Abstract
Dendritic cells play a key role in activation of the immune system as potent antigen-presenting cells. This pivotal position, along with the ability to generate dendritic cells from monocytes and ready uptake of antigen, makes them an intriguing vehicle for immunotherapy for a variety of indications. Since the first reported trial using dendritic cells in 1995, they have been used in trials all over the world for a plethora of indications. Monocyte-derived dendritic cells are generated from whole blood or apheresis products by culturing enriched monocytes in the presence of interleukin (IL)-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF). A variety of methods can be used for enrichment of monocytes for generation of clinical-grade dendritic cells and are summarized herein.
Main Text
In the 2018 Annual Regenerative Medicine Data Report, the Alliance for Regenerative Medicine reported that 1,028 clinical trials utilizing specific regenerative medicine or advanced therapeutic technologies were underway worldwide. Of those trials, 263 were classified as cellular therapy products, and another 362 were categorized as gene-modified cellular therapy products.1 While chimeric antigen receptor (CAR) T cell therapies and adeno-associated virus (AAV) vector therapies have thrust immunotherapy into the spotlight of popular media, it is important to note that the first US Food and Drug Administration (FDA)-approved cellular therapy was a dendritic cell (DC) vaccine, sipuleucel-T, or Provenge. This vaccine was approved by the FDA in 2010 and was marketed by Dendreon.2,3
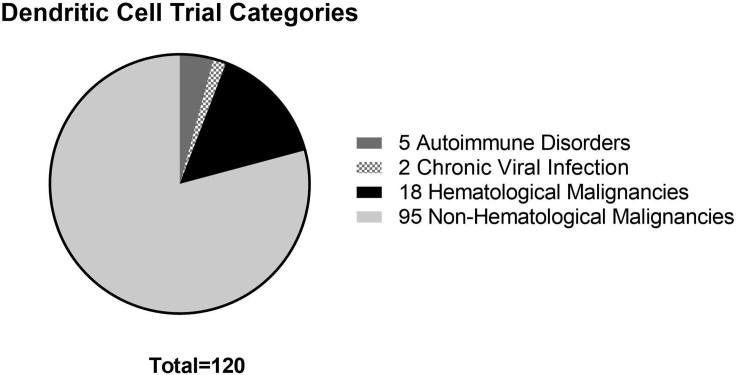
The first article describing vaccination with a DC vaccine was published in 1995, and while no major therapeutic response was noted, peptide-specific T cells were induced at the site of immunization and at distant sites.4 By 2003, more than 1,000 patients had received experimental DC vaccines for various cancer indications.5 More than 16 years later, the website ClinicalTrials.gov had 120 trials listed world-wide using DCs as a biologic drug (as of August 2019). Search criteria included “dendritic cell” with the following filters applied: active; recruiting, not active; and interventional.6 Data gathered from this search was further filtered to remove trials that did not include DCs as a biologic drug. Most trials include some combination of treatment modalities, from hematopoietic stem cell (HSC) transplant, to monoclonal antibodies (checkpoint inhibitors), to chemotherapy, to CAR T cells. Figure 1 shows that the vast majority of these trials are using DCs as a treatment for cancers; however, a few trials are looking at DCs for treatment of autoimmune disorders, such as multiple sclerosis or Crohn’s disease, or for treatment of chronic viral infection, specifically HIV.
Figure 1.
Categories of Current DC Trials Where the Drug Includes a DC as a Biologic Component of Treatment
A total of 120 trials met the criteria. Most of them (n = 113) were for malignancies, further defined as non-hematological malignancies (n = 95), and hematological malignancies (n = 18). Trials for non-malignant indications include autoimmune disorders (n = 5) and chronic viral infections (n = 2).
