Abstract
Current strategies for the management of OSA reflect a one-size-fits-all approach. Diagnosis and severity of OSA are based on the apnea-hypopnea index and treatment initiated with CPAP, followed by trials of alternatives (eg, oral appliances) if CPAP “fails.” This approach does not consider the heterogeneity of individuals with OSA, reflected by varying risk factors, pathophysiological causes, clinical manifestations, and consequences. Recently, studies using analytic approaches such as cluster analysis have taken advantage of this heterogeneity to identify OSA phenotypes, or subtypes of patients with unique characteristics, that may enable more personalized approaches to prognostication and treatment. Examples include symptom-based subtypes such as “excessively sleepy” and “disturbed sleep” with differing impact of CPAP on symptoms and health-related quality of life. Polysomnographic subtypes, distinguished by respiratory event association with hypoxemia, arousals, or both, exhibit varying risks of cardiovascular disease and response to therapy. This review summarizes the findings from recent cluster analysis studies in sleep apnea and synthesizes common themes to describe the potential role (and limitations) of phenotypic subtypes in precision medicine for OSA. It also highlights future directions, including linking of phenotypes to clinically relevant outcomes, rigorous and transparent assessment of phenotype reproducibility, and need for tools that categorize patients into subtypes, to prospectively validate phenotype-based prognostication and treatment approaches. Finally, we highlight the critical need to include women and more racially/ethnically diverse populations in this area of research if we are to leverage the heterogeneity of OSA to improve patient lives.
Key Words: cluster analysis, phenotype, precision medicine, sleep apnea
Abbreviations: AHI, apnea-hypopnea index; CAD, coronary artery disease; CHD, coronary heart disease; CVD, cardiovascular disease; ESS, Epworth Sleepiness Scale; HR, hazard ratio; PLMS, periodic limb movements of sleep; PSG, polysomnography; QOL, quality of life; REM, rapid eye movement; T90%, time spent at arterial oxygen saturation below 90%
OSA is a heterogeneous syndrome, with varied predisposing factors,1 pathophysiological mechanisms,2 clinical presentations,3,4 and consequences of respiratory events.5, 6, 7, 8 Importantly, the efficacy of OSA treatment and its impact on outcomes may also vary depending on these characteristics.9, 10, 11 However, the current paradigm for diagnosis and management of OSA largely reflects a one-size-fits-all approach whereby polysomnographic data are reduced to a single metric (the apnea-hypopnea index [AHI]) and patients are managed by trial and error initially with CPAP, followed by attempts at other treatments (eg, oral appliances) for CPAP therapeutic failure. Approaching a heterogeneous condition such as OSA in this way may have important consequences, including suboptimal treatment adherence and effectiveness leading to inefficient use of the health-care system and patient resources. Moreover, this approach may be one reason that the field’s large, multicenter clinical trials of CPAP therapy have shown modest or no risk reduction in cardiovascular disease, death, or improvement of neurocognitive outcomes.12, 13, 14, 15
An alternative approach is to leverage the heterogeneity of OSA by classifying it into smaller, more homogeneous disorder subtypes, sometimes referred to as phenotypes.16 Features used to identify such phenotypes may come from a variety of observable and measurable OSA characteristics such as signs, symptoms, demographic characteristics, polysomnographic and physiologic metrics, or comorbidities. The expected benefit of this approach is that separate phenotypes could allow for a more specific diagnostic and treatment strategy, which may lead to improved patient outcomes and successful clinical trials. For example, an OSA anatomical phenotype defined by lack of complete concentric palatal collapse has helped identify responders to airway neurostimulation, enabling a new treatment option.17 Such approaches are an important step toward developing precision medicine for OSA, whereby treatments are targeted to the needs of individual patients on the basis of genetic, biomarker, phenotypic, or psychosocial characteristics that distinguish a given patient from others with the same disorder.18
Phenotyping strategies can be broadly grouped into two analytic approaches: hypothesis-driven (or supervised) and hypothesis-generating (or unsupervised).19 Identification of the airway collapse phenotype described earlier is an example of a hypothesis-driven approach using traditional regression methods, which are suited for evaluation of individual or a few disorder features with outcomes of interest. In contrast, hypothesis-generating approaches, using unsupervised learning methods such as cluster analysis, focus on discovering emerging patterns within the data by grouping subjects into homogeneous categories on the basis of unique associations between subject features. This is accomplished by assessing similarity (or dissimilarity) between subjects by using metrics such as correlation or distance based on the features used to characterize each individual. Ideally, members of each cluster are as similar as possible to each other and as different as possible from those in other clusters. Cluster analysis techniques, such as hierarchical, K-means, or latent class analysis, have been used by several groups to identify symptom,20 polysomnographic,21 and comorbidity22 clusters among patients with OSA, with differing quality of life (QOL),20 treatment use and benefit,23 and risk for developing cardiovascular disease.21,24,25
The purpose of the current review was to: (1) summarize the findings and synthesize the themes from cluster analysis studies that aim to identify OSA phenotypes; (2) describe the role of phenotypic subtypes of OSA in precision medicine approaches to sleep apnea; and (3) highlight important future directions and unanswered questions on this topic. Discussions of precision medicine in OSA not specific to phenotypic clusters have also been recently explored.26, 27, 28, 29
Examples of OSA Patient Clusters and Associated Outcomes
In the last decade, many clusters have been identified among study participants evaluated for sleep apnea. These studies vary dramatically in terms of individuals included (eg, population, clinical, administrative cohorts), sample size (n = 161-72,217), patient features used to identify the clusters (eg, symptoms, polysomnographic indices), and outcomes (eg, cardiovascular disease, CPAP use). A summary of the designs and main findings are noted in Table 1, and the following sections describe the findings according to the domain of features used.
Table 1.
Summary of Cluster Analysis in Patients With Sleep Apnea (or Referred for Sleep Apnea Evaluation) According to Variable Domain Used for Classification
| Phenotypic Feature | Study/Year | Study Population/Sample Size/OSA Severity | Clustering Method | Main OSA Cluster Findings (Prevalence, %) | Outcomes Associated With Phenotypes/Comments |
|---|---|---|---|---|---|
| Symptoms (14 questions) ESS | Mazzotti et al,24 2019 | Population (SHHS, US) N = 1,207, PSGAHI ≥ 15Hypopnea: not defined |
LCA | 4 clusters: 1: Disturbed sleep (12%)—predominant insomnia symptoms 2: Minimally symptomatic (33%)—lowest symptom burden of all clusters 3: Excessively sleepy (17%)—predominant sleepy, involuntary sleep, drowsy driving 4: Moderately sleepy (39%)—snoring, napping |
Outcomes: Prevalent (OR) and incident CVD (HR) Adjusted for: age, sex, BMI, AHI, presence of DM, HTN, cholesterol, triglycerides, smoking status, alcohol usage, race, ethnicity, and lipid-lowering medication Prevalent:
|
| Symptoms (16 questions) ESS Comorbidities (CVD, HTN, and DM) |
Kim et al,31 2018 | Population (South Korea) N = 422 HSATAHI ≥ 15Hypopnea: 30% flow decrement with 4% desaturation |
LCA | 3 clusters: 1. Disturbed sleep (14%) 2. Minimally symptomatic (56%) 3. Excessively sleepy (30%) |
No outcomes reported No differences between clusters in AHI or BMI HTN highest among “Disturbed sleep” |
| Symptoms (19 questions) ESS Comorbidities (CVD, HTN, DM, and COPD) |
Ye et al,20 2014 | Clinical (Iceland) N = 822 HSATAHI ≥ 15Hypopnea: 30% flow reduction with 4% desaturation |
LCA | 3 clusters 1. Disturbed sleep (33%) 2. Minimally symptomatic (25%) 3. Excessively sleepy (42%) |
