Abstract
The present investigation aimed to look at the effects of biotic and abiotic elicitors during Soybean seed development and cell suspension culture in isoflavones accumulation. The expression levels of four major genes viz., CHS7, CHS8, IFS2, and IFS1 involved on isoflavones biosynthesis during seed developmental stages from R5L–R7 was seen in both MAUS-2 and JS-335 Soybean varieties. The R7 stage showed 1.24-fold upregulation of IFS1transcript level and considered as the control for Soybean seed development. Both varieties during R6−R8 stages responded differently to the foliar application of 10 µM SA, 10 µM MJ and 0.1% Aspergillus niger. The IFS2 transcripts were upregulated by SA at the R7 stage with 5.21- and 4.68-fold in JS-335 and MAUS-2, respectively. IFS1 expression was significantly increased by A. niger treatment at R7 stage with 3.98- and 3.21-fold in MAUS-2 and JS-335, respectively. The expression of CHS7 and CHS8 by 10 μM SA at R7 level revealed maximum up-regulation of 0.51- and 1.01-fold in MAUS-2; 0.37- and 0.82-fold in JS-335, respectively. In the soybean callus suspension culture, biosynthetic genes were used to validate the effects of elicitor on isoflavones. Both biotic and abiotic treatments contribute to the upregulation of IFS1 and IFS2 expression, that in turn, leads to the accumulation of isoflavone in seed development as well as in suspension cultures. These data further suggested that the IFS2 is the key gene responsible for the isoflavone accumulation during elicitor treatment.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2065-1) contains supplementary material, which is available to authorized users.
Keywords: Augmentation, Callus suspension, Elicitation, Isoflavones, qPCR
Introduction
Isoflavones are polyphenolic secondary plant metabolite found in seedlings, flowers, roots and they are especially abundant in seeds and leaves of Soybean. Within seeds, diverse tissues have the ability to synthesize isoflavones (Dhaubhadel et al. 2003). The soybean development is influenced by multigenic abiotic responses, and highly variable across the plant, organ, tissue and also vary with respect to the environmental conditions (Dhaubhadel et al. 2007; Devi et al. 2018). Isoflavones are synthesized through the phenylpropanoid pathway branch (Fig. 1) which leads to the synthesis of anthocyanins, lignin, and other significant secondary metabolites (Yu and McGonigle 2005). Phenylalanine ammonia lyase (PAL) enzyme is the initial enzyme which converts amino acid L-phenylalanine into cinnamic acid. The first vital enzyme for synthesis of flavonoid is chalcone synthase (CHS), which exists as a multigene family in Soybean, though not all copies are expressed at detectable levels in seeds (Dhaubhadel et al. 2007). Other key enzymes involved in the isoflavones synthesis pathway are chalcone reductase (CHR), for daidzein and glycitein formation and chalcone isomerase (CHI), which converts chalcones to flavanones. On the other hand, the enzyme that exclusively differentiates isoflavone-synthesizing plant species from other species is isoflavone synthase (IFS), a cytochrome P450 monooxygenase, that catalyzes 2,3 aryl ring migration of flavanones to their respective isoflavones (Akashi et al. 1999; Jung et al. 2000). IFS is present in two copies, as IFS1 and IFS2 in Soybean genome and differ from each other by few amino acids. They convert naringenin and liquiritigenin to genistein and daidzein, respectively. Both IFS1 and IFS2 contribute to the isoflavones level in Soybean seeds and are regulated differentially at the transcriptional level (Cheng et al. 2008). The expression of IFS2 increases, while the expression of IFS1 remains constant during late seed developmental stages (Dhaubhadel et al. 2007). Furthermore, only IFS2 was found to be induced in Soybean transgenic roots and hypocotyls in response to the attack of pathogens (Subramanian et al. 2004).
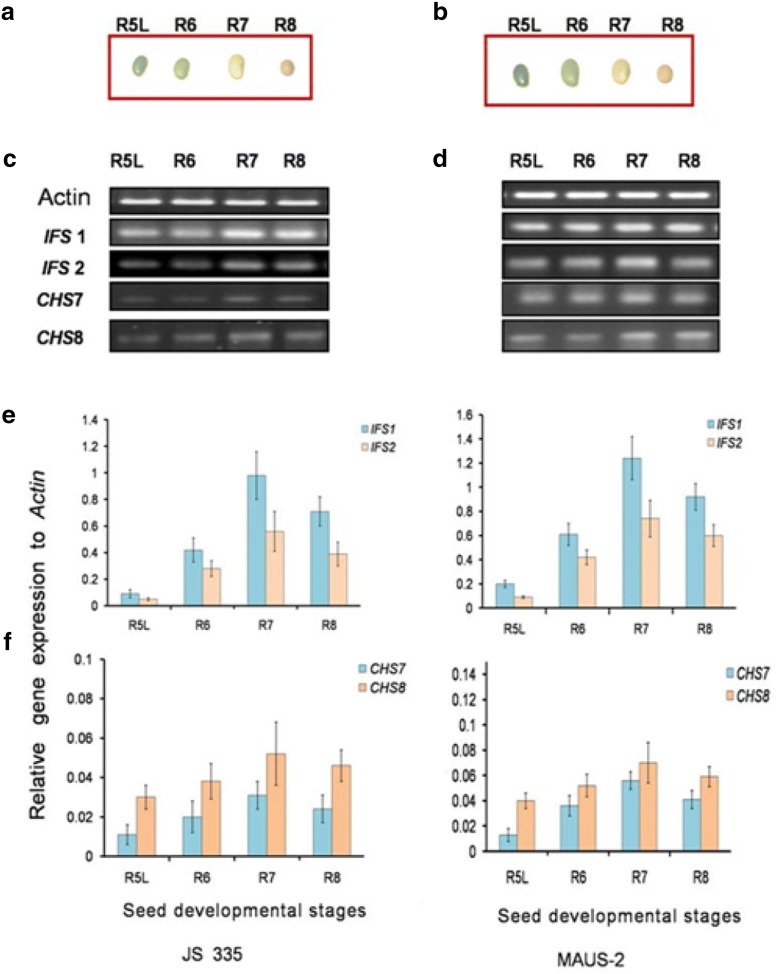
Fig. 1.
Expression analysis of isoflavones biosynthetic genes during seed developmental stages in soybean cv.JS-335 and cv.MAUS-2 variety. a Seed developmental stages in JS-335. b Seed developmental stages in MAUS variety. c, d Expression pattern of Actin, IFS1, IFS2, CHS7 and CHS8 in JS-335 MAUS-2 variety (semi-quantitative method). e Relative expression pattern of IFS1 and IFS2 was normalized to Actin in JS-335 and MAUS-2 variety (qPCR). f Relative expression pattern of CHS7 and CHS8 was normalized to Actin in JS-335 and MAUS-2 variety (qPCR). Data shown are the mean ± SD
The seed isoflavones are greatly influenced by various environmental stresses during the seed developmental stages (Lozovaya et al. 2005; Kim and Chung 2007; Dhaubhadel et al. 2007). Gutierrez-Gonzalez et al. (2010) have reported that the reduction in seed isoflavones concentration observed during a period of water stress was correlated with the expression of three key genes involved in isoflavone synthesis (i.e., CHS7, CHS8, and IFS2). Similarly, four key genes involved in isoflavones synthesis (IFS1, IFS2, CHS7, and CHS8) of seed isoflavones during seed development (seed maturity stage) were expressed at a higher temperature. But there was no clear correlation between isoflavones concentration and gene expression (Chennupati et al. 2012). A few reports investigated the alteration in the expression pattern of isoflavones biosynthetic genes under the influence of elicitors. Chen et al (2009) investigated gene expression alteration of 14 genes encoding isoflavone in Soybean sprouts (three cultivars), as well as isoflavones concentrations, with chitosan treatment.
