Abstract
Systemic cancer and ischemic stroke are common conditions and two of the most frequent causes of death among the elderly. The association between cancer and stroke has been reported worldwide. Stroke causes severe disability for cancer patients, while cancer increases the risk of stroke. Moreover, cancer-related stroke is expected to increase due to advances in cancer treatment and an aging population worldwide. Because cancer and stroke share risk factors (such as smoking and obesity) and treatment of cancer can increase the risk of stroke (e.g., accelerated atherosclerosis after radiation therapy), cancer may accelerate conventional stroke mechanisms (i.e., atherosclerosis, small vessel disease, and cardiac thrombus). In addition, active cancer and chemotherapy may enhance thrombin generation causing stroke related to coagulopathy. Patients with stroke due to cancer-related coagulopathy showed the characteristics findings of etiologic work ups, D-dimer levels, and infarct patterns. In this review, we summarized the frequency of cancer-related stroke among patients with ischemic stroke, mechanisms of stroke with in cancer patients, and evaluation and treatment of cancer-related stroke. We discussed the possibility of cancer-related stroke as a stroke subtype, and presented the most recent discoveries in the pathomechanisms and treatment of stroke due to cancer-related coagulopathy.
Keywords: Stroke, Neoplasms, Coagulopathy, Subtype, Thrombosis, Cancer
Introduction
Systemic cancer and ischemic stroke are common conditions and two of the most frequent causes of death among the elderly. The steadily increasing number of elderly people in the world is predicted to result in an increase of new cancer cases. In addition, improvements in treatment practice (cancer medicine) have the potential to improve survival, and the number of people living with cancer is expected to rise. Despite accumulated knowledge on the association between cancer and stroke, the underlying mechanisms (both molecular and macroscopic) and appropriate therapeutic strategies remain unclear.
The purpose of this review is to discuss the possibility of cancer-related stroke as a stroke subtype, and to present the most recent discoveries in the pathomechanisms and treatment of stroke due to cancer-related coagulopathy. Recently emerging data linking cancer to ischemic stroke are discussed, together with current knowledge gaps and potential research strategies to address them. We did not discuss individual shared risk factors and characteristics of stroke in cancer patients with stroke in depth, since these topics have been reviewed elsewhere [1].
Cancer-related stroke: an emerging subtype of ischemic stroke
Stroke has many etiologies. Some etiologies are frequent and potent, while others are less frequent but potent, or frequent but less potent. Most etiological classifications divide stroke patients into four groups, atherosclerotic, cardioembolism, small vessel disease, and other etiologies, as these are frequent and potent etiologies [2-4]. To be a stroke subtype, the etiology should have the following features. First, the etiology is strongly associated with ischemic stroke. Second, the etiology is relatively common in stroke patients. In the North Dublin population stroke study, the proportions of atherosclerotic, cardioembolic, and small vessel-origin were 9% to 12.9%, 33% to 36.5%, and 10% to 18.4%, respectively [5]. Third, stroke mechanisms in patients with one etiology differ from those with other etiologies. Lastly, there are unique therapeutic strategies for the stroke etiology.
Cerebrovascular disease commonly occurs in cancer patients, and the association between cancer and stroke has been reported worldwide. Nationwide studies in Europe [6,7], Asia [8,9], and United States [10,11] showed that the risk of ischemic stroke increased during the first few months (up to 1 year) after cancer diagnosis. A recent large population study showed that increased risk of arterial thromboembolic events begins 5 months before cancer is officially diagnosed and peaks 1 month prior to the diagnosis [12].
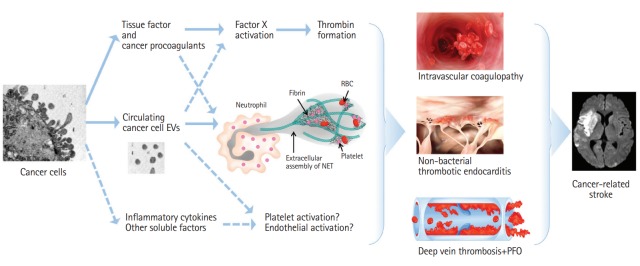
It has been estimated that one in seven to eight patients with ischemic stroke have a known or hidden cancer, and that in 40% of them, cancer-related coagulopathy is the mechanism of stroke (Figure 1) [13-15]. Among patients with ischemic stroke, 10% had known cancer and an additional 3% had hidden cancer. Nationwide inpatient data in the United States showed that about one in 10 hospitalized ischemic stroke patients has comorbid cancer, and there was a significant decrease in ischemic stroke hospitalization in the cancer-negative group, but a steady increase in ischemic stroke hospitalization with a cancer diagnosis [14]. Among stroke patients without cancer, hidden cancer was diagnosed during follow-up after acute ischemic stroke in 2.8% (20.4% in embolic stroke of unknown sources [ESUS]) of Korean patients [16], 2.1% (5.3% in ESUS) of Spanish patients [17], 4.3% of Norwegian patients [18], and 3.0% [19] of Japanese patients, suggesting that hidden malignancy could be a cause of ischemic stroke.
Figure 1.
