Abstract
The beating of eukaryotic flagella (also called cilia) depends on the sliding movements between microtubules powered by dynein. In cilia/flagella of most organisms, microtubule sliding is regulated by the internal structure of cilia comprising the central pair of microtubules (CP) and radial spokes (RS). Chlamydomonas paralyzed-flagella (pf) mutants lacking CP or RS are non-motile under physiological conditions. Here, we show that high hydrostatic pressure induces vigorous flagellar beating in pf mutants. The beating pattern at 40 MPa was similar to that of wild type at atmospheric pressure. In addition, at 80 MPa, flagella underwent an asymmetric-to-symmetric waveform conversion, similar to the one triggered by an increase in intra-flagella Ca2+ concentration during cell’s response to strong light. Thus, our study establishes that neither beating nor waveform conversion of cilia/flagella requires the presence of CP/RS in the axoneme.
Subject terms: Cellular motility, Cilia
Introduction
Cilia and flagella are beating organelles that propel cells through fluids or produce fluid flows over the cell surface. The internal structure of cilia and flagella, the axoneme, has an evolutionally conserved “9 + 2” structure, composed of nine peripheral doublet microtubules and two central microtubules (central pair: CP) (Fig. 1a). The nine outer doublets and the CP interact with each other through radial spokes (RS) projecting from each doublet microtubule. Adjacent doublets are crosslinked by a protein complex called the nexin/dynein regulatory complex (N-DRC)1. The outer doublet also attaches inner-arm dynein (IAD) and outer-arm dynein (OAD) projecting toward the adjacent doublet, which drive sliding between outer doublets to produce axonemal beating2.
Figure 1.
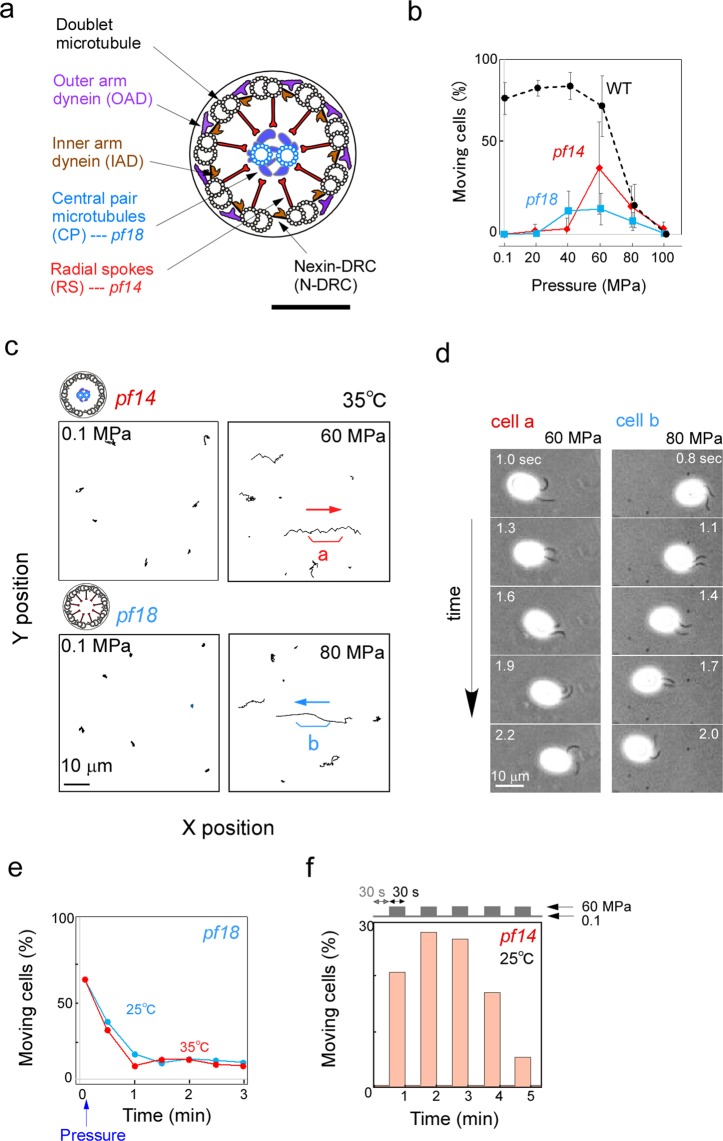
Flagella of Chlamydomonas paralyzed-flagella (pf) mutants beat at high pressure. (a) Schematic illustration of the Chlamydomonas flagellum in cross section. Mutant flagella lacking the central pair (CP) (blue) or radial spokes (RS) (red) are paralyzed under physiological conditions. Bar: 100 nm. (b) Percentage of motile cells under different pressure conditions. Cells of pf14(Red), pf18(blue), and WT (black) were examined. Mean ± SD for more than 20 cells were examined in WT and pf mutants, respectively. Temperature: 25 °C. (c) Typical swimming trajectories of pf cells for three seconds at atmospheric and high pressures. (Upper panel) pf14 lacking radial spokes. (Lower panel) pf18 lacking the central pair. At high pressure, a number of cells randomly swam for short distances, while some cells swam straight (cell a and b). Temperature: 35 °C. (d) (Left) Asymmetric bending pattern in a forward-swimming cell of pf14 (cell a in c). Bending of the two flagella is asynchronous. (Right) Symmetric bending pattern in a backward-swimming cell of pf18 (cell b in c). Bending of the two flagella is synchronous. Cells were observed by high-speed video microscopy. Numbers on the left or right indicate the time after the onset of recording. (e) Time course of the pf18 cell movement after being activated by pressure application. The fraction of moving cells reached a peak within 10 seconds, and then gradually decreased. More than 40 cells were examined for each time point. The time course was similar at 25 and 35 °C. A small percentage of cells continued to swim for more than 3 minutes. (f) Reversibility of the pressure-induced motility in pf14. A high pressure (60 MPa) and the atmospheric pressure (0.1 MPa) were alternately applied each for 30 sec. The number of moving cells 10 sec after the pressure change was shown in the graph. Cells stopped and resumed movements, both within 1 sec after the release from and application of high pressure, respectively. More than 120 cells were examined for each time point. Temperature: 25 °C.
