Abstract
Owing to early diagnosis and rapid development of treatments for cancers, the five-year survival rate of all cancer types has markedly improved worldwide. Over time, however, there has been an increase in the number of cancer patients who develop coronary artery disease (CAD) due to different causes. First, many risk factors are shared between cancer and CAD. Second, inflammation and oxidative stress are common underlying pathogeneses in both disorders. Lastly, cancer therapy can result in endothelial injury, coronary artery spasm, and coagulation, thereby increasing the risk of CAD. As more cancer patients are being diagnosed with CAD, specialized cardiac care should be established to minimize the cardiovascular mortality of cancer survivors.
Keywords: Cancer, Coronary artery disease, Cardio-oncology
Owing to the unparalleled growth in cancer therapy over the past decades, the average five-year survival rate of cancer patients has reached 67% in developed countries.1 However, the number of cancer patients with coronary artery disease (CAD) continues to increase because of the cardiac toxicity exhibited by anticancer drugs and the common pathogenesis between cancer and CAD. Presently, there are no guidelines for cancer patients with CAD. Therefore, multidisciplinary collaboration is needed to formulate reasonable strategies for the diagnosis and treatment of CAD in cancer patients.
Epidemiology of cancer-related CAD
Over the last 40 years, the 10-year survival rate of early breast cancer has increased from 40% to 80%, and a similar growth has been found in other cancers, such as solid cancers and hematologic cancers.2, 3, 4 Unfortunately, improvements in cancer prognosis have been achieved at the cost of cardiovascular toxicity. Thus, cancer survivors have an increased medium-to long-term risk of CAD development.5 In newly diagnosed cancer patients, the 6-month cumulative incidence of myocardial infarction was found to be markedly higher than that of matched control patients (HR = 2.9).6 A similar issue could also be present in childhood cancer survivors. A prospective study of 7289 childhood cancer survivors revealed that the cumulative incidence of CAD was approximately 10% at 10 years from cancer diagnosis.7 There has also been an increase in the incidence of cancer in patients with acute coronary syndrome (ACS). A prospective study with 17 years of follow-up demonstrated that the incidence of malignant tumor was approximately three times higher in ACS patients than the general population.8 Data from a retrospective trial of 12,785 patients who underwent percutaneous coronary intervention (PCI) revealed that cancer survivors accounted for a high proportion of PCI patients (one in every 13 patients).9
Cancer survivors with CAD have poor prognosis even after receiving the optimal medical therapy and PCI. Yusuf et al10 found that the one-year estimated survival rate of cancer patients with non-ST elevation myocardial infarction (non-STEMI) was only 26% after medical treatment or PCI, while that of cancer patients with ST elevation myocardial infarction (STEMI) was 22%. Overall survival was even worse in patients with a history of lymphoma/leukemia, chest radiotherapy, chemotherapy, and advanced cancer. The BleeMACS study was a multicenter observational registry involving patients with ACS undergoing PCI. In this study, cancer patients accounted for 6.4% of all the enrolled patients, and cancer was the strongest independent predictor of death and re-infarction (HR = 2.1), and bleeding (HR = 1.5).11 Notably, CAD in cancer patients does not often result from the toxicity of cancer therapy, and it may be related to aging or an exacerbation of the underlying cardiovascular disease. Thus, early identification and management of CAD in cancer patients are critical for maintaining the survival benefits of modern cancer therapy.
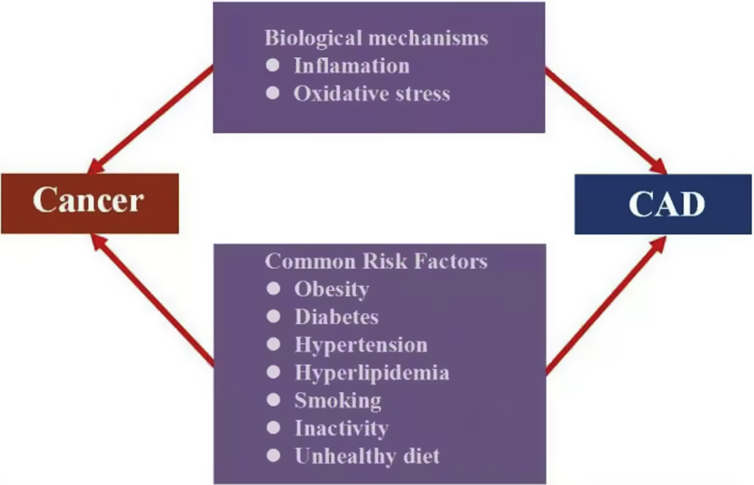
Common risk factors and pathogeneses between cancer and CAD
Common risk factors
Growing evidence has indicated that cancer and CAD share common risk factors, including obesity, diabetes, hypertension, hyperlipidemia, smoking, inactivity, and unhealthy diet. Obesity is associated with multiple cancers, and every 5% increase in body mass index increases the risk of thyroid, esophageal, endometrial, and gallbladder cancers by 33%–59%.12 A study comprising of 2943 patients with breast cancer found that an increase in visceral or intramuscular adiposity was associated with the risk of cardiovascular disease (CVD).13 Obesity is accompanied by insulin resistance, atherogenic dyslipidemia, and inflammation, which contribute to the occurrence of cancer and CAD. Diabetes is considered to be one of the most important risk factors for CVD and has been established as a risk factor for breast cancer. Besides insulin resistance and lipid metabolism disorders, hyperglycemia may also lead to intestinal flora disorder for the induction of inflammation, ultimately promoting carcinogenesis and tumor progression.14
Hypertension and dyslipidemia are related to the development of cancers. Compared to normotensive patients, the risk of renal cancer was increased by 94% in patients with a systolic blood pressure >160 mmHg and 75% in those with a diastolic blood pressure >90 mmHg.15 By examining 244 breast cancer patients, Rodrigues et al16 found that compared to patients with lower LDL cholesterol (<117 mg/dL), patients with higher LDL cholesterol had significantly larger tumor volume and lower survival rate. The cholesterol metabolite, 27-hydroxycholesterol, was also proven to induce the proliferation and metastasis of breast cancer cells in an experimental mouse model.17 Smoking and drinking have also been linked to the occurrence of cancers. Smoking may produce carcinogens (benzopyrene and nitrosamine), proinflammatory substances, and oxidation to facilitate tumorigenesis while drinking significantly increases the risk of esophageal, oral, throat, and breast cancers and cancer-related mortality.18
In addition, active physical exercise and a healthy diet can improve cellular immune function, maintain energy metabolism, and prevent tumorigenesis.19 Compared to individuals that performed less exercise, the risk of esophageal cancer, liver cancer, and lung cancer was found to be respectively reduced by 42%, 27%, and 26% in individuals that participated in regular exercise.20 The Framingham risk scale (FRS)-predicted 10-year risk of CVD was reduced by 11% in persons that participated in aerobic and resistance exercise.21 Based on the effects of diet on cancer, the intake of vegetables and fruit per day was found to reduce the risk of cancer,22 and low-carbohydrate diet was positively associated with cancer mortality (RR = 1.08).23 Besides, substituting red meat and processed meat protein with plants to serve as the protein source can reduce cancer-related (HR = 0.61) and CVD-related (HR = 0.58) mortality.24
Common pathogenesis
The role of inflammation and oxidative stress in atherosclerosis has been previously established.25 A cohort study of 7178 patients with stable CAD found that chronic systemic low-grade inflammation (C-reactive protein (CRP) ≤10 mg/L) was related to the incidence of cancer (HR = 1.35) during the 12-year follow-up.26 The CANTOS trial comprising of 10,061 patients with pre-existing myocardial infarction found that patients treated with canakinumab, a therapeutic monoclonal antibody targeting the inflammatory cytokine, IL-1b, had a lower rate of recurrent cardiovascular events than placebo patients. More importantly, canakinumab led to a significant decrease in lung cancer incidence, lung cancer death, and total cancer mortality.27,28 Oxidative stress and reactive oxygen species are involved in the pathological process of cancer. The activity of Zn-superoxide dismutase (SOD) is highly elevated in patients with colorectal cancer.29 Additionally, chronic inflammation may induce oxidative stress, facilitating the development of cancer and CAD. The common risk factors and the pathogenesis between cancer and CAD are shown in Fig. 1.
