Abstract
Objective
Post–bariatric surgery hypoglycemia (PBH) is defined as the presence of neuroglycopenic symptoms accompanied by postprandial hypoglycemia in bariatric surgery patients. Recent clinical studies using continuous glucose monitoring (CGM) technology revealed that PBH is more frequently observed in vertical sleeve gastrectomy (VSG) patients than previously recognized. PBH cannot be alleviated by current medication. Therefore, a model system to investigate the mechanism and treatment is required.
Methods
We used CGM in a rat model of VSG and monitored the occurrence of glycemic variability and hypoglycemia in various meal conditions for 4 weeks after surgery. Another cohort of VSG rats with CGM was used to investigate whether the blockade of glucagon-like peptide-1 receptor (GLP-1R) signaling alleviates these symptoms. A mouse VSG model was used to investigate whether the impaired glucose counterregulatory system causes postprandial hypoglycemia.
Results
Like in humans, rats have increased glycemic variability and hypoglycemia after VSG. Postprandial hypoglycemia was specifically detected after liquid versus solid meals. Further, the blockade of GLP-1R signaling raises the glucose nadir but does not affect glycemic variability.
Conclusions
Rat bariatric surgery duplicates many features of human post–bariatric surgery hypoglycemia including postprandial hypoglycemia and glycemic variability, while blockade of GLP-1R signaling prevents hypoglycemia but not the variability.
Keywords: Continuous glucose monitoring, Vertical sleeve gastrectomy, Post–bariatric surgery hypoglycemia, Glycemic variability, Exendin 9-39, Glucagon-like peptide-1 receptor
Abbreviations: PBH, post–bariatric surgery hypoglycemia; CGM, continuous glucose monitoring; VSG, vertical sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; OGTT, oral glucose tolerance test; Ex9, exendin 9-39; GLP-1R, glucagon-like peptide-1 receptor; DPP-4, dipeptidyl peptidase-4; 2DG, 2-deoxyglucose; PYY, peptide YY; GIP, gastric inhibitory polypeptide; MAG, mean absolute glucose; NE, norepinephrine; Epi, epinephrine
Highlights
-
•
VSG causes glycemic variability during ad lib feeding condition.
-
•
Single liquid meal ingestion causes post-VSG hypoglycemia.
-
•
Blockade of GLP-1 receptor prevents post-VSG hypoglycemia.
1. Introduction
Vertical sleeve gastrectomy (VSG) is currently the most frequently performed bariatric surgery in the world [1,2]. VSG surgically removes approximately 80% of the stomach along the greater curvature and results in 81% excess body weight loss and a 31% remission rate of type 2 diabetes mellitus, as demonstrated by a dramatic reduction in glycosylated hemoglobin (HbA1c%; 9.5% to 6.6%) within 1 year after surgery [3].
With the rising use of bariatric surgery, there is also greater awareness of associated complications. One increasingly recognized complication is post–bariatric surgery hypoglycemia (PBH) [4]. Given that adrenergic and cholinergic symptoms in the postprandial state can be nonspecific, it has recently been proposed that PBH be strictly defined as the presence of neuroglycopenic symptoms (difficulty thinking, weakness, fatigue) with concomitant hypoglycemia (<54 mg/dL = 3 mmol/L) [4] that is relieved within minutes of carbohydrate ingestion. Episodes of hypoglycemia impair cognition and increase the risk for syncope, cardiac arrhythmias, seizures, coma, and even death. Moreover, many patients are disabled because of the inability to perform job-related tasks and inability to safely operate a motor vehicle. While hypoglycemia can frequently occur in both type 1 and type 2 diabetic patients, medication or lifestyle modifications can be effectively used to reduce recurrence. This is not the case for bariatric surgery patients.
Early studies regarding PBH have focused on postprandial hypoglycemia seen after bariatric surgery. Initially, the prevalence of hypoglycemia after bariatric surgery was thought to be rare; <1% of patients were hospitalized for hypoglycemia [5]. However, the use of continuous glucose monitoring (CGM) in bariatric surgery patients has led to the finding that postprandial hypoglycemia occurs more frequently than previously recognized and occurs in both Roux-en-Y gastric bypass (RYGB) and VSG. A recent study demonstrated that postprandial hypoglycemia was detected in ~55% of VSG patients when assessed by CGM, whereas it was detected in ~24% when assessed using the glucose responses to an oral glucose tolerance test (OGTT) or via questionnaires [6]. Another study using CGM found that postprandial hypoglycemia occurred in 75% of RYGB patients, while in the same population, a mixed-meal tolerance test induced a hypoglycemic event in 29% of patients [7]. These results underscore the importance of CGM in diagnosing PBH and highlights that the incidence is much higher than previously recognized.
The mechanisms for PBH remain unknown, and the fact that the symptoms take months or even years to arise make the condition even more perplexing. Increased gastric emptying (i.e., dumping syndrome) has been implicated, but PBH is not always associated with meals [4]. The elevated gastric emptying contributes to increased peak glucose levels and consequently insulin levels, two parameters that have been found to be even greater in PBH-susceptible patients after RYGB [8,9]. Thus, reducing postprandial peak glucose levels and concomitant insulin levels by reducing simple carbohydrates in the diet or by adding acarbose to slow carbohydrate absorption are common initial strategies [10]. Pharmacological strategies have been applied to specifically reduce postprandial insulin levels, including somatostatin receptor analogues [11] or diazoxide [12]. However, these strategies are beneficial in only some patients. Unfortunately, all of these therapies are limited by side effects or incomplete efficacy, even in combination. Although there was initial enthusiasm for partial pancreatectomy to reduce islet mass, recurrence of hypoglycemia with time was observed, underscoring the complex interactions beyond insulin secretion that contribute to hypoglycemia [13].
The increased postprandial levels of insulin following VSG and RYGB (reviewed in [14]) have been highly associated with increased postprandial levels of the incretin glucagon-like peptide-1 (GLP-1) [15], which has also been found to be greater in PBH-susceptible patients after RYGB [8,9]. Furthermore, multiple studies have shown that administration of exendin 9-39 (Ex9), a potent GLP-1 receptor (GLP-1R) antagonist, blunted postprandial hypoglycemia [16]. Blocking GLP-1R with Ex9 also corrected the PBH-associated autonomic symptoms, neuroglycopenic symptoms, and symptoms of malaise [17].
We hypothesized that CGM in a rat model of VSG will repeat the increased glycemic variability and occurrence of hypoglycemia that occurs clinically. Using multiple paradigms of meal intake studies and pharmacological studies that induce glucose counterregulation or that antagonize GLP-1R, we attempted to unveil the mechanisms underlying the glycemic variability and postprandial hypoglycemia seen after VSG.
2. Materials and methods
2.1. Study design
We conducted 3 separate studies. 1) We used CGM to determine whether the rat VSG model recapitulates human PBH and glycemic variability and whether the type of meal or feeding paradigm exacerbated hypoglycemia and/or glycemic variability. 2) As we found postprandial hypoglycemia after VSG, we then determined whether VSG alters glucose counterregulatory hormonal responses. 3) Lastly, we investigated whether GLP-1R signaling is necessary for PBH and glycemic variability.