DCs are professional antigen-presenting cells. These cells bridge the gap between innate and adaptive immunity and are able to ingest proteins and present antigens in complex with both major histocompatibility complex (MHC) class I and MHC class II molecules. As such, they play a key role in regulation of the immune response through both activation and tolerance.7, 8, 9 In the absence of danger signals, immature DCs are capable of inducing peripheral tolerance to self-antigens; however, in the presence of danger signals recognized by pattern recognition receptors, DCs undergo maturation. Mature DCs migrate to the lymph nodes and have increased expression of co-stimulatory molecules, such as CD40, CD80, and CD86, and MHC-peptide complexes on their surface. The additional secretion of inflammatory cytokines allows mature DCs to fully activate T cells with production of all three signals by the same cell: signal 1 (antigen presented in the context of MHC), signal 2 (co-stimulation through upregulation of CD86 and others), and signal 3 (pro-inflammatory cytokine secretion).7,10,11
The breadth of indications using DCs as a treatment modality reflects their important role in eliciting immunity. Various methods for generating clinical DCs have been developed since their discovery and characterization as a morphologically unique cell type with a capacity to activate naive lymphocytes.12, 13, 14 While DCs may be isolated from the blood or generated from hematopoietic progenitor cells,15 the most common method is generation from monocytes using a combination of granulocyte-macrophage colony-stimulation factor (GM-CSF) and interleukin (IL)-4.15, 16, 17, 18 The focus of this review is on the methods for monocyte enrichment used to generate these monocyte-derived DCs.
Generation of DCs from Human Monocytes
Generation of monocyte-derived DCs (MoDCs) for immunotherapy begins with an apheresis collection of peripheral blood mononuclear cells from the patient or donor. The monocytes are enriched using a variety of methods, including both open and closed manipulations, then cultured for approximately 6 days in the presence of IL-4 and GM-CSF to generate immature DCs. Culturing of the cells can be done in either flasks or bags and has recently been discussed elsewhere.19 Additionally, Corning Life Sciences recently launched a novel closed, automated perfusion cell culture system, MicroDEN, developed by Flaskworks.20 Immature DCs generated in the MicroDEN system are phenotypically and functionally similar to plate-generated immature DCs, while manual steps and risk of contamination are vastly reduced.21 Shorter culture times, as little as 18–24 h, have been used to generate immature DCs, with an additional 24-h incubation to induce maturation of DCs.22,23 Cultured DCs are exposed to the protein or antigen of choice through a variety of methods, including transfection, transduction using a variety of viral vectors, or simple addition of tumor lysate or peptides to the culture medium (peptide pulsing), to fusion of DCs with tumor cells through co-culture with the addition of polyethylene glycol.24 Immature or mature DCs are harvested and cryopreserved or administered as a fresh product. Samples of each product are removed for quality control testing and sterility testing prior to patient administration.
Open System Methods for Enrichment
Peripheral blood monocytes must be enriched prior to culturing to deplete contaminating lymphocytes, red blood cells, and platelets. Monocyte enrichment from peripheral blood mononuclear cells (PBMCs) by plastic adherence with or without prior density purification is one of the classic procedures used in research laboratories for cell enrichment.25 Ficoll density purification is particularly important when a high amount of granulocytes and RBCs are present in the starting material. The tube Ficoll method is commonly used for small scale applications, however, this is not easily adapted to large scale processes.26 Large-scale Ficoll gradient methods have been developed for use in clinical trial, providing good recoveries and a closed system.27,28 Apheresis products contain fewer red blood cells (RBCs) and granulocytes than peripheral blood; however, performing a density purification on these products can result in higher purity DCs.