Outcomes: QOL (SF-12 physical and mental components) QOL highest for “Minimally symptomatic” No differences between clusters in AHI or BMIComorbidities highest in “Minimally symptomatic” |
| Symptoms (17 questions) ESS 3 comorbidities (CVD, HTN, and DM) |
Keenan et al,30 2018 and Pien et al,23 2018 | Clinical (Iceland) N = 215 (Keenan et al30) N = 706 (Pien et al23)HSATAHI ≥ 15Hypopnea: 30% flow reduction with 4% desaturation |
LCA | 3 clusters: 1. Disturbed sleep (33%) 2. Minimally symptomatic (29%) 3. Excessively sleepy (38%) Nearly identical to Ye et al20/2014 regarding age, BMI, AHI, and comorbidity distribution, which did not differ among clusters |
Outcomes (Pien et al23): changes in symptoms, QOL, comorbidities, anthropometrics over time; CPAP adherence Effect of CPAP on symptoms was most notable in “Excessively sleepy” on the sleepiness symptoms (eg, drowsy driving, falling asleep during the day) Both CPAP users and nonusers improved in “Disturbed sleep.” CPAP users improved in sleepiness and insomnia symptoms QOL improved in “Excessive sleepy” only “Minimally symptomatic” with highest rate of HTN and CVD at follow-up |
| Symptoms (17 questions), ESS 3 comorbidities (CVD, HTN, and DM) |
Keenan et al,30 2018 | Clinical (multi-ethnic, multinational) N = 757 PSG/HSATAHI ≥ 15Hypopnea:30% flow reduction with 4% desaturation |
LCA | 5 clusters: 1. Disturbed sleep (19%) 2. Minimally symptomatic (20%) 3. Upper airway with sleepiness (similar to “Excessively sleepy” from Icelandic studies) (22%) 4. UA symptoms (19%) 5. Sleepiness-dominant (similar to moderately sleepy in SHHS study) (20%) New clusters (4 and 5) composed of patients in “Minimally symptomatic” and “Excessively sleepy” in Icelandic study |
Similar trends in age, BMI, and AHI among three common clusters to above studies “Upper airway with sleepiness” is younger, more obese, and sleepy than others; no clinical difference in AHI “Disturbed sleep” with highest proportion of women and highest rates of comorbidities (not consistent with SHHS, South Korean, or Icelandic studies) |
| Multiple variable domains: Symptoms Comorbidities (HTN, DM, CVD, and others) Anthropometrics (Age, sex, and BMI) AHI not included |
Bailly et al,32 2016 | Clinical (registry in France) N = 18,263 Sleep assessment not specifiedAHI ≥ 15Hypopnea: no specified |
Multiple correspondence analysis for feature selection followed by hierarchical clustering | 6 clusters: 1. Young symptomatic (10%) Low BMI, few or no comorbidities, high sleepiness, and near misses driving; medium T90% 2. Older obese (23%) Lowest ESS, few comorbidities 3. Multidisease, old, obese (19%) Symptomatic but low ESS, HTN, diabetes, CVD; highest T90% 4. Young snorers (15%) Lowest BMI, few symptoms no comorbidities; lowest T90% 5. Drowsy obese (19%) Highly symptomatic, few comorbidities 6. Multidisease, obese, symptomatic (15%) Highly symptomatic, HTN, diabetes, and CVD; high T90% |
No outcomes reported Fatigue differed by cluster. Highest among “Young symptomatic” and “Multidisease symptomatic” No difference in depression scores |
| Multiple variable domains: Anthropometrics Sleep symptoms Insomnia report Depressive symptoms Comorbidities (HTN, CVD, and DM) AHI not included |
Gagnadoux et al,34 2016 | Clinical (France) N = 5,983 PSG/HSATAHI ≥ 15Hypopnea: not defined |
LCA | 5 clusters: 1. Female OSA with insomnia (14%) Middle-aged, obese women with insomnia and comorbidities2. Male OSA with comorbidities (15%) 3. Severe sleepy OSA without comorbidities (18%) Youngest, lack of comorbidities 4. Mild sleepiness, insomnia (32%) Non-obese with minimal comorbidities 5. Older, comorbid OSA (21%) Minimally symptomatic |
Outcome: CPAP success at 6 mo (this metric defined as a combination of) Adherence (≥ 4 h daily) and (ESS decrease of ≥ 4 OR, increase of ≥ 7 points in vitality from SF-36 Adjusted for: marital, educational, and employment status; model, AHI, and baseline ESS score “Female OSA with insomnia” (OR, 0.66) “Mildly sleepy, insomnia” (OR, 0.66) and “Older, comorbid OSA” (OR, 0.38) with lower likelihood of CPAP success vs “Severely sleepy OSA without comorbidities”“Older, comorbid OSA,” despite highest CPAP use/adherence, had lowest reduction in ESS and improvement in QOLAHI differed by significance with narrow range (38-46) |
| Multiple variable domains: Sleepiness Demographic characteristics Anthropometrics Polysomnographic indices Lung function Blood gases Comorbidities (HTN, DM, CVD, and others) |
Lacedonia et al,36 2016 | Clinical (Italy) HSAT N = 198 AHI ≥ 5Hypopnea:AASM 2007 criteria (recommended or alternative not specified)Patients excluded:OHSCOPDNMD |
PCA for feature selection, Network analysis with hierarchical and local optimizing clustering | 3 clusters: 1. Severe, hypoxic OSA (50%) Most sleepy, obese, small lung function 2. Moderate, nonhypoxic OSA (51%) 3. Severe, minimally hypoxic OSA (9%) Large AHI vs ODI discrepancy Less sleepy |
No outcomes reported No differences in comorbidities, age, or sex No differences in blood gases or lung function |
| Multiple variable domains: Demographic Anthropometric Symptoms Comorbidities (CHF, pulmonary HTN, and arrhythmias) AHI not included |
Ferreira-Santos and Pereira Rodrigues,33 2018 | Clinical (Portugal) N = 211 AHI: cutoff not definedPatients excluded:Severe lung diseasesNeurological conditions |
K-modes categorical clustering | 3 clusters: 1. Nonobese, young, drowsy (55%) 2. Female, poor sleep (20%) 3. Obese, older, non-drowsy (25%) |
No outcomes reported No difference in AHI or comorbidities among clusters “Obese, older, non-drowsy” with highest Mallampati score and neck circumference “Female, poor sleep” with headaches and nonrestorative sleep |
| Multiple variable domains: 19 variables: Demographic Health habits BP AHI, T90 Comorbidities Medications |
Quan et al,25 2018 | Clinical (clinical trial, multinational) N = 2,649 Patients with CAD and/or CeVD and OSA (ODI ≥ 12) on home sleep apnea test randomized to receive CPAP or usual care |
LCA | 4 clusters: 1. CeVD and DM (9%) 2. CAD and DM (15%)3. CeVD (37%)4. CAD (39%) |
Outcomes. Primary, composite of death from any CV cause or incident MI, stroke, hospitalization for unstable angina, HF, or TIA (HR by cluster). Adjusted for: posterior probability of cluster membershipPrimary outcome: CAD and DM (HR, 2.1)CeVD and DM (HR, 1.7)CAD and DM (HR, 1.4)CAD (referent)Rate of primary outcome by < 4 h/night vs ≥ 4 h/night CPAP use:CeVD and DM (21% vs 5%; P = .015)Other clusters with no significant differences |
| Comorbidities (30 conditions, ICD-9 defined) | Turino et al,40 2017 | Clinical (Spain) N = 72,217 Patients on CPAP therapyAHI, hypopnea not reported |
Multiple correspondence for feature selection, K-means for clustering |
6 clusters: 1. Neoplastic (10%) 2. Metabolic syndrome (28%)3. Asthmatic (6%)Most women (53% of cluster)4. Musculoskeletal and joint disorders (10%)5. Few comorbidities (35%)6. Oldest CVD (10%) |
Outcomes: all-cause mortality, hospitalizations, Health-care utilization “Neoplastic” and “Oldest CVD” with highest mortality (15%) and hospitalizations (> 1 visit, 30%-37%)Lowest mortality for “Metabolic syndrome” and “Musculoskeletal and joint disorders” (< 2%) |
| Comorbidities (19 components of Charlson comorbidity index), AHI |
Vavougios et al,22 2016 | Clinical (Greece) N = 1,472 Patients referred for PSGAHI: no cutoff usedHypopnea: 50% flow reduction or 30% flow reduction with arousal or 3% desaturation |
PCA for feature selection, “Two-step clustering” (“preclustering” followed by hierarchical clustering) |
6 clusters: 1. Mild OSA, no comorbidities (20%) Increased CAD vs no OSA2. Moderate OSA, high comorbidity (7%)Older, obese, low oxygen nadirOSA3. No OSA, no comorbidities (17%)Youngest, no sleepiness4. Severe OSA, no comorbidities (31%)Obese, sleepy5. Severe, high comorbidity (10%)Older, morbidly obese, hypersomnia6. Moderate OSA, no comorbidities (15%)Mild obesity, not sleepy, high oxygen nadir |
No outcomes reported More obese, older individuals tended to be in more comorbid clusters Comorbidities cluster independently of the AHI or hypoxemia (measured by nadir oxygen saturation) |
| PSG characteristics (all from supine sleep) Mean event duration Minimum oxygen saturation Fraction of apneas Arousal ratio (respiratory/total) AHI |
Nakayama et al,37 2019 | Clinical (Japan) N = 210 PSGAHI ≥ 15Hypopnea:50% flow reduction with 3% desaturation or arousalPatients excluded:CVDPsychiatric diseaseWomenHypnotic usePLM index ≥ 15 |