In the first study, we used two separate cohorts of rats that had sham (n = 6) versus VSG (n = 8) surgery with concurrent implantation of a glucose telemetry probe (see below for more details). This number of animals was chosen based on the number of telemetry probes we had available at the time of surgery. After surgery, the rats’ glycemic values were continuously monitored for 30 days. We analyzed the data during different postoperative time points and under different feeding conditions. At day 14 postsurgery, all animals were fasted for 5 h starting at 8 AM and received a 4 mL/kg administration of dextrose (2 g/kg) via gavage. To calibrate the glucose telemetry probe, a tail vein blood sample was taken prior to and 15 min after glucose administration, and blood glucose was measured using Accu-Chek® glucose strips and glucometer (Roche Diagnostics, Indianapolis, IN, USA). At days 18–20, food was removed at 12 PM, and solid food was returned at the onset of the dark phase. Food was weighed 2 and 18 h after food was returned. At day 21, food was removed at 12 PM, but only 1.5 g of food (calculated based on food intake during 2 h over the 3 previous days) was returned at the onset of the dark phase. All animals ate 1.5 g during 2-h access, and food was returned ad libitum. At days 23–25, food was removed at 1 PM and a bottle containing a liquid diet (Ensure Plus®) was returned at the onset of the dark phase. Liquid diet intake was measured at 2 and 18 h after access, during which animals did not have access to a solid diet. At day 26, the liquid diet was removed at 1 PM, and a fixed amount of 2 mL Ensure Plus® was administered per gavage at the onset of the dark phase. Solid food was returned and available ad libitum 2 h postgavage. Like day 14, at day 28 animals received a glucose administration per gavage, and telemetry glucose levels were compared with glucose levels measured from tail blood. At postsurgery day 35, animals had been fasted overnight, and ±500 μL tail blood was collected in EDTA-coated tubes containing aprotinin and dipeptidyl peptidase-4 (DPP-4) inhibitor. Consecutively, rats received 2 mL Ensure Plus® containing dextrose (500 mg) and acetaminophen (100 mg/kg of mean body weight [BW]) via gavage (total caloric load = 4.86 kcal) and were euthanized 15 min later using CO2. A cardiac puncture was performed to collect a second blood sample, and whole blood was centrifuged at 1000 ×g for 20 min at 4 °C. Plasma was stored at −80 °C until further analysis.
In study 2, we assessed whether neuroendocrine glucose counterregulation was disrupted after VSG. For this study, we performed 12 sham and 16 VSG surgeries in mice. Four weeks after surgery, the mice were injected with saline or 2-deoxyglucose (2DG, a nonmetabolizable glucose agent that blocks glycolysis and thus imitates the physiological impact of hypoglycemia) and plasma levels of glucagon, norepinephrine (NE), and epinephrine (Epi) were measured 30 min after the 2DG injection. The mice were euthanized by CO2 gas at 10 weeks after surgery. In the last study, another cohort of rats received a sham (n = 5) or VSG (n = 7) surgery with concurrent implantation of a glucose telemetry probe. This number of animals was chosen based on the number of telemetry probes we had available at the time of surgery. One animal from the VSG group died from unknown causes during the postoperative period, resulting in n = 6 in the VSG group. Glycemic values were monitored under ad libitum feeding or during an OGTT following saline or Ex9 treatment. Thirty-five days after surgery, the rats were injected with Ex9 prior to a mixed liquid meal gavage and euthanized by CO2 gas 15 min after the gavage. Blood was taken for subsequent analysis of glucose and gut peptide levels.
All of the studies described were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee at the University of Michigan. We followed protocols outlined in the National Institutes of Health (NIH) guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.2. Surgery
2.2.1. Telemetry probe implantation
Under isoflurane anesthesia, rats were shaved on the ventral abdomen, and buprenorphine hydrochloride (0.03 mg/kg), meloxicam (0.5 mg/kg), and 0.9% saline (10 mL) were administered subcutaneously. A midline laparotomy was performed, and the intestine was repositioned to the right and covered with moistened 2 × 2-inch gauze pads. A segment of the descending aorta was isolated just below the left renal vein and approximately 1 cm below that point, taking care to determine that there were no hidden arterial branches. A suture (6-0 Silk, Ethicon Endo-Surgery, Cincinnati, OH, USA) was passed under both sites and retracted with hemostats to restrict blood flow to the isolated aortic segment. A bent 25-gauge needle was inserted into the aorta, and the glucose sensor tip of an HD-XG rat glucose telemetry implant (Data Sciences International, St. Paul, MN) was inserted and then advanced. A small “V” was notched in the end of each of two pieces of cellulose fiber patch, and one was placed above the inserted tip with the other below, and both were brought together to form a tight seal with the catheter entry point. A drop of surgical glue (Vetbond, 3M St. Paul, MN, USA) was then applied to this region and allowed to dry. The back muscle wall was exposed, and two sutures (6-0 Prolene, Ethicon Endo-Surgery) were passed through and then threaded into the suture rib of the device and tied. The reference electrode was positioned along the abdominal wall and affixed with two sutures (6-0 Prolene, Ethicon Endo-Surgery). The intestines were replaced within the cavity, and the lead attached to the battery unit was then manipulated carefully to place the unit over the intestines and close to the laparotomy incision.
2.2.2. Rat vertical sleeve gastrectomy
Immediately following the probe implantation, the stomach was identified and exteriorized through the laparotomy incision, and all ligaments were cut, allowing the mobilized stomach to be placed on saline-moistened 4 × 4-inch gauze pads. A small incision was made in the fundus at the greater curvature to allow stomach contents to be extruded onto the gauze pads (resulting in a flat stomach), and any stomach contents were wiped away from the stomach surface. An Echelon Flex-35 Vascular Stapler loaded with a 35-mm staple load (Ethicon Endo-Surgery) was introduced below the esophagus, at the angle of His, and maneuvered until a sleeve of appropriate size, spanning the stomach width, was attained. The stapler was deployed according to manufacturer's instructions, and the excised stomach tissue was discarded. The sleeve was checked for bleeding/leaks, and any remaining gauze pads were discarded. For the sham surgery, gentle pressure was placed across the stomach using a pair of forceps. The stomach (sham) or sleeve (VSG) was replaced in the abdominal cavity, taking care not to disturb the telemetry probe and its associated components. The laparotomy incision was closed with a continuous suture (4-0 Vicryl Rapide, Ethicon Endo-Surgery), and the battery unit in the abdominal cavity was secured to the body wall by suturing through the two fastening eyelets. The skin incision was closed with a running subcutaneous suture (4-0 Vicryl Rapide, Ethicon Endo-Surgery), and the animal was returned to its housing cage and placed on a heating pad until it was mobile.
2.2.3. Mouse vertical sleeve gastrectomy
Under isoflurane anesthesia, mice were shaved on the ventral abdomen and injected with buprenorphine hydrochloride (0.10 mg/kg), meloxicam (0.5 mg/kg), gentamicin (8 mg/kg), and 0.9% saline (1 mL), all administered subcutaneously. A midline laparotomy was performed, and the stomach was identified and exteriorized through the incision. All ligaments were cut, allowing the mobilized stomach to be placed on saline-moistened 2 × 2-inch gauze pads. A small incision was made in the fundus at the greater curvature to allow stomach contents to be extruded onto the gauze pads (resulting in a flat stomach), and any stomach contents were wiped away from the stomach surface. An Echelon Flex-35 vascular stapler loaded with a 35-mm staple load (Ethicon Endo-Surgery) was introduced below the esophagus, at the angle of His, and maneuvered until a sleeve of appropriate size, spanning the stomach width, was attained. The stapler was deployed according to the manufacturer's instructions, and the excised stomach tissue was discarded. The sleeve was checked for bleeding/leaks, and any remaining gauze pads were discarded. For the sham surgery, gentle pressure was placed across the stomach using a pair of forceps. The stomach (sham) or sleeve (VSG) was replaced in the abdominal cavity, and the laparotomy incision was closed with a continuous suture (5-0 Vicryl Rapide, Ethicon Endo-Surgery). The skin incision was closed with a running subcutaneous suture (5-0 Vicryl Rapide, Ethicon Endo-Surgery), and the animal was returned to its housing cage and placed on a heating pad until it was mobile.
2.3. CGM
This study was performed on two separate cohorts of male Long Evans rats (Envigo, Indianapolis, IN, USA) aged 10–11 weeks at arrival. All animals were housed individually and had ad libitum access to Tso's 45% high-fat diet (HFD; Cat. No. D03082706, Research Diets, New Brunswick, NJ, USA) and water for 20 weeks prior to surgery. Animals on average weighed 704 ± 58 g prior to surgery. In cohort 1 (n = 5), n = 3 rats underwent VSG and n = 2 underwent a sham surgery with a Data Sciences International (DSI) radio-transmitter installed in the abdominal cavity of each (as described in more detail above). Cohort 2 comprised n = 5 rats that received VSG surgery and n = 4 that received sham surgery, again in combination with radio-transmitter installment. All animals were transferred to the telemetry room, where every cage was placed under a heat lamp for 24 h postsurgery. Room temperature was set to 21 °C, and lights were set on a 6 AM:6 PM light:dark cycle. All animals received postoperative care during the first 7 days postsurgery (see details below). Every cage was placed on a receiver plate. Data collection started at 5 PM on the day of surgery and was collected until day 28 postsurgery. The same protocol (Figure 1A) was applied to both cohorts. In general, all animals had ad libitum access to food and water throughout the study unless otherwise stated.