A representative description of monocyte enrichment using plastic adherence is to seed 10–15 × 106 PBMCs per flask into 25-cm2 cell culture flasks, and incubate at 5% CO2 and 37°C for approximately 2 h in 5 mL of media to allow to adherence of the monocytes. At the end of the incubation period, the non-adherent cells are removed, and the adherent cells are carefully washed twice with media or buffered saline to remove remaining non-adherent or loosely adherent cells.29 Lymphocyte contamination in the first hour after adherence may be high, as much as 40%–50% after two washes and 30% even after five washings.30 An expected purity using similar methods at a larger scale is >67.3% with viability of >99%.31 The relative low cost of supplies, common access to required equipment, and adaptability to small volumes of starting PBMC material ensures that the process will continue to be used in research and development work. However, this monocyte adherence enrichment process is an open manual-based protocol. The risk of contamination to cell cultures when using an open multi-flask process, the technical skill required to ensure adequate removal of non-adherent cells while not inadvertently removing adherent cells, and time requirement of 4–8 h of initial processing indicate that this process is not ideal for large-scale production for clinical trials or current Good Manufacturing Practices (cGMP). Additionally, plastic adherence has been suggested to activate the monocytes, which may negatively impact DC development.32
A second open method for monocyte enrichment is elutriation using the counterflow centrifugal elutriation (CCE) cell separating technique. This method enables cells to be separated based on size. The key concept is that the larger cells will stay within the flowing buffer solution while smaller cells will be flushed though the tubing with the buffer solution. Since cell size is correlated with cell cycle stages, this method can also be used to separate cells at different stages of the cell cycle. The sedimentation property differs within the buffer solution. The cells will have sedimentation properties that differ in the various cell cycle stages. The basic principle of differentiating the cells using CCE is the balance between centrifugal force and the counterflow drag force. When cells enter the elutriation chamber they are all at the outer edge of the chamber due to centrifugal force. Then, when the flow rate of the buffer solution is increased the solution pushes the cells inward toward the middle of the CCE chamber due to the unique design of the chamber. This is called the counterflow drag force by the manufacturer. As the flow rate of the buffer solution increases, the counterflow drag force begins to out-compete the centrifugal force, and the smaller cells are driven to the chamber exit by the net force and leave the chamber first. In contrast, the larger cells will stay within the elutriation chamber. Cells that escape from the elutriation chamber can be collected in the exit of the system, which consists of a series of collection bags with clamps to direct flow, allowing segregation of different cell fractions.33
In early studies, the average yield per leukapheresis procedure was 1.5 × 109 cells, with a purity of 91% using the 40-mL separation chamber. The recovery of lymphocytes and monocytes was 82% ± 7% and 78% ± 8%, respectively. In vitro analysis of the viability and function of the purified monocytes shows that neither morphological integrity nor physiological activity was compromised.34 During the CCE cell separation technique, the cells are suspended in a buffer solution and enter a centrifuge. The whole process does not involve any chemical additives to the cells to lyse the cell membrane. Additionally, there are no physical additives such as the attachment of antibody or activation of the cells that might impact subsequent functionality of the cells. The cells effectively remain unchanged before, during, and after the separation. Therefore, the enriched cells are available for further experiments, cell culture, or additional separation and enrichment by other techniques.
Because the CCE process relies on centrifugal force and the counterflow drag force to collect the cells in fractions as they pass through the centrifuge, the speed of separation is rapid, and up to 20 × 109 PBMCs can be elutriated within 1 h.22,35 Alternatively, unlike plastic adherence, the CCE cell separation technique requires specific and expensive equipment to allow for processing. The Beckman J-5.0 rotor (Beckman Coulter, Fullerton, CA), which is an open system, is the current standard.36 There are multiple small parts that are subject to wear and cracking during handling, so back-up parts must be available for use. Additionally, although the process is quick, the setup, decontamination, and subsequent reassembly of the chamber add at least 4 h to processing. The processing technique can be challenging to master and can require at least two trained operators to ensure effective and efficient processing. The elutriation process also requires a substantial volume of starting material such as a leukapheresis collection for adequate separation. Lastly, as the system remains open and supplies are not single use, the risk of contamination of a product or between products is high. Validation of equipment sterilization is required to help control the risk of cross-contamination. This limits the process usefulness in large-scale production for clinical trials or cGMP manufacturing.
Closed-System Methods of Enrichment
CCE provides a more pure monocyte preparation than does standard plastic adherence; however, the open aspect of the system, as well as the required sterilization of the chamber and tubing, drastically increases the risk of product contamination. To address this, Terumo BCT (Lakewood, CO, USA) offers a closed system called the Elutra. This instrument allows monocyte enrichment directly from leukapheresis products using counterflow elutriation. The Elutra incorporates single-use, functionally sealed disposable sets that contain a 40-mL elutriation chamber. The equipment can be programed to increase enrichment for different cell types in each fraction and also offers a high degree of flexibility with the ability to perform multistep separations. Similar to CCE, the Elutra passes fluid through the cell layer established by centrifugal force. Varying the flow of fluid allows collection of particles based primarily on size, from smallest to largest, and based on density, from lower to higher. After priming the Elutra, the leukapheresis product is loaded into the elutriation chamber using the cell inlet pump. The centrifuge speed is set at 2,400 rpm and held constant. The flow of elutriation media is programed to increase slowly in a stepwise fashion to enhance the elutriation of the specific cell fractions into the pre-attached collection bags.