Various lines of evidence indicate that the microtubule sliding is regulated by CP and RS3. Chlamydomonas mutants lacking the CP or RS are non-motile under physiological conditions, and are called paralyzed-flagella (pf) mutants4,5. In contrast to the axonemes isolated from wild type (WT), which undergo beating upon addition of ATP, axonemes from pf mutants lacking the CP/RS do not beat under the same conditions5. However, the flagella and axonemes of pf mutants can beat under certain genetic and chemical conditions. First, in the background of a certain mutation (suppressor mutation) in N-DRC, IAD or OAD, the pf flagella can beat without recovering the missing structure6. This and other observations suggest that N-DRC and CP/RS cooperate in regulating dynein activities in the axoneme7. Second, axonemes from pf mutants, while unable to beat in the normal reactivation buffer containing physiological concentrations of ATP, can beat in reactivation solutions with low concentrations of ATP or with ATP plus ADP8. Regulatory nucleotide binding by dyneins may elicit this phenomenon9–11. Axonemes of pf mutants can also beat when appropriate concentrations of salts or organic compounds are added to the normal reactivation buffer12. The wide range of effective chemicals led to the hypothesis that a change in the solvation of axonemal proteins underlies the beating of the pf axonemes12. Experiments using various dynein-deficient mutants indicated that OAD, but not IAD, is essential for generation of beating. Thus, the change in solvation or non-physiological nucleotide conditions may produce axonemal beating by modulating the OAD activity.
In the present study we examined the effect of hydrostatic pressure on live Chlamydomonas wild type and mutants, and unexpectedly found that pf mutants become motile at high pressures. In addition, at higher pressure, cells changed swimming direction from forward to backward by changing the flagellar waveform. Thus, at high pressure, neither flagellar beating nor waveform conversion requires the CP/RS.
Results and Discussion
Pressure-induced flagellar beating in Chlamydomonas non-motile mutants
Using high-pressure microscopy13, we examined the motility of wild type (WT) and two kinds of non-motile paralyzed-flagella (pf) mutants, pf18 lacking CP and pf14 lacking RS (Fig. 1a), at various pressures up to 100 MPa. In WT, the number and speed of swimming cells decreased with increasing pressure (Fig. 1b; Fig. S1). All cells stopped swimming at 100 MPa. Such an inhibitory effect of pressure on the beating of motile cilia and flagella is consistent with previous reports14,15. However, the pf mutants displayed peculiar responses to high pressure. When the pressure was raised to 80 MPa, many pf18 and pf14 cells started to swim within several seconds (Fig. 1c,d, Movie S1). The fraction of moving cells immediately after pressure application was about 60% but decreased to 10% in 3 min (Fig. 1e). When released from high pressure, all cells stopped swimming. Upon re-application of 60 MPa, a fraction of cells started to move again (Fig. 1f). Thus, the pressure-induced motility induction is reversible at least partially.
In a series of analysis under varying hydrostatic pressure (from 0.1 to 100 MPa) and temperature (from 5 to 35 °C), the fraction of the moving cells peaked at lower pressures with decrease in temperature (Fig. S2). This behavior is consistent with the idea that the beating and non-beating states are in equilibrium, and that the equilibrium changes with pressure and temperature; we can think that the pf mutants are non-motile under physiological conditions because the equilibrium is somehow shifted to the non-beating state.
Eukaryotic flagella have two types of axonemal dyneins, inner-arm and outer-arm dyneins (IAD and OAD) (Fig. 1a). Previous studies showed that IAD is important for generating strong bending of flagella, while OAD is important for generating high beat frequency16–18. To explore the mechanism of pressure-induced activation of pf mutants, we investigated the motility of pf18 cells with the background of oda1 or ida5, mutation causing loss of the entire OAD17,18 or several IAD species16,19 (Table S1). Like WT, these dynein-deficient mutants gradually decreased their motility with increase in pressure (Fig. 2a). The double mutant pf18ida5 displayed vigorous flagellar beating at 80 MPa. The optimal pressure for motility induction in pf18ida5 was higher than in pf18, suggesting that some IAD species facilitate the induction of beating in the pf mutants at high pressure, although they are not prerequisite for motility. In contrast, pf18oda1 displayed no movements at any pressure (Fig. 2b; Table S1). Thus, OAD seems to be critical for flagellar beating of pf18 at high pressure.
Figure 2.
