Abstract
Here, we investigated whether the optimal threonine (Thr) to lysine (Lys) ratio in high Lys diet improves the growth performance of modern broiler chickens at finisher period and determined the possible mechanism underlying improvement in the growth performance of chickens fed with high Lys or Lys + Thr diet using metabolome analyses. Eighteen 21-day-old chickens housed in individual cages were randomly divided into three groups of six chickens fed with different diets as follows: control diet, high Lys diet (150% Lys content of National Research Council requirement), and high Lys + Thr diet (0.68 of Thr/Lys in high Lys diet). Body weight gain (BWG) increased in chickens receiving high Lys diet as compared with those fed with the control diet (P<0.05); no significant difference was observed in BWG of chickens from high Lys + Thr and high Lys groups. Feed conversion ratio (FCR) was lower in chickens fed with high Lys or high Lys + Thr diet than in those on the control diet. Serotonin concentration increased in the plasma of chickens fed with high Lys diet as compared to those fed with other diets. A negative correlation was observed between plasma serotonin concentration and FCR. These results provide the first evidence on the use of high Lys in broiler diets to reduce FCR during finisher period, which may be associated with change in plasma serotonin concentration. These findings suggest that high Lys content in finisher diet, but not high Thr + Lys diet, may affect the peripheral serotonergic metabolism and improve FCR. Thus, plasma serotonin may serve as a biomarker of FCR in broilers.
Keywords: amino acid, broiler chicken, lysine, metabolome analysis, serotonin, threonine
Introduction
The National Research Council (NRC, 1994) determined the nutritional requirements of broiler chickens, including amino acid content of diet. Nutritional requirements of modern broiler chickens have undergone changes owing to improvements in genetic selection and breeding, resulting in further improvements in growth rate, feed conversion ratio (FCR), and breast meat yield (Razaei et al., 2004; Sterling et al., 2004). Some leading breeding companies such as Aviagen have revealed the nutritional composition of the feed used to induce maximum performance in broiler chickens. However, body weight gain (BWG) and FCR in broiler chickens may be improved with diets supplemented with amino acids (Kidd et al., 2004b; Dozier et al., 2007, 2008). The contents and compositions of dietary amino acids for modern broiler chickens may need further improvement. Therefore, expansion of the relevant knowledge of amino acid contents and compositions for broiler chickens is desirable (Dozier et al., 2008).
Lysine (Lys) is the second limiting amino acid in broiler chicken diets based on corn-soybean. An optimum Lys content of 1.09% higher than NRC levels was reported to improve FCR, breast meat yield, and BWG with low abdominal fat pad weight (Leclercq, 1998). The growth performance improved in broiler chickens fed with finisher diet supplemented with Lys (Leclercq, 1998; Razaei et al., 2004; Sterling et al., 2004). Hence, Lys may be considered as an important amino acid that increases meat production and efficiency of broiler chickens.
Threonine (Thr) is the third limiting amino acid in corn-soybean-based diet of chickens. BWG, FCR, and immune function improved with an increase in Thr contents in the diet of broiler chickens (Leclercq, 1998; Kidd, 2000, 2001, 2004a; Ciftci and Ceylan, 2004; Corzo et al., 2009; Star et al., 2012; Mejia et al., 2012; Mohammad, 2013; Sigolo et al., 2017). In a former meta-analysis, 0.73% and 0.75% of Thr contents in the finisher diets, higher than the requirements of NRC (3–6 week 0.74% and 6–8 week 0.68%), demonstrated maximum BWG and FCR, respectively (Ahmadi et al., 2010). Thr requirements of broiler chickens are dependent on environmental conditions such as heat stress (Mohammad, 2013). Thus, Thr also serves as an important amino acid that may improve meat production in broilers, as observed with Lys.
While each amino acid improves the performance of chickens, limited information is available on the effect of the combination of high Lys and Thr in finisher diet on BWG, feed intake (FI), and FCR in broiler chickens. Everett et al. (2010) evaluated the effect of Lys (2 levels) and Thr (4 levels) factorial responses in commercial male broilers (28 to 42 days of age) and suggested that high levels of Lys and Thr in diet may improve some live performance parameters. In starting broiler chickens, the ideal ratio of digestible Thr to digestible Lys was 70% for BWG and 66% for FCR (Mehri et al., 2012). However, the appropriate Thr content at higher Lys levels in finisher diet of broiler chickens is unknown. The growth performance during finisher period is an important characteristic for meat production.
In the present study, we evaluated the effect of an optimal Thr-to-Lys ratio (68%) in high Lys diet on growth performance indicators such as BWG and FCR in modern broiler chickens during finisher period. We used metabolome analysis to examine the possible mechanism underlying the improvement in growth performance of broiler chickens.