The cell inlet pump speed found to be most advantageous for monocyte enrichment is at a pump flow rate of 37 mL/min and media pump speed of 37–97.5–103.4–103.9 mL/min, and approximately 975 mL of elutriation media per fraction. The centrifuge is stopped and the cells are pumped at 103.9 mL/min into the final collection bag, or “rotor off” from the elutriation chamber. The total processing time is approximately 60–90 minutes.37 The mean monocyte recovery using an Elutra has be found as high as 94.3%–98.53%.37,38 The average purity was 73% ± 9% to 82.95% ± 6.01%.37,39
One of the biggest advantages of the Elutra process is the closed, single-use disposable system that allows for fast isolation of monocytes in large quantities within a closed system, decreasing the risk of contamination and increasing turn-over time for next patient preparation. Similar to CCE, the cells are also untouched by chemical or physical additives. In contrast to CCE, the training time and skill level for operators is consistent with general laboratory techniques. No advanced training or multiple staff operation is required. The process is readily adaptable to large-scale production for clinical trials or cGMP manufacturing. One drawback to sustainable research laboratory processing using Elutra for monocyte enrichment directly is the cost of the sole source supplies and maintenance support. Additionally, the volume of starting material for adequate separation is targeted at a full apheresis product and not smaller peripheral blood collections.
A second closed system for monocyte enrichment is CD14+ cell selection on the CliniMACS cell selection system (Miltenyi Biotec, Auburn, CA, USA). Similar to the Elutra, operation of the CliniMACS does not require advanced training for personnel. The CliniMACS system allows users to perform large-scale magnetic enrichment of target cells, or depletion of unwanted cells, in a closed and sterile environment. The system comprises the CliniMACS Plus instrument, CliniMACS tubing sets, CliniMACS reagent, and CliniMACS PBS/EDTA buffer. The tubing sets are single use and have the separation column integrated within the tubing set to maintain a closed system. They have been designed to process a specific amount of total cells in a closed and sterile fluid path.40 Installation of the tubing set is completed by following the image on the CliniMACS instrument and by following the on-screen instructions, making it user-friendly.
The CD14 antigen is a high-affinity receptor for the lipopolysaccharide (LPS) and LPS-binding protein (LBP) complex.41 It is strongly expressed on most human monocytes and macrophages. Purification of monocytes on the CliniMACS system is achieved using anti-CD14 monoclonal antibodies conjugated to superparamagnetic iron dextran particles.42 The apheresis product is magnetically labeled with CD14 reagent. The CD14 molecule does not have a cytoplasmic domain and acts as a co-receptor with Toll-like receptor 4 (TLR4). Due to the lack of a cytoplasmic domain, binding of the antibody to CD14 should not trigger activation of monocytes; however, in recent years, CD14 was found to activate NFAT (nuclear factor of activated T cells) independent of TLR4.43 Given these findings, enrichment of monocytes through binding of CD14 may have the potential to activate the cells. The bag containing the labeled cell suspension is connected to the buffer-primed tubing set and loaded onto the CliniMACS instrument while the column is exposed to the magnetic field. The magnetically bound CD14+ cells are retained within the column while the CD14− cells are washed off the column and are segregated into a waste bag. After extensive washing, the magnetic field is turned off and the CD14+ cells are pumped through the closed tubing into a positive fraction bag.44 Initial descriptions of CD14 positive selection using magnetic beads resulted in high purity of approximately 96%–97%, but a variable, yet low yield, ranging from 5% to 60% in two studies.16,45 Recent reports show purity of 94% ± 6.1%, with a recovery of 89% ± 23%.46
