Abstract
Background
Relative enlargement of the pulmonary artery (PA) on chest CT imaging is associated with respiratory exacerbations in patients with COPD or cystic fibrosis. We sought to determine whether similar findings were present in patients with asthma and whether these findings were explained by differences in ventricular size.
Methods
We measured the PA and aorta diameters in 233 individuals from the Severe Asthma Research Program III cohort. We also estimated right, left, and total epicardial cardiac ventricular volume indices (eERVVI, eELVVI, and eETVVI, respectively). Associations between the cardiac and PA measures (PA-to-aorta [PA/A] ratio, eERVVI-to-eELVVI [eRV/eLV] ratio, eERVVI, eELVVI, eETVVI) and clinical measures of asthma severity were assessed by Pearson correlation, and associations with asthma severity and exacerbation rate were evaluated by multivariable linear and zero-inflated negative binomial regression.
Results
Asthma severity was associated with smaller ventricular volumes. For example, those with severe asthma had 36.1 mL/m2 smaller eETVVI than healthy control subjects (P = .003) and 14.1 mL/m2 smaller eETVVI than those with mild/moderate disease (P = .011). Smaller ventricular volumes were also associated with a higher rate of asthma exacerbations, both retrospectively and prospectively. For example, those with an eETVVI less than the median had a 57% higher rate of exacerbations during follow-up than those with eETVVI greater than the median (P = .020). Neither PA/A nor eRV/eLV was associated with asthma severity or exacerbations.
Conclusions
In patients with asthma, smaller cardiac ventricular size may be associated with more severe disease and a higher rate of asthma exacerbations.
Trial Registry
ClinicalTrials.gov; No.: NCT01761630; URL: www.clinicaltrials.gov
Key Words: asthma, CT imaging, heart
Abbreviations: ACT, Asthma Control Test; AT%, percentage of lung occupied by air trapping; eELVVI, estimated epicardial left ventricular volume index; eERVVI, estimated epicardial right ventricular volume index; eETVVI, estimated epicardial total ventricular volume index; eRV/eLV, estimated right ventricular-to-estimated left ventricular volume ratio; LAA%, percentage of lung occupied by low-attenuation area; PA, pulmonary artery; PA/A, pulmonary artery-to-aorta diameter ratio; SARP, Severe Asthma Research Program
FOR EDITORIAL COMMENT, SEE PAGE 243
In patients with COPD or cystic fibrosis, a larger diameter of the pulmonary artery (PA) on chest CT imaging may be a marker of pulmonary hypertension and adverse outcomes.1, 2, 3 Similarly, the ratio of the right ventricular volume to the left ventricular volume may be used as a marker of disease severity in COPD.4, 5, 6 However, little work has been done to explore these relationships in patients with asthma.
We have shown that loss of the peripheral pulmonary vasculature, a finding termed pulmonary vascular pruning, is associated with asthma severity and exacerbations.1,7 Pulmonary vascular pruning is also present in patients with emphysema, and may be one of several underlying causes for the changes seen in the heart and central pulmonary vasculature of patients with COPD.1,8 These findings raise the question of whether patients with asthma may also have measurable changes to their cardiac ventricles and central pulmonary vasculature on chest CT imaging.
We have developed an automated method to estimate epicardial cardiac chamber size on noncontrast, non-ECG-gated chest CT scans.4,6 We hypothesized that both the pulmonary artery-to-aorta diameter ratio (PA/A) and the ratio of the estimated right ventricular volume to the estimated left ventricular volume (eRV/eLV) measured by this approach may be associated with disease severity and respiratory exacerbations in patients with asthma. We also sought to explore whether any association between eRV/eLV and asthma severity was driven by relative enlargement of the right ventricle or by a relative decrease in size of the left ventricle.
Materials and Methods
Cohort Description
The Severe Asthma Research Program (SARP) is a prospective, multicenter investigation designed to improve the understanding of severe asthma. For this study we used data from adult participants with both severe and nonsevere asthma from the third phase of SARP (SARP III) as well as from a smaller group of participants characterized as healthy control subjects.9 Additional details regarding the cohort, including clinical definitions, are available in the online article (Supplemental Methods, e-Appendix 1).9, 10, 11, 12 All participants provided informed consent, and the study was approved by the institutional review board at each center (e-Table 1).
CT Image Acquisition and Analysis
Volumetric, noncontrast, non-ECG-gated CT scans of the chest were obtained as previously described.7,13,14 Similarly, lung segmentation and the assessment of imaging covariates including the percentage of lung occupied by low-attenuation area (LAA%), the percentage of lung occupied by air trapping (AT%), PA diameter, aorta diameter, and the presence of emphysema were performed according to previously published methods. Additional details regarding these methods are available in the online article (Supplemental Methods, e-Appendix 1).