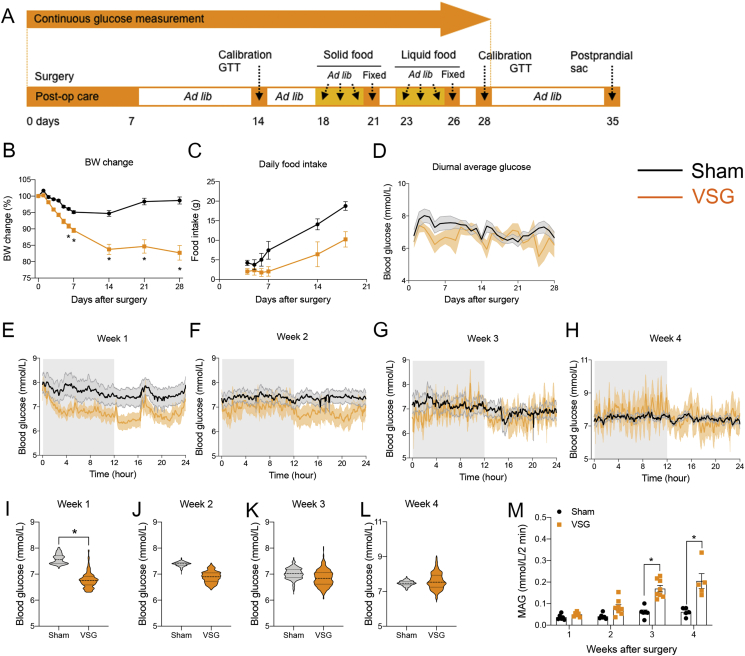
Figure 1.
CGM demonstrates glycemic variability after VSG in rat. (A) Schematic experimental timeline during CGM after surgery. (B–D) Body weight changes (B), daily food intake (C), and average glucose levels (D) across the 28 days after surgery. Mean ± SEM. *P < 0.05 for sham vs. VSG (mixed-effects model; time × surgery). (E–H) Diurnal average glucose levels during the first (E), second (F), third (G), and fourth (H) weeks after surgery. The shaded gray area reflects glucose levels during the dark cycle. Mean ± SEM. (I–L) Violin plots represents the median, quartiles, minimum, maximum, and distribution of diurnal glucose levels during the first (I), second (J), third (K), and fourth (L) weeks after surgery. *P < 0.05. (M) Mean absolute glucose (MAG) after surgery. Mean ± SEM. *P < 0.05 (mixed-effects model; time × surgery). All data were obtained from n = 6 sham and n = 8 VSG animals, except for the observations from the fourth week, which included n = 4 sham and n = 5 VSG animals.
We validated the CGM telemetry system with a conventional glucometer at 14 and 28 days after surgery. Briefly, rats were fasted overnight, and 2 mL of 50% dextrose solution was administered orally. At fasting (0 min) and 15 min after the dextrose gavage, blood glucose levels measured by telemetry (arterial glucose) versus glucometer (tail vein) were similar on both days (Supplementary Figure S1A and S1B). The glycemic responses to the dextrose gavage in sham and VSG rats were not different between 14 and 28 days after surgery (Supplementary Figure S1C).
2.4. Effect of Ex9 on glucose homeostasis in rats following VSG
Male Long Evans rats (n = 12, 10–12 weeks of age) were housed individually at arrival and had ad libitum access to Tso's 45% HFD and water for 10 weeks. Animals weighed 579 ± 37 g before VSG (n = 6) or sham (n = 5) surgery in combination with installment of DSI radio-transmitter glucose probe sensor. Following surgery, rats were housed and received the same postoperative treatment and glucose probe calibration as described above. At day 16, we injected saline or Ex9 to the rats that received sham or VSG surgery 15 min prior into the dark phase. The glycemic change was measured by the CGM system for 12 h under ad libitum feeding condition. We repeated this procedure for 3 consecutive days, and the glucose levels over the 12-h postinjection period for each of the 3 days were averaged. Nineteen days after surgery, the food was removed 6 h prior to the dark phase, and saline or Ex9 was administered to the rats 15 min prior to a glucose gavage (2 g/kg; 50% mass/volume ratio; 2 g/kg, 50% solution) and immediately before the dark phase. The glycemic patterns were measured by the CGM system, and the food was returned 2 h after glucose administration. The rats had a 4-day washout period, after which the drug treatments were crossed over, and the above experiment was repeated from days 24–27.
2.5. Mouse VSG 2DG-glucose administration
Four weeks after surgery, sham or VSG mice were given an Epi intraperitoneal (IP) injection of saline or 2DG (250 mg/kg, Sigma, St. Louis, MO, USA) after 4 h of fasting. Blood glucose was measured using Accu-Chek® glucose strips and glucometer (Roche Diagnostics) at 0, 30, 60, 90, and 120 min. Blood samples were collected at 0 and 30 min in EDTA-coated tubes containing aprotinin and a DPP-4 inhibitor to measure plasma levels of glucagon, Epi, and NE levels.
2.6. Determination of plasma epi and NE
To determine plasma Epi and NE concentrations, 9 μL of plasma was spiked with 1 μL of 12.5 mM ascorbic acid and 1 μL of a mixture containing 1 μM d6-Epi and d6-NE as internal standards to normalize for extraction efficiency and mass spectrometry ionization efficiency. Proteins were removed by the addition of 39 μL of ice-cold acetonitrile, followed by centrifugation for 10 min at 12,100 ×g. A 20-μL aliquot of the supernatant was removed and benzoylated by sequential addition of 10 μL of 100 mM sodium carbonate, 10 μL of benzoyl chloride (2% [v/v] in acetonitrile), and 10 μL of sulfuric acid (1% [v/v] in 20% [v/v] acetonitrile in water) as previously described [18,19]. Calibration solutions of Epi and NE were prepared in artificial cerebrospinal fluid, which is similar in salt composition to plasma without protein, to create a calibration range of 0.1–50 nM. Calibration standards were spiked with the internal standard, diluted with acetonitrile, and derivatized as described above. Calibration curves were prepared based on the peak area ratio of the calibration to the internal standard by linear regression. All samples and standards were analyzed in triplicate using a Phenomenex Kinetex C18 chromatography column (100 × 2.1 mm, 1.7 μm, 100 Å) on a Vanquish ultra-high-pressure liquid chromatograph (Thermo Fisher Scientific, Gemering, Germany) interfaced to a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Fisher Scientific, San Jose, CA). Mobile phase A was 10 mM ammonium formate with 0.15% (v/v) formic acid in water. Mobile phase B was acetonitrile. The gradient used was as follows: initial, 5% B; 0.01 min, 19% B; 0.68 min, 26% B, 1.05 min, 75% B; 1.8 min, 100% B; 2.8 min, 100% B; 4 min, 5% B; 5.0 min, 5% B at 600 μL/min. Benzoylated NE was eluted at 1.77 min, and benzoylated Epi was eluted at 1.81 min. The sample injection volume was 5 μL. The autosampler was kept at ambient temperature, and the column was held at 30 °C in still-air mode. Electrospray ionization was used in positive mode at 4 kV. The capillary temperature was 400 °C, the vaporizer temperature was 350 °C, the sheath gas was 10, and the auxiliary gas was 5. Ions were detected in tandem mass spectrometry mode. For Epi and d6-Epi, the precursor ions were m/z 478 and 484, respectively, with the tube lens set to 93 and collision energy of 26. For NE and d6-NE, the precursor ions were m/z 464 and 470, respectively, with a tube lens value of 81 and a collision energy of 19. The product ion was m/z 105 for all analytes. Automated peak integration was performed using XCalibur 3.0 MS software. All peaks were visually inspected to ensure proper integration.
2.7. Enzyme-linked immunosorbent assay (ELISA)
Rat plasma total GLP-1 (MesoScale Discovery, Rockville, MD, USA; Cat. No. K150JVC) and glucagon (MesoScale Discovery, Cat. No. K150HCC) were assayed using a sandwich ELISA assay kit, while total GIP (Millipore, Burlington, MA, USA; Cat. No. EZRMGIP-55K), total PYY (Crystal Chem, Elk Grove Village, IL, USA; Cat. No. 81502), and insulin (Crystal Chem, Cat. No. 90060) were assayed using standard ELISA assay kits. Plasma acetaminophen was assayed by a kit purchased from Sekisui Diagnostics (Lexington, MA, USA).
