Abstract
This study includes development, characterization, and optimization of herbal ethosomal formulation. The aim of the present study is to develop drug loaded ethosomes capped with Azadirachta indica (neem) which, was further incorporated in Carbopol 934 K thereby, resulting in the formation of ethosomal gel. The formulation is aimed to express effective treatment against fungal infection. The build was formulated using drug (Luliconazole), soyalecithin, ethanolic neem extract and propylene glycol. In total nine ethosomal, formulations of distinct concentrations of ingredients were processed, to determine out the optimized formulation among the all. Further the prepared drug loaded ethosomes were subjected to various evaluation parameters like particle size, zeta potential, polydispersity index (PDI) and % entrapment efficiency. For the evaluation of its surface morphology, transmission electron microscopy was executed whereas, atomic force microscopy was carried out which contributes in detail and depth information of surface morphology. For the analysis of thermal behavior Thermal gravimetric analysis graph was obtained for luliconazole, soyalecithin, neem extract, physical mixture and optimized formulation (LF5). Attenuated total internal reflection Fourier transforms infra-red spectroscopy was performed for luliconazole, soyalecithin, neem extract, physical mixture, and optimized formulation (LF5) to examine the interaction between the drug and the excipients. Viscosity, pH, spreadability and extrudability of the ethosomal gel were calculated to determine the suitability of the formulation for topical application. In vitro drug permeation study and antifungal activity was executed out with the aid of Wistar albino rat skin model and tube dilution assay respectively. The complete study wrap up, that this herbal ethosomal approach provides advanced sustained and targeted delivery of luliconazole. On analyzing the results, ethosomal formulation LF5 was found to be optimized one, due to its optimum concentration of soyalecithin (300 mg) and ethanol (35%). Hence it has maximum entrapment efficiency of 86.56 ± 0.74%. Optimum vesicle size, zeta potential, and PDI were found to be 155.30 ± 1.2 nm, − 42.20 ± 0.3 mV, and 0.186 ± 0.07 respectively. In vitro drug permeation study expresses release of 83.45 ± 2.51 in 24 h whereas; the in vivo activity proved that LF5 is more active and effective against Candida parapsilosis in comparison to Aspergillus niger. In the end, it was estimated that ethosomal suspension and lyophilized ethosomal suspension was utmost stable at 4 °C/60 ± 5 RH. The complete study clearly indicates that the buildup of ethosomal formulation with luliconazole and neem extract show synergistic effect thereby, expressing excellent result against the treatment of fungal infection.
Keywords: Ethosomes, Luliconazole, Antifungal, Dermal, Transdermal delivery
Introduction
In the aspect of advanced drug delivery systems, transdermal drug delivery expresses number of advantages, as it utilizes skin as route of administration for drug through passive diffusion at a predetermined and controlled rate thereby, providing optimal systemic action. Transdermal patch regulates the delivery of drug through both hydrophilic and lipophilic polymer. Ethosomes are basically novel, ingenious and invasive carrier vesicle for lipid based systems which, was first reported by Touitou et al. (2000). They are spongy, soft, and malleable in nature meant for advanced and improved delivery system of the drug. They are fundamentally phospholipid vesicles and are predominantly used for dermal and transdermal drug delivery systems. It chiefly compromises multiple and concentric layer of pliable phospholipid, ethanol, and water whereas, in case of liposomes it includes cholesterol instead of ethanol. It is bilayer (aqueous and lipid) vesicular system having affinity for both hydrophobic and lipophilic drug, ultimately enhancing the bioavailability. It consists of high concentration of ethanol in comparison to that of liposome thereby, boosting up topical delivery of drug and also aid in softening of lipid bilayer. As a result, unlike liposomal drug delivery systems, it increases the permeation though stratum corneum barrier thereby, directing the permeation into deep seated layers of the skin. This is not only achieved, as because of the operation of ethanol on stratum corneum whereas, deformability and permeability of vesicles contributes too. In addition its interaction to that of skin lipid adds up in the enhancement of skin permeation, hence resulting in steady state of transdermal flux of approximately 1 mg/cm2/h. The mechanism behind the drug permeation into the skin through ethosomes is till dated not understood. Whereas, the mechanism of high permeability of drug into the deep rooted layers of skin through ethosomes carrier system can be correlated with that of high content of ethanol, vesicle made up of phospholipid and lipids of skin. In the area of polar head, ethanol along with that of lipid molecules enhances fluidity thereby aiding in the increased permeability through the membrane. Due, to its distinct special characteristics, ethosomes are considered as excellent carrier for transdermal drug delivery system. Hence, it is recognized as an advanced version of liposome. Several previous studies have concluded that ethosomes expresses increased delivery across the skin at both in-vitro and in-vivo level such as, ketoprofen, testosterone, cannabidiol, buspirone hydrochloride, erythromycin, ammonium glycyrrhizinate, ibuprofen, benzocaine, fluconazole, finasteride, lamivudine, 5-aminolevulinic acid of drug, and luliconazole. This advanced carrier is quiet easy to build up as, it does not demand for special equipments. Last but not the least, discovery of ethosomes has led to new innovation in context vesicular studies for transdermal drug delivery system. (Ainbinder et al. 2009; Shumilov and Touitou 2010; Shumilov et al. 2010; Ainbinder et al. 2010).
Luliconazole is an imidazole antifungal medication; hence used to prevent the growth of fungus (Cappelletty et al. 2007; Sutton et al. 2017; Sheehan et al. 1999; Baginski et al. 2009). The drug is basically lipophilic in nature of approximately log P of about 4.07. It is an R enantiomer whereas, double bonded beside dithiolone group in E configuration; in addition it holds single chiral center. Pharmacodynamically the drug kills the fungal organism, mainly by causing change in the cell membrane of the fungus. Accurate mechanism of action (MOA) of Luliconazole is not yet acknowledged but it is speculated that it involves inhibition of cytochrome P450 14 a-demethylase (P45014M). This enzyme is employed in sterol biosynthesis pathway that ultimately results in the formation ergosterol, which is the chief component of fungal cell membrane. Inspite of the fact that luliconazole is usually administered topically, clinical trial has revealed that after the first dose in a patient suffering from Tinea pedia, a Cmax (maximum plasma concentration) was obtained to be 0.40 ± 0.76 mg/ml (mean ± SD). The volume of distribution is still not estimated. It has an excellent protein binding that is more than 99%. Metabolism of the drug is not so far determined. The cream of luliconazole available in market causes allergic reactions like rashes, irritation, swelling (mainly on face, tongue and throat), dizziness last but not the least trouble in breathing. Hence, to overcome side effects originated by cream, nanonized herbal ethosomal gel loaded with luliconazole drug was formulated. As, ethosomal gel will enhance the drug permeability though skin due to increased transdermal flux thereby, increasing the bioavaibility and are very much effective with least side effects.
In the proposed study, luliconazole drug was incorporated in herbal ethosomal system, which was formulated with the aid of ethanolic neem extract. Subsequently, the drug loaded ethosomes was incorporated in carbopol gel, thereby revealing sustained transdermal delivery of the drug. Further the formulation was subjected to various in-vitro characterization like particle size, zeta potential, PDI, % entrapment Efficiency, TEM, AFM, total internal ATR-FTIR, in vitro drug permeation study and in vivo characterization though tube dilution assay thereby, providing evidence for its suitability and effectiveness for the therapy.
Methods
Materials
The luliconazole drug was collected from Aurochem Laboratries Limited, Solan as a gift sample. Chemicals like soya lecithin, ethanol, methanol, isopropylene alcohol, and propylene glycol were obtained from Sigma-Aldrich Chemicals, USA. Carbopol 934 K was attained from Himedia laboratory Mumbai, India; whereas, rest of the ingredients utilized in the research work were pure and of good quality with analytical grade.
Preparation of ethanolic neem extract
Fresh leaves of neem (Azadirachta indica) were collected from the campus of Banasthali Vidyapith. The leaves were washed properly with aid of water to remove dirt and were further subjected to drying. Weighed quantity of neem leaves was soxheleted using 80% hydro-alcoholic solvent (80% ethanol, 20% double distilled water, v/v). Alcohol was completely evaporated at low temperature and the residue was weighed and was further dissolved in dimethyl sulfoxide (DMSO) for future use in the study (Pandey et al.2014).
