Abstract
Objective
To improve the diagnosis and treatment of anti-GABAB receptor (anti-GABABR) encephalitis and prevent misdiagnosis or non-diagnosis.
Methods
We retrospectively examined the chief clinical manifestations, auxiliary examination results, treatment strategies, treatment efficacy, and long-term follow-up results of seven consecutive patients with anti-GABABR encephalitis.
Results
Epileptic seizures were the first symptom in 100% of the patients; 85.7% had memory deficit in the hospital, 42.8% had residual symptoms of cognitive impairment at discharge, and 28.6% had cognitive impairment at the end of follow-up; 71.4% of the patients had psychosis in the hospital, 57.1% had residual symptoms of psychosis at discharge, and 14.3% still had psychosis at the end of follow-up. However, the clinical symptoms (psychiatric disorders, cognitive decline) and signs (consciousness disturbance) at onset and after follow-up were not significantly different (P > 0.05). In 71.4% of the patients, anti-GABABR antibody serum levels were higher than those in the cerebrospinal fluid (especially in patients with lung cancer). Magnetic resonance imaging in 71.4% of patients indicated that the marginal lobe demonstrated encephalitis lesions. The average modified Rankin Scale score (2.0 ± 2.31) at follow-up was significantly better than that (3.86 ± 0.90) at the time of admission (P < 0.05).
Conclusion
The clinical characteristics of anti-GABABR encephalitis were refractory epilepsy, psychiatric disorders, and cognitive impairment. Multiple antiepileptic drugs are crucial for the treatment of intractable epilepsy. Clinicians should eliminate the possibility of small-cell lung cancer in patients with high anti-GABABR antibody levels. Early active immunotherapy is effective, and the long-term prognosis is good for patients without tumors.
Keywords: anti-gamma-aminobutyric acid B receptor encephalitis, clinical features, treatment, prognosis
Introduction
Anti-GABAB receptor (anti-GABABR) encephalitis is a newly reported form of autoimmune encephalitis associated with anti-neuron surface antigen antibodies [1]. The rate of misdiagnosis is high, since only a few cases have been reported to date and the clinical manifestations and prognosis of anti-GABABR encephalitis have yet to be investigated systematically [2]. We analyzed the clinical manifestations, auxiliary examination results, treatment strategies, and long-term prognoses in seven consecutive patients with anti-GABABR encephalitis, to improve the diagnosis and treatment of anti-GABABR encephalitis.
Clinical materials and methods
Patient characteristics
The study included seven consecutive patients (women: 3, men: 4; age: 44.7 ± 14.09 years, range: 27–64 years) with anti-GABABR encephalitis, who were treated at the Department of Neurology at the First Affiliated Hospital of Guangxi Medical University (China). The duration of the follow-up period was 16.14 ± 4.41 months and ranged from 10 months to 2 years. No patient had a relevant family history of genetic abnormalities, immunodeficiency history, or developmental abnormalities.
Methods
Diagnostic criteria
All patients met the following diagnostic criteria [3] for anti-GABABR encephalitis:
acute or subacute onset, progressive aggravation; (2) clinical symptoms in accordance with the characteristics of marginal encephalitis; (3) slightly elevated lymphocyte levels and/or normal white blood cell (WBC) count in the cerebrospinal fluid (CSF); (4) presence of anti-GABABR antibodies in the serum and/or CSF; abnormal signals in the unilateral/bilateral medial temporal lobe or an absence of lesions on brain magnetic resonance imaging (MRI); and (5) abnormal electroencephalogram (EEG) findings. Patients with lesions suggestive of herpes simplex encephalitis, toxic encephalopathy, acute disseminated encephalomyelitis, or multiple sclerosis were excluded.
Experimental detection and treatment methods
Serum and CSF samples were collected and sent to Guangdong Jinyu Inspection Company (Guangzhou, China) for commercial testing to detect the following autoimmune encephalitis antibodies: anti-GABABR antibodies, anti-glutamate receptor (including NMDA, AMPA1, and AMPA 2) IgG antibodies, anti-leucine-rich glioma-inactivated protein 1 IgG antibodies, and anti-contact protein associated protein 2 IgG antibodies. The antibodies were detected using an indirect immunofluorescence test, which is widely accepted as the most suitable method. All patients received corticosteroid pulse therapy (methylprednisolone 1 g/day for 5 days, 0.5 g/day for 5 days, 0.25 g/day for 5 days, 0.125 g/day for 5 days, and subsequent oral administration), which was accompanied by intravenous gamma globulin treatment (0.4 g/kg/day for 5 days) in 28.5% of patients. Rehabilitation efficacy was assessed according to the modified Rankin Scale (mRS) scores, as a measure of global disability [4].
