR. The nymphal stages were unambiguously identified based on the lengths of the whole body, hind tibia and abdomen48. Sexes were discriminated by examining the morphologies of the last two abdominal sternites49. The criteria were applicable nymphs of Periplaneta species including P. americana30,42,49.
Field emission scanning electron microscopy
After anesthetising nymphal and adult cockroaches on ice, whole antennae were isolated using a razor blade. The isolated antennae were immersed in 50% acetone and ultrasonically cleaned for 1 min. Antennae were dehydrated in an ascending acetone series (50% to 100%) and dried at 60 °C for more than 3 hours. Thereafter, each antenna was attached to an aluminium stub using water-soluble glue. After drying again, antennae were coated with platinum-palladium using an ion sputter (E-1045; Hitachi, Tokyo, Japan). Observations were performed using a field emission SEM (S-4800; Hitachi, Tokyo, Japan). After counting the number of flagellomeres, the external structures of the sensilla on a given flagellomere were thoroughly examined, and their digital images were obtained. The obtained images were processed using Adobe Photoshop CS3 and Illustrator CS3 (Adobe Systems, San Jose, CA, USA). Regarding the sensillar nomenclature, we referred to previous studies of adult P. americana15,17,18,28.
Single sensillum recording
The method used for extracellular recording from OSNs in single sensilla of the nymphs was modified from the method used for adult P. americana22. In this study, the fourth instar males and females were used for the electrophysiological experiments. For a SSR, the ice-anesthetised nymph was immobilised ventral-side-up on an acrylic plate that had been covered with a thin layer of low-melting point wax. To prevent movement, the body and legs were gently mounted on the plate using low-melting wax, the neck was immobilised with small acrylic plates, and the antennae were gently fixed using the wax on the plate.
The plate was placed on the stage of a light microscope (AZ100, Nikon, Tokyo, Japan). The antenna was observed through the microscope at 500 × magnification. We performed SSRs from arbitrarily selected olfactory sensilla on the ventral surface of the flagellum. For recording, a silver wire indifferent electrode was manually inserted into the head capsule near the ipsilateral compound eye, and a tungsten recording electrode, which had been electrolytically sharpened in saturated KNO2 solution, was inserted into the basal cavity of the sensillum using a micromanipulator (Fig. 2A1–C1). After observing the spontaneous spike activities of OSNs, olfactory stimuli were presented to the antenna. Electrical signals were processed by a preamplifier with high input impedance (MEZ-8201; Nihon Kohden, Tokyo, Japan), amplified by a main AC/DC amplifier (EX-1; Dagan Corporation, Minneapolis, MN, USA), and displayed on an oscilloscope. Signals were digitised and recorded with a Power Lab data acquisition system at a sampling rate of 20 kHz (Power Lab 8/35; AD Instruments Japan Inc., Nagoya, Japan).
To identify the sensillum type, the recorded sensillum was observed with a SEM (Fig. 2A2–C2). After recording of olfactory responses, the recorded sensillum was marked by removing the surrounding bristles by manipulating the electrode. Then, an antenna fragment that contained the recorded sensillum was observed in detail using a SEM. The sensillar types were unambiguously identified based on the shape of the cuticular apparatus and olfactory pores as described in the result section.
Olfactory stimulation
We used 15 different odourants and their solvents as well as purified substances of the main components of the sex pheromone (PB) and the aggregation pheromone (PLD-E) that were synthesised previously as odour stimuli (Table 1)14,50. Six odourants effectively attract nymphal cockroaches5,35–37, and eight odourants are used for classification of OSN types in adults22. Both PB and PLD-E were diluted in hexane at a concentration of 0.1 ng/µl as stock solutions. Faecal substances were extracted by immersing 5 g of dry faeces from cockroaches fed an agar/sugar diet in 10 ml of dichloromethane for 30 min (faecal extract)13. A gas chromatograph assay revealed that 16.6 µl of the faecal extract contained 2 ng of PLD-E (data not shown). Cuticular hydrocarbons were crudely extracted by immersing 40 fourth instar nymphs in dichloromethane for 1 hour (Cr-CHCs). Each odourant was dissolved in hexane or dichloromethane, placed on an aluminium plate (15 × 5 mm), and dried 3 min to evaporate the solvent. Therefore, their concentrations were denoted as dry weights in this study. Other odourants were diluted in paraffin oil at a concentration of 10 mM, and 20 µl of odourant solution was added to a piece of filter paper (15 × 5 mm). Immediately before recordings, the aluminium plates and the filter papers loaded with odourants were separately inserted into glass pipettes.
