Abstract
Background
Clinical trials have demonstrated a mortality benefit from lung cancer screening by low-dose CT (LDCT) in current or past tobacco smokers who meet criteria. Potential harms of screening mostly relate to downstream evaluation of abnormal screens. Few data exist on the rates outside of clinical trials of imaging and diagnostic procedures following screening LDCT. We describe rates in the community setting of follow-up imaging and diagnostic procedures after screening LDCT.
Methods
We used Clinformatics Data Mart national database to identify enrollees age 55 to 80 year who underwent screening LDCT from January 1, 2016, to December 31, 2016. We assessed rates of follow-up imaging (diagnostic chest CT scan, MRI, and PET) and follow-up procedures (bronchoscopy, percutaneous biopsy, thoracotomy, mediastinoscopy, and thoracoscopy) in the 12 months following LDCT for lung cancer screening. We also assessed these rates in an age-, sex-, and number of comorbidities-matched population that did not undergo LDCT to estimate rates unrelated to the screening LDCT. We then reported the adjusted rate of follow-up testing as the observed rate in the screening LDCT population minus the rate in the non-LDCT population.
Results
Among 11,520 enrollees aged 55 to 80 years who underwent LDCT in 2016, the adjusted rates of follow up 12 months after LDCT examinations were low (17.7% for imaging and 3.1% for procedures). Among procedures, the adjusted rates were 2.0% for bronchoscopy, 1.3% for percutaneous biopsy, 0.9% for thoracoscopy, 0.2% for mediastinoscopy, and 0.4% for thoracotomy. Adjusted rates of follow-up procedures were higher in enrollees undergoing an initial screening LDCT (3.3%) than in those after a second screening examination (2.2%).
Conclusions
In general, imaging and rates of procedures after screening LDCT was low in this commercially insured population.
Key Words: chest imaging, computed tomography, follow up, health-care utilization, imaging, LDCT, lung cancer screening, NLST, procedures
Abbreviations: CPT, Current Procedural Terminology; LCS, lung cancer screening; LDCT, low-dose CT; NLST, National Lung Screening Trial
FOR EDITORIAL COMMENT, SEE PAGE 247
Lung cancer is the leading cause of cancer-related mortality in the United States, with an estimated 154,050 attributable deaths in 2018.1 A large US trial, The National Lung Screening Trial (NLST), published in 2011 demonstrated lower mortality from lung cancer and from all causes using annual low-dose CT (LDCT) screening.2 Subsequently the US Preventive Services Task Force issued a Grade B recommendation in 2013 for lung cancer screening (LCS) with LDCT.3 In 2018, another large European trial with 10-year follow up showed even larger reductions in lung cancer mortality with LDCT screening.4
The generalizability of NLST results to the community is uncertain for many reasons. Compared with the estimated 8 million eligible Americans, the demographics of the NLST cohort consisted of a higher number of former smokers (vs current), were more educated, and more likely to be white and without pulmonary disease.5 The NLST sites were either National Cancer Institute-designated cancer centers and/or large academic medical centers with specialized thoracic radiologists who underwent specific training and quality control in the image interpretation. The rate of invasive testing after positive screens was lower than had been previously reported and with lower complication rates. A concern is whether LDCT LCS performed in the community may have higher false-positive rates and higher rates of subsequent evaluations and complications.6, 7, 8 If the rate of invasive procedures performed in the broader community is greater than in NLST, the same net benefit of LCS may not be realized.
Use of follow-up testing in the community setting is largely unknown. Questions remain if widespread implementation in the community can achieve similarly low rates of follow-up testing after LDCT LCS outside a rigorously conducted clinical trial setting. We report follow-up evaluation after LDCT LCS in a national commercial insurance database. We hypothesized that use of invasive follow-up testing would be higher than that shown in NLST.
Methods
Source of Data
This was a retrospective analysis using the Clinformatics Data Mart database from July 1, 2014, to December 31, 2017. Clinformatics Data Mart is one of the nation’s largest commercial health insurance databases, with more than 18 million enrollees. The data sources include the Member Eligibility Tables, with information on members enrolled, and the Medical Claims Table, which contains claims for inpatient and outpatient professional services including services such as outpatient surgery, laboratory, and radiology. Persons in the south and those aged 21 to 64 years are overrepresented in the data set.