Cardiac ventricular size was estimated on the basis of a previously described statistical model of the heart that uses 50 modes of variation of the cardiac structure to describe anatomical variability.4,6,15 Briefly, the model was developed with anatomical segmentations of four-dimensional (three-dimensional plus time) data from 138 subjects in an independent cohort that included individuals with and without a broad range of diseases. A trained operator begins the supervised segmentation by manually fitting the average shape model to the CT scan. Surface fitting is then performed by an active shape model method that deforms the model according to a probabilistic map of the boundary between pericardial fat and myocardium (ie, the epicardial surface). Because of limitations related to the lack of intravenous contrast, the ventricular volumes were defined on the basis of epicardial surface fitting and therefore included both the wall and chamber volumes.4,6 The eRV/eLV was defined as the estimated epicardial right ventricular volume divided by the estimated epicardial left ventricular volume. All of the cardiac volumes were normalized by patient body surface area (in square meters) to yield the estimated volume indices, that is, the estimated epicardial right ventricular volume index (eERVVI), the estimated epicardial left ventricular volume index (eELVVI), and the estimated epicardial total ventricular volume index (eETVVI).16
Statistical Analysis
Univariate associations of PA/A, eRV/eLV, eERVVI, eELVVI, and eETVVI with duration of disease (age at enrollment – age at diagnosis), inspiratory CT scan-measured lung volume (normalized by height), LAA%, AT%, prebronchodilator percent predicted FEV1, prebronchodilator percent predicted FVC, Asthma Control Test (ACT) score, peripheral percent eosinophils, and bronchoreversibility [defined as (postalbuterol FEV1 − prealbuterol FEV1)/(prealbuterol FEV1)] were analyzed by Pearson correlation. In addition, associations between the pulmonary vascular and cardiac measures with childhood diagnosis of asthma (defined as patient-reported diagnosis of asthma at age < 18) were assessed by t tests.
Associations between asthma severity and the PA/A and cardiac measures were analyzed by multivariable linear regression with adjustments for height-normalized, CT scan-measured lung volume as well as for age, sex, race, prebronchodilator percent predicted FEV1, BMI, and systolic BP. Associations between exacerbations (both retrospective [in the year enrollment] and prospective [during follow-up]) and the PA/A and cardiac measures (dichotomized as described above) were evaluated by zero-inflated negative binomial regression with adjustments for the same covariates as the linear regression analyses as well as for ACT score and asthma severity category.2,17 Analyses of prospective exacerbations included a time scale factor and were additionally adjusted for a history of asthma exacerbation in the year before enrollment.18 The exacerbation analyses were performed both in the entire cohort and in the subgroup with severe asthma.
Multiple secondary analyses were performed as detailed in the online article (Supplemental Methods, e-Appendix 1). These included the following: multivariable logistic regression analyses to evaluate the association between PA/A (dichotomized at 1) and cardiac measures (dichotomized at their medians) and the presence of severe asthma (yes/no), multivariable linear regression analyses to evaluate the association between cardiac size and corticosteroid dose, and secondary analyses of these and the primary outcomes in the subgroup of participants without visually defined emphysema.
All statistical tests were two-sided, and P values < .05 were considered to indicate statistical significance. The analyses were performed with R version 3.5.0.19
Results
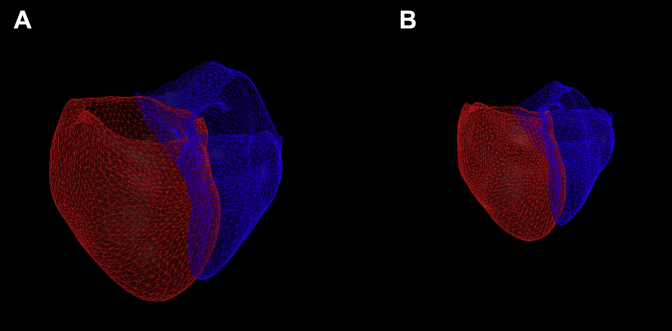
Two hundred and thirty-seven participants had clinical and imaging data available for analysis. Of those, cardiac segmentation was successfully performed on 233, 211 of whom had longitudinal follow-up data available and 185 of whom had expiratory imaging available (Fig 1). Sample cardiac segmentation images are shown in Figure 2. The cohort had a mean age of 46.2 years, was largely white, and had a female predominance (Table 1).
Figure 1.
CONSORT diagram. CONSORT = Consolidated Standards of Reporting Trials.
Figure 2.
Sample cardiac segmentation images. Sample heart segmentation images from participants with mild/moderate asthma (A; cardiac volume, 362.8 mL) and severe asthma (B; cardiac ventricular volume, 152.6 mL). The red surface represents the epicardial surface of the left ventricle, and the blue surface represents the epicardial surface of the right ventricle. Note that the images are shown to scale.
Table 1.
Clinical and Radiologic Characteristics of the Cohort
| Characteristic | Mean | SD | No. | Characteristic | No | % |
|---|---|---|---|---|---|---|
| Clinical Characteristics | ||||||
| Age, y | 46.2 | 14.7 | 233 | Female | 153 | 65.7 |
| Duration of disease, y | 27.6 | 15.0 | 233 | Nonwhite | 93 | 39.9 |
| Height, m | 1.7 | 0.1 | 233 | Healthy control subjects | 10 | 4.3 |
| BMI | 32.9 | 9.0 | 233 | Mild/moderate asthma | 66 | 28.3 |
| Systolic BP, mm Hg | 124.7 | 15.9 | 233 | Severe asthma | 157 | 67.4 |
| Heart rate | 72.8 | 12.0 | 233 | Exacerbation in year before enrollment | 126 | 54.1 |
| Prebronchodilator FEV1 % predicted | 72.9 | 20.8 | 233 | Exacerbation during follow-up | 125 | 53.7 |
| Prebronchodilator FVC % predicted | 84.6 | 18.0 | 233 | |||
| Asthma Control Test score | 16.3 | 5.0 | 223 | |||
| Duration of follow-up, y | 2.9 | 0.8 | 211 | |||
| Prospective exacerbation rate, per year | 0.9 | 1.2 | 211 | |||
| Radiologic Characteristics | ||||||
| Percentage of lung with density < –950 HU, LAA% | 2.1 | 2.3 | 233 | Visual paraseptal emphysema | 7 | 0.9 |
| Pulmonary artery-to-aorta diameter ratio | 0.9 | 0.1 | 233 | Visual centrilobular emphysema | 5 | 2.2 |
| CT-measured lung volume, L | 5.1 | 1.2 | 233 | |||
| Normalized by height, m | 3.0 | 0.6 | 233 | |||
| Left ventricular volume, mL | 173.3 | 43.8 | 233 | |||
| Normalized by body surface area, mL/m2, eELVVI | 104.3 | 24.2 | 233 | |||
| Right ventricular volume, mL | 106.2 | 28.7 | 233 | |||
| Normalized by body surface area, mL/m2, eERVVI | 63.9 | 16.1 | 233 | |||
| Total ventricular volume, mL | 279.5 | 70.8 | 233 | |||
| Normalized by body surface area, mL/m2, eETVVI | 168.2 | 39.2 | 233 | |||
| RV volume-to-LV volume ratio, eRV/eLV | 0.61 | 0.07 | 233 |
eELVVI = estimated epicardial left ventricular volume index; eERVVI = estimated epicardial right ventricular volume index; eETVVI = estimated epicardial total ventricular volume index; eRV/eLV = estimated right ventricular volume to estimated left ventricular volume ratio; HU = Hounsfield unit; LAA = low-attenuation area.
