Abstract
Background and Purpose
Depression is common and debilitating illness accompanying many neurological disorders including non-traumatic subarachnoid hemorrhage (SAH). The aim of this systematic review was to identify and critically appraise all published studies that have reported the frequency, severity and time course of depression after SAH, the factors associated with its development and the impact of depression on patients’ quality of life after SAH.
Methods
The PubMed database was searched for studies published in English that recruited at least 40 patients (>18 years old) after SAH who were also diagnosed with depression.
Results
Altogether 55 studies covering 6,327 patients met study entry criteria. The frequency of depression ranged from 0% to 61.7%, with a weighted proportion of 28.1%. Depression remained common even several years after the index SAH. Depression after SAH was associated with female sex, premorbid depression, anxiety, substance use disorders or any psychiatric disorders, and coping styles. Comorbid cognitive impairment, fatigue, and physical disability also increased the risk of depression. Aneurysmal SAH and infarction may be related to depression as well. Depression reduces the quality of life and life satisfaction in patients after SAH.
Conclusions
Depression is common after SAH and seems to persist. Further research is needed to clarify its time course and identify the neuroendocrine and neurochemical factors and brain circuits associated with the development of post-SAH depression. Randomized controlled treatment trials targeting SAH-related depression are warranted.
Keywords: Subarachnoid hemorrhage, Depression, Systematic review
Introduction
Subarachnoid hemorrhage (SAH) is a relatively uncommon and severe type of stroke. As patients are affected by SAH at a mean age of 55 years, they can lose many years of productive life. The rupture of an intracranial aneurysm is the underlying cause in 85% of SAH cases [1]. Approximately 55% of patients survive SAH and regain independent functioning, whereas 19% remain dependent and 26% die [1]. Many survivors of SAH have long-term deficits in cognition, and decreased quality of life [2]. Neuropsychiatric disturbances such as depression, anxiety, post-traumatic stress disorder, and fatigue are not uncommon, yet often neglected in patients with SAH [3].
Depression is common in patients with neurological diseases such as Alzheimer’s disease, Parkinson’s disease, traumatic brain injury and stroke [4]. Depression is a frequent consequence of head injury, affecting up to 61% of patients. Depression is associated with worse global outcomes, impaired social functioning, difficulty performing activities of daily living, and a lower quality of life [5].
Post-stroke depression contributes to disability and increased mortality following stroke. Depression is increasingly becoming a standard part of post-stroke assessment and rehabilitation [6]. However, there is still a lack of methodically sound psychopharmacological and psychosocial treatment trials on SAH-related depression.
The aims of this systematic review were as follows: (1) to determine the frequency, severity and time course of depression after SAH; (2) to identify the factors associated with the development of depression after SAH, including patients’ demographic data, baseline characteristics of SAH, psychological factors including anxiety and cognitive impairment, somatic complications related to SAH (neuroendocrine changes, infarcts, and preexisting and post-SAH medical comorbidities); and (3) to evaluate the impact of depression on patients’ quality of life following SAH.
Methods
Literature search
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. The principal author (W.K.T.) searched the PubMed, EMBASE, PsycINFO, and Ovid Nursing databases on December 5, 2018, using the keywords “depression” or “mood” or “depressive” and “subarachnoid.” Two authors (W.K.T. and L.W.) read every title and abstract, obtained the full texts of potentially relevant papers, and applied inclusion and exclusion criteria to each text. Any uncertainties were discussed. W.K.T. also scrutinized the reference lists of included papers to identify further studies.
Inclusion criteria
Studies were included in the review if they (1) were written in English, (2) were published in peer-reviewed journals, (3) included 10 or more patients who had survived non-traumatic SAH and were older than 18 years [7], and (4) assessed patients for depression using a validated single or multiple item self-report instrument or diagnostic interview.
Exclusion criteria
Publications were excluded if they were (1) case reports, (2) pediatric studies (patients <18 years old), (3) dissertations, or (4) articles with no primary data (reviews, editorials letters, etc.), (5) sample size <40, (6) poor quality (a Strengthening the Report of Observational Studies in Epidemiology [STROBE] checklist score ≤13, i.e., 60% of maximum score).
Data extraction
Two authors (W.K.T. and L.W.) independently extracted the following data from the studies included in the review: study characteristics (aims/objectives, study design, inclusion and exclusion criteria, criteria for and measurement of depression), participants’ characteristics (definition of the study population, age, gender, number, ethnicity, and socio-economic status of the patients at the beginning and end of the study, the number of deaths due to SAH, drop-outs, and patients lost to follow-up before the end of study, first or recurrent SAH, severity of SAH, comorbidities and complications) and results (characteristics of patients’ subgroups, outcome data, and relationship between depression and patients’ characteristics or SAH characteristics and/or outcomes).
Quality assessment
We used STROBE statement for quality assessment of the included papers [8]. It consists of 22 items. We scored each item 1 point. The maximum possible score is 22.
Data synthesis
Statistical analyses were performed in Software R (package metaphor & meta, R Foundation for Statistical Computing, Vienna, Austria). The results are presented as a narrative review and are also tabulated. Frist, the weighted proportion of the frequency of depression was calculated. We conducted a metaanalysis of frequency of depression, using the variance-stablizing double-arscine method transformation [9]. Pooled estimates in both the overall (and subgroup) analyses were calculated using the Hartung-Knapp-Sidik-Jonkman method, under the random effect model [10]. Statistical heterogeneity among the trials was assessed, and P<0.1 was considered as statistical significance [11]. Level of heterogeneity was assessed by I2, which describes the percentage of total variation across studies because of heterogeneity rather than chance alone. A randomeffects model for the trials with statistically significant heterogeneity was used. Subgroup analyses were performed according to data collection settings, i.e., interview and questionnaire. Second we conducted a meta-analysis of frequency of depression, using the variance-stablizing double-arscine method transformation [9]. Pooled estimates in both the overall (and subgroup) analyses were calculated using the Hartung-Knapp-Sidik-Jonkman method, under the random effect model [10]. Publication bias was examined by Funnel plot and Egger’s regression test. Data from the identical cohorts was reported only once. Where depression was assessed with more than one method in a study, only the results with the most commonly used assessment method were considered. Where data from two or more time points after SAH were available, data from the earlier time point was included in the analysis.
Results
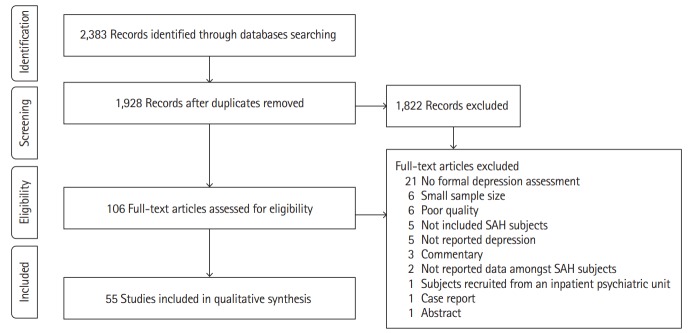
The electronic search identified 2,383 publications potentially eligible for the review. One hundred and six full texts were retrieved for detailed evaluation, of which 51 studies were excluded (Figure 1). Fifty-five studies covering 6,327 patients (range, 40 to 1,181) in 47 cohorts (data from five cohorts were used in 13 publications [12-24]) met the inclusion criteria (Table 1). The majority of studies (46 of 55) included more female than men. Quality of included studies was very different and the STROBE score varied from 14 to 22 (Supplementary Table 1).
Figure 1.
Flow-chart diagram presenting the selection of eligible studies. SAH, subarachnoid hemorrhage.
Table 1.