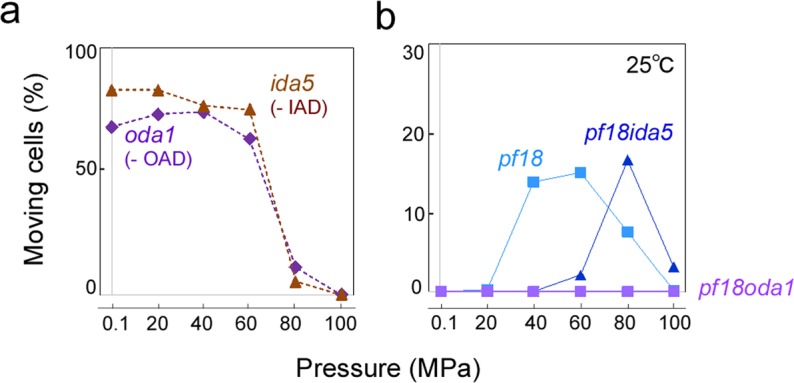
Percentage of moving cells in mutants lacking central pair and different types of dyneins. Motility of (a) dynein deficient mutants and (b) double mutants lacking central pair and dyneins at high pressure. The mutant oda1 lacks the entire outer-arm dynein (OAD), whereas the mutant ida5 lacks several inner-arm dynein species (IAD) (Table S1). The number of moving cells decreased with the increase of pressure in the single dynein mutants, as observed in WT. While pf18ida5 moved at high pressures like pf18, pf18oda1 displayed no movement at any pressure. More than 20 cells were examined for each data point. Temperature: 25 °C.
The requirement of OAD for pressure-induced flagellar beating in pf mutants is reminiscent of previous studies. One study showed that mechanical stimulation of live pf mutants induced temporary flagellar beating (oscillation lasted only for <10 cycles), and that OAD was indispensable for this motility20. Other studies showed that some of the suppressor mutations that restore flagellar beating in pf mutants6 have mutations in the β or γ heavy chains (HCs) of OAD21,22, suggesting that modulation of these HCs could induce beating of pf flagella. Although previous studies have thus observed flagellar beating in pf mutants under certain conditions, our present study is the first to show fairly stable flagellar beating in live pf mutants without any additional mutation.
The activation of flagellar motility in the pf mutants may be brought about through a direct action of pressure on the axoneme. Alternatively, motility may be induced through some vital cellular function. To distinguish between the two possibilities, we performed in vitro assays at high pressures using isolated axonemes23. In a reactivating buffer containing 1 mM ATP and 1 mM EGTA, at atmospheric pressure, WT axonemes displayed vigorous beating with asymmetric waveform, but pf14 axonemes displayed no movements5. However, application of 40 MPa pressure induced beating in some axonemes (Fig. 3a; Movie S2). The fraction of beating axonemes in the total axonemes reached 10–30% at 40–60 MPa. Similar results were obtained with pf18 axonemes (Movie S2). These observations strongly suggest that the applied pressure induced flagellar movements in pf cells by directly acting on the axoneme.
Figure 3.
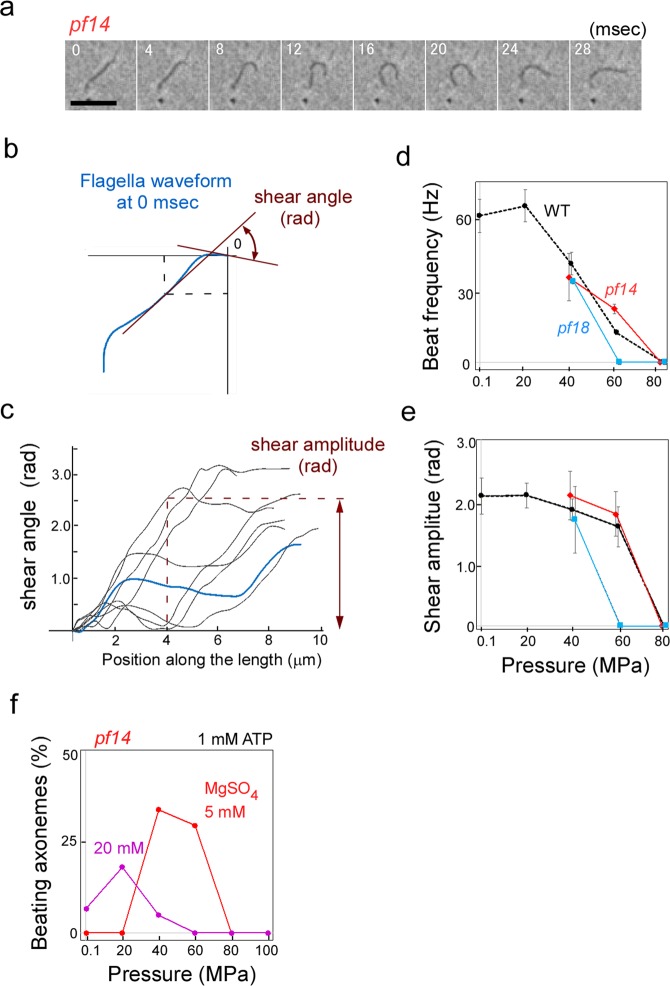
Reactivated motility of axonemes at high pressure. (a) Sequential photographs of a pf14 axoneme beating with an asymmetric waveform in the presence of 1 mM ATP and 1 mM.EGTA at 40 MPa. Temperature: 25 °C (b) A trace of waveform in (a). The angle between the tangents to the proximal segment (position 0, at upper right position) and multiple positions every 0.25 μm along the axoneme were measured. The angle (shear angle) is proportional to the sliding distance between doublet microtubules at each position50. (c) The shear angle at every 0.25 μm is plotted along the length of axoneme (shear curve). Shear curves for waveforms at different time points in (a) were overlaid; the shear curve at 0 ms is drawn in blue. Shear amplitude at 4 μm from the proximal end (indicated by the dashed line) is used for waveform comparison. (d,e) Beat frequency and shear amplitude in the beating axonemes of WT and pf mutants at different pressures. Mean ± SD were measured in 10 axonemes each for WT and mutants. Temperature: 25 °C. (f) Pressure-induced beating of pf14 axoneme at different MgSO4 concentrations. Optimal pressure for pf14 axonemes were lower at 20 mM MgSO4 than at 5 mM MgSO4. More than 50 cells were examined for each data point.
