Abstract
Background and Purpose
Although onset-to-treatment time is associated with early clinical recovery in acute ischemic stroke (AIS) patients treated with intravenous tissue plasminogen activator (tPA), the effect of the timing of tPA-induced recanalization on functional outcomes remains debatable.
Methods
We conducted a multicenter, prospective observational cohort study to determine whether early (within 1-hour from tPA-bolus) complete or partial recanalization assessed during 2-hour real-time transcranial Doppler monitoring is associated with improved outcomes in patients with proximal occlusions. Outcome events included dramatic clinical recovery (DCR) within 2 and 24-hours from tPA-bolus, 3-month mortality, favorable functional outcome (FFO) and functional independence (FI) defined as modified Rankin Scale (mRS) scores of 0–1 and 0–2 respectively.
Results
We enrolled 480 AIS patients (mean age 66±15 years, 60% men, baseline National Institutes of Health Stroke Scale score 15). Patients with early recanalization (53%) had significantly (jos-2019-01648P<0.001) higher rates of DCR at 2-hour (54% vs. 10%) and 24-hour (63% vs. 22%), 3-month FFO (67% vs. 28%) and FI (81% vs. 39%). Three-month mortality rates (6% vs. 17%) and distribution of 3-month mRS scores were significantly lower in the early recanalization group. After adjusting for potential confounders, early recanalization was independently associated with higher odds of 3-month FFO (odds ratio [OR], 6.19; 95% confidence interval [CI], 3.88 to 9.88) and lower likelihood of 3-month mortality (OR, 0.34; 95% CI, 0.17 to 0.67). Onset to treatment time correlated to the elapsed time between tPA-bolus and recanalization (unstandardized linear regression coefficient, 0.13; 95% CI, 0.06 to 0.19).
Conclusions
Earlier tPA treatment after stroke onset is associated with faster tPA-induced recanalization. Earlier onset-to-recanalization time results in improved functional recovery and survival in AIS patients with proximal intracranial occlusions.
Keywords: Thrombolysis, Stroke, Reperfusion, Outcomes
Introduction
Intravenous thrombolysis (IVT) with tissue plasminogen activator (tPA) remains the only approved systemic therapy for acute ischemic stroke (AIS), with onset-to-treatment time being directly associated with improved short-term and long-term clinical outcomes [1-3]. Since recanalization following IVT is directly associated with early clinical recovery [4], it is logical to assume that faster recanalization is the mechanism why earlier treatment with tPA can lead to improved 3-month functional outcomes [5-8]. However, the potential correlation between onset-to-tPA treatment time and elapsed time between tPA-bolus and beginning of recanalization has not been established yet. Furthermore, the influence of timing of tPA-induced recanalization on functional outcomes and risk of symptomatic intracerebral hemorrhage (sICH) in AIS remains debatable.
In view of these considerations, we designed the ‘PROspective multi-national Combined Lysis of Thrombus in Brain Ischemia Using Transcranial Ultrasound and Systemic tPA (CLOTBUST) collaboration on reperfusion therapies for stroke’ (CLOTBUST-PRO) [9] that sought to determine whether early recanalization (within 1-hour from tPA-bolus), assessed with real-time transcranial Doppler (TCD) monitoring, is independently associated with improved 3-month outcomes in AIS patients with proximal intracranial arterial occlusions. We also investigated the association between the elapsed time between symptom onset and tPA-bolus with the elapsed time between tPA-bolus and beginning of recanalization. We hypothesized the early tPA delivery may result to swift recanalization which in turn may translate into better clinical outcomes.
Methods
Study design
We conducted this prospective, open-label, observational cohort study in 12 participating tertiary care stroke centers in North America, South America, Europe, and Asia. The rationale for the study, design details, and eligibility criteria have been previously published [9]. Exclusion criteria together with other detail on the methodology of our trial are available in the Supplementary Material.
Study population
We included patients with AIS attributable to a proximal intracranial occlusion eligible for IVT, who presented within 4.5 hours to the participating sites [9]. The study was approved by the Ethics Committees and the Institutional Review Boards of participating Institutions as previously described. All patients or their substitute decision-makers provided written consent prior to participation [9].
Insonation protocol
All eligible patients received continuous 2-MHz TCD or transcranial color-coded duplex (TCCD) assessment of recanalization up to 2 hours following tPA-bolus by experienced sonographers. The duration of continuous TCD monitoring varied between 60 and 120 minutes, because if there was no ultrasonographic evidence of recanalization within 60 minutes from tPA-bolus and the patient was considered by the treating physician as a candidate for additional revascularization therapies (intra-arterial thrombolysis or mechanical thrombectomy), TCD monitoring was discontinued and an additional TCD evaluation was performed at the end of endovascular reperfusion therapies. This therapeutic approach towards endovascular reperfusion therapies was based on the lack of available randomizedcontrolled clinical trial data supporting the clinical efficacy of mechanical thrombectomy at the time of study design and patient enrollment (2008 to 2014).
In patients with clinical deterioration following initial improvement within the first 24 hours from symptom onset, an additional TCD recording was performed to assess the presence of delayed re-occlusion.
TCD findings were interpreted using previously validated criteria, including the thrombolysis in brain ischemia (TIBI) flow grade definitions [10,11].
Outcomes
The main hypothesis and primary efficacy outcome of the study was to determine whether early tPA-induced partial or complete recanalization, achieved during the first hour of TCD monitoring following tPA-bolus, was independently associated with more favorable early and long term clinical outcomes. The primary outcome of interest was the difference in the favorable functional outcome (FFO; defined modified Rankin Scale [mRS] score of 0–1 at 3-month) rates in patients with early complete or partial recanalization compared to those with persisting occlusion at 1-hour TCD monitoring. The exact time of the beginning, duration, timing and amount of recanalization and re-occlusion were recorded for all patients. Beginning of recanalization was marked as the time point in which at least 1 point improvement in TIBI score was noticed during the TCD monitoring [12]. A table summarizing the definitions of the aforementioned outcomes is provided in the Supplementary Table 1.