The odour stimulation device used in this study has been reported in a previous study38. Fresh air from outdoors was transported via a diaphragm air pump and was cleaned and dried with charcoal and silica-gel filters. The air stream was maintained at 1 l/min using a flowmeter. The main tube was connected to a three-way solenoid valve, which was operated by a stimulator (SEN7203, Nihon Kohden, Tokyo, Japan). During the inter-stimulus period, the constant air in the tube, which was connected to one outlet of the valve, passed through the blank glass pipette and flowed over the antenna. During stimulation period, the constant air stream was stopped and the air stream from the other outlet of the valve was passed through the glass pipette containing a given odourant. The tips of glass pipettes were positioned approximately 3 cm apart from the targeted sensillum. The air around the preparation was continuously exhausted through a duct behind the recording electrode. We regarded the timing of the solenoid valve gating as the onset of odour stimulation. The stimulus period was set to 1 or 2 sec. Each odourant was presented two to five times with >30 sec intervals. After stimulation, a new glass pipette containing another odorant was attached.
Data analysis
In each SSR, several different shapes of spikes from different OSNs were concurrently recorded. Although the spike amplitudes were distinguishable in several traces, the spikes were not easily distinguishable in many recordings. Therefore, we grouped spikes with different shapes in each SSR and calculated the increase in total spike frequency from the spontaneous frequency as follows; R−R0, where R and R0 were the total numbers of spikes during the 1-sec period before and after the onset of odour stimulation, respectively. The average response was defined as the response intensity to a given odorant.
To evaluate the response properties of each OSN in single sensilla, we sorted spikes with different shapes in several recordings using the spike sorting function of Spike 2 ver. 8.08 (CED, Cambridge, UK). We performed principal component analysis on the recorded spikes according to the spike amplitude and duration. Each spike was plotted in a three-dimensional space using the first three principal components, and the spikes were clustered (Fig. 3C). The response intensity was represented as R − R0. Statistical analyses were performed using the free software R v.3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Acknowledgements
The authors thank Dr. Tanaka of Kyoto Univ. for offering cockroach faecal extracts and Dr. Matsuo of Dainihon Jochugiku Co. Ltd. for synthesising PLD-E. We also thank Dr. Ai and Dr. Nakagawa of Fukuoka Univ. and Dr. Nishino and Dr. Mizunami of Hokkaido Univ. for experimental advices. We thank Lisa Kreiner, PhD, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript. This research was supported by JSPS KAKENHI Grant Number JP19K06775 (to HW) and JP16K07446 (to FY) and funding from the Central Research Institute of Fukuoka University, Grant number 197103 (to HW).
Author contributions
K.T., F.Y. and H.W. designed the experiments. K.T. performed the experiments. Y.N. and M.S. prepared the materials. K.T. and H.W. analysed the data and wrote the manuscript. F.Y. contributed the revisions of the manuscript. All authors reviewed the manuscript.
Data availability
The electrophysiological datasets used in the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wyatt, T. D. Pheromones and Animal Behavior: chemical signals and signatures. 2nd edn, (Cambridge University Press, Cambridge. 2014).
- 2.Cardé R. T.& Millar J. G. Advances in Insect Chemical Ecology Ch. 6: Sex pheromone of cockroaches (eds Gemeno, C. & Schal, C.). 179-247 (Cambridge University Press, 2004).