Almost all (~99%) axonemes of WT and mutants beating in the reactivation buffer displayed asymmetric pattern (Fig. 3a; Movie S2) at any pressure. At 40 MPa, the beat frequency of pf14 was 35 ± 10 Hz (mean ± SD, n = 14), which was about half of the WT frequency at atmospheric pressure, 0.1 MPa (63 ± 7 Hz, n = 10) (Fig. 3d). The shear amplitude of pf14 at 40 MPa was 2.2 ± 0.4 rad (n = 14), which was similar to that of WT at 0.1 MPa, 2.1 ± 0.2 rad (n = 10) (Fig. 3e). Thus, overall, the beating pattern of pf mutant axonemes at 40 MPa is similar to that of WT under physiological conditions.
We previously showed that the presence of ATP plus salts (such as MgSO4) or organic compounds induced WT-like beating in pf mutant axonemes in vitro, and proposed that those chemicals induced axonemal motility by changing protein solvation in the axoneme12. In a pressure-application experiment, we found that pf axonemes started to beat at lower pressure when the MgSO4 concentration was increased from the standard 5 mM to 20 mM (Fig. 3f). High MgSO4 concentrations and high pressure are thus apparently additive in the effect to induce motility in pf axonemes. Both may cause a perturbation of the protein solvation in the axoneme. In fact, both high pressure and the addition of salt or organic compounds are known to change protein conformation through a change in solvation24–27. Since OAD was necessary for motility induction by pressure as well as by salt12, we surmise that a change in protein solvation might change the manner of interaction between OAD and doublet microtubules.
The OAD HCs specifically affected by high pressure may be identified by examining the motile properties of isolated OAD HCs and microtubules, using an in vitro system similar to the one used to analyze the properties of kinesin under high pressure28. Information about specific OAD HC may also be obtained from experiments applying pressure on mutants lacking a specific HC, such as oda11, oda4-S7, and oda2-t29–31.
Although high pressure could directly affect the activity of dynein HCs, naturally it could also affect the hydration and conformation of all axonemal proteins. Their changes may induce large-scale changes in the axoneme, leading to a modulation of dynein activity. For example, a model of axonemal beating mechanism called the Geometric Clutch model postulates that a change in distance between adjacent outer-doublet microtubules switches the dynein-doublet interaction on and off 32,33. In accordance with this model, many suppressor mutations, i.e. mutations that restore motility in pf mutants, have mutations in N-DRC34,35, IAD36, or OAD21,22, structures that may critically affect the inter-doublet distance. We could imagine that the high hydrostatic pressure, as well as high salts, induces motility in the pf mutants by also affecting the inter-doublet distance through a change in protein solvation.
The mechanism that produces oscillatory bending movements in cilia and flagella is still not established even though various models, including Geometric Clutch model, have gained certain experimental supports. Our observations allow us to rule out any hypothesis that postulates an essential role of CP and RS in the generation of axonemal beating.
Switching of the swimming direction by pressure
In the above experiments, we noticed that some cells under high-pressure conditions swam backward while some swam forward. Chlamydomonas cells usually swim forward by beating the two flagella with asymmetric waveforms but, when stimulated by light or other environmental factors, transiently swim backward by changing the waveform to a symmetric pattern37,38. As shown in Fig. 1d, forward-swimming pf mutant cells displayed an asymmetric flagellar waveform, whereas backward-swimming cells displayed a symmetric waveform (Movie S3). Many other cells displayed jiggling movements such that a cell swam only for a short distance comparative to its body size during recording for a few seconds (Movie S3). WT cells also displayed backward swimming at 80 MPa (Movie S4). The forward swimming velocity of pf14 cells at 60 MPa was about 10 times slower than the WT velocity at the same pressure, which decreased with increasing pressure (pf14, 8.3 ± 4.2 μm/s, WT, 85 ± 14 μm/s; 35 °C) (Fig. 4a). Slow swimming velocity in pf14 probably resulted from uncoordinated flagellar beating, such as the asymmetric flagellar beating interrupted by a short period of symmetric beating (e.g., Fig. 1d at 1.9 sec), or simultaneous occurrence of two types of waveforms in the two flagella on a single cell (Fig. 1d at 2.2 sec). In contrast, in the backward-swimming mode at 80 MPa, pf14 cells swam at a velocity comparable to that of WT (pf14, 5.7 ± 2.7 μm/s; WT, 7.3 ± 4.1 μm/s; 35 °C) (Fig. 4a). Similar results were obtained with the pf18 cells (Fig. 4a).
Figure 4.