2.8. Data analysis
All CGM data were obtained using DSI software. GraphPad Prism 8.0 (GraphPad software, San Diego, CA, USA) or STATA 15 were used for the statistical analysis. Data were considered significant when P < 0.05. Where applicable, a two-tailed t-test (for data with a single factor, such as glucose incremental area under the curve levels between sham vs. VSG rats), an ordinary two-way analysis of variance (ANOVA; for data with two independent factors, such as glucose nadir between sham vs. VSG rats under liquid vs. solid diets), and a repeated-measures (RM) ANOVA (2DG data) were applied to determine significant main effects and interactions between variables (surgery, drug, and/or time). If there was a significant main effect without a significant interaction, this is indicated in the figure legend, and the P value is indicated within the figure. Significant interactions are indicated in the figure legends, and between-group differences were determined by Holm-Sidak's post hoc testing. Separate linear mixed-effects regression models were used to compare BW, food intake, and mean absolute glucose (MAG) by surgery and by time (main effects). The interaction between surgery and time was also included in each model to assess group difference over time. Animals were assigned as random intercepts in all models to account for between-animal variability, and a random slope was included in the model for BW to account for variation in slopes between animals.
3. Results
3.1. CGM reveals glycemic variability after VSG
We performed sham or VSG surgery and concurrently placed a CGM telemetry probe in HFD-fed male rats (n = 6 for sham, n = 8 for VSG; 631.6 ± 30.8 vs. 632.5 ± 24.3 g of BW in sham vs. VSG at the time of surgery). Using the CGM system, blood glucose levels were monitored starting 4 h after surgery and continuously thereafter for 28 days. The experimental design is outlined in Figure 1A. VSG rats had significantly lower body mass compared with their sham-surgery counterparts starting at day 4, and this persisted throughout the study (Figure 1B). Food intake in VSG rats was significantly lower compared with the sham group starting at 6–23 days postoperatively, but it was gradually restored to the level of the sham animals by 5 weeks after surgery (Figure 1C). The line graphs for the average diurnal glucose levels across the 28-day period are shown in Figure 1D, and the weekly glucose levels are shown in Figure 1E–L. During the first week after surgery, VSG rats had significantly lower diurnal blood glucose levels compared with sham animals (median glucose ± SEM; 7.5 ± 0.4 vs. 6.7 ± 0.2 mmol/L in sham vs. VSG, respectively; Figure 1E, I). By the second (7.4 ± 0.3 vs. 6.9 ± 0.3 mmol/L in sham vs. VSG) week and continuing through the third (7.1 ± 0.3 vs. 6.8 ± 0.3 mmol/L in sham vs. VSG) and fourth (7.5 ± 0.1 vs. 7.5 ± 0.8 mmol/L in sham vs. VSG) weeks, there were no longer any significant differences in the average glucose values between the two surgical groups (Figure 1E–L). This is despite the fact that daily food intake was still significantly lower in VSG animals for 3 weeks postoperatively (Figure 1C). The violin plots in Figure 1I–L display the distribution of the glucose values during weeks 1–4. The MAG value was calculated across both the light and dark cycles according to the formula MAG = ΔGlucose/ΔTime [20], as an indicator of glycemic variability [8,21]. The MAG value of the VSG rats was not significantly different during the first 2 weeks after surgery but became significantly higher than sham rats during postoperative weeks 3 and 4 (Figure 1M). Overall, these data demonstrate that glycemic variability (as indicated by MAG) starts as early as 3 weeks postoperatively, and interestingly, this is the time point when the median diurnal glucose levels were restored to the level of sham rats.
3.2. Glycemic patterns in response to meals in VSG rats
Solid foods empty from the stomach at a slower rate compared with liquid foods. We previously found that the gastric-emptying rate of both liquid and solid food is increased in VSG versus sham rats, an effect we believe links to higher or similar peak glucose values despite greater insulin secretion and sensitivity after VSG [22]. In this experiment, we investigated whether the glycemic patterns after VSG were different in response to solid versus liquid meals.
The rats were fasted for 6 h prior to each experiment, and food was given just before the start of the dark phase. Average blood glucose values were 6.4 ± 0.2 versus 6.4 ± 0.2 mmol/L in sham versus VSG rats, respectively, after 6-h fasting and prior to solid food access (Supplementary Table 1). During the first 30 min of the ad libitum feeding period (45% HFD), VSG rats had a significantly higher peak (8.0 ± 0.3 vs. 6.7 ± 0.3 mmol/L in VSG vs. sham rats) and average (7.1 ± 0.4 vs. 6.4 ± 0.2 mmol/L in VSG vs. sham rats) blood glucose levels compared with sham rats (Supplementary Table 1). The blood glucose levels returned to sham levels at 30 min (6.2 ± 0.2 vs. 6.3 ± 0.4 mmol/L in sham vs. VSG rats at 30 min) but increased again with another peak at 72 min after the start of the meal (6.3 ± 0.2 vs. 7.1 ± 0.3 mmol/L in sham vs. VSG rats at 72 min; P = 0.042, t-test; Figure 2A). This dynamic glycemic pattern in the VSG rats during ingestion of solid food was repeatedly observed for 12 h and resulted in a significantly increased MAG value in VSG versus sham rats (Figure 2B, inset).
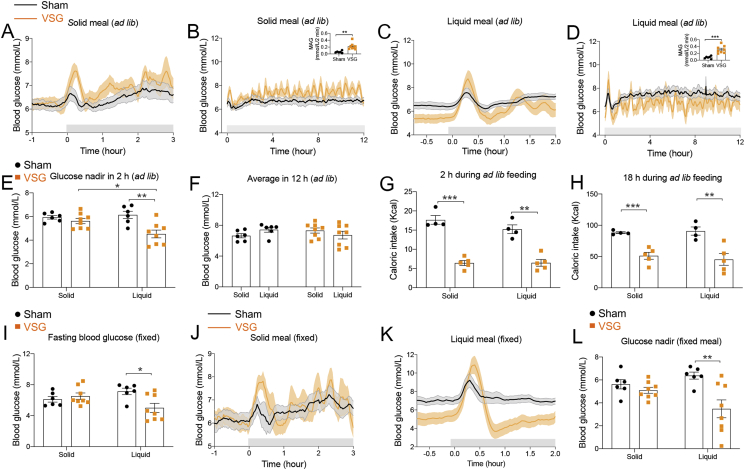
Figure 2.
VSG causes low glycemic responses under liquid meal ingestion. (A–B) Blood glucose levels during 3 h (A) and 12 h (B) of solid ad libitum meal feeding. Data are represented as the average of 3 consecutive days of blood glucose measurements. (B, inset) Mean absolute glucose (MAG) during the 12-h observation was significantly greater in VSG versus sham (**P < 0.01) animals. (C–D) Blood glucose levels during 2 h (C) and 12 h (D) of liquid ad libitum meal (Ensure Plus®) feeding. Data are represented as the average of 3 consecutive days of blood glucose measurements. (D, inset) MAG change during the 12-h observation was calculated (***P < 0.001). (E) The glucose nadir after solid versus liquid ad libitum meal feeding. *P < 0.05; **P < 0.01 (diet × surgery). (F) The average blood glucose levels during 12 h after solid versus liquid ad libitum meal feeding. (G–H) Caloric intake during 2 h (G) and 18 h (H) of solid versus liquid ad libitum meal ingestion. Data represent the average of 3 consecutive days of food intake measurements. **P < 0.01, ***P < 0.001 (diet × surgery). (I) Fasting glucose levels before solid versus liquid fixed meal ingestion. *P < 0.05 (diet × surgery). (J–K) Blood glucose levels after solid fixed (J; 1.5 g, 45% HFD) meal or liquid fixed (K; 2 mL single gavage, Ensure plus®) feeding. (L) Glucose nadir after solid versus liquid fixed meal feeding. **P < 0.01 (diet × surgery). All data are represented as mean ± SEM. All rats were fasted for 6 h prior to the food returns. Food was returned at the onset of the dark phase. Sham (n = 6), VSG (n = 8).