Considering the gene expression analysis described above, there is not much information available for the regulation of biosynthesis of isoflavones under the influence of elicitors, mainly during seed development, where isoflavones are actively accumulated. Though there are few reports on the effect of stress factors on isoflavones regulation in in vitro cell cultures of Fabaceae but there are no reports in Soybean cell cultures. With this, the purpose of present study was to profile the expression of isoflavones biosynthetic genes during seed developmental stages in JS-335 and MAUS-2 varieties and also differential expression analysis of isoflavones biosynthetic genes under the influence of selective elicitors in JS-335 and MAUS-2 variety and callus suspension cultures.
Materials and methods
Chemicals
HPLC standards for daidzin, glycitin, genistin, daidzein, glycitein, and genistein were purchased from Sigma-Aldrich, India. High-performance liquid chromatography (HPLC) grade methanol, acetonitrile, HCl and acetic acid were purchased from Rankem Laboratories, India. Deionized water from Milli-Q (Millipore Co., India) was used for all the extraction and quantification purposes.
Plant material and cultivation
Soybean seeds (Glycine max L.) varieties namely, JS-335 and MAUS-2 were collected from AICRP Soybean, Gandhi KrishiVignana Kendra, Bangalore, India. For the present study, uniform size seeds were handpicked and used for isoflavones analysis. To establish in vitro seedlings for explants preparation and subsequent callus induction, seeds were subjected to surface sterilization procedure as described by us earlier (Akitha Devi and Giridhar 2014).
Callus induction and suspension culture initiation
The cotyledonary node leaves from three-week-old seedlings of JS-335 variety were used as an explant (9–10 mm) for callus induction on MS medium, supplemented with 9.51 µM 2,4-Dichlorophenoxyacetic acid (2,4-D), 29.64 µM α-Naphthalene acetic acid (NAA), and 1.63 µM kinetin (Kn). The pH was adjusted to 5.8 and autoclaved for 20 min at 121 °C. The cultures were maintained at the light intensity of 57 μmol m– 2 s–1 (illumination supplied by cool white fluorescent tubes) with a 16 h photoperiod at 25 ± 2 °C. The callus induced from explants were separated and subcultured onto the same medium twice at a 15-day interval to get a friable callus. Suspension cultures were initiated with 200 mg from the established friable callus in 150 mL Erlenmeyer flasks containing 40 mL of MS liquid medium supplemented with 3% sucrose (w/v), growth regulators, 9.51 µM 2,4-D, 29.64 µM α-NAA, and 1.63 µM Kn. Cultures were incubated on a rotary shaker at 100 rpm with 16 h light/8 h dark photoperiod at 25 ± 1 °C. After two subcultures on the same medium at a 4 week interval, cultures were uniform and used for elicitor studies.
Establishment of plants in polyhouse
To establish plants in polyhouse (in vivo), the seeds of selective varieties (JS335 and MAUS-2) were then imbibed in double-deionized water for 1 h and then sown in pots containing 6 kg of air-dried red silt loam (fine-silty) at 2-cm depth for germination. Seed at various developmental stages (Table 1, Fig. 2) such as R5L (50d), R6 (65d), R7 (75d), and R8 (88d) was collected from JS-335 variety and R5L (60d), R6 (75d), R7 (85d), and R8 (97d) stage seeds from MAUS-2 variety for analyzing isoflavones content. After harvesting, the samples were immediately frozen in liquid nitrogen and stored at − 80 °C for later use.
Table 1.
Isoflavones concentration in different seed growth stages of selected soybean varieties (micrograms per gram of dry weight) under abiotic elicitor 10 µM Salicylic acid treatment
| Varietya | Growth stage | Salicylic acid 10 µM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total daidzein (µg/g (dw)) | Total glycitein (µg/g (dw)) | Total genistein (µg/g (dw)) | Total isoflavone content (µg/g (dw)) | ||||||
| C | E | C | E | C | E | C | E | ||
| JS-335 | R5 (e) | 80.6e | 108.4g | 12.6e | 17.39d | 50.2e | 67.91f | 138.4e | 193.7g |
| R6 | 105.6de | 220.55f | 40.6bc | 94.56c | 166.9d | 393.66ef | 283.6d | 708.77ef | |
| R7 (m) | 165.9c | 570.45d | 37.8cd | 125.87c | 272.2c | 922.34d | 462.5c | 1618.7d | |
| R8 | 270.3ab | 1352.0b | 39.5c | 199.88b | 443.5b | 2214.8b | 753.5b | 3766.6b | |
| MAUS -2 | R5 (e) | 114.2d | 195.24f | 17.6d | 30.075d | 70.3de | 118.36f | 196.3de | 343.67fg |
| R6 | 158.8cd | 386.56e | 65.5a | 147.14bc | 225cd | 558.05e | 376.4cd | 1091.7e | |
| R7 (m) | 267.9b | 1052.3c | 49.8b | 195.46b | 467.8ab | 1795.3c | 780.5ab | 3043.0c | |
| R8 | 359.2a | 1939.5a | 62.3ab | 336.42a | 626.9a | 3386.0a | 1048.6a | 5661.9a | |
Different alphabets above the bars indicate a significant difference between the treatment. Treatment showing the same alphabets is not significantly different at p-value 0.05. Values calculated using one way ANOVA followed by Tukey’s multiple range test
C Control, E Elicitor treatment
Fig. 2.
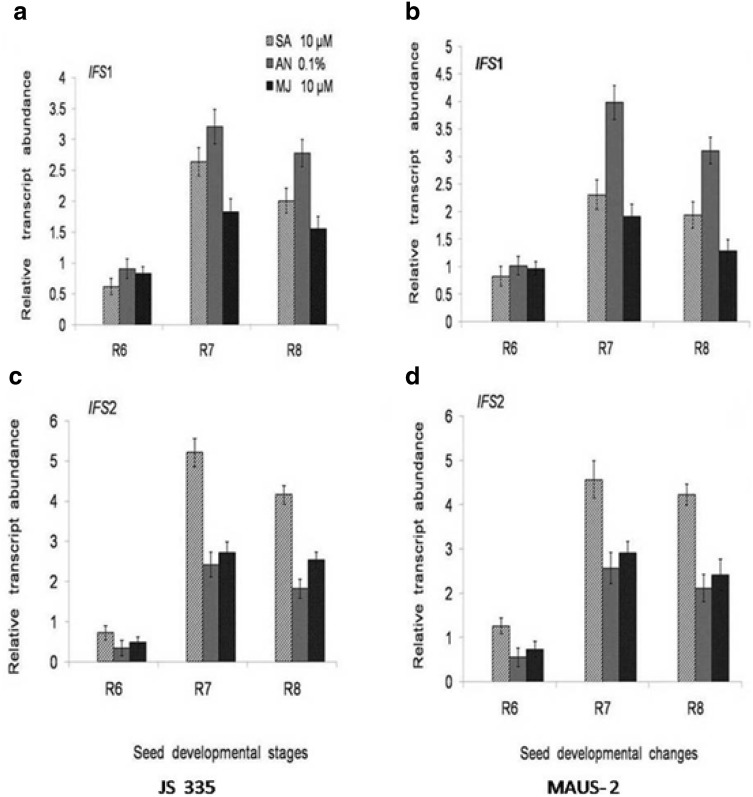
Expression analysis of isoflavones biosynthetic genes during elicitor treatment in soybean varieties. a, b Relative transcript abundance of IFS1 in response to elicitors; SA (10 µM), A. niger (0.1%) and MJ (10 µM) in JS-335 and MAUS-2 seed developmental stages. c, d Relative transcript abundance of IFS2 in response to elicitors; SA (10 µM), A. niger (0.1%) and MJ (10 µM) in JS-335 and MAUS-2 seed developmental stages
Elicitor preparation
Biotic elicitors
The biotic elicitor was prepared using fungal culture (Giridhar and Parimalan 2010), Aspergillus niger (AN) that was obtained from the Microbiology and Fermentation Technology Department (CSIR-CFTRI). Fresh cultures of AN were grown on potato dextrose agar (PDA) medium (HiMedia, Mumbai) slants and then incubated in the dark at 37 °C for 7 days. Further respective fungal spores were selected for spore suspension preparation in 0.1% sodium lauryl sulfate (w/v) and diluted with sterile distilled water under sterile conditions to obtain a spore density of ~ 2.5 × 106 spores mL−1. Afterward, the same spores were then inoculated in 40 mL of potato dextrose broth prepared in 150 mL Erlenmeyer conical flasks and then incubated for 10 days at dark. Subsequently, incubated cultures were autoclaved and mycelium was separated from the culture broth using filtration, and fresh weight was recorded. The aqueous extract was prepared by homogenization. The extract was then filtered (using Whatman no. 1 filter paper) and set aside as the stock solution. From the stock, broad working concentration (fungal mycelium wet weight in 1 L of distilled water) range of fungal mycelial extract was prepared in sterile water and used for conducting elicitor experiments. Similarly, Rhizopus oligosporus was cultures and mycelial extract was prepared in the same manner as explained for A. niger. Chitosan (Sigma-Aldrich, India) was prepared by dissolving 1% (w/v) in 0.5% acetic acid solution, and then adjusted the final volume to 100 mL using distilled water and then the pH adjusted to 5.8 with NaOH.