Formulation of ethosomes incorporated with Luliconazole
Luliconazole incorporated ethosomes were formulated by considering the approach discussed by Touitous et al. (2000). All required necessities were weighed in distinct concentrations as tabulated in Table 1 for the preparation of ethosomal formulation. At the outset, ethanolic neem extract and propylene glycol were utilized to mix the drug and soya lecithin, and then the resulting solution was heated at temperature of 30 °C on a water bath. In addition, distilled water was supplemented in the form of fine flow in the solution. With the use of magnetic stirrer (Remi equipment, Mumbai) the solution was subjected to endless stirring, at rpm of 700 in a sealed vessel. Then, the obtained solution was kept at a temperature of 4 °C and subsequently with aid of probe sonicator, sonication was carried out for three times, each for approximately 5 min along with 5 min gap between the two (Touitou et al. 2000; Dave et al. 2017a, b).
Table 1.
Composition of luliconazole loaded ethosomes
| Composition | LF1 | LF2 | LF3 | LF4 | LF5 | LF6 | LF7 | LF8 | LF9 |
|---|---|---|---|---|---|---|---|---|---|
| Luliconazole (mg) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Soyalecithin (mg) | 200 | 200 | 200 | 300 | 300 | 300 | 400 | 400 | 400 |
| Ethanolic neem extract (%) | 30 | 35 | 40 | 30 | 35 | 40 | 30 | 35 | 40 |
| Propylene glycol (ml) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Water | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s |
Incorporation of Luliconazole loaded ethosomes in gel
Carbopol 934 K, 0.75% w/v was dissolved slowly and gradually in small quantity of distilled water, with continuous stirring with the use of magnetic stirrer, for a period of 1 h. Thereby, the polymer was allowed to swell and in addition 20 ml of ethosomal suspension loaded with luliconazole was supplemented into it, with constant agitation. Stirring was endless at the speed and temperature of 700 rpm and 30 °C respectively, in a closed vessel till homogenous ethosomal gel like texture was obtained. Further, 7.4 ml of triethanol amine was added (so that the pH get neutralized) at slow stirring thereby, facilitating formation of gel. In the end, glycerine was added which acts as a humectant hence, it increases skin hydration thus boosting up the permeation of drug through skin. By following above methods, nine ethosomal formulations were formulated of different concentration and were further used for several evaluation parameters (Anand et al. 2010; Paliwal et al. 2019a, b).
Characterization of ethosomes incorporated with Luliconazole
Determination of particle size, polydispersity index (PDI) and zeta potential
Important specifications like particle size, polydispersity index (PDI), and zeta potential is very much necessary to determine. These parameters were evaluated by the utilization of Malvern zetasizer nano ZS which, is laid the groundwork of dynamic light scattering. To proceed with the estimation, 1 ml of ethosomal formulation was dispersed in high performance liquid chomatography (HPLC) water. Further, readings were determined at a scattering angle of 90° at 25 °C with 0.8862 viscosity of the medium and refractive index of 1.36. Distribution of particle size was estimated by the evaluation of PDI which concludes about the width of size distribution. Whereas, zeta potential was determined through the use of both laser Doppler velocimetry and phase analysis light scattering (PALS). Reading for particle size, PDI, and zeta potential were obtained in triplicates (Paliwal et al. 2019a, b; Li et al. 2012).
Morphology of luliconazole loaded ethosomes though transmission electron spectroscopy (TEM) and atomic force microscopy (AFM)
Transmission electron spectroscopy (TEM) was carried out to determine morphology of the formulation. TEM, (AIIMS, Delhi) was considered for the estimation of internal composition, morphology and crystallization of sample. Magnification of 9000× and 200 kV was required to conduct the experiment. 20 µl of formulation was diluted ten times in a nutshell with help of deionized water, and further 2% w/v phosphotungstic acid was utilized for staining (approximately 30 s). Eventually the specimens were kept on a grid laminated with copper and were left for drying. For each specimen, two grids were set up and were frequently observed (Pathana et al. 2017).
Atomic force microscopy (AFM) is a modernized technology for imaging to evaluate height, diameter, and other surface properties of the buildup formulation, which cannot be easily obtained through SEM and TEM imaging. In addition, at high resolution it provides information about swelling dynamics of the ingredients in aspect to each other and its real behavior. Evaluation was done by cautiously placing 1 ml of ethosomal suspension on a dirt free sliced mica sheet and was allowed to incubate for a period of 5 min. Thickness of the prepared slide was adjusted manually by adding more or less drop of formulation consequently. Then it was rinsed by deionized water to remove unbound ethosomes. Eventually, the sample was allowed to dry at room temperature and was kept under the lens and analyzed at distinct magnification to get three-dimensional structure. Scanning was performed with the aid of Advance Integrated Scanning tool for Nano technology (AIST- NT) Model: Smart SPM 1000, (NIFP, Russia) in tapping mode. For each specimen scanning was performed at a resolution of 5 µm × 5 µm and ultimately the images were visualized one by one, by expressing height, amplitude, and phase signal of the cantilever in the trace direction (Dave et al. 2017a, b).
Determination though attenuated total internal reflection Fourier transforms infra-red spectroscopy (ATR-FTIR)
Infrared spectra of pure drug (luliconazole), physical mixture (1:1), soyalecithin, and of optimized ethosomal formulation loaded with that of luliconazole was scanned with the aid of Bruker EQUINOX 55 FTIR spectrophotometer. The instrument is provided with liquid nitrogen cooled mercury cadmium telluride (MCT) detector at a resolution of approximately 2 cm−1. Diamond is acknowledged as an essential feature for the determination of internal reflection and hence was placed at an incidence angle of 45°. At last the 32 and 21 resolutions were considered as speed of scans rate for single internal reflection. The procedure involves dispersing of sample in KBr (200–400 mg) and further was subjected to compression by applying pressure of 5 tons for 5 min to mould the sample into pellet. Finally, the pellet was placed in the path of light and ultimately the spectra were obtained. From the frequency range of 4000–400 cm−1, the spectra was scanned and further advanced ATR correction was used to obtain the spectrum. Fitting of all peaks was executed out with the aid of software named opus (Venkatesh et al. 2015; Dave et al. 2019).
Determination though thermo-gravimetric analysis (TGA)
Physiochemical information like weight loss, sublimation vaporization, absorption, adsorption etc. was obtained though TGA execution. Thermal nature of drug, polymer, and lyophilized formulation were obtained with the aid of TG-4000 Perkin Elmer with Manager Software. TGA involves expression of specific characteristics of the sample which either shows weight loss or weight gain due to decomposition. First of all, the weight of empty crucible and with that of specimen was recorded thereby, initiating the execution of characterization. Further analysis of specimens were performed in the assembly and the thermo-gravimetric graph was examined, reported, and sketched by considering temperature and present weight loss on X and Y axis respectively (Babu et al. 2003).
Determination of entrapment efficiency, EE (%)
Entrapment efficiency, EE (%) is calculated so as to estimate the quantity of drug entangled within the colloidal system. Initially small amount of formulation was taken in Eppendorf tube and then it was subjected to centrifugation at a speed of 14,000 rpm at a temperature of 4 °C for 15 min. The process was rerun until and unless cloudless form of supernatant was obtained by aid of ultracentrifuge (Remi) which is furnished with TLA-45 rotor. The cloudless supernatant was attained and the concentration of drug was measured with the help of UV/visible spectrophotometer at wavelength of 299 nm for ultimate result, further quantity of drug entangled in the colloidal system, was calculated though following formula. Each and every sample was estimated in triplicates (Ling et al. 2010; Shah et al. 2012).
Estimation of pH, viscosity, spreadability, and extrudability
Digital pH meter was utilized for the determination of the formulation. The glass electrode was immersed entirely into the ethosomal gel and finally the readings were displayed and recorded. Brookfield viscometer (model LVDV-II Pro) spindle of S 96, was immersed into the beaker containing ethosomal gel at 10, 15, 20 rpm for various interval at lower, middle and upper case to estimate the viscosity of the formulation. Whereas, the room temperature was maintained during the entire process execution (Mandal et al. 2013).
For estimation of spreadability, 1 g of ethosomal gel was accurately weighed and was retained between two glass slides of 8 cm in length. Disparate weights were tied to the pulley and weight at which the glass slide shifted from its position and the time needed to pull the upper slide and lead into further extension of gel to the lower slide was recorded. The measurement was taken thrice and lastly the readings for spreadability were measured from the succeeding formula:
where S, M, L and T stands for spreadability for ethosomal gel, weight tied to the upper slide (g), distance moved by the slide (cm) and time taken by the upper slide to move downwards (s) respectively.