Conversely, depletion of non-monocytes with a cocktail of antibodies can be used to generate a monocyte population that is not directly attached to magnetic particles. Depletion is achieved by indirectly labeling non-monocytes, such as T cells, natural killer (NK) cells, B cells, DCs, and basophils, with biotin-conjugated antibodies and then binding the biotin-labeled cells with anti-biotin conjugated to superparamagnetic iron dextran particles. This method is not widely used for large-scale generation of DC products. The initial large-scale depletions were performed on the Isolex 300i and removed T cells and B cells using anti-CD2 and anti-CD19. This method had statistically significant lower purity of starting monocytes, slightly more than 60%, compared to selection, with typical purities around 95%. Two out of three studies had a purity below 50%, which is even lower than DCs manufactured using plastic adherence.16,45 One study was able to generate mature DCs with a median purity of 72%; however, in this study, cells were matured with peptide (gp100) and LPS, not LPS and interferon (IFN)-γ.47
Comparisons of Monocyte Enrichment Methods
Direct comparison of monocyte enrichment methods are summarized in Table 1. Plastic adherence is the only technique described here that can be performed using standard laboratory equipment and is not limited by the amount of starting material; however, it provides a lower purity final product, as well as low recovery based on the total starting monocytes.16,45,47 Larger volumes of starting material increase the processing time as well as the risk of contamination. CCE requires specialized equipment that is not single use; however, it provides excellent recovery and purity of monocytes, which translates into some of the highest purity and recovery of final products.16 While enrichment of monocytes is fast, decontamination of the equipment takes approximately 4 h. Other than plastic adherence, all other methods for monocyte enrichment have the added benefit of easily moving enriched cells into a functionally closed culture system, by collecting the monocytes into bags, limiting the risk of contamination during the culture period. Closed-system methods, such as the Elutra and CD14 selection with the CliniMACS, provide high-purity products but lower recoveries.16,45 These methods also require specialized equipment and sole-source consumables, increasing the cost of materials. Depletion of CD2 and CD19 cells, as described on the Isolex 300i, also requires specialized equipment and expensive consumables, but unfortunately cannot meet the purities shown with positive selection on the CliniMACS.16,45,47
Table 1.
Monocyte Enrichment Method Comparison
| Monocytes |
Immature DCs |
Mature DCs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Publication | Cell Source | Method | Purity (%) | Recovery (%) | Purity (%) | Recovery (%) | Purity (%) | Recovery (%) | Maturation Method |
| Eyrich et al.46 | healthy donors, high-grade glioma patients | CD14 selection | 94 ± 6.1 | 89 ± 23 | N/A | N/A | N/A | 15.3 ± 5.3 | tumor lysate 1,000 U/mL TNF-α, 2,000 U/mL IL-1β |
| Elutra | 89 ± 1.5 | 95 ± 24 | N/A | N/A | N/A | 14.5 ± 8 | |||
| Dohnal et al.16 | healthy donors, cancer patients | adherence | n.d | n.d. | N/A | N/A | 62 ± 5 | 6 ± 2 | 50 ng/mL IFN-γ, 1–1,000 ng/mL LPS |
| elutriation | 82 ± 3 | 87 ± 7 | N/A | N/A | 93 ± 2 | 16 ± 2 | |||
| CD14 selection | 96 ± 2 | 59 ± 4 | N/A | N/A | 97 ± 0 | 4 ± 1 | |||
| depletiona | 61 ± 4 | 41 ± 3 | N/A | N/A | 42 ± 8 | 15 ± 3 | |||
| Felzmann et al.45 | healthy donors, cancer patients | adherence | n.d | n.d. | 72 ± 4 | 25 ± 5 | 69 ± 6 | 12 ± 3 | 50 ng/mL IFN-γ, 200 U/mL LPS |
| CD14 selection | 94 ± 4 | 40 ± 9 | 97 ± 1 | 8 ± 3 | 97 ± 1 | 4 ± 2 | |||
| depletiona | 61 ± 5 | 56 ± 7 | 42 ± 10 | 21 ± 6 | 31 ± 8 | 16 ± 6 | |||
| Pullarkat et al.47 | melanoma patients | adherence | n.d | n.d. | N/A | N/A | 63 ± 14 | 2.7 ± 0.96 | 10 μg/mL gp100, 1 μg/mL LPS |
| depletiona | 52 ± 11 | 40 ± 9 | N/A | N/A | 72 ± 11 | 4.84 ± 2.65 | |||
N/A, not available (results were not reported); n.d., not done (no data available because adherent cells would have to be disrupted to determine purity or recovery).
Depletion performed on the Isolex 300i magnetic cell selector (Nexell, Irvine, CA, USA).