Abiotic elicitors
Stock solutions of salicylic acid (SA) was prepared by dissolving in sterile distilled water, filter-sterilized and diluted to different concentrations. Methyl jasmonate (MJ) (Sigma Chemical Co., St. Louis, USA) was dissolved in 70% (v/v) ethanol and prepared as a stock solution. Further dilution was done using deionized water. Solution was filtered through a microfilter (0.2 mm) before being dispensed into cell culture at various concentrations. Control cultures were treated with sterile water was added instead of elicitor.
Foliar application of elicitors in in vivo Glycine max plants
Germinated soybean seedlings were maintained for control and treatment as quadruples at the Plant Cell Biotechnology Department's polyhouse. Initial studies were conducted with different concentrations of SA (2.5, 5, 10, 25 and 50 µM), MJ (2.5, 5, 10, 25 and 50 µM) and AN (0.05, 0.1, 0.5, 1 and 2) for the evaluation of isoflavones in the seeds (data not shown). Among them, 10 µM SA, 10 µM MJ and 0.1% AN were selected for the present study according to initial results. All the elicitors were sprayed onto the fully opened flower of JS-335 and MAUS-2 plants between 10 and 11 AM. Respective biotic elicitor (A. niger) and abiotic elicitors (Salicylic acid-SA and methyl jasmonate-MJ) was administered on the flowers for the evaluation of isoflavones production in the seeds and control plants were sprayed with an equal quantity of distilled H2O. Both treated and control seeds were harvested from various developmental stages such as R5,R6, R7 and R8 from both JS-335 and MAUS-2 varieties. For gene expression studies, the harvested Soybean seeds were frozen immediately and stored at − 80 °C for later use.
Elicitor treatment in callus suspension cultures
Biotic and abiotic elicitors were added to G. max callus suspension culture with concentration of SA (2.5, 5.0 and 10 µM), MJ (2.5, 5.0 and 10 µM) and A. niger-AN (0.1, 0.5 and 1.0%), R. oligosporus (0.1, 0.5 and 1.0%) and chitosan (2.0, 5.0 and 10 µg) for the evaluation of isoflavones. Culture with equal volumes of water without elicitor was maintained as a control. Cultures were harvested at 12 to 24th days (for isoflavones) after the addition of elicitors. The experiment was repeated thrice for the analysis of growth parameters and metabolites. To study the gene expression, suspension cultures were harvested on the 18th day based on the peak levels of isoflavones content and frozen immediately and stored at −80 °C (Akitha Devi and Giridhar 2014). Expression of genes selected viz., CHS7, CHS8, IFS1, and IFS2 from the isoflavones biosynthetic pathway were quantified by qRT-PCR.The gene specific primers were designed using the Integrated DNA technology (IDT) online software and synthesized from IDT (Belgium). The list of gene-specific primers used is shown in Table 2.
Table 2.
Primer pairs used in quantitative RT-PCR
| Gene | Enzyme name | Forward primer (5′–3′) | Reverse primer(5′–3′) | ||
|---|---|---|---|---|---|
| CHS7 | Chalcone synthase 7 | AACCCACCAAACCGTGTTGAT | CTTGTCACACATGCGCTGAAAT | ||
| CHS8 | Chalcone synthase 8 | GCACACCTTCATTTCAACCTC | ACATGCGCTGGAATTTCTCT | ||
| IFS1 | Isoflavone synthase 1 | GGGCCCTCAAGGACAAATA | CTGCGATGGCAAGACACTAC | ||
| IFS2 | Isoflavone synthase 2 | AAACCAAGGACGAGAACACG | TGGCCACTGAGCTATCATAG | ||
| Actin | Actin | TCCCAGTATTGTTGGCCGA | TTCCATGTCATCCCAGTTGCT | ||
Isoflavones extraction
The Soybean isoflavones were extracted from seeds and callus as described by Sakthivelu et al. (2008) with minor modifications. 2 g of Soybean seed with the seed coat was mixed with 2 mL of 0.1 N HCl and 10 mL of acetonitrile (ACN) in a 125 mL screw-top flask, stirred for 2 h at room temperature, and filtered through Whatman no. 42 filter paper. The filtrate was dried in a vacuum evaporator and re-dissolved in 10 mL of 80% HPLC grade methanol and re-dissolved samples were filtered through 0.45 μm filter unit (Cameo 13 N syringe-filter, nylon).
Extraction of isoflavones from callus cultures: Callus mass (400 mg) from each treatment was ground finely and then extracted with 2 mL concentrated HCl and 10 mL ethanol in a boiling water bath for 2 h using a standard method (Vyn et al. 2002). The resultant suspensions were then cooled and centrifuged at 111800 g for 10 min, and the supernatant was further filtered by a syringe filter (Whatmann 0.5 μm, 13 mm diameter).
HPLC analysis
The HPLC analysis was conducted as described by Sakthivelu et al. (2008). The Shimadzu LC 20-AD high-pressure liquid chromatograph equipped with a dual pump and a UV detector (model SPD-10A) was used to separate, identify, and quantify isoflavones from the samples. Separation of isoflavones was achieved by a Bondapak C18 reversed phase HPLC column (150 mm × 4.6 mm and 5 μm internal diameter), and 20 μL samples were injected using a Rheodyne 7125 injector. A linear HPLC gradient was used with solvent A (0.1% glacial acetic acid and 5% acetonitrile in water) and solvent B (0.1% glacial acetic acid in acetonitrile). HPLC program Solvent B was initiated from 10 to 14% B over 10 min, then increased to 20% over 2 min, maintained at 20% for 8 min continued to increase to 70% over 10 min, maintained at 70% for 3 min and then returned to 10% at the end of the 34 min running time. The flow rate of the solvent was kept at 1 mL/min. The wavelength of the ultraviolet (UV) detector was set at 260 nm. Solvent ratios were expressed on a volume basis.
Calibration curves were obtained for each standard with high linearity (r > 0.995), by plotting the standard concentration as a function of the peak area, obtained from HPLC analyses with 20 µL injections. Triplicate injections were analysed for each concentration of standards.