Materials and Methods
Birds
One-day-old male broiler chickens (Ross 308 strain) obtained from a local hatchery (Mori breeding farm Co., Ltd, Koriyama, Japan) were used in the present study. The birds were housed in electrically-heated battery brooders and fed with a corn-soybean meal-based diet (basal diet; 220 g of crude protein [CP] /kg and 3.0 Mcal metabolizable energy [ME]/kg from 0 to 7 days of age and 200 g/kg of CP and 3.1 Mcal/kg of ME from 8 to 21 days of age) ad libitum for 21 days. Selected 21 day-old chickens had similar body weights to ensure body-weight uniformity and were individually reared in stainless-steel wire cages (one bird in one cage) in a temperature- (25°C) and light (23 h/day) -controlled room. All basal diets were formulated to contain essential nutrients that met or exceeded recommended levels (NRC, 1994). All procedures were approved by the Animal Care and Use Committee of the Tokyo University of Agriculture and Technology.
Experimental Diets and Blood Sampling
Eighteen chickens (21 day of age) in individual cages were randomly divided into three groups of six chickens, and each group was provided with one of three experimental diets for 17 days ad libitum. The experimental diets are shown in Table 1. The control diet was a corn-soybean meal-based diet containing 180 g/kg of CP and 3.2 Mcal/kg of ME supplemented with 100% essential amino acid contents recommended by NRC (1994), including Lys and Thr requirements of 10.0 and 6.8 g/kg of diet, respectively. The high Lys diet contained 150% of Lys recommended by NRC (1994). This additional level of 150% Lys was determined from the findings of our previous study (Kobayashi et al., 2011). Based on the results of our previous study, we included high Lys + Thr diet (0.68 of Thr/Lys), which was reported to optimize broilers' performance at 21 to 42 days of age (Kidd et al., 2004a). The CP level of high Lys and high Lys + Thr diets was maintained by the dietary addition of l-glutamic acid. All other nutrients such as essential amino acids, vitamins, and minerals were adjusted in three experimental diets as per NRC requirements.
Table 1. Test diets for the 21 to 38 d period (% as is basis)1.
| Ingredients | Control | High Lys | High Lys + Thr |
|---|---|---|---|
| Corn | 65.70 | 66.09 | 66.13 |
| Soybean meal | 22.52 | 22.52 | 22.52 |
| Canola meal | 3.00 | 3.00 | 3.00 |
| Yellow grease | 4.35 | 4.35 | 4.35 |
| CaCO3 | 0.67 | 0.67 | 0.67 |
| Ca3(PO4)2 | 1.66 | 1.66 | 1.66 |
| NaCl | 0.16 | 0.16 | 0.16 |
| Choline Cl | 0.03 | 0.03 | 0.03 |
| Vitamins and minerals2 | 0.10 | 0.10 | 0.10 |
| L-Lysine-HCl | 0.12 | 0.75 | 0.75 |
| DL-Methionine | 0.25 | 0.25 | 0.25 |
| L-Threonine | 0.04 | 0.04 | 0.38 |
| L-Gultamic acid3 | 1.40 | 0.38 | — |
| Total | 100 | 100 | 100 |
| Nutrient analysis | |||
| ME, Mcal/kg | 3.2 | 3.2 | 3.2 |
| CP | 18.00 | 18.00 | 18.00 |
| Ca | 0.89 | 0.89 | 0.89 |
| Available P | 0.40 | 0.40 | 0.40 |
| Lysine | 1.00 | 1.50 | 1.50 |
| Threonine | 0.68 | 0.69 | 1.02 |
| Arginine | 1.12 | 1.12 | 1.12 |
High Lys is the amount of 1.5% lysine in test diet. High Lys + Thr is the amount of 1.5% Lys and 1.02% Threonine in test diet (Thr / Lys=0.68).
Premix provided the following per kilogram of diet: vitamin A (vitamin A acetate) 9,200 IU; cholecalciferol 4,200 IU; vitamin E (source unspecified) 15 IU; menadione, 2.9 mg; B12, 13 µg; choline, 38 mg; riboflavin, 6.7 mg; niacin, 50 mg; D-biotin, 0.13 mg; pyridoxine, 3.4 mg; manganese, 130 mg; zinc, 100 mg; iron, 40 mg; copper, 16 mg; iodine, 1.3 mg.
Corrected using glutamic acid to satisfy CP of caluculation in each test feed.
Blood samples were collected from the wing vein of six chickens from each dietary group after treatment and plasma was stored at −80°C until further analysis.