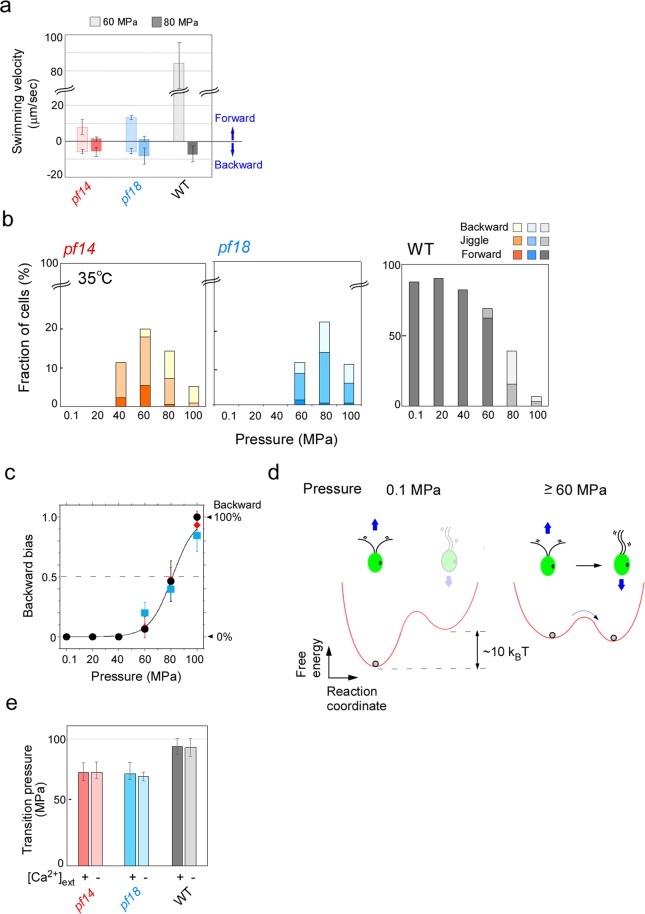
Backward-swimming cells increase at high pressure. (a) Comparison of forward- and backward-swimming velocities in WT and pf mutant cells. Swimming velocities of more than five cells were measured under respective pressure conditions. Mean ± SD for more than five cells were examined in WT and pf mutants, respectively. Temperature: 35 °C. (b) The number ratio of forward-swimming, backward-swimming, and jiggling cells under high-pressure conditions for pf and WT cells at 35 °C. More than 50 cells were examined for each data point. Similar results were obtained at 15 and 25 °C (Fig. S3). (c) Increase in number of backward-swimming cells with the increase of pressure. Backward bias, nbackward/(nforward + njiglling + nbackward), was calculated, where nforward, njiglling, nbackward are the numbers of the cells in the forward, jiggling and backward swimming states, respectively. Data for cells whose flagella were stopped were excluded from the calculation. The mean ± SEM of three independent experiments were shown for pf14 (red), pf18 (blue), and WT (black). (d) Two-state equilibrium model of pressure-induced change of flagellar waveform. The forward-moving (asymmetrically beating) state and backward-moving (symmetrically beating) state would be in the equilibrium. At ambient pressure (0.1 MPa), forward-moving state is prevailing because its free energy potential is lower than that of backward-moving state. High pressure decreases the free-energy potential difference between the two states, and increases the number of backward-moving cells with symmetric beating pattern. Following a two-state model41, the backward bias is thermodynamically given by (1 + exp((ΔG + PΔV)/kBT))−1, where ΔG is standard free energy, P is pressure, ΔV is a pressure-dependence parameter (reaction volume), kB is the Boltzman’s constant, and T is temperature. The best-fit result was obtained with ΔG = 9.4 kBT, ΔV = −0.49 nm3 for pf mutant and WT cells (Solid line in c). A gray ball in the left figure indicates that all cells swim forward at atmospheric pressure. In contrast, two gray balls in the right figure indicates that, at high pressure (≥60 MPa), the two states are in equilibrium and that the number of cells moving backward increases with the increase of pressure (arrow). (e) The transition pressure (the pressure at which 50% of moving cells displayed backward swimming) for the three strains in the culture medium containing either 0.35 mM Ca2+ or 2 mM EGTA. Backward bias with or without Ca2+ was analyzed for more than 40 cells, as in Fig. 4b and 4c. The mean ± SEM of the transition pressure in three independent experiments were shown for pf14 (red), pf18 (blue), and WT (gray). All three strains showed almost the same transition pressure in the two media.
We classified the types of cell movement into forward swimming, backward swimming, jiggling, and non-motile types by eye. As shown in Fig. 4b, at ≤20 MPa and 35 °C, all pf14 cells were non-motile. At 40–60 MPa, some cells became motile, either swimming forward or jiggling in a small area. At 80–100 MPa, a significant fraction of moving cells swam backward. Similar results were obtained with the pf18 cells (Fig. 4b). In the case of WT cells, all motile cells swam forward at ≤40 MPa. At higher pressure, some cells displayed backward swimming and the fraction of backward swimming cells increased with pressure (Fig. 4b). At lower temperatures (15 and 25 °C), cells started backward swimming at lower pressure (Fig. S3). To characterize the pressure-induced change in swimming direction, we calculated the probability of motile cells to swim backward (backward bias) by nbackward/(nforward + njiglling + nbackward), where nforward, njiglling, nbackward are the numbers of cells in the forward swimming, jiggling and backward swimming states, respectively (Fig. 4c). Non-motile cells were not included in the calculation. The backward bias value increased steeply with the pressure increase, and reached 0.5 at ~80 MPa. Notably, the backward bias increased with pressure similarly for all strains. The observation that flagella can undergo asymmetrical-symmetrical waveform conversion even without CP or RS is consistent with the results reported by a previous study using mutant axonemes reactivated under non-physiological nucleotide conditions at varied Ca2+ concentrations39.