RNA isolation and cDNA synthesis
Total RNA was isolated from 100 mg samples with TRIZOL reagent as per manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Prior to DNase, the RNA concentration and quality was determined using NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA), and the total RNA purity was determined with A260/A230 and A260/A280 ratios. The first-strand cDNA synthesis was carried out using 1 μg of DNase-treated total RNA in 20 μl reaction mix was performed using the GeNei™ M-MuLV RT-PCR Kit (GeNei, Bangalore, India).
Quantitative real-time PCR (qPCR) analysis
qPCR was carried out in a 10 µl reaction volume, which includes 2 µl of diluted cDNA, each primer at 5 µM, and 5 µl of 2X SsoFastEvaGreenSupermix (Bio-Rad Inc., CA, USA) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Inc., CA, USA). The qPCR program steps included an initial denaturation step of 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. A melt curve was obtained from 65 to 95 °C, with an increment of 0.5 °C at every 5 s. To normalize the gene expression, Actin was used as an internal control. Relative gene expression was calculated according to a 2−ΔΔCT method (Livak and Schmittgen 2001). The fold change in each target gene was compared with a control, which was set to 1 and also the qPCR was done in triplicates.
Statistical analysis
Isoflavones analysis in Soybean seed developmental stages by HPLC was performed in triplicates. The data were analysed statistically by SPSS 17.0 using one-way analysis of variance (ANOVA), and the difference between the means of the sample was analysed by the least significant difference (LSD) test at a probability level of 0.05.
Results and discussion
The isoflavones content (on a dry weight basis) of control seeds (untreated) is found to be maximum in MAUS-2 soybean variety, wherein the highest levels of daidzein (359.2 μg/g), glycitein (62.3 μg/g) and genistein (626.9 μg/g) were recorded, respectively. A positive correlation of total isoflavones (TI) content and growth stages was found during the control seed development (Table 1). These results obtained in our study were further supported by the report of Kim and Chung (2007), who noticed an elevation in TI content during the growth advancement stages (R5–R8). Significant rise in total isoflavones concentration was observed for JS-335 (from 138.4 to 753.5 μg/g which is 5.4-fold) and MAUS-2 (from 196.3 to 1048.6 μg/g which is 5.3-fold), respectively, from R5 to R8 stage. In early R5 stage, MAUS-2 had the maximum total isoflavones (196.3 μg/g), total daidzein (114.2 μg/g), total glycitein (17.6 μg/g) and total genistein (70.3 μg/g). However, JS-335 had the lowest level of total isoflavones (138.4 μg/g), total daidzein (80.6 μg/g), total glycitein (12.6 μg/g) and total genistein (50.2 μg/g). The same trend was observed in R6, and R7, until complete maturity, where the level of most isoflavones forms was at the highest in MAUS-2 than JS-335.
The R6 and R7 stage seed of MAUS-2 showed an increase of 2.8 and 1.3 fold TI in comparison to matured seeds. The data presented in Table 3 showed a maximum level of isoflavones forms were present between R5 and R6 stages, where the minimal increase of isoflavones forms was found between R7 stage and complete maturity.
Table 3.
Isoflavones concentration in different seed growth stages of selected soybean varieties (micrograms per gram of dry weight) under abiotic elicitor 10 µM Methyl jasmonate treatment
| Varietya | Growth stage | Methyl jasmonate 10 µM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total daidzein (µg/g (dw)) | Total glycitein (µg/g (dw)) | Total genistein (µg/g (dw)) | Total isoflavone content (µg/g (dw)) | ||||||
| C | E | C | E | C | E | C | E | ||
| JS-335 | R5 (e) | 80.6e | 88.44f | 12.6e | 13.75d | 50.2e | 55.395e | 138.4e | 157.59e |
| R6 | 105.6de | 159.65f | 40.6bc | 73.795 cd | 166.9d | 262.66de | 283.6d | 496.1de | |
| R7 (m) | 165.9c | 456.17d | 37.8 cd | 105.48bc | 272.2c | 709.68c | 462.5c | 1271.3c | |
| R8 | 270.3ab | 1012.4b | 39.5c | 148.13b | 443.5b | 1663.4b | 753.5b | 2823.9b | |
| MAUS-2 | R5 (e) | 114.2d | 132.19f | 17.6d | 20.82d | 70.3de | 82.185de | 196.3de | 235.19e |
| R6 | 158.8cd | 264.94e | 65.5a | 123.13bc | 225cd | 387.58d | 376.4cd | 775.64d | |
| R7 (m) | 267.9b | 861.76c | 49.8b | 153.54ab | 467.8ab | 1482.5b | 780.5ab | 2497.8b | |
| R8 | 359.2a | 1496.1a | 62.3ab | 218.8a | 626.9a | 2448.7a | 1048.6a | 4163.5a | |
Different alphabets above the bars indicate a significant difference between the treatment. Treatment showing the same alphabets is not significantly different at p-value 0.05. Values calculated using one way ANOVA followed by Tukey’s multiple range test
C Control, E Elicitor treatment
Influence of elicitor treatment on isoflavones content of soybean seeds
Biotic elicitor A. niger at 0.1% triggered total isoflavone content of MAUS-2 seeds by 3.1, 3.6 and 5.9 folds compared to control R6 (1166.84 µg/g DW), R7 (2809.8 µg/g DW) and R8 (6186.74 µg/g DW) stage seeds (Table 4). However, the response was slightly less for 10 µM SA treatment followed by 10 µM MJ elicitation. The overall total isoflavone content of JS-335 seeds was significantly low compared to that of MAUS-2 at respective elicitor treatment. Interestingly, for total isoflavones content 10 µM SA showed highest performance (2.8, 3.2 and 4.6 folds compared to control) that to that of 0.1% AN (2.5, 3.5 and 5 folds compared to control) followed by MJ (1.75, 2.75 and 3.75 folds compared to control) as in case of JS-335 seeds at R6 to R8 stages. The trend for total daidzein, total glycitein and total genistein under respective elicitor treatments were appears to be same to that of total isoflavones content.
Table 4.
Isoflavones concentration in different seed growth stages of selected soybean varieties (micrograms per gram of dry weight) under biotic elicitor 0.1% Aspergillus niger treatment
| Varietya | Growth stage | Aspergillus niger 0.1% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total daidzein (µg/g (dw)) | Total glycitein (µg/g (dw)) | Total genistein (µg/g (dw)) | Total isoflavone content (µg/g (dw)) | ||||||
| C | E | C | E | C | E | C | E | ||
| JS-335 | R5 (e) | 80.6e | 59.94g | 12.6e | 61.31f | 50.2e | 44.895f | 138.4e | 166.14g |
| R6 | 105.6de | 276.99ef | 40.6bc | 108.29c | 166.9d | 409.71e | 283.6d | 794.99f | |
| R7 (m) | 165.9c | 531.665d | 37.8cd | 120.98bc | 272.2c | 828.16d | 462.5c | 1480.80d | |
| R8 | 270.3ab | 1305.865b | 39.5c | 180.62b | 443.5b | 1980.36b | 753.5b | 3466.85b | |
| MAUS -2 | R5 (e) | 114.2d | 156.185fg | 17.6d | 23.32f | 70.3de | 94.68f | 196.3de | 274.19g |
| R6 | 158.8 cd | 408.44de | 65.5a | 179.12b | 225cd | 580.71e | 376.4 cd | 1166.27e | |
| R7 (m) | 267.9b | 961.26c | 49.8b | 179.54b | 467.8ab | 1668.5c | 780.5ab | 2809.3c | |
| R8 | 359.2a | 2860.0a | 62.3ab | 48.8a | 626.9a | 2841.5a | 1048.6a | 6186.3a | |
Different letters are significant among cultivars (p < 0.05). C Control, E Elicitor treatment
Differential expression of isoflavone biosynthetic genes during seed developmental stages in G. max
Soybean seeds are the major source of isoflavones for human consumption, most of the research has been focused on understanding the biosynthesis and the accumulation of these isoflavones in seeds (Dhaubhadel et al. 2007). In the present study, two varieties, JS-335 (widely cultivated) and MAUS-2 (high isoflavonoid content) were selected to analyse the differential expression of isoflavones biosynthetic genes during late seed developmental stages (R5–R8). Expressions of major genes of isoflavones biosynthesis, such as CHS7, CHS8, IFS1 and IFS2 were primarily analysed using qPCR. Actin was selected as a house-keeping gene in the study due to its stability in the gene expression (Chen et al. 2009).