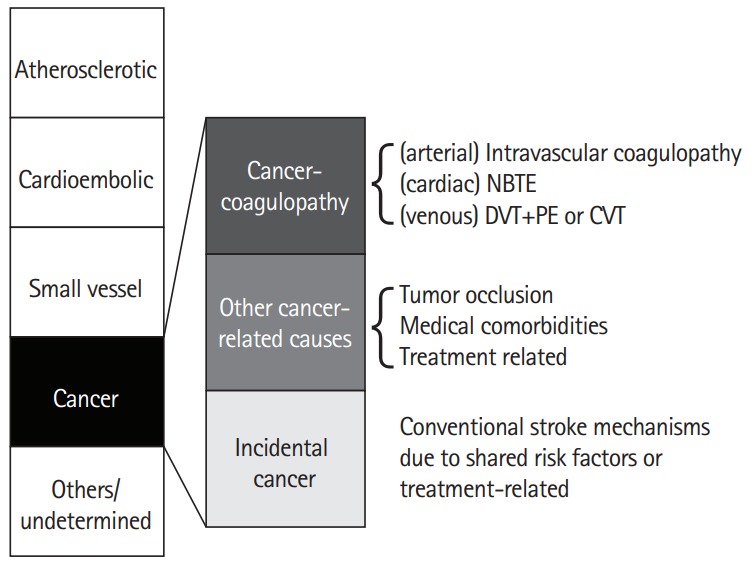
Stroke subtypes, including cancer-related stroke. NBTE, non-bacterial thrombotic endocarditis; DVT, deep vein thrombosis; PE, paradoxical embolism; CVT, cerebral venous thrombosis.
Mechanisms of stroke related to cancer-coagulopathy
The controversies regarding the characteristics of stroke in patients with cancer may be due to the involvement of both cancer-unrelated (by conventional stroke mechanisms [CSMs]) and cancer-related mechanisms in the development of stroke in cancer patients [13]. Besides cancer-related coagulopathy, cancer and its treatment may accelerate CSMs (i.e., atherosclerosis, small vessel disease, and cardiac thrombus) [1]. Cancer and ischemic stroke share risk factors such as smoking, obesity, and inflammation [20]. In addition, treatment of cancer can increase the risk of stroke, e.g., accelerated atherosclerosis after radiation therapy [21,22]. Rarely, occlusion of brain vessels by tumor cells (tumor embolism) occurs in patients with solid tumors of the lung or heart (e.g., cardiac myxoma) that access arterial circulation by invading pulmonary veins or cardiac chambers, and in patients with intravascular lymphoma, a subset of diffuse large B-cell lymphoma [23,24].
Stroke due to cancer-related coagulopathy is a definable disease entity, as unique characteristics of this condition were reported among studies from different populations [13,25,26]. Patients with stroke due to cancer-related coagulopathy show ESUS on extensive work ups and often show unique characteristics in diffusion-weighted image (DWI) patterns of multiple lesions involving multiple arterial territories and laboratory findings suggesting coagulopathy, such as elevated D-dimer levels. Stroke can be caused by clots originating from the venous system (paradoxical embolism from deep vein thrombosis [DVT]), cardiac valves (nonbacterial thrombotic endocarditis [NBTE]), or arteries (intravascular coagulopathy). Several factors are associated with coagulopathy in stroke patients, including characteristics of cancer, such as the type (primary cancer and pathologic type) and extent of the cancer, and the time interval from diagnosis to onset of stroke [13]. Among the pathologic types of lung cancer, adenocarcinoma was significantly more prevalent in cancer-related stroke; about 70% of patients with cancer-related stroke had adenocarcinoma, whereas about 70% of patients with cancer-unrelated stroke had non-adenocarcinoma types. Moreover, metastasis at the time of stroke was more prevalent in cancer-related stroke. Chemotherapy may enhance thrombin generation by the release of extracellular vesicles (EVs) and cell-free DNA from cancer cells and increase the risk of stroke [27]. Angiogenesis inhibitors (e.g., bevacizumab, a vascular endothelial growth factor-A inhibitor), which interfere with these normal processes, worsen conditions like stroke, coronary artery disease, and peripheral artery disease [28,29].
Intravascular coagulopathy
This is a common mechanism of stroke in cancer patients. Characteristics of thrombi of this type of cancer-related coagulopathy are occurrence within the vessel in the absence of gross nidus for thrombus formation/propagation, such as embolic sources in the cardiac valve or deep vein. As a result, strokes caused by intravascular coagulopathy typically occur as numerous small infarcts in multiple territories. A transcranial duplex (TCD) monitoring study showed that embolisms caused by coagulopathy could be the main pathomechanism underlying cancer-related stroke [30]. While embolic signals on TCD were detected in only 5.7% of unselected stroke patients [31], the frequency was very high (58%) in cancer-related stroke patients [30]. Moreover, the number of embolic signals was correlated with the D-dimer levels in patients with cancer-related stroke, but decreased after anticoagulation therapy [30].