In contrast to the solid food, feeding the animals a liquid nutrient diet (Ensure Plus®) resulted in a significant reduction in the fasting blood glucose level in VSG (5.6 ± 0.3 mmol/L) compared with sham (6.7 ± 0.2 mmol/L) rats (Figure 2C, Supplementary Table 1). In contrast to the solid meal, VSG rats did not show any significant differences in peak glucose or average glucose values during the 30-min to 2-h observation period (Supplementary Table 1). However, similar to the solid meal, liquid diet resulted in increased glycemic variability in VSG rats (Figure 2D). The glucose nadir for 2 h following the meal was not different between sham and VSG rats during solid meal ingestion (Figure 2E). However, during ingestion of a liquid meal, the glucose nadir was significantly lower in VSG rats (4.5 ± 0.3 mmol/L) compared with sham rats (6.1 ± 0.3 mmol/L; Figure 2E). Although the VSG rats showed increased glycemic variability during both solid and liquid meal ad libitum ingestion (Figure 2B, D), the average blood glucose levels between sham and VSG rats were not significantly different (Figure 2F).
Caloric intake for the 2 groups, up to 18 h after the return of solid and liquid foods, was identical within the surgical group but was decreased overall in the VSG group compared with the sham rats (Figure 2G–H). These results indicate that both sham and VSG rats consumed the same calories regardless of the type of the meal.
To understand if the reduction in caloric intake in VSG versus sham rats results in postprandial hypoglycemia, we also examined the glycemic patterns after a fixed amount (1.5 g per rat) of solid food (45% HFD) and liquid nutrients (2 mL per rat, Ensure plus®). As with ad libitum feeding of the mixed liquid meal, VSG rats had reduced fasting blood glucose levels (7.1 ± 0.4 vs. 5.0 ± 0.6 mmol/L in sham vs. VSG rats; Figure 2I). All animals ate all of the solid food provided within the 2-h observation period. Even with the equal food intake, the glycemic pattern during the fixed solid meal (Figure 2J) was similar to that observed during ad libitum ingestion of solid food (Figure 2A). Within 30 min after the fixed solid meal, VSG rats showed significantly increased peak blood glucose and average blood glucose levels compared with the sham rats (Supplementary Table 1).
As with the feeding of the ad libitum mixed liquid meal, VSG rats had similar peak blood glucose levels compared with the sham rats (Figure 2K, Supplementary Table 1) but had significantly decreased blood glucose levels from 30 min to 120 min following the fixed-amount liquid meal (Supplementary Table 1). Blood glucose levels returned to the level of the sham rats at the 141-min time point (7.8 ± 0.4 vs. 7.7 ± 0.6 mmol/L in sham vs. VSG rats at 141 min; P = 0.913).
The glucose nadir for 2 h following the meal was not different between sham and VSG rats after ingestion of the fixed-amount solid meal (Figure 2L). However, after ingestion of the fixed-amount liquid meal, the glucose nadir was significantly lower in VSG rats (3.5 ± 0.8 mmol/L) compared with sham rats (6.4 ± 0.3 mmol/L; Figure 2L). Our CGM data demonstrated a significantly lower glucose nadir (Figure 2L) and a prolonged period of time at lower glucose values during the fixed-amount liquid but not after a fixed-amount solid meal (Figure 2J–K, Supplementary Table 1) in VSG rats, indicating that liquid meals exacerbate postprandial hypoglycemia after VSG.
Surprisingly, we observed animal variability in the glycemic pattern during the liquid meal (Supplementary Figure S2A–B) that was not observed during the solid meal (Supplementary Figure S2C–D) in VSG rats. We observed that 4 of 8 VSG rats (VSG-hypo) showed significantly reduced fasting blood glucose levels compared with the other 4 VSG rats and compared with the sham rats (3.7 ± 0.2 vs. 6.4 ± 0.6 and 7.1 ± 0.4 mmol/L in VSG-hypo vs. VSG-normal and sham rats, respectively) before the fixed liquid meal (Supplementary Figure S2E). These same rats also had a significantly reduced glucose nadir (1.6 ± 0.5 vs. 5.3 ± 0.6 and 6.4 ± 0.3 mmol/L in VSG-hypo vs. VSG-normal and sham rats, respectively; Supplementary Figure S2F). However, the blood glucose levels averaged over the 12 h following either the liquid or solid meals were not significantly different in these rats, indicating that the animals were able to recover from the hypoglycemia once returned to ad libitum feeding (Supplementary Figure S2G).
3.3. Endocrine changes after VSG causes postprandial hypoglycemia
Thirty-five days after surgery, the rats were orally administered a liquid mixed meal (Ensure Plus®) with the addition of dextrose (total caloric load = 4.86 kcal) and acetaminophen. Acetaminophen is passively absorbed into the system, and the assessment of levels in the plasma provides an index of the gastric-emptying rate. We measured plasma levels of acetaminophen and multiple gut peptides before and 15 min after the meal. Consistent with our previous results [22,23], VSG rats had significantly increased plasma acetaminophen levels after the liquid meal, indicating that the gastric-emptying rate was increased after VSG (Supplementary Figure S3A). There were no significant differences in basal levels, but the postprandial levels of glucose, insulin, total GLP-1, total GIP, and total PYY were significantly greater in VSG than in sham rats (Supplementary Figure S3B–F). The postprandial levels of glucagon were also significantly greater in VSG than in sham rats (Supplementary Figure S3G).
3.4. Glucose counterregulation is normal after VSG
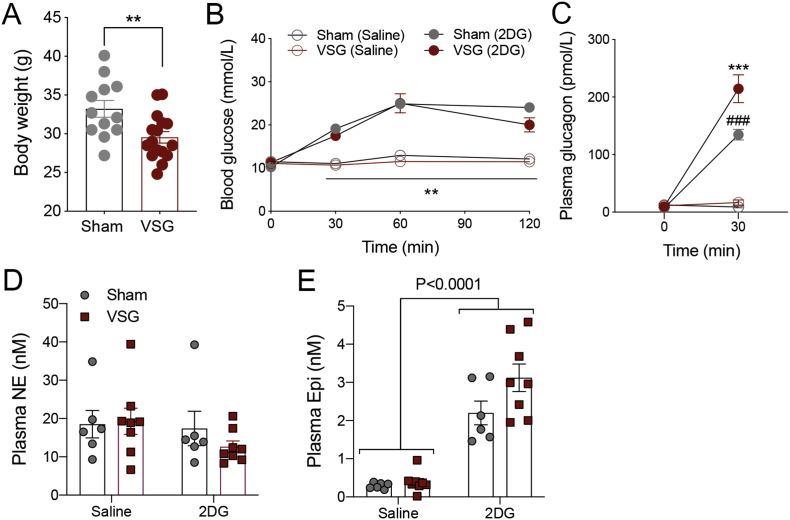
To test whether the lower glucose nadirs after the liquid meals might be due to impaired counterregulation, we measured the glucose and neuroendocrine responses to 2DG, a compound that competes with glucose for entry into the cell, causing glucoprivation, in a cohort of mice that had sham versus VSG surgery. VSG successfully reduced BW compared with sham animals (Figure 3A). Interestingly, blood glucose responses to 2DG were similar between sham and VSG mice (Figure 3B; RM ANOVA; time × surgery interaction). The 2DG injection significantly increased plasma glucagon levels in both sham and VSG mice, but the increment was significantly higher in VSG than in sham (Figure 3C; RM ANOVA; time × surgery interaction). The 2DG injection did not significantly increase plasma levels of NE (Figure 3C) but did significantly increase plasma Epi (Figure 3D) in both sham and VSG mice (Figure 3D). These data indicate that hypoglycemia counterregulation is normal 4 weeks after VSG.
Figure 3.