Metabolome Analysis of Plasma
Metabolite levels in plasma were measured using capillary electrophoresis time-of-flight mass spectrometry (CE-TOF/MS) system (Agilent Technologies Inc., Santa Clara, CA, USA). A fused silica capillary (50 µm×80 cm) was used. Detected peaks were analyzed by an automatic integration software (MasterHands ver. 2. 16. 0. 15; Keio University, Tokyo, Japan).
Measurement of Free Amino Acids
The characteristic peaks of plasma amino acids were measured with metabolome analysis. The free amino acid levels in blood were measured as described by Imanari et al. (2007) using amino acid analyzer (JLC-500/V; JEOL, Tokyo, Japan). A multi-segment tandem packed column (LC-500 AC4016, Li type, 4 mm diameter×160 mm; JEOL, Tokyo, JAPAN) was used. The detection wavelengths were 440 and 570 nm.
Measurement of Serotonin Concentrations in Plasma
Detailed analysis was conducted on plasma serotonin (5-hydroxytryptamine, 5-HT), as its concentration in the Lys group was more than the double of that in Lys + Thr group in metabolome analysis. 5-HT levels in blood were measured using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's guidelines (BA E-8900; ImmuSmol SAS, Pessac, France).
Statistical Analysis
The SPSS application software package was used for statistical calculations (PASW Statistics 18.0, IBM, NY 10504). The group data for multiple comparisons were analyzed with analysis of variance (ANOVA) using a general linear model procedure followed by Tukey-Kramer test. Results are expressed as mean±standard deviation (SD). Statistical significance was interpreted as values of P<0.05. Correlation analysis was performed between the factors measured in the present study.
Results
Growth Performance
BWG increased in chickens that received high Lys diet as Compared with those receiving the control diet, while no significant difference was observed in BWG of high Lys + Thr and other two groups (Table 2). Although FI was unchanged among different treatment groups, both high Lys and high Lys + Thr diets resulted in an improvement in FCR (Table 2).
Table 2. Live performance measurements of male broiler as affected by high level of Lys and Lys + Thr administrered from 21–38 d of age.
| Control | High Lys | High Lys + Thr | |
|---|---|---|---|
| Initial BW (g) | 1010.8±7.2 | 1012.7±10.6 | 1013.6±6.2 |
| BWG (g) | 1507.1±153.3b | 1673.9±39.9a | 1534.1±124.1ab |
| Feed intake (g) | 3180.6±292.4 | 3294.8±65.1 | 3080.5±210.2 |
| FCR | 1.686±0.056b | 1.584±0.044a | 1.609±0.040a |
Values are means±SD for 6 birds.
Values with different superscripts are significantly different (a, b, c P<0.05)
Lys, lysine; Thr, threonine; BWG, body weight gain; FCR, feed conversion ratio.
Free Amino Acid Concentration in Plasma
The Lys content in the plasma of chickens fed with high Lys or high Lys + Thr diets was higher than that in the plasma of the control group, while arginine (Arg) content was lower in the plasma of chickens fed with high Lys diet than in plasma of those fed with the control diet (P=0.074) (Table 3). The concentration of Thr increased in the plasma of chickens fed with high Lys + Thr diet (Table 3). No significant differences were observed in the levels of other free amino acids in the plasma of chickens from the three groups.
Table 3. Effect of high Lys diet and high Lys + Thr diet on free amino acid concentration in plasma on broilers (nmol/mL plasma).
| Control | High Lys | High Lys + Thr | |
|---|---|---|---|
| Thr | 401.3±72.9A | 334.9±103.1A | 2585.0±772.4B |
| Ser | 486.3±50.3a | 551.8±118.8ab | 673.6±89.8b |
| Glu | 174.1±18.8 | 185.2±46.3 | 134.8±14.5 |
| Gln | 1151.3±180.6 | 1348.1±152.9 | 1378.5±96.6 |
| Gly | 592.3±42.0 | 557.1±136.6 | 613.7±76.7 |
| Ala | 1074.1±205.4 | 1068.4±234.6 | 991.0±159.5 |
| Val | 135.4±23.1 | 135.4±26.3 | 135.7±16.5 |
| Met | 82.5±8.4 | 98.7±13.1 | 94.9±19.8 |
| Cys | 50.5±2.6 | 49.0±3.6 | 48.9±7.8 |
| Ile | 65.9±15.2 | 70.4±19.9 | 67.4±11.9 |
| Leu | 275.0±33.5 | 257.5±33.5 | 277.8±23.8 |
| Tyr | 225.6±51.4 | 269.7±40.6 | 267.1±41.6 |
| Phe | 133.0±16.7 | 119.0±17.4 | 121.5±8.0 |
| His | 86.9±46.9 | 46.2±12.2 | 52.6±3.6 |
| Lys | 113.8±73.9A | 636.9±177.5B | 547.6±64.9B |
| Arg | 313.0±102.3 | 199.6±72.7† | 208.2±48.2 |
Values are means±SD, and values in each row with different susperscripts are significantly different at A, B P<0.01, or a, b P<0.05.
tend to be lower than in the control group (P=0.074).
