Abstract
Purpose
To determine the impact on clinical management of patients with high-risk (HR) prostate cancer at diagnosis and patients with biochemical recurrence (BCR) using a new kit form of 68Ga-prostate-specific membrane antigen (PSMA), namely tris(hydroxypyridinone) (THP)-PSMA, with positron emission tomography-computed tomography (PET-CT).
Methods
One hundred eighteen consecutive patients (50 HR, 68 BCR) had management plans documented at a multidisciplinary meeting before 68Ga-THP-PSMA PET-CT. Patients underwent PET-CT scans 60-min post-injection of 68Ga-THP-PSMA (mean 159 ± 21.2 MBq). Post-scan management plans, Gleason score, prostate-specific antigen (PSA) and PSA doubling time (PSAdt) were recorded.
Results
HR group: 12/50 (24%) patients had management changed (9 inter-modality, 3 intra-modality). Patients with PSA < 20 μg/L had more frequent management changes (9/26, 34.6%) compared with PSA > 20 μg/L (3/24, 12.5%). Gleason scores > 8 were associated with detection of more nodal (4/16, 25% vs 5/31, 16.1%) and bone (2/16, 12.5% vs 2/31, 6.5%) metastases. BCR group: Clinical management changed in 23/68 (34%) patients (17 inter-modality, 6 intra-modality). Forty out of 68 (59%) scans were positive. Positivity rate increased with PSA level (PSA < 0.5 μg/L, 0%; PSA 0.5–1.0 μg/L, 35%; PSA 1.0–5.0 μg/L, 69%; PSA 5.0–10.0 μg/L, 91%), PSAdt of < 6 months (56% vs 45.7%) and Gleason score > 8 (78.9% vs 51.2%).
Conclusions
68Ga-THP-PSMA PET-CT influences clinical management in significant numbers of patient with HR prostate cancer pre-radical treatment and is associated with PSA. Management change also occurs in patients with BCR and is associated with PSA and Gleason score, despite lower scan positivity rates at low PSA levels < 0.5 μg/L.
Keywords: Prostate-specific membrane antigen, Prostate cancer, PSMA-PET-CT, Management impact, 68Ga-THP-PSMA
Introduction
Prostate cancer is the most commonly diagnosed malignancy in men and is a leading cause of cancer-related death [1, 2]. Accurate staging of prostate cancer before radical treatment and for the detection of recurrence is vital for directing treatment and predicting prognosis [3]. This has conventionally been dependent upon digital rectal examination, prostate-specific antigen (PSA) testing and prostate biopsy, complemented with imaging, including multiparametric MRI (mpMRI), computed tomography (CT) and bone scintigraphy [4].
More recently, positron emission tomography-computed tomography (PET-CT), with tracers such as 18F-choline, has increasingly played a role in the management of these patients [5, 6]. However, sensitivity is only moderate for detecting nodal metastases [5] or in those with low PSA levels at biochemical recurrence (BCR) [7]. This has led to the investigation of more prostate-specific tracers with greater diagnostic accuracy.
Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein with significantly increased expression in prostate cancer cells compared with benign tissue [8]. It has emerged as a potential disease biomarker for imaging and targeted treatment. Several enzymes with structural and functional homology to PSMA have been identified leading to the possibility of exploiting these small molecules for the imaging and treatment of prostate cancer via PSMA-targeting [9].
The clinical breakthrough of PET imaging with PSMA ligands was achieved with 68Ga-HBED-CC-PSMA, also referred to as 68Ga-PSMA-11 [10, 11]. This compound has a strong binding affinity for PSMA and highly efficient internalisation into the prostate cancer cell. Since 2011, the use of PSMA PET ligands, predominantly 68Ga-HBED-CC-PSMA, has gained rapid acceptance with excellent diagnostic performance in BCR, with an ability to detect lesions at low PSA levels, but also in primary staging [9, 12, 13].
A novel cold-kit formulation, 68Ga-tris (hydroxypyridinone)-PSMA (68Ga-THP-PSMA), has been introduced that allows more rapid radiolabelling using a single-step kit [14, 15]. It features the THP ligand, which forms complexes with 68Ga rapidly at a low concentration at room temperature and over a wide pH range. Evaluations have demonstrated a favourable bio-distribution and affinity for targeting PSMA [14–16]. Whilst 68Ga-HBED-CC-PSMA PET-CT has been shown to impact management in between 39 and 62% of patients with BCR of prostate cancer after previous definitive treatment and in 21% of patients undergoing primary staging, no such data has yet been reported for 68Ga-THP-PSMA [17, 18].
We hypothesised that 68Ga-THP-PSMA PET-CT impacts clinical management in prostate cancer in high-risk (HR) patients before planned curative treatment and following BCR.
Our aim was to prospectively record changes in intended treatment plans in both groups of patients in a tertiary prostate cancer service.