Glucose and neuroendocrine responses to glucoprivation in sham versus VSG mice. (A) Body weights at 10 weeks after sham versus VSG in C57BL/6 mice. **P < 0.01 (repeated-measures ANOVA; drug × surgery × time). (B) An IP injection of 2DG increases blood glucose levels at 4 weeks after sham and VSG surgery. **P < 0.01, in saline versus 2DG in both surgery groups at each indicated time point (repeated-measures ANOVA; drug × time). (C) An IP injection of 2DG increases plasma glucagon levels in both sham and VSG mice. ***P < 0.001, 0 min versus 30 min in both surgery groups after 2DG injection; ###P < 0.001, significant difference observed between sham and VSG mice after 2DG injection at the 30-min time point (repeated-measures ANOVA; drug × surgery × time). (D) Plasma norepinephrine (NE) was not significantly increased after 2DG in either surgical group. (E) Epinephrine (Epi) was significantly increased 30 min after 2DG in both sham and VSG mice (main effect of drug). All data are represented as mean ± SEM. Sham-Sal (n = 6), Sham-2DG (n = 6), VSG-Sal (n = 8), VSG-2DG (n = 8).
3.5. The role of GLP-1R signaling in PBH and glycemic variability
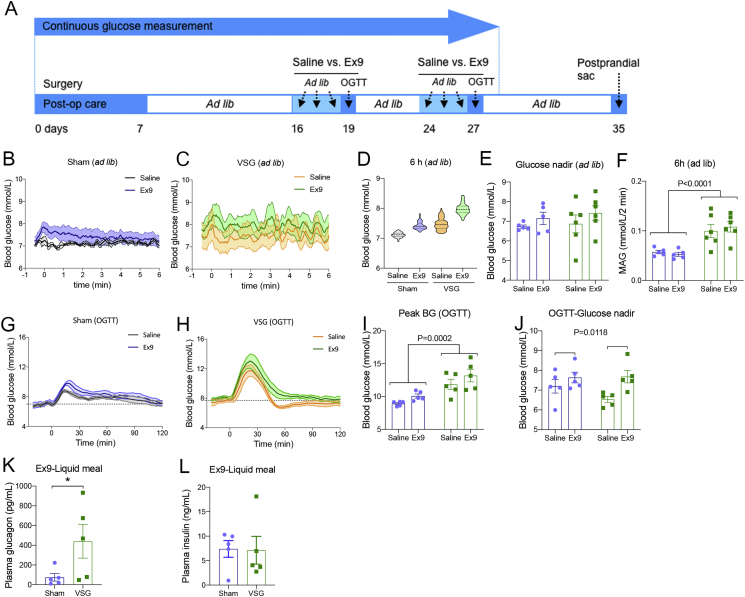
To test the degree to which GLP-1 and concomitant insulinemia were the causes of the hypoglycemia in our animal model, we measured the glycemic pattern in response to saline or Ex9, administered immediately before the dark phase for 4 days in an additional cohort of sham versus VSG rats that had CGM telemetry probes implanted at the time of surgery. On days 16–19 after surgery, we measured the blood glucose levels from the saline or Ex9-treated rats under ad libitum feeding for 3 consecutive days, and on the fourth day, we performed an OGTT following 6 h of fasting and Ex9 (or saline) administration. On days 24–27 after surgery, the drug treatments were crossed over, and results were pooled (Figure 4A). Thus, each animal was exposed on one occasion to either saline or Ex9. The pharmacokinetics profile of a subcutaneous injection of Ex9 in rodents after bariatric surgery has not been described. However, one study demonstrated that the plasma concentration of Ex9 was elevated up to 6 h after a single subcutaneous injection in patients with PBH [24]; therefore, we analyzed glucose variability during the 6-h time frame following Ex9 injections. Glycemic patterns during 6 h after the saline or Ex9 treatment under ad libitum feeding are shown at Figure 4B–C (average of 3 days of monitoring). Median blood glucose levels during the 6 h after saline or Ex9 treatment during the ad libitum feeding period were not significantly different between surgeries or drug treatments (7.1 ± 0.0 vs. 7.4 ± 0.3 mmol/L in sham-saline vs. sham-Ex9; 7.4 ± 0.4 vs. 7.9 ± 0.5 mmol/L in VSG-saline vs. VSG-Ex9; Figure 4D). The glucose nadir during the 2 h following the saline or Ex9 injection was not different between sham and VSG rats nor between saline and Ex9-treated rats (Figure 2E). Glycemic variability as assessed by the MAG calculation was significantly increased with VSG surgery, and Ex9 treatment had no significant impact on this result (Figure 4F). During the OGTT, Ex9 treatment did not significantly increase the peak blood glucose levels in sham or VSG rats (Figure 4G–I). However, the Ex9 injection significantly increased the glucose nadir in both sham and VSG rats (Figure 4J). At the time of sacrifice, we injected Ex9 15 min prior to a liquid mixed-meal gavage and assessed plasma hormone levels 15 min later. Plasma levels of glucagon (Figure 4K), total GLP-1 (Supplementary Figure S4A), total GIP (Supplementary Figure S4B), and total PYY levels (Supplementary Figure S4C) were all increased in VSG rats with the liquid meal. However, we found that, unlike our previous data in untreated animals (Supplementary Figure S3C), the postprandial plasma insulin levels were identical between sham and VSG rats after Ex9 treatment (Figure 4L). These data suggest that the blockade of GLP-1R signaling prevents postprandial increases in insulin and consequently reduces drops in glucose in VSG rats.
Figure 4.
Blockade of GLP-1 receptor prevents post-VSG hypoglycemia but not glycemic variability after an oral glucose load. (A) Schematic experimental timeline during CGM after surgery. (B–C) Blood glucose levels during 6 h of ad libitum feeding in saline or Ex9-treated sham (B) or VSG (C) rats. (D) Violin plots represent the median, quartiles, minimum, maximum, and distribution of glucose levels during 6 h of the ad libitum feeding condition. (E) The glucose nadir during 2 h of ad libitum feeding ingestion. (F) Mean absolute glucose (MAG) during 6 h of ad libitum feeding. Mean ± SEM (main effect of surgery). (G–H) Blood glucose levels during 120 min after an oral glucose load in saline or Ex9-treated sham (G) or VSG (H) rats. Mean ± SEM. (I) Peak glucose levels after an oral glucose load. Mean ± SEM. Main effect of surgery was observed in both drug groups (main effect of surgery). (J) Glucose nadir after an oral glucose load. Mean ± SEM (main effect of drug). (K–L) Ex9 treatment increased plasma glucagon level but not plasma insulin levels of VSG versus sham rats 15 min after a liquid mixed-meal gavage. *P < 0.05. All data are represented as mean ± SEM except (D). Saline or Ex9 (50 μg per kg average rat BW) was cross-injected to the rats 15 min into the dark phase during ad libitum feeding (B–E) or 15 min prior to oral glucose administration (F–I). Data obtained from the ad libitum feeding condition are represented as an average of 3 consecutive days of measurements. The cross-injection studies were performed at 3–4 weeks, and there was a 4-day washout period. Sham (n = 5), VSG (n = 6). One VSG rat showing low glycemic response under a single oral glucose administration was omitted (F–K).
4. Discussion
With increasing awareness and diagnostic tools such as CGM, it is apparent that PBH is a condition that affects more patients than previously recognized [4]. Although limiting carbohydrate intake and/or absorption helps some patients, for many the treatment options are limited, especially given the unknown etiology. Preclinical models can help advance mechanistic understanding of clinical phenomenon. Here, using CGM in a rat model of bariatric surgery, we demonstrate that rodents also experience glycemic variability and hypoglycemia. Like patients, these results in rats vary depending on the experimental conditions, with solid food ingestion driving glycemic variability and liquid meals driving postprandial hypoglycemia. In addition, we demonstrate that glucose hormonal counterregulation is normal and that postprandial drops in glucose, but not the glycemic variability, can be prevented by blocking GLP-1R signaling.
Here, we used CGM in a rat model of VSG. An important question to address is the extent to which the preclinical model can be used to study mechanisms for PBH. With CGM, it has been reported that VSG patients also have a substantial risk for PBH [25], supporting the relevance of this work. Although the degree to which the rodent model is experiencing neuroglycopenic systems cannot be gauged and is an important limitation to this model, hypoglycemia and glycemic variability, also hallmark symptoms of PBH, are apparent. Importantly, there are many parallels in the glucoregulatory responses to bariatric surgery between humans and rodents. Both humans and rodents have increased gastric-emptying rate, and high peak postprandial glucose, plasma GLP-1, and insulin responses to bariatric surgery. In addition, like humans [8,26,27], using CGM, we saw a high degree of glycemic variability in rodents after VSG (as indexed by the MAG calculation). We could also exacerbate postprandial hypoglycemia with a gavage of a mixed-nutrient liquid meal. Importantly, this level of hypoglycemia is not detected during a standard OGTT in rats [28] or mice [29,30]. As tail bleeding is stressful, in particular for mice [31], we predict that this method limits the detection of postprandial hypoglycemia with VSG. In contrast, in humans, CGM was more readily able to detect hypoglycemia during activities of daily living compared with after a standardized mixed-meal tolerance test in the lab [7].