Hierarchical and K-means | 3 clusters: 1. Hyper-severe OSA, hypoxemic (20%) Obese, highest NREM 1 stage sleep, most arousals respiratory2. Severe OSA, long event durationNonobese, low NREM 1 stage, most arousals respiratory, non-hypoxemic3. Severe OSA, short event durationOverweight, higher central apneas, low fraction of apneas, low NREM 1 stage, nonhypoxemic |
No outcomes reported |
| PSG characteristics (AHI metrics stratified by position and sleep state [REM vs NREM]), arousals, age, BMI, sex, ESS | Joosten et al,35 2012 | Clinical (Australia)N = 1,064 PSGAHI 5-30 per hour Hypopnea: > 50% reduction in the oronasal pressure signal, or a smaller reduction in association with oxygen desaturation of 3% or an arousal |
K-means | 6 Clusters: 1. Mild supine predominant OSA (32%) Youngest, nonobese2. Moderate supine predominant OSA (21%)Older3. Moderate supine isolated OSA (4%)Younger, nonobese4. REM predominant OSA (12%)Most female, most obese5. Mild REM-supine OSA (20%)Oldest6. Moderate REM-supine OSA (13%)Younger |
No outcomes reported |
| PSG characteristics only 29 variables in domains of: Respiratory disturbance Sleep architecture Autonomic dysfunction Hypoxia |
Zinchuk et al,21 2018 | Clinical (US veterans) N = 1,247 Patients referred for OSA evaluationPSGAHI: no cutoff usedHypopnea: > 30% reduction in nasal pressure with a 4% desaturation |
PCA and hierarchical clustering for feature selection, K-means for clustering |
7 clusters: 1. Mild (43%) Lowest apneas/hypopneas2. PLMS (20%)3. NREM and poor sleep (15%)Highest ratio of arousals per AHI, minimal hypoxemia4. REM and hypoxia (15%)Relatively preserved sleep architecture5. Hypopnea and hypoxia (6%)6. Arousal and poor sleep (3%)Highly fragmented sleep, minimal hypoxemia7. Combined severe (10%)Apneas with arousals and desaturations, severe hypoxemia |
Outcome: incident CVD or death by cluster (HR, compared with “Mild” cluster) Adjusted for: Framingham risk score, regular CPAP use, ethnicity, alcohol use, home oxygen useMultiple clusters in each conventional severity category:Mild: 1 and 2Moderate: 3 and 4Severe: 5, 6, and 7“PLMS” (HR, 2.0)“Hypopnea and hypoxia” (HR, 1.7)“Combined severe” (HR, 1.7)Risk of outcome in regular vs nonregular CPAP users, cluster“PLMS” (OR, 0.38)“Hypopnea and hypoxia” (OR, 0.22) |
| CPAP adherence trajectories Hours of CPAP use per day by each patient over 180 d |
Babbin et al,45 2015 | Clinical (clinical trial, multinational) N = 161 AHI ≥ 5Hypopnea: not defined |
Time series analysis and dynamic cluster analysis | 4 Clusters 1. Great users (17%) 2. Good users (33%)3. Low users (23%)4. Slow decliners (27%) |
Outcomes: CPAP adherence (hours/night), symptoms (ESS), QOL (FOSQ), attention (PVT) “Good users” more vigilant (FOSQ) vs “Low users” or “Slow decliners” “Good users” with higher productivity (FOSQ) vs “Low users” and “Great users”“Great users” and “Good users” higher sleep quality vs “Low users”Over time, self-efficacy waned in “Low-users” |
OR and hazard ratio (HR) reported only for significant associations between clusters and outcome. AASM = American Academy of Sleep Medicine; AHI = apnea-hypopnea index; CAD = coronary artery disease; CeVD = cerebrovascular disease; CHD = coronary heart disease (myocardial infarction; coronary revascularization procedure); CHF = congestive heart failure; CV = cardiovascular; CVD = cardiovascular disease (CHD, stroke, and heart failure); DM = diabetes mellitus; ESS = Epworth Sleepiness Scale; FOSQ = Functional Outcomes of Sleep Questionnaire; HF = heart failure; HSAT = home sleep apnea testing; HTN = hypertension; ICD-9 = International Classification of Diseases, Ninth Revision; LCA = latent class analysis; MI = myocardial infarction; NMD = neuromuscular disease; NREM = non-rapid eye movement; ODI = oxygen desaturation index; OHS = obesity hypoventilation syndrome; PCA = principal component analysis; PLMS = periodic limb movements of sleep; PVT = Psychomotor vigilance test; QOL = quality of life; REM = rapid eye movement; SF = Short-form quality of life questionnaire; SHHS = Sleep Heart Health Study; T90% = percent recording time spent at arterial oxygen saturation below 90%; TIA = transient ischemic attack; UA = unstable angina.
Patient Symptoms
Assessment of the heterogeneity of OSA by using cluster analysis has been most consistently applied to patient symptoms.20,23,24,30,31 Individuals studied include both clinical20,23,30 and population24,31 cohorts with AHI ≥ 15 events per hour, assessed with the same questionnaires on symptoms of sleepiness (eg, drowsy driving), insomnia (eg, early awakenings), nighttime disturbance (eg, restless sleep), upper airway symptoms (eg, snoring), and other related reports (eg, morning headaches). In a seminal study by Ye et al,20 in the predominantly male Icelandic Sleep Apnea Cohort (n = 822), three clusters of patients were identified, confirming that sleepiness captures only a part of the OSA symptomatic spectrum.3 The “Excessively sleepy” cluster (prevalence of 42%), with its highest Epworth Sleepiness scale (ESS) scores, falling asleep involuntarily, and drowsy driving, was consistent with the “Classic OSA” cluster and comprised the youngest patients. Additional clusters were termed “Disturbed sleep” (33%), characterized predominantly by insomnia-related symptoms, restless sleep, and gasping awakenings; and “Minimally symptomatic” (25%), comprising the oldest patients and the most rested upon awakening, with spouse-disturbing snoring and apneic episodes at night as the most common symptoms.20 Notably, the traditional OSA characteristics of AHI and BMI did not differ among the patient groups, whereas potential consequences of OSA (mental and physical QOL) did (highest for the “Minimally symptomatic” cluster).
These same clusters were also identified in a study by Keenan et al,30 in separate Icelandic (n = 215)23,30 and international (n = 757) clinical cohorts, although in the latter, additional symptom patient subgroups (“Upper airway symptoms” and “Sleepiness dominant”) were noted (Table 1).30 Similar clusters were detected in OSA populations by Mazzotti et al24 and Kim et al,31 with differences in prevalence likely reflecting higher frequency of less symptomatic patients in these samples. Other studies incorporating demographic, anthropometric, AHI, and comorbidity data in addition to symptoms (“Multidomain” clusters in Table 1) identified analogous patient subgroups32, 33, 34; however, given the diversity of features, patients, and inclusion of AHI, cluster types also vary widely in these reports.
Overall, it is notable that: (1) the symptoms in these clusters do not track with AHI; (2) the traditional sleepiness measured by using ESS only captures part of the phenotypic spectrum; and (3) some patients, like those in the “Minimally symptomatic” group who exhibit disturbing snoring and require naps, may not be diagnosed until later in life. Other implications were gleaned from a longitudinal two-year analysis by Pien et al23 showing that the effect of CPAP treatment on symptoms and QOL differed according to symptom cluster. Notably, not only the “Excessively sleepy” patients benefited from treatment. Significant improvements were also observed in the “Disturbed sleep” group (less restless sleep, more restful awakenings, and lower sleepiness) and in the “Minimally symptomatic” group (less fatigue and fewer apneic episodes) among those who used CPAP regularly. A recent analysis by Mazzotti et al24 in the Sleep Heart Health Study revealed that risk of incident cardiovascular disease (CVD) (coronary heart disease [CHD], heart failure, stroke, or cardiovascular mortality) differed according to cluster. The “Excessively sleepy” group was at increased risk of incident CVD (hazard ratio [HR] of 2.2–2.4, driven by CHD and heart failure) compared with the other clusters. This finding was not present among those with the same symptoms but without OSA.
Features of Polysomnography
It has long been recognized that overall AHI does not capture the diversity of the polysomnographic presentations of OSA as reflected by a priori categorizations according to sleep stage (eg, rapid eye movement [REM] sleep predominant), position (eg, supine predominant), and others proposed over the years.16 Joosten et al35 confirmed these clinical observations by identifying six patient clusters (Table 1) in an analysis of clinic patients (n = 1,064) with mild to moderate OSA (AHI 5 to < 30) incorporating age, BMI, sex, AHI, and sleep stage/position. Clusters were based on REM or position predominance and stratified according to either mild (AHI 5 to < 15) or moderate (AHI 15 to < 30) severity.