The University of Texas Medical Branch Institutional Review Board approved the research (IRB 17-109) and waived informed consent because of nature of the research.
Cohorts
The cohort consisted of beneficiaries aged 55 to 80 years of age who underwent LDCT LCS from January 1, 2016, through December 31, 2016, and who had complete insurance enrollment in 1 year before receipt of the first screening LDCT (e-Fig 1). LDCT LCS has specific Current Procedural Terminology (CPT) codes (G0297 or S8032).
We generated a control cohort of enrollees who did not have LDCT testing in 2016 to estimate the background rates for chest imaging and procedures not secondary to LDCT LCS testing. The control patients were matched for age (±1 year), sex, and number of comorbidities (0-1, 2-3, 4+) using Elixhauser comorbidity scores generated from all claims in the 12 months before each study year and categorized according to number of comorbidities.9
Finally, we created a cohort comparing rates of follow-up testing stratified by whether the enrollee had undergone a prior chest CT vs the LDCT LCS being an initial screen. This cohort included enrollees with continuous health plan coverage for ≥18 months before the index LDCT LCS, with a 12-month follow-up period (n = 6,993; e-Fig 1). Because there was not a separate CPT code for LDCT LCS for the entire time between July 1, 2014, and December 31, 2015, we stratified enrollees by whether there was a CPT code for any chest CT scan during the 18 months before the studied LDCT LCS.
Outcomes
Imaging outcomes were receipt of diagnostic CT scan, PET, or MRI of the chest. Outcomes for invasive testing were bronchoscopy, percutaneous biopsy, thoracoscopy, thoracotomy and mediastinoscopy (e-Table 1 provides CPT and Procedure codes for each outcome). We did not include the code for LDCT scan in assessing follow-up chest CT scan examinations in the main analyses because we could not distinguish whether early repeat LDCT scan might be secondary to a technical problem in the initial LDCT or whether late LDCT might be the next routine yearly follow-up examination performed somewhat early. We did include any follow-up LDCT examination as part of follow-up chest imaging in sensitivity analyses. Each enrollee could contribute once to each specific outcome (eg, CT scan, MRI, bronchoscopy, biopsy). Repeats of the same test or procedure were not counted.
Statistical Analyses
We assessed the rate of each imaging and diagnostic procedure for each enrollee in the 12 months following the index date of a LDCT screening examination from January 1, 2016, to December 31, 2016. The index and date of LDCT was the first LDCT received in 2016. We calculated the number of beneficiaries with a charge for each of the outcomes and repeated the analyses for the control group. For the control patients, we assigned each the same date as the LDCT examination for their matched case, and followed for 12 months after that. We assessed whether patients had imaging or a procedure during the 12-month follow-up period. Each test and procedure was conducted separately. For those who received the same test or procedure multiple times in the follow-up period, we only counted them once in calculating rates. The non-LDCT control group was matched by age, sex, number of comorbidities, and index month in those patients not undergoing LDCT and with complete insurance coverage in 12 months after the first day of the index month. The proportion of patients with CT scan, PET, MRI, bronchoscopy, percutaneous biopsy, mediastinoscopy, thoracoscopy, and thoracotomy was compared between the LDCT and control group. The 95% CIs for those proportions and adjusted rate was obtained by Wald method. We also assessed rates of follow-up imaging and procedures stratified by enrollee characteristics.
Finally, we created a cohort of patients undergoing LDCT in which we could separate them by whether the LDCT scan was the initial LDCT vs a subsequent follow-up test. We created a cohort of those who received LDCT in 2016 and who had insurance coverage in the 18 months before LDCT imaging (n = 6,993). We then stratified by whether the enrollees had any chest imaging in the 18 months before the index LDCT and calculated rates of imaging and procedures in the subsequent 12 months for each group. Initial LDCT scan (n = 5,971) was defined as an index LDCT without chest CT scan, MRI, or PET imaging in 18 months before the index date. Follow-up LDCT had at least one type of chest imaging completed 18 months before the index LDCT.