Fig. 1.
Common risk factors and pathogenesis between cancer and coronary artery disease.
Cancer therapy-induced CAD
Approximately 50% of the cardiovascular toxicity caused by anticancer therapy is presented as vascular injury, which increases the risk of CAD. All types of cancer therapies contribute to the development of CAD, including chemotherapy, radiotherapy, and targeted drug therapy. The pathophysiological mechanisms of myocardial ischemia related to cancer therapy are summarized in Table 1.
Table 1.
Pathophysiological mechanisms of CAD related to cancer therapy.
| Agents | Pathophysiological mechanism | Risk of myocardial ischemia |
|---|---|---|
| Fluorouracil drugs (5-fluorouracil and capecitabine) |
|
|
| Platinum drugs (Cisplatin) |
|
|
| Immune checkpoint inhibitors |
|
|
| Radiotherapy |
|
|
| Targeted drug therapy |
|
|
ACS: acute coronary syndrome; CAD: coronary artery disease; VEGF: Vascular endothelial growth factor.
Fluorouracil drugs
Fluorouracil is widely used in the treatment of digestive tract tumors and other solid tumors. 5-fluorouracil and its oral pro-drug capacitance are notoriously associated with myocardial ischemia, and the occurrence rates can be as high as 68% and 9%, respectively.30 The underlying mechanisms of cardiotoxicity induced by fluorouracil are associated with coronary artery vasospasm, vascular endothelial injury, direct cardiotoxic effects, and thrombogenicity.31 The reported incidence of coronary spasm caused by fluorouracil ranges from 1% to 68%.32 In addition, 5-fluorouracil is a radiosensitizer that increases the risk of radiation-induced thrombosis.33 Lestuzzi et al34 examined 358 cancer patients administered 5-fluorouracil. As a result, they found that 5.9% of patients had rest ischemia. Among those patients, 228 underwent the treadmill exercise test, which revealed that the rate of effort induced myocardial ischemia was approximately 6.9%. Angina and ischemic ECG changes usually occur within a few days after 5-fluorouracil administration, and sometimes may persist following treatment cessation. The propensity of 5-fluorouracil to induce acute myocardial infarction has been well established,35 and people with cardiovascular risk factors, such as smoking, hypercholesterolemia, and diabetes, are more likely to suffer from cardiotoxicity induced by this drug. Calcium channel blockers and nitrates are used in high-risk patients to prevent and treat coronary artery spasm caused by 5-fluorouracil.36 However, coronary spasm may recur after 5-fluorouracil administration due to the limited effects of this vasodilator.
Platinum drugs
Platinum drugs are effective therapies for numerous solid tumors such as ovarian cancer, lung cancer, and testicular cancer; however, they increase the risk of adverse cardiovascular events. The long-term risk of CAD is 1.5–7.0 times higher in patients receiving platinum-based chemotherapy than controls.37, 38, 39, 40 Cisplatin has been reported to be closely associated with acute coronary thrombosis, and even multiple coronary thrombi may occur after cisplatin treatment.41 Approximately 2% of patients treated with cisplatin suffer from myocardial ischemia due to arterial thrombosis.42 The proposed mechanisms underlying the cardiotoxicity induced by platinum drugs are direct endothelial toxicity, platelet activation and aggregation, and thrombogenesis.43, 44, 45 In a study with testicular cancer survivors, compared to patients receiving surgery alone, those administered cisplatin were found to have a significant increase in the risk of CAD (HR = 2.6) during the 20-year follow-up.46 Moore et al42 also observed that 18.1% of patients treated with cisplatin had venous and arterial thromboembolic events.
Immune checkpoint inhibitors
Programmed death molecule 1 (PD-1) and cytotoxic T lymphocyte-cell associated antigen 4 (CTLA-4) are major immune checkpoints that can prevent the overactivation of the human immune system and avoid the occurrence of autoimmune diseases. Under the stimulation of the tumor microenvironment, the immune checkpoint pathway is over-activated, leading to immune escape.47 Immune checkpoint inhibitors significantly improve the prognosis of many cancers, but also increase the risk of CAD.48 Immune checkpoint inhibitors play a critical role in atherosclerosis by regulating the activation and proliferation of T cells, macrophages, and platelets.49 Immune checkpoint inhibitors can contribute to the formation and rupture of unstable atherosclerotic plaques by inducing different inflammatory cytokines and atherosclerotic cytokines related to overactivated T cells.50 A meta-analysis showed that the incidence of myocardial infarction was approximately 1% in cancer patients treated with immune checkpoint inhibitors.51
Radiotherapy
Radiotherapy is considered to be the primary treatment for solid tumors and hematological malignancies. Thus, more than 50% of cancer patients have been administered this therapy. Numerous studies have confirmed that radiotherapy can damage vascular endothelial cells, and this is followed by plaque rupture and thrombosis formation, which might be accompanied by coronary spasm, ultimately causing CAD.52 Radiotherapy leads to the formation of cholesterol plaques and thrombosis within a few days in experimental models.53,54 Radiotherapy on the left breast or chest often impairs the left anterior descending branch and diagonal branch, especially the ostium of the vessel. Besides, fibrosis occurs in all three layers of the vascular wall followed by accelerated atherosclerosis.55,56 In addition to macrovascular disease, radiotherapy results in microvascular dysfunction, causing the reduction of coronary flow reserve (CFR) and myocardial ischemia. CAD usually appears after more than 10 years of radiotherapy, and young patients with curable malignancies suffer the most from radiation-induced cardiotoxicity. CAD has been found in nearly 20% of young people receiving radiotherapy with 20 as the average age.57 Mediastinal radiation for Hodgkin disease and left-sided breast cancer are the main factors that lead to a greater propensity toward CAD in young cancer patients. In a retrospective study of 2524 survivors of Hodgkin's lymphoma, Van Nimwegen et al58 found that compared to the non-mediastinal radiotherapy group, the mediastinal radiotherapy group had a 4- to 6-fold increase in the risk of CAD. A chest radiation dose greater than 30 Gy was also previously proven to cause cardiovascular injury.59 Individuals with cardiovascular risk factors are more susceptible to CAD induced by radiotherapy.60 By analyzing 3964 Hodgkin lymphoma patients with pre-existing heart disease, Myrehaug et al61 concluded that a prior history of heart disease was the strongest predictor of cardiac toxicity after radiotherapy (HR = 3.98, P < 0.001).