Statistical analysis
We retrospectively analyzed the mode of onset, initial symptoms, main clinical manifestations, auxiliary examination results, treatment strategies, and long-term prognosis for each patient. The data were analyzed using the SAS 9.3 statistical software. The measured data exhibited normal distribution and were expressed as mean ± standard deviation. The Student t test, for paired data, was used to compare the respective mRS scores at follow-up and admission. The clinical symptoms and signs at onset and after follow-up were compared using the exact probability method.
Results
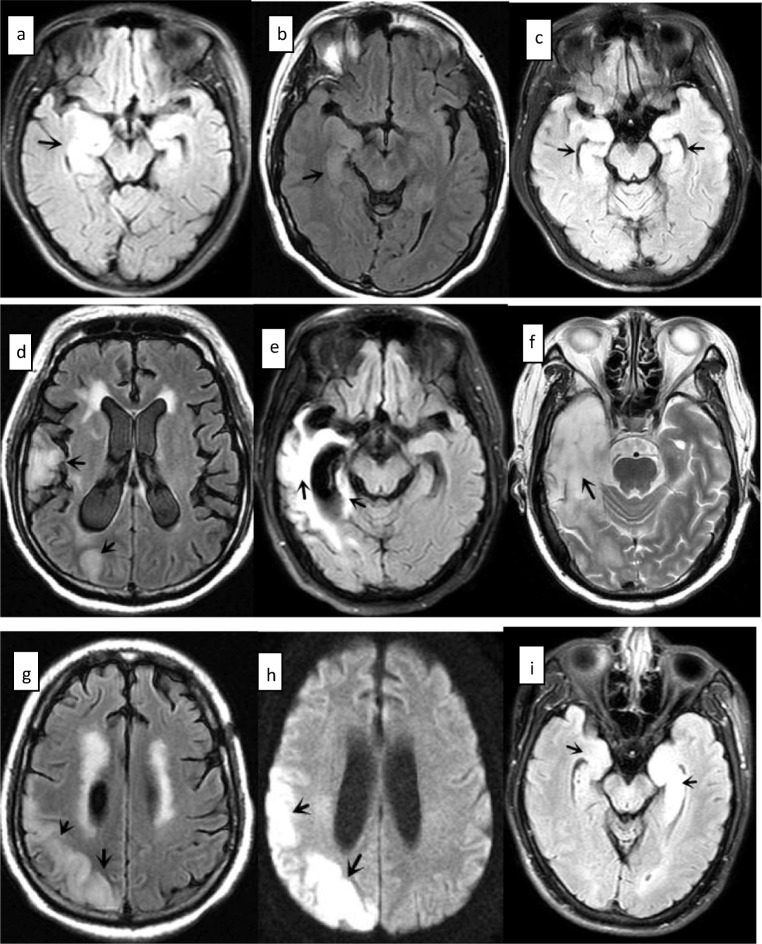
The clinical symptoms, auxiliary examination results, treatment strategies, and long-term follow-up results in patients with anti-GABABR encephalitis are presented in Table 1. No symptoms of infection, including cold, diarrhea, fever, or vomiting, were observed in 85.7% of patients prior to onset. Epileptic seizures were the first symptom in 100% of patients. Memory deficits were observed in 85.7% of patients in the hospital, 42.8% had residual symptoms of cognitive impairment at discharge, and 28.6% still had cognitive impairment at the end of the follow-up period. Furthermore, 71.4% of patients had psychosis in the hospital, 57.1% had residual symptoms of psychosis at discharge, and 14.3% still had psychosis at the end of the follow-up period. No significant difference was observed in the clinical symptoms (psychiatric disorders and cognitive decline) and signs (consciousness disturbance) at onset and after follow-up (P > 0.05). The CSF WBC count and protein levels were both slightly elevated in 14.3% of patients. Serum anti-GABABR antibody levels were higher than those in the CSF in 71.4% of patients, especially in the two patients with lung cancer. MRI demonstrated encephalitis lesions in the marginal lobe of 71.4% of patients. Low-intensity or equisignal lesions on T1-weighted imaging and high-intensity lesions were observed on T2-weighted imaging. None of the lesions showed significant enhancement (Fig. 1). The average mRS score at follow-up (2.0 ± 2.31) was significantly better than that (3.86 ± 0.90) at admission (P < 0.05). No recurrence of epilepsy or encephalitis was observed in any patient during the follow-up period.
Table 1.