Functionally, there are minimal differences reported regardless of the method of monocyte enrichment. One publication, not shown in Table 1, compared plastic adherence to magnetic-activated cell sorting (MACS) methods and found that DCs generated from monocytes enriched using plastic adherence had a higher phagocytic activity than did MACS-enriched monocytes; however, the mean fluorescence intensity of phagocytic cells in the MACS-enriched monocytes was higher.29 Of the publications reviewed in Table 1, only Eyrich et al.46 showed a difference in the quality of DCs based on the method of purification, and only in the mature DCs. Their results showed that matured DCs generated from CD14 enrichment had statistically significant higher expression of DC markers (CD80, CD83, CCR7, PD-L1) and CD14 compared to matured DCs generated from elutriation.46 Immature DCs did not show this difference, however, and the publication did not discuss the impact of increased expression.
Discussion
DCs continue to be used in clinical trials as a means to boost the immune response. Monocyte enrichment is a key step to generating monocyte-derived DCs for downstream applications, and various methods for enrichment have been discussed. The method for enrichment appears to have little impact on the quality of the resultant DCs. The decision of which method to use for enrichment should consider various factors, including quantity and quality of starting material, purity required, maturation status of the final product, experience of personnel manufacturing the DCs, equipment currently available, and phase of the clinical trial.
While the focus of this review is monocyte-derived DCs, it is evident that other sources of DCs are being explored for clinical translation, including generation of DCs from CD34+ progenitor cells, and even DCs from induced pluripotent stem cells.15,48 Regardless of the source of DCs, manufacturing strategies need to be reproducible and robust, with the ultimate goal of providing safe, high-quality products.
Author Contributions
E.L.H. wrote, edited, reviewed, coordinated, and submitted the paper, and C.C. wrote, edited, and reviewed the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The authors thank Linda Kelley (Moffitt Cancer Center) for her support and assistance with this manuscript. This work was supported in part by NCI 3P30CA076292.
References
- 1.Alliance for Regenerative Medicine . 2018. Annual regenerative medicine data report 2018.https://alliancerm.org/wp-content/uploads/2019/02/ARM_AR2018_Report.pdf [Google Scholar]
- 2.US Food and Drug Administration . 2019. Provenge (sipuleucel-T)https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/provenge-sipuleucel-t [Google Scholar]
- 3.Anassi E., Ndefo U.A. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P&T. 2011;36:197–202. [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherji B., Chakraborty N.G., Yamasaki S., Okino T., Yamase H., Sporn J.R., Kurtzman S.K., Ergin M.T., Ozols J., Meehan J. Induction of antigen-specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide-pulsed autologous antigen presenting cells. Proc. Natl. Acad. Sci. USA. 1995;92:8078–8082. doi: 10.1073/pnas.92.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–886. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- 6.US National Library of Medicine . 2019. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/results?cond=&term=“dendritic+cell”&type=Intr&rslt=&recrs=a&recrs=d&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&lupd_s=&lupd_e=&sort= [Google Scholar]
- 7.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Merad M., Sathe P., Helft J., Miller J., Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waisman A., Lukas D., Clausen B.E., Yogev N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 2017;39:153–163. doi: 10.1007/s00281-016-0583-z. [DOI] [PubMed] [Google Scholar]
- 10.Steinman R.M., Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 11.Shortman K., Caux C. Dendritic cell development: multiple pathways to nature’s adjuvants. Stem Cells. 1997;15:409–419. doi: 10.1002/stem.150409. [DOI] [PubMed] [Google Scholar]
- 12.Steinman R.M. Dendritic cells and immune-based therapies. Exp. Hematol. 1996;24:859–862. [PubMed] [Google Scholar]