Characteristics of the studies included in the systematic review
| Study | Design | Participants |
Aetiology of SAH | Timing after SAH | Tool used to diagnose depression | ||
|---|---|---|---|---|---|---|---|
| No. | Age (mean±SD) | Male sex (%) | |||||
| Ljunggren et al. (1985) [63] | Cross | 40 | NR | 42.5 | Aneu | 40–45 mo | Interview |
| Hütter et al. (1995) [20,21] | Cross | 58 | 46 (median) | 43 | Aneu | 1–5 yr | BDI |
| Hellawell et al. (1999) [39] | Long | 44 | 49.7±14.6 | 45 | Aneu | 6 mo (T1) | HADS |
| 1 yr (T2) | |||||||
| 2 yr (T3) | |||||||
| Fertl et al. (1999) [51] | Cross | 40 | 51 (SD NR) | 40 | Aneu | Average 22 mo (range, NR) | BDI |
| Carter et al. (2000) [61] | Cross | 182 | 52 (SD NR) | NR | Aneu | 1–5 yr | ZDS |
| Powell et al. (2002) [18] and Powell et al. (2004) [19] | Long | 52 | 46.9±10.4 | 33 | Aneu | 3 mo (T1) | BDI & HADS |
| 9 mo (T2) | |||||||
| 18 mo (T3) | |||||||
| Fontanella et al. (2003) [50] | Long | 37 | 55.3±8.8 | 35 | Aneu | 6 mo | BDI |
| Bellebaum et al. (2004) [49] | Long | 32 | 54.4±12.5 | NR | Aneu | 23–28 mo | BDI |
| Morris et al. (2004) [40] | Cross | 70 | 45.2±15.2 | 39 | Aneu (81%) | 14–23 mo | BDI & HADS |
| Salmond et al. (2006) [48] | Cross | 20 | 58.6±2.1 | 20 | Aneu | 14–99 mo | BDI |
| Wermer et al. (2007) [38] | Cross | 610 | 54.3±9.0 | 36 | Aneu | 2.3–18.8 yr | HADS |
| Kreitschmann-Andermahr et al. (2007) [47] | Cross | 40 | 43.8 (SD NR) | NR | Aneu | 12–66 mo | BDI |
| Preiss et al. (2007) [46] | Long | 75 | 45.7 (SD NR) | 33 | Aneu | 1 yr | BDI |
| Orbo et al. (2008) [45] | Long | 42 | 48 (SD NR) | 40.4 | Aneu | 1 yr | BDI |
| Visser-Meily et al. (2009) [36] | Cross | 141 | 54.1±12.3 | 33.3 | Aneu | 2–4 yr | HADS |
| Haug et al. (2009) [55] | Long | 46 | 53 (median) | 37 | Aneu | 1 yr | MADRS |
| King et al. (2009) [37] | Long | 178 | 54.7±12.6 | 26 | Aneu | NR | HADS |
| Mukerji et al. (2010) [35] | Cross | 77 | 54.3±10.8 | 32.5 | Aneu (84%) | 12–266 mo | HADS |
| Passier et al. (2010) [14], Passier et al. (2011) [15], Passier et al. (2012) [16] | Long | 111 | 52.8±13.0 | 18 | Aneu | 3 mo | BDI |
| Meyer et al. (2010) [44] | Long | 113 | 54.4±14.1 | 32.7 | Aneu | At discharge (T1) | BDI |
| 12 mo (T2) | |||||||
| Caeiro et al. (2011) [54] | Long | 108 | 53.5±14.2 | 30 | Aneu (56%) | ≤4 day | MADRS |
| Alfieri et al. (2008) [17] | Long | 38 | 44.3±13.7 | 42 | Aneu (47%) | On admission (T1) | BDI |
| 1 mo (T2) | |||||||
| 1 yr (T3) | |||||||
| 3 yr (T4) | |||||||
| 5 yr (T5) | |||||||
| Hedlund et al. (2011) [62] | Long | 83 | 52±9 | 36 | NR | 10 day (T1) | Structured clinical interview for DSM-IV axis I disorders |
| 7 mo (T2) | |||||||
| Wong et al. (2012) [57] | Long | 90 | 45±11 | 76 | Aneu | 3 mo | GDS |
| Latimer et al. (2013) [33] | Cross | 23 | 52.7 (SD NR) | NR | Aneu | 40–45 mo | HADS |
| von Vogelsang et al. (2013) [34] | Cross | 217 | 50.6±12 | 29 | Aneu | 8.8–12 yr | HADS |
| Kreiter et al. (2013) [53] | Long | 216 | 51.2±13.8 | 36 | Aneu (87%) | 3 mo (T1) | CESD |
| 1 yr (T2) | |||||||
| Wong et al. (2013) [56] | Long | 120 | 51 (median) | 68 | Aneu | 1 yr | GDS |
| Vetkas et al. (2013) [59] | Cross | 114 | 54±13 | 32 | Aneu | 1–10 yr | Emotional State Questionnaire |
| Noble et al. (2014) [32] | Cross | 414 | 44.6 (SD NR) | 25 | Aneu (68.4%) | 0–34 yr | HADS |
| Wong et al. (2014) [3] | Cross | 103 | 55±10 | 29 | Aneu | 1–4 yr | Neuropsychiatric inventory |
| Gill et al. (2015) [31] | Cross | 93 | 49.67±10.05 | 20.43 | NR | 2–58 mo | HADS |
| Hütter et al. (2014) [43] | Cross | 45 | 47.1±10.7 | 44 | Aneu (64%) | 3–5 yr | BDI |
| Boerboom et al. (2014) [22] and Boerboom et al. (2016) [23,24] | Long | 76 | 53.8±11.5 | 31.6 | Aneu | 0.4 yr (T1) | CESD |
| 3.9 yr (T2) | |||||||
| von Vogelsang et al. (2015) [29] | Long | 88 | 52.6±14.2 | 34.1 | Aneu | 6 mo (T1) | HADS |
| 1 yr (T2) | |||||||
| 2 yr (T3) | |||||||
| Buunk et al. (2015) [30] | Cross | 200 | 58.7 (SD NR) | 36.5 | Aneu | 2–10 yr | HADS |
| Brand et al. (2015) [65] | Long | 21 | 58.8 (SD NR) | 19 | Aneu | 5–9 mo | Depression Skala |
| Scherfler et al. (2016) [28] | Long | 14 | 46.1±12 | 43 | Aneu (36%) | 1 yr | HADS |
| Gerber et al. (2016) [42] | Cross | 15 | 52.7±9.8 | 27 | Aneu | 44 mo | BDI |
| Taufique et al. (2016) [52] | Long | 1181 | 52.3±12.8 | NR | NR | 1 yr | CESD |
| Kronvall et al. (2016) [60] | Long | 51 | NR | NR | Aneu | 3–6 mo (T1) | Psychological general well-being |
| 6–12 mo (T2) | |||||||
| 12–24 mo (T3) | |||||||
| Pačić-Turk et al. (2016) [64] | Long | 72 | 46±9.2 | 39 | Aneu | 11 mo (T1) | Cornell personality questionnaire |
| 12–48 mo (T2) | |||||||
| Colledge et al. (2017) [12,13] | Cross | 15 | 57.3±8.9 | 27 | Aneu | 44 mo | BDI |
| Ackermark et al. (2017) [41] | Long | 93 | 50.3±11.8 | 19.4 | Aneu | 3 mo (T1) | BDI |
| 1 yr (T2) | |||||||
| 2–5 yr (T3) | |||||||
| Buunk et al. (2018) [26] | Cross | 221 | 57.0±10.0 (Aneu group), 55.4±10.2 (other group) | 31.3 (Aneu group), 58.2 (other group) | Aneu (75%) | 3–10 yr | HADS |
| Tölli et al. (2018) [25] | Long | 45 | 57.4±9.9 | 22.9 | NR | 3 mo (T1) | HADS |
| 6 mo (T2) | |||||||
| 12 mo (T3) | |||||||
| Bründl et al. (2018) [58] | Long | 21 | 42.0–59.8 (mean) | 33.3–50 | Aneu (71%) | 11–35 day (T1) | ICD-10-Symptom-Rating Questionnaire |
| 6 mo (T2) | |||||||
SD, standard deviation; SAH, subarachnoid hemorrhage; Cross, cross-sectional; NR, not reported; Aneu, aneurysmal; BDI, Beck Depression Inventory; Long, longitudinal; HADS, Hospital Anxiety Depression Scale; ZDS, Zung Self-rating Depression Scale; MADRS, Montgomery Åsberg Depression Rating Scale; DSMIV, Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; GDS, Geriatric Depression Scale; CESD, Center for Epidemiologic Studies Depression; ICD-10, International Classification of Diseases 10th Edition.
Fifty-one of 55 studies (93%) used one of the following screening or rating scales to ascertain the presence of depression: Hospital Anxiety Depression Scale (HADS) [18,19,25-40], Beck Depression Inventory (BDI) [12-21,40-51], Center for Epidemiologic Studies Depression (CESD) [22-24,52,53], Montgomery Åsberg Depression Rating Scale (MADRS) [54,55], Geriatric Depression Scale (GDS) [56,57], International Classification of Diseases 10th Edition (ICD-10)-Symptom-Rating questionnaire [58], Neuropsychiatric Inventory [3], Emotional State Questionnaire [59], Psychological General Well-Being Index [60], and the Zung Depression Scale (ZDS) [61]. Less commonly used measures of depression included the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) axis I disorders [62], clinical interview [63], Cornell personality questionnaire [64], and Depression Skala [65] (Table 1).
Twenty-seven studies recruited patients early after SAH and assessed them for depression later. Twenty-six studies recruited patients and collected data at one or more defined time points after SAH (range, 4 days to 10 years) (Table 1).
Frequency, severity, and time course of depression after SAH
Frequency of depression after SAH
Thirty-seven studies (n=5,340) reported the frequency of depression after SAH. The frequency ranged widely depending on the assessment method and how long it was performed after the index SAH.
The frequency of depression reported in the 35 studies (n=5,217) that applied rating instruments/questionnaires was in the range of 0% to 61.7%, with a weighted frequency of 28.1%. Depression was assessed at 4 days to 10.1 years after ictus (Table 2). The weighted frequency of depression at ≤1 and >1 year after ictus were 32.2% and 27.3%, respectively. The frequency in the two studies (n=123) that used interviews to diagnose depression found 20% to 25% (weighted proportion=21.6%) of patients depressed (Table 2) [62,63].
Table 2.