While we observed a high degree of glycemic variability, we did not observe hypoglycemia in animals fed a solid HFD. It is not clear whether the incidence of hypoglycemia with solid food would occur later postoperatively or whether it relates to the increased gastric-emptying rate associated with a liquid compared with a solid meal. One potential factor in understanding the impact of surgery on glycemic variability in free-living conditions is that VSG animals take in more frequent meals. However, to compensate for the increased frequency, meal size is typically lower. This pattern would be expected to decrease rather than increase peak glucose levels in VSG compared with sham animals. In an attempt to control for the differences in meal patterns, we performed an additional feeding experiment in which the animals were administered the same amount of HFD for 2 h. Even with the equal intake, the glycemic patterns remained the same, with VSG animals having a higher peak and average glucose values over the study period.
Although often simplified to a postprandial problem associated with factors that drive postprandial insulin, the etiology of PBH is likely more complex. Understanding the mechanisms of PBH has been difficult since it is underdiagnosed and the onset of PBH is months to years after bariatric surgery [4]. Some have reported that susceptible patients have greater postprandial GLP-1 levels [8,9,17], but not all studies show this effect [32]. An increase in insulin sensitivity in PBH-susceptible patients has also been suggested, but several studies do not support this contention [9,32,33]. Greater insulin secretion [9], less suppression of insulin with falling glucose [32], reduced insulin clearance [32], and increased insulin-independent glucose disposal [33] have all been reported in patients with documented (but not with CGM) hypoglycemia after surgery. The problem with these associative findings is that all VSG and RYGB patients have increased peak glucose, increased insulin sensitivity, and increases in postprandial GLP-1 and consequently insulin, and most of these changes occur within days of surgery [34,35]. Lastly, the timeline of early changes in these parameters but later onset of symptoms is incongruent. Although very complicated, a prospective study that follows patients from surgery to onset of symptoms would lead to a better understanding of these types of findings.
One potential mechanism for PBH is impairment of glucose counterregulation. To test this, we assessed counterregulatory hormonal responses to acute administration of a glucoprivic agent (i.e., 2DG) 4 weeks after surgery in mice. VSG mice had similar glucose, glucagon, and catecholamine responses to 2DG, suggesting that glucose counterregulation is normal after surgery. Although this study was done in mice and the CGM is done in rats, rats and mice have similar qualitative responses to 2DG, including increased feeding, glucose, and counterregulatory hormones. However, we cannot rule out the possibility that VSG may induce a different response to 2DG in rats than in mice.
GLP-1 has long been implicated as a mechanism for the metabolic success of bariatric surgery. Given that some data suggest it is also higher in PBH patients, it has also been implicated as a cause of PBH. Multiple studies have administered Ex9 during a meal tolerance test to determine the role of GLP-1R signaling in PBH. Indeed, multiple studies show that Ex9 reduces peak insulin and raises the glucose nadir in response to a meal [16,17] and also prevents neuroglycopenic symptoms [17], suggesting the involvement of GLP-1R signaling in PBH. Consistent with these data, our rat VSG model demonstrated that Ex9 treatment increased the glucose nadir. However, we did not see any impact of Ex9 on glycemic variability. Blockade of the GLP-1R also increased the glucose nadir in sham rats, which raises the question of whether GLP-1 signaling is an actual mechanism that underlies postprandial hypoglycemia, which is one important characteristic of PBH, or whether it is a therapeutic target but does not address the actual cause of postprandial hypoglycemia. Ex9 also prevented the typically larger insulin response in VSG compared with sham animals, supporting the idea that the blockade of GLP-1R can be a treatment option for postprandial hypoglycemia occurring after VSG, if future work is able to directly link the increase in insulin to the hypoglycemia.
CGM has only recently been more readily available for diagnosing PBH, meaning that in previous studies, the delineation between the symptomatic and asymptomatic patient populations may not have been as clear cut. These limitations to understanding PBH are underscored by the fact that we have only recently begun to understand that the problem is far more widespread. In fact, CGM during activities of daily living detects hypoglycemia in patients to a much greater extent than doing a standardized mixed-meal tolerance test in the clinic [7]. CGM also provides a greater number of parameters to assess regarding glucose control. In fact, CGM is increasingly being used as a tool to study glucose variation in diabetic individuals. The standard biomarker for glucose control is HbA1c%, which provides an index of the average glucose values over the previous 3 months. A recent direct comparison of using HbA1c% versus CGM found that HbA1c% correlated with time spent in hyper- but not time spent in hypoglycemia glucose ranges determined by CGM [36]. In fact, hypoglycemia was detected in patients with HbA1c% of greater than 8.5% (clinical target for HbA1c% is ∼7–8%), suggesting that simply recommending tighter glucose control (which increases the risk for hypoglycemia in and of itself) is not an effective strategy to target these patients and that CGM-derived metrics should be included in routine glucose care discussions. This discussion may also be relevant for the wide range of standards used in published papers to define diabetes resolution after bariatric surgery and highlights the risk that at least a subset of patients may not have diabetes resolution but may have a greater incidence of hypoglycemia.
5. Conclusion
In this study, we propose that animal models of VSG replicate many aspects of PBH in humans. We found that VSG increases glycemic variability, and specifically in response to a mixed-meal liquid gavage, hypoglycemia is readily detectable. Lastly, blockade of GLP-1R signaling prevents falls in the glucose nadir but does not improve glycemic variability. These responses were seen very early after surgery and therefore offer an experimental model of PBH that could be examined over a reasonable time frame.
Author contributions
SSE and KSK were responsible for executing the rat experiments. NB was responsible for executing mouse experiments. MJS, YK, and RTK were responsible for determination of plasma epinephrine and norepinephrine concentrations. SEW and DEM supported setting up the CGM telemetry system. AGL and DF were responsible for executing VSG and CGM telemetry implantation surgeries. SSE, KSK, RJS, and DAS were responsible for the design of experiments, analysis and interpretation of data, and drafting of the manuscript. DAS provided final approval of the submitted manuscript.
Acknowledgements
The authors thank Mouhamadoul Toure and Andriy Myronovych for conducting rat and mouse VSG. This work is supported in part by NIH awards DK107282 (DAS), DK107652 (RJS), DK046960 (RTK), 5T32DK071212-12 (NB), 5T32Dk108740 (NB), and DK093848 (RJS) and by an American Diabetes Association grant (1-19-IBS-252; DAS). This work is also supported in part by the NIH-funded Michigan Diabetes Research Center (P30 DK020572) including the CGM laboratory (directed by DEM) within the Animal Studies Core (directed by DAS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.12.011.