Results
Table 1 summarizes the characteristics of the 11,520 beneficiaries who underwent LDCT testing from January 1, 2016, to December 31, 2016. Approximately one-half were men; 30% were age 55 to 64 years and 70% were 65 to 80 years; and more than one-half had more than two comorbidities. The South Atlantic region was overrepresented with 30% of the total, reflecting both the higher number of enrollees in that area and their higher rate of LDCT screening.10
Table 1.
Characteristics of Patients Who Underwent LDCTa for Lung Cancer Screening in 2016
| Characteristic | No. Enrollees With LDCT (%) |
|---|---|
| All | 11,520 (100) |
| Age, y | |
| 55-59 | 1,479 (12.84) |
| 60-64 | 1,922 (16.68) |
| 65-69 | 3,902 (33.87) |
| 70-74 | 3,198 (27.76) |
| 75-77 | 902 (7.83) |
| 78-80 | 117 (1.02) |
| Sex | |
| Female | 5,651 (49.05) |
| Male | 5,869 (50.95) |
| Region | |
| New England | 884 (7.67) |
| Middle Atlantic | 1,119 (9.71) |
| South Atlantic | 3,503 (30.41) |
| East North Central | 1,799 (15.62) |
| West North Central | 1,458 (12.66) |
| East South Central | 637 (5.53) |
| West South Central | 514 (4.46) |
| Mountain | 762 (6.61) |
| Pacific | 811 (7.04) |
| Unknown | 33 (0.29) |
| No. comorbiditiesb | |
| 0 | 2,424 (21.04) |
| 1 | 2,788 (24.20) |
| 2 | 2,245 (19.49) |
| 3 | 1,509 (13.10) |
| 4+ | 2,554 (22.17) |
LDCT, low-dose CT.
LDCT for lung cancer screening was identified by Current Procedural Terminology codes G0297 or S8032.
Elixhauser comorbidity score is the number of any of the following listed comorbidities: chronic pulmonary disease, congestive heart failure, valvular disease, pulmonary circulation disorders, peripheral vascular disorders, hypertension, paralysis, other neurological disorders, diabetes-uncomplicated, diabetes-complicated, hypothyroidism, renal failure, liver disease, peptic ulcer disease excluding bleeding, AIDS, lymphoma, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis/collagen vascular diseases, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychoses, and depression.9
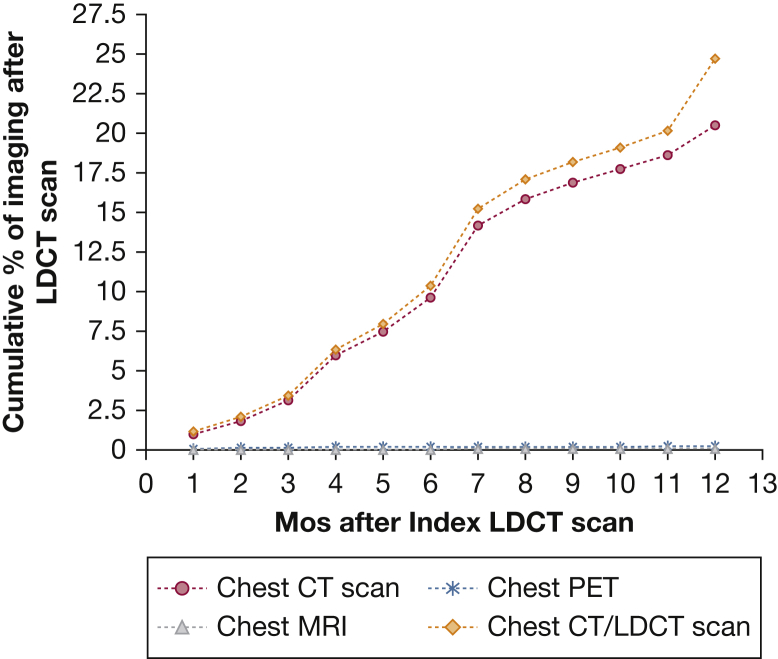
Figure 1 graphs the percent of those receiving LDCT who underwent diagnostic CT, MRI, or PET scanning of the chest in the 12 months after the index LDCT scan. More than 98% of the follow-up imaging was CT scans, with 20.7% of enrollees receiving CT scans, 0.3% chest PET scans, and 0.05% receiving chest MRI scans in the year after LDCT testing. The largest increases in follow-up CT scans were between 3 and 4 months and 6 and 7 months after the LDCT.