Clinical symptoms and long-term follow-up results of seven patients with anti-GABABR encephalitis
| Assessment | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
|
Prodromal symptomsa (14.3%) |
No | Yes | No | No | No | No | No |
| Psychiatric disorders (5/7, 71.4%) | No | Yes | Yes | Yes | Yes | Yes | No |
| Cognitive decline (6/7, 85.7%) | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Consciousness disturbance (2/7, 28.6%) | No | No | Yes | No | No | No | Yes |
| Epilepsy (100%) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Involuntary movement (2/7, 28.6%) | No | No | No | Yes | No | No | Yes |
| CSF pressure (mmH2O) | – | – | 220 | – | 210 | – | – |
| WBC in CSFb | – | – | – | 67 × 106/L | – | – | – |
| CSF proteinc | – | – | – | – | 594 mg/L | – | – |
| EEG | Epileptic waves | Moderate abnormality | Mild or moderate abnormality | --- | epileptic waves | Epileptic waves | Moderate abnormality |
| Lesions on brain MRI | Right hippocampus and amygdala | Right temporal lobe | Bilateral hippocampus and medial temporal lobe | No lesion | Right temporal lobe and occipital lobe | No lesion | Bilateral hippocampus |
| Tumors | – | Cervical cancer | Lung cancer | Lung cancer | – | – | – |
| Serum antibody titer | 1:100 | 1:10 | 1:320 | 1:320 | 1:10 | 1:10 | 1:100 |
| CSF antibody titer | 1:100 | 1:32 | 1:10 | 1:32 | Negative | Negative | 1:10 |
| mRS score at admission (3.86 ± 0.90) | 3 | 4 | 5 | 5 | 3 | 3 | 4 |
| Corticosteroid pulse therapy | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| IVIg | No | No | No | No | No | Yes | Yes |
| AED treatment in hospital | CBZ, BDZ | CBZ, BDZ | TPM, VPA | VPA, BDZ | VPA, LEV | PB, OXC, TPM | VPA, BDZ |
| Residual symptoms at discharge | Hypophrenia | Hypophrenia and psychosis | Psychosis | Psychosis | Hypophrenia | Psychosis | No |
| Follow-up period | 19 months | 16 months | 14 months | 10 months | 14 months | 16 months | 24 months |
| Consciousness (0%) | Clear | Clear | Clear | Clear | Clear | Clear | Clear |
| Cognitive function (2/7, 28.6%) | Normal | Normal | Normal | Normal | Memory deficits | Normal | Memory deficits |
| Psychiatric disorders (1/7, 14.3%) | No | No | No | Yes | No | No | No |
| mRS score at follow-up (2.0 ± 2.31 | 0 | 0 | 5 | 5 | 3 | 0 | 1 |
| Prednisone treatment after discharge | 1 month | 3 months | < 1 month | < 10 months | < 1 month | 3 months | 6 months |
| Azathioprine treatment after discharge | 1 month | 1 year | 2 months | < 10 months | < 1 month | 1 year | 1 year |
| AED treatment after discharge | CBZ × 1 month | CBZ × 3 months | TPM VPA < 1 month | VPA × 10 months | VPA, LEV × 14 months | PB, OXC, TPM × 16 months | VPA × 6 months |
Note: The modified Rankin scores at baseline/admission and after follow-up are denoted as the mean ± standard deviation (t = 3.240, P = 0.018 < 0.05). The clinical symptoms (psychiatric disorders, cognitive decline) and signs (consciousness disturbance) at onset and after follow-up did not exhibit a statistically significant difference (total P > 0.05). “–” stands for normal
CSF cerebrospinal fluid, WBC white blood cell, EEG electroencephalography, MRI magnetic resonance imaging, IVIg intravenous immunoglobulin, mRs modified Rankin scale, AED antiepileptic drug, CBZ carbamazepine, LEV levetiracetam, OXC oxcarbazepine, PB phenobarbitone, VPA valproic acid, BDZ benzodiazepines, TPM topiramate, SD standard deviation
aProdromal symptoms refer to headache, vomiting, fever, and diarrhea
bNormal range of CSF WBC count: 0–8 × 106/L
cNormal range of CSF proteins: 150–450 mg/L
Fig. 1.