Extrudability was preceded by packing 20 g of formulated ethosomal gel in collapsible tube and further pressure was applied normally. Clamp was attached to the tube, to avoid the back flow of the content. In the end, the tube was opened and the amount of gel extruded out from the tube till the pressure persists was measured and recorded (Panigrahi et al. 2006).
Determination of stability studies
Stability testing of the formulation was done to determine the draining and drug amassing properties of lipid layers which, are the considerable issues with the stability of ethosomal formulation during the time of storage. Drug holding capacity of optimized ethosomal formulation was determined though stability testing as per ICH guidelines. The experiment was carried out for three months at distinct temperatures. Both of the formulations that is, lyophilized ethosomes and ethosomal suspension were segregated into two and were stored in a closed vial of 10 ml at 4 °C/60 ± 5 RH in refrigerator and at room temperature of 25 °C/60 ± 5 RH. The samples were examined as the function of time after 15, 30, 60, and 90 days of hoarding and drug entangled in the formulation was determined with the aid of centrifugation hence, same procedure was adopted as that of entrapment efficiency (Kim et al. 2006).
In vitro study for skin permeability
The animal experimental studies were in accordance with institutional animal ethics committee (IAEC) protocol number BV//2018-19/3769. Male albino Wistar rat of weight approximately (150–200 g) was considered for the execution of in-vitro skin permeation study. At outset, hairs of rat from the dorsal region of the skin were trimmed by applying hair removal cream. Physiologically salt solution (PSS) and distilled water were used to clean the subcutaneous fat and connective tissues of the skin to remove debris and other excessive materials. Cleaned skin was encased in aluminum foil and was hoarded in a refrigerator at 4 °C until it was used, for experiment execution. The thickness of the skin was estimated to be 2.6 ± 0.2 mm though digital micrometer. Franz diffusion cell was setup and the prepared skin was brought to normal temperature. The skin acts as a semi-permeable membrane which was hooked up at the bottom of the donor section, whereas the receptor section was repleted with 10 ml phosphate buffer of pH 7.4. Constant stirring was provided at 200 rpm and the degree of hotness was setup constant at 37 ± 1 °C with the aid of magnetic stirrer. 1 g of luliconazole loaded ethosomal gel was weighed accurately and was supplemented into the donor compartment, on the skin. Aliquots of 1 ml of specimen was taken out from the receptor region at prearranged intervals, i.e., at 1, 2, 3, 4 …0.24 h and was replaced by fresh media to maintain sink condition. Further, the withdrawn specimens were percolated though the membrane of 0.45 µm size. The solutions were subjected to UV/visible spectrophotometer readings at 299 nm against suitable blank. Similar procedure was repeated for all formulations and finally graph was sketched between % cumulative amount of drug release and time. For all ethosomal formulation, the experiment was performed thrice and the readings were expressed in the form of mean ± SD (Agrawal et al. 2013; Dave et al. 2010).
Anti-fungal activity
Pure culture of C. parapsilosis (MTCC2512) and A. niger (MTCC8652) was gathered from microbial type culture collection, Chandigarh (India). Culture of C. parapsilosis was evaluated on yeast malt agar (YMA) medium plate whereas, A. niger were cultivated on, potato dextrose agar (PDA) medium plate. Agar slants were prepared and kept overnight at a temperature of 4 °C, and was used for the study. The prepared media temperature was made lower at about 45–50 °C and was transferred into different petri dishes thereby, initiating solidification of the media. Further. 0.1 ml of the considered strain was poured through micro pipette over the solidified media and spreading was done with the aid of L-tube. Three wells of diameter 6 mm in each plate was punched on the surface of agar with the aid sterilized cork or borer for pouring the formulation into it. The prepared petri dishes were kept in an incubator at 30 °C for about 72–96 h. Further the dishes were observed for zone of inhibition hence, measurement and comparison with that of standard formulation were executed out. For half inhibitory concentration (IC50) of formulation was evaluated with the use of tube dilution assay and hence it was correlated to that of the standard ketoconazole. (Hi-media laboratories Pvt. Ltd, India) which was used as a reference agent.
Tube dilution assay
Tube dilution method with some changes was considered, to evaluate half inhibitory concentration (IC50) of optimized formulation. Number of culture tubes containing 10 ml of PDA (A. niger) medium and YMA (C. parapsilosis) medium were prepared in sterilized glass ware. 200 µl of diluted drug and 1 ml of fungal culture (suspension was regulated to 0.5 Mc Farland standard turbidity, approximately 108 CFU/ml) were supplemented in each and every tube. Subsequently all tubes were incubated at 25 °C for 24–48 h, so as to promote fungal growth. The tubes which were not treated were considered as a control. Lastly concentration of fungus in the incubated culture tube were analyzed using UV spectrophotometer, hence absorbance was calculated at wavelength of 580 nm and ultimately IC50 was estimated using the formula:
where, AC and AS represents absorbance for control and absorbance for treated sample respectively.
Results
Determination of particle size, polydispersity index (PDI), and zeta potential
Estimation of particle size was done on the basis of dynamic light scattering (DLS) and the outcomes for particle size and PDI were listed in Table 2. Particle size range of ethosomal formulation varies between 155.3 ± 1.2 and 196.4 ± 2.4 nm and PDI range varies between 0.186 ± 0.07 and 0.413 ± 0.073. Formulation LF5 exhibit maximum entrapment efficiency in comparison to the rest of the formulation, with optimum particle size and PDI of 155.3 ± 1.2 nm and 0.186 ± 0.07 respectively. Whereas, formulation LF9 exhibit maximum value of particle size and PDI that is 196.4 ± 2.4 nm and 0.413 ± 0.073 respectively. Size of the vesicle plays very crucial role in case of topical drug delivery. In the investigation, it has been noticed that on increasing the concentration of soyalecithin and ethanolic neem extract above 300 mg and 35% respectively there is sudden increase in the particle size of the formulation. Thereby, from the above statement it can be concluded that on altering the ratio of lipid and ethanol effects, the physical properties of formulation in one or other way. In addition, it can be stated that size of particle and concentration of ethanol affects the rate of penetration of formulation into the skin. As, smaller is the size of the vesicle, more competently it delivers the content into the rooted layers of the skin. In account of above outcomes the formulation LF 5 showed optimum particle size of about 155.3 ± 1.2 hence, expressed excellent appropriateness for topical application. The order for particle size was obtained as—LF5 < LF4 < LF3 < LF2 < LF6 < LF1 < LF7 < LF8 < LF9 whereas, of PDI the order was—LF5 < LF1 < LF4 < LF6 < LF3 < LF8 < LF7 < LF2 < LF9. Zeta potential is considered for determining the stability of ethosomal formulation. The values of zeta potential varied between − 23.5 ± 1.6 and − 42.2 ± 0.03 mV as listed in Table 2. Highest value of zeta potential is displayed by LF5 that is − 42.2 ± 0.03 mV whereas; lowest value of it is expressed by LF8 of about 38.5 ± 0.7 mV. Formulations LF 1, LF2, and LF 3 displayed no meaningful change in the values of zeta potential that is − 29.5 ± 0.6, − 30.3 ± 1.5 and − 32.5 ± 0.8. Whereas, formulations LF4, LF5, and LF8 showed increased value of zeta potential − 37.5 ± 0.5, − 42.2 ± 0.3 and − 38.5 ± 0.7. From the above data it can be stated that the particles present in the suspension carries charge of anionic nature which, ultimately increases on increasing and hence prevent aggregation of particles. The order for Zeta potential can be expressed as—LF5 < LF8 < LF4 < LF3 < LF7 < LF2 < LF1 < LF6 < LF9 (Verma et al. 2003; Plessis et al. 1994).
Table 2.