As shown in Table 2, PA/A was weakly inversely correlated with the duration of disease and AT%, and weakly directly correlated with prebronchodilator percent predicted FVC. The left, right, and total ventricular volumes were weakly inversely correlated with CT-measured lung volume and LAA%. Notably, no statistically significant associations were present between AT% and cardiac volume. In addition, no association was found between childhood diagnosis of asthma and any of the cardiac measures (e-Figs 1, 2).
Table 2.
Univariate Associations
| Clinical/Radiologic Characteristic | PA/A |
eRV/eLV |
eELVVI |
eERVVI |
eETVVI |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Duration of disease | –0.19 | .006 | –0.09 | .197 | –0.09 | .197 | 0.13 | .048 | 0.12 | .081 |
| CT scan-measured lung volume | 0.03 | .654 | 0.02 | .784 | –0.23 | < .001 | –0.26 | < .001 | –0.26 | < .001 |
| Percentage of lung with density < –950 HU, LAA% | –0.07 | .321 | 0.04 | .537 | –0.27 | < .001 | –0.31 | < .001 | –0.30 | < .001 |
| Air trapping | –0.17 | .024 | 0.04 | .573 | –0.12 | .117 | –0.13 | .079 | –0.13 | .084 |
| Percent predicted FEV1 | 0.08 | .197 | 0.05 | .462 | –0.06 | .354 | –0.10 | .123 | –0.09 | .184 |
| Percent predicted FVC | 0.13 | .041 | 0.10 | .111 | –0.09 | .188 | –0.15 | .020 | –0.13 | .049 |
| Asthma Control Test score | 0.002 | .979 | 0.11 | .117 | –0.02 | .760 | –0.64 | .339 | –0.05 | .474 |
| Peripheral percent eosinophils | –0.09 | .187 | –0.04 | .512 | –0.11 | .095 | –0.12 | .067 | –0.12 | .075 |
| Bronchoreversibility | 0.06 | .387 | 0.07 | .311 | 0.03 | .637 | 0.06 | .390 | 0.042 | .520 |
CT scan-measured lung volume normalized by height in meters; all cardiac volumes normalized (indexed) by body surface area in square meters; bronchoreversibility defined as (postalbuterol FEV1 − prealbuterol FEV1)/prealbuterol FEV1. PA/A = pulmonary artery-to-aorta diameter ratio. See Table 1 legend for expansion of other abbreviations.
In the multivariable analyses, there was no association between asthma severity and PA/A or eRV/eLV (Table 3). However, participants with severe asthma had smaller left ventricular, right ventricular, and biventricular volumes than healthy control subjects and smaller ventricular volumes than those with mild/moderate asthma (Table 4). In addition, those with mild/moderate asthma also had smaller right ventricular volume than healthy control subjects (Table 4). Similarly, smaller ventricular size was associated with higher odds of having severe asthma (e-Table 2).
Table 3.
Multivariable Associations Between Asthma Severity, Pulmonary Artery-to-Aorta Ratio, and Right Ventricular Volume-to-Left Ventricular Volume Ratio
| Asthma Severity | Pulmonary Artery/Aorta Diameter |
Estimated Right Ventricular/Estimated Left Ventricular Volume |
||||
|---|---|---|---|---|---|---|
| Difference | 95% CI | P Value | Difference | 95% CI | P Value | |
| Healthy Control Subjects as Reference | ||||||
| Healthy control subjects | Reference | Reference | ||||
| Mild/moderate asthma | –0.031 | –0.126 to 0.063 | .512 | –0.023 | –0.066 to 0.021 | .306 |
| Severe asthma | –0.057 | –0.153 to 0.039 | .246 | –0.031 | –0.075 to 0.013 | .171 |
| Mild/Moderate Asthma as Reference | ||||||
| Mild/moderate asthma | Reference | Reference | ||||
| Severe asthma | –0.025 | –0.069 to 0.019 | .260 | –0.008 | –0.028 to 0.012 | .427 |
Multivariable models adjusted for age, sex, race, BMI, systolic BP, percent predicted FEV1, the percentage of lung occupied by low-attenuation area, and height-normalized, CT scan-measured lung volume.