The seed developmental stages, R5L (late stage), R6, R7 and R8 of JS-335 and MAUS-2, are depicted in Fig. 1a, b, respectively. The expression pattern of CHS7, CHS8, IFS1, and IFS2 were analysed by qPCR in seed developmental stage of both varieties (Fig. 1e, f). Increase in the progression of four genes expression in the order of CHS7, CHS8, IFS1, and IFS2 during developmental stages from R5L- R7 was observed in both the varieties. (Fig. 1e, f).Among the varieties, the up-regulation of 1.24-fold was observed in IFS1 expression at the R7 stage of MAUS-2 (Fig. 1e). In JS-335 variety, the expression of IFS1 (0.98-fold) reaches maximum at the R7 stage, followed by IFS2 (0.56-fold). Besides, there was a decline in the expression of all the four genes in the matured seeds (R8) (Fig. 1e, f). Likewise, in MAUS-2 variety, there was a stepwise increase in the expression level from R5L to R7 seed developmental changes (Fig. 1e, f) and the expression level was maximum for IFS1, followed by IFS2, CHS8 and CHS7. An up-regulation of CHS7, CHS8, IFS1 and IFS2 expression was observed at R7 stage of MAUS-2 than JS-335 variety (Fig. 1e, f).
The up-regulation of genes during the R7 developmental stage revealed that the accumulation of isoflavones in Soybean embryos increases as seeds matures. After R7, Soybean seed slowly starts losing water, shrinks its size and attains dormant condition. The level of seed isoflavones reaches the maximum level at mature seeds (Dhaubhadel et al. 2003).
CHS7 and CHS8 expression in the seed developmental stages were higher in MAUS-2 and JS-335, suggested that these two genes have a critical role in the accumulation of isoflavones. The same phenomenon is observed in gene expression analysis during the embryo development in Soybean. Where the gene expression profiles of two Soybean cultivars (RCAT Angora-high isoflavonoid cultivar and Harovinton-low isoflavonoid cultivar) that contrasted in seed isoflavonoid content were compared (Dhaubhadel et al. 2007). The highest level of IFS1 and IFS2 expression pattern was observed in MAUS-2 than JS335 variety and this confirms the higher accumulation of total isoflavones in MAUS-2. Though, the earlier studies have reported that the higher IFS1 expression was observed in the root and seed coat and IFS2 was expressed in embryos and pods, and also in elicitor-treated or pathogen-challenged tissues (Dhaubadhal et al. 2003).
Differential expression of isoflavones biosynthetic genes under the influence of elicitors in G. max developing seeds
To study the correlation of isoflavones accumulation and regulation of isoflavones biosynthesis which occur during elicitor treatment, the expression profiling of major genes were investigated. The phenylpropanoid pathway genes for qPCR analysis were chosen on the basis of earlier literature (Chen et al. 2009; Gutierrez-Gonzalez et al. 2010; Chennupati et al. 2012).
The key genes that encode enzymes leading to isoflavones production were selected for qPCR analysis. Each primer set designed to amplify a specific gene under the optimized qPCR conditions and the amplicon was sequenced. The sequence identity was confirmed through BLAST. The BLAST showed 100% homology for CHS7, CHS8, IFS2 except for IFS1, which of showed 99.9% homology in JS-335 variety. The sequence of IFS1 has been submitted to the NCBI database (Accession No. JZ845698).
The expression level of four key genes (CHS7, CHS8, IFS1, and IFS2) were investigated after foliar application of selected abiotic (SA 10 µM and MJ 10 µM) and biotic (A. niger 0.1%) elicitors in two Soybean varieties (JS-335 and MAUS-2) during R6−R8 stages (Figs. 2, 3). Both the varieties have responded differently under the influence of elicitors. IFS2 expression was up-regulated under the influence of SA, at R7 stage with 5.21-fold and 4.68 fold in JS-335 and MAUS-2, respectively (Fig. 2c, d). However, IFS1 abundant expression was observed due to the A. niger treatment at R7 stage with 3.98- and 3.21-fold in MAUS-2 and JS-335, respectively (Fig. 2a, b).
Fig. 3.
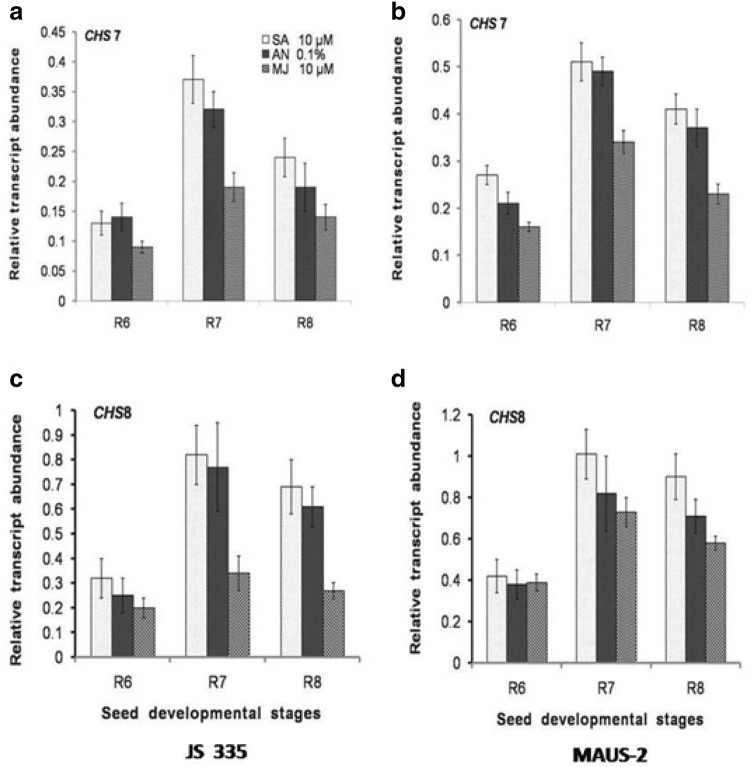
Expression analysis of isoflavones biosynthetic genes (CHS7 and CHS8) during elicitor treatment in Soybean varieties. a, b Relative transcript abundance of CHS7 in response to elicitors; SA (10 µM), A. niger (0.1%) and MJ (10 µM) in JS-335 and MAUS-2 seed developmental stages. c, d Relative transcript abundance of CHS8 in response to elicitors; SA (10 µM), A. niger (0.1%) and MJ (10 µM) in JS-335 and MAUS-2 seed developmental stages
Expression of CHS7 and CHS8 showed maximum up-regulation at R7 stage in presence of SA 10 µM, 0.51-fold and 1.01-fold in MAUS-2 variety (Fig. 3b, d). At R7 stage of JS-335 variety, the expression of CHS7 and CHS8 were upregulated with a fold increase of 0.37 and 0.82-fold (SA 10 µM) (Fig. 3a, c). Similar to the differential expression pattern observed during seed development, there is a stepwise increase in the expression of CHS7, CHS8, IFS1, and IFS2 from R6 to R7 followed by a decline in R8 in both varieties.