Early clinical outcome was assessed with the rates of dramatic clinical recovery (DCR), defined by a change of equal or more than 10 points in the National Institutes of Health Stroke Scale (NIHSS) or total NIHSS score of less or equal to 3 within 2 and 24 hours after tPA-bolus [11]. Finally, we prospectively followed all patients up to 3 months to record also functional independence (FI; defined as 3-month mRS of 0–2) and deaths from any cause during the 3-month follow-up period.
sICH with clinical worsening (≥4 NIHSS points) that in the opinion of the treating physician was causatively and temporally related to neurological deterioration during the first 24 hours following tPA infusion [9], was considered as the primary safety endpoint.
For all patients, serial assessments on NIHSS scores were obtained at baseline (pre-tPA-bolus), within 10 minutes of the beginning of recanalization or re-occlusion, at the end of TCD monitoring and at 24-hour after symptom onset by vascular neurologists certified in the NIHSS scoring [9]. Functional outcome was evaluated at 3-month by the on-site investigators (certified in the assessment of mRS) who were unaware of TCD monitoring findings. All radiological examinations were independently evaluated by institutional radiologists/neuroradiologists who were blinded to both TCD findings and patient clinical data.
Statistical analyses
We enrolled 480 patients to identify at least 120 cases of early complete recanalization and have power of 82.4% to detect a statistically significant difference of at least 15% in FI rates at 3-month between patients with early recanalization and persisting occlusion/delayed recanalization [5]. Dichotomous variables were presented with their absolute values and corresponding percentages, while continuous variables were presented as mean values with their corresponding standard deviations for normally distributed data, or median values with their corresponding interquartile ranges (IQRs) when distributions were skewed. We compared baseline characteristics and outcomes between patients with complete or partial recanalization and those with persisting occlusion with the Pearson’s chi-square or Mann-Whitney-Wilcoxon test where appropriate. The distributions on the mRS-score between groups at 3-month follow-up were compared with the Cochran-Mantel-Haenszel test, as previously described [13].
Univariable and multivariable logistic regression analyses were performed to evaluate the associations between baseline characteristics and outcomes (sICH, 3-month FFO and mortality). We also performed additional sensitivity analyses after dichotomizing time from symptom onset to beginning of recanalization using the variable’s observed median value as threshold (>178 and ≤178 minutes) and persisting occlusion set as reference category. Only the factors proven significant (P<0.05) at the univariable analyses were entered into the multivariable models. We also performed a post hoc subgroup analysis by including only patients with middle cerebral artery (MCA) occlusions.
Apart from the analysis in the total sample, a nested analysis (univariable and multivariable logistic regression analysis) was performed in the subgroup of patients with sustained complete or partial early recanalization at the end of 2-hour TCD-monitoring. The same approach was followed as in the total sample, with the exception of time from symptom onset to the beginning of recanalization, which was treated in increments of 30 minutes. In this subgroup of patients, the potential correlations between onset-to-treatment time (elapsed time between symptom onset and tPA-bolus) and baseline characteristics, including elapsed time between tPA-bolus and beginning of recanalization, were assessed with simple and multiple regression analyses, adjusting for potential confounders. Statistical analyses were performed with STATA/SE version 13 (Stata Corp, College Station, TX, USA).
Data availability statement
De-identified patient dataset will be made available upon reasonable request from the corresponding author.
Results
The predetermined sample size of 480 AIS patients (mean age 66±15 years, 60% men) with proximal intracranial occlusions, who underwent continuous 2-hour TCD-monitoring following tPA-bolus, was enrolled during a 7-year period (January 2008 to December 2014) from 12 participating tertiary care stroke centers from North America (n=2), South America (n=1), Europe (n=7), and Asia (n=2). The complete list of participating centers is available in the Supplementary Material. The median admission NIHSS-score was 15 points (IQR, 10 to 19) and the median onset to treatment time was 140-minute (IQR, 116 to 170). Baseline characteristics of the study population are presented in Supplementary Table 2. The distribution of proximal intracranial occlusions was as follows: M1-MCA 48% (n=231), M2-MCA 29% (n=141), terminal internal carotid artery (ICA) 9% (n=44), tandem ICA/MCA 5% (n=24), basilar artery 7% (n=61), intracranial vertebral artery 0.2% (n=1), and posterior cerebral artery 1% (n=6). No patient was lost during the 3-month follow-up.
Complete or partial recanalization was detected in 180 (38%) and 71 (15%) patients at 1-hour TCD monitoring, respectively, and in 211 (44%) and 90 (19%) patients at the 2-hour TCD-monitoring point, respectively. Sustained complete or partial recanalization was achieved in 201 (42%) and 85 (18%) patients, respectively at the end of 2-hour TCD-monitoring, while re-occlusion was documented in 18 cases (4%). The median onset to recanalization and the median bolus to recanalization times were 178-minute (IQR, 145 to 214) and 24-minute (IQR, 22 to 53), respectively. Comparisons of the baseline characteristics between patients with persisting occlusion or any (partial or complete) recanalization within the first hour of TCD monitoring are presented in Table 1. Patients with complete or partial early recanalization during the first hour of TCD-monitoring as compared to patients with persisting occlusion/delayed recanalization were younger (63±14 years vs. 69±15 years, P<0.001), more likely to be men (65% vs. 55%, P=0.048) and had lower prevalence of isolated ICA or tandem ICA/MCA occlusion (10% vs. 19%, P=0.006). Patients with early recanalization had shorter median onset-to-treatment times (135-minute vs. 145-minute, P=0.002), higher pre-treatment systolic (160±26 mm Hg vs. 154±26 mm Hg, P=0.022), and diastolic blood pressure levels (90±15 mm Hg vs. 85±16 mm Hg, P<0.001). sICH rates were not different between patients with early complete or partial recanalization and patients with persisting occlusion (2% vs. 6%, P=0.065). Patients with early recanalization had significantly (P<0.001) higher rates of DCR at 2-hour (54% vs. 10%) and 24-hour (63% vs. 22%), 3-month FFO (67% vs. 28%) and 3-month FI (81% vs. 39%). Three-month mortality rates (6% vs. 17%) and distribution of 3-month mRS scores (1 [0 to 2] vs. 3 [1 to 5]) were significantly lower in the early recanalization subgroup. The respective comparisons of the baseline characteristics and outcomes between patients with persisting occlusion and those with any (partial or complete) recanalization within 2 hours of TCD monitoring are available in Supplementary Table 3.