Ala, alanine; Arg, arginine; Cys, cystine; Gln, glutamine; Glu, glutamic acid; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; Phe, phenylalanine; Ser, serine; Thr, threonine; Tyr, tyrosine; Val, valine.
Metabolome Analysis in Plasma
To elucidate the effect of high Lys and high Lys + Thr diets on plasma metabolites, we determined metabolite levels using the CE-TOF/MS system. A total of 149 compounds were detected in the plasma of each group; these compounds were divided into three groups as follows: upregulated (>1.2-fold), downregulated (<0.8-fold), and unchanged (0.8–1.2) (Table 4). The extracted metabolites changed more than 1.5 times or less than 0.7 times among different groups (Table 5). General Lys metabolites such as 2-aminoadipic acid, β-alanine, and pipecolic acid increased in the plasma of broilers fed with high Lys or high Lys + Thr diets as compared with the plasma of broilers from the control group. The metabolites related to urea cycle, including Arg, ornithine, citrulline, urea, and uric acid, decreased in the high Lys group as compared with the control group. The metabolites of creatine pathway also showed changes in response to high Lys or high Lys + Thr diet. Characteristic changes in the plasma serotonin concentrations were detected (Table 5). The analysis of plasma serotonin level was confirmed by ELISA. Serotonin content significantly increased in the plasma of chickens fed with high Lys diet as compared with those from the control and high Lys + Thr groups (Fig. 1).
Table 4. Classification of metabolites by fold-change.
| Fold-change of high Lys vs. control | Total | Fold-change of high Lys + Thr vs. control | Total | Fold-change of high Lys + Thr vs. high Lys | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.8 (down regulated) |
0.8–1.2 (unchanged) |
1.2< (up regulated) |
<0.8 (down regulated) |
0.8–1.2 (unchanged) |
1.2< (up regulated) |
<0.8 (down regulated) |
0.8–1.2 (unchanged) |
1.2< (up regulated) |
||||
| Number of metabolites | 21 | 93 | 35 | 149 | 32 | 92 | 22 | 146 | 36 | 99 | 13 | 148 |
| % of total metabolites | 14.1 | 62.4 | 23.5 | 100 | 21.9 | 63.0 | 15.1 | 100 | 24.3 | 66.9 | 8.8 | 100 |
Chicks were fed the control, high Lys or high Lys + Thr diet from 21 to 38 days old.
All samples in each group were pooled.
Metabolites are classified into three groups: up-regulated (>1.2-fold), down-regurated (<0.8-fold) and unchanged(0.8–1.2).
Table 5. Effect of high Lys or high Lys + Thr diets on detected metabolites in blood of broilers at 38 days.
| Compound name | Relative Area |
Comparative analysis (fold-change) |
||||
|---|---|---|---|---|---|---|
| Control | High Lys | High Lys + Thr | High Lys vs. control | High Lys + Thr vs. control | High Lys + Thr vs. high Lys | |
| Lysine metabolites | ||||||
| Lysine | 3.49×10−2 | 2.05×10−1 | 1.74×10−1 | 5.9 | 5.0 | 0.8 |
| 2-Aminoadipic acid | 2.98×10−4 | 9.04×10−4 | 7.53×10−4 | 3.0 | 2.5 | 0.8 |
| Pipecolicacid | 1.26×10−3 | 2.98×10−3 | 2.91×10−3 | 2.4 | 2.3 | 1.0 |
| N6-Acetyllysine | 1.41×10−3 | 3.50×10−3 | 3.76×10−3 | 2.5 | 2.7 | 1.1 |
| N-Acetyllysine | 2.77×10−4 | 4.85×10−4 | 5.25×10−4 | 1.8 | 1.9 | 1.1 |
| Carboxymethyllysine | 3.36×10−4 | 3.06×10−4 | 3.83×10−4 | 0.9 | 1.1 | 1.3 |
| Metabolites increased more than ≧ 1.5 fold-change | ||||||