- 13.Steinman R.M., Cohn Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman R.M., Witmer M.D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc. Natl. Acad. Sci. USA. 1978;75:5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell J.D., Piechaczek C., Winkels G., Schwamborn E., Micheli D., Hennemann S., Schmitz J. Isolation and generation of clinical-grade dendritic cells using the CliniMACS system. Methods Mol. Med. 2005;109:55–70. doi: 10.1385/1-59259-862-5:055. [DOI] [PubMed] [Google Scholar]
- 16.Dohnal A.M., Graffi S., Witt V., Eichstill C., Wagner D., Ul-Haq S., Wimmer D., Felzmann T. Comparative evaluation of techniques for the manufacturing of dendritic cell-based cancer vaccines. J. Cell. Mol. Med. 2009;13:125–135. doi: 10.1111/j.1582-4934.2008.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romani N., Reider D., Heuer M., Ebner S., Kämpgen E., Eibl B., Niederwieser D., Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 18.Sabado R.L., Miller E., Spadaccia M., Vengco I., Hasan F., Bhardwaj N. Preparation of tumor antigen-loaded mature dendritic cells for immunotherapy. J. Vis. Exp. 2013;78:e50085. doi: 10.3791/50085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fekete N., Béland A.V., Campbell K., Clark S.L., Hoesli C.A. Bags versus flasks: a comparison of cell culture systems for the production of dendritic cell-based immunotherapies. Transfusion. 2018;58:1800–1813. doi: 10.1111/trf.14621. [DOI] [PubMed] [Google Scholar]
- 20.Corning . 2019. Corning launches Corning MicroDEN automated system for generating dendritic cells.https://www.corning.com/worldwide/en/products/life-sciences/news-events/news-releases/2019/10/corning-launches-corning-microden-automated-system-for-generating-dendritic-cells.html [Google Scholar]
- 21.Kozbial A., Bhandary L., Collier B.B., Eickhoff C.S., Hoft D.F., Murthy S.K. Automated generation of immature dendritic cells in a single-use system. J. Immunol. Methods. 2018;457:53–65. doi: 10.1016/j.jim.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarnjak-Jankovic S., Hammerstad H., Saebøe-Larssen S., Kvalheim G., Gaudernack G. A full scale comparative study of methods for generation of functional dendritic cells for use as cancer vaccines. BMC Cancer. 2007;7:119. doi: 10.1186/1471-2407-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koski G.K., Koldovsky U., Xu S., Mick R., Sharma A., Fitzpatrick E., Weinstein S., Nisenbaum H., Levine B.L., Fox K. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J. Immunother. 2012;35:54–65. doi: 10.1097/CJI.0b013e318235f512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasir B., Borges V., Wu Z., Grosman D., Rosenblatt J., Irie M., Anderson K., Kufe D., Avigan D. Fusion of dendritic cells with multiple myeloma cells results in maturation and enhanced antigen presentation. Br. J. Haematol. 2005;129:687–700. doi: 10.1111/j.1365-2141.2005.05507.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennett S., Breit S.N. Variables in the isolation and culture of human monocytes that are of particular relevance to studies of HIV. J. Leukoc. Biol. 1994;56:236–240. doi: 10.1002/jlb.56.3.236. [DOI] [PubMed] [Google Scholar]
- 26.GE Healthcare . 2019. Isolation of mononuclear cells. Methodology and applications.https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/General_Information/1/ge-isolation-of-mononuclear-cells.pdf [Google Scholar]
- 27.Janssen W.E., Ribickas A., Meyer L.V., Smilee R.C. Large-scale Ficoll gradient separations using a commercially available, effectively closed, system. Cytotherapy. 2010;12:418–424. doi: 10.3109/14653240903479663. [DOI] [PubMed] [Google Scholar]
- 28.Law P., Dooley D.C., Alsop P., Smith D.M., Landmark J.D., Meryman H.T. Density gradient isolation of peripheral blood mononuclear cells using a blood cell processor. Transfusion. 1988;28:145–150. doi: 10.1046/j.1537-2995.1988.28288179019.x. [DOI] [PubMed] [Google Scholar]
- 29.Delirezh N., Shojaeefar E., Parvin P., Asadi B. Comparison the effects of two monocyte isolation methods, plastic adherence and magnetic activated cell sorting methods, on phagocytic activity of generated dendritic cells. Cell J. 2013;15:218–223. [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett S., Por S.B., Stanley E.R., Breit S.N. Monocyte proliferation in a cytokine-free, serum-free system. J. Immunol. Methods. 1992;153:201–212. doi: 10.1016/0022-1759(92)90323-l. [DOI] [PubMed] [Google Scholar]