- 3.Nojima S. Identification of the Sex Pheromone of the German Cockroach, Blattella germanica. Science. 2005;307(5712):1104–1106. doi: 10.1126/science.1107163. [DOI] [PubMed] [Google Scholar]
- 4.Sakuma M, Fukami H. Aggregation arrestant pheromone of the German cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae): isolation and structure elucidation of blattellastanoside-A and -B. J. Chem. Ecol. 1993;19:2521–2541. doi: 10.1007/BF00980688. [DOI] [PubMed] [Google Scholar]
- 5.Imen S, Christian M, Virginie D. & Colette, R. Intraspecific signals inducing aggregation in Periplaneta americana (Insecta: Dictyoptera) Environ. Entomol. 2015;44:713–723. doi: 10.1093/ee/nvv035. [DOI] [PubMed] [Google Scholar]
- 6.Persoons CJ, Verwiel PEJ, Talman E, Ritter FJ. Sex pheromone of the American cockroach, Periplaneta americana. J. Chem. Ecol. 1979;5:221–236. doi: 10.1007/bf00988237. [DOI] [Google Scholar]
- 7.Adams MA, et al. Sex pheromone of the American cockroach: absolute configuration of periplanone-B. J. Am. Chem. Soc. 1979;101:2495–2498. doi: 10.1021/ja00503a049. [DOI] [Google Scholar]
- 8.Persoons CJ, Ritter FJ, Verwiel PEJ, Hauptmann H, Mori K. Nomenclature of American cockroach sex pheromones. Tetrahedron Lett. 1990;31:1747–1750. doi: 10.1016/S0040-4039(00)88871-1. [DOI] [Google Scholar]
- 9.SEELINGER GÜNTER, GAGEL SIGRID. On the function of sex pheromone components in Periplaneta americana: improved odour source localization with periplanone-A. Physiological Entomology. 1985;10(2):221–234. doi: 10.1111/j.1365-3032.1985.tb00038.x. [DOI] [Google Scholar]
- 10.Tobin TR, Seelinger G, Bell WJ. Behavioral responses of male Periplaneta americana to periplanone B, a synthetic component of the female sex pheromone. J. Chem. Ecol. 1981;7:969–979. doi: 10.1007/BF00987621. [DOI] [PubMed] [Google Scholar]
- 11.Chow YS, Wang SF. Attraction responses of the American cockroach to synthetic periplanone-B. J. Chem. Ecol. 1981;7:265–272. doi: 10.1007/BF00995749. [DOI] [PubMed] [Google Scholar]
- 12.Lihoreau M., Costa J. T., Rivault C. The social biology of domiciliary cockroaches: colony structure, kin recognition and collective decisions. Insectes Sociaux. 2012;59(4):445–452. doi: 10.1007/s00040-012-0234-x. [DOI] [Google Scholar]
- 13.Tanaka M, Daimon T. Tissue localization of aggregation pheromones in the American cockroach, Periplaneta americana (Blattodea: Blattidae) Appl. Entomol. Zool. 2018;53:447–452. doi: 10.1007/s13355-018-0573-9. [DOI] [Google Scholar]
- 14.Nishimura, Y. et al. Cockroach attraction-aggregation substance, cockroach aggregation attractant and cockroach controlling agent. United States patent. US 10,167,269 B2, Jan 1 (2019).