Studies of frequency and/or severity of depression after SAH
| Study | Study design and setting | Timing after SAH | Cut off point | Proportion of participants with depression (%) | Depressive symptom scores (mean±SD) | Limitations | |
|---|---|---|---|---|---|---|---|
| Depression measured by rating instruments/questionnaires | |||||||
| Alfieri et al. (2008) [17] | Longitudinal study, hospitalized patients | On admission (T1) | NR | NR | T1: 17.1±6.5 | Small sample size | |
| 1 mo (T2) | T2: 22.1±3.6 | ||||||
| 1 yr (T3) | T3: 19.9±4.8 | ||||||
| 3 yr (T4) | T4: 14.3±3.8 | ||||||
| 5 yr (T5) | T5: 13.2±3.8 | ||||||
| Caeiro et al. (2011) [54] | Prospective study, consecutive admissions to an academic neurosurgery center | ≤4 day | MADRS ≥7 | 45 | 9.2±7.3 | Sample biased towards SAH of mild severity, depression not assessed at subacute and chronic stage of SAH | |
| Meyer et al. (2010) [44] | Longitudinal study | At discharge (T1) | BDI >10 | T1: 24.8 | NR | Patients with aphasia excluded, possible confounders not measured. | |
| 12 mo (T2) | T2: 61.7 | ||||||
| Passier et al. (2010) [14], Passier et al. (2011) [15], Passier et al. (2012) [16] | Cross-sectional study, all subjects treated with clipping or coiling | 3 mo | BDI ≥10 | 40 | 9.6±6.9 | Nursing home patients not included. | |
| Powell et al. (2002) [18] and Powell et al. (2004) [19] | Cross-sectional study, consecutive admissions to a neurovascular service | 3 mo (T1) | BDI >10 | T1: 9.1 | T1: 9.6±6.2 | Small sample size | |
| 9 mo (T2) | T2: 11.4 | T2: 9.2±6.9 | |||||
| 18 mo (T3) | T3: 16.3 | T3: 9.4±7.3 | |||||
| Ackermark et al. (2017) [41] | Longitudinal study, subjects recruited from a clinic | 3 mo (T1) | BDI ≥10 | T1: 39 | T1: 8.9±7.0 | Nursing home patients not included, no data of previous mental health problems, locus of control, optimism or social support, self-report of depressive symptoms. | |
| 1 yr (T2) | T2: 41 | T2: 9.3±7.1 | |||||
| 2–5 yr (T3) | T3: 54 | T3: 11.2±8.0 | |||||
| Kronvall et al. (2016) [60] | Prospective study in an academic neurosurgery unit | 3–6 mo (T1) | NR | NR | T1: 15.0±3.5 | Small sample size, validity of the mood assessment uncertain | |
| 6–12 mo (T2) | T2: 15.3±2.9 | ||||||
| 12–24 mo (T3) | T3: 15.8±2.9 | ||||||
| Wong et al. (2012) [57] | Prospective multi-center study of consecutive admissions | 3 mo | NR | NR | 7 (median) | Lack of gold standard measure of depression | |
| Kreiter et al. (2013) [53] | Prospective study, consecutive admissions to an academic neurosurgery center | 3 mo (T1) | CESD ≥16 | T1: 38 | NR | Less severely affected subjects more likely to complete follow-up, subjects treated for SAH a decade ago, confounders not considered. | |
| 1 yr (T2) | T2: 33 | ||||||
| Boerboom et al. (2014) [22] and Boerboom et al. (2016) [23,24] | Prospective study, consecutive admissions to an academic neurosurgery center | 0.4 yr (T1) | CESD ≥16 | T2: 26.7 | T1: 13.7±1.2 | Subjects assessed at different time points following SAH. | |
| 3.9 yr (T2) | T2: 11.9±1.2 | ||||||
| Fontanella et al. (2003) [50] | Cross-sectional study, all subjects had treated anterior communicating artery bleeding aneurysm | 6 mo | NR | NR | 13.8 (SD NR) | Small sample size, limited generalizability to patients with other type of SAH | |
| von Vogelsang et al. (2015) [29] | Longitudinal study, hospitalized subjects | 6 mo (T1) | HADS ≥8 | T1: 25.0 | T1: 5.0 | No data on previous history of depression or use of antidepressants during the follow-up period | |
| 1 yr (T2) | T2: 27.6 | T2: 4.0 | |||||
| 2 yr (T3) | T3: 29.4 | T3: 5.0 (median) | |||||
| Hellawell et al. (1999) [39] | Longitudinal study, subjects recruited from a neurosurgical unit | 6 mo (T1) | NR | T1: 8 | NR | Small sample size, high attrition rate | |
| 1 yr (T2) | T2: 9 | ||||||
| 2 yr (T3) | T3: 5 | ||||||
| Brand et al. (2015) [65] | Case-control study, all subjects had treated SAH | 5–9 mo | NR | NR | 1.42±0.29 | Small sample size, validity of the mood assessment uncertain, confounders not measured. | |
| Pačić-Turk et al. (2016) [64] | Prospective study in an academic neurosurgery unit | 11 mo (T1) | NR | NR | T1: 1.93 | Modest sample size, validity of the mood assessment uncertain | |
| 12–48 mo (T2) | T2: 2.65 | ||||||
| Scherfler et al. (2016) [28] | Longitudinal study, subjects recruited from a neurological intensive care unit | 1 yr | HADS >10 | 0 | 1 (median) | Small sample size, selected inclusion of patients without visually detectable structural lesions on MRI | |
| Tölli et al. (2018) [25] | Longitudinal study, subjects recruited from a neruointensive care unit | 1 yr | HADS ≥8 | 23 | NR | Single center study, small sample size | |
| Orbo et al. (2008) [45] | Longitudinal study, all subjects treated with clipping | 1 yr | BDI ≥14 | 5 | 6 (SD NR) | Small sample size | |
| Preiss et al. (2007) [46] | Longitudinal study, all subjects treated with coiling or clipping | 1 yr | NR | NR | 9.4 | Relative small sample size, potential selection bias | |
| Haug et al. (2009) [55] | Prospective study, all subjects treated for anterior or middle cerebral artery bleeding aneurysm | 1 yr | NR | NR | 5.5 (SD NR) | Small sample size, subjects with other locations of aneurysms excluded. | |
| Taufique et al. (2016) [52] | Prospective study, consecutive admissions to an academic neurosurgery center | 1 yr | CESD ≥16 | 33.3 | NR | Poor grade patients more likely to be lost to follow-up. | |
| Mukerji et al. (2010) [35] | Retrospective subject recruitment, all subjects received an angiogram | Median 13 mo (range, 12–266) | NR | 13 | NR | Subjects recruited at different time points following SAH, small sample size | |
| Morris et al. (2004) [40] | Cross-sectional study, method and site of recruitment not reported | Average 16 mo (range, 14–23) | BDI ≥10 | 50 | NR | Small sample size, opportunity samples, subjects recruited at different time points following SAH | |
| Gill et al. (2015) [31] | Cross-sectional study, subjects recruited from neuropsychology services, charities and online support network | Average 21.1 mo (range, 2–58) | HADS ≥8 | 51 | NR | No data on those refused to participate, self-report data of SAH, severity of injury not recorded, subjects, recruited at different time points following SAH. | |
| Fertl et al. (1999) [51] | Cross-sectional study, all subjects treated for SAH | Average 22 mo (range, NR) | ≥12 | 28 | NR | Small sample size, subjects recruited at different time points following SAH | |
| Kreitschmann-An-dermahr et al. (2007) [47] | Cross-sectional study, method and site of recruitment not reported | Average 27.3 mo (range, 12–66) | BDI >10 | 37.5 | 8.33±5.85 | Small sample size, subjects recruited at different time points following SAH | |
| Wong et al. (2014) [3] | Cross-sectional four centers study, hospitalized subjects | Average NR (range, 1–4 yr) | - | 13 | NR | Attrition, subjects recruited at different time points following SAH, confounders not measured, reporting bias | |
| Visser-Meily et al. (2009) [36] | Cross-sectional study, all subjects had been treated with coiling or clipping | Average 3 yr (range, 2–4) | HADS ≥8 | 23 | 4.8±3.9 | Selection bias as only patients still alive included, subjects recruited at different time points following SAH. | |
| Noble et al. (2014) [32] | Cross-sectional study, subjects recruited from support groups | Median 3 yr (range, 1–5) | HADS ≥8 | 45.2 | NR | Only patients had access to internet included, no data on those refused to participate, lack of psychiatric interviews, self-report data of SAH details, subjects recruited at different time points following SAH. | |
| Hütter et al. (1995) [20,21] | Cross-sectional study, all subjects operated for SAH | Median 3 yr (range, 1–5) | BDI >10 | 30 | NR | Small sample size, subjects recruited at different time points following SAH, excluded subjects had worse SAH grading. | |
| Carter et al. (2000) [61] | Cross-sectional study, consecutive admissions to a tertiary medical center | Average NR (range, 1–5 yr) | ZDS ≥50 | 36 | 45.6 (SD NR) | Subjects recruited at different time points following SAH | |
| Latimer et al. (2013) [33] | Retrospective subject recruitment, all subjects had anterior circulatory area SAH | 40–45 mo | NR | NR | 6.7 | Retrospective subject recruitment, selective sample, small sample size | |
| Colledge et al. (2017) [12,13] and Gerber et al. (2016) [42] | Cross-sectional study, almost all subjects treated with clipping | 44 mo | NR | NR | 8.9±6.6 | Small sample size, subjects recruited at different time points following SAH, lack of psychiatric interview | |
| Vetkas et al. (2013) [59] | Retrospective study of a single academic center | Average 4.5 yr (range, 1–10) | GDS ≥12 | 30 | 8.4±6.9 | Retrospective study, subjects assessed at different time points following SAH | |
| Buunk et al. (2015) [30] | Cross-sectional study, all subjects had been treated by coiling or clipping | Average 4.6 yr (range, 2–10) | HADS ≥8 | 23 | 4.2±4.3 | Selective sample, subjects recruited at different time points following SAH | |
| Boerboom et al. (2017) [27] | Cross-sectional study, hospitalized subjects | Average 4.7 yr (range, NR) | HADS ≥8 | 15.2 | 3.5 (SD NR) | Selection bias, small sample size, subjects recruited at different time points following SAH | |
| Salmond et al. (2006) [48] | Cross-sectional study, method and site of recruitment not reported | Average 68 mo (range, 14–99) | NR | NR | 6.5±1.4 | Small sample size, subjects recruited at different time points following SAH | |
| Wermer et al. (2007) [38] | Cross-sectional study, all subjects treated with clipping | Average 8.9 yr (range, 2.3–18.8) | HADS >10 | 9.4 | 6.2±3.1 | Only patients treated with clipping and regained functional independence included, relative young age of the subjects, subjects recruited at different time points following SAH. | |
| von Vogelsang et al. (2013) [34] | Retrospective subject recruitment, subjects recruited from a neurosurgical clinic | Average 10.1 yr (range, 8.8–12) | HADS ≥8 | 23.5 | 4.0 (median) | Subjects recruited at different time points following SAH, no data on previous history of depression or use of antidepressants during the followup period | |
| King et al. (2009) [37] | Cross-sectional study, subjects recruited from neurosurgery clinics | NR | HADS >10 | 9 | 4.8±3.4 | A single academic center study, some eligible patients not participated, Caucasians over-represented | |
| Depression measured by interview | |||||||
| Ljunggren et al. (1985) [63] | Cross-sectional study, all subjects had treated SAH and good neurological recovery | Average 3.5 yr (range, 14 mo–7 yr) | - | 25 | NR | Attrition, sampling bias, subjects recruited at different time points following SAH, self-report of depression | |
| Hedlund et al. (2011) [62] | Prospective study, all subjects had treated SAH and good neurological outcome | 7 mo | - | 21 | NR | Attrition, sampling bias | |
SAH, subarachnoid hemorrhage; SD, standard deviation; NR, not reported; MADRS, Montgomery Åsberg Depression Rating Scale; BDI, Beck Depression Inventory; CESD, Center for Epidemiologic Studies Depression; HADS, Hospital Anxiety Depression Scale; MRI, magnetic resonance imaging; ZDS, Zung Depression Scale; GDS, Geriatric Depression Scale.