Table 1.
Baseline characteristics and outcomes of study population (n=480) according to early recanalization status achieved during the first hour of TCD-monitoring
| Variable | Early complete or partial recanalization (n=251) | Delayed recanalization or Persisting occlusion (n=229) | P | |
|---|---|---|---|---|
| Baseline characteristic | ||||
| Age (yr) | 63±14 | 69±15 | <0.001* | |
| Male sex | 162 (65) | 127 (55) | 0.048† | |
| NIHSS-score (point) | 14 (9–19) | 15 (10–20) | 0.112* | |
| Hypertension | 164 (65) | 161 (71) | 0.217† | |
| Diabetes mellitus | 60 (24) | 56 (25) | 0.867† | |
| Atrial fibrillation | 69 (27) | 76 (33) | 0.174† | |
| Hypercholesterolemia | 108 (43) | 78 (35) | 0.073† | |
| Coronary artery disease | 64 (25) | 55 (24) | 0.707† | |
| Baseline TIBI score 0–1 | 138 (55) | 113 (49) | 0.217† | |
| Isolated ICA or tandem ICA/MCA occlusion | 25 (10) | 43 (19) | 0.006† | |
| Systolic blood pressure before tPA-bolus (mm Hg) | 160±26 | 154±26 | 0.022* | |
| Diastolic blood pressure before tPA-bolus (mm Hg) | 90±15 | 85±16 | <0.001* | |
| Serum glucose before tPA-bolus (mg/dL) | 130±53 | 135±47 | 0.140* | |
| Time from symptom onset to tPA-bolus (min) | 135 (110–165) | 145 (120–180) | 0.002* | |
| Endovascular reperfusion therapies | 4 (2) | 8 (3) | 0.183‡ | |
| Outcome | ||||
| Symptomatic intracerebral hemorrhage | 6 (2) | 13 (6) | 0.065 † | |
| Dramatic clinical recovery at 2-hr§ | 129 (54) | 21 (10) | <0.001† | |
| Dramatic clinical recovery at 24-hr∥ | 155 (63) | 46 (22) | <0.001† | |
| 3-mo favorable functional outcome mRS 0–1 | 169 (67) | 63 (28) | <0.001† | |
| 3-mo functional independence mRS 0–2 | 203 (81) | 89 (39) | <0.001† | |
| 3-mo mortality | 16 (6) | 39 (17) | <0.001† | |
| 3-mo mRS score | 1 (0–2) | 3 (1–5) | <0.001¶ | |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
TCD, transcranial Doppler; NIHSS, National Institutes of Health Stroke Scale; TIBI, thrombolysis in brain ischemia; ICA, internal carotid artery; MCA, middle cerebral artery; tPA, tissue plasminogen activator; mRS, modified Rankin Scale.
P-value derived from Mann-Whitney-Wilcoxon test for independent samples;
P-value derived from Pearson’s chi-square;
P-value derived from Fisher’s exact test;
Missing data on 2-hour NIHSS for 27 patients;
Missing data on 24-hour NIHSS for 24 patients;
P-value derived from Cochran–Mantel–Haenszel test.
In multivariable analyses adjusting for potential confounders, early complete or partial recanalization within the first hour after tPA-bolus was independently associated with higher odds of 3-month FFO (odds ratio [OR], 6.19; 95% confidence interval [CI], 3.88 to 9.88; P<0.001) (Table 2) and lower likelihood of 3-month mortality (OR, 0.34; 95% CI, 0.17 to 0.67; P=0.002) (Table 3) compared to delayed recanalization or persisting occlusion.
Table 2.
Association of early (within 1-hour from tPA-bolus) recanalization with 3-month favorable functional outcome in the study population (n=480)
| Variable | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age (/1 yr increase) | 0.96 (0.95–0.97) | <0.001 | 0.97 (0.95–0.99) | 0.001 |
| Male sex | 1.64 (1.13–2.37) | 0.009 | 1.70 (1.06–2.71) | 0.027 |
| NIHSS-score (/1 point increase) | 0.86 (0.83–0.89) | <0.001 | 0.85 (0.82–0.89) | <0.001 |
| Hypertension | 0.64 (0.44–0.95) | 0.026 | 1.18 (0.71–1.99) | 0.518 |
| Diabetes mellitus | 0.83 (0.54–1.26) | 0.372 | - | |
| Atrial fibrillation | 0.73 (0.49–1.07) | 0.108 | - | |
| Hypercholesterolemia | 1.08 (0.75–1.56) | 0.687 | - | |
| Coronary artery disease | 0.98 (0.65–1.48) | 0.913 | - | |
| Baseline TIBI score 0–1 | 0.64 (0.45–0.92) | 0.015 | 1.05 (0.64–1.72) | 0.842 |
| Isolate ICA or tandem ICA/MCA occlusion | 0.30 (0.17–0.55) | <0.001 | 0.53 (0.26–1.09) | 0.083 |
| Systolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.96 (0.90–1.03) | 0.275 | - | |
| Diastolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.98 (0.87–1.11) | 0.749 | - | |
| Mean serum glucose before tPA-bolus (/10 mg/dL increase) | 0.96 (0.93–1.00) | 0.050 | - | |
| Time from symptom onset to tPA-bolus (/10 min increase) | 0.97 (0.93–1.01) | 0.128 | - | |
| Early recanalization | 5.43 (3.67–8.04) | <0.001 | 6.19 (3.88–9.88) | <0.001 |
| Endovascular reperfusion therapies | 2.18 (0.65–7.33) | 0.209 | - | - |
tPA, tissue plasminogen activator; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; TIBI, thrombolysis in brain ischemia; ICA, internal carotid artery; MCA, middle cerebral artery.