Table 4.
Multivariable Associations Between Asthma Severity and Cardiac Ventricular Volumes
| Asthma Severity | eELVVI |
eERVVI |
eETVVI |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference | 95% CI | P Value | Difference | 95% CI | P Value | Difference | 95% CI | P Value | |
| Healthy Control Subjects as Reference | |||||||||
| Healthy control subjects | Reference | Reference | Reference | ||||||
| Mild/moderate asthma | –12.1 | –26.3 to 2.1 | .095 | –9.9 | –19.7 to –0.1 | .047 | –22.0 | –45.3 to 1.24 | .063 |
| Severe asthma | –20.3 | –34.8 to –5.9 | .006 | –15.8 | –25.8 to –5.8 | .002 | –36.1 | –59.8 to –12.5 | .003 |
| Mild/Moderate Asthma as Reference | |||||||||
| Mild/moderate asthma | Reference | Reference | Reference | ||||||
| Severe asthma | –8.3 | –14.9 to –1.6 | .015 | –5.9 | –10.4 to –1.3 | .012 | –14.1 | –24.9 to –3.3 | .011 |
Multivariable models adjusted for age, sex, race, BMI, systolic BP, percent predicted FEV1, percentage of lung occupied by low-attenuation area, and height-normalized, CT scan-measured lung volume. See Table 1 legend for expansion of abbreviations.
PA/A and eRV/eLV were not associated with the exacerbation rate in the year before enrollment or during follow-up (Tables 5, 6). However, those individuals with smaller ventricular volumes generally had higher rates of asthma exacerbations. This was true both in the year before enrollment and during follow-up, and these relationships were present in the entire cohort (Table 7) and in the subgroup of individuals with severe asthma (Table 8).
Table 5.
Multivariable Associations Between PA/A and eRV/eLV Measures and Exacerbations in the Entire Cohort
| Radiologic Measure | Incident Rate Ratioa | 95% CI | P Value |
|---|---|---|---|
| Exacerbations in the Year Before Enrollment | |||
| Pulmonary artery-to-aorta diameter ratio | 0.87 | 0.48-1.57 | .635 |
| Estimated right ventricular-to-estimated left ventricular volume ratio | 1.38 | 0.96-1.96 | .079 |
| Exacerbations During Follow-Up | |||
| Pulmonary artery-to-aorta diameter ratio | 1.05 | 0.62-1.79 | .860 |
| Estimated right ventricular-to-estimated left ventricular volume ratio | 0.98 | 0.68-1.42 | .921 |
Multivariable models adjusted for age, sex, race, BMI, systolic BP, low-attenuation area, height-normalized CT scan-measured lung volume, percent predicted FEV1, Asthma Control Test score, and asthma severity (mild/moderate vs severe). Prospective analyses also adjusted for exacerbation reported in the year before enrollment. See Table 1 and 2 legends for expansion of abbreviations.
Incident rate ratios are expressed as those with high vs those with low eRV/eLV (greater than the median vs less than the median) and high vs low PA/A (greater than 1 vs less than 1).
Table 6.
Multivariable Associations Between PA/A and eRV/eLV Measures and Exacerbations in the Subgroup With Severe Asthma
| Radiologic Measure | Incident Rate Ratioa | 95% CI | P Value |
|---|---|---|---|
| Exacerbations in the Year Before Enrollment | |||
| Pulmonary artery-to-aorta diameter ratio | 0.81 | 0.45-1.45 | .480 |
| Estimated right ventricular-to-estimated left ventricular volume ratio | 1.42 | 0.99-2.04 | .057 |
| Exacerbations During Follow-Up | |||
| Pulmonary artery-to-aorta diameter ratio | 1.67 | 1.00-2.81 | .052 |
| Estimated right ventricular-to-estimated left ventricular volume ratio | 0.84 | 0.59-1.20 | .342 |
Multivariable models adjusted for age, sex, race, BMI, systolic BP, low-attenuation area, height-normalized CT scan-measured lung volume, percent predicted FEV1, and Asthma Control Test score. Prospective analyses also adjusted for exacerbation reported in the year before enrollment. See Table 1 and 2 legends for expansion of abbreviations.
Incident rate ratios are expressed as those with high eRV/eLV vs those with low eRV/eLV (greater than the median vs less than the median) and high vs low PA/A (greater than 1 vs less than 1).
Table 7.
Multivariable Associations Between Cardiac Volume Measures and Exacerbations in the Entire Cohort
| Radiologic Measure | Incident Rate Ratioa | 95% CI | P Value |
|---|---|---|---|
| Exacerbations in the Year Before Enrollment | |||
| Estimated epicardial left ventricular volume index | 1.41 | 0.97-2.05 | .069 |
| Estimated epicardial right ventricular volume index | 1.72 | 1.21-2.44 | .003 |
| Estimated epicardial total ventricular volume index | 1.60 | 1.10-2.33 | .015 |
| Exacerbations During Follow-Up | |||
| Estimated epicardial left ventricular volume index | 1.48 | 1.03-2.13 | .035 |
| Estimated epicardial right ventricular volume index | 1.39 | 0.96-2.02 | .079 |
| Estimated epicardial total ventricular volume index | 1.57 | 1.08-2.28 | .020 |
Multivariable models adjusted for age, sex, race, BMI, systolic BP, low-attenuation area, height-normalized CT scan-measured lung volume, percent predicted FEV1, Asthma Control Test score, and asthma severity (mild/moderate vs severe). Prospective analyses also adjusted for exacerbation reported in the year before enrollment.
