Abstract
Glycyrrhetinic acid monoglucuronide (GAMG) is a novel and low-calorie sweetener that is widely applied in the food industry. This study aimed to enhance the production of fungal β-d-glucuronidase (GUS) via a novel fermentation technique by evaluating the effects of the various microparticles on Chaetomium globosum DX-THS3 GUS production. Results showed that the silica microparticle greatly affected the morphology of DX-THS3 strain relative to the other microparticles. Microbial structure imaging results showed that the smallest average diameter of fungal pellets was achieved (0.7 ± 0.1 mm) by adding 10 g/L (600 mesh) of silica. The diameter of the control was 3.0 ± 0.5 mm in shake flask fermentation. The GUS activity and biomass of DX-THS3 reached 680 U/mL and 4.2 g/L, respectively, with the use of 10 g/L of silica microparticles, whereas those of the control were 210 U/mL and 2.8 g/L via shake flask fermentation. The findings in this study may provide a potential strategy for designing the morphology of filamentous fungi using microparticles in the industrial production of GAMG.
Keywords: β-d-glucuronidase, Chaetomium globosum DX-THS3, Microparticles, Morphology, Fermentation
Introduction
Filamentous fungal fermentation is widely used to produce useful products, such as organic acids, enzymes, antibiotics, and cholesterol-lowering drugs (statins) (Johanna et al. 2016; Manzoni and Rollini 2002; Sathesh-Prabu et al. 2019; Kun et al. 2019). The complex morphology of filamentous fungi hinders their application in production and is regarded as a typical problem in their fermentation (Driouch et al. 2010). Most previous studies reported that fungal growth in pellet form, relative to other morphologies (e.g., mycelia and clumps), is an effective alternative to fungal fermentation used in production, because it not only allows the reuse of fungal biomass but also improves culture rheology, which results in enhanced mass and oxygen transfer during fermentation and in low-energy consumption for aeration and agitation (Zhang and Hu 2012; Zhang and Zhang 2015). For example, the pellet form of filamentous fungi could considerably increase citric acid (Wang et al. 2017) or itaconic acid (Gyamerah 1995) production relative to other forms of filamentous fungi. Furthermore, the strong viscosity of culture broth greatly reduces mass transfer and results in difficulties in product purification (Johansen et al. 2008). Unfortunately, fungi morphology is difficult to control, especially when seeking superior production performance. In a pioneering study, inorganic microparticles added to cultures were found to influence fungal morphology; another study also showed that the addition of talc or alumina microparticles into culture media could strongly affect the morphological development of Aspergillus niger or other filamentous microorganisms (Kaup et al. 2008, Driouch et al. 2011). This finding could be further elaborated in the targeted engineering of fungal morphology towards elevated enzyme production in different strains based on micromaterials (Driouch et al. 2012). Thus, a novel strategy can be used for the engineering of the morphology of A. niger or other filamentous fungi to produce corresponding products using microparticles.
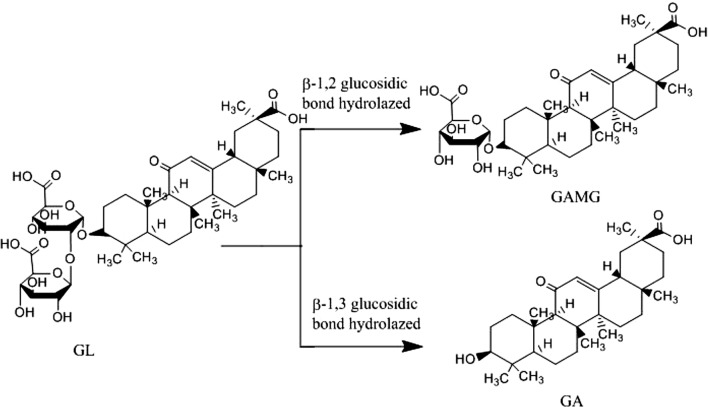
Glycyrrhizin (18β-glycyrrhetinicacid-3-O-β-d-glucuronopyranosyl-2-β-d-glucuronide, GL) is a pentacyclic triterpene glycoside abundant in liquorice (Glycyrrhiza glabra) root and rhizome (Zou et al. 2013). Many studies reported that GL shows abundant bioactivities, including anti-inflammatory (Li et al. 2018; Farag et al. 2015), antitumor (Sun et al. 2019), liver protection (Huo et al. 2018), and antiviral effects (Ashfaq et al. 2011). GL is also a low-calorie sweetener used in the food industry. However, GL contains two molecules of the glucuronic acid group, resulting in its strong polarity and poor solubility in water, thus, GL cannot easily penetrate cell membranes, and its bioavailability is low (Ahmad and Panda 2013). Glycyrrhetinic acid 3-O-mono-β-d-glucuronide (GAMG) is an important derivative of GL that hydrolyzes one of the glucuronidic acid moieties by β-d-glucuronidase (GUS, EC 3.2.1.31) (Fig. 1). It exhibits considerably stronger physiological properties and bioactivities than GL. GAMG, as a sugar with a high sweetness and low-caloric value, has 5- and 950-fold higher sweetness than GL and sucrose, respectively. The LD50 value of GAMG (5000 mg/kg) is much higher than that of GL (805 mg/kg), showing that GAMG is safer to consume than GL. These results show that GAMG is an effective alternative for glycyrrhizic medicines (Huang et al. 2015; Han et al. 2014).