In the meta-analysis, the overall pooled frequency of depression was 26.3% (95% confidence interval [CI], 21.0% to 32.0%). The frequency of depression in the interview and questionnaire studies were 25.0% (95% CI, 12.6% to 39.7%) and 26.3% (95% CI, 20.8% to 32.3%), respectively (Figure 2). The Funnel plot did not suggest any publication bias (P=0.434) (Figure 3).
Figure 2.
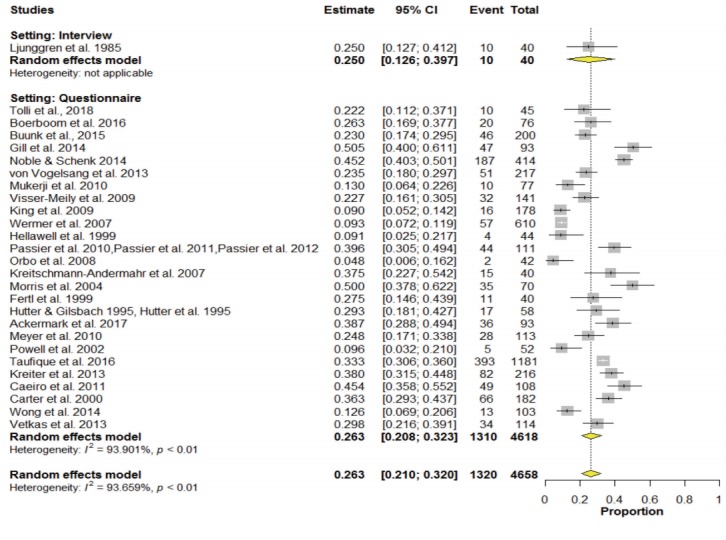
Meta-analysis of frequency of depression. CI, confidence interval.
Figure 3.
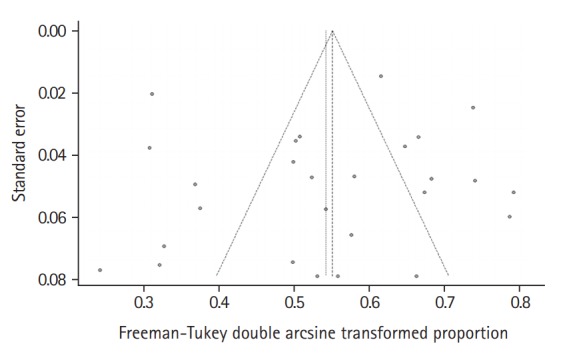
Funnel plot of included studies.
Severity of depression after SAH
Twelve studies (n=634) assessed the severity of depression using the BDI [14-16,18-21,40,41,44,45,47]. The weighted mean BDI value was 9.9 less than 1 year after SAH and 10.1 1-year or more after; these figures indicate mild to moderate depression [15]. Nine studies (n=1,280) evaluated the severity of depression using the HADS [27-30,33,34,36-38]. The weighted mean HADS value was 5 less than 1 year after SAH and 5.4 1-year or more after; according to a previous validation study [66], both values can be interpreted as an absence of depression [27]. Less commonly used mood scales were the CESD [22-24], MADRS [54,55], GDS [56,57], and one study each used the ZDS [61], Depressions Skala [65], Emotional State Questionnaire [59], Cornell Personality Scale [64], and the Psychological General Well-Being Scale [60] (Tables 1 and 2, Supplementary Table 2).
Time course of depression after SAH
Eight studies (n=539) assessed depression at more than one time point (Supplementary Table 3) [18,19,23,25,29,41,44,64]. In one study (n=113), the proportion of patients with depression increased from 24.8% at discharge to 61.7% at the 12-month follow-up [44]. In another study, 72% of patients with depressive symptoms at 3 months still had symptoms at 2 to 5 years [41]. One study reported an increase in depressive symptoms from 11 months to 12 to 48 months follow-up [64]. On the contrary, five studies (n=261) found no change in depressive symptoms between the first assessment at 3, 6, or 9 months and subsequent follow-up(s) at 6, 9 months, 1, 1.5, 2, or 4 years after SAH [18,19,23,25,29].
In a cross-sectional study, the proportion of patients with depression was not statistically different from patients 2 to 5, 5 to 10, and even more than 10 years after SAH, with 8.3%, 10.7%, and 8.6%, respectively [37]. The weighted frequency of depression was 33% up to a year after SAH and 28% a year or more after [51,53]. There was no relationship between length of follow-up and the severity of depressive symptoms at 39 months after SAH in one study [67].
Association between demographic factors, baseline characteristics of SAH, and depression
Demographic characteristics
Two studies explored the association between age and depression after SAH, all with negative findings [20,40]. One study reported a significant correlation between female sex and depression [54]. The association between sex and depression was not significant in the other three studies (n=314) [34,46]. One study found no association between depression and educational level [65]. Another study reported that non-white ethnicity predicted depression (Table 3) [53].
Table 3.
Studies of associations between clinical features and complications of subarachnoid hemorrhage, comorbidities, biomarkers, and depression
| Study | Risk factors | Associations with depression | Confounders controlled using multivariate analysis | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Hütter et al. (1995) [20] | Age | No association | No | |
| Morris et al. (2004) [40] | Age | No association | No | |
| Caeiro et al. (2011) [54] | Sex | Female sex (P=0.003) | No | |
| Preiss et al. (2007) [46] | Sex | No association | No | |
| von Vogelsang et al. (2013) [34] | Sex | No association | No | |
| Kreiter et al. (2013) [53] | Ethnicity | Non-white ethnicity, (OR, 2.7; 95% Cl, 1.4–5.4; P=0.005); non-fluency in English (OR, 3.7; 95% Cl, 1.7–8.2; P=0.001) | No | |
| Brand et al. (2015) [65] | Education | No association | No | |
| Premorbid conditions | ||||
| Caeiro et al. (2011) [54] | Psychiatric history | Past mood disorder (P=0.007), absence of pre-SAH dementia (P=0.05) | No | |
| Kreiter et al. (2013) [53] | Psychiatric history | History of depression (OR, 3.1; 95% Cl, 1.2–7.6; P=0.016) | Yes | |
| Hedlund et al. (2011) [62] | Psychiatric history | Lifetime affective disorder (OR, 11.9; 95% Cl, 3.0–46, P=0.001), anxiety disorder (OR, 6.5; 95% Cl, 1.6–26; P=0.008), substance use disorder (OR, 9.8; 95% Cl, 1.5–66; P=0.019), or any psychiatric disorders (OR, 14.1; 95% Cl, 3.0–47; P=0.001) | Yes | |
| Kreiter et al. (2013) [53] | Psychiatric history | Nicotine use (OR, 2.4; 95% Cl, 1.3–4.5; P=0.006) | Yes | |
| Ackermark et al. (2017) [41] | Premorbid personality traits | Passive coping was correlated with depressive symptoms (ρ=0.576, P<0.001). | Yes | |
| Clinical features and complications of SAH | ||||
| Hütter et al. (1995) [20,21] | Neurological outcomes | No association | No | |
| Morris et al. (2004) [40] | Neurological outcomes | No association | No | |
| Bründl et al. (2018) [58] | subtypes of SAH | Depression symptoms were more common in aneurysmal SAH patients treated with microsurgury and endovascular aneurysm occlusion than those with perimensencephalic SAH (P=0.035 and P=0.016 respectively). | No | |
| Boerboom et al. (2014) [22] | subtypes of SAH | Aneurysmal SAH patients had a higher mean CESD score (13.9±8.7 vs. 5.0±4.9, P=0.006) and higher rate of depression (44.4% vs. 0%, P=0.035) than perimensencephalic SAH. | No | |
| von Vogelsang et al. (2013) [34] | Location of aneurysms | Rupture of posterior circulation aneurysms, compared to anterior circulation aneurysums, was related to a higher level of depression (P=0.036). | No | |
| Hütter et al. (1995) [20] | subtypes of SAH | No association | No | |
| Kreiter et al. (2013) [53] | Infarctions | SAH-related infarction predicted depression (OR, 2.1; 95% Cl, 1.1–4.0; P=0.026). | Yes | |
| Hütter et al. (1995) [21] | Infarctions | Parietal and/or frontal infarcts were negatively correlated with depression (n=58; F=5.03, t=2.57, P=0.03). | No | |
| Bellebaum et al. (2004) [49] | SAH treatment | Patients treated with clips had more depressive symptoms than those treated with coils (U=73.50; P=0.039). | No | |
| Preiss et al. (2007) [46] | SAH treatment | No difference between clips and coils | No | |
| Fontanella et al. (2003) [50] | SAH treatment | No difference between clips and coils | No | |
| Latimer et al. (2013) [33] | SAH treatment | No difference between clips and coils | No | |
| Comorbidities | ||||
| Boerboom et al. (2017) [27] | Cognitive function | Self-rated cognitive function (r=0.372) and memory function (r=–0.427) | No | |
| Fertl et al. (1999) [51] | Cognitive function | Cognitive impairment (P<0.01) | No | |
| Passier et al. (2010) [14] | Cognitive function | depressive symptoms predicted cognitive complaints (β=0.40, P<0.001) | Yes | |
| Wong et al. (2012) [57] | Cognitive function | MoCA (Kendall’s tau b coefficient 0.191; P=0.027) and MMSE (Kendall’s tau b coefficient 0.198; P=0.024) | No | |
| Brand et al. (2015) [65] | Cognitive function | No association | No | |
| Tölli et al. (2018) [25] | Cognitive function | No association | No | |
| Orbo et al. (2008) [45] | Cognitive function | No association | No | |
| Ljunggren et al. (1985) [63] | Fatigue | Correlated with depressive symptoms (r=0.597) | No | |
| Buunk et al. (2018) [26] | Fatigue | Correlated with depressive symptoms (r=0.58) | No | |
| Hütter et al. (2014) [43] | Post-traumatic stress disorder | Severity of depression was correlated with scores on the IES avoidance and intrusion subscales (r=0.45 and r=0.52, respectively). | No | |
| Gill et al. (2015) [31] | Post-traumatic stress disorder | Higher rate of depression predicted greater symptoms of post-traumatic stress disorder (β=0.38, t=5.74, P<0.001). | Yes | |
| Boerboom et al. (2017) [27] | Physical comorbidity | Correlated with depressive symptoms (r=0.419) | No | |
| Functioning | ||||
| Ackermark et al. (2017) [41] | Disability | Correlated with depressive symptoms (ρ=–0.343, P=0.001) | Yes | |
| Fertl et al. (1999) [51] | Reduced working capacity | Depression was more frequent in patients with reduced working capacity (P<0.001). | No | |
| Biomarkers | ||||
| Colledge et al. (2017) [13] | Hair cortisol level | Correlated with depressive symptoms (r=0.56) | No | |
| Kreitschmann-Andermahr et al. (2007) [47] | Basal cortisol value | Correlated with (r=–0.56, P<0.01) and predicted depression (R2=0.30) | Yes | |
| Alfieri et al. (2008) [17] | APOE-ε4 | Correlated with depressive symptoms (P<0.05) | No | |
OR, odds ratio; SAH, subarachnoid hemorrhage; CESD, Center for Epidemiologic Studies Depression; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; IES, Impact of Event Scale; APOE-ε4, apolipoprotein E ε4.