Incident rate ratios are expressed as those with lower volume compared with those with higher volume dichotomized at the median.
Table 8.
Multivariable Associations Between Cardiac Volume Measures and Exacerbations in the Subgroup With Severe Asthma
| Radiologic Measure | Incident Rate Ratioa | 95% CI | P Value |
|---|---|---|---|
| Exacerbations in the Year Before Enrollment | |||
| Estimated epicardial left ventricular volume index | 1.59 | 1.21-2.51 | .012 |
| Estimated epicardial right ventricular volume index | 1.82 | 1.28-2.59 | .001 |
| Estimated epicardial total ventricular volume index | 1.90 | 1.33-2.73 | < .001 |
| Exacerbations During Follow-Up | |||
| Estimated epicardial left ventricular volume index | 1.35 | 0.94-1.95 | .104 |
| Estimated epicardial right ventricular volume index | 1.41 | 0.99-2.00 | .054 |
| Estimated epicardial total ventricular volume index | 1.55 | 1.08-2.22 | .017 |
Multivariable models adjusted for age, sex, race, BMI, systolic BP, low-attenuation area, height-normalized, CT scan-measured lung volume, percent predicted FEV1, and Asthma Control Test score. Prospective analyses also adjusted for exacerbation reported in the year before enrollment.
Incident rate ratios are expressed as those with lower volume compared with those with higher volume dichotomized at the median.
The distribution of annual average corticosteroid dose is shown in e-Table 4A. In general, there was a trend toward high-dose corticosteroid use being associated with smaller ventricular size, but this reached statistical significance only for eERVVI and eRV/eLV.
Similar results for all of the analyses were found when those with visually defined emphysema were excluded (e-Tables 3, 4B, 5B, 6-12).
Discussion
In this study we found that there was no association between PA/A or eRV/eLV and asthma exacerbations in patients with asthma, nor was PA/A or eRV/eLV associated with asthma severity. However, smaller ventricular volumes were independently associated with an increased rate of both retrospective and prospective asthma exacerbations and with asthma severity.
The lack of association between PA/A and respiratory exacerbations in asthma runs counter to findings in COPD and cystic fibrosis, in which a PA/A ratio greater than 1 has been associated with respiratory exacerbations.2,3,5 This suggests that it may be that parenchymal destruction and regional hypoxemia, which are more prominent features of COPD and emphysema, are the driving forces behind enlargement of the PA/A in COPD, and that regional hyperinflation and decreased ventilation/perfusion matching associated with asthma are insufficient to result in central changes to the pulmonary vasculature.2,5,7,20, 21, 22
Perhaps the more interesting findings in our study are the associations between smaller estimated cardiac ventricular size, asthma severity, and exacerbations. Prior work using echocardiography has shown that asthma is associated with right and left ventricular dysfunction, especially in children.23,24 Clinically, asthma has long been associated with small cardiac size on chest radiography.25,26 Although this latter finding has largely been attributed to air trapping and lung hyperinflation, several autopsy studies have suggested that severe asthma may be associated with an anatomically smaller heart independent of the functional effect of expiratory airflow limitation.25,27,28
There are several reasons why our findings are also unlikely to be related to air trapping and hyperinflation alone. Hyperinflation due to airway disease is thought to result in smaller cardiac size on chest radiography for two primary reasons. The first is a visual effect caused by the lowering of the diaphragm, which results in an anterior rotation of the cardiac apex and clockwise rotation (as viewed from above) of the heart that leads to a smaller visible cross-section on a standard posterior-anterior or anterior-posterior chest radiograph.26,29 This effect is mitigated in our study by the use of three-dimensional ventricular volumes. The second is through increased intrathoracic pressure and decreased venous return.26 In our study, estimated ventricular volume was negatively correlated with CT scan-measured lung volume, and this effect likely does play some role in explaining our findings. However, the strength of that association was very weak, and the estimated ventricular volumes were not associated with air trapping on expiratory CT imaging. In addition, our findings remained present in multivariable analyses adjusted for lung volume, suggesting there may be an explanation for the relationship between heart size and disease severity beyond the physiologic effect of hyperinflation.
One conceivable, albeit speculative, anatomical explanation for the associations between heart size and asthma severity in our study may be that smaller heart size is anatomically associated with smaller airway caliber. This hypothesis might also help explain the lack of correlation between the cardiac measures and other measures of disease severity such as the ACT score, eosinophilia, and bronchoreactivity, as it may be that those patients with asthma with smaller hearts have an asthma subtype primarily related to anatomy and those with eosinophilia may have a subtype more driven by inflammation.30, 31, 32 Arguing against this hypothesis is the lack of association in our study between childhood diagnosis of asthma and cardiac size, as well as prior work demonstrating an association between childhood asthma diagnosis and higher left ventricular mass in asymptomatic adults.33 Additional work, including incorporation of quantitative airway analyses as well as analyses of the peripheral pulmonary vasculature with arterial/venous segmentation, is needed to investigate this more fully.7,34 Another possibility is that either asthma alone, or its treatment with chronic corticosteroids, is associated with cardiac wasting. Although limited, our finding of a possible relationship between steroid dose and ventricular size support this latter hypothesis, as does prior work using a large record linkage database showing the association between corticosteroid use and cardiovascular disease.35 Finally, given the low rate of visually defined emphysema in this cohort and the fact that our findings did change minimally when those with emphysema were excluded, it does not appear that undiagnosed emphysema is the etiology of the findings in this study.