Table 3.
Association of early (within 1-hour from tPA-bolus) recanalization with 3-month mortality in the study population (n=480)
| Variable | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age (/1 yr increase) | 1.05 (1.03–1.08) | <0.001 | 1.04 (1.01–1.06) | 0.003 |
| Male sex | 0.65 (0.37–1.14) | 0.131 | - | |
| NIHSS-score (/1 point increase) | 1.16 (1.11–1.22) | <0.001 | 1.16 (1.10–1.22) | <0.001 |
| Hypertension | 1.07 (0.58–1.96) | 0.834 | - | |
| Diabetes mellitus | 1.47 (0.79–2.72) | 0.220 | - | |
| Atrial fibrillation | 1.79 (1.00–3.17) | 0.048 | 1.16 (0.60–2.24) | 0.649 |
| Hypercholesterolemia | 0.85 (0.47–1.54) | 0.592 | - | |
| Coronary artery disease | 1.72 (0.94–3.12) | 0.078 | - | |
| Baseline TIBI score 0–1 | 1.20 (0.68–2.12) | 0.521 | - | |
| Isolated ICA or tandem ICA/MCA occlusion | 2.35 (1.20–4.59) | 0.013 | 1.32 (0.63–2.77) | 0.462 |
| Systolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.93 (0.84–1.04) | 0.195 | - | |
| Diastolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.89 (0.74–1.07) | 0.221 | - | |
| Mean serum glucose before tPA-bolus (/10 mg/dL increase) | 1.02 (0.96–1.07) | 0.536 | - | |
| Time from symptom onset to tPA-bolus (/10 min increase) | 1.00 (0.94–1.06) | 0.923 | - | |
| Early recanalization | 0.33 (0.18–0.61) | <0.001 | 0.34 (0.17–0.67) | 0.002 |
| Endovascular reperfusion therapies | Not estimable* | >0.999† | - | |
tPA, tissue plasminogen activator; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; TIBI, thrombolysis in brain ischemia; ICA, internal carotid artery; MCA, middle cerebral artery.
Not estimable due to lack of events (deaths) in the subgroup of patients receiving endovascular treatment;
P-value derived from Fisher’s exact test.
These associations were reproduced in our sensitivity analyses stratifying patients with complete or partial recanalization during the 2-hour TCD monitoring by the median of symptom onset to recanalization time (178 minutes). More specifically, a more pronounced beneficial effect on 3-month FFO was documented in patients with initiation of recanalization within the first 178 minutes after symptom onset (OR [vs. the reference group of persisting occlusion], 14.70; 95% CI, 7.90 to 27.33; P<0.001) (Table 4) compared to patients with a more delayed recanalization after 178 minutes from symptom onset (OR [vs. the reference group of persisting occlusion], 6.72; 95% CI, 3.78 to 11.92; P<0.001) (Table 4). An independent association with lower likelihood of 3-month mortality was detected for both groups of early (≤178-minute from symptom onset; OR, 0.37; 95% CI, 0.16 to 0.85; P=0.018) or delayed (>178-minute from symptom onset; OR, 0.22; 95% CI, 0.10 to 0.51; P<0.001) recanalization versus persisting occlusions (Table 5). In the subgroup analysis of patients with MCA occlusions (n=374) similar differences in the outcomes of interest were documented between patients with early complete or partial recanalization compared to patients with delayed recanalization or persisting occlusion (Supplementary Table 4). This subgroup analysis confirmed the associations documented in our primary analysis including all patients.
Table 4.
Association of elapsed time between symptom onset and beginning of recanalization with 3-month favorable functional outcome in the study population (n=480)
| Variable | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age (/1 yr increase) | 0.96 (0.95–0.97) | <0.001 | 0.97 (0.95–0.99) | 0.002 |
| Male sex | 1.64 (1.13–2.37) | 0.009 | 1.55 (0.96–2.52) | 0.076 |
| NIHSS-score (/1 point increase) | 0.86 (0.83–0.89) | <0.001 | 0.85 (0.81–0.89) | <0.001 |
| Hypertension | 0.64 (0.44–0.95) | 0.026 | 1.33 (0.77–2.31) | 0.303 |
| Diabetes mellitus | 0.83 (0.54–1.26) | 0.372 | - | |
| Atrial fibrillation | 0.73 (0.49–1.07) | 0.108 | - | |
| Hypercholesterolemia | 1.08 (0.75–1.56) | 0.687 | - | |
| Coronary artery disease | 0.98 (0.65–1.48) | 0.913 | - | |
| Baseline TIBI score 0–1 | 0.64 (0.45–0.92) | 0.015 | 1.21 (0.73–2.01) | 0.460 |
| Isolate ICA or tandem ICA/MCA occlusion | 0.30 (0.17–0.55) | <0.001 | 0.57 (0.27–1.21) | 0.143 |
| Systolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.96 (0.90–1.03) | 0.275 | - | |
| Diastolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.98 (0.87–1.11) | 0.749 | - | |
| Mean serum glucose before tPA-bolus (/10 mg/dL increase) | 0.96 (0.93–1.00) | 0.050 | - | |
| Time from symptom onset to tPA-bolus (/10 min increase) | 0.97 (0.93–1.01) | 0.128 | - | |
| Endovascular reperfusion therapy | 2.18 (0.65, 7.33) | 0.209 | - | - |
| Time from symptom onset to beginning of recanalization | ||||
| Persisting occlusion (reference category) ≤178 min | 13.23 (7.82–22.39) | <0.001 | 14.70 (7.90–27.33) | <0.001 |
| Persisting occlusion (reference category) >178 min | 5.55 (3.39–9.10) | <0.001 | 6.72 (3.78–11.92) | <0.001 |
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; TIBI, thrombolysis in brain ischemia; ICA, internal carotid artery; MCA, middle cerebral artery; tPA, tissue plasminogen activator.
