Abstract
Sleep-disordered breathing (SDB), including OSA and central sleep apnea, is highly prevalent in patients with heart failure (HF). Multiple studies have reported this high prevalence in asymptomatic as well as symptomatic patients with reduced left ventricular ejection fraction (HFrEF), as well as in those with HF with preserved ejection fraction.
The acute pathobiologic consequences of OSA, including exaggerated sympathetic activity, oxidative stress, and inflammation, eventually could lead to progressive left ventricular dysfunction, repeated hospitalization, and excessive mortality. Large numbers of observational studies and a few small randomized controlled trials have shown improvement in various cardiovascular consequences of SDB with treatment. There are no long-term randomized controlled trials to show improved survival of patients with HF and treatment of OSA. One trial of positive airway pressure treatment of OSA included patients with HF and showed no improvement in clinical outcomes. However, any conclusions derived from this trial must take into account several important pitfalls that have been extensively discussed in the literature. With the role of positive airway pressure as the sole therapy for SDB in HF increasingly questioned, a critical examination of long-accepted concepts in this field is needed. The objective of this review was to incorporate recent advances in the field into a phenotype-based approach to the management of OSA in HF.
Key Words: adaptive servo-ventilation, central sleep apnea, heart failure, Hunter-Cheyne-Stokes breathing, noninvasive ventilation, oxygen, positive airway pressure
Abbreviations: AHI, apnea-hypopnea index; CSA, central sleep apnea; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LG, loop gain; PAP, positive airway pressure; RCT, randomized controlled trial; SDB, sleep-disordered breathing
Heart failure (HF) is a major and growing global public health problem affecting an estimated 26 million people worldwide.1 It is a progressive and highly prevalent disorder, associated with substantial morbidity, mortality, and economic cost. Patients with HF frequently experience worsening symptoms of dyspnea and fatigue, resulting in exercise intolerance and hospitalization.2 HF is the most frequent cause of hospitalization in US Medicare beneficiaries,3 accounting for > 1 million admissions annually representing 1% to 2% of all hospitalizations.4 The economic impact of HF is profound, with the total costs of HF estimated to increase from $31 billion in 2012 to $70 billion in 2030 in the United States alone.5 These observations underscore the need to identify and treat CHF and its associated comorbidities, which also contribute to disease burden.
In this regard, multiple comorbidities have been identified in patients with HF, including sleep-disordered breathing (SDB). Although many of the comorbidities have been recognized and targeted for therapy for many years, SDB has generally been overlooked as a target for diagnosis and treatment, even though both forms of SDB, OSA and central sleep apnea (CSA), are highly prevalent in both forms of HF, namely HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction.6 Although OSA and CSA differ in pathophysiology, they share similar adverse sleep consequences common to SDB, including hypoxia/reoxygenation, marked swings in Paco2, and recurrent arousals.
In this review, the first of the series, we discuss the relationships between HF and OSA; in the second part, we discuss the characteristics peculiar to CSA.
Prevalence, Clinical Presentation, and Consequences of OSA in HF
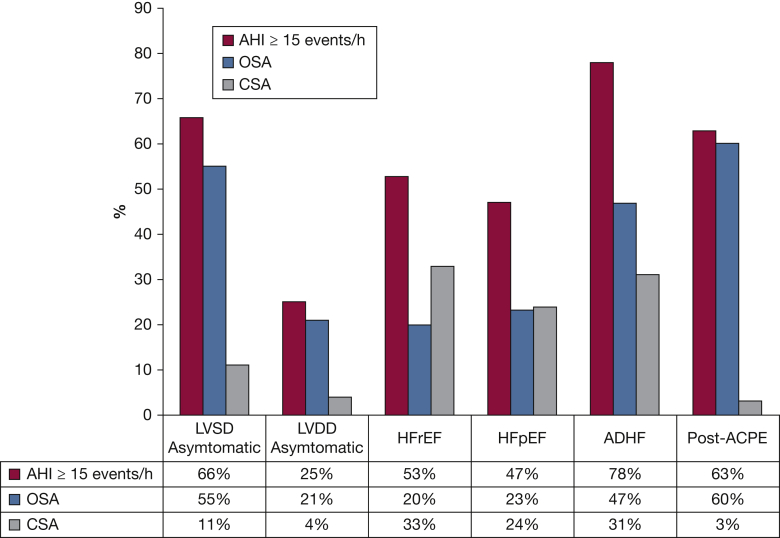
The high prevalence of SDB in individuals with both HFrEF and HF with preserved ejection fraction is well established7 and is summarized in Figure 1. In terms of the clinical presentation of OSA, there are important differences between its presentation in the general population and that in patients with HF. These are discussed in detail in e-Appendix 1. Briefly, sleepiness, an important symptom of OSA in the general population, is not an established presentation of OSA in patients with HF. In these patients, there is dissociation between patient-reported sleepiness and objectively measured sleepiness.8 The underreporting of sleepiness likely contributes to the underrecognition of OSA in HF.