Materials and methods
Patient data and management
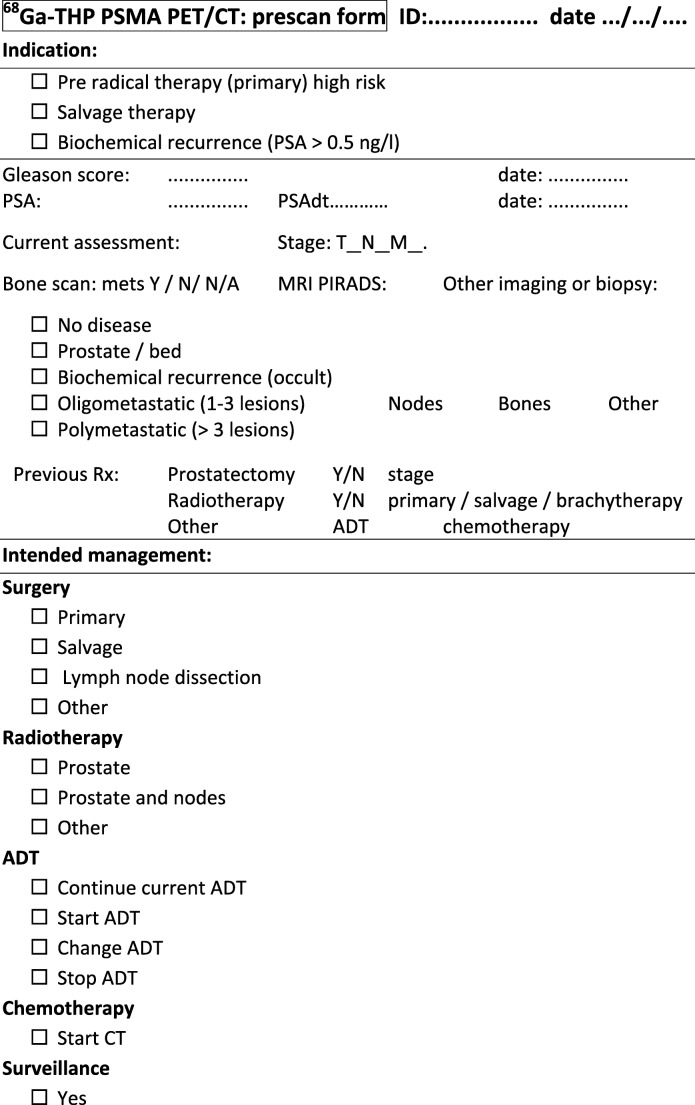
A questionnaire was adapted from Roach et al. to record management plans before and after 68Ga-THP-PSMA PET-CT [17] (Appendix 1). Patients with high-risk (HR) prostate cancer (defined by the D’Amico classification) before surgery or radiation therapy, or patients with BCR, defined by the American Society for Therapeutic Radiology and Oncology (ASTRO) criteria, were eligible [19, 20]. This service evaluation was granted institutional approval by the Guy’s and St Thomas’ Service Evaluation committee and the requirement for consent for data use that was anonymised before analysis was waived.
A pre-PET-CT management plan was prospectively documented on the questionnaire at a multidisciplinary meeting (MDM) consisting of urologists, oncologists, histopathologists, radiologists and a nuclear medicine physician. PSA at diagnosis or at recurrence, PSA doubling time (PSAdt) in those with BCR and Gleason score at diagnosis or at recurrence were recorded. Results of conventional prior imaging were used to inform management. All patients undergoing primary staging have a multiparametric MRI as part of their routine diagnostic evaluation. Any subsequent validation information for the 68Ga-THP-PSMA PET-CT results, including histology and post-PSMA PET-CT conventional imaging up to 6 months, was evaluated.
The post-68Ga-THP-PSMA PET-CT management plan resulting from MDM discussion or clinical review was recorded on the questionnaire. Any cases where the management plan remained unclear were reviewed by a clinical oncologist who assessed the post-68Ga-THP-PSMA PET-CT scan treatment plan according to local and national treatment guidelines. The management change was recorded as either intra-modality or inter-modality. Inter-modality change was defined as an alteration in the type of management (e.g. cancellation of salvage radiotherapy (RT) due to poly-metastatic disease demonstrated on 68Ga-THP-PSMA PET-CT, whereas intra-modality change was defined as a modification of dose/site/strategy that was previously indicated.
Radiochemistry
68Ga-THP-PSMA was prepared using a kit preparation (GalliProst™) containing 40.0 μg tris(hydroxypyridinone)-Glu-urea-Lys(Ahx), sodium bicarbonate, mannitol and phosphate buffer.
Gallium-68 from a Ge-68/Ga-68 generator with a Ge-68 breakthrough of ≤ 0.001 % was directly eluted into the kit vial. The generator was eluted within 5 mL of 0.1 M HCl(aq). The kit solution was incubated for 5 min at room temperature for complete labelling with a vent needle in situ to clear excess carbon dioxide produced during the labelling process.
68Ga-THP-PSMA was analysed according to the monographs 2462 (gallium chloride) and 2482 (gallium edotreotide) of the European Pharmacopeia. Radioanalytic thin layer chromatography (TLC) was performed using glass microfiber chromatography paper impregnated with a silica gel (Agilent technologies) and 10% ammonium acetate in water/methanol (030:70) and also with citrate cugger pH 5. The radio-iTLCs were analysed on a MiniGita thin-layer chromatography scanner.
High-performance liquid chromatography (radio-HPLC) was performed on a Varian ProStar high-pressure gradient system equipped with an ultraviolet-visible detector (Varian ProStar 335) and a radiodetector using a RP-18 column. Radiochemical purity and yield were > 95%.
68Ga-THP-PSMA PET-CT scan
No specific patient preparation was required except bladder voiding immediately before imaging. All patients were injected intravenously with 68Ga-THP-PSMA (mean 159 ± 21.2 MBq). At 60 min, a scan was acquired from pelvis to skull base at 4 min per bed position with an axial field of view of 15.7 cm and an 11-slice overlap between bed positions, using a GE Discovery 710 PET-CT scanner (GE Healthcare, Chicago, USA) at our institution. A low-dose CT scan (140 kV, mA 15–100, noise index 40, 0.5-s rotation time and 40 mm collimation) was performed at the start of imaging to provide attenuation correction and an anatomical reference. PET image reconstruction included scanner-based corrections for radiotracer decay, scatter, randoms and dead-time. Emission sinograms were reconstructed with an ordered subset expectation maximisation algorithm (2 iterations, 24 subsets) with the scatter correction performed using the absolute scaling of the scatter sinogram.