Figure 1.
Cumulative percentage of chest CT, MRI, and PET scans in the 12 months after LDCT. LDCT = low-dose CT.
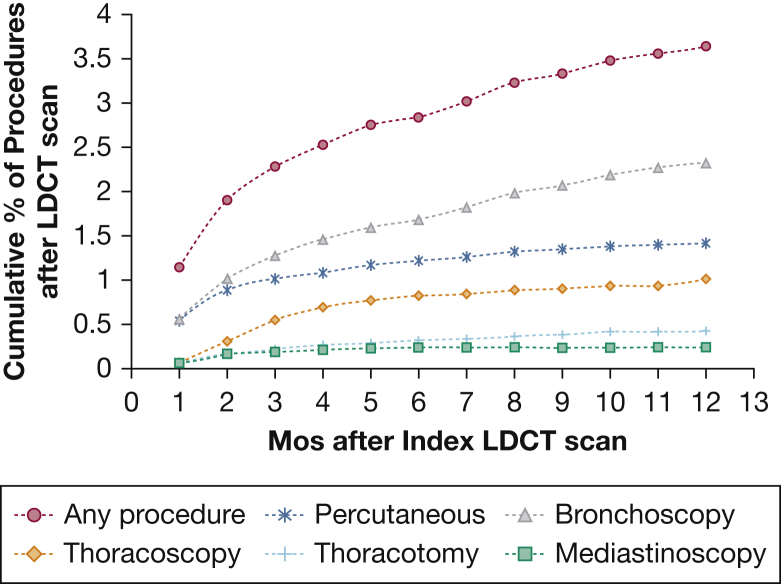
Figure 2 graphs the rate of chest procedures in the 12 months after LDCT, including bronchoscopy, percutaneous biopsy, thoracoscopy, mediastinoscopy, and thoracotomy. The cumulative rate of any of those procedures was 3.7% within a year of LDCT. Bronchoscopy was highest at 2.3% and mediastinoscopy was lowest at 0.2%. As a comparison, we also examined the rates of chest imaging and procedures in age-, sex-, and comorbidity-matched enrollees who did not undergo LDCT. The 12-month rates of follow-up imaging or procedures for the LDCT and control groups are presented in Table 2. The final column in Table 2 presents the adjusted rates, which are the rates in the LDCT population minus the rates in the non-LDCT control patients. The adjusted rate of any chest imaging, not including repeat LDCT scan, was 13.8%. In a sensitivity analysis, we included a repeat LDCT scanning as part of the follow-up imaging. In that case, the adjusted rate for any chest imaging was 17.7%. The adjusted rate of undergoing any invasive procedure was 3.1% (Table 2).
Figure 2.
Cumulative percentage of bronchoscopy, thoracotomy, thoracoscopy, mediastinoscopy, or percutaneous biopsy in 12 months after LDCT scan. See Figure 1 legend for expansion of abbreviation.
Table 2.