Figure 1.
Prevalence of moderate to severe sleep apnea (AHI ≥ 15 events per hour) in asymptomatic LVSD or LVDD, HFpEF or HFrEF, ADHF, and ACPE. ACPE = acute cardiogenic pulmonary edema; ADHF = acutely decompensated heart failure; AHI = apnea-hypopnea index; CSA = central sleep apnea; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVDD = left ventricular diastolic dysfunction; LVSD = left ventricular systolic dysfunction. Reprinted with permission of Elsevier from Javaheri et al.7
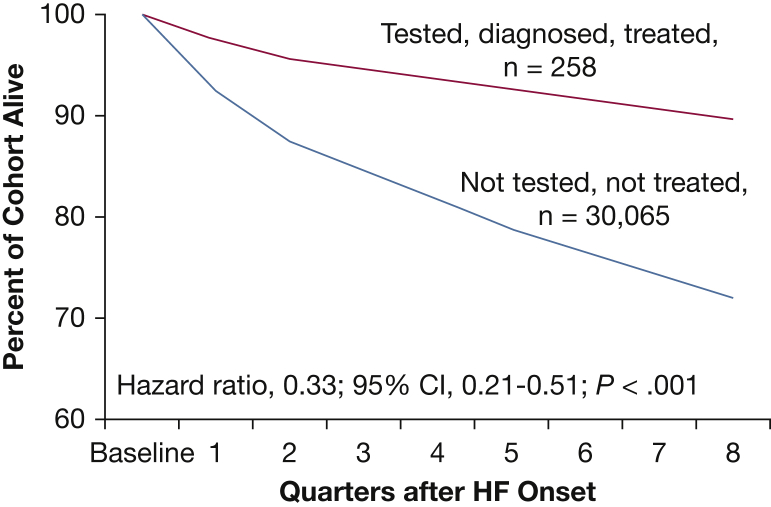
e-Appendix 1 contains a detailed discussion of the immediate effects (e-Fig 1) and the cardiovascular consequences of HF OSA in HF. It is important to note that large cohort studies from the United States have shown that OSA is independently associated with excess rehospitalization and premature mortality.9, 10, 11 In contrast, those patients with HF who were treated with CPAP had improved survival (Fig 2).
Figure 2.
Impact of sleep apnea on survival in heart failure (HF). Survival of patients with HF treated for sleep apnea (n = 258) and patients with HF not tested or treated for sleep apnea (n = 30,065). Kaplan-Meier survival curves adjusted according to age, sex, and Charlson Comorbidity Index. (Reprinted with permission of Am J Respir Crit Care Med. from Javaheri et al11)
Treatment of OSA in HF
Therapy of OSA with CPAP is considered the treatment of choice. Unfortunately, a significant number of patients with OSA reject CPAP, either initially or in the long term. Given the unacceptably low adherence to CPAP, identification of alternative treatment approaches is critical. Improved understanding of the pathophysiology of OSA in recent years provides an opportunity for an approach to individualizing therapies based on subpopulations and mechanisms.7 This approach has been considered for OSA in the general population. The following sections describe a phenotype-based therapeutic approach to OSA in HF.
Pathophysiologic Phenotypes of OSA and Their Therapeutic Implications
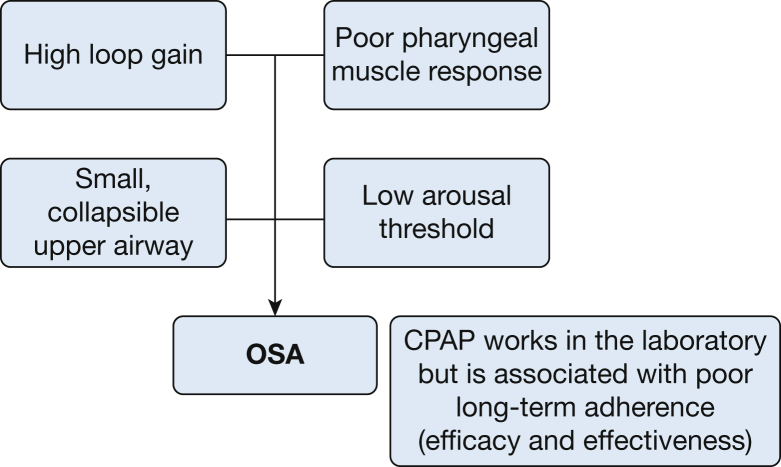
OSA is a heterogeneous disorder with both anatomic and nonanatomic mechanisms, either singularly or in combination, contributing to upper airway occlusion via upper airway dilator muscle relaxation, the ultimate common pathway. Several endotypic/phenotypic traits (Fig 3) have been identified in patients with OSA in the general population, with each phenotype being a target for a specific therapy.12, 13 These include high loop gain (LG), a condition that can occur in the feedback control system of breathing that promotes system instability; reduced pharyngeal dilator muscle activity during sleep; an anatomically narrow pharyngeal airway; and a low respiratory arousal threshold leading to recurrent awakenings. These traits have been best studied in individuals with OSA and no known HF. However, results from these investigations can be informative with respect to the pathogenesis and treatment of SDB in HF.