Glimpse of characterization of different ethosomal formulation
| Characterization | LF1 | LF2 | LF3 | LF4 | LF5 |
|---|---|---|---|---|---|
| Particle size | 178.3 ± 2.4 | 172.6 ± 3.2 | 166.2 ± 4.6 | 162.7 ± 5.3 | 155.3 ± 1.2 |
| PDI | 0.252 ± 0.05 | 0.302 ± 0.04 | 0.292 ± 0.05 | 0.263 ± 0.02 | 0.186 ± 0.07 |
| Zeta potential | − 29.5 ± 0.6 | − 30.3 ± 1.5 | − 32.5 ± 0.8 | − 37.5 ± 0.5 | − 42.2 ± 0.3 |
| pH | 6.1 | 6.4 | 5.7 | 5.9 | 5.2 |
| Viscosity | 6849 ± 0.5 | 7321 ± 1.3 | 7063 ± 2.8 | 7521 ± 1.9 | 7232 ± 1.2 |
| Spearability | 5.90 ± 0.8 | 6.20 ± 1.5 | 6.60 ± 0.7 | 7.83 ± 0.3 | 8.28 ± 0.6 |
| Extrudability | ++ | ++ | ++ | ++ | +++ |
| Characterization | LF6 | LF7 | LF8 | LF9 |
|---|---|---|---|---|
| Particle size | 178.6 ± 4.1 | 182.8 ± 2.7 | 189.3 ± 5.3 | 196.4 ± 2.4 |
| PDI | 0.298 ± 0.02 | 0.341 ± 0.06 | 0.37 ± 0.04 | 0.413 ± 0.073 |
| Zeta potential | − 27.4 ± 0.5 | − 31.3 ± 0.4 | − 38.5 ± 0.7 | − 23.2 ± 1.6 |
| pH | 5.3 | 6.7 | 6.1 | 5.8 |
| Viscosity | 8528 ± 2.4 | 6532 ± 1.6 | 6723 ± 1.8 | 7651 ± 0.5 |
| Spearability | 8.05 ± 1.5 | 5.80 ± 1.8 | 5.5 ± 0.7 | 4.8 ± 1.6 |
| Extrudability | ++ | ++ | ++ | +++ |
Morphology of luliconazole loaded ethosomes though transmission electron microscopy (TEM) and atomic force microscopy (AFM)
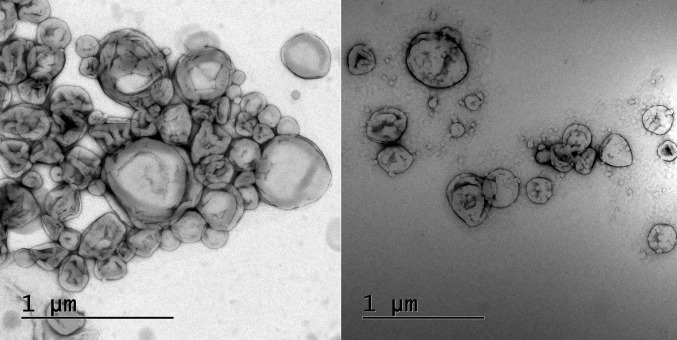
The surface morphology of the prepared, optimized LF5 buildup was expressed with the aid of TEM in (Fig. 1). Along with surface morphology of the formulation, TEM images also provide information about spherical shape of particle in the formulation. Images obtained though TEM expressed that the ethosomes were of round shape, smooth in appearance and free from crystalline nature of drug. TEM add up in the confirmation of surface morphology of optimized ethosomal formulation.
Fig. 1.
Surface morphology obtained by TEM
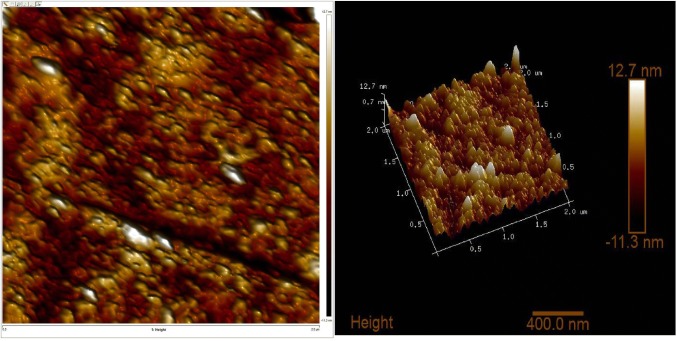
AFM images are displayed in (Fig. 2). An AFM image provides such deep information about the morphological characteristics, behavior of the formulation, and swelling dynamics among each other which, is quite a difficult task to obtain from TEM images. In addition, it justifies about the external morphology in the aspect of height, diameter, and area of the molecule. The image clearly expresses that the parameters like height and diameter are in appropriate range. The height, area, and diameter of the ethosomes are found to be 1.067 nm, 548.813 nm2, and 26.434 nm respectively. AFM displayed absence of cracks and pinholes in ethosomes (Bratu et al. 2011; Muller et al. 2002).
Fig. 2.
Surface morphology obtained by AFM
Determination though attenuated total internal reflection Fourier transforms infra-red spectroscopy (ATR-FTIR)
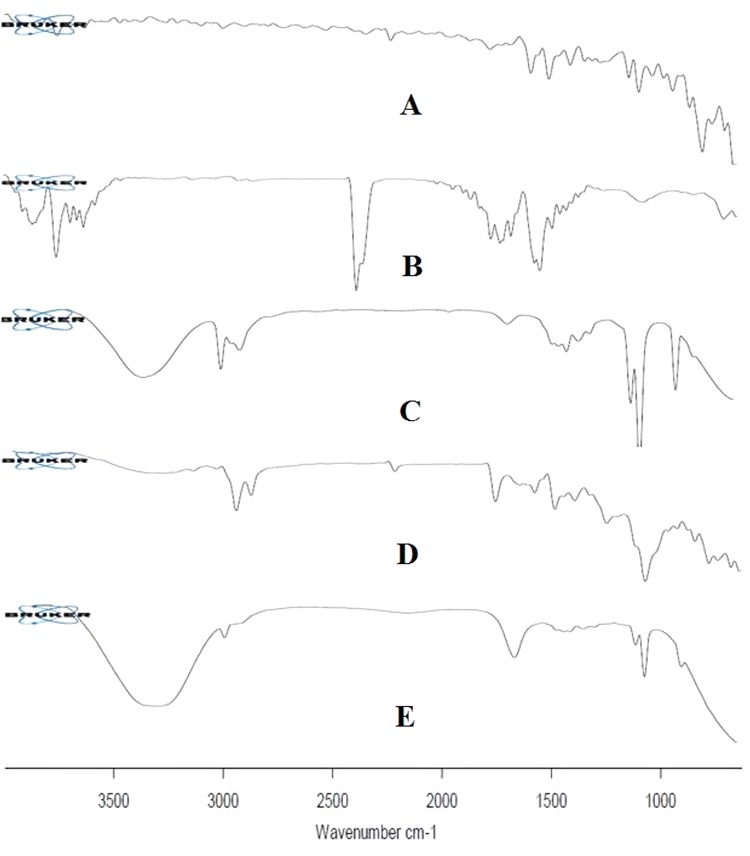
ATR-FTIR of pure drug, soyalecithin, neem extract, physical mixture, and LF5 was performed and its results were evaluated from (Fig. 4). Spectrum obtained though ATR-FTIR spectroscopy for drug, represented number of peaks significantly at 2200–2250 cm−1, 1640–1680 cm−1, 1030–1230 cm−1, 1550–1650 cm−1, 600–800 cm−1, 685–698 cm−1 and 836.36 cm−1 due to C≡N, C=C, C–N, C=N, C–Cl, C–S and distribution of aromatic protons. The formulation with highest entrapment efficiency was considered to be optimized. Hence LF5 with 86.56 ± 0.74% of entrapment efficiency was considered to optimize and its prominent peaks appear at 3300 cm−1, 1700 cm−1 and 1200 cm−1. The ATR-FTIR peaks of luliconazole and optimized formulation showed no modification hence, it clearly depicts that there was no interaction between drug and polymer. (Ahmad et al. 2019a, b; Venkatesh et al. 2015; Harun et al. 2014). The spectra for IR spectroscopy are shown in (Fig. 3).
Fig. 4.
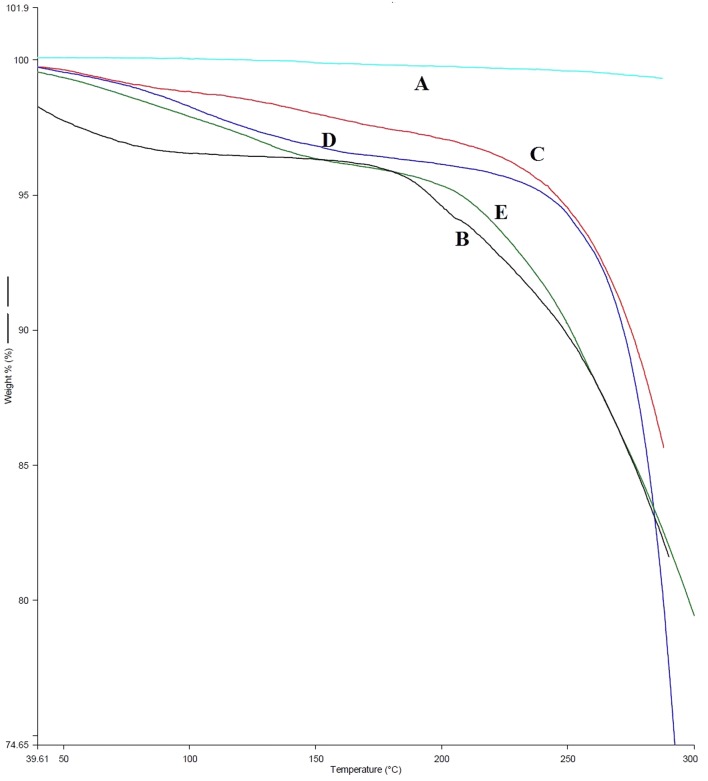
Overlapped TGA obtained graph (a) Luliconazole, (b) soyalecithin (c) neem extract (d) physical mixture and (e) optimized formulation (LF5)
Fig. 3.