A wider scope of polysomnography (PSG) indices (29 measures) was used by Zinchuk et al21 in 1,247 US veterans referred for OSA evaluation. The study included metrics of degree of flow reduction, association of events with hypoxemia vs arousals or both, sleep architecture, periodic limb movements of sleep (PLMS), sleep fragmentation, and detailed assessment of hypoxemia. Multiple clusters were observed within each conventional AHI severity stratum (AHI 0 to < 15, “Mild” and “PLMS”; AHI 15 to < 30, “NREM and poor sleep” and “REM and hypoxia”; and AHI ≥ 30, “Hypopnea and hypoxia,” “Arousal and poor sleep,” and “Combined severe”), potentially reflecting different mechanistic pathways. For example, in the severe OSA stratum, the “Arousal and poor sleep” cluster, characterized by marked sleep fragmentation and apneas associated with arousals only, exhibited minimal hypoxemia (median percentage of sleep below oxygen saturation of 90% [T90%] of 0%) despite a high frequency of events (AHI of 68). In contrast, the “combined severe” cluster with a high AHI of 84 exhibited events associated with both arousals and desaturations (T90% of 20%).
A study by Lacedonia et al36 used the AHI, oxygen desaturation index, T90%, and multiple non-PSG characteristics for analysis of 198 clinic patients. The investigators observed three clusters, including a subgroup (cluster 3) with markedly elevated AHI and low level of hypoxemia (analogous to the “Arousal and poor sleep” cluster described earlier) and a high frequency of events associated with severe hypoxemia (cluster 1, analogous to the “Combined severe” cluster described earlier). Using respiratory event duration, apnea fraction, respiratory arousal fraction, and nadir oxygen saturation in patients with AHI ≥ 15, Nakayama et al37 identified three clusters, including a patient subgroup with AHI > 30, short event duration, and no hypoxemia and severe OSA with high fraction of apneas and hypoxia, analogous to the aforementioned clusters.
Few studies examining PSG clusters report associations with outcomes, and thus in some cases, implications can be inferred from data on isolated PSG features. For example, studies examining the REM-AHI indices show an association with prevalent and incident hypertension,7,38 which raises the importance of CPAP adherence during the second half of the night when REM sleep occurs.39 In the US veterans study that examined outcomes, risk of incident CVD and death was increased for the “PLMS,” “Hypopnea and hypoxia,” and “Combined severe” clusters. In contrast, stratifying patients according to traditional AHI-based severity (or continuous AHI) was not associated with CVD. Furthermore, only some clusters (“PLMS” and “Hypopnea and hypoxia”) benefited from CPAP treatment in terms of CVD risk reduction. Specifically, among the clusters with AHI < 15, the “PLMS” group was at twofold increased risk vs the “Mild” cluster, and regular CPAP use was associated with attenuated risk for CVD (OR, 0.4).
Comorbidities
Given that OSA is common and associated with a host of disorders that can also affect symptoms, function, and prognosis, some studies have attempted to identify comorbidity patterns among patients with OSA. In one study, Vavougios et al22 used the AHI and 19 comorbidities, including cardiovascular, metabolic, liver, renal, and pulmonary disorders and malignancies. They found high and low comorbidity burden clusters within both moderate and severe OSA groups. As anticipated, age, BMI, daytime oxygen saturation, and hypertension predicted inclusion in the high vs low comorbidity clusters. Quan et al25 used cardiovascular and cerebrovascular comorbidity data (in addition to other clinical features) from the Sleep Apnea Cardiovascular Endpoints (SAVE) trial14 to identify four clusters: coronary artery disease (CAD), CAD and diabetes, cerebrovascular disease, and cerebrovascular disease and diabetes. The risk of incident composite cardiac and stroke outcomes was increased in all clusters (HR, 1.4–2.1) compared with those with cerebrovascular disease alone.
In a population of 72,217 CPAP-treated patients in Spain using 30 diagnoses in an administrative database, Turino et al40 identified six clusters of comorbidity (Table 1). A predominantly female subgroup of patients with asthma was identified, exhibiting higher rates of hospitalization than clusters with minimal or musculoskeletal comorbidities. Such findings are consistent with previous studies suggesting a bidirectional OSA-asthma relationship with a combined adverse impact on health outcomes.41,42 In addition, a cluster with cancer and one with cardiovascular disease (eg, heart failure, stroke), both oldest and with previously reported associations with hypoxemia in OSA,43,44 exhibited the highest rates of mortality and health-care resource use. Whether age and the aforementioned comorbidities alone or in conjunction with OSA determined health-care use and mortality was not assessed.
Multidomain and Other Features
Several studies included multiple measures of symptoms, anthropometrics, and comorbidities in clustering analyses among patients with AHI ≥ 15. Bailly et al32 identified six clusters (Table 1). Two subgroups of young, nonobese patients with snoring and equivalent OSA metrics (AHI, oxygen desaturation index, and T90%) differed primarily according to presence of sleepiness, fatigue, and drowsy driving. Clusters with high burdens of hypertension, diabetes, CVD, COPD, and depression (“Older, comorbid OSA” and “Multidisease, old, obese” clusters) were primarily distinguished from others by marked8,32 nocturnal hypoxemia (T90%, 49%-59%). Gagnadoux et al34 reported five clusters, including a “Female OSA” characterized by insomnia, a “Mildly symptomatic OSA” with low sleepiness but nocturnal and insomnia symptoms, and a “Comorbid OSA” comprising older patients. These three clusters exhibited significantly lower rates of CPAP success (OR, 0.36-0.66) than the patients in the “Severe OSA” cluster who were younger and exhibited higher sleepiness (ESS score > 10).
Babbin et al45 have taken advantage of longitudinal CPAP use data to identify patterns of adherence by using individual time series analysis combined with clustering. Among 161 patients with AHI ≥ 5 initiated on CPAP in a clinical trial, the investigators identified four subgroups of adherence: “Great users,” “Good users,” “Low users,” and “Slow decliners.” Notably, in some groups, such as “Slow decliners,” adherence changed over time, highlighting that an established marker of long-term CPAP use, early adherence,46,47 is not a consistent predictor in > 25% of patients and that increased vigilance following early CPAP success is warranted in some. Moreover, although all patient subgroups started with the same level of treatment self-efficacy (ie, a modifiable psychological determinant of adherence48,49), this coping mechanism eroded by 3 to 6 months in “Low users,” offering an opportunity for intervention in this group. CPAP use trajectory analyses have identified other clinically relevant OSA subgroups. Using a large CPAP telemonitoring dataset, Liu et al50 noted four groups with increasing risks of therapy termination: OSA (lowest), treatment transient, persistent, and emergent central sleep apneas (highest). Pepin et al51 found that switching patients with increased residual events, including high frequency of central apneas, to adaptive servo-ventilation improved treatment efficacy and adherence.
Common Themes
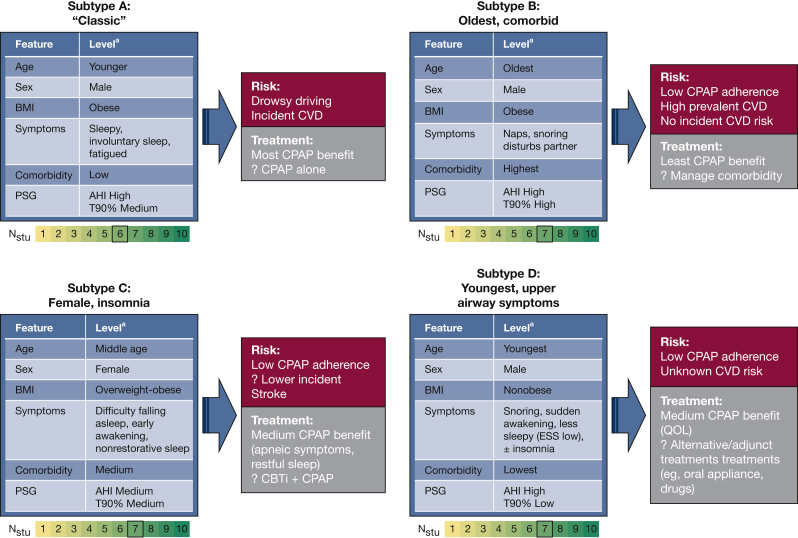
Although direct comparison between results of the aforementioned studies in OSA are not possible due to large discrepancies in populations, OSA features, analytic techniques, and outcomes studied, we attempted (in the following sections) to synthesize the common themes. We first focused on potential OSA subtypes based on relative differences between clusters in age, BMI, sex, symptoms, and comorbidities (Fig 1),52 followed by OSA physiology as assessed by using PSG (Fig 2).
Figure 1.