There are a number of limitations to our work. Perhaps most important was the use of an automated technique for the measurement of estimated ventricular volume based on noncontrast, non-ECG-gated CT imaging.4,6 We have found in prior work that the ventricular volume measurements made by the CT scan-based method are correlated with cardiac MRI-based measurements and clinical outcomes in COPD.4,6 However, they are not the same as the measurements made when using the more established techniques of MRI and transthoracic echocardiography.6 Of particular note is the fact that because the CT scans were noncontrast, detection of the intraventricular septum is likely to be one of the least accurate aspects of the CT scan technique, thus limiting inferences drawn from our eRV/eLV findings. In addition, the absolute values of the ventricular estimates differ both from those obtained by MRI and transthoracic echocardiography, likely because the CT scan-based measurements in this study are epicardial and include structures such as the papillary muscles, and also because the CT images are non-ECG gated, leading to what is likely a temporal average of the ventricular volume across the cardiac cycle instead of the typically reported end-diastolic values.36,37 For these reasons we also cannot comment on whether any of the differences seen in cardiac size are primarily due to changes in this endocardial volume or the myocardial thickness/volume. Despite these limitations, should this technique be validated in other cohorts and diseases, then it may be a useful adjuvant tool for the assessment of cardiac size from imaging studies acquired for other indications. This may be of particular use as an epidemiologic tool in large population-based studies in which CT imaging of the chest is acquired but no dedicated cardiac imaging is performed.
Other limitations include the lack of expiratory imaging for all of the participants and the use of inspiratory rather than expiratory imaging for cardiac image analyses. Also, because the CT images used were acquired at several institutions, albeit using a standard research protocol, we cannot exclude the fact that the results may vary due to institution and scanner differences. In addition, because the CT scans were not always obtained on the same day as a study visit in which questions about recent exacerbations were asked, we are unable to account for effects related to a recent asthma exacerbation. It should also be noted that, although the associations between ventricular size and exacerbations were generally consistent, several did not reach statistical significance. Finally, because of limitations in the event rate and overall size of the study, other possible confounders such as bronchoreactivity and eosinophilia could not be controlled for in the multivariable analyses. The study size also limited our investigation into relationships between our findings and other biologic variables such as sex. A variety of future work is needed to overcome the limitations described above, including studies of larger or combined cohorts that use multimodal imaging of the heart and chest, as well as clinical and laboratory measures of disease severity, muscle wasting, and inflammation.38
Conclusions
In summary, our findings in the SARP III cohort, using objective analysis of noncontrast, non-ECG-gated images, suggest that neither PA/A nor eRV/eLV is associated with asthma severity or exacerbations, but that smaller estimated ventricular volume indices may be associated with both asthma severity and exacerbations. If our findings are replicated, they may suggest that severe asthma, or its treatments, are associated with changes in cardiac morphology, potentially opening new avenues of investigation for the etiology and treatment of asthma.
Acknowledgments
Author contributions: S. Y. A., G. V. S.-F., and R. S. J. E. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S. Y. A., G. V. S.-F., G. R. W., E. I., and R. S. J. E. are responsible for the study concept and design. G. V. S.-F., F. N. R., and R. S. J. E. developed the cardiac segmentation tool. S. Y. A. performed the statistical analyses. G. R. W., E. I., and R. S. J. E. supervised the study. All authors contributed to the acquisition, analysis, or interpretation of data. All authors contributed to the drafting of the manuscript. All authors contributed to the intellectual content. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Financial/nonfinancial disclosures: The authors reported to CHEST the following: E. R. B. reports having undertaken clinical trials through his employer, Wake Forest School of Medicine and the University of Arizona, for AstraZeneca, MedImmune, Boehringer Ingelheim, Cephalon/Teva, Genentech, Johnson & Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme. He has also served as a paid consultant for AstraZeneca, MedImmune, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Regeneron, and Sanofi Genzyme outside the submitted work. M. C. reports grants from the National Institutes of Health (NIH) and the American Lung Association during the conduct of the study; personal fees from Aviragen, Boehringer Ingelheim, Boston Scientific, Elsevier, Genentech, GlaxoSmithKline, Nuvaira, and Teva; grants from Amgen, Boehringer Ingelheim, Genentech, Gilead, GlaxoSmithKline, Invion, Medimmune, Sanofi-Aventis, and Vectura; all outside the submitted work. J. V. F. reports receiving consultant fees from Boehringer Ingelheim, Pieris, Entrinsic Health Solutions, and Sanofi Genzyme; all outside the submitted work. G. R. W. reports grants from the NIH, grants and other from Boehringer Ingelheim, other from Genentech, other from Quantitative Imaging Solutions, other from PulmonX, other from Regeneron, other from ModoSpira, grants from BTG Interventional Medicine, grants and other from Janssen Pharmaceuticals, other from Toshiba, other from GlaxoSmithKline; all outside the submitted work; and G. R. W.’s spouse works for Biogen, which is focused on developing therapies for fibrotic lung disease. E. I. reports personal fees from AstraZeneca, personal fees from Novartis, personal fees from Philips Respironics, personal fees from Regeneron Pharmaceuticals, personal fees from Teva Specialty Pharmaceuticals, grants from Genentech, nonfinancial support from Boehringer Ingelheim, nonfinancial support from GlaxoSmithKline, nonfinancial support from Merck, nonfinancial support from Sunovion, nonfinancial support from Teva, grants from Sanofi, personal fees from Bird Rock Bio, personal fees from Nuvelution Pharmaceuticals, personal fees from Vitaeris, grants from Boehringer Ingelheim, nonfinancial support from Teva Specialty Pharmaceuticals, personal fees from Sanofi, personal fees from Merck, personal fees from Entrinsic Health Solutions, personal fees from GlaxoSmithKline, other from Vorso Corp., personal fees from Pneuma Respiratory, personal fees from 4D Pharma, outside the submitted work. S. B. F. reports grants from GE Healthcare, outside the submitted work; and served as a paid consultant advising on quantitative CT protocols for the COPD Gene Project. E. A. H. is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. S. E. W. reports grants and personal fees from AstraZeneca, grants from Boehringer Ingelheim, grants from GlaxoSmithKline, grants and personal fees from Sanofi, grants from Novartis, personal fees from Pieris, personal fees from Up to Date, outside the submitted work. None declared (S. Y. A., G. V. S.-F., M. L. S., F. N. R., A. R., C. E. C., J. C. R., N. N. J., B. M. G., B. D. L., and A. G. C.).