| Specificallys in high Lys vs. control | ||||||
| Serotonin | 2.10×10−4 | 8.89×10−4 | 6.47×10−5 | 4.2 | 0.3 | 0.1 |
| Ethanolamine | 1.05×10−3 | 1.63×10−3 | 1.18×10−3 | 1.5 | 1.1 | 0.7 |
| Specificallys in high Lys + Thr vs. control | ||||||
| Threonine | 9.63×10−2 | 8.46×10−2 | 5.66×10−1 | 0.9 | 5.9 | 6.7 |
| 2-Hydroxy-4-methylvaleric acid | 2.38×10−4 | 1.78×10−4 | 3.47×10−4 | 0.7 | 1.5 | 2.0 |
| Common high Lys and high Lys + Thr vs. control | ||||||
| β-Alanine | 7.13×10−3 | 1.55×10−2 | 1.11×10−2 | 2.2 | 1.6 | 0.7 |
| Homocitrulline | 7.33×10−5 | 1.08×10−4 | 1.12×10−4 | 1.5 | 1.5 | 1.0 |
| Metabolites increased less than ≦ 0.7 fold-change | ||||||
| Specificallys in high Lys vs. control | ||||||
| 2-Hydroxy-4-methylvaleric acid | 2.38×10−4 | 1.78×10−4 | 3.47×10−4 | 0.7 | 1.5 | 2.0 |
| Ornithine | 5.29×10−3 | 3.90×10−3 | 4.30×10−3 | 0.7 | 0.8 | 1.1 |
| Citrulline | 1.59×10−3 | 1.15×10−3 | 1.44×10−3 | 0.7 | 0.9 | 1.3 |
| Uricacid | 1.93×10−2 | 1.39×10−2 | 1.52×10−2 | 0.7 | 0.8 | 1.1 |
| 2-Hydroxyisobutyric acid | 3.78×10−4 | 2.56×10−4 | 3.68×10−4 | 0.7 | 1.0 | 1.4 |
| Taurocholicacid | 4.37×10−4 | 2.41×10−4 | 4.28×10−4 | 0.6 | 1.0 | 1.8 |
| 3-Methylhistidine | 1.60×10−2 | 7.55×10−3 | 6.52×10−3 | 0.5 | 0.4 | 0.9 |
| Specificallys in high Lys + Thr vs. control | ||||||
| N-Acetylmuramic acid | 2.69×10−4 | 2.29×10−4 | 1.84×10−4 | 0.8 | 0.7 | 0.8 |
| Sulfotyrosine | 1.61×10−4 | 1.37×10−4 | 1.08×10−4 | 0.9 | 0.7 | 0.8 |
| Ectoine | 4.88×10−4 | 4.46×10−4 | 3.21×10−4 | 0.9 | 0.7 | 0.7 |
| γ-Butyrobetaine | 8.05×10−4 | 7.43×10−4 | 5.19×10−4 | 0.9 | 0.6 | 0.7 |
| Diethanoiamine | 3.64×10−4 | 3.50×10−4 | 2.30×10−4 | 1.0 | 0.6 | 0.7 |
| Nicotinamide | 9.74×10−4 | 9.71×10−4 | 5.95×10−4 | 1.0 | 0.6 | 0.6 |
| Hypoxanthine | 1.32×10−3 | 1.50×10−3 | 7.77×10−4 | 1.1 | 0.6 | 0.5 |
| Taurine | 1.30×10−2 | 1.50×10−2 | 6.25×10−3 | 1.2 | 0.5 | 0.4 |
| 3-Methylhistidine | 1.60×10−2 | 7.55×10−3 | 6.52×10−3 | 0.5 | 0.4 | 0.9 |
| Serotonin | 2.10×10−4 | 8.89×10−4 | 6.47×10−5 | 4.2 | 0.3 | 0.1 |
| Common to high Lys and high Lys + Thr vs. control | ||||||
| 2-Hydroxyglutaric acid | 3.20×10−4 | 2.28×10−4 | 2.11×10−4 | 0.7 | 0.7 | 0.9 |
| Arginine | 1.19×10−1 | 8.04×10−2 | 8.18×10−2 | 0.7 | 0.7 | 1.0 |
| Dyphylline | 3.56×10−3 | 2.64×10−3 | 1.69×10−3 | 0.7 | 0.5 | 0.6 |
| Glyceric acid | 2.00×10−3 | 1.46×10−3 | 1.18×10−3 | 0.7 | 0.6 | 0.8 |
| Glycerol | 1.20×10−2 | 8.64×10−3 | 4.70×10−3 | 0.7 | 0.4 | 0.5 |
| Creatine | 1.35×10−2 | 9.69×10−3 | 5.39×10−3 | 0.7 | 0.4 | 0.6 |
| Carnitine | 2.30×10−3 | 1.61×10−3 | 1.20×10−3 | 0.7 | 0.5 | 0.7 |
| Xanthine | 3.64×10−4 | 2.42×10−4 | 2.09×10−4 | 0.7 | 0.6 | 0.9 |
| Lauric acid | 1.73×10−3 | 1.08×10−3 | 1.28×10−3 | 0.6 | 0.7 | 1.2 |
| Urea | 8.06×10−3 | 4.83×10−3 | 4.31×10−3 | 0.6 | 0.5 | 0.9 |
| Histidine | 2.73×10−2 | 1.30×10−2 | 1.45×10−2 | 0.5 | 0.5 | 1.1 |
| Metabolites increased more than twice in high Lys + Thr vs. high Lys | ||||||
| Threonine | 9.63×10−2 | 8.46×10−2 | 5.66×10−1 | 0.9 | 5.9 | 6.7 |
| Metabolites decreased to less than half in high Lys + Thr vs. high Lys | ||||||
| Taurine | 1.30×10−2 | 1.50×10−2 | 6.25×10−3 | 1.2 | 0.5 | 0.4 |
| Serotonin | 2.10×10−4 | 8.89×10−4 | 6.47×10−5 | 4.2 | 0.3 | 0.1 |
Chicks were fed the high Lys or high Lys + Thr diet from 21 to 38 days.