Figure 3.
The various phenotypic/endotypic traits of OSA. (Courtesy of Dr David White, with permission.)
Increased LG and Chemical Chemosensitivity in OSA
LG is a measure of the sensitivity of a negative feedback system to changes in the controlled variables. When this concept is applied to the respiratory system, it defines the magnitude of the change in respiratory drive in response to a given disturbance (eg, a minor decrease in ventilation).14 Thus, in the case of excessive LG, a minor decrease in ventilation (and thus a minor increase in Paco2, the controlled variable) will result in an excessive increase in ventilatory drive and ventilation (which may suction the upper airway muscles inward, causing pharyngeal closure, particularly in the presence of predisposing anatomy) and a consequent fall in Paco2. The latter results in an exaggerated reduction in ventilatory drive and ventilation, even an apnea, if it drops below the apneic threshold Pco2, collectively contributing to an increase in Paco2. The ventilatory control system is unstable and oscillates between periods of excessive ventilatory drive and periods of abnormally low, or even absent, output. The other component of respiratory control instability is increased LG below eupnea reflected in the difference between eupneic Paco2 and the apnea threshold, also known as the CO2 reserve. A low CO2 reserve makes it more likely that the ventilatory overshoot will bring the Paco2 to the apnea threshold precipitating central apnea.
The role of exaggerated LG in the pathogenesis of CSA has been well established.15, 16 Younes et al17 showed that increased LG contributes to the severity of OSA. It is not clear whether the high LG is the result of a genetic predisposition or is the consequence of severe OSA itself. To address this question, we measured hypoxic and hypercapnic ventilatory responses of the immediate family members of hypercapnic or normocapnic patients with OSA.18 There were no differences in chemical sensitivity to hypoxia or hypercapnia in the family members, suggesting that diminished chemosensitivity in patients with OSA was an acquired rather than an inherited trait. Interestingly, in a follow-up study, Loewen et al19 arrived at a similar conclusion by showing that in patients with severe OSA, increased drive declined after treatment of OSA with CPAP. We suggest that, regardless of the time course as to when high LG is acquired, its presence could contribute to worsening SDB and be a therapeutic target.
Two medications, oxygen20, 21 and acetazolamide,22, 23 have been used to treat OSA (without HF). Both were previously used to treat CSA associated with HF.24, 25 Acetazolamide reduces LG by decreasing the plant gain but increases CO2 chemosensitivity; the cumulative effect of these opposing tendencies determines responsiveness. Edwards et al23 performed detailed physiological measurements in 13 patients with OSA who received acetazolamide at 500 mg bid for 1 week. LG was reduced significantly in association with reduction in the non-rapid-eye-movement sleep apnea-hypopnea index (AHI) with a modest correlation between reductions in LG and AHI. Interestingly, increased carbonic anhydrase activity is reportedly associated with severity of OSA and related hypoxemia.26 As noted earlier, severe OSA is the form associated with increased LG. Based on the aforementioned studies, it is conceivable that acetazolamide may be most effective in the severe OSA subgroup, and a trial in OSA/HF is warranted, potentially with triple effects: decreasing plant gain; being a mild diuretic; and moving the alkalotic pH toward normal values, further improving periodic breathing.15
The other drug therapy that downregulates LG is oxygen, which has been studied extensively in HFrEF patients with CSA. There are few studies on the use of oxygen to treat OSA patients without HF.20, 21 In a recent study,27 36 patients with severe OSA (average AHI 57 events per hour) completed two nights of polysomnography on supplemental oxygen (40%) vs sham (air). OSA traits were quantified from the air-night polysomnography. Nine of 36 patients (25%) responded to supplemental oxygen (AHI decreased from 59 to 12 events per hour of sleep).
It remains to be determined whether acetazolamide, oxygen, or their combination could effectively treat OSA in patients with HF and elevated LG. Given that patients with both HF and OSA are frequently not reporting hypersomnolence and do not experience symptomatic improvement with positive airway pressure (PAP) (and consequently exhibit poor adherence), a pharmaceutical solution may represent an attractive alternative.