Targeted drug therapy
Targeted drugs kill cancer cells by specifically binding to carcinogenic sites without affecting the biological function of peripheral normal cells. However, many targeted therapies, especially monoclonal antibodies and tyrosine kinase inhibitors, have been demonstrated to interfere with the cell signaling pathway closely related to CAD.62 Vascular endothelial growth factor (VEGF) inhibitors, which are a type of chemotherapeutics for multiple solid tumors, predispose patients to the increased risk of thrombosis. In fact, patients treated with the VEGF pathway inhibitors are at a 2 to 6 times greater risk of ACS.63,64 Compared to chemotherapy alone, the incidence of arterial thromboembolism is significantly increased with the combination of bevacizumab and chemotherapy (HR = 2).65 The incidence of arterial thromboembolism is 1.7% in patients treated with sorafenib and 1.4% in those treated with sunitinib. A decrease in CFR occurs in 72% of patients receiving sunitinib therapy, especially in patients administered long-term treatment.66 Sunitinib also contributes to microvascular dysfunction67 and the simultaneous rarefication of microvascular pericytes and capillaries in the laboratory.68 Sorafenib was reported to induce vasospasm and impair multiple vessels at a rate that is more profound than that of sunitinib.69, 70, 71 Besides, sorafenib contributes to the progression of CAD and atherosclerotic plaque rupture by impairing endothelial healing.72 Nilotinib and ponatinib have been found to be associated with the progression of atherosclerosis.73,74
Diagnosis of CAD in cancer patients
Typical chest pain is usually absent in cancer patients with CAD. However, dyspnea is the principal clinical manifestation, resulting from analgesic treatment or neurotoxicity of radiotherapy and chemotherapy. Only 30.3% of cancer patients have chest pain and 44% of those with CAD have dyspnea.10 However, its atypical clinical presentation poses a great challenge when CAD is being diagnosed in cancer patients. The assessment and examination of myocardial ischemia are thus key to diagnosing silent CAD. If cancer patients have dyspnea, attention should be paid to the screening of CAD. In fact, symptoms, risk factors, cardiac biomarkers, electrocardiogram (ECG), and anticancer therapy should be assessed according to the ACC and AHA guidelines.75 Anticancer therapies not only cause coronary artery injury, but also coronary microcirculation dysfunction.76 If the angina symptom of cancer patients is typical, but abnormality is not evident by coronary CT angiography (CCTA) or coronary angiography, coronary microcirculation resistance index or myocardial contrast echocardiography (MCE) should be performed.
Approximately 10% of cancer patients with ACS have takotsubo cardiomyopathy, and most are treated with 5-fluorouracil, capecitabine, cytarabine, axitinib, sunitinib, bevacizumab, rituximab, trastuzumab, or combretastatin.77 The prevalence of cancer is also high (23.7%) in patients with takotsubo cardiomyopathy.78 Early identification of takotsubo cardiomyopathy can prevent the risk of bleeding caused by antithrombotic treatment.79 Takotsubo cardiomyopathy can be diagnosed according to the Mayo Center diagnostic criteria: (a) weakened left ventricular middle wall motion, dyskinesia or no movement with or without apical wall motion abnormalities; the range of the wall motion abnormalities should be larger than that of a single coronary artery distribution, and stress stimulus factors often occur simultaneously; (b) no evidence of coronary artery stenosis or plaque rupture; (c) new ECG changes (ST segment elevation or T wave inversion in precordial leads); and (d) no pheochromocytoma, myocarditis, or other diseases, leading to left ventricular dysfunction.
Cancer-related CAD should be distinguished from Kounis syndrome (KS), which is defined as a special type of ACS that occurs secondary to allergic reaction by inflammatory mediators mainly released from activated mast cells.80,81 Anticancer agents have hapten characteristics. As previously reported, approximately 42% of patients display hypersensitivity reaction, 2% have severe allergy after paclitaxel treatment, and 19.27% of patients treated with capecitabine, oxaliplatin, or bevacizumab have allergic reactions. There are 3 types of KS according to the different pathogeneses: Type I, ACS secondary to coronary spasm in patients without underlying CAD; Type II, ACS secondary to coronary spasm or plaque rupture in patients with underlying CAD; and Type III, ACS secondary to coronary thrombosis, including stent thrombosis. KS can be diagnosed according to the following criteria: (a) ACS after infusion of anticancer drugs that may cause allergy; (b) symptoms, signs, and laboratory findings of acute allergic diseases; (c) eosinophils and mast cells identified through histological examination of thrombus; and (d) exclusion of other diseases (such as thrombophilia, systemic lupus erythematosus (SLE), and polycythemia). Besides the above diseases, others that must be excluded when considering cancer-related CAD include myocarditis, pericarditis, and pulmonary embolism.
Cardiovascular imaging for the detection of CAD in cancer patients
Cardiovascular imaging is required for the diagnosis and therapeutic monitoring of CAD in cancer patients and echocardiography is primarily used to examine cardiac function and structure. As CAD patients often exhibit normal wall motion, except for critical ischemia or myocardial infarction, normal resting echocardiography may not exclude the possibility of CAD. Thus, stress echocardiography has a great sensitivity and specificity for CAD diagnosis, especially in patients with negative exercise ECG.82,83
CCTA is broadly used as an ideal imaging modality to evaluate suspected patients with CAD. As most cancer patients may suffer from silent ischemia, CCTA may be used as an effective tool to assess coronary arteries, despite the lack of typical symptoms.84 CCTA can also improve the accuracy of CAD estimation for asymptomatic patients by using the coronary artery calcification score.85 However, further large-scale trials are needed to explore the feasibility of CCTA in predicting long-term cardiovascular outcomes.86 Currently, coronary angiography remains the “gold standard” for diagnosing CAD.
Cardiac MRI can detect CAD and accurately quantify the degree of ischemia. Theoretically, cardiac perfusion MRI may be used to detect the insufficiency of myocardial perfusion during or after anticancer therapy.87 Positron emission tomography (PET) and Single-Photon Emission Computed Tomography (SPECT) are also used to assess myocardial perfusion abnormalities for the diagnosis, risk stratification, and therapy guidance of cancer patients with suspected CAD.88
Biomarkers for the detection of CAD in cancer patients
Specific biomarkers are particularly important in the early identification and progression monitoring of CAD in cancer patients. Thus, several cardiac biomarkers have been examined in the field of cardio-oncology. A 7-year follow-up study of breast cancer patients undergoing radiotherapy revealed that B-type natriuretic peptide (BNP) levels were related to radiation doses, but were irrelevant to the onset of CAD.89 A prospective study comprising of 555 patients with cancer as their primary diagnosis showed that the serum level of high-sensitive troponin T was increased in cancer patients before the initiation of any cardiotoxic anticancer therapy, and was strongly related to advanced cancer stage and all-cause mortality.90 A cohort study also found that troponin I was increased in cancer patients shortly after high-dose chemotherapy, indicating that it can be used as a strong predictor of poor cardiac outcome in cancer patients receiving high-dose chemotherapy.91 Other biomarkers of cardiotoxicity, including myocardial ischemia markers (heart-type fatty acid binding protein [H-FABP] and glycogen phosphorylase isoenzyme BB [GPBB]), and inflammatory and oxidative stress markers (Interleukin, high-sensitivity C-reactive protein [hs-CRP], and glutathione peroxidase) have been reported to be significantly changed during chemotherapy in small-sample studies.92,93 As H-FABP and GPBB change 2–4 h after myocardial injury, they are considered to be sensitive early biomarkers for cardiotoxicity. GPBB can also be used for risk stratification in cancer patients with CAD as it is independently associated with mortality94 while hs-CRP is a risk factor for CVD and cancer. A retrospective study involving 2867 stable CAD patients undergoing PCI suggested that hs-CRP was closely related to the higher risk of cancer death.95 However, further research is needed to accurately evaluate the predictive value of these cardiac biomarkers in cancer patients.