Several researchers have studied the expression pattern of isoflavones biosynthetic genes in various biotic, environmental and physical stresses in Soybean (Gutierrez-Gonzalez et al. 2010; Chennupati et al. 2012). Gutierrez-Gonzalez et al. (2010) reported that the reduction in seed isoflavones concentration observed during a period of water stress was correlated with the expression of three key genes involved in isoflavones synthesis (CHS7, CHS8, and IFS2). Chennupati et al. (2012), stated that the effect of high-temperature stress leads to Soybean isoflavones accumulation during the seed development, as well as the expression of four key genes involved in isoflavones synthesis (IFS1, IFS2, CHS7, and CHS8). However, the results suggested that there was no clear correlation between isoflavones concentration and gene expression. A very few reports have explained the alteration in the expression pattern of isoflavones biosynthetic genes under the influence of elicitors. This is the first report that investigated the regulation of the genes encoding key enzymes of isoflavones biosynthesis by SA, MJ and A. niger treatments, leading to the gene induction in Indian Soybean cultivars. Previously, Chen et al. (2009) investigated the gene expression alteration of 14 genes encoding isoflavones in Soybean sprouts (three cultivars), as well as isoflavone concentrations, following treatment with chitosan. In general, MJ and SA are the key signaling molecules, modulating several physiological events such as defense response to the environmental stresses in plants (Creelman and Mullet 1997; Draper 1997). Significant evidences have showed that the exogenous application of MJ has led to the increase of various classes of secondary metabolites in several plants (Modolo et al. 2002; Wei 2010). As suggested by earlier reports, the use of MJ activates the genes of phenylpropanoid (PP) pathway (Dixon and Paiva 1995). The various expression of IFS genes (IFS1 and IFS2) is responsible for the changes in the level of isoflavones in seeds under the elicitor treatment (Dhaubhadel et al. 2007; Cheng et al. 2008). Overall, the results of the present correlate isoflavones augmentation and up-regulation of biosynthetic genes under the elicitor treatments.
The expression of IFS1 (relative transcript abundance) was prominent for 0.1% AN treatment in both JS-335 and MAUS-2 varieties followed by 10 µM SA and 10 µM MJ at R6 to R8 stages. But, IFS2 expression was more for SA treatment followed by MJ and AN at R6 to R8 stage of seed development in both JS-335 and MAUS-2 varieties. It is quite interesting to note that, both CHS7 and CHS8 genes transcript abundance was more under SA treatment followed by AN and MJ elicitor treatments in both soybean varieties at R6-R8 stages. A glance at all these selected genes expression pattern at different stages of seeds development and their total isoflavones content appears to be having similar trend. As the IFS1 and IFS2 genes expression was very high compared to CHS7 and CHS8, they might be having a pivotal role in overall isoflavones content changes. The overall, relative transcript abundance of CHS7 and CHS8 was quite low compared to IFS1 and IFS2 genes; however, the difference was moderate among the three elicitors viz, SA, MJ and AN.
Influence of elicitors on isoflavones content of in vitro cell suspension cultures of G. max
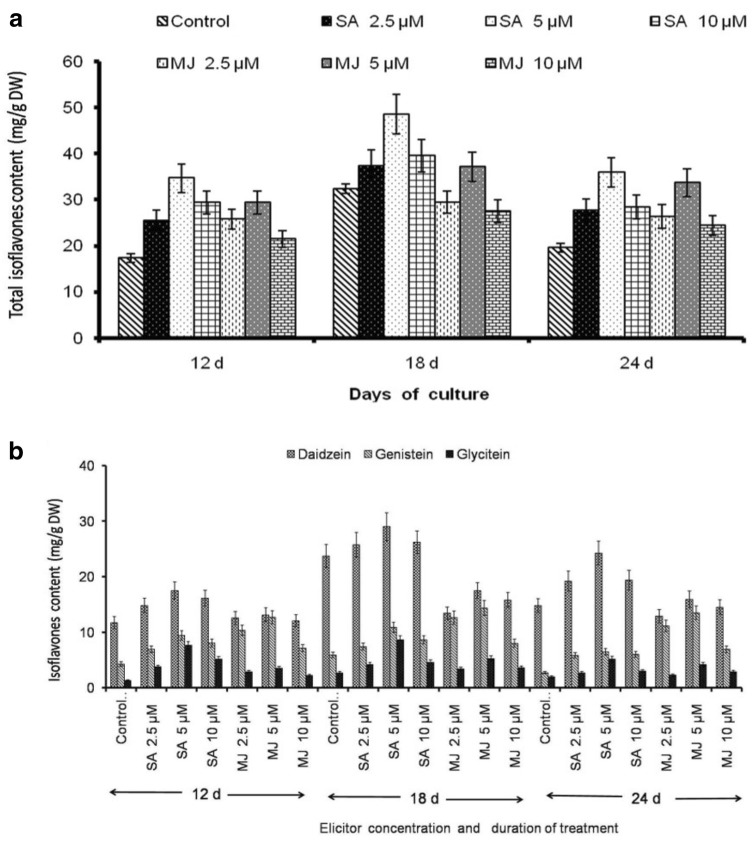
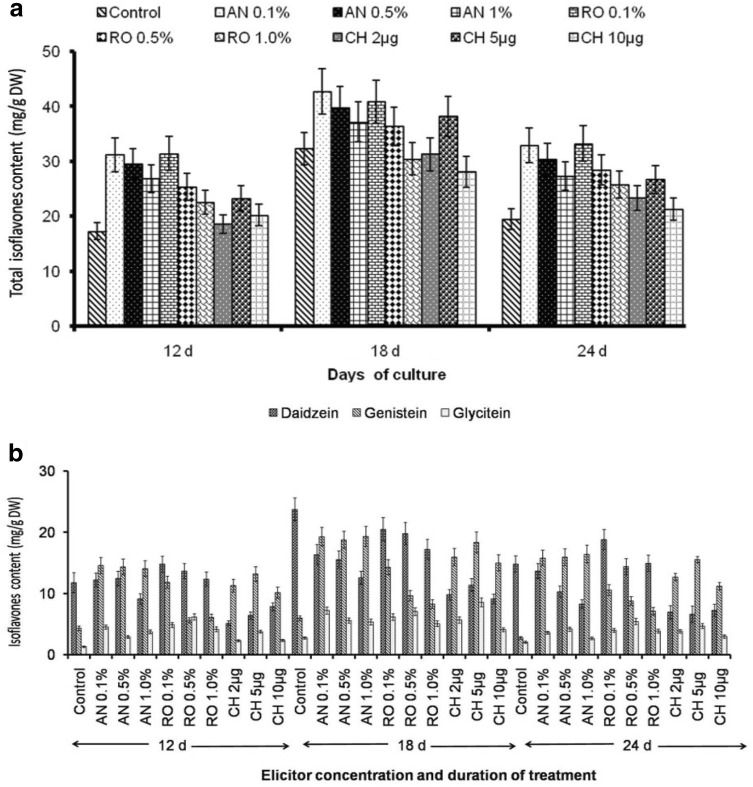
All the elicitors significantly influenced isoflavones content in callus suspension cultures at different time intervals when compared to control. Highest isoflavones content was found in 18th day old cultures. Abiotic elicitors SA and MJ positively influenced the TI content than biotic elicitor A. niger. This is obviously evident from the results that amongst the various elicitors, TI content was considerably high in SA supplemented cultures than control. SA (5 µM) treated cultures showed a maximum TI content (48.55 ± 3.89 mg/g DW) (Fig. 4a) that includes daidzein (23.74 ± 2.07 mg/g DW), genistein (5.92 ± 0.48 mg/g DW and glycitein (2.71 ± 0.24 mg/g DW) (Fig. 4b). Therefore, an increase of 39% TI than control (32.37 ± 2.93 mg/g DW) cultures were examined (Fig. 4a). Lower concentration of SA significantly influences the TI content and higher concentration of SA (10 µM) mimic the enhancement. In contrast, a remarkable increase in the isoflavones content was exhibited with an increase in MJ concentration. This was evident with a rise of 13.72% and 27.45% in TI content in 5 µM and 10 µM MJ, respectively (Fig. 4a). Results of the present study suggested that the elicitor concentration, incubation time as well as growth conditions were crucial for elicitation strategy as per earlier reports (Gueven and Knorr 2011; Sivanandhan et al. 2013; Theboral et al. 2014). Still the results were in contrast with earlier reports that MJ was more efficient than SA for the isoflavones accumulation soybean callus suspension cultures (Gueven and Knorr 2011; Theboral et al. 2014).