Reduced Pharyngeal Dilator Muscle Tone and OSA
In patients with dilator muscle activity insufficient to maintain an open airway while asleep, hypoglossal nerve stimulation is currently somewhat popular as the target of therapy. This approach has been used to treat OSA in the general population, in PAP-intolerant subjects, or in those who refuse PAP. In one such study,28 the mean AHI decreased from 32 ± 12 events per hour to 15 ± 16 events per hour following 12 months of hypoglossal stimulation. The study most likely included responsive and nonresponsive patients given the substantial variability in AHI response at 12 months. Patients were not preselected based on poor dilator muscle activity. It may be hypothesized that screening for this trait could allow for the prediction of suitable candidates; further studies are required to examine this strategy. Two of the patients in the aforementioned trial had HF, but whether either of them was a treatment success or failure was not reported. Given the overall risk of anesthesia and implantation in HF, it would be important to treat only the OSA phenotype that would be most responsive to stimulation.
In addition to hypoglossal nerve stimulation, treatment of OSA with noradrenergic agonists represents another therapeutic approach, as noradrenergic withdrawal is believed to be the main cause of pharyngeal hypotonia in non-rapid-eye-movement sleep.29, 30 However, in view of their potential cardiotoxicity,31 tricyclic antidepressant use in the HF population is not warranted.
Anatomically Narrow Airway
Oral appliances have been used to treat OSA both in the general population and in patients with HF, usually without screening for any particular anatomic compromise. In a preliminary observational study of 25 patients with HF, Eskafi32 showed that AHI decreased nearly 50% after 4 to 6 weeks of therapy. Eskafi et al33 also evaluated oral appliances in a small controlled study in patients with HF and found that the range of AHI response varied from a decrease of two to 34 events per hour, suggesting that, just as for OSA in the general population, some patients with HF are responsive and some are nonresponsive to this therapeutic modality. A large trial in patients with HF and well-characterized OSA, likely limited to those with anatomically narrow upper airway, is warranted.
Low Respiratory Arousal Threshold
Proponents of this counterintuitive treatment modality theorize that, if arousal from sleep could be delayed (e-Fig 1), dilator muscles of the upper airway will eventually be recruited, thus opening the upper airway while the patient remains asleep, finally reestablishing sustained normal breathing. This idea has been tested in patients with OSA but without HF. Two different hypnotic agents, trazodone and eszopiclone, have been tested with limited success.34, 35 If eventually a very effective drug is found, inherent in this approach is the virtual certainty that obstructive events would lengthen, resulting in greater degrees of negative swings in intrathoracic/juxta-cardiac pressure, hypoxemia, hypercapnia, and respiratory acidosis such that adverse cardiovascular consequences, including arrhythmias, could ensue. Moreover, these more profound derangements in gas exchange and acid/base status may well promote excessive hyperventilation once the upper airway does finally open, although, if the drug happens to also downregulate chemical ventilatory response, it could attenuate postapnea hyperventilation. We must emphasize, however, that the effects of benzodiazepines on ventilatory response are variable.36 Overall, our concern is that a drug-induced prolonged arousal trait could be converted into an equally undesirable high LG trait. It can be anticipated that such an approach could be particularly hazardous in patients with cardiovascular disorders such as HF.
Therapies Targeting More Than One Pathway of OSA
Exercise and OSA
Exercise has been shown to improve OSA in the general population.37 We recently performed a controlled trial38 in which 65 subjects with OSA/HFrEF were randomized to one of four arms: usual guideline-based care alone, or that combined with either exercise only, CPAP plus exercise, or CPAP alone. After 3 months of follow-up, the mean AHI did not change significantly in the usual care group, decreased in the exercise group (28 to 18), and decreased significantly more in the CPAP group (32 to 8) and in the exercise plus CPAP group (25 to 10). Importantly, both exercise and CPAP improved subjective excessive daytime sleepiness, quality of life, and New York Heart Association functional class. The reduction in AHI in patients with HF in the exercise arm (a 35% reduction) is consistent with a study by Ueno et al39 utilizing a similar supervised exercise protocol in a comparable group of patients with HF and OSA. These two studies lend support to a treatment strategy involving either exercise alone or exercise plus PAP that would have a salutary effect on OSA in HF and improve the quality of life of these patients. Consistent with the results of these 2 polysomnographic studies are studies showing that, in patients with HF, exercise and cardiac rehabilitation programs (which inevitably involve programmed exercise) attenuate exertional oscillatory ventilation.38, 40
The mechanism by which exercise improves OSA is likely multifactorial. Possible pathways include factors such as weight loss, decreasing lower extremity edema and consequent cephalad fluid translocation when the patient reclines for sleep,41 or stabilization of ventilatory control. Exercise stabilizes ventilatory control both in animal models of HF40 and in human experiments. In a study in man, Tomita et al42 showed that postmyocardial infarction exercise training downregulates the hypercapnic ventilatory response, attenuating LG, which should decrease the probability of developing apnea during sleep.