Premorbid conditions
Three studies (n=411) examined the role of history of depression prior to SAH. Associations were found between depression and previous mood disorders [54]; lifetime history of major depression, or of anxiety or substance use disorder [62], and self-reported history of depression [53]. One study reported that passive coping predicted post-SAH depressive symptoms (Table 3) [41]. Other premorbid conditions were examined in three studies.
One found that depression was associated with lifetime psychiatric comorbidity [62]. The second reported that non-fluency in English and nicotine use predicted depression [53]. The third study observed a borderline association between depression and absence of pre-SAH dementia (Table 3) [54].
Association between clinical features and complications of SAH, comorbidities, biomarkers, and depression
Clinical features and complications of SAH
Two studies (n=128) examined the relationship between neurological outcomes and depression, with negative results (Table 3) [20,40].
Three studies (n=124) compared subtypes of SAH with respect to depression. Depressive symptoms were more common in patients with aneurysmal SAH treated by endovascular treatment or microsurgical clipping than in patients with perimesencephalic SAH [58]. Similarly, aneurysmal SAH patients had a higher mean CESD score and higher rate of depression than their perimensencephalic counterparts in a cohort study [22]. No difference in depressive symptoms between aneurysm versus other bleeding source was observed in a third study (Table 3) [20].
Two studies (n=274) examined the effect of infarction on the frequency of depression following SAH. SAH-related infarction predicted depression [53]. Parietal and/or frontal infarcts were negatively correlated with depression (Table 3) [20].
Four studies (n=167) investigated the effect of SAH treatment. Patients treated with clips had more depressive symptoms than those treated with coils [49]. The other three studies (n=135) reported no difference in depressive symptom scores between patients who were treated by surgical clipping and by endovascular coiling [33,46,50]. One study reported no significant association between measures of depression scores and time from admission until surgery [40]. In a second study, rupture of posterior circulation aneurysms was related to a higher level of depression (Table 3) [34].
Comorbidities
Seven studies (n=399) examined the impact of cognitive function on post-SAH depression. Two studies reported an association between depressive symptoms and objective and self-rated cognitive function and memory function [27,57]. Another study found depression to be more frequent in cognitively impaired patients [51]. In the fourth study, depressive symptoms were significant determinants of cognitive complaints [14]. In contrast, three studies found no association between depressive symptoms and cognitive performance or impairment [25,45,65]. Two studies explored the role of fatigue in post-SAH depression. Both reported a positive correlation between depressive symptoms and fatigue (Table 3) [13,63].
Other comorbid conditions were examined in five studies. Severity of depression was correlated with symptoms of posttraumatic stress disorder in two studies [31,43]. A third study found an association between depressive symptoms and overall physical comorbidity [27]. In the fourth study, disability predicted depressive symptoms [41]. Depression was more frequent in patients with reduced working capacity in the fifth study (Table 3) [51].
Biological markers
Three studies (n=93) assessed the relationship between biological markers and depression following SAH. One reported an association between hair cortisol level and depressive symptoms [13]. Another found that depression was correlated with basal cortisol value, which also predicted depression [47]. The third study found a positive correlation between apolipoprotein E ε4 (APOE-ε4) levels and depressive symptoms (Table 3) [17].
Impact of depression after SAH on patients’ lives
Five studies (n=685) examined the impact of depression on work-related issues. Two studies reported that depression or depressive symptoms were more frequent in patients who were unemployed or with reduced working capacity [30,51]. Lifetime history of major depression and/or post-traumatic stress disorder reduced the likelihood of returning to gainful employment [62]. Two studies concluded that depression or depressive symptoms predicted unemployment (Table 4) [24,61].
Table 4.
Summary of studies reporting impact of depression after subarachnoid hemorrhage on patients’ lives
| Study | Outcomes | Associations with outcomes | Confounders adjusted for | |
|---|---|---|---|---|
| Work | ||||
| Buunk et al. (2015) [30] | Unemployment | Unemployed patients had higher level of depression (HADS-D score: 4.98±4.57 vs. 3.01±3.41, P<0.05). | None | |
| Hedlund et al. (2011) [62] | Unemployment | Patients with a lifetime history of depression has higher rate of unemployment (χ2=5.5, P=0.019). | None | |
| Boerboom et al. (2016) [24] | Unemployment | Depression predicted unemployment (OR, 1.126; 95% CI, 1.01–1.25; P=0.031) | Age, gender, cognitive function | |
| Carter et al. (2000) [61] | Unemployment | Depression predicted unemployment (OR, 10.5; 95% CI, 3.3–33.7; P<0.001) | Age, physical disability, neurological deficits | |
| Fertl et al. (1999) [51] | Reduced work capacity | Depression was more common amongst patients with reduced work capacity (P<0.001). | None | |
| HRQOL and related outcomes | ||||
| Passier et al. (2012) [16] | HRQOL | Depressive symptoms did not predict HRQOL. | Gender, education level, aneurysm location, discharge destination, cognitive function, level of impairment | |
| Taufique et al. (2016) [52] | HRQOL | Depression predicted poor HRQOL (OR, 2.3; 95% CI, 1.7–7.3; P=0.02). | Age, ethnicity, education level, history of anxiety, neurological assessments, dmission CT scan grading, complications | |
| Vetkas et al. (2013) [59] | HRQOL | Depressive symptoms were related to lower mental health component score of HRQOL (β=–8.8, SE=1.6, P<0.05). | Anxiety, agoraphobia-panic, fatigue and insomnia symptoms | |
| Meyer et al. (2010) [44] | HRQOL | Depression predicted poor HRQOL (β=–1.80, 95% CI, –4.01 to –0.06, P=0.03). | Gender, marital status, education, clinical status on admission, functional disability | |
| King et al. (2009) [37] | HRQOL | Depressive symptoms was correlated with lower HRQOL (ρ=–0.52, P<0.001). | Disability, anxiety symptoms | |
| Brand et al. (2015) [65] | HRQOL | No association | None | |
| Fertl et al. (1999) [51] | Satisfaction in life | Depressive symptoms was correlated with lower satisfaction in life (r=–0.46, P<0.01). | None | |
| Functional outcomes | ||||
| Wong et al. (2013) [56] | Functional outcomes | Depression predicted unfavorable outcome (OR, 1.24; 95% CI, 1.1–1.3; P<0.001). | Cognitive deficits, neurological deficits | |
| Buunk et al. (2018) [26] | Functional outcomes | No association | Fatigue and anxiety symptoms | |
| Hütter et al. (1995) [20] | Functional impairment in daily life | Depression was correlated with functional impairment in daily life (r=0.63, P<0.001). | None | |
| Other outcomes | ||||
| Buunk et al. (2015) [30] | Leisure and social activities | HADS-D score correlated with problems in leisure (r=0.45, P<0.01) and social (r=0.51, P<0.01) activities. | None | |
| Carter et al. (2000) [61] | Reintegration to normal living | Depression predicted reintegration to normal living (OR, 15.2; 95% CI, 6.4–36.2; P<0.001). | Age, physical disability, neurological deficits | |
| Passier et al. (2011) [15] | Fatigue | Depressive symptoms predicted severity of fatigue (F=4.10, P=0.046). | Level of impairment | |
HADS-D, Hospital Anxiety Depression Scale-depression subscale; OR, odd ratio; HRQOL, health-related quality of life; CT, computed tomography; SE, standard error.
Seven studies (n=1,642) looked at health-related quality of life (HRQOL) or life satisfaction. Two studies found a negative association between depressive symptoms and HRQOL or satisfaction with life [16,51]. Four studies found that depression and depressive symptoms predicted poor overall HRQOL or the mental health component of HRQOL [37,44,52,59]. One small-scale study reported no association between depression scores and quality of life (Table 4) [65].
Three studies (n=399) explored how depression influenced functional outcomes. Depression was correlated with self-rated functional impairment in daily life and with the impact of these impairments [20]. Depression predicted poor functional outcomes a year after SAH [56], but this was not confirmed 3 to 10 years after SAH (Table 4) [26].