Fig. 4.
a Influence of abiotic elicitors SA and MJ on total isoflavones content and b. Effect ondaidzein, genistein and glycitein content in callus suspension cultures of G.max. Values are means ± S.D with significant at p < 0.05
The various biotic elicitors, 0.1% A. niger treated cultures showed an increase of 27.63% than control (Fig. 5a). The TI content in A. niger treated cultures were 42.75 ± 4.12 mg/g DW that includes daidzein (16.31 ± 1.68 mg/g DW), genistein (19.26 ± 1.54 mg/g DW) and glycitein (7.18 ± 0.58 mg/g DW) (Fig. 5b). In contrast to abiotic elicitors, A. niger and chitosan positively influence the genistein content. Also, it is evident from the study that the lower concentration of A. niger and R. oligosporus has an augmenting effect than the higher concentration. All concentration of chitosan excluding 10 µg, negatively influence the TI content. (Komaraiah et al. 2002) reported that Rhizopus and Aspergillus sp. elicit twofold to threefold plumbagin production in Plumbago rosea L. suspension cultures. In the present study, we found that the A. niger elicitor proved to be better for the induction of isoflavones than R. oligosporus.
Fig. 5.
a Influence of biotic elicitors on total isoflavones content. b Effect ondaidzein, genistein and glycitein content in callus suspension cultures of G.max. Values are means ± S.D with significant at p < 0.05
Differential expression of isoflavones biosynthetic genes under the influence of elicitors in in vitro cultures of G. max
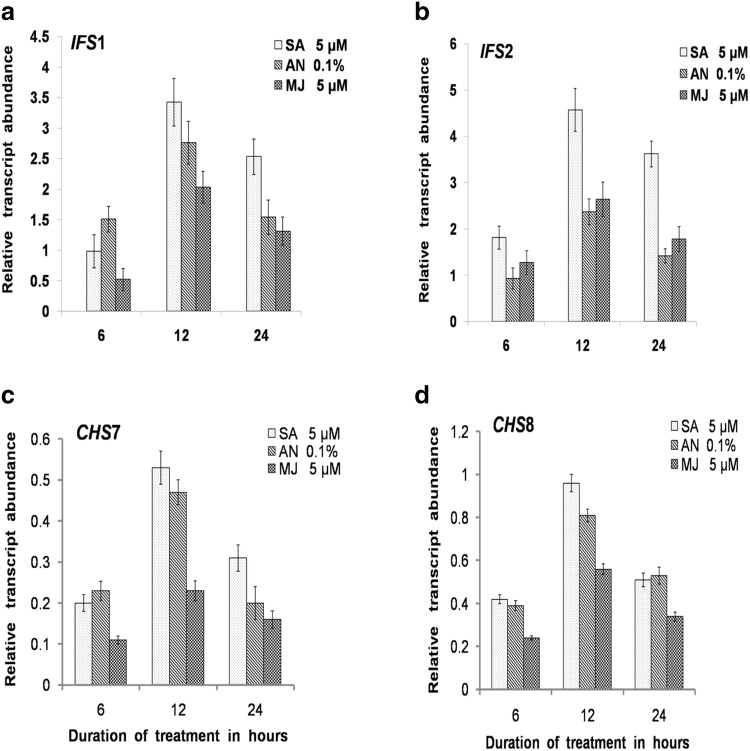
In the present investigation, accumulation of isoflavones was correlated with the expression of isoflavones biosynthetic genes during elicitor treatment in callus suspension cultures of JS-335 by qPCR. To determine the optimum elicitors, concentration for isoflavones augmentation and its influence on isoflavones gene expression were investigated. The up-regulation of isoflavones biosynthetic genes during elicitation in in vitro cultures was due to the elicitors or other environmental factors. On the basis of preliminary experiments carried out in this regard, the best concentration of abiotic elicitors SA (5 µM), MJ (5 µM) and biotic elicitor A. niger (0.1%) were selected.
Production of the isoflavones was maximum on the 18-day-old callus suspension culture in control as well as elicitor-treated cultures. On this basis, the expression of four genes CHS7, CHS8, IFS1 and IFS2 were quantified on the 18-day-old culture at 6 h, 12 h, and 24 h intervals. Expression of four genes was detected at all the three intervals, wherein the up-regulation was maximum at 12 h for all the genes and the treatments (Fig. 6). The IFS2 and IFS1 expression were up-regulated to the maximum, with 4.57- and 2.76-fold with SA 5 µM (12 h), A. niger 0.1% (12 h), respectively (Fig. 6a, b). A maximum up-regulation of 0.53-fold and 0.96-fold was observed in CHS7 and CHS8 under the SA 5 µM (12 h) treatment (Fig. 6c, d). The results also revealed that the up-regulation of isoflavones biosynthetic genes was correlated with the isoflavones accumulation under the influence of elicitors in callus suspension cultures. Furthermore, yeast extract elicits the accumulation of isoflavones via elevated level of L-phenylalanine ammonia lyase and chalcone synthase expression in the callus suspension of Medicago truncatula (Suzuki et al., 2005). In contrast, long-term drought stress in Soybean plants has been established to result in the down-regulation of IFS2 gene coinciding with a decrease in isoflavone content (Gutierrez-Gonzalez et al. 2010). Similarly, drought stress exhibits profound influence on variations in physiological responses and isoflavone content in soybean (Akitha Devi and Giridhar 2015). Various biotic and abiotic elicitors are reported to be efficient in triggering metabolites production in soybean suspension culture as shown recently for folic acid (Akitha devi et al. 2018).
Fig. 6.