Overlapped ATR-FTIR obtained graph (a) luliconazole (b) soyalecithin (c) neem extract (d) physical mixture (e) ethosomal formulation
Determination though thermal gravimetric analysis (TGA)
TGA study was executed out to determine change in mass of sample in accordance with change in temperature. TGA was done for (A) luliconazole, (B) soyalecithin, (C) neem extract, (D) physical mixture and (E) optimized formulation (LF5), so as to investigate their thermal property. The execution of experiment was carried out at controlled temperature and in controlled environment for all specimens. The percentage of weight loss (%) of luliconazole, soyalecithin, physical mixture, and optimized formulation was estimated to be 100.01%, 96%, 97%, 98% and 95% at a melting temperature of 310 °C, 305 °C, 300 °C, 290 °C, and 320 °C, respectively. The optimized formulation showed abrupt decrease in % weight loss of 91% between 150 °C and 200 °C and becomes a constant till 250 °C. From the TGA evaluation, it is depicted that soyalecithin along with the mixture of drug enhance the stability of the formulation. In addition, the results clearly indicate that there was no or minimal opposing effect on the formulation in the aspect of excipients and moisture. (Babu et al. 2003). Superimposed TGA spectrum is displayed in (Fig. 4).
Estimation of entrapment efficiency, EE (%)
Entrapment efficiency is one of the essential specifications, as it tells about the delivery capacity of the buildup formulation. The value of entrapment efficiency of different ethosomal formulation loaded with Luliconazole is recorded in Table 2. The graph was plotted for all the formulations as shown in (Fig. 5). From the graph it was depicted that the formulation LF5 has highest entrapment efficiency of 86.56 ± 0.74% whereas, LF9 has minimum entrapment efficiency of 48.93 ± 1.23%. Maximum entrapment efficiency of the optimized formulation EF5 is due the optimum amount of lipid and ethanol that is about 300 mg and 35% respectively. Whereas, formulation LF1, LF2, LF3, LF4, LF6, LF7 and LF8 showed entrapment efficiency of 66.23 ± 0.14%, 73.48 ± 0.29%, 61.75 ± 0.63%, 78.19 ± 1.63%, 63.39 ± 2.12%, 56.23 ± 0.86% and 52.23 ± 0.59% respectively (Shumilov et al. 2010; Sutton et al. 2017; Verma et al. 2003). Formulation LF1, LF3 and LF6 show no significant difference in the values of entrapment efficiency 66.23 ± 0.14%, 61.75 ± and 63.39 ± 2.12. Further decreased value of entrapment efficiency is expressed by LF7, LF8 and LF9 182.8 ± 2.7, 189.3 ± 5.3 and 48.93 ± 1.23%. From the results, it was investigated that ethanolic content upto 35% showed increased entrapment efficiency whereas, on increasing the concentration above 35% ultimately leads in the decreased value of entrapment efficiency. The entrapment efficiency increased due to increase in the concentration of lecithin till 300 mg but as the concentration is increased above it the entrapment value starts decreasing. As, because of high permeation characteristics due to presence of ethanolic content leads in the outflow of lipid bilayer. But the statement is contraindicated in case of LF6, as the concentration of soyalecithin is 300 mg and shows decreased entrapment efficiency of 63.39 ± 2.12% due, to the high concentration of ethanol of about 40%. In addition it is well known that high content of ethanol does not coexist with that of lipid vesicle. Hence, it can be concluded that concentration of soyalecithin and ethanol significantly affect the particle size and entrapment efficiency of ethosomal formulation. The increasing order of entrapment efficiency for all nine ethosomal formulation was established as—LF5 > LF4 > LF2 > LF1 > LF6 > LF3 > LF7 > LF8 > LF9 (Ling et al. 2010; Shah et al. 2012).
Fig. 5.
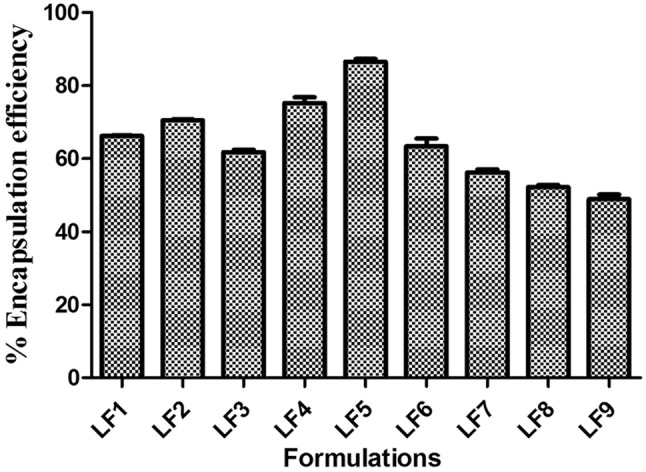
EE (%) of ethosomal formation LF1 to LF9
Determination pH, viscosity spreadability and extrudability
The parameters like pH and viscosity of all ethosomal formulation was found to be ranged between 6.7 to 5.2 and 8528 ± 2.4 to 6532 ± 1.6 cps respectively. The results of all formulations are listed in Table 2. The pH and viscosity of optimized formulation (LF5) was found to be 5.2 and 7232 ± 1.2 cps, respectively (Dave et al. 2017a, b). The results of spreadability and extrudability show excellent range of values which were recorded in Table 2. The outcomes of optimized formulation that is LF5 for spreadability and extrudability were found to be 8.28 ± 0.6 cm and +++, respectively (Panigrahi et al. 2006). The results showed that the formulation is very much suitable for topical application. The optimized formulation LF expressed optimum value of pH, viscosity, and spreadability which indicates good gelling property of ethosomal gel in the aspect of topical application. Hence, the ratio of ethanol and lipid for formulation LF5 was found to be optimized on considering all parameters mentioned in (Table 2). It can be stated that high content of ethanol plays an important role in the formulation.
Stability studies
The capacity of ethosomal system to cling with the drug was estimated though stability studies. The study was carried out at distinct temperatures continuously for 3 months at different temperatures. The chief issues with that of ethosomal formulation are drainage and aggregation of drug in/from the lipid bilayer. The optimized formulation LF5 was scrutinized for stability testing and the outcomes were listed in Table 3. In this study, two batches of lyophilized ethosomal suspension and ethosomal suspension were sealed in a vial and was kept at 4 °C/60 ± 5 RH (n = 3) and at 25 °C/60 ± 5 RH for the initiation of study. On 7, 15, 30, 60, and 90 days of hoarding, sampling was done. Millipore water was utilized to redisperse the obtained lyophilized sample and was subjected to sonication for 1–2 min. And then further various evaluations like particle size, zeta potential, PDI, and % entrapment efficiency were performed. The procedure was executed thrice to get accurate outcomes and the outcomes were expressed in form of mean ± SD (Kim et al. 2006). From the results of stability testing, it can be concluded that entrapment efficiency value decreases when ethosomal suspension and lyophilized ethosomal suspension are stored at 25 °C/60 ± 5 RH whereas, no significant change is noticed when the sample are stored at 4 °C/60 ± 5 RH. Hence, lastly it can be declared that 4 °C/60 ± 5 RH was considered as suitable and appropriate condition storage condition for ethosomal formulation LF5.
Table 3.