SARP Investigators: Elliot Israel MD, Bruce Levy MD, George Washko MD, Manuela Cernadas MD, Wanda Phipatanakul MD, Sally Wenzel MD, Merritt Fajt MD, Benjamin Gaston MD, James Chmiel MD, W. Gerald Teague MD, Anne-Marie Irani MD, Serpil Erzurum MD, Sumita Khatri MD, Suzy Comhair MD, Raed Dweik MD, Kristie Ross MD, Ross Myers MD, Wendy Moore MD, Deborah Meyers PhD, Eugene Bleecker MD, Stephen Peters MD, Annette Hastie MD, Victor Ortega MD, Greg Hawkins PhD, Xingan Li MD, Anne Fitzpatrick PhD, Nazar Jarjour MD, Loren Denlinger MD PhD, Sean Fain PhD, Ronald Sorkness PhD, Mario Castro MD MPH, Leonard Bacharier MD, David Gierada MD, Kenneth Schechtman PhD, Jason Woods PhD, John Fahy MD, Prescott Woodruff MD MPH, Ngoc Ly MD MPH, David Mauger PhD.
Additional Information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Dr Ash and Dr Vegas Sanchez-Ferrero contributed equally to this work (co-first authors).
Dr Israel and Dr San Jose Estepar contributed equally (co-senior authors).
FUNDING/SUPPORT: Supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), to the Severe Asthma Research Program (SARP) Principal Investigators, Clinical Centers, and Data Coordinating Center as follows: U10 HL109164 (E. R. B.), U10 HL109257 (M. C.), U10 HL109250 (B. M. G.), U10 HL109146 (J. V. F.), U10 HL109250 (B. M. G.), U10 HL109172 (E. I. and B. D. L.), U10 HL109168 (N. N. J.), U10 HL109152 (S. E. W.), and U10 HL109086 (D. T. M.). In addition, this program is supported through the following NIH National Center for Advancing Translational Sciences awards: UL1 TR000448 (Washington University), UL1 TR001420 (Wake Forest University), UL1 TR000427 (University of Wisconsin), and UL1 TR001102 (Harvard University). Additional support was provided by R01 HL116473 (R. S. J. E. and G. R. W.), K23 HL136905 (F. N. R.), K23 HL114735 (C. E. C.), K23 AI125785 (J. C. C), T32 HL007633 (S. Y. A.), and K08 HL145118 (S. Y. A).
Contributor Information
Samuel Y. Ash, Email: syash@bwh.harvard.edu.
SARP Investigators:
Elliot Israel, Bruce Levy, George Washko, Manuela Cernadas, Wanda Phipatanakul, Sally Wenzel, Merritt Fajt, Benjamin Gaston, James Chmiel, W. Gerald Teague, Anne-Marie Irani, Serpil Erzurum, Sumita Khatri, Suzy Comhair, Raed Dweik, Kristie Ross, Ross Myers, Wendy Moore, Deborah Meyers, Eugene Bleecker, Stephen Peters, Annette Hastie, Victor Ortega, Greg Hawkins, Xingan Li, Anne Fitzpatrick, Nazar Jarjour, Loren Denlinger, Sean Fain, Ronald Sorkness, Mario Castro, Leonard Bacharier, David Gierada, Kenneth Schechtman, Jason Woods, John Fahy, Prescott Woodruff, Ngoc Ly, and David Mauger
Supplementary Data
References
- 1.Schiebler M.L., Bhalla S., Runo J. Magnetic resonance and computed tomography imaging of the structural and functional changes of pulmonary arterial hypertension. J Thorac Imaging. 2013;28(3):178–193. doi: 10.1097/RTI.0b013e31828d5c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells J.M., Washko G.R., Han M.K. COPDGene Investigators; ECLIPSE Study Investigators. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells J.M., Farris R.F., Gosdin T.A. Pulmonary artery enlargement and cystic fibrosis pulmonary exacerbations: a cohort study. Lancet Respir Med. 2016;4(8):636–645. doi: 10.1016/S2213-2600(16)30105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S.P., Vegas-Sanchez-Ferrero G., Rahaghi F.N. Cardiac morphometry on computed tomography and exacerbation reduction with β-blocker therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(11):1484–1488. doi: 10.1164/rccm.201702-0399LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells J.M., Iyer A.S., Rahaghi F.N. Pulmonary artery enlargement is associated with right ventricular dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circ Cardiovasc Imaging. 2015;8(4) doi: 10.1161/CIRCIMAGING.114.002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahaghi F.N., Vegas-Sanchez-Ferrero G., Minhas J.K. Ventricular geometry from non-contrast non-ECG-gated CT scans: an imaging marker of cardiopulmonary disease in smokers. Acad Radiol. 2017;24(5):594–602. doi: 10.1016/j.acra.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ash S.Y., Rahaghi F.N., Come C.E. SARP Investigators. Pruning of the pulmonary vasculature in asthma: the Severe Asthma Research Program (SARP) cohort. Am J Respir Crit Care Med. 2018;198(1):39–50. doi: 10.1164/rccm.201712-2426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estepar R.S., Kinney G.L., Black-Shinn J.L. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore W.C., Bleecker E.R., Curran-Everett D. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarjour N.N., Erzurum S.C., Bleecker E.R. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185(4):356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 12.National Asthma Education and Prevention Program Expert panel report: guidelines for the diagnosis and management of asthma: update on selected topics—2002. J Allergy Clin Immunol. 2002;110(5 suppl):S141–S219. [PubMed] [Google Scholar]