Potential OSA Subtypes (A-D) based on common themes in the cluster analysis studies focused on demographic characteristics, anthropometrics, comorbidities, and symptoms. aLevel is based on relative difference for that variable/feature within each study. Studies and clusters corresponding to potential OSA subtypes (X [Cluster #], where X is first initial of the study cited): Subtype A: B [1 or 5], F [1], G [3], Ke [3], M [3], Y [3]; Subtype B: B [3], G [5], Ke [2], Ki [2], M [2], V [5], Y [2]; Subtype C: G [1], F [2]; Ke [1], Ki [1], M [1], P [1], Y [1]; and Subtype D: B [4], G [4], Ke [4], V [6]. Study cited: B = Bailly et al,32 2016; G = Gagnadoux et al,34 2016; F = Ferreira-Santos and Pereira Rodrigues,33 2018; Ke = Keenan et al,30 2018; Ki = Kim et al,31 2018; M = Mazzotti et al,24 2019; Y = Ye et al,20 2014. AHI = apnea-hypopnea index; CBTi = cognitive behavioral therapy for insomnia; CVD = cardiovascular disease (coronary heart disease, heart failure, and stroke); ESS = Epworth Sleepiness Scale; Nstu = number of studies that identified analogous clusters, with 0 to 10 range selected for demonstration; PSG = polysomnography; QOL = quality of life (measured by using the Short Form-36 Health Survey); T90% = percent of total sleep (or recording) time spent with oxygen saturation below 90%.
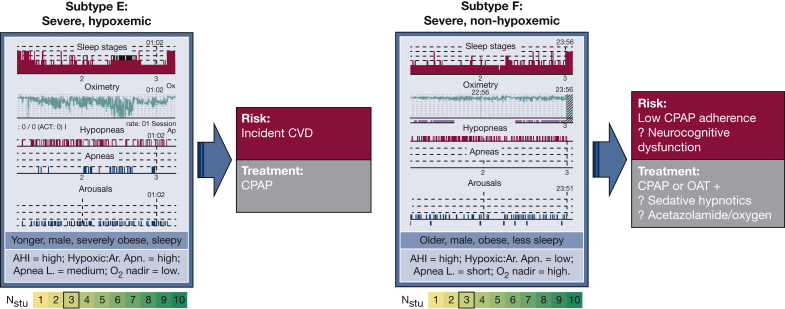
Figure 2.
Potential OSA Subtypes (E and F) based on common themes in the cluster analysis studies focused on polysomnographic features. Characteristics described in the text-box for each subtype are based on relative differences for that variable/feature within each study. Studies and clusters corresponding to potential OSA subtypes (X [Cluster #] where X is first initial of the study cited): Subtype E: L [1], N [1], Z [7]; and Subtype F: L [3], N [3], Z [6]. Study cited: L = Lacedonia et al,36 2016; N = Nakayama et al,37 2019; Z = Zinchuk et al,21 2018. Apn. L. = apnea length; CVD = cardiovascular disease (acute coronary syndrome, stroke, or death), Hypoxic:Ar Apn. = ratio of apneas with 4% desaturation only to apneas with arousal only; OAT = oral appliance therapy. See Figure 1 legend for expansion of other abbreviations. (Hypnogram images modified and adapted from Cooksey et al.52)
Subtype A. This group includes younger, obese, predominantly male individuals with severe OSA and “classic” symptoms (eg, sleepiness, drowsy driving). Analogous clusters were identified in six studies.20,24,30,32,34 The younger age of these patients may reflect an earlier diagnosis given their recognizable symptoms. This subtype tended to benefit most from CPAP treatment in terms of improving symptoms and QOL.23,34,53 These patients were at highest risk of drowsy driving (18%-30%) and, based on the “Excessively sleepy” cluster in a study by Mazzotti et al,24 were at an increased risk (HR, 2.2-2.4) of incident CVD (CHD and heart failure). As such, this subtype may represent a high-yield group in which to initiate treatment, although the impact of CPAP on CVD has not been assessed given the exclusion of the hyper-sleepy patients from previous randomized clinical trials.14,15
Subtype B. This group consists of older, obese, predominantly male subjects with minimal to moderate symptom burden (sleepiness, disturbed sleep, and consistent snoring), frequent comorbidities, and severe OSA with marked hypoxemic burden (T90%, 32%-59%). Older age at diagnosis may reflect unrecognized symptoms of OSA or development of the disorder later in life. The high prevalence of hypertension, diabetes, and CVD in this group is consistent with both older age and impact of profound, ongoing desaturation.44,54 Gagnadoux et al34 found such patients to have lowest rates (OR, 0.36) of CPAP success (defined as a composite of adherence, reduction in ESS score, and improvement in QOL), although fatigue and apneic episodes improved with CPAP in such a group in the study by Pien et al.23 This scenario suggests that if treatment is initiated in such a subtype, conventional sleepiness metrics alone (eg, ESS) are not sufficient to assess for potential benefits, and attention should be paid to snoring, apneic episodes, and fatigue. Notably, incidence of CVD was not increased in this group in the study by Mazzotti et al.24 This finding is consistent with some,55 but not all,56 previous reports that elderly subjects with OSA are not at an increased risk of incident CVD. Effects of CPAP on recurrence in older populations with established CVD are not known, although subanalyses from the SAVE trial suggest lack of benefit for secondary prevention.14
Subtype C. This group includes middle-aged, mildly obese, predominantly female subjects with symptoms of insomnia (difficulty falling and staying asleep, early awakenings, and nonrestorative sleep). In six studies,20,24,30,31,33,34 10% to 35% of patients exhibited analogous symptoms with moderate to severe OSA and hypertension, diabetes, and CVD prevalence that were generally higher than for Subtype A and lower than for Subtype C. Although CPAP success was lower in this group than in younger sleepy patients (OR, 0.66),34 regular CPAP users exhibited significant symptomatic improvements in self-reported sleepiness (rather than ESS) and restful sleep but not insomnia.23 Previous studies showed that patients with comorbid OSA and insomnia exhibit lower CPAP adherence vs those with OSA alone and that middle-of-the-night insomnia, rather than sleep onset or early awakenings, tends to respond to CPAP.57,58 Such findings suggest that in patients with OSA and insomnia, combination therapy such as cognitive behavioral therapy for sleep initiation insomnia and CPAP for middle insomnia (rather than cognitive behavioral therapy or CPAP alone) could be explored. The “Disturbed sleep” cluster in the Sleep Heart Health Study (SHHS) exhibited reduced risk of stroke compared with other OSA subtypes (HR, 0.19-0.26).24 These findings require confirmation and highlight the importance of objective sleep duration measurement given that insomnia with short sleep duration may be a risk factor for CVD.59
Subtype D. Patients in this group are younger nonobese, predominantly male subjects with primarily upper airway symptoms (snoring, sudden awakening, and cessation of breathing). Sleepiness (as measured by using the ESS or other questions) was not a predominant feature, with ESS scores consistently < 10 in four studies that identified similar patients.22,30,32,34 Insomnia symptoms occurred in a minority (13%-26%).30,34 The AHIs were in the traditional severe range (38-51 events/hour) and comparable to other clusters. With the exception of the cluster from Vavougios et al,22 these exhibited the lowest metrics of hypoxemia among other clusters (T90%, 10%-15%).32,34 The most prevalent comorbidity was hypertension (< 10% in all but the study of Vavougios et al), with overall lowest rates of associated comorbidities. Only Gagnadoux et al34 reported outcomes with lower CPAP success (OR, 0.66) for cluster 4, driven by lower improvements in ESS reduction and QOL (similar to Subtype C).
Although many other clusters were identified in studies using the feature domains described here, commonalities between those clusters were less apparent in the current review, recognizing the limitations of the qualitative approach and cluster implications that require validation.
Among PSG metric-based studies, two subtypes appear in most, both predominantly male (Fig 2). The first (Subtype E) includes patients with hyper-severe OSA (AHI, 66–84), highest BMIs (33-38 kg/m2), and most marked hypoxemia among the clusters (T90%, 20%–45%).21,36,37 They reported the highest ESS scores compared with other PSG clusters. A high fraction of apneas (89%–98%) may be suggestive of increased collapsibility, and marked obesity may predispose to hypoxia with respiratory events in this subtype.60,61 In the study by Zinchuk et al,21 such patients exhibited increased risk of incident CVD or death (HR, 1.91). Although high sleepiness and obesity are similar to Subtype A, lack of more detailed polysomnographic data in that cluster and detailed symptom data in the study by Zinchuk et al precludes direct comparisons.
The second common PSG phenotype (Subtype F) includes those with severe OSA (AHI, 34-68), lower BMIs (28-38 kg/m2), and notably lowest degrees of hypoxemia for a given AHI (T90%, 0%–12%).21,36,37 ESS scores (9–10) in these clusters were lower compared with other clusters of similar OSA severity. In study by Nakayama et al,37 such a subgroup exhibited the shortest respiratory event duration. Although speculative, such findings may suggest that in addition to pharyngeal collapsibility, low arousal threshold and/or elevated loop gain may contribute to pathogenesis in such patients.62,63 In the study by Zinchuk et al,21 the “Arousal and poor sleep” cluster exhibited the lowest rate of regular CPAP use (29%) among clusters with AHI ≥ 15 and did not exhibit a significant reduction in CVD or death with CPAP use (OR, 0.55; P > .05). For reasons similar to those noted earlier, direct comparisons with demographic/symptom/comorbidity-based clusters are difficult; however, both Subtype C (predominant insomnia, a disorder of hyperarousal) and Subtype D (less sleepy, with increased sudden awakenings and lower hypoxemia) may be connected with this polysomnographic subtype.