Rates of Testing 12 Mo in the LDCT Cohort and in a Matched Control Cohort That Did Not Undergo LDCT
| Test | LDCT (n = 11,520)a | Control Patients (n = 11,520)b | Adjusted Rate (95% CIc) |
|---|---|---|---|
| Any chest imaging (not including LDCT) | 20.66 (19.92-21.40) | 6.90 (6.43-7.37) | 13.76 (12.88-14.64) |
| Any chest imaging (including LDCT) | 24.86 (24.07-25.65) | 7.15 (6.68-7.63) | 17.71 (16.78-18.64) |
| CT | 20.56 (19.81-21.30) | 6.79 (6.32-7.25) | 13.77 (12.89-14.65) |
| PET/MRI | 0.29 (0.18-0.39) | 0.16 (0.08-0.23) | 0.13 (0.00-0.26) |
| Any procedure | 3.71 (3.36-4.06) | 0.57 (0.43-0.72) | 3.13 (2.75-3.51) |
| Bronchoscopy | 2.33 (2.05-2.61) | 0.32 (0.21-0.43) | 2.01 (1.70-2.31%) |
| Percutaneous biopsy | 1.48 (1.26-1.71) | 0.19 (0.11-0.28) | 1.29 (1.05-1.54) |
| Mediastinoscopy | 0.23 (0.14-0.33) | 0.01 (0.00-0.03) | 0.23 (0.13-0.32) |
| Thoracoscopy | 1.01 (0.82-1.19) | 0.07 (0.02-0.12) | 0.94 (0.74-1.13) |
| Thoracotomy | 0.43 (0.30-0.55) | 0.04 (0.00-0.09) | 0.38 (0.25-0.52) |
Data are presented as percent (95% CI). See Table 1 legend for expansion of abbreviation.
LDCT cohort consisted of LDCT identified by Current Procedural Terminology codes G0297 or S8032. Enrollees were aged 55 to 80 y and had complete insurance coverage 12 mo after LDCT.
Control patients: among those patients with no LDCT scan in 2016, subjects were matched to the LDCT group by age, sex, and number of comorbidities.
95% CI for difference was calculated by Wald method.
We repeated the analyses of adjusted rates for follow-up imaging and procedures, stratified by enrollee characteristics. Rates of imaging were higher in the oldest enrollees, women, and those with fewer comorbidities, and rates also differed by region (e-Table 2). There were no significant differences in rates of follow-up procedures by enrollee characteristics (e-Table 3).
We also examined the rates of follow-up imaging and procedures stratified by whether the index LDCT was an initial LDCT or a follow-up LDCT examination (Table 3). This analysis was limited to the enrollees who had insurance in the 18 months before LDCT testing. The adjusted rates of any imaging follow-up within 12 months was 15.3% after an initial LDCT vs 13.7% after a follow-up LDCT scan. For any procedure, the adjusted rate was 3.3% after an initial LDCT and 2.3% after a follow-up LDCT scan.
Table 3.
Comparison of Follow-Up Imaging and Procedures Within 12 Months on the Basis of Initial or Follow-Up LDCT
| Test | LDCT (n = 6,993)a |
|||
|---|---|---|---|---|
| Initial LDCT (n = 5,971)b |
Follow-Up LDCT (n = 1,022)c |
|||
| % With Further Evaluation | Adjusted Rate (95% CI)d | % With Further Evaluation | Adjusted Rate (95% CI)d | |
| Any chest imaging (not including LDCT) | 20.70 (19.67-21.73) | 15.26 (14.06-16.45) | 18.79% (16.39-21.19) | 13.70% (10.85-16.54) |
| Any chest imaging (including LDCT) | 24.77% (23.67-25.87) | 19.13% (17.87-20.38) | 23.78% (21.16-26.39) | 18.10% (15.03-21.17) |
| CT | 20.60% (19.57-21.63) | 15.27% (14.08-16.46) | 18.69% (16.29-21.08) | 13.80% (10.97-16.63) |
| PET/MRI | 0.29% (0.14-0.22) | 0.10% (-0.09 to 0.29) | 0.20% (0-0.47) | 0% (-0.48 to 0.48) |
| Any procedures | 3.94% (3.43-4.44) | 3.32% (2.77-3.86) | 2.94% (1.85-4.02) | 2.25% (1.00-3.50) |
| Bronchoscopy | 2.46% (2.06-2.86) | 2.04% (1.60-2.49) | 1.86% (1.03-2.69) | 1.37% (0.34-2.40) |
| Percutaneous | 1.67% (1.34-2.01) | 1.49% (1.13-1.85) | 0.78% (0.24-1.33) | 0.68% (0.01-1.36) |
| Mediastinoscopy | 0.27% (0.13-0.40) | 0.27% (0.12-0.42) | 0.10% (0-0.29) | 0.10% (-0.19 to 0.39) |
| Thoracoscopy | 1.22% (0.94-1.51) | 1.17% (0.87-1.47) | 0.59% (0.11-1.06) | 0.49% (-0.11 to 1.09) |
| Thoracotomy | 0.54% (0.35-0.73) | 0.50% (0.29-0.71) | 0.20% (0-0.47) | 0.20% (-0.17 to 0.56) |
Data are presented as percent (95% CI). See Table 1 legend for expansion of abbreviation.