- 13.Sieren J.P., Newell J.D., Jr., Barr R.G. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi S., Hoffman E.A., Wenzel S.E. Quantitative computed tomographic imaging-based clustering differentiates asthmatic subgroups with distinctive clinical phenotypes. J Allergy Clin Immunol. 2017;140(3) doi: 10.1016/j.jaci.2016.11.053. 690-700.e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoogendoorn C., Duchateau N., Sanchez-Quintana D. A high-resolution atlas and statistical model of the human heart from multislice CT. IEEE Trans Med Imaging. 2013;32(1):28–44. doi: 10.1109/TMI.2012.2230015. [DOI] [PubMed] [Google Scholar]
- 16.Mosteller R.D. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 17.McCoy K., Shade D.M., Irvin C.G. Predicting episodes of poor asthma control in treated patients with asthma. J Allergy Clin Immunol. 2006;118(6):1226–1233. doi: 10.1016/j.jaci.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Miller M.K., Lee J.H., Miller D.P., Wenzel S.E., TENOR Study Group Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481–489. doi: 10.1016/j.rmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 19.RStudio Team . RStudio, Inc.; Boston, MA: 2015. RStudio: Integrated Development for R. [computer program]www.rstudio.com/ [Google Scholar]
- 20.Matsuoka S., Washko G.R., Dransfield M.T. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol. 2010;17(1):93–99. doi: 10.1016/j.acra.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuoka S., Washko G.R., Yamashiro T. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2010;181(3):218–225. doi: 10.1164/rccm.200908-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka S., Yamashiro T., Diaz A. The relationship between small pulmonary vascular alteration and aortic atherosclerosis in chronic obstructive pulmonary disease: quantitative CT analysis. Acad Radiol. 2011;18(1):40–46. doi: 10.1016/j.acra.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozdemir O., Ceylan Y., Razi C.H., Ceylan O., Andiran N. Assessment of ventricular functions by tissue Doppler echocardiography in children with asthma. Pediatr Cardiol. 2013;34(3):553–559. doi: 10.1007/s00246-012-0493-3. [DOI] [PubMed] [Google Scholar]
- 24.Uyan A.P., Uyan C., Ozyurek H. Assessment of right ventricular diastolic filling parameters by Doppler echocardiography. Pediatr Int. 2003;45(3):263–267. doi: 10.1046/j.1442-200x.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 25.Rubin E.L. The size of the heart in asthma and emphysema. Lancet. 1936;228(5906):1089–1093. [Google Scholar]
- 26.Baratto O., Muehsam G.E. Heart size in pulmonary emphysema. JAMA. 1968;203(4):293–295. [PubMed] [Google Scholar]
- 27.Rackemann F.M. Fatal asthma: report of a case with autopsy. Boston Med Surg J. 1926;194(12):531–534. [Google Scholar]
- 28.Götzl A., Kienböck R. [Bronchial asthma and cardiac atrophy] [article in German] Wien Klin Wochenschr. 1908;21(36):1261–1265. [Google Scholar]
- 29.Palmieri G.C., Jr. [Morphology of the heart in emphysema; comparison of radiographic and plastic model data] [article in Italian] Bull Sci Med (Bologna) 1958;130(2):185–189. [PubMed] [Google Scholar]
- 30.Denlinger L.C., Phillips B.R., Ramratnam S. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Israel E., Reddel H.K. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965–976. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 32.Lange P., Celli B., Agusti A. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 33.Sun D., Wang T., Heianza Y. A history of asthma from childhood and left ventricular mass in asymptomatic young adults: the Bogalusa Heart Study. JACC Heart Fail. 2017;5(7):497–504. doi: 10.1016/j.jchf.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washko G.R., Nardelli P., Ash S.Y. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease: a longitudinal observational study. Am J Respir Crit Care Med. 2019;200(4):454–461. doi: 10.1164/rccm.201811-2063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei L., MacDonald T.M., Walker B.R. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141(10):764–770. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 36.Ostenfeld E., Flachskampf F.A. Assessment of right ventricular volumes and ejection fraction by echocardiography: from geometric approximations to realistic shapes. Echo Res Pract. 2015;2(1):R1–R11. doi: 10.1530/ERP-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawel-Boehm N., Maceira A., Valsangiacomo-Buechel E.R. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald M.L., Diaz A.A., Ross J.C. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease: a cross-sectional study. Ann Am Thorac Soc. 2014;11(3):326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.