Screening for CAD in cancer survivors
After anticancer therapy, the risk of CAD is considerably increased in cancer patients. Thus, growing emphasis has been placed on the identification of cancer survivors who are most vulnerable to CAD. However, more effective evaluation measures are required for early detection of cardiotoxicity to optimize the cardioprotective strategies and enhance personalized medical therapy. Identifying patients with pre-existing CAD and cardiovascular risk factors is critical before the initiation of anticancer therapy. The patient's history of CVD should also be acquired before initiating potentially cardiotoxic anticancer therapy; high-risk factors of CAD should be screened; and the patient's treatment process (e.g., cumulative dose of chemotherapy and radiotherapy) must be recorded in-depth. The risk of recurrent and secondary malignant tumors and adverse cardiovascular events are markedly increased in cancer survivors. Therefore, regular annual follow-up of high-risk individuals, such as survivors of Hodgkin's lymphoma, is recommended according to the Clinical Practice Guidelines for Oncology (NCCN).
The best time to initiate the surveillance of cardiotoxic manifestations remains unclear due to the divergent opinions of experts and the lack of official guidelines. Patients with a history of systemic radiotherapy, cardiotoxic chemotherapy, total mediastinal radiotherapy ≥20 Gy, and combined chemotherapy are at a high risk of developing cardiotoxic events, and they are recommended to have an annual follow-up and physical examination by the Children's Oncology Group.96 For patients who receive a total anthracycline dosage of 300 mg/m2 or chest radiation on both sides, an annual serial ECG is recommended. Serial ECG can be performed every 2–5 years for patients at lower risk. Asymptomatic patients at high risk should undergo stress testing and echocardiography for surveillance of CAD 5–10 years after anticancer therapy. If no new symptoms arise, a reassessment every 5 years is recommended.97 CCTA is superior to functional stress tests for assessing the coronary artery. Ultimately, coronary angiography remains the best method to detect CAD, and imaging modalities and functional tests should be considered if necessary. In 2016, the Society of Cardiovascular Angiography Intervention (SCAI) guidelines98 proposed that patients undergoing chemotherapy or radiotherapy should perform CCTA every 5 years, especially in high-risk populations. Irradiated patients who are older than 60 years with one or more cardiovascular risk factors or established CAD, should reexamine CCTA 2 years after treatment.
Management of CAD in cancer patients
As the health condition of cancer patients is complex and changeable, the management of CAD in cancer patients needs multidisciplinary cooperation to formulate a reasonable and individualized plan.
Special considerations with anticancer therapy
The risk of myocardial ischemia caused by fluorouracil and capecitabine is relatively high; thus, patients with angina should exercise caution when taking these drugs and myocardial ischemia should be closely monitored via regular ECG and cardiac biomarkers. Once symptomatic ischemia occurs, the drugs should be immediately terminated. Anthracycline drugs should also be used with caution in patients with myocardial infarction or heart failure. Imaging tests and monitoring with cardiac biomarkers are also recommended. In cancer patients with unstable or acute decompensated heart diseases (e.g., ACS or heart failure), temporary cessation of anticancer therapy with cardiotoxicity should be considered. For patients with old myocardial infarction and compensated cardiac function, if the cardiotoxic anticancer therapy is the first-line treatment, communicating with patients and obtaining informed consent are crucial before application. Additionally, patients should be closely monitored. Reducing the dosage of radiation therapy and chemotherapeutic agents, or switching to less cardiotoxic chemotherapy drugs aids in reducing cardiotoxicity.
Pharmacotherapy
Cancer patients with stable angina prefer to receive drugs that can relieve angina and improve tumor prognosis. Many agents are reported to have cardioprotective effects in the laboratory; however, whether those agents can alleviate the cardiac symptoms induced by chemotherapy and radiotherapy remain unclear. Agents, such as aspirin, beta-blockers, statins, and angiotensin antagonists, have been demonstrated to be cardioprotective in cancer patients. Among those agents, aspirin and beta-blocker are beneficial for cancer patients even after adjustments are made for confounders.99 An analysis of 456 cancer patients with acute myocardial infarction showed that long-term aspirin (HR = 0.77) and beta blocker (HR = 0.64) treatment significantly reduced mortality.100 Further, a Danish study indicated that cancer-related mortality was significantly lower in long-term statin users compared to non-statin users (HR = 0.85).101 Raebel et al102 found that hypertensive patients taking angiotensin converting enzyme inhibitors had a significantly lower risk of breast cancer (HR = 0.55) than patients taking calcium channel blockers. Metformin was reported to reduce the risk of multiple cancers, including gastric cancer, lung cancer, and breast cancer.103, 104, 105 A dose-dependent association was found between the use of hydrochlorothiazide and the increased risk of squamous cell carcinoma.106 The cumulative hydrochlorothiazide dose >200,000 mg increases the risk of basal cell tumors by 54% and squamous cell carcinoma by 6-fold.107 Besides the above drugs, the use of insulin glargine is associated with an increased risk of breast cancer in women with type 2 diabetes.
PCI
Generally, cancer patients with stable CAD should receive medical therapy as the first treatment. When angina (CCS III or IV) is present despite the optimal medical therapy, revascularization therapy should be considered in stable patients. Importantly, the severity of CAD, malignant tumor staging, and patient status should be considered when exploring revascularization strategies.108 The minimally invasive procedure, PCI, is the preferred revascularization strategy for most cancer patients with CAD, especially if their malignancy is aggressive or widespread. Furthermore, if STEMI or high risk non-STEMI occurs in cancer patients with an expected life span of <1 year, PCI should also be considered.109 A lower mortality rate was found among cancer patients undergoing PCI regardless of cancer types. In addition, a study comprising of 49,515 cancer patients with ACS found that the in-hospital mortality rate of patients undergoing PCI was significantly lower than that of patients receiving conservative medical therapy.110 Thus, hemoglobin, platelet count, and coagulation tests should be timely assessed in cancer patients before PCI. Cancer patients diagnosed within 6 months prior to ACS presentation appear to have the highest risk of mortality among all patients. They are also more susceptible to hemodynamic disorder and often need hemodynamic support.