Fig. 1.
Schematic of hydrolyzing GL into GAMG and GA
Recently, our group found that Chaetomium globosum DX-THS3 can produce GUS for the highly efficient hydrolysis of GL into GAMG (as the sole product) with high substrate specificity. Some GUSs that could transform GL into GAMG have been found from numerous microorganisms, such as Penicillium purpurogenum Li-3 (Huang et al. 2015), Aspergillus. sp (Kang et al. 2012), and A. niger (Quan et al. 2015). However, the catalytic activity of PGUS is only 14 U/mL after the optimization of fermentation conditions by P. purpurogenum Li-3; this value is considerably lower than our result (210 U/mL) (Huang et al. 2015). Such GUS activities, including our results, are not suited for industrial applications. Many previous studies have shown that the engineering of fungal morphology can increase the production of enzymes in fermentation. Thus, the pelleted form of C. globosum DX-THS3 may obtain higher product yields than the dispersed mycelial form. However, reports for this species of Chaetomium are few. The present study investigated the fungal morphology, GUS activity, and microparticles of C. globosum DX-THS3 to develop a potential approach for its pellet formation and to further enhance GUS production via the fermentation of the DX-THS3 strain. To the best of our knowledge, this report is the first to investigate the feasibility and advantages of using silica as microparticles for controlling fungal morphology in Chaetomium fermentation. Our results may serve as a possible strategy for optimizing the fermentation of filamentous microorganisms.
Materials and methods
Strains and medium
Chaetomium globosum DX-THS3 was previously isolated from Dong Xiang wild rice and used in this study (Wang et al. 2015). The DX-THS3 strain was maintained on a potato dextrose agar (PDA) slant. The inoculum culture medium was potato dextrose broth (PDB) containing 200 g of potato, 20 g of glucose, and 0.2 g of yeast extract in 1 L of distilled water. The fermentation culture medium contained 5 g of sucrose, 3.2 g of KH2PO4, 5 g of NH4NO3, 0.5 g of NaCl, 0.05 g of yeast extract, 0.5 g of MgSO4·7H2O, 0.0029 g of ZnSO4·7H2O, 0.00024 g of NaMoO4·2H2O, 0.002 g of MnSO4·H2O, 0.014 g of CaCl2, and 0.00003 g of H3BO3 in 1 L of distilled water. The microparticles of silica (5, 10, 15, and 20 g/L) in different size fractions (300, 400, 600, and 1000 mesh), aluminum oxide (10 g/L, 300 mesh), talcum powder (10 g/L, 300 mesh), and glass bead (10 g/L, 2 mm) were obtained as dry powder from Sigma-Aldrich, USA. The pH of the medium was 5.50 before sterilization and 5.30 after sterilization.
Cultivation and fermentation of C. globosum DX-THS3 for GUS production
Chaetomium globosum DX-THS3 was initially grown on PDB medium in a 500 mL flask with 200 mL of seed culture medium at 28 °C on a rotary shaker at 150 rpm for 3 days. The sample was then transferred (3% inoculum, v/v) into a 250 mL flask containing 50 mL of fermentation medium supplemented with different microparticles, including aluminum oxide (10 g/L, 300 mesh), talcum powder (10 g/L, 300 mesh), silica (10 g/L, 300 mesh), and glass bead (10 g/L, 2 mm diameter). The culture was grown at 28 °C in a shaker with a shaking speed of 150 r/min for 7 days to produce GUS. The samples were collected from each flask every 12 h to determine the dry cell weight and enzyme activity.