Other polysomnographic subtypes, such as those with REM-predominant OSA, were identified in more than one study and have been previously considered a potentially relevant clinical phenotype of OSA.64
A Potential Role for Phenotypic Clusters in a Precision Medicine Approach to OSA
There is growing evidence and consensus that the one-size-fits-all approaches are insufficient for the diagnosis and management of individuals with sleep apnea.2,16,26,27 Ideally, caring for patients with such a complex disorder would incorporate genetic, pathophysiologic, biomarker, phenotypic, and treatment response characteristics that form the foundation of precision medicine approaches. Targeted prevention, prognostication, and treatment selection based on phenotypes, including the potential phenotypic clusters described earlier, may represent a first step in that direction. For example, improved prognostication may be achieved by stratifying patients at risk of CVD based on a constellation of symptoms, such as those observed in the excessively sleepy subtype of OSA, rather than on AHI and ESS alone. Notably, inclusion of patients at different risk of adverse outcomes can bias clinical trials of a potentially useful therapy toward the null hypothesis (false negative).65 Phenotypes may therefore also be used to design more successful clinical trials of CPAP therapy by targeting subtypes of OSA with the highest risk of adverse health outcomes and largest symptomatic benefit from CPAP, such as the excessively sleepy OSA, a group excluded from previous trials of CPAP therapy in CVD.14,15
Deeper characterization of individuals exhibiting a particular phenotype may also reveal pathophysiological mechanisms, genetic risk factors, and new potential treatments. For example, patients with OSA and profound sleep fragmentation whose respiratory events are predominantly associated with arousals may exhibit short event duration.37 This scenario, in turn, has been associated with certain genetic loci66 and may reflect specific pathophysiological mechanisms such as easy arousability and/or increased loop gain.62,63 Treatment in these patients might include attenuating arousability with non-myorelaxant hypnotic agents and reducing loop gain with acetazolamide,67,68 alone or in combination with some form of upper airway stabilization (oral appliance or CPAP). The former has been previously reported to improve adherence and AHI in unselected patients with OSA.69,70
Limitations of Current Literature, Unanswered Questions, and Future Directions
The examples discussed here highlight the potential utility of phenotypic clusters. However, the studies reviewed also reflect a number of limitations to phenotype-based approaches, in general, and those using unsupervised learning methods, in particular, that will need to be addressed prior to such approaches being used in personalized treatment of patients with OSA. For example, phenotypes based on clinical or polysomnographic features may not reflect unique pathophysiological or biological mechanisms (ie, endotypes) or genetic risk, and thus may limit the ability to identify new mechanism-based treatments or improve prognostication.16 In fact, similar to other heterogeneous disorders such as COPD and asthma, it is likely that an OSA phenotype may be an end-result of multiple mechanisms with several genetic risk factors.71 One way to ensure that phenotype-based approaches have utility is for future research to consistently link the phenotypes to clinically meaningful outcomes (eg, symptoms, response to therapy, health outcomes, QOL) (Table 2). Focus on outcomes beyond AHI reduction (eg, treatment adherence, BP reduction, cognitive performance) to define treatment success is prudent given the examples in the literature that lowering AHI does not translate to benefit10 or may even be harmful.72 In addition, research focusing on outcomes beyond “hard” end points such as risk of CVD or mortality to include patient-centered outcomes such as OSA-specific QOL and functional status73 may help tailor evaluation of treatment response for each patient subtype and thus personalized care. For example, although in sleepy patients (Subtype A, “Classic”), assessing response to treatment using sleepiness metrics maybe sufficient, for others such as Subtype B (“Oldest-comorbid”) or Subtype C (“Female, insomnia”), assessing snoring, fatigue, and QOL, or early awakenings, sleep quality, and function, respectively, may be needed. Targeting patient outcomes based on phenotypes requires prospective assessment and validation.
Table 2.
Summary of Gaps and Future Directions
|
Currently, very limited data are available on reproducibility of phenotypic clusters within the same datasets (ie, consistency of classifying OSA individuals into a given subtype) and across cohorts (eg, clinical, population). Assessing cluster stability by using established approaches such as the Rand or Jaccard index74 to ensure that the phenotypes are reproducible is needed prior to evaluating their clinical or pathophysiological implications. Notably, in other heterogeneous disorders such as COPD, examining clusters based on common clinical and physiological variables across 10 independent cohorts showed that clustering results were only modestly reproducible.75 Although the “Disturbed sleep,” “Excessive sleepiness,” and “Minimally symptomatic” clusters, as assessed in both clinical and population samples,24,30,31 represent a first step in establishing generalizability of phenotypic subtypes, studies that evaluate reproducibility across populations are needed.
One barrier to addressing these challenges has been the lack of large datasets and consistency in features and clustering methods. Identifying and validating unique (and clinically relevant) OSA phenotypes require large, multidimensional datasets compiled by using validated and transparent methods. The Sleep Apnea Global Interdisciplinary Consortium and the National Sleep Research Resource (www.sleepdata.org), a data repository containing > 30,000 PSG records and various phenotype data from well-characterized cohorts, exemplify some of the resources that can be leveraged for this purpose. Harmonizing methods for characterizing OSA risk factors, symptoms, polysomnographic manifestations, and physiological traits (eg, loop gain, arousal threshold) for use as features in cluster analyses are critical to ensure consistency of identification and implication of phenotypic clusters across subjects and settings. In addition, it is unknown whether domain-specific (eg, symptoms) or multidomain (eg, risk factors, symptoms, PSG features) clustering approaches are optimal to identify unique OSA phenotypes. The domain-specific approaches are suited to interpret the implications of heterogeneity within a single domain of data (eg, symptomatic presentation) but may be difficult to integrate with other OSA characteristics for those subgroups (eg, PSG features, risk factors) not included in clustering. Conversely, the multidomain approaches provide a more holistic view of the disorder subtypes but can be difficult to interpret. Novel methods that integrate clusters across data domains in an interpretable way have been developed in the fields of cancer and lung fibrosis.76, 77, 78 Application of such strategies to multidomain OSA data may help identify OSA endotypes, linking pathogenesis, polysomnographic manifestations, and symptoms with unique clinical implications.
Although more cohorts with consistent features and clustering methods are likely to address reproducibility of phenotypic OSA clusters, studies incorporating new features are needed to refine current and elucidate novel phenotypic expressions of OSA with clinical implications. For example, incorporating new measures such as hypoxic burden,79,80 respiratory event duration,81 arousal intensity, and heart rate response82 could more precisely define a current polysomnographic OSA phenotype with frequent events, fragmented sleep, and lack of hypoxemia. Continuous measures of physiologic signals, such as cardiopulmonary coupling, may enable longitudinal assessment of its clinical implications (eg, sleep quality).83 Lack of reproducible and precisely defined phenotypes has been identified as a barrier to linking the phenotypes to pathogenic mechanisms or endotypes (eg, high pharyngeal collapsibility, low arousal threshold) upon which treatment can be personalized, a key gap in the field.26 Ultimately, coupling phenotypic expression (cluster-based or defined by using supervised analytic methods) with endotypes and genomic and genetic data as well as biomarkers is needed to define disorder subtypes and develop precision medicine approaches.
A clear opportunity exists to incorporate biomarker profiles in OSA (blood, salivary, urinary, or other) to better understand the sequelae imposed by the current subtypes, assess response to treatment, and monitor progression. For example, evaluating inflammatory [eg, C-reactive protein, interleukin-8, tyrosine(Y) lysine(K) leucine(L)-40 peptide], metabolic (eg, insulin, fatty acids), and sympathetic activation (eg, norepinephrine/epinephrine) biomarkers in the OSA subtypes (Figs 1 and 2) may provide mechanistic insights into the profiles of risk for CVD, neurocognitive, or liver dysfunction84, 85, 86, 87, 88 among these subtypes. Incorporating potential biomarker profiles into multidomain, unsupervised learning analyses that also include symptoms and/or polysomnographic features may help identify new phenotypes with biomarker relations to sleepiness and insomnia and/or intermittent hypoxia and sleep fragmentation. Because some biomarkers exhibit responses to treatment,9,86 they may be used to predict response to treatment or monitor risk over time. One example includes a cluster of micro-RNAs that predicts BP response to CPAP in a clinical phenotype of patients with OSA and resistant hypertension.9
Given that some phenotypic clusters, such as “Excessively sleepy,” exhibit potential prognostic (risk of CVD) and treatment (CPAP response) utility, prediction models of how to categorize patients into such subtypes without clustering are needed. Ideally, predictors would include readily obtainable clinical, sleep study, and potentially biomarker data. This method can enable prospective validation of clinical outcomes in observational studies and randomized clinical trials, necessary before such approaches can be applied to caring for those with sleep apnea.