Thrombocytopenia has been identified in most patients with acute leukemia, lymphoma, and multiple myeloma. However, 10%–25% of patients with solid tumors after chemotherapy may also have this condition111 and because of the effects of chemotherapy, hypohemoglobin is also possible.112 If thrombocytopenia and hypohemoglobin occur simultaneously, the cessation of antiplatelet and invasive therapy would be required. Although thrombocytopenia cannot prevent the development of myocardial ischemia in cancer patients, it can contribute to thrombus formation.113,114 Therefore, thrombocytopenia is not an absolute contraindication for intervention therapy. When platelet count is > 50,000/mL and coagulation abnormality does not occur, the standard dose of heparin (50–70 U/kg) or bivalirudin can be used as the anticoagulation treatment during PCI; however, if platelet count is < 50,000/mL, the initial dose of heparin should be reduced (30–50 U/kg) and an additional heparin dose be added when ACT is less than 250 s during the procedure.115,116 Currently, preventive platelet transfusion is not recommended; instead, it should be considered when: (a) platelet count is < 20,000/mL with at least one of the following situations (high fever, leukocytosis, rapid decrease of platelet count, and other abnormal coagulation function); and (b) patients with solid tumors have a platelet count <20,000/mL. Therapeutic platelet transfusion is only recommended in thrombocytopenia patients who develop bleeding during or after cardiac catheterization.
Radial access is the primary choice for PCI as it reduces bleeding complications. However, femoral artery access is the preferred choice for patients that fail both arms of the Allen's test, are on regular hemodialysis, and have undergone bilateral mastectomy.117 Moreover, balloon dilatation alone is preferred for patients requiring cancer surgery in the near future, and stent placement can be performed after the surgery, if necessary. To prevent the increased risk of bleeding from long-term dual antiplatelet therapy (DAPT), bare metal stents are preferred; however, the risk of restenosis is higher with the stents.118 Currently, the duration of DAPT can be remarkably shortened following the implantation of the new generation of drug eluting stents (DES). Therefore, it is recommended that the new generation DES119 be selected for cancer patients. However, drug coated balloon (DCB) can also be attempted to shorten the DAPT duration in some patients. Intravascular imaging, including intravascular ultrasound (IVUS) or optical coherence tomography (OCT), should be performed to optimize PCI, and sometimes, functional tests, including flow fraction reserve (FFR) and instant flow reserve (iFR), should be performed to determine the hemodynamic relevance of coronary artery stenoses in cancer patients. As anticancer therapy often leads to an impaired immune system, vascular closure devices should be avoided in cancer patients to prevent local infection.120 However, rotational atherectomy is recommended for severe calcified lesions.
The current SCAI consensus for cancer patients with CAD provides the following recommendations: aspirin administration when platelet count is >10,000/mL; DAPT with aspirin and clopidogrel administration if platelet count is >30,000/mL; and the administration of the new potent ADP receptor antagonists, such as ticagrelor and prasugrel, only when platelet count is >50,000/mL.121 Previously, we found that a half dose of ticagrelor exhibited the same antiplatelet effect as its standard dose,122 and 1/4 of its standard dose displayed a significantly better antiplatelet effect than the standard dose of clopidogrel.123 Therefore, the dosage of new antiplatelet drugs should be reduced in cancer patients with CAD, but the optimal dosage must be confirmed via future studies.
Generally, PCI is safe in cancer patients with a platelet count >50,000/mL; however, balloon dilatation alone, bare metal stent (BMS), DCB, and the new generation DES are the preferred choices of treatment. DAPT duration can be appropriately shortened in cancer patients with a high risk of bleeding after PCI. However, for patients that undergo balloon dilatation alone, BMS implantation or DCB, and the new generation DES implantation, DAPT should be administered for 2 weeks, 4 weeks, and 6 months, respectively, if platelet count is <50,000/mL and optimal stent expansion has been confirmed by IVUS or OCT.124 Intravascular imaging modalities and functional tests aid in PCI optimization. If the endothelialization of stents is delayed and the risk of stent thrombosis is increased by active chemotherapy, prolonging DAPT for an appropriate duration may be considered. After PCI, the antithrombotic therapy administered to cancer patients using cisplatin and thalidomide should be strengthened because of the pro-thrombosis effects.
Coronary artery bypass grafting (CABG)
For cancer patients displaying good survival and are appropriately indicated, CABG should be considered to reduce the accompanied cardiac complications during or after noncardiac surgery. Additionally, the stage of cancer and the general health condition of the patient are important considerations prior to CABG. CABG can be simultaneously performed with lung cancer surgery via the same incision.125,126 The simultaneous performance of CABG and tumor resection is beneficial as repeated thoracotomy is avoided, which leads to reduced complications and hospitalization costs. However, to avoid the risk of mediastinitis, the resection of gastrointestinal tumors should not be simultaneously performed with CABG.127 CABG may be difficult in patients administered radiotherapy as they have a high incidence of mediastinal fibrosis. Because of this condition, the use of internal mammary artery as a bridging vessel should be avoided in patients administered radiotherapy.128
Conclusions
Cancer and CAD share some common risk factors. In addition, they have the same underlying pathogenesis. Although substantial advancements have been made in cancer treatment, the number of cancer patients with concomitant CAD has been increasing. Owing to the complex condition of these patients, it is imperative that the oncology department and cardiovascular department work together to derive optimal specialized cardiac care for patients. Moreover, current guidelines are not suited for cancer patients with CAD. Thus, both basic and clinical research are required to develop diagnostic and therapeutic guidelines for cancer patients with CAD.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No.81830012).
Conflict of interest
None.
Edited by Yan-Gang Ren and Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Miller K.D., Siegel R.L., Lin C.C. Cancer treatment and survivorship statistics, 2016. CA A Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Lyon A.R. Cardiovascular disease following breast cancer treatment: can we predict who will be affected? Eur Heart J. 2019;40:3921–3923. doi: 10.1093/eurheartj/ehz598. [DOI] [PubMed] [Google Scholar]
- 3.May D., Wandl U., Becher R., Niederle N., Schmidt C.G. [Cardiac side effects of 5-fluorouracil] Dtsch Med Wochenschr. 1990;115:618–621. doi: 10.1055/s-2008-1065055. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA A Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 5.Strongman H., Gadd S., Matthews A. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navi B.B., Reiner A.S., Kamel H. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna A., Pequeno P., Gupta S. Increased risk of all cardiovascular disease subtypes among childhood cancer survivors: population-based matched cohort study. Circulation. 2019;140:1041–1043. doi: 10.1161/CIRCULATIONAHA.119.041403. [DOI] [PubMed] [Google Scholar]