Enzymatic assay
The collected samples from the shake flask were passed through a vacuum filter and the resultant cells were washed with distilled water and filtered again. According to our previous study, GUS is an intracellular enzyme that shows no activity in broth during fermentation. Then, the cells were used for GUS activity analyses, and the supernatant was utilized for other analyses. The GUS activity was detected by GL hydrolysis (Zou et al. 2013). The enzymatic reaction containing 800 µL of GL solution (2 mg/mL dissolved in 20 mmol/L of acetate buffer with pH of 6.0) and 200 µL of crude enzymes was incubated for 2 h at 45 °C. Then, the reaction was terminated by boiling water for 10 min. GAMG was detected by ultra-performance liquid chromatography (ACQUITY UPLC, Waters) equipped with a vacuum degasser, a quaternary pump, an autosampler, a thermostated column compartment, and a PDA detector. The injection volume was 10 µL and the flow rate was 1 mL/min. The column temperature was constant at 35 °C and the mobile phase was composed of 80% methanol and 20% water (with 0.5% acetic acid). The detection wavelength was 254 nm. The obtained data were used to calculate the activity unit of GUS (U/mL), which was defined as the generated 0.1 μg of GAMG/h under the set assay conditions.
Other assays
Fungal mycelium was collected from a whole flask by filtration, and the cell dry weight was determined after washing the cells with distilled water and dried at 70 °C for 72 h (measurement was constant using analytical balance). The phenol sulfuric acid method was used to analyze the total sugar consumption. Fungal pellets collected from the shake flasks with various microparticle concentrations were rinsed with distilled water to remove excess microparticles and medium components. During fermentation, the morphological changes in the pellets were photographed under a light microscope (BA310-Digital) with attached image analysis software. All of the assays were analyzed with three parallel treatments.
Results and discussion
Microparticles affect the GUS activity of C. globosum DX-THS3
Many studies have shown that the morphology of filamentous microorganisms is one of the key factors in product production (Veiter et al. 2018). Studies have shown that the fungal morphology is mainly influenced by medium compositions, inoculum, pH, medium shear, additives, culture temperature, and medium viscosity (Liu et al. 2010; Darah et al. 2015; Zhang et al. 2015); among them, various types of microparticles, such as silicates, titanates, aluminas, and talc are frequently and effectively used in the study of engineering fungal morphology (Krull et al. 2013; Walisko et al. 2012; Driouch et al. 2010). Herein, different microparticles, including glass bead, alumina, talcum powder, and silica, were added into the medium to culture the DX-THS3 strain and investigate the effects of the microparticles on GUS activity in fermentation. Our results demonstrated that relative to the control, four types of microparticles increased the GUS activity of C. globosum DX-THS3 after fermentation for 168 h. As shown in Table 1, the GUS activities increased by 26%, 44%, 61%, and 79% with the addition of glass bead (265 U/mL), aluminum oxide (304 U/mL), talcum powder (338 U/mL), and silica (375 U/mL) relative to the control.
Table 1.