Scan analysis
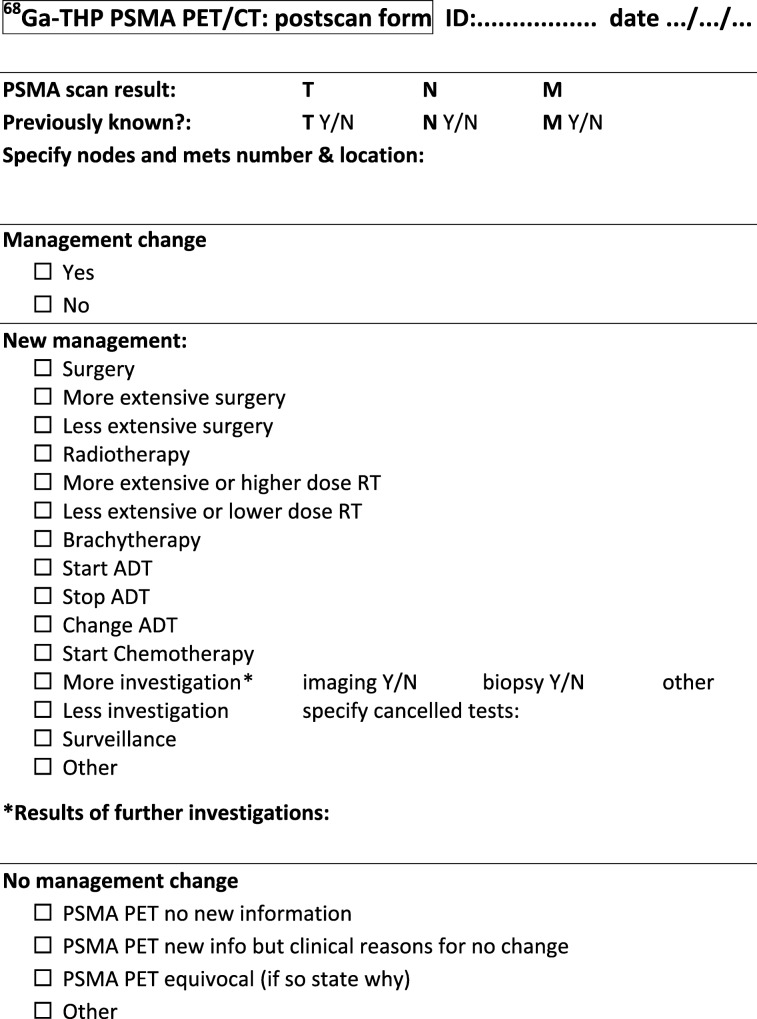
All scans were assessed by two PET specialists (GC/VW) in consensus using Hermes Hybrid Viewer (version 2.6, Hermes Medical Solutions, Stockholm, Sweden). To enable us to evaluate real life conditions, the reviewers had knowledge of the patients’ relevant clinical details including other imaging. A positive scan was recorded if prostate, prostate bed, lymph node, bone or focal visceral uptake was present above adjacent background tissues and could not be accounted for by physiological or normal variation uptake [14]. Consensus was reached on all scans between the two readers without the need to use a third reader. Scan results were made available to the referring clinician and prostate cancer MDM only after a pre-scan management plan had been documented.
Statistical analysis
Data were tested for normality using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± standard deviation. Non-normally distributed data were log-transformed before comparison or displayed as median with range. Statistical analyses were conducted using IBM SPSS statistics software (version 24). A p value of < 0.05 was used for statistical significance.
Results
Patient demographics
Fifty patients with high-risk, newly diagnosed prostate cancer and 68 patients with BCR were consecutively evaluated. Mean patient age was 65 years (range 48–85 years).
Demographic and pathological data are found in Table 1. Gleason score for the HR group is taken from prostate biopsy, whilst those from the BCR cohort are taken from biopsy and/or prostatectomy specimen as applicable. Ten cases were reviewed by a clinical oncologist, who provided clarification of the management plans.
Table 1.
Patient and disease characteristics of 118 scanned patients
| Characteristic | HR group | BCR group |
|---|---|---|
| Age in years | 65 years (48–85) | 68.2 years (49–85) |
| PSA level (μg/L) | 38.72 (1.61–265.1) | 4.44 (0.16–71.02) |
| Gleason score | 7 (3 + 3 to 5 + 5) | 7 (3 + 3 to 5 + 4) |
| 6 | 2 | 5 |
| 7 | 32 | 42 |
| 8 | 5 | 6 |
| 9 | 9 | 15 |
| 10 | 2 | 0 |
| Pathological stage | ||
| T2a | 7 | 5 |
| T2b | 9 | 20 |
| T2c | 6 | 10 |
| T3a | 10 | 16 |
| T3b | 18 | 15 |
| T4 | 0 | 2 |
| PSA doubling time | N/A | 9.06 months (1–40.9) |
Data are means with ranges in parentheses
HR group
The information acquired from 68Ga-THP-PSMA PET-CT in 12 of the 50 scans (24 %) led to a change in management. Nine were inter-modality and 3 were intra-modality (Table 2) (Fig. 1).
Table 2.
Type of management change in primary diagnosis high-risk cohort following 68Ga-THP-PSMA PET-CT scan
| Initial management plan | Revised management plan (n = 12) |
|---|---|
| RARP with PLND | RARP without PLND (n = 3) |
| RARP without PLND | RARP and PLND (n = 1) |
| RT & ADT (n = 1) | |
| ADT alone (n = 1) | |
| Radiotherapy (including in combination with ADT) | Brachytherapy (n = 1) |
| ADT alone (n = 2) | |
| ADT and chemotherapy (n = 1) | |
| ADT alone | RT and ADT (n = 1) |
| ADT and chemotherapy (n = 1) |
Fig. 1.
68Ga-THP-PSMA PET-CT scan of a man diagnosed with high-risk prostate cancer. a Maximum intensity projection image. b The primary tumour shows focal activity in the left peripheral zone (arrow). c There is evidence of retroperitoneal nodal disease (arrow) and d bone metastases (arrow). The detection of metastatic disease changed management from a surgical to systemic therapy approach with hormones and chemotherapy
All 68Ga-THP-PSMA PET-CT scans demonstrated uptake within the prostate. The SUVmax was significantly higher (p < 0.0001) in prostate cancer lesions (mean 7.08; 95% CI 5.67–8.50) than in corresponding normal prostatic tissue (mean 1.89; 95% CI 1.68–2.09). The median ratio of SUVmax in prostate cancer lesions to normal tissue in the same patient was 3.1 (range 1.6–16.4).