- 8.Berton G., Cordiano R., Cavuto F., Bagato F., Segafredo B., Pasquinucci M. Neoplastic disease after acute coronary syndrome: incidence, duration, and features: the ABC-4* Study on Heart Disease. J Cardiovasc Med (Hagerstown) 2018;19:546–553. doi: 10.2459/JCM.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 9.Landes U., Kornowski R., Bental T. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis. 2017;28:5–10. doi: 10.1097/MCA.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S.W., Daraban N., Abbasi N., Lei X., Durand J.B., Daher I.N. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35:443–450. doi: 10.1002/clc.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannaccone M., D'Ascenzo F., Vadalà P. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care. 2018;7:631–638. doi: 10.1177/2048872617706501. [DOI] [PubMed] [Google Scholar]
- 12.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 13.Cespedes Feliciano E.M., Chen W.Y., Bradshaw P.T. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J Clin Oncol. 2019;37:2528–2536. doi: 10.1200/JCO.19.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher E.J., LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95:727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanfilippo K.M., McTigue K.M., Fidler C.J. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension. 2014;63:934–941. doi: 10.1161/HYPERTENSIONAHA.113.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues Dos Santos C., Fonseca I., Dias S., Mendes de Almeida J.C. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Canc. 2014;14:132. doi: 10.1186/1471-2407-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cedó L., Reddy S.T., Mato E., Blanco-Vaca F., Escolà-Gil J.C. HDL and LDL: potential new players in breast cancer development. J Clin Med. 2019;8:853. doi: 10.3390/jcm8060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagnardi V., Rota M., Botteri E. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. 2013;24:301–308. doi: 10.1093/annonc/mds337. [DOI] [PubMed] [Google Scholar]
- 19.Hojman P., Gehl J., Christensen J.F., Pedersen B.K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metabol. 2018;27:10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Moore S.C., Lee I.M., Weiderpass E. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K., Tripathy D., Demark-Wahnefried W. Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 2019;5:710–714. doi: 10.1001/jamaoncol.2019.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aune D., Giovannucci E., Boffetta P. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazidi M., Katsiki N., Mikhailidis D.P., Sattar N., Banach M. Lower carbohydrate diets and all-cause and cause-specific mortality: a population-based cohort study and pooling of prospective studies. Eur Heart J. 2019;40:2870–2879. doi: 10.1093/eurheartj/ehz174. [DOI] [PubMed] [Google Scholar]
- 24.Budhathoki S., Sawada N., Iwasaki M. Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern Med. 2019;179:1509–1518. doi: 10.1001/jamainternmed.2019.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abolhasani S., Shahbazloo S.V., Saadati H.M., Mahmoodi N., Khanbabaei N. Evaluation of serum levels of inflammation, fibrinolysis and oxidative stress markers in coronary artery disease prediction: a cross-sectional study. Arq Bras Cardiol. 2019;113:667–674. doi: 10.5935/abc.20190159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van't Klooster C.C., Ridker P.M., Hjortnaes J. The relation between systemic inflammation and incident cancer in patients with stable cardiovascular disease: a cohort study. Eur Heart J. 2019;40:3901–3909. doi: 10.1093/eurheartj/ehz587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 28.Ridker P.M., MacFadyen J.G., Thuren T. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 29.Zińczuk J., Maciejczyk M., Zaręba K. Antioxidant barrier, redox status, and oxidative damage to biomolecules in patients with colorectal cancer. Can malondialdehyde and catalase Be markers of colorectal cancer advancement. Biomolecules. 2019;9 doi: 10.3390/biom9100637. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarantini L., Gulizia M.M., Di Lenarda A. ANMCO/AIOM/AICO Consensus Document on clinical and management pathways of cardio-oncology: executive summary. Eur Heart J Suppl. 2017;19:D370–D379. doi: 10.1093/eurheartj/sux019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polk A., Vistisen K., Vaage-Nilsen M., Nielsen D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:47. doi: 10.1186/2050-6511-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong J.H., Ghosh A.K. Coronary artery vasospasm induced by 5-fluorouracil: proposed mechanisms, existing management options and future directions. Interv Cardiol. 2019;14:89–94. doi: 10.15420/icr.2019.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fajardo L.F., Stewart J.R. Pathogenesis of radiation-induced myocardial fibrosis. Lab Investig. 1973;29:244–257. [PubMed] [Google Scholar]
- 34.Lestuzzi C., Vaccher E., Talamini R. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol. 2014;25:1059–1064. doi: 10.1093/annonc/mdu055. [DOI] [PubMed] [Google Scholar]
- 35.Kosmas C., Kallistratos M.S., Kopterides P. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008;134:75–82. doi: 10.1007/s00432-007-0250-9. [DOI] [PubMed] [Google Scholar]
- 36.Saif M.W., Shah M.M., Shah A.R. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8:191–202. doi: 10.1517/14740330902733961. [DOI] [PubMed] [Google Scholar]
- 37.Gietema J.A., Meinardi M.T., Messerschmidt J. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet. 2000;355:1075–1076. doi: 10.1016/s0140-6736(00)02044-4. [DOI] [PubMed] [Google Scholar]
- 38.Huddart R.A., Norman A., Shahidi M. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513–1523. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 39.van den Belt-Dusebout A.W., Nuver J., de Wit R. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2006;24:467–475. doi: 10.1200/JCO.2005.02.7193. [DOI] [PubMed] [Google Scholar]
- 40.Feldman D.R., Schaffer W.L., Steingart R.M. Late cardiovascular toxicity following chemotherapy for germ cell tumors. J Natl Compr Cancer Netw. 2012;10:537–544. doi: 10.6004/jnccn.2012.0051. [DOI] [PubMed] [Google Scholar]
- 41.Karabay K.O., Yildiz O., Aytekin V. Multiple coronary thrombi with cisplatin. J Invasive Cardiol. 2014;26:E18–E20. [PubMed] [Google Scholar]
- 42.Moore R.A., Adel N., Riedel E. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jafri M., Protheroe A. Cisplatin-associated thrombosis. Anti Cancer Drugs. 2008;19:927–929. doi: 10.1097/CAD.0b013e3283100e9c. [DOI] [PubMed] [Google Scholar]
- 44.Dieckmann K.P., Gerl A., Witt J., Hartmann J.T., German Testicular Cancer Study Group Myocardial infarction and other major vascular events during chemotherapy for testicular cancer. Ann Oncol. 2010;21:1607–1611. doi: 10.1093/annonc/mdp597. [DOI] [PubMed] [Google Scholar]
- 45.Ito D., Shiraishi J., Nakamura T. Primary percutaneous coronary intervention and intravascular ultrasound imaging for coronary thrombosis after cisplatin-based chemotherapy. Heart Vessel. 2012;27:634–638. doi: 10.1007/s00380-011-0222-5. [DOI] [PubMed] [Google Scholar]
- 46.Hjelle L.V., Gundersen P.O., Oldenburg J. Long-term platinum retention after platinum-based chemotherapy in testicular cancer survivors: a 20-year follow-up study. Anticancer Res. 2015;35:1619–1625. [PubMed] [Google Scholar]
- 47.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salem J.E., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomita Y., Sueta D., Kakiuchi Y. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann Oncol. 2017;28:2893–2895. doi: 10.1093/annonc/mdx326. [DOI] [PubMed] [Google Scholar]