Patient 1: a T2-FLAIR showing hyperintensity in the right hippocampus, parahippocampal gyrus, and amygdala. Patient 2: b T2-FLAIR showing hyperintense lesions in the right temporal lobe. Patient 3: c T2-FLAIR showed hyperintensities in the hippocampus, bilaterally, and in the medial temporal lobe. Patient 5: d T2-FLAIR showing abnormal signals in the right temporal lobe. e T2-FLAIR showing abnormal signals in the right temporal lobe and hippocampus. f T2-weighted sequence showing hyperintense lesions in the right temporal lobe. g T2-FLAIR showing hyperintensity in the right temporal occipital lobes. h Diffusion-weighted imaging sequence showing hyperintensity in the right temporal and occipital lobes. The lesions described above are marked with arrows. Patient 7: i T2-FLAIR showing abnormal signal shadows in the hippocampus, bilaterally (more obvious on the left). Patient 4 and patient 6 did not show any encephalitis lesions on magnetic resonance imaging. FLAIR: fluid-attenuated inversion recovery
Discussion
Clinical manifestations
Viral infection is among the principal causes of autoimmune encephalitis [5]. However, only a few patients in our study had prodromal symptoms of infection; infection was not a trigger for the onset in most patients. Epilepsy is a prominent and critical clinical manifestation of anti-GABABR encephalitis, and epilepsy or refractory epilepsy is often the first symptom [6]. In a previous study by Guan et al., 17 of the 18 patients presented with new refractory epilepsy or status epilepticus [7], while all the patients in our study had epilepsy. Herpes simplex encephalitis is perhaps most frequently associated with epilepsy, of the types of sporadic viral encephalitis, with an incidence of about 50% in cases of combined epilepsy [8]. The risk of seizures in population-based cohorts of survivors of central nervous system infections is between 6.8 and 8.3% [9]. Three-quarters of patients with anti-GABABR antibodies developed refractory seizures [10]. In short, these results indicate that the incidence of epilepsy in anti-GABABR encephalitis was significantly higher than that in viral, infectious, or autoimmune encephalitis. High levels of anti-GABABR antibodies are associated with seizures, refractory status epilepticus, or both [11]. Anti-GABABR encephalitis epilepsy occurs due to an immune reaction to neuronal elements, driven by an underlying malignancy. Our study showed that memory deficit is common in these patients and that complete recovery is difficult, which may be closely related to damage to the marginal lobe. Psychiatric disorders are common (64.7%) [7] in patients with anti-GABABR encephalitis, and this result is roughly in line with our finding. Mental disorders are also common symptoms that tend to persist until the patient is discharged. No statistically significant difference was observed (P > 0.05) between the clinical symptoms (psychiatric disorders, cognitive decline) and signs (consciousness disturbance) at onset and after follow-up, which may be attributed to the small sample size.
Auxiliary examination results
Non-specific inflammatory changes, including slight increases in lymphocyte count and protein levels in the CSF, were observed in our patients. These were similar to the observations in viral encephalitis. Our results suggest that anti-GABABR encephalitis cannot be excluded in patients without lesions on MRI. Most EEG results revealed moderate to severe abnormalities, such as increases in slow waves and epileptic waves. Routine EEG should be performed in patients with suspected anti-GABABR encephalitis. Our research suggests that anti-GABABR antibodies are first produced in the blood, and subsequently enter the CSF by crossing the blood-brain barrier. Serum antibody levels should be evaluated to avoid a misdiagnosis, if patients test negative for anti-GABABR antibodies in the CSF. The pathogenesis of anti-GABABR encephalitis may be related to immune dysfunction caused by tumors. Small-cell lung cancer is associated with limbic encephalitis [2], and 50% of patients with anti-GABABR encephalitis were also diagnosed with small-cell lung cancer [12]. Therefore, patients with anti-GABABR encephalitis should be regularly screened for tumors (mainly small-cell lung cancer), especially when the serum antibody levels are significantly higher than those in the CSF.
Treatment and long-term prognosis
Early immunotherapy is recommended for treatment of anti-GABABR encephalitis [3]. Early immune modulation may prevent serious potential consequences [13] and may result in a good prognosis [2], except for patients with malignant tumors. First-line immunotherapy includes corticosteroid pulse therapy, plasma exchange, and intravenous immunoglobulin therapy. We recommend the use of corticosteroid pulse therapy, because it is less expensive and easy to administer. Combined intravenous immunoglobulins may be effective, if corticosteroid pulse therapy is ineffective. Second-line immunotherapy includes cyclophosphamide and/or rituximab [14], which can be administered in case of suboptimal results with the first-line treatment. Our research showed that most patients exhibited some residual symptoms at discharge, with varying degrees of memory deficit, even at the end of the follow-up. Monotherapy was used in 54.1% of patients with epilepsy [15]. All the patients in our study required treatment with two or more antiepileptic drugs (AEDs), which were gradually reduced or even discontinued in several patients, during follow-up. Patients with autoimmune encephalitis have a high rate of seizure remission, and the long-term use of AEDs may be unnecessary to control seizures [10]. The long-term effects of active treatment were good, despite the difficulty in treating early epilepsy in anti-GABABR encephalitis.