Compared with systemic exercise with pleotropic effects on sleep apnea, oropharyngeal exercise likely exerts a local effect in maintaining airway patency43 during sleep.
PAP Devices for Treatment of OSA
PAP devices, including CPAP and bilevel PAP, remain the most effective treatment option for OSA both in the general population and in patients with HF. Although acceptance and long-term adherence remain problematic, there is no dispute that, when used, PAP therapy suppresses OSA. In addition to elimination of the upper airway obstruction, the positive end-expiratory pressure has multiple physiological effects relevant to the patients with HF (as discussed in the following sections).
PAP has been used extensively for the treatment of respiratory failure due to decompensated HF and pulmonary edema.44 In these patients, PAP improves gas exchange, increases left ventricular ejection fraction, and reduces left ventricular filling pressure.45 In one study, a few hours of CPAP administration reduced myocardial muscle energy consumption without decreasing cardiac contractile efficiency.46 Furthermore, CPAP reduced respiratory and cardiac muscle work load within < 2 h of administration.47 An important effect of CPAP in patients with a failing heart is the increase in the intrathoracic pressure and subsequent reduction in the transmural cardiac pressure gradient resulting in reduced myocardial work load and myocardial oxygen consumption.48, 49 However, a potential adverse effect associated with excessive increases in intrathoracic pressure is a reduction in venous return and right ventricular preload, plus an increase in right ventricular afterload as lung volume is increased. Such hemodynamic consequences of PAP therapy, particularly with added inspiratory pressure support (with bilevel and adaptive servo-ventilation), could be deleterious if the right ventricle is preload dependent and is facing increased afterload due to existing pulmonary hypertension.
One small randomized controlled trial (RCT) evaluated the effect of CPAP for the treatment of OSA during hospitalization for decompensated HF. in this setting, CPAP improved discharge left ventricular ejection fraction in patients with HFrEF.50 Other small studies evaluated the effect of CPAP in the treatment of OSA in patients with stable HF. CPAP was found to improve sympathetic overactivity,51, 52 including cardiac sympathetic tone and energetics, cardiac vagal tone,53 and cardiac afterload.54 In two small RCTs, several weeks of treatment with CPAP in patients with HFrEF and severe OSA resulted in improvement in left ventricular ejection fraction.55 In large observational cohort studies, patients with HF who were treated for SDB had improved survival9, 11 (Fig 3).
These aforementioned studies provided support for the safety and effectiveness of CPAP in patients with HF and OSA. Only more recently has a large RCT evaluated the effect of treating OSA with CPAP on cardiovascular events. The Sleep Apnea Cardiovascular Endpoints (SAVE) study conducted an international, multicenter, randomized trial that evaluated whether CPAP prevented major cardiovascular events in patients with OSA.56 The study randomly assigned 2,717 patients between ages 45 and 75 years with moderate to severe OSA and coronary or cerebrovascular disease to receive CPAP plus usual care or usual care alone. After a mean follow up of 3.7 years, there was no significant effect on prevention of cardiovascular events. We have emphasized57 a number of limitations to the SAVE trial that may have affected the results; for example, inclusion of a number of nonsleepy patients with OSA who may have been less likely to benefit from the CPAP effect on OSA-related cardiovascular outcomes. We also noted the low average adherence to CPAP of 3.3 h per night, which may be insufficient to improve cardiovascular outcomes. As it relates to the current review, SAVE excluded patients with HF in New York Heart Association functional class III and IV, and enrolled only those in class I and II.
Practical Implications and Multimodality Treatment Strategies
In the preceding section, we individually considered the mechanisms implicated in the pathogenesis of OSA with or without HF, and illustrated lessons learned for possible treatment strategies ranging from OSA without HF to OSA with HF. We justify this extensive discussion of non-PAP therapeutic approaches because adherence to PAP is commonly inadequate in patients with HF. In the future, personalized therapy for OSA in HF based on endotype/phenotype may achieve a place in the treatment paradigm for OSA in HF, obviating the difficulties in achieving PAP adherence. Clearly, only limited data are available concerning the phenotypes/endotypes of OSA in HF, and treatment not involving PAP remains speculative. At present, PAP therapy is the mainstay of treatment for OSA patients with or without HF with its known limitations.