Effects of different microparticles on the morphology of C. globosum DX-THS3
| Types | Average pellet size diameter (mm) | Enzyme activity (U/mL) |
|---|---|---|
| Without | 3.0 ± 0.5 | 210 ± 8 |
| Glass bead | 2.8 ± 0.1 | 265 ± 12 |
| Alumina | 2.5 ± 0.1 | 304 ± 16 |
| Talcum powder | 0.9 ± 0.1 | 338 ± 10 |
| Silica | 0.7 ± 0.1 | 375 ± 13 |
The measurements were performed on the culture broth after 156 h of cultivation
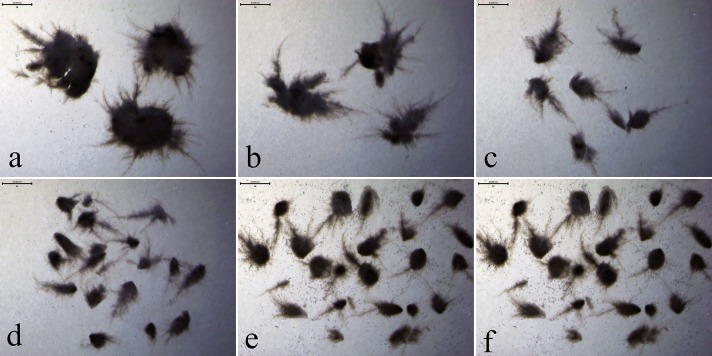
In addition, we further investigated the morphology of C. globosum DX-THS3 with addition of different microparticles. The morphology of the DX-THS3 strain added with different microparticles was analyzed. As shown in Fig. 2, the average pellet diameter of C. globosum DX-THS3 was smaller, and the mycelial surface was significantly reduced in comparison with that of the control. The average pellet diameter decreased (Fig. 2b–e), as summarized in Table 1. The following morphologies of DX-THS3 were observed: glass bead, gray thorny pellet with 2.8 ± 0.1 mm of diameter; aluminum oxide, gray thorny pellet with 2.5 ± 0.1 mm diameter; talcum powder, gray thorny pellet with 0.9 ± 0.1 mm diameter; and silica, gray thorny pellet with 0.7 ± 0.1 mm diameter. Thus, silica is the best microparticle for producing GUS via C. globosum DX-THS3 fermentation. Many studies have reported that production is increased by the addition of microparticles to control fungal morphology (Kaup et al. 2008, Driouch et al. 2011). Wang et al. (2017) produced citric acid using filamentous fungi and found that citric acid production increases with the addition of microparticles. Fungi grown in submerged cultures take on several different morphological forms such as mycelia, clumps or pellets (Niu et al. 2015; Verónica et al. 2013). Compared with other fungal morphologies, previous studies have shown that the pellet form is better than other fungal morphologies in production. The pellet form of fungal mycelium can promote mass transfer. In the present study, microparticles, including glass bead, talcum powder, alumina, and silica, significantly increased GUS production, and the DX-THS3 strain formed pellets through microparticles.
Fig. 2.
Image analysis without microparticle (a) and with glass bead (b), 10 g/L of talcum powder (c), 10 g/L of alumina (d), and 10 g/L of silica (e) and their effects on the morphology of C. sp. DX-THS3 in submerged culture obtained from shaken flasks. Image analysis by light microscopy after 168 h of cultivation. The ruler is 2 mm
Silica increased the GUS activity of C. globosum DX-THS3 by controlling fungal morphology
The results showed that silica is a favorable microparticle for GUS production. The effects of silica on GUS activities were investigated. The GUS activities during the fermentation of C. globosum DX-THS3 with different concentrations and mesh of silica were studied. As shown in Fig. 3, the GUS activities produced by fermentation via the DX-THS3 strain with silica were significantly increased relative to those in the control. Specifically, the following increases in GUS activities were noted: 300 mesh of silica, 98% (398 U/mL); 400 mesh of silica, 129% (462 U/mL); 600 mesh of silica, 160% (524 U/mL); and 1000 mesh of silica, 130% (463 U/mL). The best GUS enzymatic activity was achieved using 600 mesh of silica (Fig. 3). Meanwhile, the GUS activities of the DX-THS3 strain with different concentrations of silica for fermentation were analyzed. As shown in Table 2, the GUS activities with the addition of 0, 5, 10, 15, and 20 g/L of silica were better than those without silica. The following GUS activities were detected: 210 U/mL for 0 g/L of silica, 387 U/mL for 5 g/L of silica, 576 U/mL for 10 g/L of silica, 474 U/mL for 15 g/L of silica, and 267 U/mL for 20 g/L of silica. The best GUS activities were achieved with the addition of 10 g/L of silica (Table 2). Our results are similar to those of previous studies. Coban et al. (2015) reported that the presence of 0–25 g/L talcum in media could enhance the production of phytase during A. niger fermentation. However, a high concentration of talcum decreased the enzymatic activity of phytase.
Fig. 3.
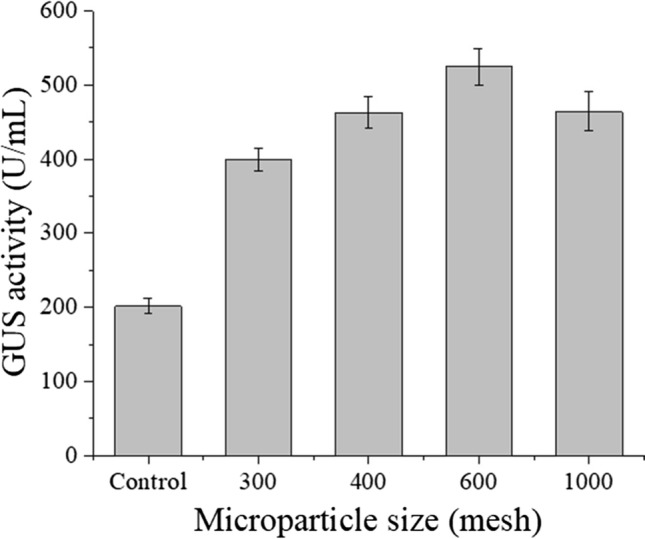
GUS activity of C. globosum DX-THS3 at varying sizes of 10 g/L silica
Table 2.