Finally, most phenotypic clustering studies include predominantly middle-aged to older white men. This population does not reflect the heterogeneity of OSA as affected by age, sex, and race/ethnicity. For example, the role of anatomical vs nonanatomical risk factors for OSA differs between younger and older individuals,89 men and women,90 as well as Asian, African-American, and Caucasian race/ethnicities.91, 92, 93 Sleep symptoms also vary, with the highest prevalence of excessive sleepiness in African-American individuals,1,94 findings that may in part be explained by epigenetic changes.95 Lastly, implications of OSA differ according to race/ethnicity and age. Higher prevalence of nocturnal BP nondipping in African-American subjects vs Caucasian subjects and differences in risk of CVD between men and women with OSA are just some examples.96 If we are to embrace heterogeneity of sleep apnea to develop equitable and individualized approaches for improving the lives of patients, studies must include a broader spectrum of age, more women, and racially and ethnically diverse populations.
Altogether, identifying and validating clinically relevant phenotypic subtypes of OSA may be a promising avenue toward applying precision medicine tools to patients with sleep apnea. Such approaches are bound to inform prognosis, provide insight into mechanisms, and allow for the design of more rigorous clinical trials that will lead to personalized treatments for patients.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. Z. is supported by the Parker B. Francis Fellowship Award. H. K. Y. is supported by the NIH/NHLBI K24 HL 132093 Mentoring in Sleep Research and Sleep Interventions in Heart Disease and Stroke.
References
- 1.Chen X., Wang R., Zee P. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carberry J.C., Amatoury J., Eckert D.J. Personalized management approach for OSA. Chest. 2018;153(3):744–755. doi: 10.1016/j.chest.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Bjorvatn B., Lehmann S., Gulati S., Aurlien H., Pallesen S., Saxvig I.W. Prevalence of excessive sleepiness is higher whereas insomnia is lower with greater severity of obstructive sleep apnea. Sleep Breath. 2015;19(4):1387–1393. doi: 10.1007/s11325-015-1155-5. [DOI] [PubMed] [Google Scholar]
- 4.Conwell W., Patel B., Doeing D. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16(2):519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 5.Ayappa I., Rapaport B.S., Norman R.G., Rapoport D.M. Immediate consequences of respiratory events in sleep disordered breathing. Sleep Med. 2005;6(2):123–130. doi: 10.1016/j.sleep.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Koch H., Schneider L.D., Finn L.A. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea. Sleep. 2017;40(11) doi: 10.1093/sleep/zsx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokhlesi B., Finn L.A., Hagen E.W. Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roca G.Q., Redline S., Claggett B. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort. Circulation. 2015;132(14):1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-de-la-Torre M., Khalyfa A., Sanchez-de-la-Torre A. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol. 2015;66(9):1023–1032. doi: 10.1016/j.jacc.2015.06.1315. [DOI] [PubMed] [Google Scholar]
- 10.Barbe F., Duran-Cantolla J., Sanchez-de-la-Torre M. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 11.Edwards B.A., Andara C., Landry S. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194(11):1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb D.J., Punjabi N.M., Mehra R. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushida C.A., Nichols D.A., Holmes T.H. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35(12):1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEvoy R.D., Antic N.A., Heeley E. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 15.Peker Y., Glantz H., Eulenburg C., Wegscheider K., Herlitz J., Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 16.Zinchuk A.V., Gentry M.J., Concato J., Yaggi H.K. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev. 2017;35:113–123. doi: 10.1016/j.smrv.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strollo P.J., Jr., Soose R.J., Maurer J.T. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 18.Jameson J.L., Longo D.L. Precision medicine—personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 19.Pillai R.A., Calhoun W.J. Introduction to asthma and phenotyping. Adv Exp Med Biol. 2014;795:5–15. doi: 10.1007/978-1-4614-8603-9_1. [DOI] [PubMed] [Google Scholar]
- 20.Ye L., Pien G.W., Ratcliffe S.J. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–1607. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinchuk A.V., Jeon S., Koo B.B. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73(5):472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vavougios G.D., George D.G., Pastaka C., Zarogiannis S.G., Gourgoulianis K.I. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res. 2016;25(1):31–38. doi: 10.1111/jsr.12344. [DOI] [PubMed] [Google Scholar]
- 23.Pien G.W., Ye L., Keenan B.T. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic Sleep Apnea Cohort. Sleep. 2018;41(3) doi: 10.1093/sleep/zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzotti D.R., Keenan B.T., Lim D.C., Gottlieb D.J., Kim J., Pack A.I. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. doi: 10.1164/rccm.201808-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan W., Zheng D., Douglas McEvoy R. High risk characteristics for recurrent cardiovascular events among patients with obstructive sleep apnoea in the SAVE study. EClinicalMedicine. 2018;2:59–65. doi: 10.1016/j.eclinm.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards B.A., Redline S., Sands S.A., Owens R.L. More than the sum of the respiratory events: personalized medicine approaches for obstructive sleep apnea. Am J Respir Crit Care Med. 2019;200(6):691–703. doi: 10.1164/rccm.201901-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim D.C., Sutherland K., Cistulli P.A., Pack A.I. P4 medicine approach to obstructive sleep apnoea. Respirology. 2017;22(5):849–860. doi: 10.1111/resp.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Garcia M.A., Campos-Rodriguez F., Barbe F., Gozal D., Agusti A. Precision medicine in obstructive sleep apnoea. Lancet Respir Med. 2019;7(5):456–464. doi: 10.1016/S2213-2600(19)30044-X. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland K., Almeida F.R., de Chazal P., Cistulli P.A. Prediction in obstructive sleep apnoea: diagnosis, comorbidity risk, and treatment outcomes. Expert Rev Respir Med. 2018;12(4):293–307. doi: 10.1080/17476348.2018.1439743. [DOI] [PubMed] [Google Scholar]
- 30.Keenan B.T., Kim J., Singh B. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep. 2018;41(3) doi: 10.1093/sleep/zsx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J., Keenan B.T., Lim D.C., Lee S.K., Pack A.I., Shin C. Symptom-based subgroups of Koreans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(3):437–443. doi: 10.5664/jcsm.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailly S., Destors M., Grillet Y. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira-Santos D., Pereira Rodrigues P. Phenotyping obstructive sleep apnea patients: a first approach to cluster visualization. Stud Health Technol Inform. 2018;255:75–79. [PubMed] [Google Scholar]
- 34.Gagnadoux F., Le Vaillant M., Paris A. Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest. 2016;149(1):288–290. doi: 10.1016/j.chest.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Joosten S.A., Hamza K., Sands S., Turton A., Berger P., Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17(1):99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 36.Lacedonia D., Carpagnano G.E., Sabato R. Characterization of obstructive sleep apnea-hypopnea syndrome (OSA) population by means of cluster analysis. J Sleep Res. 2016;25(6):724–730. doi: 10.1111/jsr.12429. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama H, Kobayashi M, Tsuiki S, Yanagihara M, Inoue Y. Obstructive sleep apnea phenotypes in men based on characteristics of respiratory events during polysomnography [published online ahead of print January 29, 2019]. Sleep Breath. https://doi.org/10.1007/s11325-019-01785-8. [DOI] [PubMed]
- 38.Appleton S.L., Vakulin A., Martin S.A. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest. 2016;150(3):495–505. doi: 10.1016/j.chest.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Aurora R.N., Crainiceanu C., Gottlieb D.J., Kim J.S., Punjabi N.M. Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med. 2018;197(5):653–660. doi: 10.1164/rccm.201706-1112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turino C., Bertran S., Gavalda R. Characterization of the CPAP-treated patient population in Catalonia. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0185191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens R.L., Macrea M.M., Teodorescu M. The overlaps of asthma or COPD with OSA: a focused review. Respirology. 2017;22(6):1073–1083. doi: 10.1111/resp.13107. [DOI] [PubMed] [Google Scholar]
- 42.Teodorescu M., Barnet J.H., Hagen E.W., Palta M., Young T.B., Peppard P.E. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015;313(2):156–164. doi: 10.1001/jama.2014.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos-Rodriguez F., Martinez-Garcia M.A., Martinez M. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 44.Kendzerska T., Mollayeva T., Gershon A.S., Leung R.S., Hawker G., Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18(1):49–59. doi: 10.1016/j.smrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Babbin S.F., Velicer W.F., Aloia M.S., Kushida C.A. Identifying longitudinal patterns for individuals and subgroups: an example with adherence to treatment for obstructive sleep apnea. Multivariate Behav Res. 2015;50(1):91–108. doi: 10.1080/00273171.2014.958211. [DOI] [PubMed] [Google Scholar]