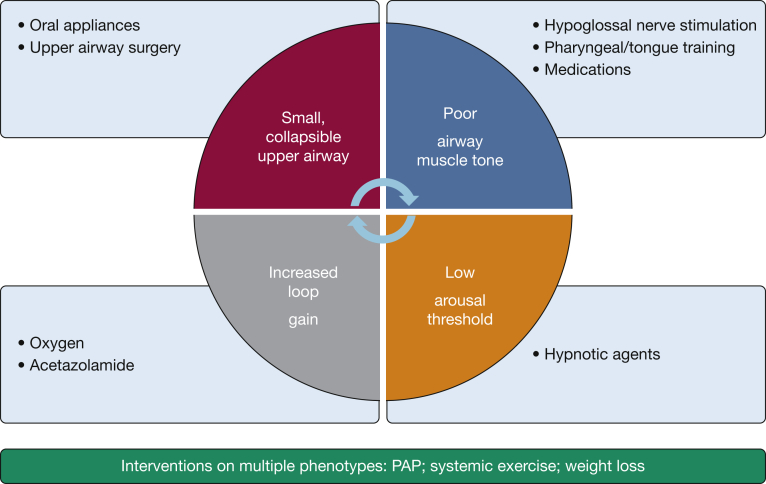
In this context, it is important for the clinician to determine the treatment target, especially when non-PAP approaches are considered. PAP devices can generally normalize the AHI into a range well below 5 events per hour. However, given that most trials reported < 4 h of use per night, the patient experiences the benefit of treatment only for one-half of the night. If an alternative approach that is tolerated for most of the sleep time can achieve a reduction in AHI into the mild rage (< 15 events per hour), the patient may then have a better overall reduction in AHI and desaturation burden than with PAP. Another consideration in the treatment of OSA, and SDB in general, in the HF population is achieving a decrease in oxygen saturation time < 90%, a parameter that was shown to correlate with excess mortality in patients with HFrEF.58 Examples of combination phenotype-based therapies include any mixture of weight reduction, supine preclusion, oral appliance, oxygen, hypoglossal stimulation, and acetazolamide. Figure 4 summarizes the phenotype-directed approach to treatment.
Figure 4.
A conceptual approach to phenotype-guided treatment for OSA in patients with heart failure. PAP = positive airway pressure.
Conclusions and Future Directions
Acute and chronic pathophysiologic consequences of OSA in the setting of HF are well known. Data from multiple observational studies show that OSA is associated with rehospitalization and mortality. Thus far, there is no adequately powered RCT evaluating CPAP, the most effective therapy for OSA, in patients with HF. Because patients with HF do not report sleepiness at the same rate as patients without HF, there should be less ethical concern regarding randomization to receive either CPAP or usual care. However, lack of subjective sleepiness will likely be associated with limited symptomatic improvement with CPAP, consequently contributing to poor adherence. One alternative is to randomize only eligible subjects, defined as those who used sham CPAP at least 5 h per night for several weeks. Use of alternative comparative therapies such as an oral appliance vs CPAP may fail to answer the ultimate question; that is, whether OSA is a cause of rehospitalization and mortality.
RCTs to evaluate the effect of therapy based on OSA phenotypes are needed. An example would be an RCT with an oral appliance in subjects with the phenotype of narrow upper airway anatomy. Pilot studies with oxygen and acetazolamide vs placebo for OSA/HF patients with high LG, compared with low LG, would be a reasonable next step as proof of concept. Meanwhile, in the absence of large RCTs showing clinically significant improvement in morbidity and mortality of patients with HF treated for OSA, what should the clinicians do in everyday practice? We heartily concur that evidence-based medicine is the most important source to guide therapy of any disorder. The available data do not provide any guidance on the best course of action in these patients. An approach that incorporates the phenotype-guided therapy is likely to be the next direction of this field. Thus far, clinicians are left to make individual decisions based on best judgment in a certain clinical scenario.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: L. K. B. has participated in advisory panels for Philips Respironics concerning adaptive servo-ventilation for patients with HF. He will be a collaborating investigator in a National Heart, Lung, and Blood Institute grant studying low-flow oxygen treatment in patients with HF and CSA. He has received grant support in the past from the National Science Foundation-funded Smart Lighting Engineering Research Center studying human circadian phase estimation and a related Innovation and Commercialization Award from the University of New Mexico National Institutes of Health Clinical and Translational Science Center for a study to advance human circadian phase utilizing programmed lighting. He co-edits the sleep and respiratory neurobiology section of Current Opinion in Pulmonary Medicine; wrote on CPAP treatment for OSA in UpToDate and on OSA in Clinical Decision Support: Pulmonary Medicine and Sleep Disorders; and co-edited an issue of Sleep Medicine Clinics on PAP therapy. He serves on the Polysomnography Practice Advisory Committee of the New Mexico Medical Board and chairs the New Mexico Respiratory Care Advisory Board for the State of New Mexico. None declared (S. J., W. T. A., R. K.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix and e-Figure can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by a grant [1 UG3 HL140144-01] from National Institutes of Health the National Heart, Lung, and Blood Institute (Drs Abraham, Javaheri, and Khayat).