Effects of different concentrations of silica on the morphology of C. globosum DX-THS3
| Concentration of silica (g/L) | Average pellet size diameter (mm) | Enzyme activity (U/mL) |
|---|---|---|
| 0 | 3.0 ± 0.5 | 210 ± 8 |
| 1 | 2.5 ± 0.5 | 289 ± 13 |
| 5 | 1.1 ± 0.5 | 387 ± 13 |
| 10 | 0.7 ± 0.1 | 576 ± 12 |
| 15 | 0.7 ± 0.1 | 474 ± 19 |
| 20 | 0.7 ± 0.1 | 267 ± 15 |
The measurements were performed on the culture broth after 156 h of cultivation
Furthermore, the GUS activities of fermentation using C. globosum DX-THS3 which increased by silica was investigated. The morphology of DX-THS3 fermented with different concentrations of silica (0, 1, 5, 10, 15, and 20 g/L of silica) was also analyzed. As shown in Fig. 4, the following fungal pellet diameters were achieved: 3.0 ± 0.5 mm, without silica (Fig. 4a); 2.5 ± 0.5 mm, 1 g/L of silica (Fig. 4b); 1.1 ± 0.5 mm, 5 g/L of silica (Fig. 4c); 0.7 ± 0.1 mm, 10 g/L of silica (Fig. 4d); 0.7 ± 0.1 mm, 15 g/L of silica; and 0.7 ± 0.1 mm, 20 g/L of silica (Figs. 4e–f). Adding more than 10 g/L of silica microparticles did not affect the fungal pellet size. Overall, a full range of macro-morphological pellet forms from large dense pellets to small-sized pellets could be generated. The GUS activities were controlled by the pellet diameter of DX-THS3 and a fungal pellet diameter of 0.7 mm was deemed best for the production of GUS (in Table 2). These results indicate that silica can increase the GUS activities of DX-THS3 by controlling the fungal morphology during fermentation.
Fig. 4.
Analysis of C. globosum DX-THS3 morphology with different concentrations of silica: 0 g/L (a), 1 g/L (b), 5 g/L (c), 10 g/L (d), 15 g/L (e), and 20 g/L (f). Image analysis by light microscopy after 168 h of cultivation. The ruler is 2 mm
Growth and enzymatic production performance
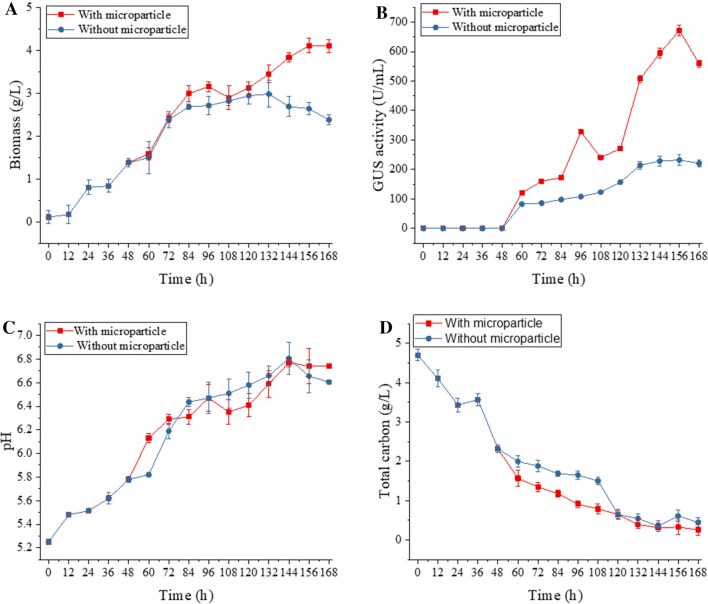
Our results indicate that the engineering of the morphology of C. globosum DX-THS3 is possible via the addition of microparticles. Hence, the effects of silica microparticles on the growth and enzyme production of C. globosum DX-THS3 were investigated. The biomass, GUS activity, pH, and total sugar were analyzed during the fermentation of DX-THS3 in the presence or absence of silica microparticles (Fig. 5). As shown in Fig. 5a, the accumulated biomass with microparticles increased from 2.8 to 4.2 g/L and the maximum biomass was achieved at 168 h for 4.2 g/L (Fig. 5a). In the absence of silica microparticles, the maximum biomass was observed at 132 h for 2.8 g/L. Thus, the presence or absence of microparticles can enhance or delay the effects of C. globosum DX-THS3 growth, respectively.