Conflict of interest
The authors declare the following potential conflicts of interest. RJS has received research support from Ethicon Endo-Surgery, Zafgen, Novo Nordisk, Kallyope, AstraZeneca, and Pfizer. RJS has served on scientific advisory boards for Ethicon Endo-Surgery, Novo Nordisk, Sanofi, Janssen, Kallyope, Scohia, Ironwood Pharma, and GuidePoint Consultants. RJS is a stakeholder of Zafgen and Redesign Health. DAS has received research support from MedImmune and Novo Nordisk. The remaining authors have no potential conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ali M., El Chaar M., Ghiassi S., Rogers A.M., American Society for Metabolic and Bariatric Surgery Clinical Issues Committee American Society for Metabolic and Bariatric Surgery updated position statement on sleeve gastrectomy as a bariatric procedure. Surgery for Obesity and Related Diseases – Official Journal of the American Society for Bariatric Surgery. 2017;13(10):1652–1657. doi: 10.1016/j.soard.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Welbourn R., Hollyman M., Kinsman R., Dixon J., Liem R., Ottosson J. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obesity Surgery. 2018:1–14. doi: 10.1007/s11695-018-3593-1. [DOI] [PubMed] [Google Scholar]
- 3.Schauer P.R., Kashyap S.R., Wolski K., Brethauer S.A., Kirwan J.P., Pothier C.E. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New England Journal of Medicine. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salehi M., Vella A., Mclaughlin T., Patti M., Society E. Hypoglycemia after gastric bypass surgery: current concepts and controversies. The Journal of Clinical Endocrinology & Metabolism. 2018;103(8):2815–2826. doi: 10.1210/jc.2018-00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C.J., Wood G.C., Lazo M., Brown T.T., Clark J.M., Still C. Risk of post-gastric bypass surgery hypoglycemia in nondiabetic individuals: a single center experience. Obesity (Silver Spring, Md. 2016;24(6):1342–1348. doi: 10.1002/oby.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capristo E., Panunzi S., De Gaetano A., Spuntarelli V., Bellantone R., Giustacchini P. Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: a randomized trial. The Journal of Clinical Endocrinology & Metabolism. 2018;103(6):2136–2146. doi: 10.1210/jc.2017-01695. [DOI] [PubMed] [Google Scholar]
- 7.Kefurt R., Langer F.B., Schindler K., Shakeri-Leidenmühler S., Ludvik B., Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surgery for Obesity and Related Diseases. 2015;11(3):564–569. doi: 10.1016/j.soard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Tharakan G., Behary P., Wewer Albrechtsen N.J., Chahal H., Kenkre J., Miras A.D. Roles of increased glycaemic variability, GLP-1 and glucagon in hypoglycaemia after Roux-en-Y gastric bypass. European Journal of Endocrinology. 2017;177(6):455–464. doi: 10.1530/EJE-17-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldfine A.B., Mun E.C., Devine E., Bernier R., Baz-Hecht M., Jones D.B. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. Journal of Clinical Endocrinology and Metabolism. 2007;92(12):4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 10.Suhl E., Anderson-Haynes S.E., Mulla C., Patti M.E. Medical nutrition therapy for post-bariatric hypoglycemia: practical insights. Surgery for Obesity and Related Diseases. 2017;13(5):888–896. doi: 10.1016/j.soard.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilarrasa N., Goday A., Rubio M.A., Caixàs A., Pellitero S., Ciudin A. Hyperinsulinemic hypoglycemia after bariatric surgery: diagnosis and management experience from a Spanish multicenter registry. Obesity Facts. 2016;9(1):41–51. doi: 10.1159/000442764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spanakis E., Gragnoli C. Successful medical management of status post-roux-en-Y-Gastric-Bypass hyperinsulinemic hypoglycemia. Obesity Surgery. 2009;19(9):1333–1334. doi: 10.1007/s11695-009-9888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderveen K.A., Grant C.S., Thompson G.B., Farley D.R., Richards M.L., Vella A. Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery. 2010;148(6):1237–1246. doi: 10.1016/j.surg.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K.-S., Sandoval D.A. vol. 7. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2017. pp. 783–798. (Endocrine function after bariatric surgery Comprehensive Physiology). [DOI] [PubMed] [Google Scholar]
- 15.Scrocchi L.A., Brown T.J., Maclusky N., Brubaker P.L., Auerbach A.B., Joyner A.L. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon–like peptide 1 receptor gene. Nature Medicine. 1996;2(11):1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 16.Salehi M., Gastaldelli A., D'Alessio D.A. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–680.e2. doi: 10.1053/j.gastro.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig C.M., Liu L.-F., Deacon C.F., Holst J.J., McLaughlin T.L. Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia. 2017;60(3):531–540. doi: 10.1007/s00125-016-4179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong J.-M.T., Malec P.A., Mabrouk O.S., Ro J., Dus M., Kennedy R.T. Benzoyl chloride derivatization with liquid chromatography–mass spectrometry for targeted metabolomics of neurochemicals in biological samples. Journal of Chromatography A. 2016;1446:78–90. doi: 10.1016/j.chroma.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song P., Mabrouk O.S., Hershey N.D., Kennedy R.T. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography–mass spectrometry. Analytical Chemistry. 2012;84(1):412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermanides J., Vriesendorp T.M., Bosman R.J., Zandstra D.F., Hoekstra J.B., Devries J.H. Glucose variability is associated with intensive care unit mortality. Critical Care Medicine. 2010;38(3):838–842. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 21.Kohnert K.-D., Heinke P., Fritzsche G., Vogt L., Augstein P., Salzsieder E. Evaluation of the mean absolute glucose change as a measure of glycemic variability using continuous glucose monitoring data. Diabetes Technology and Therapeutics. 2013;15(6):448–454. doi: 10.1089/dia.2012.0303. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni B.V., LaSance K., Sorrell J.E., Lemen L., Woods S.C., Seeley R.J. The role of proximal versus distal stomach resection in the weight loss seen after vertical sleeve gastrectomy. American Journal of Physiology – Regulatory Integrative and Comparative Physiology. 2016;311(5):R979–R987. doi: 10.1152/ajpregu.00125.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers A.P., Smith E.P., Begg D.P., Grayson B.E., Sisley S., Greer T. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. AJP Endocrinology and Metabolism. 2014;306(4):E424–E432. doi: 10.1152/ajpendo.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig C.M., Liu L.F., Nguyen T., Price C., Bingham J., McLaughlin T.L. Efficacy and pharmacokinetics of subcutaneous exendin (9-39) in patients with post-bariatric hypoglycaemia. Diabetes Obesity and Metabolism. 2018;20(2):352–361. doi: 10.1111/dom.13078. [DOI] [PubMed] [Google Scholar]
- 25.Papamargaritis D., Koukoulis G., Sioka E., Zachari E., Bargiota A., Zacharoulis D. Dumping symptoms and incidence of hypoglycaemia after provocation test at 6 and 12 Months after laparoscopic sleeve gastrectomy. Obesity Surgery. 2012;22(10):1600–1606. doi: 10.1007/s11695-012-0711-3. [DOI] [PubMed] [Google Scholar]
- 26.Hanaire H., Bertrand M., Guerci B., Anduze Y., Guillaume E., Ritz P. High glycemic variability assessed by continuous glucose monitoring after surgical treatment of obesity by gastric bypass. Diabetes Technology and Therapeutics. 2011;13(6):625–630. doi: 10.1089/dia.2010.0203. [DOI] [PubMed] [Google Scholar]
- 27.Jiménez A., Ceriello A., Casamitjana R., Flores L., Viaplana-Masclans J., Vidal J. Remission of type 2 diabetes after Roux-en-Y gastric bypass or sleeve gastrectomy is associated with a distinct glycemic profile. Annals of Surgery. 2015;261(2):316–322. doi: 10.1097/SLA.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 28.Chambers A.P., Jessen L., Ryan K.K., Sisley S., Wilson-Pérez H.E., Stefater M.A. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pressler J.W., Haller A., Sorrell J., Wang F., Seeley R.J., Tso P. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes. 2014 doi: 10.2337/db14-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mul J.D., Begg D.P., Haller A.M., Pressler J.W., Sorrell J., Woods S.C. MGAT2 deficiency and vertical sleeve gastrectomy have independent metabolic effects in the mouse. American Journal of Physiology Endocrinology and Metabolism. 2014;307(11):E1065–E1072. doi: 10.1152/ajpendo.00376.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayala J.E., Samuel V.T., Morton G.J., Obici S., Croniger C.M., Shulman G.I. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Disease Models and Mechanisms. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salehi M., Gastaldelli A., D'Alessio D.A. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. Journal of Clinical Endocrinology & Metabolism. 2014 doi: 10.1210/jc.2013-2686. Epub 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti M.E., Li P., Goldfine A.B. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity. 2015;23(4):798–807. doi: 10.1002/oby.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umeda L.M., Silva E.A., Carneiro G., Arasaki C.H., Geloneze B., Zanella M.T. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obesity Surgery. 2011;21(7):896–901. doi: 10.1007/s11695-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen N.B., Jacobsen S.H., Dirksen C., Bojsen-Moller K.N., Naver L., Hvolris L. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. AJP Endocrinology and Metabolism. 2012;303(1):E122–E131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch I.B., Welsh J.B., Calhoun P., Puhr S., Walker T.C., Price D.A. Associations between HbA 1c and continuous glucose monitoring-derived glycaemic variables. Diabetic Medicine DME. 2019:14065. doi: 10.1111/dme.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.