Table 5.
Association of elapsed time between symptom onset and beginning of recanalization with 3-month mortality in the study population (n=480)
| Variable | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age (/1 yr increase) | 1.05 (1.03–1.08) | <0.001 | 1.04 (1.01–1.06) | 0.002 |
| Male sex | 0.65 (0.37–1.14) | 0.131 | - | |
| NIHSS-score (/1 point increase) | 1.16 (1.11–1.22) | <0.001 | 1.16 (1.10–1.23) | <0.001 |
| Hypertension | 1.07 (0.58–1.96) | 0.834 | - | |
| Diabetes mellitus | 1.47 (0.79–2.72) | 0.220 | - | |
| Atrial fibrillation | 1.79 (1.00–3.17) | 0.048 | 1.21 (0.62–2.34) | 0.574 |
| Hypercholesterolemia | 0.85 (0.47–1.54) | 0.592 | - | |
| Coronary artery disease | 1.72 (0.94–3.12) | 0.078 | - | |
| Baseline TIBI score 0–1 | 1.20 (0.68–2.12) | 0.521 | - | |
| Isolate ICA or tandem ICA/MCA occlusion | 2.35 (1.20–4.59) | 0.013 | 1.26 (0.59–2.70) | 0.546 |
| Systolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.93 (0.84–1.04) | 0.195 | - | |
| Diastolic blood pressure before tPA-bolus (/10 mm Hg increase) | 0.89 (0.74–1.07) | 0.221 | - | |
| Mean serum glucose before tPA-bolus (/10 mg/dL increase) | 1.02 (0.96–1.07) | 0.536 | - | |
| Time from symptom onset to tPA-bolus (/10 min increase) | 1.00 (0.94–1.06) | 0.923 | - | |
| Endovascular reperfusion therapy | Not estimable* | >0.999† | - | - |
| Time from symptom onset to beginning of recanalization | ||||
| Persisting occlusion (reference category) ≤178 min | 0.28 (0.14–0.59) | 0.001 | 0.37 (0.16–0.85) | 0.018 |
| Persisting occlusion (reference category) >178 min | 0.26 (0.12–0.55) | 0.001 | 0.22 (0.10–0.51) | <0.001 |
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; TIBI, thrombolysis in brain ischemia; ICA, internal carotid artery; MCA, middle cerebral artery; tPA, tissue plasminogen activator.
Not estimable due to lack of events (deaths) in the subgroup of patients receiving endovascular treatment;
P-value derived from Fisher’s exact test.
In the nested subgroup analysis (Supplementary Table 5) of patients with sustained complete or partial recanalization at the end of 2-hour TCD-monitoring (n=301), an independent association was detected between increasing elapsed time from symptom onset to the beginning of recanalization and reduced odds of 3-month FFO (OR per 30-minute increase, 0.84; 95% CI, 0.73 to 0.97; P=0.015) (Figure 1). Moreover, among all baseline characteristics only onset-to-treatment time correlated positively to the elapsed time between tPA-bolus and beginning of recanalization (unstandardized linear regression coefficient, 0.13; 95% CI, 0.06 to 0.19; P<0.001) (Figure 2) in the subgroup of patients with complete or partial recanalization at the end of TCD-monitoring (Supplementary Table 6). In a post hoc sensitivity analysis after adjusting for predefined potential confounders (baseline age, NIHSS score, presence of tandem occlusion, and endovascular treatment), a similar correlation of elapsed time from symptom onset to tPA-bolus with elapsed time from tPA-bolus to the beginning of recanalization documented (unstandardized linear regression coefficient, 0.13; 95% CI, 0.06 to 0.20; P<0.001).
Figure 1.
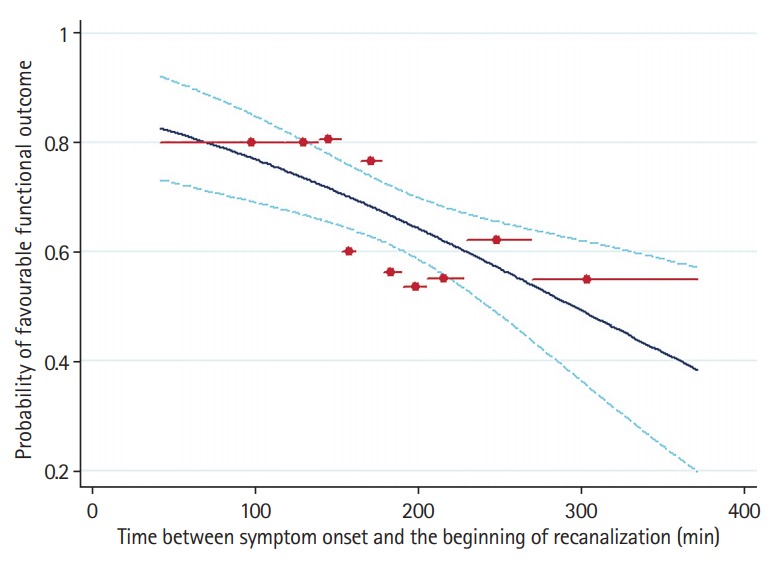
Frequency of favorable functional outcome by elapsed time between symptom onset and the beginning of recanalization, as predicted by unadjusted analysis in patients with sustained complete or partial recanalization at the end of transcranial Doppler-monitoring (n=301). The recanalization cohort (including patients with complete or partial sustained recanalization) was divided into groups of about 30 on the basis of deciles in the time to recanalization. The red circles show the proportion with favorable functional outcome and mean time to recanalization in each group, and the red horizontal lines depict the range of time included in that group. The solid curve shows the model results from the logistic regression analysis, with 95% confidence interval prediction bands shown in dashed curves. Favorable functional outcome was defined as a modified Rankin Scale score of ≤1.