Nonbacterial thrombotic (marantic) endocarditis
This involves the deposition of small sterile vegetations on the heart valve leaflets and is thought to be an important cause of stroke in patients with cancer, but its exact prevalence in cancer patients with ischemic stroke is unknown. Patients with NBTE have typical multiple, widely distributed, large and small infarcts lesions in multiple territories [32]. In our data of patients with active cancer and no CSMs, disseminated small lesions were the most frequent DWI lesion pattern, and transesophageal echocardiogram (TEE) did not commonly reveal vegetations [13]. Merkler et al. [33] reported that TEE was superior to transthoracic echocardiogram (TTE) in detecting NBTE in cancer patients with ischemic stroke, but almost 70% of patients with active cancer and no CSMs showed negative results on TEE. These findings suggest that intravascular clot formation is one of the main sources of such embolisms, and NBTE is less common than in previous autopsy data [34].
Paradoxical embolism
This is another important mechanism of stroke in cancer patients because one of four cancer patients have patent foramen ovale (PFO), and one of five cancer patients have venous thromboembolism (VTE) (in autopsy data, half of cancer patients had VTE) [35,36]. Therefore, patients with PFO and DVT could be at risk of paradoxical embolism [37]. Testing for the presence of PFO should be considered in patients with DVT, and vice versa.
Pathomechanisms of cancer-coagulopathy
There have been various efforts to understand the pathomechanisms underlying VTE in cancer patients [38,39] and to develop standardized risk assessments that identify patients at high risk for cancer-associated VTE [40-42]. Biomarker study results for VTE in cancer patients cannot be directly translated to cancer-related stroke. While an elevated platelet count is strongly and independently associated with VTE in cancer patients [38,43], an elevated D-dimer is most consistently and independently associated with intravascular coagulopathy-related stroke. On the contrary, the added value of serial biomarker monitoring in improving VTE prediction and patient care is unclear, because there was no continuous increase in D-dimer levels before VTE occurrence [44]. Such discrepancy may be derived from differences in the mechanism of thrombus formation between arteries and veins, shear wall stress, and dysfunction of endothelial cells that could influence arterial thrombus formation [45].
The Optimal Anticoagulation Strategy In Stroke related to cancer (OASIS-Cancer) study is ongoing to evaluate biological markers for intravascular coagulopathy causing stroke and for monitoring the effects of anticoagulation therapy in patients with active cancer and stroke (clinicalTrial.gov, identifier NCT02743052). Up to 400 patients with acute ischemic stroke and active cancer will be enrolled in this study, and control groups include healthy subjects, stroke patients without active cancer, and active cancer patients without stroke. In this study, serial levels of circulating biomarkers, such as cancer cell-derived EVs, cargo procoagulant proteins, and microRNAs, and neutrophil extracellular traps (NETs), as well as D-dimer, were prospectively evaluated before and after anticoagulation therapy.
In the OASIS-Cancer study, cancer cell-derived EV levels were elevated in patients with cancer-related stroke, and cancer cell-derived EV levels were correlated with D-dimer levels, a nonspecific marker of hypercoagulability [46]. Furthermore, the effects of these EVs may be mediated via tissue factor-independent mechanisms [46]. More recently, we showed that circulating EV levels were higher in patients with lung adenocarcinoma, which is a well-known cancer type for cancer-coagulopathy, than in those with other cell types (such as squamous type) [47]. In addition, coagulation assays demonstrated a direct role of cancer cell-derived EVs in clotting [47]. Compared to treatment of EVs obtained from stroke patients with CSMs or squamous lung cancer cell lines, treatment of EVs obtained from cancer-related stroke patients and adenocarcinoma cell lines resulted in dose-dependent reduction of clotting times.
NETs are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens [48]. Our study and other studies showed that NETosis markers (e.g., circulating DNAs and citrullinated histone H2) were increased in patients with cancer-related stroke [49,50]. Increased circulating cancer cell-derived EVs could increase the formation of NETs in cancer-associated thrombosis [49,51]. Neutrophils, upon activation, release de-condensed chromatin for the formation of NETs, that promote thrombosis by the formation of a scaffold for adhesion of platelets, red blood cells, and platelet adhesion molecules such as fibrinogen, and activate both intrinsic and extrinsic coagulation pathways.
Through the work of the OASIS-Cancer and other studies (Figure 2), we anticipate that mechanism-based diagnosis/theranostics and molecular target-guided monitoring of treatment effects will be possible in patients with cancer-related stroke. In addition, a better understanding of molecular targets underlying coagulopathy related to stroke will help determine the risk for stroke in stroke-free cancer patients, etiological stroke subtype in stroke patients with cancer, and assist in monitoring the effects of therapies for cancer-related stroke.
Figure 2.
Overview of some of the pathways involved in the pathogenesis of cancer-related coagulopathy in stroke patients. Dashed lines represent the possible mechanisms involved in venous thrombosis, but have not been reported in cancer-related stroke. EV, extracellular vesicle; NET, neutrophil extracellular trap; RBC, red blood cell; PFO, patent foramen ovale.
Evaluation for the diagnosis of cancer-related stroke
Appropriate work ups might be difficult in patients with active cancer, due to poor medical conditions. Moreover, appropriate investigations might differ for stroke patients with and without cancer. Purposes of work ups in patients with presumed cancer-related stroke are as follows.