All samples in each group were pooled (pooled by n=6).
Fold-change is the ratio of the reactive areas (between 2 groups in each group)
The metabolite of up-regulated or down-regulated was shown separately except for general lysine metabolites.
Fig. 1.
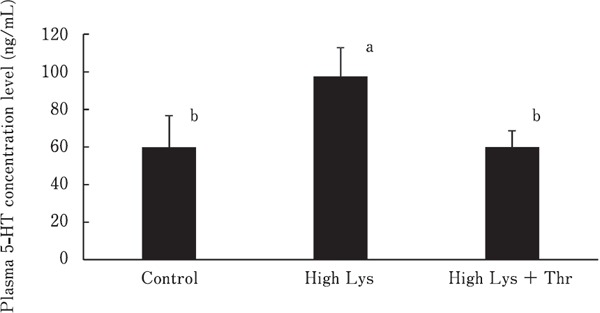
Effect of high Lys and high Lys + Thr diets on plasma serotonin (5-hydroxytryptamine, 5-HT) concentrations in broilers at 38 days. Bars indicate the SD of the mean (n=6). Different superscripts indicate significant differences, P<0.01.
Correlation Analysis between Serotonin and Other Factors
Similar experiment was conducted to evaluate the correlation between plasma serotonin concentration and BWG, FI, and FCR (n=30). A negative correlation was detected between serotonin level and FCR (P<0.008, r=−0.51) (Fig. 2). No significant correlation was observed between other factors and plasma serotonin concentration.
Fig. 2.
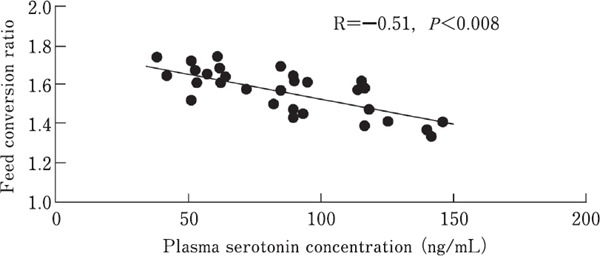
Correlation analysis between plasma serotonin concentration and feed conversion ratio in broilers at 38 days. The treatment protocol is described in Materials and Methods (n=30).
Discussion
Lys is well known as the second limiting amino acid in corn-soybean-based diets. Here, we demonstrate that the supplementation of chicken diets with Lys at 15 g/kg diet concentration, which is 150% of NRC requirement (high Lys diet in the present study) resulted in an improvement in BWG and FCR (Table 2). These results suggest that dietary supplementation with Lys increases the meat production in birds. The supplementation of Lys in diet as per the NRC requirement may improve BWG, FI, and FCR in broiler chickens (Leclercq, 1998; Razaei et al., 2004; Sterling et al., 2004). It was reported that 128% Lys content in finisher diet of broilers significantly increased BWG as compared with supplementation with 100% Lys in 28 to 42-day-old chicken (Everett et al., 2010). However, no report has evaluated the effect of 150% Lys content (high Lys content) in finisher diet on the performance of broiler chickens, while no significant improvement was observed in the broiler chickens fed with a diet containing 150% Lys at 14–24 days of age.