Patients undergoing LDCT are aged 55 to 80 y, with complete insurance coverage in 18 mo before and 12 mo after LDCT.
Initial LDCT: those without chest imaging (chest CT, chest MRI/PET) in 18 mo before the index LDCT in 2016.
Follow-up LDCT: those with chest imaging (chest CT, chest MRI/PET) in 18 mo before the index LDCT in 2016.
Adjusted rate is the difference between the LDCT cohort and the control cohort.
Discussion
After adjusting for the background rates of testing, we estimated that 13.8% of enrollees in a commercial insurance plan underwent additional chest imaging and 3.1% underwent chest procedures in the 12 months after LDCT testing in the community. If repeat LDCT examinations are included in the count of additional chest imaging, the estimate of additional chest imaging increased to 17.7%.
Our data may overestimate the true rates of imaging and procedures solely related to findings on LDCT scan. For instance, some of the follow-up imaging and procedures in our cohort presumably were for reasons unrelated to the LDCT imaging. To help account for this, we estimated the “background rate” of imaging and procedures by assessing an age-, sex-, and comorbidity-matched control population that did not undergo LDCT and subtracted those rates from the observed rates in the LDCT population to obtain adjusted rates. In addition, some follow-up testing may have been obtained to evaluate an incidental finding on LDCT imaging unrelated to possible lung cancer. We could not estimate the rate of incidental findings because we had no access to the LDCT readings. Previous studies of LDCT screening have suggested rates of “actionable incidental findings” in the range of 8% to 20%, which can lead to other types of follow-up testing such as imaging and procedural tests of cardiovascular status.11, 12, 13, 14
The NLST reported follow-up testing (21.7% additional imaging, 0.6% percutaneous biopsy, 1.2% bronchoscopy, 0.2% mediastinoscopy, 0.3% thoracoscopy, and 0.8% thoracotomy) for the 12 months following an LDCT.15 Our findings on adjusted rates of chest imaging were lower than in the NLST (13.8% for follow-up CT scan, MRI, or PET vs 21.7% reported in the NLST). For procedures, however, the adjusted rates of follow-up in our study for bronchoscopy, percutaneous biopsy, and thoracoscopy were 58% to 210% higher than in the NLST, whereas mediastinoscopy was similar and thoracotomy was 43% lower. However, direct comparisons of our results to those of the NLST are problematic for several reasons. First, the rates of follow-up testing for the NLST were following the initial LDCT. Second, the NLST only reported follow-up testing that occurred to evaluate a positive LDCT screen for possible malignancy. Any evaluation following an LDCT classified as “negative with minor abnormalities” or with incidental findings, “negative with clinically significant abnormalities not suspicious for lung cancer,” were not included. Also, any test that was unrelated to LDCT findings was not included in the estimates of follow-up testing in the NLST.