Exclusion of CSMs
Stroke patients who have active cancer and CSMs should be classified as cancer-unrelated mechanisms and treated accordingly. Cancer-specific mechanisms were unlikely to play a role in the development of stroke among patients exhibiting CSMs, given that the distribution of stroke subtype among cancer patients with CSMs was similar to that in stroke patients without cancer [13]. The role of anticoagulation is uncertain and treatment strategies for CSMs (medical management and carotid intervention for atherosclerotic stroke and oral anticoagulants for cardioembolism) would be beneficial [13,52]. Routine vascular and electro-/echo-cardiographic studies are needed to rule out CSMs in patients with cancer and stroke.
Detection of coagulopathy
Multicenter studies in Korea and Italy showed that ESUS and hypercoagulability (elevated D-dimer level or presence of VTE) were associated with active cancer in patients with ischemic stroke [13,53]. The principal lesion patterns on DWI are multiple small cortical lesions extending to multiple vascular territories (in about 40% [13,54]) and multi-staged acute and subacute ischemic lesions [55,56]. The level of D-dimer, a plasmin-derived degradation product of cross-linked fibrin, is a direct measure of activated coagulation. In cancer-related stroke, hypercoagulability as assessed by serial D-dimer levels was associated with early neurological deterioration, stroke recurrence and poor survival after stroke in patients with active cancer, suggesting that D-dimer level can be used in monitoring the effect of anticoagulation therapy [55,57-60]. However, D-dimer levels for detection and monitoring of hypercoagulability should be used with caution, as they are nonspecific and may change with treatment or comorbidity such as infection.
Identification of embolic source or conditions
TTE is the first choice of technique for patients with stroke, whereas TEE is considered the gold standard in the evaluation of ESUS, being superior in detecting NBTE and primary cardiac cancer, such as cardiac myxoma that can cause distal tumor embolism. Therefore, TEE may be indicated in cases with multiple large (>10 mm) infarcts. However, TEE is often impossible due to coagulopathy, bleeding tendency, mental change, or acute illness related to systemic cancer or stroke itself. In addition, work ups for the presence of right-to-left shunt with the echocardiographic bubble test or TCD shunt test are needed, and anticoagulation with non-vitamin K oral antagonists (NOACs) should be considered in cases with DVT on leg Duplex scans.
Screening for hidden malignancy
Patients with covert cancer may present characteristic symptoms and infarct patterns suggesting cancer-related stroke and show elevated D-dimer levels [61]. In our retrospective analysis of patients with ESUS, patients with active cancer were prevalent (71 of 348 patients; 20%) and had distinctive D-dimer levels (>20×higher than those without cancer) and infarct patterns (multiple lesions in multiple vascular territories) [16]. Interestingly, in this study, among 10 patients who showed such characteristics but had no prior diagnosis of cancer, workup for hidden malignancy revealed hidden cancer in all patients. Therefore, in patients who had characteristic infarct patterns, elevated D-dimer levels, and no apparent etiology for their index stroke, screening for hidden malignancy (by serological or radiological tests) should be considered (Figure 3). Thoraco-abdomino-pelvic computed tomography or position emission tomography can be considered initially as cancers in lung, pancreas, genito-urinary (prostate, ovarian, or renal), gastrointestinal (colorectal and gastric), or breast are the most frequent types (in order of frequency) of both known and hidden cancers in patients with cancer-related stroke [7,8,10,11,13,16,17,26].
Figure 3.
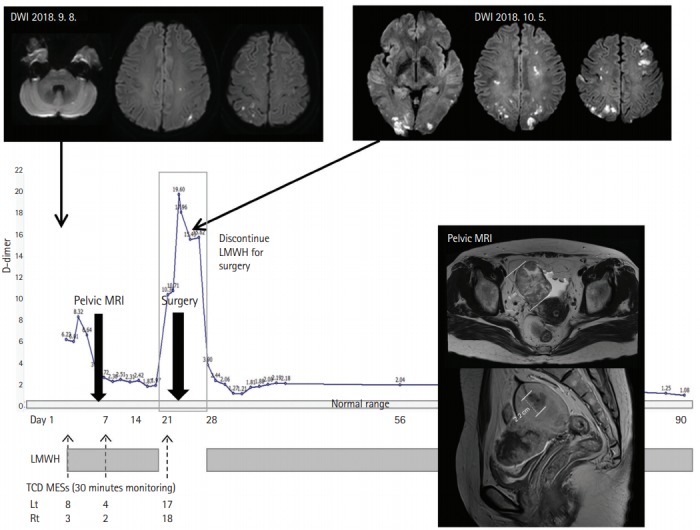
Hidden cancer and stroke caused by cancer-related coagulopathy. A 49-year-old woman presented with recurrent left arm weakness and sensory changes. Vascular study and transthoracic echocardiogram were negative. Microembolic signals were detected during transcranial Doppler monitoring. TCD shunt test showed right-to-left shunt and leg duplex revealed occlusion of right leg veins. Serum D-dimer level was elevated to 6.23 μg/mL (normal <0.5 μg/mL) and CA-125 level was 489.6 U/mL (normal range up to 35 U/mL). Abdominopelvic magnetic resonance image (MRI) showed a 6 cm right ovarian cancer and a 2.2 cm endometrial cancer, both at stage 1a with no metastasis. D-dimer level was lower after anticoagulation with enoxaparin. The patient experienced recurrent stroke with elevation of D-dimer levels during the period of discontinuation for tumor resection. After surgery, anticoagulation therapy was restarted and she remained stable without stroke recurrence. Anticoagulation therapy was discontinued after 6 months of surgery, and D-dimer levels remained in the normal range. DWI, diffusion-weighed image; LMWH, low-molecular weight heparin; TCD MES, transcranial duplex microembolic signal.