- 31.Wahl L.M., Wahl S.M., Smythies L.E., Smith P.D. Isolation of human monocyte populations. Curr. Protoc. Immunol. 2006;Chapter 7 doi: 10.1002/0471142735.im0706as70. [DOI] [PubMed] [Google Scholar]
- 32.Haskill S., Johnson C., Eierman D., Becker S., Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J. Immunol. 1988;140:1690–1694. [PubMed] [Google Scholar]
- 33.Schwartz C. Beckman Coulter; 2014. Optimizing Cell Separation with Beckman Coulter’s Centrifugal Elutriation System. [Google Scholar]
- 34.Faradji A., Bohbot A., Schmitt-Goguel M., Siffert J.C., Dumont S., Wiesel M.L., Piemont Y., Eischen A., Bergerat J.P., Bartholeyns J. Large scale isolation of human blood monocytes by continuous flow centrifugation leukapheresis and counterflow centrifugation elutriation for adoptive cellular immunotherapy in cancer patients. J. Immunol. Methods. 1994;174:297–309. doi: 10.1016/0022-1759(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 35.Bauer J. Advances in cell separation: recent developments in counterflow centrifugal elutriation and continuous flow cell separation. J. Chromatogr. B Biomed. Sci. Appl. 1999;722:55–69. doi: 10.1016/s0378-4347(98)00308-9. [DOI] [PubMed] [Google Scholar]
- 36.Faradji A., Bohbot A., Schmitt-Goguel M., Dumont S., Eischen A., Wiesel M.L., Stierle A., Follea G., Eber M., Bergerat J.P. Apheresis-elutriation program for adoptive immunotherapy with autologous activated monocytes in cancer patients. Int. J. Artif. Organs. 1991;14:304–312. [PubMed] [Google Scholar]
- 37.Meital L.T., Coward A.S., Windsor M.T., Bailey T.G., Kuballa A., Russell F.D. A simple and effective method for the isolation and culture of human monocytes from small volumes of peripheral blood. J. Immunol. Methods. 2019;472:75–78. doi: 10.1016/j.jim.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Repnik U., Knezevic M., Jeras M. Simple and cost-effective isolation of monocytes from buffy coats. J. Immunol. Methods. 2003;278:283–292. doi: 10.1016/s0022-1759(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 39.Seager Danciger J., Lutz M., Hama S., Cruz D., Castrillo A., Lazaro J., Phillips R., Premack B., Berliner J. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J. Immunol. Methods. 2004;288:123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Miltenyi Biotec . 2019. Installation instructions: CliniMACS tubing sets for research use.https://www.miltenyibiotec.com/_Resources/Persistent/d3101721e7e0599b5debf8add42757e0b57be447/Installation-instruction-tubing-sets.pdf [Google Scholar]
- 41.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 42.Miltenyi Biotec . 2019. CliniMACS CD14 product line.https://www.miltenyibiotec.com/US-en/products/cell-manufacturing-platform/clinimacs-reagents/clinimacs-r-cd14-product-line.html#200-070-121 [Google Scholar]
- 43.Wu Z., Zhang Z., Lei Z., Lei P. CD14: biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019;48:24–31. doi: 10.1016/j.cytogfr.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Miltenyi Biotec . 2019. CD14 reagent (human) package insert. [Google Scholar]
- 45.Felzmann T., Witt V., Wimmer D., Ressmann G., Wagner D., Paul P., Hüttner K., Fritsch G. Monocyte enrichment from leukapharesis products for the generation of DCs by plastic adherence, or by positive or negative selection. Cytotherapy. 2003;5:391–398. doi: 10.1080/14653240310003053. [DOI] [PubMed] [Google Scholar]
- 46.Eyrich M., Schreiber S.C., Rachor J., Krauss J., Pauwels F., Hain J., Wölfl M., Lutz M.B., de Vleeschouwer S., Schlegel P.G., Van Gool S.W. Development and validation of a fully GMP-compliant production process of autologous, tumor-lysate-pulsed dendritic cells. Cytotherapy. 2014;16:946–964. doi: 10.1016/j.jcyt.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Pullarkat V., Lau R., Lee S.M., Bender J.G., Weber J.S. Large-scale monocyte enrichment coupled with a closed culture system for the generation of human dendritic cells. J. Immunol. Methods. 2002;267:173–183. doi: 10.1016/s0022-1759(02)00181-3. [DOI] [PubMed] [Google Scholar]
- 48.Cai S., Hou J., Fujino M., Zhang Q., Ichimaru N., Takahara S., Araki R., Lu L., Chen J.M., Zhuang J. iPSC-derived regulatory dendritic cells inhibit allograft rejection by generating alloantigen-specific regulatory T cells. Stem Cell Reports. 2017;8:1174–1189. doi: 10.1016/j.stemcr.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]