- 46.Chai-Coetzer C.L., Luo Y.M., Antic N.A. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36(12):1929–1937. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luyster F.S., Strollo P.J., Jr., Thunstrom E., Peker Y. Long-term use of continuous positive airway pressure therapy in coronary artery disease patients with nonsleepy obstructive sleep apnea. Clin Cardiol. 2017;40(12):1297–1302. doi: 10.1002/clc.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakker J.P., Wang R., Weng J. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest. 2016;150(2):337–345. doi: 10.1016/j.chest.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stepnowsky C.J., Marler M.R., Palau J., Annette Brooks J. Social-cognitive correlates of CPAP adherence in experienced users. Sleep Med. 2006;7(4):350–356. doi: 10.1016/j.sleep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Liu D., Armitstead J., Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751–760. doi: 10.1016/j.chest.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pepin J.L., Woehrle H., Liu D. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med. 2018;14(1):57–63. doi: 10.5664/jcsm.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooksey J., Mokhlesi B. Postoperative complications in obesity hypoventilation syndrome and hypercapnic OSA: CO2 levels matter! Chest. 2016;149(1):11–13. doi: 10.1016/j.chest.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Crawford M.R., Espie C.A., Bartlett D.J., Grunstein R.R. Integrating psychology and medicine in CPAP adherence—new concepts? Sleep Med Rev. 2014;18(2):123–139. doi: 10.1016/j.smrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Stone K.L., Blackwell T.L., Ancoli-Israel S. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39(3):531–540. doi: 10.5665/sleep.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottlieb D.J., Yenokyan G., Newman A.B. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Garcia M.A., Campos-Rodriguez F., Catalan-Serra P. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909–916. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 57.Bjornsdottir E., Janson C., Sigurdsson J.F. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901–1909. doi: 10.5665/sleep.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallace D.M., Sawyer A.M., Shafazand S. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath. 2018;22(1):5–15. doi: 10.1007/s11325-017-1605-3. [DOI] [PubMed] [Google Scholar]
- 59.Javaheri S., Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. doi: 10.1016/j.chest.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gleadhill I.C., Schwartz A.R., Schubert N., Wise R.A., Permutt S., Smith P.L. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 61.Peppard P.E., Ward N.R., Morrell M.J. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180(8):788–793. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sands S.A., Terrill P.I., Edwards B.A. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep. 2018;41(1) doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sforza E., Boudewijns A., Schnedecker B., Zamagni M., Krieger J. Role of chemosensitivity in intrathoracic pressure changes during obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154(6 pt 1):1741–1747. doi: 10.1164/ajrccm.154.6.8970364. [DOI] [PubMed] [Google Scholar]
- 64.Varga A.W., Mokhlesi B. REM obstructive sleep apnea: risk for adverse health outcomes and novel treatments. Sleep Breath. 2019;23(2):413–423. doi: 10.1007/s11325-018-1727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwashyna T.J., Burke J.F., Sussman J.B., Prescott H.C., Hayward R.A., Angus D.C. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192(9):1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cade B.E., Chen H., Stilp A.M. Genetic associations with obstructive sleep apnea traits in Hispanic/Latino Americans. Am J Respir Crit Care Med. 2016;194(7):886–897. doi: 10.1164/rccm.201512-2431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eckert D.J., Owens R.L., Kehlmann G.B. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120(12):505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards B.A., Sands S.A., Eckert D.J. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590(pt 5):1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eskandari D., Zou D., Grote L., Hoff E., Hedner J. Acetazolamide reduces blood pressure and sleep-disordered breathing in patients with hypertension and obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2018;14(3):309–317. doi: 10.5664/jcsm.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lettieri C.J., Shah A.A., Holley A.B. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151(10):696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- 71.Agusti A. Phenotypes and disease characterization in chronic obstructive pulmonary disease. Toward the extinction of phenotypes? Ann Am Thorac Soc. 2013;10(suppl):S125–S130. doi: 10.1513/AnnalsATS.201303-055AW. [DOI] [PubMed] [Google Scholar]
- 72.Castro-Grattoni A.L., Torres G., Martinez-Alonso M. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: the predictive value of 24-h ambulatory blood pressure monitoring. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.00651-2017. [DOI] [PubMed] [Google Scholar]
- 73.Billings M.E., Rosen C.L., Auckley D. Psychometric performance and responsiveness of the functional outcomes of sleep questionnaire and sleep apnea quality of life instrument in a randomized trial: the HomePAP study. Sleep. 2014;37(12):2017–2024. doi: 10.5665/sleep.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Luxburg U. Clustering stability: an overview. Found Trends Mach Learning. 2010;2(3):235–274. [Google Scholar]
- 75.Castaldi P.J., Benet M., Petersen H. Do COPD subtypes really exist? COPD heterogeneity and clustering in 10 independent cohorts. Thorax. 2017;72(11):998–1006. doi: 10.1136/thoraxjnl-2016-209846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S., Herazo-Maya J.D., Kang D.D. Integrative phenotyping framework (iPF): integrative clustering of multiple omics data identifies novel lung disease subphenotypes. BMC Genomics. 2015;16:924. doi: 10.1186/s12864-015-2170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen T., Tagett R., Diaz D., Draghici S. A novel approach for data integration and disease subtyping. Genome Res. 2017;27(12):2025–2039. doi: 10.1101/gr.215129.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang B., Mezlini A.M., Demir F. Similarity network fusion for aggregating data types on a genomic scale. Nat Methods. 2014;11(3):333–337. doi: 10.1038/nmeth.2810. [DOI] [PubMed] [Google Scholar]
- 79.Azarbarzin A., Sands S.A., Stone K.L. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40(14):1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kulkas A., Duce B., Leppanen T., Hukins C., Toyras J. Severity of desaturation events differs between hypopnea and obstructive apnea events and is modulated by their duration in obstructive sleep apnea. Sleep Breath. 2017;21(4):829–835. doi: 10.1007/s11325-017-1513-6. [DOI] [PubMed] [Google Scholar]
- 81.Butler M.P., Emch J.T., Rueschman M. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study. Am J Respir Crit Care Med. 2019;199(7):903–912. doi: 10.1164/rccm.201804-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Azarbarzin A., Ostrowski M., Hanly P., Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014;37(4):645–653. doi: 10.5665/sleep.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas RJ, Wood C, Bianchi MT. Cardiopulmonary coupling spectrogram as an ambulatory clinical biomarker of sleep stability and quality in health, sleep apnea and insomnia [published online ahead of print December 9, 2019]. Sleep. https://doi.org/10.1093/sleep/zsx196. [DOI] [PMC free article] [PubMed]
- 84.Baril A.A., Carrier J., Lafreniere A. Biomarkers of dementia in obstructive sleep apnea. Sleep Med Rev. 2018;42:139–148. doi: 10.1016/j.smrv.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Luca Canto G., Pacheco-Pereira C., Aydinoz S., Major P.W., Flores-Mir C., Gozal D. Biomarkers associated with obstructive sleep apnea and morbidities: a scoping review. Sleep Med. 2015;16(3):347–357. doi: 10.1016/j.sleep.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 86.Jullian-Desayes I., Joyeux-Faure M., Tamisier R. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: a systematic review from sham CPAP randomized controlled trials. Sleep Med Rev. 2015;21:23–38. doi: 10.1016/j.smrv.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Mesarwi O.A., Shin M.K., Drager L.F. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep. 2015;38(10):1583–1591. doi: 10.5665/sleep.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peres B.U., Hirsch Allen A.J., Fox N. Circulating biomarkers to identify cardiometabolic complications in patients with obstructive sleep apnea: a systematic review. Sleep Med Rev. 2019;44:48–57. doi: 10.1016/j.smrv.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Edwards B.A., Wellman A., Sands S.A. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37(7):1227–1236. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pengo M.F., Won C.H., Bourjeily G. Sleep in women across the life span. Chest. 2018;154(1):196–206. doi: 10.1016/j.chest.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dudley K.A., Patel S.R. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2016;18:96–102. doi: 10.1016/j.sleep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee R.W., Vasudavan S., Hui D.S. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee R.W.W., Sutherland K., Sands S.A. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology. 2017;22(5):1015–1021. doi: 10.1111/resp.13022. [DOI] [PubMed] [Google Scholar]
- 94.Baldwin C.M., Ervin A.M., Mays M.Z. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6(2):176–183. [PMC free article] [PubMed] [Google Scholar]
- 95.Barfield R., Wang H., Liu Y. Epigenome-wide association analysis of daytime sleepiness in the Multi-Ethnic Study of Atherosclerosis reveals African-American-specific associations. Sleep. 2019;42(8) doi: 10.1093/sleep/zsz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ancoli-Israel S., Stepnowsky C., Dimsdale J., Marler M., Cohen-Zion M., Johnson S. The effect of race and sleep-disordered breathing on nocturnal BP "dipping": analysis in an older population. Chest. 2002;122(4):1148–1155. doi: 10.1378/chest.122.4.1148. [DOI] [PubMed] [Google Scholar]