Prevention of stroke in cancer coagulopathy
All major consensus treatment guidelines, including from the American College of Chest Physicians, the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the International Clinical Practice Guidelines, recommend low-molecular weight heparin (LMWH) for the initial and longterm treatment of cancer patients with VTE, although the duration of anticoagulant therapy varies from 3 months to indefinitely, as long as there is clinical evidence of active malignancy [62-66]. More recently, NOACs have been shown to be as effective as conventional anticoagulant therapy for the treatment of cancer-related VTE [67]. A recent randomized controlled trial comparing the effects of LMWH and NOACs showed that NOACs could be an alternative option for the treatment of cancer patients with VTE [68]. Recent guidelines recommended NOAC for maintenance therapy in selected patients to prevent VTE [63,66,69].
Although the number of publications on cancer-related stroke has recently increased, there are no evidence-based guidelines for treatment of cancer-related stroke. Like VTE, anticoagulation therapy is also important in patients with active cancer and ischemic stroke. Effective correction of hypercoagulability played a protective role in the survival of cancer-related stroke patients [57]. Vitamin K antagonists (VKA) have been used in cancer patients with stroke but have several important limitations, in that they are often less effective for cancer-associated VTE than for cancer-unrelated VTE, probably because cancer cells release procoagulants which activate factor X independent of factor VII, and bleeding risk is high in cancer patients [70]. In addition, both advanced cancer and stroke are associated with a poor quality of anticoagulation control with VKA, due to medical comorbidity and neurological disability. A meta-analysis showed that LMWH was significantly superior to VKA with respect to reducing the risk of recurrent VTE, and its safety was comparable to VKA [71]. Our retrospective study of cancer-related stroke patients showed that LMWH was more effective than VKA for lowering D-dimer levels, and that recurrent strokes were more frequent in VKA users (16%) than in LMWH users (3.4%) [72].
However, LMWHs require injection and may not be suitable for long-term maintenance therapy for preventing the reoccurrence of stroke. Patient persistence with anticoagulation to prevent VTE was lower in the LMWH group than in the VKA and NOAC groups [73]. In addition, a recent trial comparing LMWH and aspirin in patients with active cancer and acute ischemic stroke, 60% patients randomized to LMWH crossed over to aspirin due to discomfort [74]. NOACs could be an alternative option for treatment of cancer-related stroke. The effects of NOACs were reported in only few cases, but the efficacy and safety of NOACs in cancer-related stroke have not been documented by comprehensive studies yet [75]. A randomized control trial comparing NOAC and LMWH in cancer-related stroke is currently underway (edoxaban for the treatment of coagulopathy in patients with active cancer and acute ischemic stroke trial, clinicalTrial.gov, identifier NCT03570281). In this trial, factor Xa activity and antifactor Xa activity are serially measured, before and after anticoagulant use, to investigate the possibility of patient-specific individualized anticoagulation therapy.
In the setting of acute ischemic stroke, recanalization therapy remains the principal therapeutic approach. The use of thrombolysis and endovascular therapy is not contraindicated in cancer patients under current guidelines for acute stroke therapy. However, the response to recanalization therapies may differ between stroke patients with and without cancer [55,76]. A recent report of thrombectomy specimens in patients with stroke and active cancer (n=16) showed that retrieved thrombi were mostly platelet-risk, suggesting the role of antiplatelet in prevention of cancer-related stroke [77]. However, further studies with a larger cohort are needed and a recent study showed that although retrieved thrombi may provide useful information on the treatment strategy, the components of retrieved thrombi could be changed during the thrombectomy procedure [78].
Conclusions and perspectives
Stroke severely impacts cancer patients, while cancer increases the number of strokes. Cancer-related stroke is as common as established stroke subtypes, such as atherosclerotic and lacunar stroke. Stroke subtype ratios differ among populations and could change with adequate control of risk factors and changes in lifestyle; for example, the progression of lacunar stroke and severity of intracranial atherosclerosis decreased significantly with adequate control of hypertension and anti-atherogenic strategies, respectively [79-81]. On the contrary, cancer-related stroke is expected to increase in people living with cancer that is due to advances in cancer treatment and an aging population worldwide. The literature reviewed herein calls for the need to define cancer-related stroke as an isolated entity.
Further studies should focus to establish a standardized risk assessment and treatment guidelines for cancer-related stroke. Given that various mechanisms cause ischemic cerebrovascular disease, individualized therapeutic approaches, based on molecular and macroscopic features, are needed.
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant (No. 2018R1A2B2003489).