- 15.Toh Y. Fine structure of antennal sense organs of the male cockroach, Periplaneta americana. J. Ultrastruct. Res. 1977;60:373–394. doi: 10.1016/S0022-5320(77)80021-X. [DOI] [PubMed] [Google Scholar]
- 16.Altner H, Sass H, Altner I. Relationship between structure and function of antennal chemo-, hygro-, and thermoreceptive sensilla in Periplaneta americana. Cell. Tissue. Res. 1977;176:389–405. doi: 10.1007/BF00221796. [DOI] [PubMed] [Google Scholar]
- 17.Schaller D. Antennal sensory system of Periplaneta americana L.: distribution and frequency of morphologic types of sensilla and their sex-specific changes during postembryonic development. Cell. Tiss. Res. 1978;191:121–139. doi: 10.1007/BF00223221. [DOI] [PubMed] [Google Scholar]
- 18.Altner Helmut, Prillinger Linde. International Review of Cytology. 1980. Ultrastructure of Invertebrate Chemo-, Thermo-, and Hygroreceptors and Its Functional Significance; pp. 69–139. [Google Scholar]
- 19.Sass H. Production, release and effectiveness of two female sex pheromone components of Periplaneta americana. J. Comp. Physiol. 1983;152:309–317. doi: 10.1007/BF00606237. [DOI] [Google Scholar]
- 20.Sass H. Olfactory receptors on the antenna of Periplaneta: Response constellations that encode food odors. J. Comp. Physiol. 1978;128:227–233. doi: 10.1007/BF00656855. [DOI] [Google Scholar]
- 21.Boeckh J, Ernst KD. Contribution of single unit analysis in insects to an understanding of olfactory function. J. Comp. Physiol. 1987;161:549–565. doi: 10.1007/Bf00603661. [DOI] [Google Scholar]
- 22.Fujimura K, Yokohari F, Tateda H. Classification of antennal olfactory receptors of the cockroach, Periplaneta americana L. Zool. Sci. 1991;8:243–255. [Google Scholar]
- 23.Watanabe H, et al. Complete mapping of glomeruli based on sensory nerve branching pattern in the primary olfactory center of the cockroach Periplaneta americana. J. Comp. Neurol. 2010;518:3907–3930. doi: 10.1002/cne.22452. [DOI] [PubMed] [Google Scholar]
- 24.Burrows M, Boeckh J, Esslen J. Physiological and morphological properties of interneurones in the deutocerebrum of male cockroaches which respond to female pheromone. J. Comp. Physiol. 1982;145:447–457. doi: 10.1007/Bf00612810. [DOI] [Google Scholar]
- 25.Hösl M. Pheromone-sensitive neurons in the deutocerebrum of Periplaneta americana: receptive fields on the antenna. J. Comp. Physiol. 1990;167:321–327. doi: 10.1007/BF00192567. [DOI] [Google Scholar]
- 26.Nishino H, et al. Spatial receptive fields for odor localization. Curr. Biol. 2018;28:600–608 e3. doi: 10.1016/j.cub.2017.12.055. [DOI] [PubMed] [Google Scholar]
- 27.Schafer R, Sanchez TV. Antennal sensory system of the cockroach, Periplaneta americana: postembryonic development and morphology of the sense organs. J Comp. Neurol. 1973;149:335–354. doi: 10.1002/cne.901490304. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe H, et al. Two types of sensory proliferation patterns underlie the formation of spatially tuned olfactory receptive fields in the cockroach Periplaneta americana. J. Comp. Neurol. 2018;526:2683–2705. doi: 10.1002/cne.24524. [DOI] [PubMed] [Google Scholar]
- 29.Prillinger L. Postembryonic development of the antennal lobes in Periplaneta americana L. Cell. Tissue. Res. 1981;215:563–575. doi: 10.1007/BF00233532. [DOI] [PubMed] [Google Scholar]
- 30.Nishino H, Yoritsune A, Mizunami M. Different growth patterns of two adjacent glomeruli responsible for sex-pheromone processing during postembryonic development of the cockroach Periplaneta americana. Neurosci. Lett. 2009;462:219–224. doi: 10.1016/j.neulet.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Schafer R. The nature and development of sex attractant specificity in cockroaches of the genus Periplaneta. IV. electrophysiological study of attractant specificity and its determination by juvenile hormone. J. Exp. Zool. 1977;199:189–207. doi: 10.1002/jez.1401990204. [DOI] [PubMed] [Google Scholar]
- 32.Boeckh J, Sass H, Wharton DR. Antennal receptors: reactions to female sex attractant in Periplaneta americana. Science. 1970;168:589. doi: 10.1126/science.168.3931.589. [DOI] [PubMed] [Google Scholar]