Fig. 5.
Analysis of biomass (a), GUS activity (b), pH (c), and total sugar consumption (d) throughout the growth process of C. globosum DX-THS3 with silica or without silica. Results are from triplicate cultivations with corresponding deviation
The effects of silica on GUS production via DX-THS3 fermentation were studied. The GUS activities were detected every 12 h from 48 h to 168 h after the fermentation of DX-THS3 with or without silica. The highest GUS activity was achieved with 600 mesh and 10 g/L of silica concentration after 156 h of fermentation (Fig. 5b). Under these conditions, the resulting GUS activity (680 U/mL) was 3.2-fold higher than that in the control (210 U/mL). The achieved GUS activity (680 U/mL) was better than the GUS activity (14 U/mL) in P. purpurogenum Li-3 obtained by Huang et al. (2015). The GUS activity decreased at 96–108 h during the fermentation of DX-THS3 with silica, and the activity increased again. Meanwhile, a similar result for biomass was observed (Fig. 5a). This result suggested that biomass is closely related to enzyme activity. Previous studies reported that fungal spores generally exhibit a negative surface charge, which mainly induces fungal pellet formation and could be affected by pH (Zhang et al. 2015). High pH values are the driving factors for pellet formation. pH variation in fermentation was detected using DX-THS3 with or without silica. As shown in Fig. 5c, the pH levels of the two types of fermentation did not show an obvious difference and they all increased from 5.5 to 7. Therefore, pH did not contribute to the formation of the pellet morphology of C. globosum DX-THS3. The total sugar in the fermentation of C. globosum DX-THS3 with and without microparticles was determined (Fig. 5d). The total sugar consumption was fast when microparticles were added. Thus, a larger amount of carbon source was utilized at a higher rate by C. globosum DX-THS3 in the presence of silica than in the absence of silica. In addition, more biomass of C. globosum DX-THS3 was obtained with the addition of silica than without it. As such, microparticle addition can accelerate C. globosum DX-THS3 growth due to the consumption of sugar, resulting in a higher biomass than that in the case of absent microparticles.
Conclusion
The targeted engineering of the morphology of C. globosum DX-THS3 into small pellets was performed via the addition of silica microparticles. The fungal pellet size and structure could be precisely adjusted by adding the appropriate amount of micromaterial. This strategy could open new possibilities in the use of microparticles for tailor-made morphology design in biotechnological production. In this study, the optimized silica concentration (10 g/L) and size (600 mesh) were utilized and this strategy successfully produced a highly active biopellet form, which strongly enhanced GUS production via the fermentation of DX-THS3. Our data showed that the achieved GUS activity was 680 U/mL, which was approximately 3.2 times higher than the corresponding GUS activity in a conventional operation without silica. Meanwhile, the biomass was significantly higher than the control. Thus, our results may provide a potential strategy for optimizing the fermentation of filamentous microorganisms.
Acknowledgements
This study was supported by Natural Science Foundation of China (31260137), the Natural Science Foundation of Jiangxi Province of China (20171BAB204009), Funds of Jiangxi Science and Technology Normal University (3000990631), the Foundation of Jiangxi Educational Committee (GJJ180636), Funds of Jiangxi Science and Technology Normal University (2017XJZD004).
Footnotes
Liangqing Du and Boliang Gao contributed equally to this work.