As this observational study included a small number of patients, our findings should be interpreted with caution, and further studies are required to confirm the generalizability of our results.
Conclusion
The clinical characteristics of anti-GABABR encephalitis are refractory epilepsy, psychiatric disorders, and cognitive impairment. Tests for anti-GABABR antibodies, brain MRI, and EEG should be performed as early as possible, and corticosteroid pulse therapy is the treatment of choice. If corticosteroid pulse therapy is unsuccessful, combined treatment with intravenous immunoglobulin may be effective. Patients should undergo screening for small-cell lung cancer (especially patients with high antibody titers). Early treatment with multiple AEDs is crucial for refractory epilepsy. Active immunotherapy may ensure a good long-term prognosis for patients without tumors.
Author contribution
Jinou Zheng made an important contribution to the collection of clinical data. Wei Zeng made an important contribution to the writing of the manuscript and discussion of previous literature. Liming Cao played an important role in thesis writing, proofreading, and study conception.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
Research involving human participants and/or animals
The study design was approved by the ethical review committee of the First Affiliated Hospital of Guangxi Medical University (No. KY-E-023).
Informed consent
All study participants provided written informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA (B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TJ, Lee ST, Shin JW, et al. Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol. 2014;270:45–50. doi: 10.1016/j.jneuroim.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Neurology Branch of Chinese Medical Association Chinese expert consensus on the diagnosis and management of autoimmune encephalitis. Chin J Neurol. 2017;50:91–98. [Google Scholar]
- 4.Quinn TJ, Dawson J, Walters MR, et al. Functional outcome measures in contemporary stroke trials. Int J Stroke. 2009;4(3):200–205. doi: 10.1111/j.1747-4949.2009.00271.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao MM. The relationship between anti-N-methyl-D-aspartate receptor encephalitis and viral encephalitis. Foreign Med Sci (Section of Pediatrics) 2016;43(6):453–456. [Google Scholar]
- 6.Zhang J, Zhang Z, Fan CQ, et al. Clinical characteristics of anti-gamma- aminobutyric acid B receptor encephalitis. Chin J Neurol. 2016;49(6):439–444. doi: 10.3760/cma.j.issn.1006-7876.2016.06.004. [DOI] [Google Scholar]
- 7.Guan HZ, Ren HT, Yang XZ, et al. Limbic encephalitis associated with anti-γ-aminobutyric acid B receptor antibodies:a case series from China. Chin Med J(Engl) 2015;128(22):3023–3028. doi: 10.4103/0366-6999.168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008;49(Suppl 6):13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 9.Vezzani A, Fujinami RS, White HS, Preux PM, Blümcke I, Sander JW, Löscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131(2):211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Q, Ma M, Wei X, et al. Characteristics of seizure and antiepileptic drug utilization in outpatients with autoimmune encephalitis. Front Neurol. 2019;9:1136. doi: 10.3389/fneur.2018.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petit-Pedrol Mar, Armangue Thaís, Peng Xiaoyu, Bataller Luis, Cellucci Tania, Davis Rebecca, McCracken Lindsey, Martinez-Hernandez Eugenia, Mason Warren P, Kruer Michael C, Ritacco David G, Grisold Wolfgang, Meaney Brandon F, Alcalá Carmen, Sillevis-Smitt Peter, Titulaer Maarten J, Balice-Gordon Rita, Graus Francesc, Dalmau Josep. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. The Lancet Neurology. 2014;13(3):276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies :novel findings in anew case series of 20 patients. Neurology. 2013;81(17):1500–1506. doi: 10.1212/WNL.ObO13e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serafini A, Lukas RV, Vanhaerents S, et al. Paraneoplastic epilepsy. Epilepsy Behav. 2016;61:51–58. doi: 10.1016/j.yebeh.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 14.Nosadini M, Mohammad SS, Ramanathan S, et al. Immunetherapy in autoimmune encephalitis:a systematic review. Expert Rev Neumther. 2015;15(12):1391–1419. doi: 10.1586/14737175.2015.1115720. [DOI] [PubMed] [Google Scholar]
- 15.Yu P, Zhou D, Liao W, et al. An investigation of the characteristics of outpatients with epilepsy and antiepileptic drug utilization in a multicenter cross-sectional study in China. Epilepsy Behav. 2017;69:126–132. doi: 10.1016/j.yebeh.2016.09.021. [DOI] [PubMed] [Google Scholar]