Supplementary Data
References
- 1.Ambrosy A.P., Fonarow G.C., Butler J. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Schiff G.D., Fung S., Speroff T., McNutt R.A. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114(8):625–630. doi: 10.1016/s0002-9343(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare & Medicaid Services. 100% MEDPAR Inpatient Hospital Data for Fiscal Year 2016 Short Stay Inpatient By Diagnosis Related Groups. CMS; 2016. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/Downloads/DRG16.pdf. Accessed June 5, 2019.
- 4.Blecker S., Paul M., Taksler G., Ogedegbe G., Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidenreich P.A., Albert N.M., Allen L.A. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 7.Javaheri S., Barbe F., Campos-Rodriguez F. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehra R., Wang L., Andrews N. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med. 2017;13(12):1411–1422. doi: 10.5664/jcsm.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khayat R., Jarjoura D., Porter K. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463–1469. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khayat R., Abraham W., Patt B. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18(7):534–540. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javaheri S., Caref E.B., Chen E., Tong K.B., Abraham W.T. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183(4):539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 12.Eckert D.J., White D.P., Jordan A.S., Malhotra A., Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellman A., Edwards B.A., Sands S.A. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 2013;114(7):911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosner P. Perturbation approach to the response of a control system. IRE Transactions on Automatic Control. 1961;6(3):361–362. [Google Scholar]
- 15.Nakayama H., Smith C.A., Rodman J.R., Skatrud J.B., Dempsey J.A. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165(9):1251–1260. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- 16.Solin P., Roebuck T., Johns D.P., Walters E.H., Naughton M.T. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162(6):2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 17.Younes M. Apnea following mechanical ventilation may not be caused by neuromechanical influences. Am J Respir Crit Care Med. 2001;163(6):1298–1301. doi: 10.1164/ajrccm.163.6.pc1201b. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S., Colangelo G., Corser B., Zahedpour M.R. Familial respiratory chemosensitivity does not predict hypercapnia of patients with sleep apnea-hypopnea syndrome. Am Rev Respir Dis. 1992;145(4 pt 1):837–840. doi: 10.1164/ajrccm/145.4_Pt_1.837. [DOI] [PubMed] [Google Scholar]
- 19.Loewen A., Ostrowski M., Laprairie J. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009;32(10):1355–1365. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb D.J., Punjabi N.M., Mehra R. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellman A., Malhotra A., Jordan A.S., Stevenson K.E., Gautam S., White D.P. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162(2):144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tojima H., Kunitomo F., Kimura H., Tatsumi K., Kuriyama T., Honda Y. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax. 1988;43(2):113–119. doi: 10.1136/thx.43.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards B.A., Sands S.A., Eckert D.J. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590(5):1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javaheri S., Ahmed M., Parker T.J., Brown C.R. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep. 1999;22(8):1101–1106. doi: 10.1093/sleep/22.8.1101. [DOI] [PubMed] [Google Scholar]
- 25.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173(2):234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 26.Wang T., Eskandari D., Zou D., Grote L., Hedner J. Increased carbonic anhydrase activity is associated with sleep apnea severity and related hypoxemia. Sleep. 2015;38(7):1067–1073. doi: 10.5665/sleep.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sands S.A., Edwards B.A., Terrill P.I. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J. 2018;52(3) doi: 10.1183/13993003.00674-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strollo P.J., Jr., Soose R.J., Maurer J.T. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 29.Taranto-Montemurro L., Edwards B.A., Sands S.A. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjects. Am J Respir Crit Care Med. 2016;194(7):878–885. doi: 10.1164/rccm.201511-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taranto-Montemurro L., Sands S.A., Edwards B.A. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur Respir J. 2016;48(5):1340–1350. doi: 10.1183/13993003.00823-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acosta D., Ramos K. Cardiotoxicity of tricyclic antidepressants in primary cultures of rat myocardial cells. J Toxicol Environ Health. 1984;14(2-3):137–143. doi: 10.1080/15287398409530568. [DOI] [PubMed] [Google Scholar]
- 32.Eskafi M. Sleep apnoea in patients with stable congestive heart failure an intervention study with a mandibular advancement device. Swed Dent J Suppl. 2004;(168):1–56. [PubMed] [Google Scholar]