All patients underwent a mpMRI scan as part of routine diagnostic evaluation prior to 68Ga-THP-PSMA PET-CT.
Correlation with clinical parameters
34.6% (9/26) of patients with a management change had a PSA < 20 μg/L whilst 12.5% (3/24) of patients with a management change had a PSA > 20 μg/L (Table 3).
Table 3.
Management change (%) in primary diagnosis high-risk cohort as related to PSA and TNM stage
| PSA | < 20 (n = 9/26) 34.6% | > 20 (n = 3/24) 12.5% |
|---|---|---|
| N1 | 4 | 5 |
| M1a | 0 | 3 |
| M1b | 4 | 0 |
Gleason score did not affect the proportion of patients who had a management change (25.8% when Gleason < 8 and 25% if > 8) but Gleason score > 8 was associated with more N1 disease (25%, 4/16 vs 16.1%, 5/31) and more bone metastases (12.5%, 2/16 vs 6.5%, 2/31) (Table 4).
Table 4.
Management change (%) in primary diagnosis high-risk cohort as related to Gleason score and TNM stage
| Gleason | < 8 (n = 8/31) 25.8% | > 8 (n = 4/16) 25% |
|---|---|---|
| N1 | 16.1% | 25% |
| M1a | 6.5% | 6.3% |
| M1b | 6.5% | 15 |
Staging
68Ga-THP-PSMA PET-CT identified 42 patients with N0 disease and 8 with N1 disease compared with 41 N0 and 9 N1 on mpMRI. Regarding the presence of distant metastases, 3 extra-pelvic lymph nodes (M1a) and 4 bone (M1b) lesions were identified on 68Ga-THP-PSMA PET-CT compared with 2 M1a and 2 M1b on mpMRI and bone scan.
Treatment plan
In 8 patients, pelvic lymph node dissection (PLND) was planned at the time of radical prostatectomy, as they were identified as ‘high-risk’ as per the D’Amico classification. If pathological lymph nodes are identified on imaging, as per standard UK practice, radical surgery is generally not offered to patients, and radiotherapy or systemic treatments are recommended instead. Following the 68Ga-THP-PSMA PET-CT scan, pathological lymph nodes were identified in 2/8 patients, with the remaining 6 having negative scans for nodal disease. One patient underwent a radical prostatectomy with PLND (as per patient choice) with histologically positive nodes and the second underwent radical radiotherapy and hormone treatment. Of the remaining 6 patients (with negative 68Ga-THP-PSMA PET-CT imaging), 4 underwent a PLND due to high-risk clinical features. All removed lymph nodes were benign. Two patients did not undergo PNLD and are currently free of recurrence.
Follow-up
Follow-up is available for a median of 17 months (range 1–23 months). In 46 patients, the 68Ga-THP-PSMA PET-CT correctly staged the patient, as reflected by their current follow-up, with the majority of patients on PSA surveillance following radical treatment (robot-assisted radical prostatectomy (RARP) or RT with an undetectable PSA (defined as < 0.03 μg/L). One patient has been referred back to his original hospital and we do not have further follow-up data.
Three patients are having additional treatment. One had RARP without PLND (no nodes identified by 68Ga-THP-PSMA PET-CT). The PSA remained elevated post-operatively and the patient has commenced hormones and radiotherapy to the prostate bed on the assumption that residual disease was present. The second patient had a positive node on pre-RARP 68Ga-THP-PSMA PET-CT. PLND was conducted but post-operative PSA remained elevated and repeat 68Ga-THP-PSMA PET-CT confirmed further nodal disease. The patient is now on hormone and radiotherapy treatment. The final patient had a positive surgical margin and therefore required radiotherapy to the prostatic bed.
BCR group
Twenty-three of 68 (34%) patients had 68Ga-THP-PSMA PET-CT scans that resulted in a management change. Seventeen of the 23 (74%) were inter- rather than intra-modality management changes, and details are outlined in Table 5 (Fig. 2). Forty of the 68 68Ga-THP-PSMA PET-CT scans demonstrated positive uptake (59%). The mean time from primary treatment to BCR was 28 months (range 4–62 months) and 8 patients had a recurrence within 9 months of their initial treatment.
Table 5.
Type of management change in BCR cohort following 68Ga-THP-PSMA PET-CT
| Management plan pre-PSMA PET-CT | Patients who had management changed | New management plan post-PSMA PET-CT | Patients who did not have management changed |
|---|---|---|---|
| Surveillance | 3 | ADT (n = 1) | 11 |
| ADT and chemotherapy (n = 1) | |||
| SABR (n = 1) | |||
| RARP | 2 | Surveillance (n = 1) | 6 |
| ADT (n = 1) | |||
| Brachytherapy | 2 | Surveillance (n = 1) | 1 |
| Watchful waiting (n = 1) | |||
| SABR | 2 | EBRT (n = 1) | 5 |
| ADT (n = 1) | |||
| EBRT | 5 | Surveillance (n = 1) | 13 |
| ADT (n = 2) | |||
| SABR (n = 1) | |||
| RARP (n = 1) | |||
| ADT | 9 | Best supportive care (n = 1) | 9 |
| ADT and chemotherapy (n = 3) | |||
| Chemotherapy (n = 3) | |||
| EBRT (n = 1) | |||
| SABR (n = 1) |
ADT androgen deprivation therapy, SABR stereotactic ablative radiotherapy, EBRT external beam radiotherapy, RARP robotic-assisted radical prostatectomy
Fig. 2.