- 50.Foks A.C., Kuiper J. Immune checkpoint proteins: exploring their therapeutic potential to regulate atherosclerosis. Br J Pharmacol. 2017;174:3940–3955. doi: 10.1111/bph.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Y.B., Zhang Q., Li H.J. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2017;6:8S8–8S20. doi: 10.21037/tlcr.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darby S.C., Cutter D.J., Boerma M. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart F.A., Heeneman S., Te Poele J. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649–658. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee M.S., Finch W., Mahmud E. Cardiovascular complications of radiotherapy. Am J Cardiol. 2013;112:1688–1696. doi: 10.1016/j.amjcard.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 55.Brosius F.C., 3rd, Waller B.F., Roberts W.C. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70:519–530. doi: 10.1016/0002-9343(81)90574-x. [DOI] [PubMed] [Google Scholar]
- 56.Veinot J.P., Edwards W.D. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 57.Küpeli S., Hazirolan T., Varan A. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin's lymphoma. J Clin Oncol. 2010;28:1025–1030. doi: 10.1200/JCO.2009.25.2627. [DOI] [PubMed] [Google Scholar]
- 58.van Nimwegen F.A., Schaapveld M., Janus C.P. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 59.Cutter D.J., Schaapveld M., Darby S.C. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107:djv008. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinou M., Gaya A. Cardiac complications after radical radiotherapy. Semin Oncol. 2013;40:178–185. doi: 10.1053/j.seminoncol.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Myrehaug S., Pintilie M., Yun L. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood. 2010;116:2237–2240. doi: 10.1182/blood-2010-01-263764. [DOI] [PubMed] [Google Scholar]
- 62.Ewer M.S., Ewer S.M. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:620. doi: 10.1038/nrcardio.2015.133. [DOI] [PubMed] [Google Scholar]
- 63.Chen X.L., Lei Y.H., Liu C.F. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: a meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schutz F.A., Je Y., Azzi G.R., Nguyen P.L., Choueiri T.K. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- 65.Scappaticci F.A., Skillings J.R., Holden S.N. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 66.Sen F., Yildiz I., Basaran M. Impaired coronary flow reserve in metastatic cancer patients treated with sunitinib. J BUON. 2013;18:775–781. [PubMed] [Google Scholar]
- 67.Kappers M.H., van Esch J.H., Sluiter W., Sleijfer S., Danser A.H., van den Meiracker A.H. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–681. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 68.Chintalgattu V., Rees M.L., Culver J.C. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005066. 187ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kappers M.H., de Beer V.J., Zhou Z. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59:151–157. doi: 10.1161/HYPERTENSIONAHA.111.182220. [DOI] [PubMed] [Google Scholar]
- 70.Arima Y., Oshima S., Noda K. Sorafenib-induced acute myocardial infarction due to coronary artery spasm. J Cardiol. 2009;54:512–515. doi: 10.1016/j.jjcc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Porto I., Leo A., Miele L., Pompili M., Landolfi R., Crea F. A case of variant angina in a patient under chronic treatment with sorafenib. Nat Rev Clin Oncol. 2010;7:476–480. doi: 10.1038/nrclinonc.2010.67. [DOI] [PubMed] [Google Scholar]
- 72.Pantaleo M.A., Mandrioli A., Saponara M. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC Canc. 2012;12:231. doi: 10.1186/1471-2407-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Je Y., Schutz F.A., Choueiri T.K. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 74.Quintás-Cardama A., Kantarjian H., Cortes J. Nilotinib-associated vascular events. Clin Lymphoma, Myeloma & Leukemia. 2012;12:337–340. doi: 10.1016/j.clml.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Amsterdam E.A., Wenger N.K., Brindis R.G. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 76.Peretto G., Lazzeroni D., Sartorio C.L., Camici P.G. Cardiotoxicity in oncology and coronary microcirculation: future challenges in theranostics. Front Biosci (Landmark Ed). 2017;22:1760–1773. doi: 10.2741/4570. [DOI] [PubMed] [Google Scholar]
- 77.Munoz E., Iliescu G., Vejpongsa P. Takotsubo stress cardiomyopathy: “good news” in cancer patients. J Am Coll Cardiol. 2016;68:1143–1144. doi: 10.1016/j.jacc.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 78.Sattler K., El-Battrawy I., Lang S. Prevalence of cancer in Takotsubo cardiomyopathy: short and long-term outcome. Int J Cardiol. 2017;238:159–165. doi: 10.1016/j.ijcard.2017.02.093. [DOI] [PubMed] [Google Scholar]
- 79.da Silva Costa I., Figueiredo C.S., Fonseca S. Takotsubo syndrome: an overview of pathophysiology, diagnosis and treatment with emphasis on cancer patients. Heart Fail Rev. 2019;24:833–846. doi: 10.1007/s10741-019-09813-1. [DOI] [PubMed] [Google Scholar]
- 80.Rodríguez-Ruiz C., Puig-Carrión G., Delgado-Nieves A., López-Candales A. Kounis syndrome: a more commonly encountered cause of acute coronary syndrome. Heart Views. 2019;20:122–125. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_43_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Connor R.D., Hofland E., Latten G., Pluijms W.A., Ruiters A.W., Hoofwijk D. [Kounis syndrome, the allergic acute coronary syndrome] Ned Tijdschr Geneeskd. 2019;163 [PubMed] [Google Scholar]
- 82.Picano E., Mathias W., Jr., Pingitore A., Bigi R., Previtali M. Safety and tolerability of dobutamine-atropine stress echocardiography: a prospective, multicentre study. Echo Dobutamine International Cooperative Study Group. Lancet. 1994;344:1190–1192. doi: 10.1016/s0140-6736(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 83.Sicari R., Nihoyannopoulos P., Evangelista A. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur J Echocardiogr. 2008;9:415–437. doi: 10.1093/ejechocard/jen175. [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann U., Truong Q.A., Schoenfeld D.A. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Picano E., Sicari R., Landi P. Prognostic value of myocardial viability in medically treated patients with global left ventricular dysfunction early after an acute uncomplicated myocardial infarction: a dobutamine stress echocardiographic study. Circulation. 1998;98:1078–1084. doi: 10.1161/01.cir.98.11.1078. [DOI] [PubMed] [Google Scholar]
- 86.Mahabadi A.A., Möhlenkamp S., Lehmann N. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. JACC Cardiovasc Imaging. 2017;10:143–153. doi: 10.1016/j.jcmg.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 87.Authors/Task Force members. Windecker S., Kolh P. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS)developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 88.Imbert L., Marie P.Y. CZT cameras: a technological jump for myocardial perfusion SPECT. J Nucl Cardiol. 2016;23:894–896. doi: 10.1007/s12350-015-0216-2. [DOI] [PubMed] [Google Scholar]
- 89.Palumbo I., Palumbo B., Fravolini M.L. Brain natriuretic peptide as a cardiac marker of transient radiotherapy-related damage in left-sided breast cancer patients: a prospective study. Breast. 2016;25:45–50. doi: 10.1016/j.breast.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 90.Pavo N., Raderer M., Hülsmann M. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 91.Cardinale D., Colombo A., Sandri M.T. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 92.Cardinale D., Salvatici M., Sandri M.T. Role of biomarkers in cardioncology. Clin Chem Lab Med. 2011;49:1937–1948. doi: 10.1515/CCLM.2011.692. [DOI] [PubMed] [Google Scholar]