The present study shows that the increase in Thr content in high Lys diet negatively affected the performance of broilers, although Thr is the third limiting amino acid in corn-soybean-based diets in finisher period. The ratio of Thr and Lys in diet is an important factor that determines the performance of broilers, and the broilers between 21 and 42 days of age reguire 68% of digestible Thr to Lys ratio (Kidd et al., 1997; Mejia, 2012). We, therefore, hypothesized that high Lys + Thr diet with 68% of Thr and Lys ratio may improve the performance of broilers as compared with control and high Lys diets. The present findings, however, show that BWG in high Lys + Thr group was not significantly different from BWG in the control group, while FCR was significantly lower in the chickens fed with high Thr + Lys diet than in those fed with control diet. This result suggests that the optimal Thr-to-Lys ratio (68%) in finisher diet of broiler chickens was not the appropriate ratio for maximal production, especially for BWG, in high Lys diet. Therefore, this is the first study to demonstrate that Thr supplementation at 68% of Thr/Lys in finisher diets may be insufficient to improve BWG of broilers. Further experiments evaluating the effects of dose-dependent supplementation of Thr in high Lys diet may allow elucidation of optimum Lys and Thr contents in finisher diet to support maximum performance of broilers.
We performed metabolome analysis of the plasma obtained from the chickens fed with high Lys and high Lys + Thr diets. Although high Lys and high Lys + Thr diets induced changes in several metabolites, a characteristic change was detected in the metabolite serotonin. In mammals, there are two independent serotonin systems: one in the central nervous system and the other in the periphery. Serotonin affects feeding behaviors and obesity in the central nervous system (Leibowitz and Alexander, 1998). In contrast, the role of peripheral serotonin has not been clarified; peripheral serotonin is thought to play an important role in the regulation of glucose and lipid metabolism (Kim et al., 2011; Watanabe et al., 2016). Serotonin injection in the hypothalamus of chickens increases FI values (Tachibana et al., 2001). Whether peripheral serotonin, particularly plasma serotonin, affects glucose and lipid metabolism as well as FCR in broiler chickens is unknown. A recent study showed that supplementation of broiler chicken diet with tryptophan may improve FCR under chronic and unpredictable stress, probably owing to serotonergic metabolism (Yue et al., 2017). Therefore, the metabolic role of peripheral serotonin has been identified in broilers in the present study. High plasma serotonin concentration detected in the chickens fed with high Lys diet (Fig. 1) correlated with FCR. These results suggest that plasma serotonin may be involved in FCR of broiler chickens during finisher period. This result indicates the possibility that plasma serotonin concentration may serve as a biomarker for the improvement in FCR of broiler chickens.
In the present study, Lys metabolites such as 2-amino-adipic acid, β-alanine, and pipecolic acid increased in the blood of chickens fed with high Lys diet. Similar changes in metabolites were observed in the muscle of chickens fed with high Lys diet (Watanabe et al., 2015). The fold changes in metabolites were higher in the muscles than in blood of chickens from control and high Lys diet groups. Hence, the changes in the metabolites of Lys were similar in the muscle and blood of chickens fed with high Lys diet.
Lys-Arg antagonism is induced by feeding chickens with high Lys diet (Austic and Scott, 1970; Austic and Nesheim, 1975). The optimum Lys content per Arg content in finisher diet of broilers is 1.18 (Labandan et al., 2001). Therefore, high Lys diet may likely induce negative effects on the performance of broilers, resulting in Lys-Arg antagonism. However, the contents of Lys, which was 150% of NRC requirement, in finisher diets improved the performance of broilers as compared with the Lys content in the control diet. In addition, the metabolites related to urea cycle, including Arg, ornithine, citrulline, urea, and uric acid, tended to decrease in high Lys group as compared with control group (Table 5). Here we suggest that high Lys diet, at least 150% of NRC requirement, failed to cause Lys-Arg antagonism or affect the live performance of modern broiler chickens.
In conclusion, Lys supplementation, particularly at 150% of NRC requirement, enhanced the performance in finisher period of broiler chickens, demonstrating that high Lys diet may be a useful component in poultry industry. In contrast, Thr may not be a valuable supplement to improve meat production with finisher diet supplemented with high Lys content. The present study reveals for the first time that plasma serotonin concentration regulates FCR of broiler chickens, suggesting that plasma serotonin may serve as a novel biomarker for the improvement of FCR in chickens.
Acknowledgments
We would like to thank the laboratory members for their help with the study.