How might the increased pressure induce waveform conversion? Flagellar waveform conversion in Chlamydomonas is known to take place through an increase in intraflagellar concentration of Ca2+ from ~10−7 to ~10−4 M (refs. 23,40). Thus, applied pressure might increase intraflagellar concentration of Ca2+. Another possibility is that pressure directly modulates some key axoneme proteins to induce waveform change without changing intraflagellar Ca2+ concentration. However, the latter possibility is unlikely because, in the absence of Ca2+ in the medium, we did not observe any axonemes beating with a symmetrical waveform at high pressure (Fig. 3a).
We hypothesized that the increased Ca2+ concentration shifts the equilibrium between the asymmetrically beating state and symmetrically beating state. High pressure could decrease the free-energy potential difference between the two states, which would increase the number of backward-moving cells with symmetric beating pattern (Fig. 4d). Following the two-state model considered for a previous pressure-application experiment41, the potential difference was thermodynamically estimated to be ~10 kBT, where kB is the Boltzman’s constant, and T is temperature (Fig. 4c,d). The value is close to the chemical potential difference of Ca2+ required for flagellar waveform change (refs. 23,40), kBTln(10−7/10−4), which is ~7 kBT. This result is thus consistent with the idea that the applied pressure worked to increase the intraflagellar Ca2+ concentration.
The light-induced waveform change in live Chlamydomonas cells is triggered by Ca2+ entry from extracellular medium into flagella through a voltage-gated Ca2+ channel42–44. We thought that pressure might open the Ca2+ channel responsible for the Ca2+ entry from the extracellular medium. To test this possibility, we applied pressure in the medium containing 2 mM EGTA instead of the original culture medium that contained 0.35 mM CaCl2. To our surprise, significant fractions of pf14 and pf18 cells still displayed backward swimming at 80 MPa. The transition pressure at which 50% of moving cells swam backward remained almost the same with and without Ca2+ in all strains (Fig. 4e). This result indicates that waveform conversion took place without an influx of Ca2+ from extracellular medium. Pressure possibly works on the intracellular Ca2+ store45 to increase cytosolic Ca2+, which then induces flagellar waveform conversion.
Although exactly how hydrostatic pressure induces waveform conversion remains to be studied further, our present study clearly showed that flagella in live Chlamydomonas cells can beat without the CP and RS (Fig. 1), and that they can undergo a reversible waveform conversion at high pressure as in WT flagella under physiological conditions (Fig. 4). These observations raise the question regarding the function of CP and RS. It may be much more subtle than generally thought. However, as the flagellar movement of the pf mutants at high pressure was vigorous but unstable, we can at least say that one of their functions is to stabilize flagellar oscillatory bending.
Methods
Cell strains and culture
Chlamydomonas reinhardtii strains 137c (wild type), oda1 lacking the outer-arm dyenin17, ida5 lacking a subset of the inner-arm dyneins46, pf14 lacking the radial spokes, and pf18 lacking the central pair4,5 were used. Double mutants of the pf mutants with oda1 or with ida5 were also used. Cells were grown in Tris-acetate-phosphate (TAP) medium47 with aeration on a 12 h/12 h, light/dark cycle.
Reactivation of isolated flagellar axonemes
Flagellar axonemes were prepared by the method of Witman et al.5 using demembranation of isolated flagella in HMDEK (30 mM HEPES (pH7.4), 5 mM MgSO4, 1 mM DTT, 1 mM EGTA, 50 mM K-acetate) containing 0.2% Nonidet P-40 (Nakali tesque, Kyoto, Japan). The demembranated axonemes were reactivated with 1 mM ATP in HMDEKP (HMDEK plus 1% polyethyleneglycol (Mr 20,000; Wako chemicals, Osaka, Japan)).
High-pressure microscopy
The high-pressure microscope system used has been described elsewhere in detail13,41. The sample solution was placed in a high-pressure chamber and mounted on an inverted microscope (Ti–E, Nikon, Tokyo, Japan) equipped with a pressuring apparatus. This apparatus enabled a pressure increase by several tens of MPa within a few seconds. The temperature in the chamber was controlled within ±1 °C48,49. Microscopic images were acquired with a CCD camera (WAT–120N+, Watec,Tokyo, Japan) at 30 frame s−1 or with a high-speed camera (LRH20000B, digimo, Tokyo, Japan) at 500 frames s−1. All microscopic images were stored in a computer and analyzed offline using ImageJ software (http://imagej.nih.gov/ij/). Chlamydomonas culture medium, TAP medium, contains 0.35 mM CaCl247. To investigate the cell motility in the absence of Ca2+, Ca2+-free TAP medium containing 2 mM EGTA was used.
Assessment of axoneme motility
The movements of live cells and reactivated axonemes were examined at temperatures between 5 °C and 35 °C, while pressure was being increased from 0.1 to 100 MPa in a stepwise manner.
The beat frequency and bending amplitude of axonemes were assessed by manually-traced bending waveforms. The tangent angle at every 0.25 μm along the axoneme length was measured relative to the angle in the proximal segment (Fig. 3b). A shear curve was obtained by plotting the angles (shear angles) for the total length50 (Fig. 3c). Shear curves for specific time points were overlaid, and the angular variation in the shear curves, at a position 4 μm from the base, was regarded as the representative amplitude39 (Fig. 3c).
Supplementary information
Acknowledgements
We are very grateful to Dr. Ritsu Kamiya for critical reading this manuscript and Dr. Yoshie Harada for technical support. This work was supported in part by JSPS KAKENHI Grant Numbers JP22570157, JP26440074, JP23118706, JP15H01327 (to T.Y.) and JP23118710, JP25117511, JP16K04908, JP19H02566, 19H04679 (to M.N.).