Reasons for low follow-up rates of additional chest imaging are likely multifactorial. The Lung-RADS reporting structure was developed and refined after publication of the NLST.16,17 Implementation of Lung-RADS standardizes reporting of screen-detected nodules and recommendations for follow-up management,17 which has been shown to reduce subsequent testing,16 and may be responsible for the lower rates of follow-up imaging found in our study. The low rates of imaging may also be a function of low adherence to recommendations after LDCT scan in the community compared with clinical trials’ LDCT scan.2,4,18, 19, 20, 21 This may also account for the lower rates of follow-up testing of follow-up vs initial LDCT examinations compared with NLST data in which rates were similar after the second LDCT and lower for the third LDCT scan.2
Our study has several limitations. We can only report on follow-up testing received. It is possible that there were recommendations for further testing after LDCT scans were not completed. We did not assess the complications associated with each follow-up test. Huo et al22 recently studied commercial insurance data and found higher complication rates from invasive procedures such as bronchoscopy and percutaneous biopsy than reported in clinical trials. These rates are not directly applicable to complications after LDCT screening because the procedures could have been done for any reason, not as a follow-up to LDCT screening. Another limitation is that there was not a separate CPT code for LDCT before mid-2015 (LDCT scans shared the same code as diagnostic chest CT scans before that date). This precludes study of use of LDCT screening in the community using administrative charge data before 2016. A major limitation is that the implementation of LDCT screening has led to far fewer patients screened than initially anticipated.10,23,24 Among Medicare enrollees estimated to qualify for LCS, only about 5% underwent LDCT screening in 2016.23 This reduced sample size precludes rigorous study of downstream complications from LDCT imaging or study of variation in rates of downstream imaging and testing by facility.25,26 However, LCS uptake is expected to increase over time, with growth already seen in the number of facilities offering LCDT LCS. In 2018, there were more than 153 facilities accredited by the American College of Radiology,27 more than 600 centers with an LCS “center of excellence” designation,28 and an unknown number of LDCT scans performed at other facilities. As LDCT screening increases, follow-up should be monitored.
A major concern about widespread implementation of LCS was that even among centers participating in LCS trials, the false-positive rates, subsequent imaging, and invasive testing were highly variable and, outside a rigorous environment, even higher complication rates may occur.6, 7, 8,29 To address these concerns, the US Preventive Services Task Force recommended and Centers for Medicare & Medicaid Services adopted several LCS processes, including a mandate for a separate shared decision-making visit to ensure appropriate candidates for screening are selected, rigorous radiologic certification criteria for LDCT facilities, and participation in a national registry for all patients receiving LDCT imaging.3,26 However, it is not clear that those processes are being used. As an example, we previously reported that only 9.0% of Medicare enrollees who underwent LDCT testing in 2016 had a prior visit for shared decision making.30
To our knowledge, this is the first report of rates of follow-up testing in a national sample. It will be important to assess rates in other community samples and to assess rates of serious complications related to testing, particularly if the rates of LDCT testing increase over time. It is not clear that there are mechanisms in place to ensure follow-up of those who are enrolled in the Centers for Medicare & Medicaid Services-mandated registry of individuals undergoing LDCT. In the absence of that, observational studies using administrative charge data may provide valuable information on the effectiveness of LDCT in the community.
In conclusion, rates of subsequent testing after LDCT scan in the community are low. Without further detailed clinical information, the appropriateness of the follow-up testing in community practice and the downstream outcomes of evaluation cannot be determined. Additional studies reporting on larger populations will be required to validate these findings and explore mechanisms for these differences.
Acknowledgments
Author contributions: J. G. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. All authors contributed to the study concept and design. J. G. acquired data. All authors undertook analysis and interpretation of data. J. G. and S. P. E. N. drafted the manuscript. All authors undertook critical revision of the manuscript for important intellectual content. J. Z. and Y.-F. K. performed statistical analysis. J. G. obtained funding. J. Z. provided administrative, technical, or material support.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by the Cancer Prevention and Treatment Institute of Texas [Grant RP160674] and the National Institutes of Health [Grants K05 CA134923, P30 AG024832, and UL1TR001439].
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer V.A., US Preventive Services Task Force Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 4.NELSON study shows CT screening for nodule volume management reduces lung cancer mortality by 26 percent in men [press release]. IASLC 19th World Conference on Lung Cancer (WCLC). September 25, 2018.