Expression analysis of isoflavones biosynthetic genes during elicitor treatment in JS-335 G. max callus suspension cultures. a Relative transcript abundance of a IFS1 b IFS2 c CHS7 d CHS8 in response to elicitors SA (5 µM), A. niger (0.1%) and MJ (5 µM) treatment
Conclusion
In the present study, improvement of isoflavones content in Soybean seeds as well as callus suspension cultures was achieved in various elicitors’ treatment. Expression of IFS1, IFS2, CHS7, CHS8 differentially expressed during the seed development and selected biotic and abiotic elicitors in selected two seed varieties, is first of its kind and no such reports are known. Present studies unraveled significant expression levels of both IFS1 and IFS2 under stress, that is, a good sign for obtaining enhanced levels of isoflavones. With respect to the developed callus suspension cultures of soybean, the elicitor mediated differential expression of IFS1, IFS2 and CHS along with the three type of isoflavones augmentation under respective elicitor treatment in JS-335 which is the commercially important soybean variety. This outcome could be useful in the future to look at the functionality of identified regulatory genes involved in isoflavones biosynthetic pathway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to CSIR, New Delhi for funding this project (CSIR-CFTRI-MLP-0152). Authors MKAD and GK are grateful to CSIR, New Delhi and ICMR, New Delhi, respectively, for fellowship.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Akashi T, Aoki T, Ayabe S. Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol. 1999;121:821–828. doi: 10.1104/pp.121.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akitha Devi MK, Giridhar P. Isoflavone augmentation in Soybean cell cultures is optimized using response surface methodology. J Agric Food Chem. 2014;62:3143–3149. doi: 10.1021/jf500207x. [DOI] [PubMed] [Google Scholar]
- Akitha Devi MK, Giridhar P. Variations in physiological response, lipid peroxidation, antioxidant enzyme activities, proline and isoflavones content in Soybean varieties subjected to drought stress. Proc Natl Acad Sci, India, Sect B Biol Sci. 2015;85(1):35–44. doi: 10.1007/s40011-013-0244-0. [DOI] [Google Scholar]
- Akitha Devi MK, Kumar SS, Giridhar P. High yield production of folates from soybean callus cultures in response to elicitors. Biotech. 2018;8:80. doi: 10.1007/s13205-018-1101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Seguin P, Archambault A, Constan L, Jabaji S. Gene expression and isoflavone concentrations in Soybean sprouts treated with chitosan. Crop Sci. 2009;49:224–236. doi: 10.2135/cropsci2007.09.0536. [DOI] [Google Scholar]
- Chennupati P, Seguin P, Chamoun R, Jabaji S. Effects of high temperature stress on Soybean isoflavone concentration and expression of key genes involved in isoflavone synthesis. J Agric Food Chem. 2012;60:12421–12427. doi: 10.1021/jf3036319. [DOI] [PubMed] [Google Scholar]
- Cheng H, Yu O, Yu D. Polymorphisms of IFS1 and IFS2 gene are associated with isoflavone concentrations in Soybean seeds. Plant Sci. 2008;175:505–512. doi: 10.1016/j.plantsci.2008.05.020. [DOI] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Devi MA, Kumar SS, Giridhar P. LC–ESI–MS based characterisation of isoflavones in soybean (Glycine max (L.) Merr.) from India. J Food Sci Technol. 2018;55(12):5045–5054. doi: 10.1007/s13197-018-3443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaubhadel S, Gijzen M, Moy P, Farhangkhoee M. Transcriptome analysis reveals a critical role of CHS7 and CHS8 genes for isoflavonoid synthesis in Soybean seeds. Plant Physiol. 2007;143:326–338. doi: 10.1104/pp.106.086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaubhadel S, McGarvey BD, Williams R, Gijzen M. Isoflavonoid biosynthesis and accumulation in developing Soybean seeds. Plant Mol Biol. 2003;53:733–743. doi: 10.1023/B:PLAN.0000023666.30358.ae. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper J. Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 1997;2:162–165. doi: 10.1016/S1360-1385(97)01030-3. [DOI] [Google Scholar]
- Giridhar P, Parimalan R. A biotechnological perspective towards improvement of annatto color production for value addition—the influence of biotic elicitors. Asia Pac J Mol Biol Biotechnol. 2010;18:77–79. [Google Scholar]
- Gueven A, Knorr D. Isoflavonoid production by soy plant callus suspension culture. J Food Eng. 2011;103:237–243. doi: 10.1016/j.jfoodeng.2010.10.019. [DOI] [Google Scholar]
- Gutierrez-Gonzalez JJ, Guttikonda SK, Tran LSP, Aldrich DL, Zhong R, Yu O, Nguyen H, Sleper DA. Differential expression of isoflavone biosynthetic genes in Soybean during water deficits. Plant Cell Physiol. 2010;51:936–948. doi: 10.1093/pcp/pcq065. [DOI] [PubMed] [Google Scholar]
- Jung W, Yu O, Lau SMC, O'Keefe DP, Odell J, Fader G, McGonigle B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat Biotechnol. 2000;18:208–212. doi: 10.1038/72671. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chen F, Wang X, Rajapakse NC. Effect of chitosan on them biological properties of sweet basil (Ocimum basilicum L.) J Agric Food Chem. 2005;53:3696–3701. doi: 10.1021/jf0480804. [DOI] [PubMed] [Google Scholar]
- Kim J, Chung I. Change in isoflavone concentration of Soybean (Glycine max L.) seeds at different growth stages. J Sci Food Agric. 2007;87:496–503. doi: 10.1002/jsfa.2743. [DOI] [Google Scholar]
- Komaraiah P, Naga Amrutha R, Kavi Kishor PB, Ramakrishna SV. Elicitor enhanced production of plumbagin in suspension cultures of Plumbago rosea L. Enzyme Microb Technol. 2002;31:634–639. doi: 10.1016/S0141-0229(02)00159-X. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−∆∆C(T)) Method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lozovaya VV, Lygin AV, Ulanov AV, Nelson RL, Daydé J, Widholm JM. Effect of temperature and soil moisture status during seed development on Soybean seed isoflavone concentration and composition. Crop Sci. 2005;45:1934–1940. doi: 10.2135/cropsci2004.0567. [DOI] [Google Scholar]
- Modolo LV, Cunha FQ, Braga MR, Salgado I. Nitric oxide synthase- mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthepha seolorum f. sp. meridionalis elicitor. Plant Physiol. 2002;130:1288–1297. doi: 10.1104/pp.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthivelu G, Akitha Devi M, Giridhar P, Rajasekaran T, Ravishankar G, Nikolova M, Angelov G, Todorova R, Kosturkova G. Isoflavone composition, phenol content, and antioxidant activity of Soybean seeds from India and Bulgaria. J Agr Food Chem. 2008;56:2090–2095. doi: 10.1021/jf072939a. [DOI] [PubMed] [Google Scholar]
- Sivanandhan G, Kapil Dev G, Jeyaraj M, Rajesh M, Arjunan i, Muthuselvam M, Manickavasagam M, Selvaraj N, Ganapathi A. Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Culture (PCTOC) 2013;114:121–129. doi: 10.1007/s11240-013-0297-z. [DOI] [Google Scholar]
- Subramanian S, Hu X, Lu G, Odelland JT, Yu O. The promoters of two isoflavone synthase genes respond differentially to nodulation and defense signals in transgenic Soybean roots. J Plant Mol Biol. 2004;54:623–639. doi: 10.1023/B:PLAN.0000040814.28507.35. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Reddy MS, Naoumkina M, Aziz N, May GD, Huhman DV, Sumner LW, Blount JW, Mendes P, Dixon RA. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta. 2005;220:696–707. doi: 10.1007/s00425-004-1387-2. [DOI] [PubMed] [Google Scholar]
- Theboral J, Sivanandhan G, Subramanyam K, Arun M, Selvaraj N, Manickavasagam M, Ganapathi A. Enhanced production of isoflavones by elicitation in hairy root cultures of Soybean. Plant Cell Tissue Organ Culture (PCTOC) 2014;117:477–481. doi: 10.1007/s11240-014-0450-3. [DOI] [Google Scholar]
- Vyn TJ, Yin X, Bruulsema TW, Jackson CJC, Rajcan I, Brouder SM. Potassium fertilization effects on isoflavone concentrations in Soybean [(L.) Merr.] J Agric Food Chem. 2002;50:3501–3506. doi: 10.1021/jf0200671. [DOI] [PubMed] [Google Scholar]
- Wei S. Methyl jasmonic acid induced expression pattern of terpenoid indole alkaloid pathway genes in Catharanthusroseus seedlings. Plant Growth Regul. 2010;61:243–251. doi: 10.1007/s10725-010-9468-7. [DOI] [Google Scholar]
- Yu O, McGonigle B. Metabolic engineering of isoflavone biosynthesis. AdvAgron. 2005;86:147–190. doi: 10.1016/S0065-2113(05)86003-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.