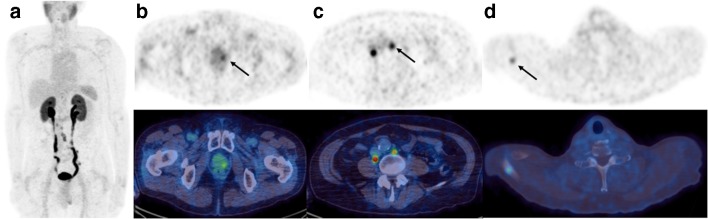
68Ga-THP-PSMA PET-CT scan of a man with a rising PSA (3 μg/L) after a previous radical prostatectomy. a Maximum intensity projection image. b There is evidence of nodal disease in the left supraclavicular fossa (arrow) and c retroperitoneum (arrow) as well as d a vertebral bone metastasis (arrow)
Correlation with clinical parameters
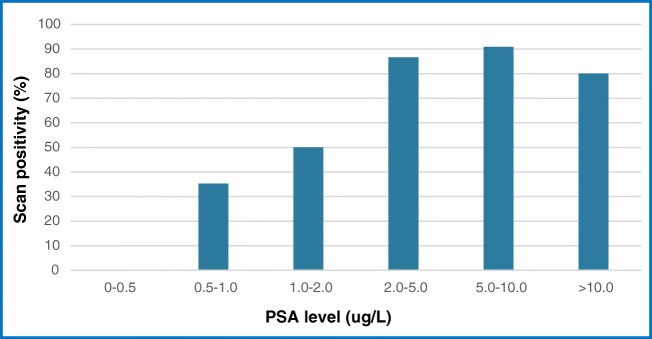
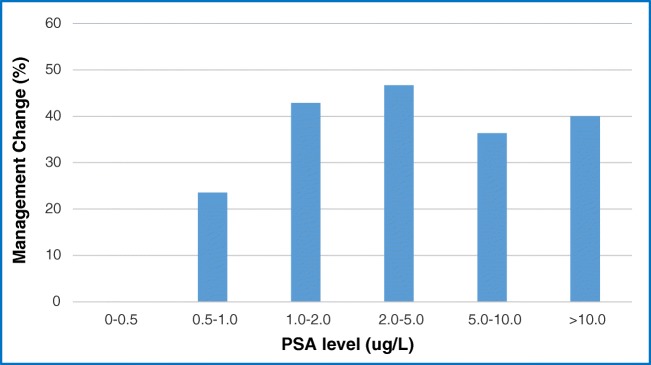
68Ga-THP-PSMA PET-CT scan positivity was compared with PSA values. Fifty nine percent of scans were positive (PSA range 0.82–71.0 μg/L (mean 6.34)) whilst 41% were negative (PSA range 0.11–14.2 μg/L mean 1.7, p < 0.001) (Fig. 3). No positive scans were identified when the PSA was < 0.5 μg/L. Management change was seen in 4/17 (23.5%) patients with PSA levels between 0.5 and 1.0 μg/L with higher but broadly similar proportions of changes in management in patients with PSA 1.0–2.0 μg/L (6/14, 42.9%), PSA 2.0–5.0 μg/L (7/15, 46.7%), PSA 5.0–10.0 μg/L (4/11, 36.4%) and PSA > 10.0 μg/L (2/5 (40%) (Fig. 4).
Fig. 3.
Scan positivity in BCR at different PSA levels
Fig. 4.
Management change as related to PSA value in BCR group
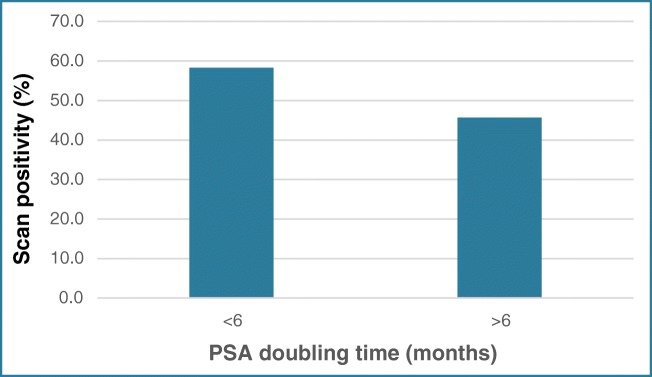
PSAdt at diagnosis was available for 60 patients and Gleason scores for 62, as a proportion of patients were diagnosed and treated in external hospitals. For those with a PSAdt < 6 months, 14/25 (56%) had a positive scan compared with 16/35 (45.7%) > 6 months (Fig. 5). Management changed was in 9/25 (36%) of those with PSAdt < 6 months and 9/35 (25.7%) of those with PSAdt > 6 months.
Fig. 5.
Scan positivity as related to PSA doubling time in BCR group
Twenty-two out of 43 (51.2%) patients with a Gleason score < 8 had a positive scan compared with 15/19 (78.9%) of those with a Gleason score > 8 (Fig. 6). Management changed was in 13/43 (30.2%) patients with Gleason < 8 and in 7/19 (36.8%) of patients with Gleason > 8.
Fig. 6.
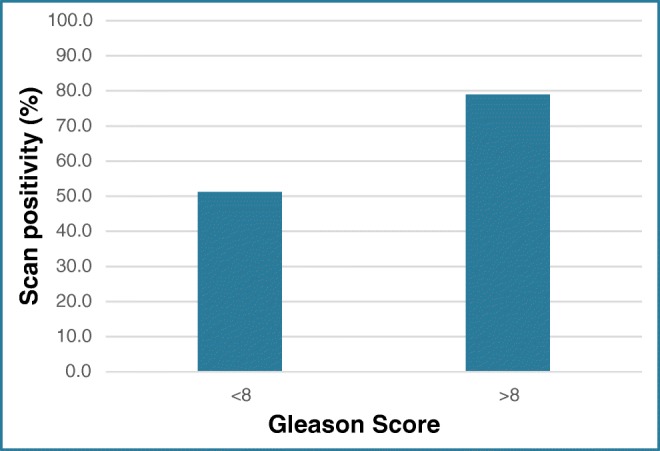
Scan positivity as related to Gleason score in BCR group
Follow-up
Follow-up is available for a median of 13 months (range 2 to 18 months) for the total cohort from the date of the 68Ga-THP-PSMA PET-CT. Currently, 27 men are on PSA surveillance, 22 on androgen deprivation therapy (ADT), 10 are having chemotherapy (2 with hormone-sensitive disease, 8 with castrate-resistant metastatic prostate cancer), 4 undergoing radiotherapy, 2 on watchful waiting, 2 having best supportive care (due to metastatic tumours of non-prostatic origin) and 1 being considered for salvage surgery. In 61 of the 68 BCR patients, subsequent follow-up has not been discordant with 68Ga-THP-PSMA PET-CT results.