Stability study of optimized formulation
| Time (days) | Microscopic evaluation | % Encapsulation efficiency |
|---|---|---|
| Ethosomal suspension (LF5), At 4 °C/60 ± 5 RH (n = 3) | ||
| Initial | Smooth spherical vesicle | 94 ± 2.9 |
| 7 | Smooth spherical vesicle | 96 ± 1.2 |
| 15 | Smooth spherical vesicle | 94 ± 1.0 |
| 30 | Smooth spherical vesicle | 90 ± 2.6 |
| 60 | Smooth spherical vesicle | 84 ± 1.7 |
| 90 | Rough spherical vesicle | 78 ± 2.1 |
| At 25 °C/60 ± 5 RH (n = 3) | ||
| Initial | Smooth spherical vesicle | 96 ± 1.0 |
| 7 | Smooth spherical vesicle | 92 ± 1.5 |
| 15 | Smooth spherical vesicle | 88 ± 2.0 |
| 30 | Smooth spherical vesicle | 81 ± 2.4 |
| 60 | Rough spherical vesicle | 67 ± 2.0 |
| 90 | Agglomerate | 61 ± 1.2 |
| Lyophilized ethosomal suspension (LF5), At 4 °C/60 ± 5 RH (n = 3) | ||
| Initial | Smooth spherical vesicle | 96 ± 1.0 |
| 7 | Smooth spherical vesicle | 95 ± 1.6 |
| 15 | Smooth spherical vesicle | 92 ± 1.9 |
| 30 | Smooth spherical vesicle | 88 ± 2.6 |
| 60 | Rough spherical vesicle | 85 ± 1.3 |
| 90 | Rough spherical vesicle | 81 ± 3.5 |
| At 25 °C/60 ± 5 RH (n = 3) | ||
| Initial | Smooth spherical vesicle | 94 ± 2.9 |
| 7 | Smooth spherical vesicle | 96 ± 1.2 |
| 15 | Smooth spherical vesicle | 94 ± 1.0 |
| 30 | Rough spherical vesicle | 90 ± 2.6 |
| 60 | Rough spherical vesicle | 84 ± 1.7 |
| 90 | Agglomerate | 78 ± 2.1 |
In-vitro study for skin permeability
The release profile was analyzed for all formulations with the aid of intact skin of Wistar albino rats. Modification in the concentration of ethanol and lipid plays crucial role in the release of the drug luliconazole. The cumulative amount of drug permeated though per unit area of rat’s skin was plotted as a function of time and the drug diffused was calculated from the slope of the obtained linear line. Cumulative drug release for all formulation (LF1–LF9) are 64.98 ± 0.63, 71.39 ± 0.45, 59.47 ± 0.02, 75.08 ± 0.65, 83.45 ± 2.51, 60.57 ± 0.61, 55.49 ± 0.07, 49.60 ± 0.85, and 45.27 ± 0.92, respectively in 24 h. The outcomes of the study for all formulations are shown in (Fig. 6). Formulation LF5 showed maximum release of drug that is 83.45 ± 2.51 due to large amount of drug entrapment in the formulation in context of the rest of the formulations. Whereas, the formulation LF9 showed least cumulative drug release of about 45.27 ± 0.92. At the start of the release the formulations expressed rapid release of the drug and then tends to sustained release due to the presence of lipid bilayer which acts as a limiting barrier thereby, reducing the amount of drug diffused. The formulations of LF5 show rapid release of drug as because of low concentration of lipid. Hence, it can be concluded that concentration of lipid and ethanol have great effect on the release of drug from the formulation. On considering all parameters, LF5 was found to have a optimized formulation and was recognized as a suitable formulation for in-vivo study (Ahmad et al. 2019a, b; Agrawal et al. 2013).
Fig. 6.
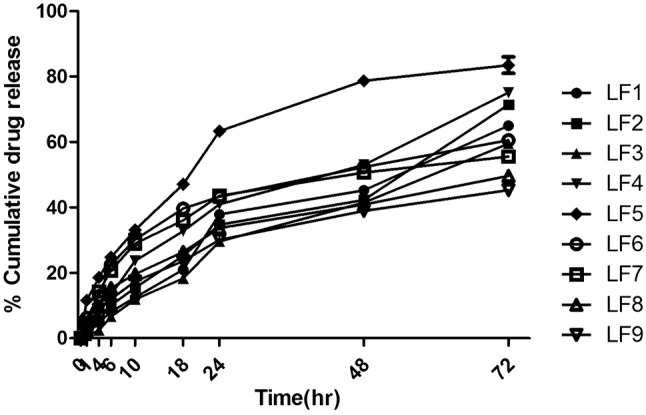
In vitro skin permeation release profile for ethosomal formation LF1 to LF9 loaded with luliconazole for 24 h
Anti-fungal activity
Anti-fungal activity was carried out to estimate efficacy of optimized formulation against the strains considered in the study. According to the outcomes of the study it can be concluded that, optimized formulation (LF5), was found to be more active and effectively opposite to C. parapsilosis strain when correlated to that of antibiotic standard. From the Figs. 7 and 8 it can be clearly depicted that the formulation LF5 is more competent toward C. parapsilosis. And this was further proved from the IC50 of the formulation against the two strain. IC50 of optimized formulation was obtained to be 15 µg/ml and 20 µg/ml against C. parapsilosis strain and 80 µg/ml and 60 µg/ml against A. niger strain. So, it can be end up by stating that the outcomes of the study undoubtedly indicate that the optimized formulation of LF5 is more powerful against C. parapsilosis in comparison to A. niger. Hence it was less effective towards the A. niger strain. The response of formulation was achieved after overnight incubation and is symbolized in Figs. 7 and 8) for C. parapsilosis and A. niger, respectively.
Fig. 7.
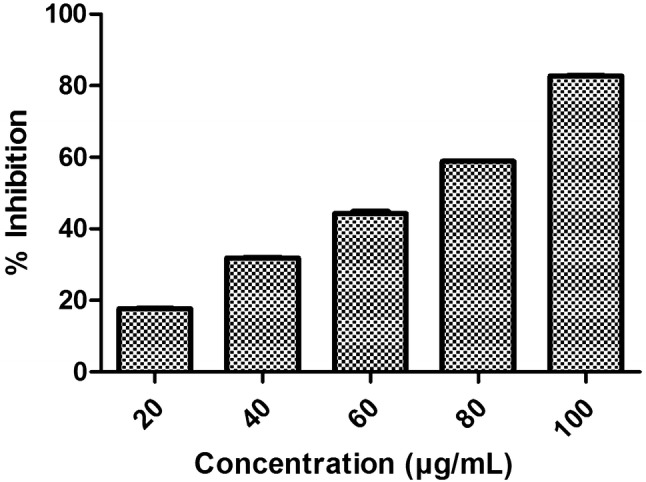
IC50 graph obtained against A. niger
Fig. 8.