Footnotes
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. 2018;83:873–883. doi: 10.1002/ana.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 4.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-SC-O (phenotypic) classification of stroke. Cerebrovasc Dis. 2009;27:502–508. doi: 10.1159/000210433. [DOI] [PubMed] [Google Scholar]
- 5.Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, et al. Stroke subtype classification to mechanismspecific and undetermined categories by TOAST, A-S-C-O, and causative classification system: direct comparison in the North Dublin population stroke study. Stroke. 2010;41:1579–1586. doi: 10.1161/STROKEAHA.109.575373. [DOI] [PubMed] [Google Scholar]
- 6.Zöller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:1875–1883. doi: 10.1016/j.ejca.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Erichsen R, Sværke C, Sørensen HT, Sandler RS, Baron JA. Risk of colorectal cancer in patients with acute myocardial infarction and stroke: a nationwide cohort study. Cancer Epidemiol Biomarkers Prev. 2013;22:1994–1999. doi: 10.1158/1055-9965.EPI-13-0444. [DOI] [PubMed] [Google Scholar]
- 8.Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population-based cohort study. Stroke. 2011;42:3034–3039. doi: 10.1161/STROKEAHA.111.615534. [DOI] [PubMed] [Google Scholar]
- 9.Jang HS, Choi J, Shin J, Chung JW, Bang OY, Kim GM, et al. The long-term effect of cancer on incident stroke: a nationwide population-based cohort study in Korea. Front Neurol. 2019;10:52. doi: 10.3389/fneur.2019.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, et al. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300. doi: 10.1002/ana.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Tagawa ST, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2019;133:781–789. doi: 10.1182/blood-2018-06-860874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke. 2010;41:798–801. doi: 10.1161/STROKEAHA.109.571356. [DOI] [PubMed] [Google Scholar]
- 14.Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis. 2013;22:1146–1150. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Selvik HA, Thomassen L, Logallo N, Næss H. Prior cancer in patients with ischemic stroke: the Bergen NORSTROKE study. J Stroke Cerebrovasc Dis. 2014;23:919–925. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Park JH, Lee MJ, Park YG, Ahn MJ, Bang OY. Clues to occult cancer in patients with ischemic stroke. PLoS One. 2012;7:e44959. doi: 10.1371/journal.pone.0044959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocho D, Gendre J, Boltes A, Espinosa J, Ricciardi AC, Pons J, et al. Predictors of occult cancer in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2015;24:1324–8. doi: 10.1016/j.jstrokecerebrovasdis.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Selvik HA, Thomassen L, Bjerkreim AT, Næss H. Cancerassociated stroke: the Bergen NORSTROKE study. Cerebrovasc Dis Extra. 2015;5:107–113. doi: 10.1159/000440730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uemura J, Kimura K, Sibazaki K, Inoue T, Iguchi Y, Yamashita S. Acute stroke patients have occult malignancy more often than expected. Eur Neurol. 2010;64:140–144. doi: 10.1159/000316764. [DOI] [PubMed] [Google Scholar]
- 20.Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, et al. Risk of malignant cancer among women with newonset atrial fibrillation. JAMA Cardiol. 2016;1:389–396. doi: 10.1001/jamacardio.2016.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 22.Parikh NS, Burch JE, Kamel H, DeAngelis LM, Navi BB. Recurrent thromboembolic events after ischemic stroke in patients with primary brain tumors. J Stroke Cerebrovasc Dis. 2017;26:2396–2403. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navi BB, Kawaguchi K, Hriljac I, Lavi E, DeAngelis LM, Jamieson DG. Multifocal stroke from tumor emboli. Arch Neurol. 2009;66:1174–1175. doi: 10.1001/archneurol.2009.172. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto A, Kikuchi Y, Homma K, O’uchi T, Furui S. Characteristics of intravascular large B-cell lymphoma on cerebral MR imaging. AJNR Am J Neuroradiol. 2012;33:292–296. doi: 10.3174/ajnr.A2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029–3034. doi: 10.1161/STROKEAHA.112.658625. [DOI] [PubMed] [Google Scholar]
- 26.Gon Y, Okazaki S, Terasaki Y, Sasaki T, Yoshimine T, Sakaguchi M, et al. Characteristics of cryptogenic stroke in cancer patients. Ann Clin Transl Neurol. 2016;3:280–287. doi: 10.1002/acn3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lysov Z, Dwivedi DJ, Gould TJ, Liaw PC. Procoagulant effects of lung cancer chemotherapy: impact on microparticles and cell-free DNA. Blood Coagul Fibrinolysis. 2017;28:72–82. doi: 10.1097/MBC.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL. A new weapon for attacking tumor blood vessels. N Engl J Med. 2008;358:2066–2067. doi: 10.1056/NEJMcibr0800272. [DOI] [PubMed] [Google Scholar]
- 29.Zuo PY, Chen XL, Liu YW, Xiao CL, Liu CY. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PLoS One. 2014;9:e102484. doi: 10.1371/journal.pone.0102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seok JM, Kim SG, Kim JW, Chung CS, Kim GM, Lee KH, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. 2010;68:213–219. doi: 10.1002/ana.22050. [DOI] [PubMed] [Google Scholar]