Other outcomes were assessed in three studies (n=489). Associations were found between depression and problems with leisure and social activities [30], failure to resume previous level of daily life [61], and fatigue (Table 4) [15].
Discussion
To the best of our knowledge, this was the first systematic review of depression after SAH. The weighted frequency of depression following SAH was 28.1%. The severity of depressive symptoms was mild to moderate. Depression after SAH seems to run a chronic course and its frequency does not decrease with time. A host of demographic variables, premorbid and comorbid conditions, as well as clinical features and complications of SAH are related to the risk of depression. Depression has negative impacts on the daily lives of patients with SAH.
The weighted frequency of depression of 28% is similar to the frequency of depression after stroke in general (31%) [68]. The frequency of depression in the included studies varied greatly, probably due to differences in the methods of assessment, patients’ characteristics, and timing of the assessment. The variation in results was more prominent in studies that used questionnaires (0% to 62%) than in those that used interviews to detect depression (20% to 25%) (Tables 2 and 4).
Interviews give a clinical diagnosis whereas questionnaires will assess depressive symptomatology rather than clinical depression. Also, the number of interview studies was considerably smaller.
Longitudinal studies confirmed that depression and depressive symptoms in the later stages of SAH were at least as frequent and severe as in the early stage. Depressive symptoms persisted for long periods after SAH in 72% of patients [41]. Depression after stroke in general is also a chronic condition, with a prevalence and incidence of around 30% and 15% at 1 to 15 years post-stroke [69].
The development of depression after SAH is associated with a variety of factors. The studies included in this review found relationships between depression and female sex, premorbid depression, anxiety, substance use, any psychiatric disorder, and coping styles. Comorbid cognitive impairment, fatigue, post-traumatic stress disorder, and physical disability also increased the risk of depression. The role of the features and complications of SAH in the development of depression was rarely explored, one study suggested that aneurysmal SAH and infarction may be related to depression. The findings on the impact of neurological deficits and treatment modalities for aneurysm repair on depression were inconclusive. Most of the above risk factors have also been found to be related to depression in stroke in general [70]. Further research on psychosocial factors such as pre-stroke life events and the quality of family and social support are warranted [70].
Pituitary dysfunction may occur in up to one in three patients after SAH [71] and could contribute to the development of depression [72]. In support of this theory, an association between low basal cortisol levels and depression has been reported in patients after SAH [47]. One small-scale study reported a possible link between the APOE-ε4 allele and depression [17]. Interestingly, hypercortisolemia, blunted cortisol awakening response [73], and APOE polymorphisms [74] increase the risk of post-stroke depression.
Post-SAH depression was significantly related to functional impairment, unemployment [75] or reduced working capacity, and poor HRQOL. Data on the impact of depression on the costs of hospitalization and mortality related to SAH are lacking [70].
In terms of treatment of post-SAH depression, only one small-scale, open label trial of mindfulness-based psychotherapy has been published. Proper randomized control trials with antidepressants and other treatment modalities are clearly needed. Antidepressants seem to be commonly prescribed in patents with SAH, in a population-based cohort of 940 patients with SAH, 27% had continuous antidepressant use [76]. On the other hand, the use of selective serotonin reuptake inhibitors, a commonly prescribed antidepressant, in general population was associated with increased risk of intracranial hemorrhage, particularly in the first 30 days of use and when used currently with anticoagulants [77]. Antidepressants are effective in the treatment [78] and prevention of post-stroke depression [79], and non-pharmacological treatment modalities including ecosystem-focused therapy, life review therapy, problem solving therapy, meridian acupressure, transcranial magnetic stimulation, music therapy, exercise, light therapy, motivational interviewing, and robotic-assisted neurorehabilitation could also be trialed [80].
This systematic review has several methodological strengths. An extensive search strategy was used so it is unlikely that relevant studies were missed. Two authors extracted pre-specified data independently, thus reducing the chance that any errors in data extraction would have gone undetected. A major limitation is the inclusion only of studies published in English.
There were several methodological shortcomings in the included studies that weakened the robustness of the review. First, the study design was heterogeneous, including longitudinal [27], cross-sectional [26], or retrospective [32] single site cohorts. Second, while most studies recruited hospitalized [26] subjects, some employed clinic [33,38,42], population [60], or support group [30,31] based sampling. Third, a number of studies involved bias sample, such as particular location [32,54,81], investigation [3] or treatment received [11,37,45], or neurological outcome [65,82] of SAH. Fourth, many studies had small sample size. Fifth, the timing of mood assessment various varied from acute [53,55] to chronic stage [33] of SAH recovery. In addition, most of them assessed depression only once; in cross-sectional studies, subjects were assessed at different time point following SAH. Sixth, most studies used scales to measure depression, whereas some employed, a single question [83], or clinical interview [61,62]. Seventh, some authors described the baseline characteristics of SAH but did not relate them to the presence or severity of depression. Eighth, the majority of studies did not measure and adjust for potential confounders, such as personality, level of social support, recent life events, or previous depression with multivariate analysis. Future studies should consider prospective multi-center design, careful and non-selective sampling method, large sample size, assessment of depression at multiple time points with structural psychiatric interview, and detailed measurement and analysis of demographic and clinical characteristics and other possible confounding factors.
Implication for clinicians
Clinicians involved in the long-term care of SAH survivors need to be aware that post-SAH depression is common, runs a chronic course, and has a negative impact on patients’ lives. Clinicians should routinely ask about depression when they review SAH patients and they should refer patients with suspected depression for psychological and/or psychiatric evaluation and treatment.
Directions for future research
More longitudinal studies are needed to determine the time course of depression after SAH using standardized diagnostic interviews. More studies assessing the relationship between depression and demographics, premorbid and comorbid conditions and complications of SAH are required to elucidate the relationship between depression and the consequences of SAH. Research on the association between depression and pituitary dysfunction, changes in neurotransmitter metabolism, and disruption of brain circuits after SAH is also warranted to clarify the pathogenesis of post-SAH depression. Finally, randomized controlled clinical trials on potential treatments for SAH-related depression are also needed.
Conclusions
Depression is common after SAH and seems to persist. The development of depression after SAH is associated with a variety of factors. Post-SAH depression had negative impacts on patients’ daily life. Further research is needed to clarify its time course and identify the neuroendocrine and neurochemical factors and brain circuits associated with the development of post-SAH depression. Randomized controlled treatment trials targeting SAH-related depression are warranted.
Footnotes
Disclosure
The authors have no financial conflicts of interest.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2019.02103.
Quality assessment of studies
Severity of depression after subarachnoid hemorrhage
Time course of depression after subarachnoid hemorrhage
References
- 1.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–666. doi: 10.1016/S0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 3.Wong GK, Lam SW, Chan SS, Lai M, Tse PP, Mok V, et al. Neuropsychiatric disturbance after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21:1695–1698. doi: 10.1016/j.jocn.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Rickards H. Depression in neurological disorders: an update. Curr Opin Psychiatry. 2006;19:294–298. doi: 10.1097/01.yco.0000218601.17722.5b. [DOI] [PubMed] [Google Scholar]
- 5.Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma. 2009;26:2383–2402. doi: 10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry. 2016;173:221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 7.Kutlubaev MA, Barugh AJ, Mead GE. Fatigue after subarachnoid haemorrhage: a systematic review. J Psychosom Res. 2012;72:305–310. doi: 10.1016/j.jpsychores.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–611. [Google Scholar]