- 33.Eskafi M., Cline C., Nilner M., Israelsson B. Treatment of sleep apnea in congestive heart failure with a dental device: the effect on brain natriuretic peptide and quality of life. Sleep Breath. 2006;10(2):90–97. doi: 10.1007/s11325-006-0053-2. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg R., Roach J.M., Scharf M., Amato D.A. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8(5):464–470. doi: 10.1016/j.sleep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Eckert D.J., Malhotra A., Wellman A., White D.P. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811–819. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohn M.A. Hypnotics and the control of breathing: a review. Br J Clin Pharmacol. 1983;16(suppl 2):245S–250S. doi: 10.1111/j.1365-2125.1983.tb02296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iftikhar I.H., Kline C.E., Youngstedt S.D. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175–184. doi: 10.1007/s00408-013-9511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Servantes D.M., Javaheri S., Kravchychyn A.C.P. Effects of exercise training and CPAP in patients with heart failure and OSA: a preliminary study. Chest. 2018;154(4):808–817. doi: 10.1016/j.chest.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Ueno L.M., Drager L.F., Rodrigues A.C. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32(5):637–647. doi: 10.1093/sleep/32.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y.L., Ding Y., Agnew C., Schultz H.D. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol (1985) 2008;105(3):782–790. doi: 10.1152/japplphysiol.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redolfi S., Yumino D., Ruttanaumpawan P. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179(3):241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 42.Tomita T., Takaki H., Hara Y. Attenuation of hypercapnic carbon dioxide chemosensitivity after postinfarction exercise training: possible contribution to the improvement in exercise hyperventilation. Heart. 2003;89(4):404–410. doi: 10.1136/heart.89.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guimaraes K.C., Drager L.F., Genta P.R., Marcondes B.F., Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179(10):962–966. doi: 10.1164/rccm.200806-981OC. [DOI] [PubMed] [Google Scholar]
- 44.Nadar S., Prasad N., Taylor R.S., Lip G.Y. Positive pressure ventilation in the management of acute and chronic cardiac failure: a systematic review and meta-analysis. Int J Cardiol. 2005;99(2):171–185. doi: 10.1016/j.ijcard.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 45.Chadda K., Annane D., Hart N., Gajdos P., Raphael J.C., Lofaso F. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002;30(11):2457–2461. doi: 10.1097/00003246-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Yoshinaga K., Burwash I.G., Leech J.A. The effects of continuous positive airway pressure on myocardial energetics in patients with heart failure and obstructive sleep apnea. J Am Coll Cardiol. 2007;49(4):450–458. doi: 10.1016/j.jacc.2006.08.059. [DOI] [PubMed] [Google Scholar]
- 47.Naughton M.T., Rahman M.A., Hara K., Floras J.S., Bradley T.D. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91(6):1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- 48.Sajkov D., Wang T., Saunders N.A., Bune A.J., McEvoy R.D. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(2):152–158. doi: 10.1164/ajrccm.165.2.2010092. [DOI] [PubMed] [Google Scholar]
- 49.Alchanatis M., Tourkohoriti G., Kakouros S., Kosmas E., Podaras S., Jordanoglou J.B. Daytime pulmonary hypertension in patients with obstructive sleep apnea: the effect of continuous positive airway pressure on pulmonary hemodynamics. Respiration. 2001;68(6):566–572. doi: 10.1159/000050574. [DOI] [PubMed] [Google Scholar]
- 50.Khayat R.N., Abraham W.T., Patt B., Pu M., Jarjoura D. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009;136(4):991–997. doi: 10.1378/chest.09-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spaak J., Egri Z.J., Kubo T. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46(6):1327–1332. doi: 10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- 52.Kaye D.M., Mansfield D., Aggarwal A., Naughton M.T., Esler M.D. Acute effects of continuous positive airway pressure on cardiac sympathetic tone in congestive heart failure. Circulation. 2001;103(19):2336–2338. doi: 10.1161/01.cir.103.19.2336. [DOI] [PubMed] [Google Scholar]
- 53.Khoo M.C., Belozeroff V., Berry R.B., Sassoon C.S. Cardiac autonomic control in obstructive sleep apnea: effects of long-term CPAP therapy. Am J Respir Crit Care Med. 2001;164(5):807–812. doi: 10.1164/ajrccm.164.5.2010124. [DOI] [PubMed] [Google Scholar]
- 54.Tkacova R., Rankin F., Fitzgerald F.S., Floras J.S., Bradley T.D. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98(21):2269–2275. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 55.Mansfield D.R., Gollogly N.C., Kaye D.M., Richardson M., Bergin P., Naughton M.T. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 56.McEvoy R.D., Antic N.A., Heeley E. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 57.Martinez-Garcia M.A., Campos-Rodriguez F., Javaheri S., Gozal D. Pro: continuous positive airway pressure and cardiovascular prevention. Eur Respir J. 2018;51(5) doi: 10.1183/13993003.02400-2017. [DOI] [PubMed] [Google Scholar]
- 58.Oldenburg O., Wellmann B., Buchholz A. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37(21):1695–1703. doi: 10.1093/eurheartj/ehv624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.