- 5.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102(23):1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzone P.J., Silvestri G.A., Patel S. Screening for lung cancer: CHEST Guideline and Expert Panel Report. Chest. 2018;153(4):954–985. doi: 10.1016/j.chest.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Bach P.B., Mirkin J.N., Oliver T.K. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopal M., Abdullah S.E., Grady J.J., Goodwin J.S. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol. 2010;5(8):1233–1239. doi: 10.1097/JTO.0b013e3181e0b977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Okereke IC NS, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis. In press. [DOI] [PMC free article] [PubMed]
- 11.Nguyen X.V., Davies L., Eastwood J.D., Hoang J.K. Extrapulmonary findings and malignancies in participants screened with chest CT in the National Lung Screening Trial. J Am Coll Radiol. 2017;14(3):324–330. doi: 10.1016/j.jacr.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 12.Rampinelli C., Preda L., Maniglio M. Extrapulmonary malignancies detected at lung cancer screening. Radiology. 2011;261(1):293–299. doi: 10.1148/radiol.11102231. [DOI] [PubMed] [Google Scholar]
- 13.Kucharczyk M.J., Menezes R.J., McGregor A., Paul N.S., Roberts H.C. Assessing the impact of incidental findings in a lung cancer screening study by using low-dose computed tomography. Can Assoc Radiol J. 2011;62(2):141–145. doi: 10.1016/j.carj.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 14.van de Wiel J.C., Wang Y., Xu D.M. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol. 2007;17(6):1474–1482. doi: 10.1007/s00330-006-0532-7. [DOI] [PubMed] [Google Scholar]
- 15.National Lung Screening Trial Research T., Church T.R., Black W.C. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinsky P.F., Gierada D.S., Black W. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–491. doi: 10.7326/M14-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip R., Henschke C.I., Yankelevitz D.F., Smith J.P. CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology. 2014;273(2):591–596. doi: 10.1148/radiol.14132950. [DOI] [PubMed] [Google Scholar]
- 18.Gould M.K., Sakoda L.C., Ritzwoller D.P. Monitoring lung cancer screening use and outcomes at four cancer research network sites. Ann Am Thorac Soc. 2017;14(12):1827–1835. doi: 10.1513/AnnalsATS.201703-237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasher P., Tanner N., Yeager D., Silvestri G. Adherence to annual lung cancer screening within the Veterans Health Administration lung cancer screening demonstration project. Chest. 2018;154(4):636A–637A. doi: 10.1016/j.chest.2020.04.063. [DOI] [PubMed] [Google Scholar]
- 20.Strengthening adherence in lung cancer screening. https://lungcanceralliance.org/wp-content/uploads/2017/09/Strengthening-Adherence.pdf
- 21.Lam V.K., Miller M., Dowling L., Singhal S., Young R.P., Cabebe E.C. Community low-dose CT lung cancer screening: a prospective cohort study. Lung. 2015;193(1):135–139. doi: 10.1007/s00408-014-9671-9. [DOI] [PubMed] [Google Scholar]
- 22.Huo J., Xu Y., Sheu T., Volk R.J., Shih Y.T. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern Med. 2019;179(3):324–332. doi: 10.1001/jamainternmed.2018.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishi S.Z.J., Kuo Y.F., Goodwin J.S. Use of lung cancer screening with low-dose computed tomography in the Medicare population. Mayo Clin Proc. 2019;179(5):716–718. doi: 10.1016/j.mayocpiqo.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen K., Schertz K., Rubin N., Begnaud A. Incidental findings in a decentralized lung cancer screening program. Ann Am Thorac Soc. 2019;16(9):1198–1201. doi: 10.1513/AnnalsATS.201812-908RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 27.ACR Designated Lung Cancer Screening Centers. 2018. https://www.acraccreditation.org/accredited-facility-search
- 28.Screening Centers of Excellence (SCOE) https://lungcanceralliance.org/risk-early-detection/screening-centers/
- 29.Wiener R.S., Gould M.K., Arenberg D.A. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192(7):881–891. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodwin J.S., Nishi S., Zhou J., Kuo Y.F. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179(5):716–718. doi: 10.1001/jamainternmed.2018.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.