- 93.Christenson E.S., James T., Agrawal V., Park B.H. Use of biomarkers for the assessment of chemotherapy-induced cardiac toxicity. Clin Biochem. 2015;48:223–235. doi: 10.1016/j.clinbiochem.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O'Donoghue M., de Lemos J.A., Morrow D.A. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–557. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 95.Endo H., Dohi T., Funamizu T. Long-term predictive value of high-sensitivity C-reactive protein for cancer mortality in patients undergoing percutaneous coronary intervention. Circ J. 2019;83:630–636. doi: 10.1253/circj.CJ-18-0962. [DOI] [PubMed] [Google Scholar]
- 96.Lancellotti P., Nkomo V.T., Badano L.P. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Lancellotti P., Nkomo V.T., Badano L.P. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 98.Levine G.N., Bates E.R., Blankenship J.C. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on clinical Practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2016;133:1135–1147. doi: 10.1161/CIR.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 99.Rubens M., Appunni S., Ramamoorthy V. Prevalence of cardiovascular risk factors among cancer patients in the United States. Metab Syndrome Relat Disord. 2019;17:397–405. doi: 10.1089/met.2018.0137. [DOI] [PubMed] [Google Scholar]
- 100.Sudhakar R. Response to treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35:646. doi: 10.1002/clc.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nielsen S.F., Nordestgaard B.G., Bojesen S.E. Statin use and reduced cancer-related mortality. N Engl J Med. 2013;368:576–577. doi: 10.1056/NEJMc1214827. [DOI] [PubMed] [Google Scholar]
- 102.Raebel M.A., Zeng C., Cheetham T.C. Risk of breast cancer with long-term use of calcium channel blockers or angiotensin-converting enzyme inhibitors among older women. Am J Epidemiol. 2017;185:264–273. doi: 10.1093/aje/kww217. [DOI] [PubMed] [Google Scholar]
- 103.Afzal M.Z., Dragnev K., Sarwar T., Shirai K. Clinical outcomes in non-small-cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag. 2019;8:LMT11. doi: 10.2217/lmt-2018-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shukla S.K., Kulkarni N.S., Chan A. Metformin-encapsulated liposome delivery system: an effective treatment approach against breast cancer. Pharmaceutics. 2019;11:E559. doi: 10.3390/pharmaceutics11110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Courtois S., Lehours P., Bessède E. The therapeutic potential of metformin in gastric cancer. Gastric Cancer. 2019;22:653–662. doi: 10.1007/s10120-019-00952-w. [DOI] [PubMed] [Google Scholar]
- 106.Pottegård A., Hallas J., Olesen M. Hydrochlorothiazide use is strongly associated with risk of lip cancer. J Intern Med. 2017;282:322–331. doi: 10.1111/joim.12629. [DOI] [PubMed] [Google Scholar]
- 107.van Veelen A., Nielen J., van Geel R., Croes S. Response to 'Hydrochlorothiazide use and risk of nonmelanoma skin cancer: a nationwide case-control study from Denmark. J Am Acad Dermatol. 2019;S0190–9622(19):30274–30279. doi: 10.1016/j.jaad.2019.01.087. [DOI] [PubMed] [Google Scholar]
- 108.Oren O., Herrmann J. Arterial events in cancer patients-the case of acute coronary thrombosis. J Thorac Dis. 2018;10:S4367–S4385. doi: 10.21037/jtd.2018.12.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vieira R.D., Pereira A.C., Lima E.G. Cancer-related deaths among different treatment options in chronic coronary artery disease: results of a 6-year follow-up of the MASS II study. Coron Artery Dis. 2012;23:79–84. doi: 10.1097/MCA.0b013e32834f112a. [DOI] [PubMed] [Google Scholar]
- 110.Guddati A.K., Joy P.S., Kumar G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J Cancer Res Clin Oncol. 2016;142:471–479. doi: 10.1007/s00432-015-2056-5. [DOI] [PubMed] [Google Scholar]
- 111.Elting L.S., Rubenstein E.B., Martin C.G. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–1146. doi: 10.1200/JCO.2001.19.4.1137. [DOI] [PubMed] [Google Scholar]
- 112.McKechnie R.S., Smith D., Montoye C. Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation. 2004;110:271–277. doi: 10.1161/01.CIR.0000134964.01697.C7. [DOI] [PubMed] [Google Scholar]
- 113.Al-Hijji M.A., Gulati R., Lennon R.J. Outcomes of percutaneous coronary interventions in patients with anemia presenting with acute coronary syndrome. Mayo Clin Proc. 2018;93:1448–1461. doi: 10.1016/j.mayocp.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 114.Sarkiss M.G., Yusuf S.W., Warneke C.L. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer. 2007;109:621–627. doi: 10.1002/cncr.22434. [DOI] [PubMed] [Google Scholar]
- 115.Yusuf S.W., Iliescu C., Bathina J.D., Daher I.N., Durand J.B. Antiplatelet therapy and percutaneous coronary intervention in patients with acute coronary syndrome and thrombocytopenia. Tex Heart Inst J. 2010;37:336–340. [PMC free article] [PubMed] [Google Scholar]
- 116.Iliescu C., Durand J.B., Kroll M. Cardiovascular interventions in thrombocytopenic cancer patients. Tex Heart Inst J. 2011;38:259–260. [PMC free article] [PubMed] [Google Scholar]
- 117.Rao S.V., Tremmel J.A., Gilchrist I.C. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention's transradial working group. Cathet Cardiovasc Interv. 2014;83:228–236. doi: 10.1002/ccd.25209. [DOI] [PubMed] [Google Scholar]
- 118.Krone R.J. Managing coronary artery disease in the cancer patient. Prog Cardiovasc Dis. 2010;53:149–156. doi: 10.1016/j.pcad.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 119.Ariotti S., Adamo M., Costa F. Is bare-metal stent implantation still justifiable in high bleeding risk patients undergoing percutaneous coronary intervention?: a pre-specified analysis from the ZEUS trial. JACC Cardiovasc Interv. 2016;9:426–436. doi: 10.1016/j.jcin.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 120.Krishnasamy V.P., Hagar M.J., Scher D.J., Sanogo M.L., Gabriel G.E., Sarin S.N. Vascular closure devices: technical tips, complications, and management. Tech Vasc Interv Radiol. 2015;18:100–112. doi: 10.1053/j.tvir.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 121.McCarthy C.P., Steg G., Bhatt D.L. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J. 2017;38:3488–3492. doi: 10.1093/eurheartj/ehx531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xue H.J., Shi J., Liu B. Comparison of half- and standard-dose ticagrelor in Chinese patients with NSTE-ACS. Platelets. 2016;27:440–445. doi: 10.3109/09537104.2015.1135890. [DOI] [PubMed] [Google Scholar]
- 123.He M., Liu B., Sun D. Corrigendum to “One-quarter standard-dose ticagrelor better than standard-dose clopidogrel in Chinese patients with stable coronary artery disease: a randomized, single-blind, crossover clinical study” [Int. J. Cardiol. 215 (2016) 209-213] Int J Cardiol. 2017;227:956. doi: 10.1016/j.ijcard.2016.04.087. [DOI] [PubMed] [Google Scholar]
- 124.Iliescu C.A., Grines C.L., Herrmann J. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of India, and sociedad Latino Americana de Cardiologıa intervencionista) Cathet Cardiovasc Interv. 2016;87(5):E202–E223. doi: 10.1002/ccd.26379. [DOI] [PubMed] [Google Scholar]
- 125.Ozsöyler I., Yilik L., Bozok S. Off-pump coronary artery bypass surgery in patients with coronary artery disease and malign neoplasia: results of ten patients and review of the literature. Heart Vessel. 2006;21:365–367. doi: 10.1007/s00380-006-0913-5. [DOI] [PubMed] [Google Scholar]
- 126.Schoenmakers M.C., van Boven W.J., van den Bosch J., van Swieten H.A. Comparison of on-pump or off-pump coronary artery revascularization with lung resection. Ann Thorac Surg. 2007;84:504–509. doi: 10.1016/j.athoracsur.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Tsuji Y., Morimoto N., Tanaka H. Surgery for gastric cancer combined with cardiac and aortic surgery. Arch Surg. 2005;140:1109–1114. doi: 10.1001/archsurg.140.11.1109. [DOI] [PubMed] [Google Scholar]
- 128.Handa N., McGregor C.G., Danielson G.K. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg. 1999;117:1136–1142. doi: 10.1016/s0022-5223(99)70250-3. [DOI] [PubMed] [Google Scholar]