In 7 patients, the 68Ga-THP-PSMA PET-CT scan was negative, but all patients have now been taken off PSA surveillance. In 5 of these patients, despite a negative 68Ga-THP-PSMA PET-CT scan, there is ongoing concern about micro-metastatic disease due to a continuing rise in PSA; 1 is having more intensive PSA surveillance (PSA 1.42 μg/L), 2 have commenced ADT (PSA 1.98 μg/L and 1.2 μg/L, respectively) and 2 are on chemotherapy (PSA 1.37 μg/L and 1.67 μg/L, respectively). In the other 2 patients, the initial 68Ga-THP-PSMA PET-CT scan was reassuring. However, due to rising PSA, the scan was repeated (at 6 and 9 months, respectively), and nodal disease has now been demonstrated. The first of these two patients is on ADT and the other on watchful waiting.
Whilst the majority of patients had multi-modal treatment and subsequently underwent a 68Ga-THP-PSMA PET-CT scan, only minor differences were identified between those with previous primary RARP compared with RT. 34.7% of patients who had a RARP had a change in management compared with 39.1% who had RT.
Discussion
Our study has demonstrated a clinically significant impact on the management of patients with high-risk prostate cancer and BCR, imaged using 68Ga-THP-PSMA PET-CT.
Primary staging in high-risk prostate cancer
In our series of patients undergoing 68Ga-THP-PSMA PET-CT for primary staging, a management change (compared with the decision made in an MDM using standard diagnostic techniques) was noted in 12 of the 50 patients (24%). Roach et al. evaluated a comparable cohort of 108 patients scanned for primary staging and reported a 21% management change [17].
We noted a higher number of management changes in patients with PSA < 20 μg/L (34.6%) compared with patients with PSA > 20 μg/L (12.5%). This implies that at high PSA levels, conventional staging with MRI, bone scan and/or CT can detect nodal or metastatic disease, but at lower PSA levels, 68Ga-THP-PSMA PET-CT has greater sensitivity and management impact. We did not find a difference in management changes between patients with Gleason score < 8 and > 8.
Perera et al. analysed 16 studies involving 1309 patients who underwent a 68Ga-PSMA PET-CT (most used 68Ga-HBED-CC-PSMA, some did not mention the specific ligand), of which 40% of scans were positive for patients undergoing primary staging (95% CI 19–64%) [12]. Four studies were evaluated for the predictive ability of PSMA PET-CT (scan positivity compared with histology), demonstrating a sensitivity of 80% and specificity of 97%.
Lymph node evaluation is integral to primary staging and there is a need for imaging which provides more sensitive detection in high-risk prostate cancer. Current practice regarding lymphadenectomy is reliant on pre-operative models using PSA levels, Gleason score and T-stage to dictate surgical planning [19].
Whilst our aim was not to specifically measure the accuracy of nodal detection, 68Ga-THP-PSMA PET-CT identified lymph nodes in 8/50 patients (16%). In 2 patients, identification of lymphadenopathy led to a management change: one to RARP with a unilateral PLND (histologically positive node) and one to radiotherapy and hormones instead of RARP.
A number of studies have evaluated the sensitivity and specificity of 68Ga-HBED-CC-PSMA PET-CT for lymph node detection. Sensitivity is reported as between 33.3–65.9% and specificity 98.9–100% [21, 22]. Budäus et al. reported that 68Ga-PSMA PET-CT scans identified 4 of 12 patients (33.3%) as node positive. In 8 patients with histologically confirmed lymph nodes, 68Ga-PSMA PET-CT was negative, giving a false-negative value of 66.7%.
Current EAU guidelines recognise that PLND are associated with worse peri- and postoperative outcomes, whilst a direct therapeutic effect is still not evident from the current literature. Thus, it is not our routine practice to offer surgery and lymph node dissection to patients with pathological nodes on imaging. A recent review of UK practice in 2017 demonstrated that most radical prostatectomies are performed for patients with Gleason grade group 2 and 3 disease (intermediate risk). Lymph node dissection rates were similar across all grade groups ranging from 13–15% [23].
In our cohort, 68Ga-THP-PSMA PET-CT detected 7 patients with metastatic disease at primary presentation (3 M1a and 4 M1b), which was greater than that identified by conventional imaging alone. Treatment choice did change as a result of these findings in 4/7 patients (57%).
Biochemical recurrence
In our BCR group undergoing 68Ga-THP-PSMA PET-CT, clinical management changed in 23 of the 68 patients (34%), with 59% of scans demonstrating positivity. In 23 scans, disease recurrence was identified locally (prostate or prostate bed), 23 in lymph nodes and in 9, metastatic disease was seen. In those where there was a management change, 10 patients were upstaged (42%) and 2 downstaged (8.3%), and in 12 scans (50%), staging was concordant with that of conventional imaging, but additional information relevant to treatment planning was identified.
Roach et al. in Australia and Afaq et al. in the UK have shown 62% and 39% change in management intent, respectively, in the BCR setting, with the use of 68Ga-HBED-CC-PSMA [17, 18]. Roach et al. have conducted the largest prospective multicentre study of 323 patients evaluating management intent. They evaluated all patients undergoing imaging for BCR, with a detectable PSA but negative conventional imaging. Overall clinical intent changed in 51% of patients following results of PSMA PET-CT. 68Ga-HBED-CC-PSMA PET-CT detected additional local disease in 27% of patients, nodes in 39% and metastatic disease in 16%.