Author contributions
T.Y. and M.N. designed and performed research; T.Y. and M.N. analyzed data; and T.Y. and M.N. wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Toshiki Yagi, Email: yagit@pu-hiroshima.ac.jp.
Masayoshi Nishiyama, Email: mnishiyama@phys.kindai.ac.jp.
Supplementary information
is available for this paper at 10.1038/s41598-020-58832-8.
References
- 1.Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 2009;187:921–933. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Summers, K. E. & Gibbons, I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc. Natl. Acad. Sci. USA68, 3092–3096 (1971). [DOI] [PMC free article] [PubMed]
- 3.Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil. Cytoskeleton. 2004;57:8–17. doi: 10.1002/cm.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warr JR, McVittie A, Randall J, Hopkins JM. Genetic control of flagellar structure in Chlamydomonas reinhardii. Genet. Res. 1966;7:335–351. doi: 10.1017/S0016672300009794. [DOI] [Google Scholar]
- 5.Witman GB, Plummer J, Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J. Cell Biol. 1978;76:729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982;28:115–24. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- 7.Loreng TD, Smith EF. The Central Apparatus of Cilia and Eukaryotic Flagella. Cold Spring Harb. Perspect. Biol. 2017;9:a028118. doi: 10.1101/cshperspect.a028118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omoto CK, Yagi T, Kurimoto E, Kamiya R. Ability of paralyzed flagella mutants of Chlamydomonas to move. Cell Motil. Cytoskeleton. 1996;33:88–94. doi: 10.1002/(SICI)1097-0169(1996)33:2<88::AID-CM2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Yagi T. ADP-dependent microtubule translocation by flagellar inner-arm dyneins. Cell Struct. Funct. 2000;25:263–7. doi: 10.1247/csf.25.263. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Shingyoji C. The roles of noncatalytic ATP binding and ADP binding in the regulation of dynein motile activity in flagella. Cell Motil. Cytoskeleton. 2007;64:690–704. doi: 10.1002/cm.20216. [DOI] [PubMed] [Google Scholar]
- 11.Kikushima K. Central pair apparatus enhances outer-arm dynein activities through regulation of inner-arm dyneins. Cell Motil. Cytoskeleton. 2009;66:272–80. doi: 10.1002/cm.20355. [DOI] [PubMed] [Google Scholar]
- 12.Yagi T, Kamiya R. Vigorous beating of Chlamydomonas axonemes lacking central pair/radial spoke structures in the presence of salts and organic compounds. Cell Motil. Cytoskeleton. 2000;46:190–199. doi: 10.1002/1097-0169(200007)46:3<190::AID-CM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama M. High-pressure microscopy for tracking dynamic properties of molecular machines. Biophys. Chem. 2017;231:71–78. doi: 10.1016/j.bpc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Otter T, Salmon ED. Hydrostatic pressure reversibly blocks membrane control of ciliary motility in Paramecium. Sci. 1979;206:358–360. doi: 10.1126/science.482945. [DOI] [PubMed] [Google Scholar]
- 15.Kitching JA. Effects of high hydrostatic pressures on the activity of flagellates and ciliates. J. Exp. Biol. 1957;34:494–510. doi: 10.1242/jeb.51.2.319. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya R, Kurimoto E, Muto E. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J. Cell Biol. 1991;112:441–447. doi: 10.1083/jcb.112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamiya R. Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J. Cell Biol. 1988;107:2253–2258. doi: 10.1083/jcb.107.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell DR, Rosenbaum JL. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J. Cell Biol. 1985;100:1228–34. doi: 10.1083/jcb.100.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagi T, Uematsu K, Liu Z, Kamiya R. Identification of dyneins that localize exclusively to the proximal portion of Chlamydomonas flagella. J. Cell Sci. 2009;122:1306–1314. doi: 10.1242/jcs.045096. [DOI] [PubMed] [Google Scholar]
- 20.Hayashibe K, Shingyoji C, Kamiya R. Induction of temporary beating in paralyzed flagella of Chlamydomonas mutants by application of external force. Cell Motil. Cytoskeleton. 1997;37:232–239. doi: 10.1002/(SICI)1097-0169(1997)37:3<232::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Porter ME, Knott JA, Gardner LC, Mitchell DR, Dutcher SK. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the beta-dynein heavy chain. J. Cell Biol. 1994;126:1495–507. doi: 10.1083/jcb.126.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rupp G, O’Toole E, Gardner LC, Mitchell BF, Porter ME. The sup-pf-2 mutations of Chlamydomonas alter the activity of the outer dynein arms by modification of the gamma-dynein heavy chain. J. Cell Biol. 1996;135:1853–65. doi: 10.1083/jcb.135.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bessen M, Fay RB, Witman GB. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol. 1980;86:446–455. doi: 10.1083/jcb.86.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luong TQ, Kapoor S, Winter R. Pressure- A gateway to fundamental insights into protein solvation, dynamics, and function. Chemphyschem. 2015;16:3555–3571. doi: 10.1002/cphc.201500669. [DOI] [PubMed] [Google Scholar]