- 31.Poppert H, Sadikovic S, Sander K, Wolf O, Sander D. Embolic signals in unselected stroke patients: prevalence and diagnostic benefit. Stroke. 2006;37:2039–2043. doi: 10.1161/01.STR.0000231644.47325.aa. [DOI] [PubMed] [Google Scholar]
- 32.Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke. 2002;33:1267–1273. doi: 10.1161/01.str.0000015029.91577.36. [DOI] [PubMed] [Google Scholar]
- 33.Merkler AE, Navi BB, Singer S, Cheng NT, Stone JB, Kamel H, et al. Diagnostic yield of echocardiography in cancer patients with ischemic stroke. J Neurooncol. 2015;123:115–121. doi: 10.1007/s11060-015-1768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64:16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi: 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- 36.Thompson CM, Rodgers LR. Analysis of the autopsy records of 157 cases of carcinoma of the pancreas with particular reference to the incidence of thromboembolism. Am J Med Sci. 1952;223:469–478. doi: 10.1097/00000441-195205000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Iguchi Y, Kimura K, Kobayashi K, Ueno Y, Inoue T. Ischaemic stroke with malignancy may often be caused by paradoxical embolism. J Neurol Neurosurg Psychiatry. 2006;77:1336–1339. doi: 10.1136/jnnp.2006.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117:219–230. doi: 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
- 39.Sousou T, Khorana AA. New insights into cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2009;29:316–320. doi: 10.1161/ATVBAHA.108.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes M, Khorana AA. Risk assessment for thrombosis in cancer. Semin Thromb Hemost. 2014;40:319–324. doi: 10.1055/s-0034-1370770. [DOI] [PubMed] [Google Scholar]
- 41.Pabinger I, van Es N, Heinze G, Posch F, Riedl J, Reitter EM, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5:e289–e298. doi: 10.1016/S2352-3026(18)30063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferroni P, Zanzotto FM, Scarpato N, Riondino S, Guadagni F, Roselli M. Validation of a machine learning approach for venous thromboembolism risk prediction in oncology. Dis Markers. 2017;2017:8781379. doi: 10.1155/2017/8781379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) J Thromb Haemost. 2010;8:114–120. doi: 10.1111/j.1538-7836.2009.03680.x. [DOI] [PubMed] [Google Scholar]
- 44.Reitter EM, Kaider A, Ay C, Quehenberger P, Marosi C, Zielinski C, et al. Longitudinal analysis of hemostasis biomarkers in cancer patients during antitumor treatment. J Thromb Haemost. 2016;14:294–305. doi: 10.1111/jth.13218. [DOI] [PubMed] [Google Scholar]
- 45.Gon Y, Sakaguchi M, Takasugi J, Mochizuki H. Ischemic stroke in cancer patients treated with direct oral anticoagulants for venous thromboembolism. Thromb Res. 2017;154:16–18. doi: 10.1016/j.thromres.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Bang OY, Chung JW, Lee MJ, Kim SJ, Cho YH, Kim GM, et al. Cancer cell-derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor-independent way: the OASIS-CANCER Study. PLoS One. 2016;11:e0159170. doi: 10.1371/journal.pone.0159170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung JW, Cho YH, Ahn MJ, Lee MJ, Kim GM, Chung CS, et al. Association of cancer cell type and extracellular vesicles with coagulopathy in patients with lung cancer and stroke. Stroke. 2018;49:1282–1285. doi: 10.1161/STROKEAHA.118.020995. [DOI] [PubMed] [Google Scholar]
- 48.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 49.Bang OY, Chung JW, Cho YH, Oh MJ, Seo WK, Kim GM, et al. Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke: the OASIS-Cancer Study. Stroke. 2019;50:2944–2947. doi: 10.1161/STROKEAHA.119.026373. [DOI] [PubMed] [Google Scholar]
- 50.Thålin C, Demers M, Blomgren B, Wong SL, von Arbin M, von Heijne A, et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. 2016;139:56–64. doi: 10.1016/j.thromres.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis. Sci Rep. 2017;7:6438. doi: 10.1038/s41598-017-06893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Addison D, Lawler PR, Emami H, Janjua SA, Staziaki PV, Hallett TR, et al. Incidental statin use and the risk of stroke or transient ischemic attack after radiotherapy for head and neck cancer. J Stroke. 2018;20:71–79. doi: 10.5853/jos.2017.01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grazioli S, Paciaroni M, Agnelli G, Acciarresi M, Alberti A, D’Amore C, et al. Cancer-associated ischemic stroke: a retrospective multicentre cohort study. Thromb Res. 2018;165:33–37. doi: 10.1016/j.thromres.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Kim JM, Jung KH, Park KH, Lee ST, Chu K, Roh JK. Clinical manifestation of cancer related stroke: retrospective casecontrol study. J Neurooncol. 2013;111:295–301. doi: 10.1007/s11060-012-1011-4. [DOI] [PubMed] [Google Scholar]
- 55.Seok JM, Kim SJ, Song P, Chung CS, Kim GM, Lee KH, et al. Clinical presentation and ischemic zone on MRI in cancer patients with acute ischemic stroke. Eur Neurol. 2012;68:368–376. doi: 10.1159/000341147. [DOI] [PubMed] [Google Scholar]