The systematic review by Han et al., evaluating management changes following 68Ga-HBED-CC-PSMA PET, was performed for BCR in 11 studies [24]. The proportion undergoing RT increased from 56 to 61%, typically with an increase in dose or target volume. There was an increase in patients undergoing salvage surgery from 1 to 7%, which included performing pelvic lymph node dissection (in our group, this decreased from 10.2 to 5.9%). The number of patients undergoing systemic treatment decreased from 26 to 12% (in our cohort, it increased from 5.9 to 10.2%). ADT was initially planned in 144 patients but was only commenced in 52 patients after 68Ga-HBED-CC-PSMA (in our group, planned in 28% and decreased to 25%). The proportion of patients with no treatment decision or on surveillance decreased from 14 to 11% (we report an increase from 29.4 to 38%). In addition, we report a small decrease in numbers undergoing radiotherapy (from 26.4 to 21%).
At PSA levels of 1.0 μg/L and above, we noted higher rates of management change (0% < 0.5 μg/L, 23% 0.5–1 μg/L, 46% 1–2 μg/L). Higher scan positivity and change in management rates were also seen in patients with short PSAdt < 6 months (56% vs 45.7% and 36.0% vs 25.7%, respectively). Similarly, those with higher Gleason scores > 8 had higher scan positivity rates (78.9% vs 51.2%) but broadly similar changes in management (36.8% vs 30.2%).
Fendler et al. using 68Ga-HBED-CC-PSMA PET-CT reported a detection rate of 75% in BCR. There was a significant increase in detection rate across PSA ranges, 38% for < 0.5 ng/mL, 57% for 0.5–< 1 ng/mL and 84% for 1.0 ng/mL [25]. There were 8 cases where the PET findings were reported as negative but histology confirmed prostate cancer.
Similar trends were identified in a retrospective series by Ceci et al. regarding factors which correlated with 68Ga-HBED-CC-PSMA PET-CT detection in patients presenting with BCR [26].
68Ga-HBED-CC-PSMA PET-CT was positive in 23 of 39 patients (59 %) with PSA < 2 μg/L. These results are consistent with data recently reported by Afshar-Oromieh et al. who reported scan positivity rates with 68Ga-HBED-CC-PSMA PET-CT of 61.1% in 90 patients with PSA < 2 μg/L [27]. PSA kinetics were evaluated, reporting that 68Ga-HBED-CC-PSMA PET-CT was positive in 85% of patients with low PSA values and short PSAdt, but in only 18.7% of patients with low PSA and long PSAdt. For Perera et al. in the BCR cohort, 68Ga-HBED-CC-PSMA PET positivity increased as serum PSA rose. For those with a PSA < 0.2 μg/L, the detection rate was 42%, which increased to 58%, 76%, and 95% for the 0.2–0.99, 1.00–1.99, and > 2.0ug/L PSA values, respectively [12]. A similar trend was demonstrated with PSAdt, with a PSMA positivity of 92% for those with a PSAdt < 6 months and 64% for those ≥ 6 months. The authors again recognise the significant heterogeneity between the groups analysed.
Several studies have determined that the clinical detection rate was dependent on the PSA level, with Derlin et al. reporting rates as high as 94.1% for PSA > 10 μg/L, 54.5% for PSA 1–2 μg/L and 20% below 0.5 μg/L [28]. Afaq et al., using 68Ga-HBED-CC-PSMA, reported an overall scan positive rate of 15.8% (PSA 0.2–0.5 μg/L), 33% (PSA 0.5–1 μg/L), 20% (PSA 1–2 μg/L) and 84.6% (PSA 2–5 μg/L) [18].
The difference in management change and scan positivity rates between these studies and ours might be explained by differences in prospective versus retrospective methodology, referral patterns and decision-making between Australian, European and UK practice. The data from Afaq et al., a UK study in the same city, is more comparable with ours and hence the closer values for management impact [18].
Whilst previous studies have reported higher detection rates with other PSMA ligands, 68Ga-THP-PSMA demonstrated reasonable detection rates in patients with BCR and PSA levels ≥ 1 μg/L. Potential explanations for the lower detection rates at very low PSA levels using this ligand compared with 68Ga-HBED-CC-PSMA include different use of ADT between studies which alters PSMA expression, faster renal clearance of THP compared with HBED and potential lower affinity of 68Ga-THP-PSMA. No comparisons have been performed to determine if this impacts on diagnostic accuracy or management.
68Ga-THP-PSMA
68Ga-THP-PSMA can be labelled to Good Manufacturing Practice criteria in 5 min at room temperature as a single-step technique and has a potential practical and cost advantage over other 68Ga-labelled PSMA radiopharmaceuticals. Hofman et al., Young et al. and Derlin et al. have evaluated this ligand for its safety and bio-distribution, whole-body radiation dose, plasma clearance and correlation of uptake with tumour PSMA expression on histopathology [14, 15]. They demonstrated that 68Ga-THP-PSMA had lower background uptake in salivary glands, liver and spleen than 68Ga-HBED-CC-PSMA. Metastatic lesions were equally identified on both HBED-PSMA and THP-PSMA-PET scans. 68Ga-THP-PSMA provides a similar effective dose to that published for 68Ga-HBED-CC-PSMA (2.07 × 10−2 mSv/MBq).
Limitations
This is a single-centre study and may not reflect practice in other units where diagnostic and treatment guidelines may differ. Nevertheless, the results reflect practice in a busy tertiary referral centre with a large surgical and oncological prostate cancer service. Management decisions were made in a multidisciplinary fashion for a consecutive cohort of patients. The study reports intended management and did not take into account actual treatment, which may on occasion differ from planned due to patient comorbidities or other patient-related factors. Not all of the PET-positive finding scans were validated by another procedure such as histological analysis of lymph nodes or visceral lesions (not always feasible) or conventional imaging.