- 33.Nishino C, Kimura R. Olfactory receptor responses of the nymphal American cockroach to sex pheromones and their mimics. Comp. Biochem. Phys. A. 1982;72:237–242. doi: 10.1016/0300-9629(82)90038-X. [DOI] [PubMed] [Google Scholar]
- 34.Schaller-Selzer L. Physiology and morphology of the larval sexual pheromone-sensitive neurones in the olfactory lobe of the cockroach, Periplaneta americana. J. Insect. Physiol. 1984;30:537–546. doi: 10.1016/0022-1910(84)90080-5. [DOI] [Google Scholar]
- 35.Saïd I, Gaertner C, Renou M, Rivault C. Perception of cuticular hydrocarbons by the olfactory organs in Periplaneta americana (L.) (Insecta: Dictyoptera) J. Insect. Physiol. 2005;51:1384–1389. doi: 10.1016/j.jinsphys.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Saïd I, Costagliola G, Leoncini I, Rivault C. Cuticular hydrocarbon profiles and aggregation in four Periplaneta species (Insecta: Dictyoptera) J. Insect. Physiol. 2005;51:995–1003. doi: 10.1016/j.jinsphys.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 37.McFarlane JE, Alli I. The effect of lactic acid and the volatile fatty acids on the aggregation behavior of Periplaneta americana (L.) Comp. Biochem. Phys C. 1987;86:45–47. doi: 10.1016/0742-8413(87)90142-3. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe H, Nishino H, Mizunami M, Yokohari F. Two parallel olfactory pathways for processing general odors in a cockroach. Front. Neural. Circuit. 2017;11:32. doi: 10.3389/fncir.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ando T, et al. Nanopore formation in the cuticle of an insect olfactory sensillum. Curr. Biol. 2019;29:1512–1520 e6. doi: 10.1016/j.cub.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Steinbrecht RA. Pore structures in insect olfactory sensilla: A review of data and concepts. Int. J. Insect. Morphol. Embryol. 1997;26:229–245. doi: 10.1016/S0020-7322(97)00024-X. [DOI] [Google Scholar]
- 41.Salecker I, Boeckh J. Embryonic development of the antennal lobes of a hemimetabolous insect, the cockroach Periplaneta americana: light and electron microscopic observations. J. Comp. Neurol. 1995;352:33–54. doi: 10.1002/cne.903520104. [DOI] [PubMed] [Google Scholar]
- 42.Nishino H, Yoritsune A, Mizunami M. Postembryonic development of sexually dimorphic glomeruli and related interneurons in the cockroach Periplaneta americana. Neurosci. Lett. 2010;469:60–64. doi: 10.1016/j.neulet.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 43.Nishino H, Iwasaki M, Mizunami M. Pheromone detection by a pheromone emitter: a small sex pheromone-specific processing system in the female American cockroach. Chem. Senses. 2011;36:261–270. doi: 10.1093/chemse/bjq122. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe H, et al. Sensillum-specific, topographic projection patterns of olfactory receptor neurons in the antennal lobe of the cockroach Periplaneta americana. J. Comp. Neurol. 2012;520:1687–1701. doi: 10.1002/cne.23007. [DOI] [PubMed] [Google Scholar]
- 45.Nikonov AA, Leal WS. Peripheral coding of sex pheromone and a behavioral antagonist in the Japanese beetle, Popillia japonica. J. Chem. Ecol. 2002;28:1075–1089. doi: 10.1023/a:1015274104626. [DOI] [PubMed] [Google Scholar]
- 46.Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. Asymmetric ephaptic inhibition between compartmentalized olfactory receptor neurons. Nat. Commun. 2019;10:1560. doi: 10.1038/s41467-019-09346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gier HT. Growth Rate in the Cockroach Periplaneta americana (Linn) Ann. Entomol. Soc. Am. 1947;40:303–317. doi: 10.1093/aesa/40.2.303. [DOI] [Google Scholar]
- 49.Saito K, Hayashi S. Some morphological characteristics for sex determination of the developing stages of cockroach, Periplaneta fuliginosa (Serville) Med. Entomol. Zool. 1973;23:181–184. doi: 10.7601/mez.23.181. [DOI] [Google Scholar]
- 50.Kuwahara Shigefumi, Mori Kenji. Synthesis of (-)-periplanone-B a sex pheromone component of the American cockroach (periplaneta americana) Tetrahedron. 1990;46(24):8075–8082. doi: 10.1016/S0040-4020(01)81464-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The electrophysiological datasets used in the current study are available from the corresponding author on reasonable request.