- 10.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials. 1986;7:267–275. doi: 10.1016/0197-2456(86)90034-6. [DOI] [PubMed] [Google Scholar]
- 12.Colledge F, Brand S, Pühse U, Holsboer-Trachsler E, Zimmerer S, Schleith R, et al. A twelve-week moderate exercise programme improved symptoms of depression, insomnia, and verbal learning in post-aneurysmal subarachnoid haemorrhage patients: a comparison with meningioma patients and healthy controls. Neuropsychobiology. 2017;76:59–71. doi: 10.1159/000486903. [DOI] [PubMed] [Google Scholar]
- 13.Colledge F, Brand S, Zimmerer S, Pühse U, Holsboer-Trachsler E, Gerber M. In individuals following aneurysmal subarachnoid haemorrhage, hair cortisol concentrations are higher and more strongly associated with psychological functioning and sleep complaints than in healthy controls. Neuropsychobiology. 2017;75:12–20. doi: 10.1159/000477966. [DOI] [PubMed] [Google Scholar]
- 14.Passier PE, Visser-Meily JM, van Zandvoort MJ, Post MW, Rinkel GJ, van Heugten C. Prevalence and determinants of cognitive complaints after aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis. 2010;29:557–563. doi: 10.1159/000306642. [DOI] [PubMed] [Google Scholar]
- 15.Passier PE, Post MW, van Zandvoort MJ, Rinkel GJ, Lindeman E, Visser-Meily JM. Predicting fatigue 1 year after aneurysmal subarachnoid hemorrhage. J Neurol. 2011;258:1091–1097. doi: 10.1007/s00415-010-5891-y. [DOI] [PubMed] [Google Scholar]
- 16.Passier PE, Visser-Meily JM, van Zandvoort MJ, Rinkel GJ, Lindeman E, Post MW. Predictors of long-term health-related quality of life in patients with aneurysmal subarachnoid hemorrhage. NeuroRehabilitation. 2012;30:137–145. doi: 10.3233/NRE-2012-0737. [DOI] [PubMed] [Google Scholar]
- 17.Alfieri A, Unterhuber V, Pircher M, Schwarz A, Gazzeri R, Reinert M, et al. Psychosocial and neurocognitive performance after spontaneous nonaneurysmal subarachnoid hemorrhage related to the APOE-epsilon4 genotype: a prospective 5-year follow-up study. J Neurosurg. 2008;109:1019–1026. doi: 10.3171/JNS.2008.109.12.1019. [DOI] [PubMed] [Google Scholar]
- 18.Powell J, Kitchen N, Heslin J, Greenwood R. Psychosocial outcomes at three and nine months after good neurological recovery from aneurysmal subarachnoid haemorrhage: predictors and prognosis. J Neurol Neurosurg Psychiatry. 2002;72:772–781. doi: 10.1136/jnnp.72.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell J, Kitchen N, Heslin J, Greenwood R. Psychosocial outcomes at 18 months after good neurological recovery from aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004;75:1119–1124. doi: 10.1136/jnnp.2002.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hütter BO, Gilsbach JM. Introspective capacities in patients with cognitive deficits after subarachnoid hemorrhage. J Clin Exp Neuropsychol. 1995;17:499–517. doi: 10.1080/01688639508405141. [DOI] [PubMed] [Google Scholar]
- 21.Hütter BO, Gilsbach JM, Kreitschmann I. Quality of life and cognitive deficits after subarachnoid haemorrhage. Br J Neurosurg. 1995;9:465–475. doi: 10.1080/02688699550041106. [DOI] [PubMed] [Google Scholar]
- 22.Boerboom W, Heijenbrok-Kal MH, Khajeh L, van Kooten F, Ribbers GM. Differences in cognitive and emotional outcomes between patients with perimesencephalic and aneurysmal subarachnoid haemorrhage. J Rehabil Med. 2014;46:28–32. doi: 10.2340/16501977-1236. [DOI] [PubMed] [Google Scholar]
- 23.Boerboom W, Heijenbrok-Kal MH, Khajeh L, van Kooten F, Ribbers GM. Long-term functioning of patients with aneurysmal subarachnoid hemorrhage: a 4-yr follow-up study. Am J Phys Med Rehabil. 2016;95:112–120. doi: 10.1097/PHM.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 24.Boerboom W, Heijenbrok-Kal MH, van Kooten F, Khajeh L, Ribbers GM. Unmet needs, community integration and employment status four years after subarachnoid haemorrhage. J Rehabil Med. 2016;48:529–534. doi: 10.2340/16501977-2096. [DOI] [PubMed] [Google Scholar]
- 25.Tölli A, Höybye C, Bellander BM, Johansson F, Borg J. The effect of time on cognitive impairments after non-traumatic subarachnoid haemorrhage and after traumatic brain injury. Brain Inj. 2018;32:1465–1476. doi: 10.1080/02699052.2018.1497203. [DOI] [PubMed] [Google Scholar]
- 26.Buunk AM, Groen RJM, Wijbenga RA, Ziengs AL, Metzemaekers JDM, van Dijk JMC, et al. Mental versus physical fatigue after subarachnoid hemorrhage: differential associations with outcome. Eur J Neurol. 2018;25:1313–1319.e113. doi: 10.1111/ene.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boerboom W, van Zandvoort MJ, van Kooten F, Khajeh L, Visser-Meily JM, Ribbers GM, et al. Long-term fatigue after perimesencephalic subarachnoid haemorrhage in relation to cognitive functioning, mood and comorbidity. Disabil Rehabil. 2017;39:928–933. doi: 10.3109/09638288.2016.1172671. [DOI] [PubMed] [Google Scholar]
- 28.Scherfler C, Schiefecker AJ, Delazer M, Beer R, Bodner T, Spinka G, et al. Longitudinal profile of iron accumulation in good-grade subarachnoid hemorrhage. Ann Clin Transl Neurol. 2016;3:781–790. doi: 10.1002/acn3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Vogelsang AC, Forsberg C, Svensson M, Wengström Y. Patients experience high levels of anxiety 2 years following aneurysmal subarachnoid hemorrhage. World Neurosurg. 2015;83:1090–1097. doi: 10.1016/j.wneu.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Buunk AM, Groen RJ, Veenstra WS, Spikman JM. Leisure and social participation in patients 4-10 years after aneurysmal subarachnoid haemorrhage. Brain Inj. 2015;29:1589–1596. doi: 10.3109/02699052.2015.1073789. [DOI] [PubMed] [Google Scholar]
- 31.Gill IJ, Mullin S, Simpson J. Are metacognitive processes associated with posttraumatic stress symptom severity following acquired brain injury? Disabil Rehabil. 2015;37:692–700. doi: 10.3109/09638288.2014.939774. [DOI] [PubMed] [Google Scholar]
- 32.Noble AJ, Schenk T. Psychological distress after subarachnoid hemorrhage: patient support groups can help us better detect it. J Neurol Sci. 2014;343:125–131. doi: 10.1016/j.jns.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 33.Latimer SF, Wilson FC, McCusker CG, Caldwell SB, Rennie I. Subarachnoid haemorrhage (SAH): long-term cognitive outcome in patients treated with surgical clipping or endovascular coiling. Disabil Rehabil. 2013;35:845–850. doi: 10.3109/09638288.2012.709909. [DOI] [PubMed] [Google Scholar]
- 34.von Vogelsang AC, Svensson M, Wengström Y, Forsberg C. Cognitive, physical, and psychological status after intracranial aneurysm rupture: a cross-sectional study of a Stockholm case series 1996 to 1999. World Neurosurg. 2013;79:130–135. doi: 10.1016/j.wneu.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 35.Mukerji N, Holliman D, Baisch S, Noble A, Schenk T, Nath F. Neuropsychologic impact of treatment modalities in subarachnoid hemorrhage: clipping is no different from coiling. World Neurosurg. 2010;74:129–138. doi: 10.1016/j.wneu.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Visser-Meily JM, Rhebergen ML, Rinkel GJ, van Zandvoort MJ, Post MW. Long-term health-related quality of life after aneurysmal subarachnoid hemorrhage: relationship with psychological symptoms and personality characteristics. Stroke. 2009;40:1526–1529. doi: 10.1161/STROKEAHA.108.531277. [DOI] [PubMed] [Google Scholar]
- 37.King JT, Jr, Tsevat J, Roberts MS. Measuring preference-based quality of life using the EuroQol EQ-5D in patients with cerebral aneurysms. Neurosurgery. 2009;65:565–573. doi: 10.1227/01.NEU.0000350980.01519.D8. [DOI] [PubMed] [Google Scholar]
- 38.Wermer MJ, Kool H, Albrecht KW, Rinkel GJ, Aneurysm Screening after Treatment for Ruptured Aneurysms Study Group Subarachnoid hemorrhage treated with clipping: long-term effects on employment, relationships, personality, and mood. Neurosurgery. 2007;60:91–98. doi: 10.1227/01.NEU.0000249215.19591.86. [DOI] [PubMed] [Google Scholar]
- 39.Hellawell DJ, Taylor R, Pentland B. Persisting symptoms and carers’ views of outcome after subarachnoid haemorrhage. Clin Rehabil. 1999;13:333–340. doi: 10.1191/026921599669500092. [DOI] [PubMed] [Google Scholar]
- 40.Morris PG, Wilson JT, Dunn L. Anxiety and depression after spontaneous subarachnoid hemorrhage. Neurosurgery. 2004;54:47–54. doi: 10.1227/01.neu.0000097198.94828.e1. [DOI] [PubMed] [Google Scholar]
- 41.Ackermark PY, Schepers VP, Post MW, Rinkel GJ, Passier PE, Visser-Meily JM. Longitudinal course of depressive symptoms and anxiety after aneurysmal subarachnoid hemorrhage. Eur J Phys Rehabil Med. 2017;53:98–104. doi: 10.23736/S1973-9087.16.04202-7. [DOI] [PubMed] [Google Scholar]
- 42.Gerber M, Colledge F, Pühse U, Holsboer-Trachsler E, Zimmerer S, Brand S. Sleep quality, sleep EEG pattern, mental well-being and cortisol secretion in patients with ruptured aneurysm post-treatment: a comparison with post-surgery meningioma patients and controls. Neuropsychobiology. 2016;73:148–159. doi: 10.1159/000444492. [DOI] [PubMed] [Google Scholar]
- 43.Hütter BO, Kreitschmann-Andermahr I. Subarachnoid hemorrhage as a psychological trauma. J Neurosurg. 2014;120:923–930. doi: 10.3171/2013.11.JNS121552. [DOI] [PubMed] [Google Scholar]
- 44.Meyer B, Ringel F, Winter Y, Spottke A, Gharevi N, Dams J, et al. Health-related quality of life in patients with subarachnoid haemorrhage. Cerebrovasc Dis. 2010;30:423–431. doi: 10.1159/000317078. [DOI] [PubMed] [Google Scholar]
- 45.Orbo M, Waterloo K, Egge A, Isaksen J, Ingebrigtsen T, Romner B. Predictors for cognitive impairment one year after surgery for aneurysmal subarachnoid hemorrhage. J Neurol. 2008;255:1770–1776. doi: 10.1007/s00415-008-0047-z. [DOI] [PubMed] [Google Scholar]