Figure 2.

Correlation of elapsed time between symptom onset and tissue plasminogen activator (tPA)-bolus (onset to treatment time) with elapsed time between tPA-bolus to the beginning of recanalization in patients with sustained complete or partial recanalization at the end of transcranial Doppler-monitoring (n=301). The 95% confidence interval (CI) forecast bands are also presented. The correlation between the two time intervals was statistically significant (unstandardized linear regression coefficient, 0.13; 95% CI, 0.06 to 0.19; P<0.001).
We detected no association between elapsed time from symptom onset and beginning of recanalization with sICH in multivariable analyses of the study population (Supplementary Table 7) and in the subgroup of patients with sustained complete or partial recanalization (n=301) at the end of TCD-monitoring (Supplementary Table 8). Three-month mortality was not associated with the timing of tPA-induced recanalization in the group of patients with sustained complete or partial recanalization at the end of 2-hour TCD-monitoring (Supplementary Table 9).
Discussion
Our prospective observational study showed that faster tPA-induced recanalization represents the link between shorter onset-to-treatment time and improved 3-month functional outcomes in AIS patients with proximal intracranial occlusions. Shorter onset-to-tPA-bolus time was associated with more prompt recanalization because of shorter tPA-bolus-to-recanalization time. Elapsed time between symptom onset and beginning of recanalization was an independent predictor of 3-month functional outcome among patients who achieved sustained early complete or partial recanalization. Finally, delayed recanalization was not related to the risk of sICH in the whole study population. Our study supports ultra-fast delivery of tPA after patient arrival to hospital. Taking also into account that the vast majority of complete or partial vessel recanalizations were found to occur within 1-hour from tPA-bolus and that very few patients recanalized after 1-hour from tPA-bolus, our results also provide unflinching support the strategy of not delaying endovascular procedures in cases of persisting occlusion after tPA administration. Therefore, our results on the association of shorter onset-to-treatment time with faster induced recanalization and improved 3-month functional outcomes, should not only apply for systemic tPA treatment, but also for endovascular therapies and align with the continuous efforts for reduction of onset-to-groin puncture and groin puncture-to recanalization times. Although TCD can be used as a very reliable tool for continuous, real-time assessment of vessel recanalization, in the era of mechanical thrombectomy it could only be useful in case of other imaging unavailability, and should not delay any systemic or endovascular therapies when employed for clinical or research purposes.
We detected sustained complete early recanalization in 42% of patients within 2 hours after tPA-bolus and DCR in 50% at the end of TCD-monitoring. Although the rates of tPA-induced recanalization are higher in our cohort than those reported for patients with large vessel occlusions (LVOs) following treatment with systemic thrombolysis [14,15], it should be highlighted that they are in accordance with the results from the CLOTBUST trial [11], reporting sustained complete recanalization in 46% and DCR in 44% of the patients receiving tPA administration coupled with continuous TCD-monitoring [11]. Our findings are also in line with a longitudinal cohort of consecutive AIS patients treated with IVT that reported sustained recanalization in 51% of the study population at the end of the 2-hour TCD-monitoring [16]. Finally, the sICH rate in our population (4%) was comparable to the treatment arm of the recently published multicenter, double-blind, phase 3, randomized controlled trial on the safety and efficacy of 2 hours continuous transcranial ultrasound insonation coupled with tPA treatment in AIS patients (3%), providing further evidence of no increase in the sICH risk with TCD insonation [17].
The timing of tPA-induced recanalization after stroke onset was a strong predictor of clinical outcomes in the whole study population and among patients with sustained recanalization at the end of TCD-monitoring. This observation is corroborated by the findings of a retrospective, single-center study of 508 AIS patients treated with IVT (54% with proximal intracranial occlusions) [18]. The investigators reported an inverse relationship between the probability of early recanalization, evaluated by TCD at 1-hour following the end of tPA infusion and elapsed time from symptom onset [18]. However, no association between onset-to-treatment and tPA-bolus to initiation of recanalization time was found [18]. The inverse relationship between onset-to-treatment time and the rate of tPA-induced recanalization has also been identified in another study of 102 AIS patients with proximal intracranial occlusions treated with IVT [19]. Early recanalization at the end of alteplase infusion (1-hour after tPA-bolus) was determined using magnetic resonance angiography (MRA). The investigators of this study reported that tPA administration within 130 minutes from symptom onset was independently associated with a 3-fold higher likelihood of complete recanalization at the end of tPA infusion [19]. Finally, our results are also in accordance with an experimental study protocol supporting that early administration of tPA after stroke onset is independently associated with better thrombus resolution [20].
The main study finding, that the beneficial effect of tPA-induced recanalization on clinical outcomes of AIS patients with proximal intracranial occlusions are time-dependent (16% decrease in the likelihood of FFO for every 30-minute delay in the elapsed time between symptom onset and initiation of recanalization) parallel the recent experience with mechanical thrombectomy in AIS patients with LVOs [21-27]. Both randomized-controlled and observational data have highlighted a time dependency of the beneficial effect of endovascular reperfusion therapies in AIS due to LVO [21-27]. More specifically, in a prespecified analysis of the Interventional Management of Stroke III (IMS III) trial, every 30-minute delay in angiographic reperfusion of AIS patients with LVO was associated with a 12% decrease in the likelihood of 3-month FI on multivariable analyses adjusting for potential confounders [26]. Similarly, the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaborators have recently reported that each 1-hour delay to reperfusion was associated with a 19% reduction in the odds of 3-month FI among LVO patients who achieved reperfusion following mechanical thrombectomy and standard therapy [27].