- 25.Boonyaratanakornkit BB, Park CB, Clark DS. Pressure effects on intra- and intermolecular interactions within proteins. Biochim. Biophys. Acta. 2002;1595:235–249. doi: 10.1016/S0167-4838(01)00347-8. [DOI] [PubMed] [Google Scholar]
- 26.Parsegian VA, Rand RP, Rau DC. Macromolecules and water probing with osmotic stress. Methods Enzymol. 1995;259:43–94. doi: 10.1016/0076-6879(95)59039-0. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber G. Kinetic studies of protein–protein interactions. Curr. Opin. Struct. Biol. 2002;12:41–47. doi: 10.1016/S0959-440X(02)00287-7. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama M, Kimura Y, Nishiyama Y, Terazima M. Pressure-induced changes in the structure and function of the kinesin-microtubule complex. Biophys. J. 2009;96:1142–50. doi: 10.1016/j.bpj.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakakibara H, Mitchell DR, Kamiya R. A Chlamydomonas outer arm dynein mutant missing the alpha heavy chain. J. Cell Biol. 1991;113:615–22. doi: 10.1083/jcb.113.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakakibara H, Takada S, King SM, Witman GB, Kamiya R. A Chlamydomonas outer arm dynein mutant with a truncated beta heavy chain. J. Cell Biol. 1993;122:653–61. doi: 10.1083/jcb.122.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, et al. Partially functional outer-arm dynein in a novel Chlamydomonas mutant expressing a truncated gamma heavy chain. Eukaryot. Cell. 2008;7:1136–45. doi: 10.1128/EC.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindemann CB. A model of flagellar and ciliary functioning which uses the forces transverse to the axoneme as the regulator of dynein activation. Cell Motil. Cytoskeleton. 1994;29:141–154. doi: 10.1002/cm.970290206. [DOI] [PubMed] [Google Scholar]
- 33.Lesich KA, DePinho TG, Dang L, Lindemann CB. Ultrastructural evidence that motility changes caused by variations in ATP, Mg, and ADP correlate to conformational changes in reactivated bull sperm axonemes. Cytoskeleton. 2014;71:649–661. doi: 10.1002/cm.21199. [DOI] [PubMed] [Google Scholar]
- 34.Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 1992;118:1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin J, et al. Building blocks of the nexin-dynein regulatory complex in Chlamydomonas flagella. J. Biol. Chem. 2011;286:29175–29191. doi: 10.1074/jbc.M111.241760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter ME, Power J, Dutcher SK. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J. Cell Biol. 1992;118:1163–76. doi: 10.1083/jcb.118.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witman GB. Chlamydomonas phototaxis. Trend. Cell Biol. 1993;3:403–408. doi: 10.1016/0962-8924(93)90091-E. [DOI] [PubMed] [Google Scholar]
- 38.Fujiu K, Nakayama Y, Iida H, Sokabe M, Yoshimura K. Mechanoreception in motile flagella of Chlamydomonas. Nat. Cell Biol. 2011;13:630–632. doi: 10.1038/ncb2214. [DOI] [PubMed] [Google Scholar]
- 39.Wakabayashi, K., Yagi, T. & Kamiya, R. Ca2+-dependent waveform conversion in the flagellar axoneme of Chlamydomonas mutants lacking the central pair/radial spoke system. Cell Motil. Cytoskeleton38, 22–28 (1997). [DOI] [PubMed]
- 40.Hyams JS, Borisy GG. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J. Cell Sci. 1978;33:235–53. doi: 10.1242/jcs.33.1.235. [DOI] [PubMed] [Google Scholar]
- 41.Nishiyama M, et al. High hydrostatic pressure induces counterclockwise to clockwise reversals of the Escherichia coli flagellar motor. J. Bacteriol. 2013;195:1809–14. doi: 10.1128/JB.02139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harz H, Hegemann P. Rhodopsin-regulated calcium currents in Chlamydomonas. Nat. 1991;351:489–491. doi: 10.1038/351489a0. [DOI] [Google Scholar]
- 43.Fujiu K, Nakayama Y, Yanagisawa A, Sokabe M, Yoshimura K. Chlamydomonas CAV2 encodes a voltage- dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 2009;19:133–9. doi: 10.1016/j.cub.2008.11.068. [DOI] [PubMed] [Google Scholar]
- 44.Geyer VF, Sartori P, Friedrich BM, Jülicher F, Howard J. Independent Control of the Static and Dynamic Components of the Chlamydomonas Flagellar Beat. Curr. Biol. 2016;26:1098–103. doi: 10.1016/j.cub.2016.02.053. [DOI] [PubMed] [Google Scholar]
- 45.Quarmby LM, et al. Inositol phospholipid metabolism may trigger flagellar excision in Chlamydomonas reinhardtii. J. Cell Biol. 1992;116:737–44. doi: 10.1083/jcb.116.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato-Minoura T, Hirono M, Kamiya R. Chlamydomonas inner-arm dynein mutant, ida5, has a mutation in an actin-encoding gene. J. Cell Biol. 1997;137:649–656. doi: 10.1083/jcb.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorman DS, Levine RP. Cytochrome F and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci.USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishiyama M, Arai Y. Tracking the Movement of a Single Prokaryotic Cell in Extreme Environmental Conditions. Methods Mol. Biol. 2017;1593:175–184. doi: 10.1007/978-1-4939-6927-2_13. [DOI] [PubMed] [Google Scholar]
- 49.Okuno D, Nishiyama M, Noji H. Single-molecule analysis of the rotation of F1-ATPase under high hydrostatic pressure. Biophys. J. 2013;105:1635–1642. doi: 10.1016/j.bpj.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brokaw, C. J. & Luck, D. J. Bending patterns of Chlamydomonas flagella I. Wild-type bending patterns. Cell Motil. 3, 131–150 (1983). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.