- 46.Preiss M, Koblihova J, Netuka D, Klose J, Charvat F, Benes V. Ruptured cerebral aneurysm patients treated by clipping or coiling: comparison of long-term neuropsychological and personality outcomes. Zentralbl Neurochir. 2007;68:169–175. doi: 10.1055/s-2007-985855. [DOI] [PubMed] [Google Scholar]
- 47.Kreitschmann-Andermahr I, Poll E, Hutter BO, Reineke A, Kristes S, Gilsbach JM, et al. Quality of life and psychiatric sequelae following aneurysmal subarachnoid haemorrhage: does neuroendocrine dysfunction play a role? Clin Endocrinol (Oxf) 2007;66:833–837. doi: 10.1111/j.1365-2265.2007.02821.x. [DOI] [PubMed] [Google Scholar]
- 48.Salmond CH, DeVito EE, Clark L, Menon DK, Chatfield DA, Pickard JD, et al. Impulsivity, reward sensitivity, and decisionmaking in subarachnoid hemorrhage survivors. J Int Neuropsychol Soc. 2006;12:697–706. doi: 10.1017/S135561770606084X. [DOI] [PubMed] [Google Scholar]
- 49.Bellebaum C, Schäfers L, Schoch B, Wanke I, Stolke D, Forsting M, et al. Clipping versus coiling: neuropsychological follow up after aneurysmal subarachnoid haemorrhage (SAH) J Clin Exp Neuropsychol. 2004;26:1081–1092. doi: 10.1080/13803390490515342. [DOI] [PubMed] [Google Scholar]
- 50.Fontanella M, Perozzo P, Ursone R, Garbossa D, Bergui M. Neuropsychological assessment after microsurgical clipping or endovascular treatment for anterior communicating artery aneurysm. Acta Neurochir (Wien) 2003;145:867–872. doi: 10.1007/s00701-003-0111-5. [DOI] [PubMed] [Google Scholar]
- 51.Fertl E, Killer M, Eder H, Linzmayer L, Richling B, Auff E. Long-term functional effects of aneurysmal subarachnoid haemorrhage with special emphasis on the patient’s view. Acta Neurochir (Wien) 1999;141:571–577. doi: 10.1007/s007010050345. [DOI] [PubMed] [Google Scholar]
- 52.Taufique Z, May T, Meyers E, Falo C, Mayer SA, Agarwal S, et al. Predictors of poor quality of life 1 year after subarachnoid hemorrhage. Neurosurgery. 2016;78:256–264. doi: 10.1227/NEU.0000000000001042. [DOI] [PubMed] [Google Scholar]
- 53.Kreiter KT, Rosengart AJ, Claassen J, Fitzsimmons BF, Peery S, Du YE, et al. Depressed mood and quality of life after subarachnoid hemorrhage. J Neurol Sci. 2013;335:64–71. doi: 10.1016/j.jns.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 54.Caeiro L, Santos CO, Ferro JM, Figueira ML. Neuropsychiatric disturbances in acute subarachnoid haemorrhage. Eur J Neurol. 2011;18:857–864. doi: 10.1111/j.1468-1331.2010.03271.x. [DOI] [PubMed] [Google Scholar]
- 55.Haug T, Sorteberg A, Sorteberg W, Lindegaard KF, Lundar T, Finset A. Cognitive functioning and health related quality of life after rupture of an aneurysm on the anterior communicating artery versus middle cerebral artery. Br J Neurosurg. 2009;23:507–515. doi: 10.1080/02688690902785701. [DOI] [PubMed] [Google Scholar]
- 56.Wong GK, Lam SW, Ngai K, Wong A, Siu D, Poon WS, et al. Cognitive domain deficits in patients with aneurysmal subarachnoid haemorrhage at 1 year. J Neurol Neurosurg Psychiatry. 2013;84:1054–1058. doi: 10.1136/jnnp-2012-304517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong GK, Lam S, Ngai K, Wong A, Mok V, Poon WS, et al. Evaluation of cognitive impairment by the Montreal cognitive assessment in patients with aneurysmal subarachnoid haemorrhage: prevalence, risk factors and correlations with 3 month outcomes. J Neurol Neurosurg Psychiatry. 2012;83:1112–1117. doi: 10.1136/jnnp-2012-302217. [DOI] [PubMed] [Google Scholar]
- 58.Bründl E, Schödel P, Bele S, Proescholdt M, Scheitzach J, Zeman F, et al. Treatment of spontaneous subarachnoid hemorrhage and self-reported neuropsychological performance at 6 months: results of a prospective clinical pilot study on good-grade patients. Turk Neurosurg. 2018;28:369–388. doi: 10.5137/1019-5149.JTN.21825-17.0. [DOI] [PubMed] [Google Scholar]
- 59.Vetkas A, Lepik T, Eilat T, Rätsep T, Asser T. Emotional health and quality of life after aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 2013;155:1107–1114. doi: 10.1007/s00701-013-1683-3. [DOI] [PubMed] [Google Scholar]
- 60.Kronvall E, Sonesson B, Valdemarsson S, Siemund R, Säveland H, Nilsson OG. Reduced quality of life in patients with pituitary dysfunction after aneurysmal subarachnoid hemorrhage: a prospective longitudinal study. World Neurosurg. 2016;88:83–91. doi: 10.1016/j.wneu.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 61.Carter BS, Buckley D, Ferraro R, Rordorf G, Ogilvy CS. Factors associated with reintegration to normal living after subarachnoid hemorrhage. Neurosurgery. 2000;46:1326–1334. doi: 10.1097/00006123-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Hedlund M, Zetterling M, Ronne-Engström E, Carlsson M, Ekselius L. Depression and post-traumatic stress disorder after aneurysmal subarachnoid haemorrhage in relation to lifetime psychiatric morbidity. Br J Neurosurg. 2011;25:693–700. doi: 10.3109/02688697.2011.578769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ljunggren B, Sonesson B, Säveland H, Brandt L. Cognitive impairment and adjustment in patients without neurological deficits after aneurysmal SAH and early operation. J Neurosurg. 1985;62:673–679. doi: 10.3171/jns.1985.62.5.0673. [DOI] [PubMed] [Google Scholar]
- 64.Pačić-Turk L, Šulentić T, Havelka Meštrović A, Paladino J, Mrak G. Personality changes following brain artery aneurysm surgery. Acta Clin Croat. 2016;55:565–578. doi: 10.20471/acc.2016.55.04.06. [DOI] [PubMed] [Google Scholar]
- 65.Brand S, Zimmerer S, Kalak N, Planta SV, Schwenzer-Zimmerer K, Müller AA, et al. Compared to controls, patients with ruptured aneurysm and surgical intervention show increase in symptoms of depression and lower cognitive performance, but their objective sleep is not affected. World J Biol Psychiatry. 2015;16:96–105. doi: 10.3109/15622975.2014.888093. [DOI] [PubMed] [Google Scholar]
- 66.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–370. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- 67.Madureira S, Canhão P, Guerreiro M, Ferro JM. Cognitive and emotional consequences of perimesencephalic subarachnoid hemorrhage. J Neurol. 2000;247:862–867. doi: 10.1007/s004150070074. [DOI] [PubMed] [Google Scholar]
- 68.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 69.Ayerbe L, Ayis S, Crichton S, Wolfe CD, Rudd AG. The natural history of depression up to 15 years after stroke: the South London Stroke Register. Stroke. 2013;44:1105–1110. doi: 10.1161/STROKEAHA.111.679340. [DOI] [PubMed] [Google Scholar]
- 70.Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131–144. doi: 10.1016/j.pharmthera.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Vespa P, Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage Endocrine function following acute SAH. Neurocrit Care. 2011;15:361–364. doi: 10.1007/s12028-011-9587-7. [DOI] [PubMed] [Google Scholar]
- 72.Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- 73.Levada OA, Troyan AS. Poststroke depression biomarkers: a narrative review. Front Neurol. 2018;9:577. doi: 10.3389/fneur.2018.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li XB, Wang J, Xu AD, Huang JM, Meng LQ, Huang RY, et al. Apolipoprotein E polymorphisms increase the risk of poststroke depression. Neural Regen Res. 2016;11:1790–1796. doi: 10.4103/1673-5374.194748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al Yassin A, Ouyang B, Temes R. Depression and anxiety following aneurysmal subarachnoid hemorrhage are associated with higher six-month unemployment rates. J Neuropsychiatry Clin Neurosci. 2017;29:67–69. doi: 10.1176/appi.neuropsych.15070171. [DOI] [PubMed] [Google Scholar]
- 76.Huttunen J, Lindgren A, Kurki MI, Huttunen T, Frösen J, von Und Zu Fraunberg M, et al. Antidepressant use after aneurysmal subarachnoid hemorrhage: a population-based casecontrol study. Stroke. 2016;47:2242–2248. doi: 10.1161/STROKEAHA.116.014327. [DOI] [PubMed] [Google Scholar]
- 77.Renoux C, Vahey S, Dell’Aniello S, Boivin JF. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. 2017;74:173–180. doi: 10.1001/jamaneurol.2016.4529. [DOI] [PubMed] [Google Scholar]
- 78.Yi ZM, Liu F, Zhai SD. Fluoxetine for the prophylaxis of poststroke depression in patients with stroke: a meta-analysis. Int J Clin Pract. 2010;64:1310–1317. doi: 10.1111/j.1742-1241.2010.02437.x. [DOI] [PubMed] [Google Scholar]
- 79.Mead GE, Hsieh CF, Lee R, Kutlubaev M, Claxton A, Hankey GJ, et al. Selective serotonin reuptake inhibitors for stroke recovery: a systematic review and meta-analysis. Stroke. 2013;44:844–850. doi: 10.1161/STROKEAHA.112.673947. [DOI] [PubMed] [Google Scholar]
- 80.Hadidi NN, Huna Wagner RL, Lindquist R. Nonpharmacological treatments for post-stroke depression: an integrative review of the literature. Res Gerontol Nurs. 2017;10:182–195. doi: 10.3928/19404921-20170524-02. [DOI] [PubMed] [Google Scholar]
- 81.Joo JH, Morales KH, de Vries HF, Gallo JJ. Disparity in use of psychotherapy offered in primary care between older African-American and white adults: results from a practicebased depression intervention trial. J Am Geriatr Soc. 2010;58:154–160. doi: 10.1111/j.1532-5415.2009.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hedlund M, Zetterling M, Ronne-Engstrom E, Ekselius L, Carlsson M. Perceived recovery after aneurysmal subarachnoid haemorrhage in individuals with or without depression. J Clin Nurs. 2010;19:1578–1587. doi: 10.1111/j.1365-2702.2009.02940.x. [DOI] [PubMed] [Google Scholar]
- 83.Krajewski K, Dombek S, Martens T, Köppen J, Westphal M, Regelsberger J. Neuropsychological assessments in patients with aneurysmal subarachnoid hemorrhage, perimesencephalic SAH, and incidental aneurysms. Neurosurg Rev. 2014;37:55–62. doi: 10.1007/s10143-013-0489-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality assessment of studies
Severity of depression after subarachnoid hemorrhage
Time course of depression after subarachnoid hemorrhage