To the best of our knowledge, CLOTBUST-PRO is the first prospective, international multicenter observational study, with adequate statistical power, a predefined protocol and no missing follow-up evaluation at 3 months, which provides realtime information on arterial recanalization dependency with shorter symptom-onset-to-treatment time linking it to 3-month functional outcomes. The correlation between earlier onset to treatment time and shorter elapsed time between tPA-bolus and initiation of recanalization has not been previously reported. Our results provide further support to the association of ultra-early tPA administration with favorable outcomes [3] and highlight the potential role of mobile stroke units in prompt tPA administration in this ultra-early time window [28].
Certain limitations of the present study need to be acknowledged. First, per study protocol, no screening log was provided from the participating centers and thus the percentage of excluded patients, with corresponding reasons for exclusion (e.g., insufficient temporal window or tPA contraindications) is not available. However, it should be highlighted that included patients from all institutions were consecutive and thus we consider the possibility of selection bias negligible. Second, we evaluated arterial recanalization only within the first 2 hours of treatment initiation. However, per our study protocol all patients experiencing clinical deterioration during the first 24 hours from symptom onset received an additional TCD recording. Third, in patients with no ultrasonographic evidence of successful recanalization at 60-minute after tPA initiation, TCD monitoring was discontinued and they were treated with additional revascularization therapies, if available at the participating centers. Although the number of patients receiving additional endovascular reperfusion therapies was limited (2% of the study population), the duration of monitoring was not consistent among the whole study population. Finally, determination of recanalization status using TCD-monitoring is challenging and highly operator-dependent. Although no central adjudication of TCD recording was available, it should be noted that the centers participating in CLOTBUST-PRO recruited skilled and experienced sonographers implementing predefined ultrasound criteria that have been shown to accurately (>80%) predict complete recanalization in real-time compared to digital subtraction angiography [29]. Moreover, we do not have data on the imaging modalities used to screen proximal intracranial occlusions and therefore we were not able to assess potential discrepancies between TCD and computed tomography angiography/MRA findings.
On the other hand, certain strengths of the present study may also be acknowledged. CLOTBUST-PRO represents a prospective, international adequately powered observational study that sought to assess and establish the association of the speed of tPA-induced recanalization with shorter symptom-onset-to-treatment time using a predefined previously published protocol [9]. To the best of our knowledge, we consider that this association represents the causative mechanism that accounts for the improved functional outcomes documented with earlier tPA delivery [1,2]. The former association has previously been established in AIS patients receiving endovascular reperfusion therapies [21-27] but has never been documented in AIS patients receiving systemic thrombolysis, since TCD represents the only imaging modality that can assess recanalization in real-time in patients receiving treatment with IVT [30].
Conclusions
In conclusion, our observational cohort study provides observational evidence that faster tPA delivery is associated with earlier tPA-induced recanalization, which in turn results in improved functional recovery and survival in AIS patients with proximal intracranial occlusions. Our study further underscores the need to arrange acute stroke services in ways that shortest possible onset-to-arrival and arrival-to-tPA-bolus time be achieved.
Acknowledgments
Robert Mikulik has been supported by the project no. LQ1605 from the National Program of Sustainability II (MEYS CR) and by the project FNUSA-ICRC no. CZ.1.05/1.1.00/02.0123 (OP VaVpI).
Footnotes
Disclosure
The authors have no financial conflicts of interest.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2019.01648.
Supplementary Material
Outcome definitions from the transcranial Doppler monitoring
Baseline characteristics of the whole study population (n=480)
Baseline characteristics and outcomes of study population (n=480) according to recanalization status during the 2-hour TCD-monitoring
Baseline characteristics and outcomes of patients with middle cerebral artery occlusion (n=374) according to early recanalization status achieved during the first hour of TCD-monitoring
Association of elapsed time between symptom onset and beginning of recanalization with 3-month favorable functional outcome in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)
Association of baseline characteristics with the elapsed time between tPA-bolus and start of recanalization in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)
Association of elapsed time between symptom onset and beginning of recanalization with symptomatic intracranial hemorrhage in the study population (n=480)
Association of elapsed time between symptom onset and beginning of recanalization with symptomatic intracranial hemorrhage in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)*
Association of elapsed time between symptom onset and beginning of recanalization with 3-month mortality in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)
References
- 1.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsivgoulis G, Katsanos AH, Kadlecová P, Czlonkowska A, Kobayashi A, Brozman M, et al. Intravenous thrombolysis for ischemic stroke in the golden hour: propensity-matched analysis from the SITS-EAST registry. J Neurol. 2017;264:912–920. doi: 10.1007/s00415-017-8461-8. [DOI] [PubMed] [Google Scholar]
- 4.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 5.Tsivgoulis G, Alexandrov AV. Does “time is brain” also mean “time is clot”? Time dependency of tissue-type plasminogen activator-induced recanalization in acute ischemic stroke. Stroke. 2014;45:2555–2556. doi: 10.1161/STROKEAHA.114.006579. [DOI] [PubMed] [Google Scholar]
- 6.Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, et al. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31:1812–1816. doi: 10.1161/01.str.31.8.1812. [DOI] [PubMed] [Google Scholar]