Conclusions
Our study of a new radiopharmaceutical PSMA formulation that can be rapidly manufactured from a 68Ga generator and cold-kit vial demonstrates that 68Ga-THP-PSMA PET-CT impacts on management decisions in HR prostate cancer prior to radical therapy and BCR, despite lower scan positivity rates at PSA levels < 0.5 μg/L. Scan positivity is related to PSA level, PSA doubling time and Gleason score.
Acknowledgements
The authors acknowledge financial support from the King’s College London/University College London Comprehensive Cancer Imaging Centres funded by Cancer Research UK and Engineering and Physical Sciences Research Council in association with the Medical Research Council and the Department of Health (C1519/A16463) and the Wellcome Trust EPSRC Centre for Medical Engineering at King’s College London (WT203148/Z/16/Z).
M Kulkarni is part funded by Theragnostics Ltd. Thanks to Dr. P Roach for allowing us to adapt the questionnaire used in the Australian study [17].
Appendix
Compliance with ethical standards
Conflict of interest
Theragnostics Ltd. provide research funding to GC. MK, PhD student, is part funded by Theragnostics. The authors declare they have no other conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required for this service evaluation of anonymised data.
Footnotes
This article is part of the Topical Collection on Oncology – Genitourinary
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer Research UK 2016. Prostate cancer incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer. Accessed 30 March 2019.
- 2.Torre A, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Foley RW, Lundon DJ, Murphy K, Murphy TB, Galvin DJ, Watson RW. Predicting prostate cancer: analysing the clinical efficacy of prostate cancer risk calculators in a referral population. Ir J Med Sci. 2015;184:701–706. doi: 10.1007/s11845-015-1291-8. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management. https://www.nice.org.uk/guidance/cg175/chapter/1-Recommendations#localised-and-locally-advanced-prostate-cancer-2. Accessed 30 March 2019.
- 5.Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol. 2013;63:1040–1048. doi: 10.1016/j.eururo.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38:305–314. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 7.Mapelli P, Incerti E, Ceci F, Castellucci P, Fanti S, et al. 11C- or 18F-Choline PET/CT for imaging evaluation of biochemical recurrence of prostate cancer. J Nucl Med. 2016;57(Suppl 3):43S–48S. doi: 10.2967/jnumed.115.169755. [DOI] [PubMed] [Google Scholar]
- 8.Donin NM, Reiter RE. Why targeting PSMA is a game changer in the management of prostate cancer. J Nucl Med. 2018;59:177–182. doi: 10.2967/jnumed.117.191874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, et al. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med. 2016;57(Suppl 3):79S–89S. doi: 10.2967/jnumed.115.170720. [DOI] [PubMed] [Google Scholar]
- 10.Eder M, Neels O, Müller M. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel) 2014;7:779–796. doi: 10.3390/ph7070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 12.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 13.von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2018;4:686–693. doi: 10.1016/j.euf.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Hofman MS, Eu P, Jackson P, Hong E, Binns D, Iravani A, et al. Cold kit for prostate-specific membrane antigen (PSMA) PET imaging: phase 1 study of (68)Ga-Tris(Hydroxypyridinone)-PSMA PET/CT in patients with prostate cancer. J Nucl Med. 2018;59:625–631. doi: 10.2967/jnumed.117.199554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young JD, Abbate V, Imberti C, Meszaros LK, Ma MT, Terry SYA, et al. 68Ga-THP-PSMA: a PET imaging agent for prostate cancer offering rapid, room temperature, one-step kit-based radiolabeling. J Nucl Med. 2017;58:1270–1277. doi: 10.2967/jnumed.117.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derlin T, Schmuck S, Juhl C, Teichert S, Zörgiebel J, Wester HJ, et al. Imaging characteristics and first experience of [(68)Ga]THP-PSMA, a novel probe for rapid kit-based Ga-68 labeling and PET imaging: comparative analysis with [(68)Ga]PSMA I&T. Mol Imaging Biol. 2018;20:650–658. doi: 10.1007/s11307-018-1160-8. [DOI] [PubMed] [Google Scholar]
- 17.Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of (68)Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med. 2018;59:82–88. doi: 10.2967/jnumed.117.197160. [DOI] [PubMed] [Google Scholar]
- 18.Afaq A, Alahmed S, Chen SH, Lengana T, Haroon A, Payne H, et al. Impact of (68)Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. doi: 10.2967/jnumed.117.192625. [DOI] [PubMed] [Google Scholar]
- 19.D’Amico AV, Whittington R, Schultz D, Malkowicz SB, Tomaszewski JE, Wein A. Outcome based staging for clinically localized adenocarcinoma of the prostate. J Urol. 1997;158:1422–1426. doi: 10.1016/S0022-5347(01)64230-7. [DOI] [PubMed] [Google Scholar]
- 20.Roach M, Hanks G, Thames H, Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–1443. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–396. doi: 10.1016/j.eururo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Calleja E, Fowler S, McGrath J, Sooriakumarana P, Aning J. Contemporary extended pelvic lymph node dissection for prostate cancer in the UK – an analysis of national practice. Eur Urol Suppl. 2017;16(3):e1866–e1867. doi: 10.1016/S1569-9056(17)31122-3. [DOI] [Google Scholar]
- 24.Han S, Woo S, Kim YJ, Suh CH. Impact of (68)Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–190. doi: 10.1016/j.eururo.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. doi: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–1294. doi: 10.1007/s00259-015-3078-6. [DOI] [PubMed] [Google Scholar]
- 27.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derlin T, Schmuck S, Juhl C, Zörgiebel J, Schneefeld SM, Walte ACA, et al. PSA-stratified detection rates for [(68)Ga]THP-PSMA, a novel probe for rapid kit-based (68)Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:913–922. doi: 10.1007/s00259-017-3924-9. [DOI] [PubMed] [Google Scholar]