- 56.Kassubek R, Bullinger L, Kassubek J, Dreyhaupt J, Ludolph AC, Althaus K, et al. Identifying ischemic stroke associated with cancer: a multiple model derived from a case-control analysis. J Neurol. 2017;264:781–791. doi: 10.1007/s00415-017-8432-0. [DOI] [PubMed] [Google Scholar]
- 57.Lee MJ, Chung JW, Ahn MJ, Kim S, Seok JM, Jang HM, et al. Hypercoagulability and mortality of patients with stroke and active cancer: the OASIS-CANCER Study. J Stroke. 2017;19:77–87. doi: 10.5853/jos.2016.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito S, Kikuchi K, Ueda A, Nagao R, Maeda T, Murate K, et al. Changes in serial D-dimer levels predict the prognoses of Trousseau’s syndrome patients. Front Neurol. 2018;9:528. doi: 10.3389/fneur.2018.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nam KW, Kim CK, Kim TJ, An SJ, Demchuk AM, Kim Y, et al. D-dimer as a predictor of early neurologic deterioration in cryptogenic stroke with active cancer. Eur J Neurol. 2017;24:205–211. doi: 10.1111/ene.13184. [DOI] [PubMed] [Google Scholar]
- 60.Shin YW, Lee ST, Jung KH, Kim DY, Park CK, Kim TM, et al. Predictors of survival for patients with cancer after cryptogenic stroke. J Neurooncol. 2016;128:277–284. doi: 10.1007/s11060-016-2106-0. [DOI] [PubMed] [Google Scholar]
- 61.Bang OY, Ovbiagele B, Kim JS. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke. 2014;45:1186–1194. doi: 10.1161/STROKEAHA.113.003720. [DOI] [PubMed] [Google Scholar]
- 62.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 63.Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 64.Mandalà M, Labianca R, European Society for Medical Oncology Venous thromboembolism (VTE) in cancer patients: ESMO clinical recommendations for prevention and management. Thromb Res. 2010;125 Suppl 2:S117–S119. doi: 10.1016/S0049-3848(10)70028-1. [DOI] [PubMed] [Google Scholar]
- 65.Streiff MB, Holmstrom B, Angelini D, Ashrani A, Bockenstedt PL, Chesney C, et al. NCCN guidelines insights: cancer-associated venous thromboembolic disease, version 2.2018. J Natl Compr Canc Netw. 2018;16:1289–1303. doi: 10.6004/jnccn.2018.0084. [DOI] [PubMed] [Google Scholar]
- 66.Farge D, Bounameaux H, Brenner B, Cajfinger F, Debourdeau P, Khorana AA, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016;17:e452–e466. doi: 10.1016/S1470-2045(16)30369-2. [DOI] [PubMed] [Google Scholar]
- 67.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147:475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 68.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 69.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654–656. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 71.Posch F, Königsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582–589. doi: 10.1016/j.thromres.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang H, Lee JJ, Lee MJ, Ryoo S, Yoon CH, Kim GM, et al. Comparison of enoxaparin and warfarin for secondary prevention of cancer-associated stroke. J Oncol. 2015;2015:502089. doi: 10.1155/2015/502089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khorana AA, McCrae KR, Milentijevic D, Fortier J, Nelson WW, Laliberté F, et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res Pract Thromb Haemost. 2017;1:14–22. doi: 10.1002/rth2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Navi BB, Marshall RS, Bobrow D, Singer S, Stone JB, DeSancho MT, et al. Enoxaparin vs aspirin in patients with cancer and ischemic stroke: the TEACH pilot randomized clinical trial. JAMA Neurol. 2018;75:379–381. doi: 10.1001/jamaneurol.2017.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Ko SB, et al. Treatment of cryptogenic stroke with active cancer with a new oral anticoagulant. J Stroke Cerebrovasc Dis. 2017;26:2976–2980. doi: 10.1016/j.jstrokecerebrovasdis.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 76.Jung S, Jung C, Kim JH, Choi BS, Bae YJ, Sunwoo L, et al. Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke. Interv Neuroradiol. 2018;24:520–528. doi: 10.1177/1591019918776207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park H, Kim J, Ha J, Hwang IG, Song TJ, Yoo J, et al. Histological features of intracranial thrombi in stroke patients with cancer. Ann Neurol. 2019;86:143–149. doi: 10.1002/ana.25495. [DOI] [PubMed] [Google Scholar]
- 78.Duffy S, McCarthy R, Farrell M, Thomas S, Brennan P, Power S, et al. Per-pass analysis of thrombus composition in patients with acute ischemic stroke undergoing mechanical thrombectomy. Stroke. 2019;50:1156–1163. doi: 10.1161/STROKEAHA.118.023419. [DOI] [PubMed] [Google Scholar]
- 79.Ornello R, Degan D, Tiseo C, Di Carmine C, Perciballi L, Pistoia F, et al. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and metaanalysis. Stroke. 2018;49:814–819. doi: 10.1161/STROKEAHA.117.020031. [DOI] [PubMed] [Google Scholar]
- 80.Van Middelaar T, Argillander TE, Schreuder FH, Deinum J, Richard E, Klijn CJ. Effect of antihypertensive medication on cerebral small vessel disease: a systematic review and metaanalysis. Stroke. 2018;49:1531–1533. doi: 10.1161/STROKEAHA.118.021160. [DOI] [PubMed] [Google Scholar]
- 81.Kimura H, Takao M, Suzuki N, Kanemaru K, Mihara B, Murayama S. Pathologic study of intracranial large artery atherosclerosis in 7260 autopsy cases. J Stroke Cerebrovasc Dis. 2017;26:2821–2827. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.056. [DOI] [PubMed] [Google Scholar]