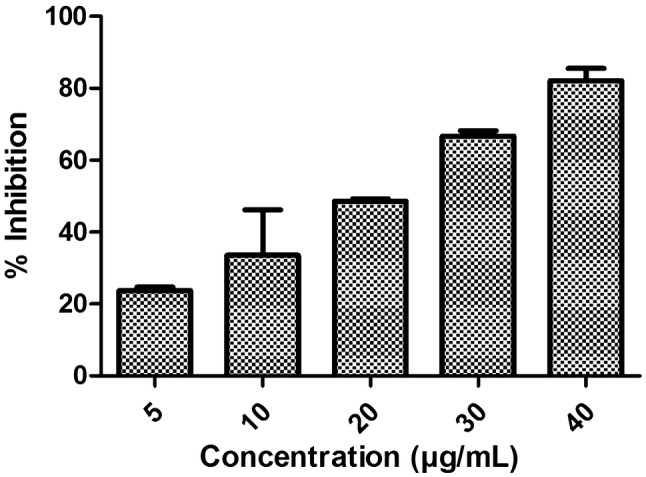
IC50 graph obtained against C. parapsilosis
Discussion
Herbal ethosomal gel was prepared with aid of ethanolic neem extract (Azadirachta indica) incorporated with that of Luliconazole (drug). The extract mainly consists of flavonoid chemical constitute which expresses excellent antifungal activity. The basic structural feature of flavonoids is the 2-phenyl-benzopyrane or flavan nucleus, which consists of two benzene rings linked with that of heterocyclic pyran ring (Brown 1980). Hence, these compounds show broad biological and pharmacological action including antifungal activities. (Kanwal et al. 2010; Niwano et al. 1998; Uchida et al. 2004) The formulated build up was subjected to various in-vitro and in-vivo characterization to reveal the evidence for its suitability and effectiveness for the therapy. In the estimation of particle size and PDI, it has been scrutinized that the concentration of soyalecthin and ethanolic neem extract are straightly linked to the size of the vesicle. An increment in the concentration of soyalecithin and ethanolic neem extract above 200 mg and 35% respectively demonstrate abrupt increase in the particle size and in addition show narrow distribution for PDI. Hence, it is concluded either increment or decrement in the proportion of lipid and ethanol, alters the physical properties of ethosomes to a large extent. The size of particles and concentration of ethanol affect the permeability of the skin, as because only particle of smaller size can go through the skin whereas, ethanol concentration usually affects the drug permeability. By taking in the above account we can come to an end, that LF5 is perfectly acceptable for topical application. In the case of zeta potential it was investigated that if the suspension carries large amount of anionic and cationic charge in the system (that is high value of negative or positive zeta potential), then the particles will experience repulsive force among themselves thereby, they will oppose agglomeration. In case, when the suspension carries less amount of anionic or cationic charge in the system (that is low value of positive or negative zeta potential), then there will be no existence of force between the particles hence they will tend to agglomerate. As a result, increase in the surface charge leads to the enhancement of ethosomes stability. From the above statement, it is depicted that LF5 shows maximum stability than the rest of the formulation as it has the highest value of zeta potential. Thereby, it can be concluded that the LF5 formulation particles will not come together easily thereby, preventing agglomeration. Hence from the study of zeta potential measurement, it can be wrapped up that the charge carried by the vesicle get effected by the alteration in the size of vesicle (Verma et al. 2003; Plessis et al. 1994). TEM images clearly depicts that the ethosomes are of round in shape, smooth in appearance and free from crystalline nature of drug. Whereas, AFM image provides deep information about the morphological characteristics, behavior of the formulation, and swelling dynamics among each other which, is quite a difficult task to obtain from TEM images. Further, the images clearly express that the parameters like height and diameter are in appropriate range thereby, adding up in the information about morphological characteristics by expressing height, area, and diameter of the ethosomes The height, area, and diameter of the ethosomes are found to be 1.067 nm, 548.813 nm2 and 26.434 nm, respectively (Bratu et al. 2011; Muller et al. 2002). The spectra of IR expresses that there is no modification in the IR peaks of pure drug and optimized formulation as, the spectra of both don’t overlap. The peak values for the drug occurs at 2200–2250 cm−1, 1640–1680 cm−1, 1030–1230 cm−1, 1550–1650 cm−1, 600–800 cm−1, 685–698 cm−1, and 836.36 cm−1 due to C≡N, C=C, C–N, C=N, C–Cl, C–S and distribution of aromatic protons. Whereas, the optimized formulation showed prominent peaks at 3300 cm−1, 1700 cm−1, and 1200 cm−1, thereby, undoubtedly expresses no interaction between drug and polymer (Venkatesh et al. 2015; Harun et al. 2014).
The TGA curves of luliconazole expresses that mass of the drug remain constant with an increase in temperature. But as soon as it reaches the melting point of the drug, the curve falls down thus, expressing loss of weight. Whereas, optimized formulation LF5 show 91% of weight loss at 150–200 °C and turn into consistent till 250 °C which concludes that the mixture of lipid and drug leads in the enhancement of stability of the formulation. From the above outcomes, it can be concluded that combination of soyalecithin with drug, enhances its stability. Thereby, in context of analysis of weight loss of the polymer and drug, TGA is considered as a fruitful method. All these results present that excipients and moisture have no or slight effect on the formulation (Babu et al. 2003). LF5 (optimized formulation) showed highest entrapment efficiency due to the reason of existence of optimum amount of lipid and ethanol that is 300 mg and 35% respectively. Among these LF1, LF3, and LF5 showed no noticeable difference in their values. However LF7 and LF8 showed decreased value of entrapment efficiency as correlated with the rest. On boosting up quantity of alcohol up to 35% in the formulation leads to the increment of entrapment efficiency. This is because of high permeation enhancing characteristics of ethanol that eventually results in the drainage of lipid bilayer. In addition, entrapment efficiency increases too, when the concentration of soyalecithin was about 200 mg. But it was examined that in LF5 the entrapment efficiency decreased, though the concentration of soyalecithin was 200 mg itself. This could be due to increased concentration of ethanol that is 40%. In general, it is well known that ethanol cannot coexist with that of lipid vesicles. From the above outcomes it can be summarized that entrapment efficiency as well as particle size have significant effect due to the varying concentration of ethanol and lipid, whereas the rest of the ingredients are kept constant. (Ling et al. 2010; Shah et al. 2012). The outcomes of pH and viscosity expresses that the formulations are of optimum pH and viscosity hence, are very much suitable for dermal therapy. The optimum range of spreadability and extrudability itself reveals that the formulation is acceptable and appropriate for dermal preparation and for dermal treatment. The spreadability results of ethosomal formulation express good gelling property for topical utilization (Panigrahi et al. 2006).
Formulation LF5 showed maximum release of drug that is 83.45 ± 2.51. Due to the existence of lipid bilayer it showed sustained release of drug, as the layer deed as a barrier thereby, restricting the rate of diffusion of the entangled drug into the formulation (Touitou et al. 2000). Whereas, the formulation LF9 showed least cumulative drug release of about 45.27 ± 0.92. Formulation LF1, LF2, and LF3 showed sudden release of drug, due to the existence of low concentration of lipid and low entrapment of the drug in the buildup. Hence, it can be wrapped up, that the modification of lipid and ethanol has forceful effect on the release profile of drug from the formulation. On analyzing, the result LF5 formulation was regarded as optimized formulation and was acknowledged appropriate for antifungal study (Agrawal et al. 2013). From the outcomes of stability study it is concluded that both lyophilized ethosomal suspension and ethosomal suspension show decrement in the % entrapment efficiency, when kept at 25 °C/60 ± 5 RH (room temperature), whereas, it does not show any meaningful modification in % entrapment Efficiency when hoarded at 4 °C/60 ± 5 RH (n = 3). Hence, it was evident that the optimized formulation LF5 storage is suitable at 4 °C/60 ± 5 RH (Kim et al. 2006). In the end, it can be concluded that the ratio of ethanol and lipid for formulation LF5 was found to be optimized on considering all parameters mentioned in (Table 2). It can be stated that high content of ethanol plays an important role in the formulation.
Conclusion
Ethosomes express themselves as an excellent carrier for the dermal delivery of luliconazole, which is entrapped between the lipid layer. It has been reported that ethosomes provide constant permeability of skin and increased drug permeability through lipid vesicle due to significant content of ethanol in its composition. Hence, this ultimately enhances the bioavaibility of drug luliconazole. The significant results of this study were optimum size of the vesicle, high entrapment efficiency, and better permeation properties of the vesicle. Thus, these properties express sustained release of drug with higher stability at 4 °C/60 ± 5 RH. Delivery of drug through ethosomal vesicle is affected by varying concentration of lipid and ethanol concentration in the formulation. Thus, in the end it can be wrapped up that ethosomes offer advanced, sustained, and targeted delivery of luliconazole thereby by providing efficacious treatment of antifungal infection. This kind of advanced approach can aid in the future for development and scaling of new formulation in the industry. Based on the present study, future investigation can be carried out to bring ethosomal gel loaded luliconazole formulation closer to clinical realization.
Acknowledgements
The author would like to give vote of thanks to Department of Pharmacy, Physics & Chemistry, Banasthali Vidyapith, Rajasthan, India for providing equipment and other required facilities for evaluation of samples.