References
- Ahmad M, Panda BP. Alginate immobilization of Escherichia coli mtcc 1652 whole cells for bioconversion of glycyrrhizinic acid and into 18-β glycyrrhetinic acid. Pak J Biol Sci. 2013;16(24):2046–2049. doi: 10.3923/pjbs.2013.2046.2049. [DOI] [PubMed] [Google Scholar]
- Ashfaq UA, Masoud MS, Nawaz Z, Riazuddin S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. J Transl Med. 2011;9(1):112. doi: 10.1186/1479-5876-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban HB, Demirci A, Turhan I. Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng. 2015;38(6):1075–1080. doi: 10.1007/s00449-014-1349-4. [DOI] [PubMed] [Google Scholar]
- Driouch H, Sommer B, Wittmann C. Morphology engineering of Aspergillus niger, for improved enzyme production. Biotechnol Bioeng. 2010;105(6):1058–1068. doi: 10.1002/bit.22614. [DOI] [PubMed] [Google Scholar]
- Driouch H, Roth A, Dersch P, Wittmann C. Filamentous fungi in good shape: microparticles for tailor-made fungal morphology and enhanced enzyme production. Bioeng Bugs. 2011;2(2):100. doi: 10.4161/bbug.2.2.13757. [DOI] [PubMed] [Google Scholar]
- Driouch H, Wittmann C, Hänsch R, Wucherpfennig T, Krull R. Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng. 2012;109(2):462–471. doi: 10.1002/bit.23313. [DOI] [PubMed] [Google Scholar]
- Farag MA, Porzel A, Wessjohann LA. Unequivocal glycyrrhizin isomer determination and comparative in vitro bioactivities of root extracts in four glycyrrhiza species. J Adv Res. 2015;6(1):99–104. doi: 10.1016/j.jare.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamerah M. Factors affecting the growth form of Aspergillus terreus nrrl 1960 in relation to itaconic acid fermentation. Appl Microbiol Biotechnol. 1995;44(3–4):356–361. doi: 10.1007/BF00169929. [DOI] [Google Scholar]
- Han YY, Yang XD, Tang XY, Li C. Simultaneous determination of glycyrrhizic acid and its fermentation products by high performance liquid chromatography. Chin J Anal Lab. 2014;9:1059–1062. doi: 10.13595/j.cnki.issn1000-0720.2014.0250. [DOI] [Google Scholar]
- Huang S, Feng XD, Li C. Enhanced production of β-glucuronidase from Penicillium purpurogenum Li-3 by optimizing fermentation and downstream processes. Front Chem Sci Eng. 2015;9(4):501–510. doi: 10.1007/s11705-015-1544-0. [DOI] [Google Scholar]
- Huo XW, Yang S, Sun XK, Meng X, Zhao Y. Protective effect of glycyrrhizic acid on alcoholic liver injury in rats by modulating lipid metabolism. Molecules. 2018;23(7):1623. doi: 10.3390/molecules23071623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D, Weloosamy H, et al. Effect of agitation speed on the morphology of Aspergillus niger hfd5a-1 hyphae and its pectinase production in submerged fermentation. World J Biol Chem. 2015;6(3):265–271. doi: 10.4331/wjbc.v6.i3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanna S, Annemarie K, Antje L, Deniz T. From discovery to production: biotechnology of marine fungi for the production of new antibiotics. Mar Drugs. 2016;14(7):137. doi: 10.3390/md14070137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen CL, Coolen L, Hunik JH. Influence of morphology on product formation in Aspergillus awamori during submerged fermentations. Biotechnol Progr. 2008;14(2):233–240. doi: 10.1021/bp980014x. [DOI] [PubMed] [Google Scholar]
- Kang LP, Li LU, Zhao Y, Yu HS, Huang HZ, Cao M. Preparation of glycyrrhetinic acid monoglucuronide by selective hydrolysis of glycyrrhizic acid via biotransformation. Chin Herbal Med. 2012;4(4):324–328. doi: 10.3969/j.issn.1674-6348.2012.04.010. [DOI] [Google Scholar]
- Kaup BA, Ehrich K, Pescheck M, Schrader J. Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng. 2008;99(3):491–498. doi: 10.1002/bit.21713. [DOI] [PubMed] [Google Scholar]