- 7.Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation. 2001;103:2897–2902. doi: 10.1161/01.cir.103.24.2897. [DOI] [PubMed] [Google Scholar]
- 8.Ribo M, Alvarez-Sabín J, Montaner J, Romero F, Delgado P, Rubiera M, et al. Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke. 2006;37:1000–1004. doi: 10.1161/01.STR.0000206443.96112.d9. [DOI] [PubMed] [Google Scholar]
- 9.Saqqur M, Tsivgoulis G, Molina CA, Demchuk AM, Garami Z, Barreto A, et al. Design of a PROspective multi-national CLOTBUST collaboration on reperfusion therapies for stroke (CLOTBUST-PRO) Int J Stroke. 2008;3:66–72. doi: 10.1111/j.1747-4949.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 10.Demchuk AM, Burgin WS, Christou I, Felberg RA, Barber PA, Hill MD, et al. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke. 2001;32:89–93. doi: 10.1161/01.str.32.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 12.Demchuk AM, Christou I, Wein TH, Felberg RA, Malkoff M, Grotta JC, et al. Accuracy and criteria for localizing arterial occlusion with transcranial Doppler. J Neuroimaging. 2000;10:1–12. doi: 10.1111/jon20001011. [DOI] [PubMed] [Google Scholar]
- 13.Saver JL, Gornbein J. Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology. 2009;72:1310–1315. doi: 10.1212/01.wnl.0000341308.73506.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378:1573–1582. doi: 10.1056/NEJMoa1716405. [DOI] [PubMed] [Google Scholar]
- 15.Tsivgoulis G, Katsanos AH, Schellinger PD, Köhrmann M, Varelas P, Magoufis G, et al. Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large-vessel occlusions. Stroke. 2018;49:232–235. doi: 10.1161/STROKEAHA.117.019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Liang S, et al. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70:353–358. doi: 10.1001/2013.jamaneurol.547. [DOI] [PubMed] [Google Scholar]
- 17.Alexandrov AV, Köhrmann M, Soinne L, Tsivgoulis G, Barreto AD, Demchuk AM, et al. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, doubleblind, phase 3, randomised controlled trial. Lancet Neurol. 2019;18:338–347. doi: 10.1016/S1474-4422(19)30026-2. [DOI] [PubMed] [Google Scholar]
- 18.Muchada M, Rodriguez-Luna D, Pagola J, Flores A, Sanjuan E, Meler P, et al. Impact of time to treatment on tissue-type plasminogen activator-induced recanalization in acute ischemic stroke. Stroke. 2014;45:2734–2738. doi: 10.1161/STROKEAHA.114.006222. [DOI] [PubMed] [Google Scholar]
- 19.Kimura K, Iguchi Y, Shibazaki K, Aoki J, Watanabe M, Matsumoto N, et al. Early stroke treatment with IV t-PA associated with early recanalization. J Neurol Sci. 2010;295:53–57. doi: 10.1016/j.jns.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Kim YD, Nam HS, Kim SH, Kim EY, Song D, Kwon I, et al. Time-dependent thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke. 2015;46:1877–1882. doi: 10.1161/STROKEAHA.114.008247. [DOI] [PubMed] [Google Scholar]
- 21.Hesselmann V, Niederstadt T, Dziewas R, Ritter M, Kemmling A, Maintz D, et al. Reperfusion by combined thrombolysis and mechanical thrombectomy in acute stroke: effect of collateralization, mismatch, and time to and grade of recanalization on clinical and tissue outcome. AJNR Am J Neuroradiol. 2012;33:336–342. doi: 10.3174/ajnr.A2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ota T, Nishiyama Y, Koizumi S, Saito T, Ueda M, Saito N. Impact of onset-to-groin puncture time within three hours on functional outcomes in mechanical thrombectomy for acute large-vessel occlusion. Interv Neuroradiol. 2018;24:162–167. doi: 10.1177/1591019917747247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazighi M, Chaudhry SA, Ribo M, Khatri P, Skoloudik D, Mokin M, et al. Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013;127:1980–1985. doi: 10.1161/CIRCULATIONAHA.112.000311. [DOI] [PubMed] [Google Scholar]
- 24.Alawieh A, Pierce AK, Vargas J, Turk AS, Turner RD, Chaudry MI, et al. The golden 35 min of stroke intervention with ADAPT: effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointerv Surg. 2018;10:213–220. doi: 10.1136/neurintsurg-2017-013040. [DOI] [PubMed] [Google Scholar]
- 25.Khatri P, Yeatts SD, Mazighi M, Broderick JP, Liebeskind DS, Demchuk AM, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a metaanalysis. JAMA. 2016;316:1279–1288. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 28.Tsivgoulis G, Geisler F, Katsanos AH, Kõrv J, Kunz A, Mikulik R, et al. Ultraearly intravenous thrombolysis for acute ischemic stroke in mobile stroke unit and hospital settings. Stroke. 2018;49:1996–1999. doi: 10.1161/STROKEAHA.118.021536. [DOI] [PubMed] [Google Scholar]
- 29.Tsivgoulis G, Ribo M, Rubiera M, Vasdekis SN, Barlinn K, Athanasiadis D, et al. Real-time validation of transcranial Doppler criteria in assessing recanalization during intra-arterial procedures for acute ischemic stroke: an international, multicenter study. Stroke. 2013;44:394–400. doi: 10.1161/STROKEAHA.112.675074. [DOI] [PubMed] [Google Scholar]
- 30.Tsivgoulis G, Alexandrov AV. Ultrasound in neurology. Continuum (Minneap Minn) 2016;22(5, Neuroimaging):1655–1677. doi: 10.1212/CON.0000000000000374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Outcome definitions from the transcranial Doppler monitoring
Baseline characteristics of the whole study population (n=480)
Baseline characteristics and outcomes of study population (n=480) according to recanalization status during the 2-hour TCD-monitoring
Baseline characteristics and outcomes of patients with middle cerebral artery occlusion (n=374) according to early recanalization status achieved during the first hour of TCD-monitoring
Association of elapsed time between symptom onset and beginning of recanalization with 3-month favorable functional outcome in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)
Association of baseline characteristics with the elapsed time between tPA-bolus and start of recanalization in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)
Association of elapsed time between symptom onset and beginning of recanalization with symptomatic intracranial hemorrhage in the study population (n=480)
Association of elapsed time between symptom onset and beginning of recanalization with symptomatic intracranial hemorrhage in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)*
Association of elapsed time between symptom onset and beginning of recanalization with 3-month mortality in patients with sustained complete or partial recanalization at the end of TCD-monitoring (n=301)
Data Availability Statement
De-identified patient dataset will be made available upon reasonable request from the corresponding author.