Author contributions
VD: He has played a vital role as a mentor, in executing out the entire research. In addition, he has also given his fruitful contribution in writing the manuscript and thereby making it worthy, to be published. In addition being a corresponding author, he has negotiated all sorts of queries regarding publishing the paper. NB: He has formulated the formulation of ethosomes and incorporated it into gel form. Besides this, he has also done all the preformulation parameters. NG: She framed the research work and expressed them into words, thereby revealing the productive knowledge across the globe. Furthermore, she has managed the data and fulfilled the required necessities to make it publishable. KT: She has made all the characterization of optimized formulation and provided data for the study thereby, making the research more productive and reliable.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
References
- Agrawal U, Meha NK, Gupta U, Jain NK. Hyperbranched dendritic nanocarriers for topical delivery of dithanol. J Drug Target. 2013;21:497–506. doi: 10.3109/1061186X.2013.771778. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Ahmad R, Al-Qudaihi A, Alaseel SE, Fita IZ, Khalid MS, Bolla SR. A novel self-nanoemulsifying drug delivery system for curcumin used in the treatment of wound healing and inflammation. 3 Biotech. 2019;9(10):1–20. doi: 10.1007/s13205-019-1885-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ahmad N, Ahmad R, Al-Qudaihi A, Alaseel SE, Fita IZ, Khalid MS, Bolla SR. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019;9(35):20192–20206. doi: 10.1039/C9RA03102B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainbinder D, Touitou E. Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv. 2010;12:297–303. doi: 10.1080/10717540500176910. [DOI] [PubMed] [Google Scholar]
- Ainbinder D, Protokin R, Chaouat M, Touitou E. Effect of honokiol and 5-FU on non-melanoma skin cancer cells. J Drug Deliv Sci Technol. 2009;19:283–287. doi: 10.1016/S1773-2247(09)50053-6. [DOI] [Google Scholar]
- Anand N, Johnson M, Arulsamy A. Antifungal properties of neem (Azadirachta indica) leaves extract to treatment hair dandruff. E-Int Sci Res J. 2010;2:244–252. [Google Scholar]
- Babu R, Kanik KN, Kikwai L, Ortega C, Andega S, Ball K, Yim S, Singh M. The influence of various methods of cold storage of skin on the permeation. J Control Release. 2003;86:49–57. doi: 10.1016/S0168-3659(02)00368-1. [DOI] [PubMed] [Google Scholar]
- Baginski M, Czub J. Amphotericin B and its new derivatives—mode of action. Curr Drug Metab. 2009;10:459–469. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- Bratu I, Borodi G, Kacso I, Moldovana Z, Filip C, Dragan F, Vasilescu M, Simon S. New solid form of Norfloxacin: structural studies. J Spectrosc. 2011;25:53–62. doi: 10.1155/2011/462913. [DOI] [Google Scholar]
- Cappelletty D, Eiselstein-McKitrick K. The echinocandins. Pharmacotherapy. 2007;27:369–388. doi: 10.1592/phco.27.3.369. [DOI] [PubMed] [Google Scholar]
- Dave V, Kumar D, Lewis S, Paliwal S. Ethosome for enhanced transdermal drug delivery of aceclofenac. Int J Drug Deliv. 2010;2:81–92. doi: 10.5138/ijdd.2010.0975.0215.02016. [DOI] [Google Scholar]
- Dave V, Sharma S, Yadav RB, Agarwal U. Herbal liposome for the topical delivery of ketoconazole for the effective treatment of seborrheic dermatitis. Appl Nanosci. 2017;7:973–987. doi: 10.1007/s13204-017-0634-3. [DOI] [Google Scholar]
- Dave V, Yadav RB, Kushwaha K. Hybrid nanoparticles for the topical delivery of norfloxacin for the effective treatment of bacterial infection produced after burn. J Microencapsul. 2017;21:67–77. doi: 10.1080/02652048.2017.1337249. [DOI] [PubMed] [Google Scholar]
- Dave V, Gupta A, Singh P, Tak K, Sharma S. PEGylated Lipova E120 liposomes loaded with celecoxib: in-vitro characterization and enhanced in-vivo anti-inflammatory effects in rat models. J Biosci. 2019;44:82–94. doi: 10.1007/s12038-019-9919-x. [DOI] [PubMed] [Google Scholar]
- Harun NY, Afzal MT, Azizan MT. TGA analysis of rubber seed kernel. Int J Eng. 2014;3:639–652. [Google Scholar]
- Kanwal Q, Hussain I, Siddiqui HL, Javaid A. Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat Prod Res. 2010;24(20):1907–1914. doi: 10.1080/14786419.2010.488628. [DOI] [PubMed] [Google Scholar]
- Kim HS, Song IH, Kim JC. In vitro and in vivo gene transferring characteristics of novel cationic lipids, DMKD (O,O 0-dimyristyl-N-lysyl aspartate) and DMKE (O, O0-dimyristyl-N-lysylglutamate) J Control Release. 2006;11:234–241. doi: 10.1016/j.jconrel.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Li G, Fan Y, Fan C, Li X, Wang X, Li M, Liu Y. Tacrolimus-loaded ethosomes: physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm. 2012;82:49–57. doi: 10.1016/j.ejpb.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Ling G, Zhang P, Zhang W, Sun J, Meng Z, Qin Y, Deng Y, He Z. Development of novel self-assembled DS-PLGA hybrid nanoparticles for improving oral bioavailability of vincristine sulphate by P-gp inhibition. J Control Release. 2010;148:241–248. doi: 10.1016/j.jconrel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Mandal B, Bhattacharjee H, Mittal SH, Balabathula P, Thoma LA, Wood GC. Core-shell type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomed Nanotechnol Biol Med. 2013;9:474–491. doi: 10.1016/j.nano.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles and nanostructured lipid carriers in cosmetics and dermatological preparations. Adv Drug Deliv Rev. 2002;54:131–155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Niwano Y, Kuzuhara N, Kodama H, Yoshida M, Miyazaki T, Yamaguchi H. In vitro and in vivo antidermatophyte activities of NND-502, a novel optically active imidazole antimycotic agent. Antimicrob Agents Chemother. 1998;42:967–970. doi: 10.1128/AAC.42.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal S, Tilaka A, Sharma J, Dave V, Sharma S, Verma K, Tak K, Raghava K, Veera SR. Flurbiprofen-loaded ethanolic liposome particles for biomedical applications. J Microbiol. 2019;161:18–27. doi: 10.1016/j.mimet.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Paliwal S, Tilak A, Sharma J, Dave V, Sharma S, Yadav RB, Patel S, Verma K, Tak K. Flurbiprofen loaded ethosomes-transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019;18:1–15. doi: 10.1186/s12944-019-1064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G, Verma KK, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta indica (neem) leaves. Int J Pharm Pharm Sci. 2014;6(2):444–447. [Google Scholar]
- Panigrahi L, Ghosal SK, Pattnaik S, Maharana L, Barik BB. Effect of permeation enhancers on the release and permeation kinetics of lincomycin hydrochloride gel formulations though mouse skin. Indian J Pharm Sci. 2006;68:205–211. doi: 10.4103/0250-474X.25716. [DOI] [Google Scholar]
- Pathana IB, Jawarea BP, Shelkeb S, Ambekarc W. Curcumin loaded ethosomes for transdermal application: formulation, optimization, in-vitro and in-vivo study. J Drug Deliv Sci Technol. 2017;44:49–57. doi: 10.1016/j.jddst.2017.11.005. [DOI] [Google Scholar]
- Plessis JD, Ramachandran C, Weiner N, Müller DG. The influence of particle size of liposomes on the deposition of drug into skin. Int J Pharm. 1994;103:277–282. doi: 10.1016/0378-5173(94)90178-3. [DOI] [Google Scholar]
- Shah PP, Desai PR, Singh M. Effect of oleic acid modified polymeric bilayered nanoparticles on percutaneous delivery of spantide II and ketoprofen. J Control Release. 2012;158:336–345. doi: 10.1016/j.jconrel.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/CMR.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumilov M, Touitou E. Buspirone transdermal administration for menopausal syndromes, in vitro and in animal model studies. Int J Pharm. 2010;387:26–33. doi: 10.1016/j.ijpharm.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Shumilov M, Bercovich R, Duchi S, Ainbinder D, Touitou E. Ibuprofen transdermal ethosomal gel: characterization and efficiency in animal models. J Biomed Nanotechnol. 2010;6:569–576. doi: 10.1166/jbn.2010.1153. [DOI] [PubMed] [Google Scholar]
- Sutton CL, Taylor ZE, Farone MB, Handy ST. Antifungal activity of substituted aurones. Bioorg Med Chem Lett. 2017;27:901–903. doi: 10.1016/j.bmcl.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Touitou E, Dayana N, Bergelsonb L, Godina B, Eliaza M. Ethosomes-novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65:403–418. doi: 10.1016/S0168-3659(99)00222-9. [DOI] [PubMed] [Google Scholar]
- Uchida K, Nishiyama Y, Yamaguchi H. In vitro antifungal activity of Luliconazole (NND-502), a novel imidazole antifungal agent. J Infect Chemother. 2004;10:216–219. doi: 10.1007/s10156-004-0327-1. [DOI] [PubMed] [Google Scholar]
- Venkatesh Shavi G, Sreenivasa Reddy M, Raghavendra R, Yogendra Nayak U, Ranjith Kumar A, Balavant Deshpande P, Udupa N, Behl G, Dave V, Kushwaha K. PEGylated liposomes of anastrozole for long-term treatment of breast cancer in vitro and in vivo evaluation. J Liposomes Res. 2015;26:1–19. doi: 10.3109/08982104.2015.1029493. [DOI] [PubMed] [Google Scholar]
- Verma DD, Verma S, Blume G, Fah A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258:141–151. doi: 10.1016/S0378-5173(03)00183-2. [DOI] [PubMed] [Google Scholar]