- Krull R, Wucherpfennig T, Esfandabadi ME, Walisko R, Melzer G, et al. Characterization and control of fungal morphology for improved production performance in biotechnology. J Biotechnol. 2013;163(2):112–123. doi: 10.1016/j.jbiotec.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Kun RS, Gomes ACS, Hildén Kristiina S, Cerez SS, Mäkelä Miia R, et al. Developments and opportunities in fungal strain engineering for the production of novel enzymes and enzyme cocktails for plant biomass degradation. Biotechnol Adv. 2019 doi: 10.1016/j.biotechadv.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Li H, Guo D, Zhang L, Feng X. Glycyrrhizin attenuates histamine-mediated muc5ac upregulation, inflammatory cytokine production, and aquaporin 5 downregulation through suppressing the nf-κb pathway in human nasal epithelial cells. Chem Biol Interact. 2018 doi: 10.1016/j.cbi.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liao W, Chen S. Study of pellet formation of filamentous fungi Rhizopus oryzae using a multiple logistic regression model. Biotechnol Bioeng. 2010;99(1):117–128. doi: 10.1002/bit.21531. [DOI] [PubMed] [Google Scholar]
- Manzoni M, Rollini M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol. 2002;58(5):555–564. doi: 10.1007/s00253-002-0932-9. [DOI] [PubMed] [Google Scholar]
- Niu K, Hu Y, Mao J, Zou SP, Zheng YG. Effect of microparticle addition on the fermentation process of Echinomycin B. Chin J Bioprocess Eng. 2015;31(7):1082–1088. doi: 10.13345/j.cjb.140578. [DOI] [Google Scholar]
- Quan Y, Wang L, Liu Y, Cong J, Xie S, Wu X. Optimization of fermentation medium for glycyrrhizin biotransformation to monoglucuronyl-glycyrrhetinic acid by Plackett-Burman and Box-Behnken design. Ko Chem Eng Res. 2015;53(3):321–326. doi: 10.9713/kcer.2015.53.3.321. [DOI] [Google Scholar]
- Sathesh-Prabu C, Shin KS, Kwak GH, Jung SK, Lee SK. Microbial production of fatty acid via metabolic engineering and synthetic biology. Biotechnol Bioprocess Eng. 2019;24(1):23–40. doi: 10.1007/s12257-018-0374-6. [DOI] [Google Scholar]
- Sun ZG, Zhao TT, Lu N, Yang YA, Zhu HL. Research progress of glycyrrhizic acid on antiviral activity. Mini Rev Med Chem. 2019;19(10):826–832. doi: 10.2174/1389557519666190119111125. [DOI] [PubMed] [Google Scholar]
- Veiter L, Rajamanickam V, Herwig C. The filamentous fungal pellet—relationship between morphology and productivity. Appl Microbiol Biotechnol. 2018;102(7):2997–3006. doi: 10.1007/s00253-018-8818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verónica LC, Mario DB, Licia MP. Tailoring fungal morphology of Aspergillus niger mya 135 by altering the hyphal morphology and the conidia adhesion capacity: biotechnological applications. Amb Express. 2013;3(1):27. doi: 10.1186/2191-0855-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walisko R, Krull R, Schrader J, Wittmann C. Microparticle based morphology engineering of filamentous microorganisms for industrial bio-production. Biotechnol Lett. 2012;34(11):1975–1982. doi: 10.1007/s10529-012-0997-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao BL, Li XX, Zhang ZB, Yan RM, Yang HL, Zhu D. Phylogenetic diversity of culturable endophytic fungi in Dongxiang wild rice (Oryza rufipogon Griff), detection of polyketide synthase gene and their antagonistic activity analysis. Fungal Biol. 2015;119:1032–1045. doi: 10.1016/j.funbio.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Wang B, Chen J, Li H, Sun F, Li Y, Shi G. Pellet-dispersion strategy to simplify the seed cultivation of Aspergillus niger and optimize citric acid production. Bioprocess Biosyst Eng. 2017;40(1):45–53. doi: 10.1007/s00449-016-1673-y. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hu B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour Technol. 2012;114:529–535. doi: 10.1016/j.biortech.2012.03.054. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang J. The filamentous fungal pellet and forces driving its formation. Crit Rev Biotechnol. 2015;36(6):1–12. doi: 10.3109/07388551.2015.1084262. [DOI] [PubMed] [Google Scholar]
- Zhang K, Yu C, Yang ST. Effects of soybean meal hydrolysate as the nitrogen source on seed culture morphology and fumaric acid production by Rhizopus oryzae. Proc Biochem. 2015;50(2):173–179. doi: 10.1016/j.procbio.2014.12.015. [DOI] [Google Scholar]
- Zou S, Liu G, Kaleem I, Li C. Purification and characterization of a highly selective glycyrrhizin-hydrolyzing β-glucuronidase from Penicillium purpurogenum li-3. Proc Biochem. 2013;48(2):358–363. doi: 10.1016/j.procbio.2012.12.008. [DOI] [Google Scholar]