References
- Ahmadi H, Golian A. The integration of broiler chicken threonine responses data into neural network models. Poultry Science, 89: 2535-2541. 2010. [DOI] [PubMed] [Google Scholar]
- Austic RE, Nedheim MC. Role of kidney arginine in variations of arginine requirement of chicks. Journal of Nutrition, 100: 855-867. 1970. [DOI] [PubMed] [Google Scholar]
- Austic RE, Scott RL. Involvement of food intake in the lysine-arginine antagonism in chicks. Journal of Nutrition, 105: 1122-1131. 1975. [DOI] [PubMed] [Google Scholar]
- Ciftci I, Ceylan N. Effects of dietary threonine and crude protein on growth performance, carcase and meat composition of broiler chickens. British Poultry Science, 2: 280-289. 2004. [DOI] [PubMed] [Google Scholar]
- Corzo A, Dozier WA, III, Loar RE, II, Kidd MT, Tillman PB. Assessing the threo-nine-to-lysine ratio of female broilers from 14 to 28 days of age. Journal of Applied Poultry Research, 18: 237-243. 2009. [Google Scholar]
- Dozier WA, III, Corzo A, Kidd MT, Branton SL. Dietary apparent metabolizable energy and amino acid density effects on growth and carcass traits of heavy broilers. Journal of Applied Poultry Research, 16: 192-205. 2007. [Google Scholar]
- Dozier WA, III, Kidd MT, Corzo A. Dietary amino acid responses of broiler chickens. Journal of Applied Poultry Research, 17: 157-167. 2008. [Google Scholar]
- Everett DL, Corzo A, Dozier WA, III, Tillman PB, Kidd T. Lysine and threonine responses in Ross TP16 male broilers. The Journal of Applied Poultry Research, 19: 321-326. 2010. [Google Scholar]
- Imanari M, Kadowaki M, Fujimura S. Regulation of taste-active components of meat by dietary leucine. British Poultry Science, 48: 167-176. 2007. [DOI] [PubMed] [Google Scholar]
- Kidd MT, Kerr BJ, Anthony NB. Dietary interactions between lysine and threonine in broilers. Poultry Science, 76: 608-614. 1997. [DOI] [PubMed] [Google Scholar]
- Kidd MT. Nutritional considerations concerning threonine in broilers. World's Poultry Science Journal, 56: 139-149. 2000. [Google Scholar]
- Kidd MT, Gerard PO, Herger J, Kerr BJ, Rowe D, Sistani K, Burnham DJ. Threonine and crude protein responses in broiler chicks. Animal Feed Science and Technology, 94: 57-64. 2001. [Google Scholar]
- Kidd MT, McDaniel CD, Branton SL, Miller ER, Boren BB, Fancher BI. Increasing amino acid density improves live performance and carcass yields of commercial broilers. Journal of Applied Poultry Research, 13: 593-604. 2004. a. [Google Scholar]
- Kidd MT, Corzo A, Hoehler D, Kerr J, Barber J, Branton SL. Threonine needs of broiler chickens with different growth rates. Poultry Science, 83: 1368-1375. 2004. b. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim JH, Hoh S, Hur HJ, Sung MJ, Hwang JT, Park JH, Yang JY, Kim MS, Kwon DY, Yoon SH. Metabolomic Analysis of Livers and Serum from High-Fat Diet Induced Obese Mice. Journal of Proteome Research, 10: 722-731. 2011. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Eguchi A, Takano W, Shibata M, Kadowaki M, Fujimura S. Regulation of muscular glutamate metabolism by high-protein diet in broiler chicks. Animal Science Journal, 82: 86-92. 2011. [DOI] [PubMed] [Google Scholar]
- Labandan MC, Jr, Hsu KN, Austic RE. Lysine and arginine requirements of broiler chickens at two to three-week intervals to eight weeks of age. Poultry Science, 80: 599-606. 2001. [DOI] [PubMed] [Google Scholar]
- Leclercq B. Specific effects of lysine on broiler production: domparison with threonine and valine. Poultry Science, 77: 118-123. 1998. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Alexander JT. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biological Psychiatry 44: 851-864. 1998. [DOI] [PubMed] [Google Scholar]
- Mehri M, Davarpanah A, Mirzaei HR. Estimation of ideal ratios of methionine and threonine to lysine in starting broiler chicks using response surface methodology. Poultry Science, 91: 771-777. 2012. [DOI] [PubMed] [Google Scholar]
- Mejia L, Tillman PB, Zumwalt CD, Corzo A. Assessment of the threonine-to-lysine ratio of male broilers from 35 to 49 days of age. Journal of Applied Poultry Research, 21: 235-242. 2012. [Google Scholar]
- Mohammad JB. Estimation of dietary threonine requirement for starter period of broilers based on the performance and immune responses criterion. International Research Journal of Applied and Basic Sciences, 5: 412-416. 2013. [Google Scholar]
- Rezaei M, Moghaddam HN, Reza JP, Kermanshahi H. The effects of dietary protein and lysine levels on broiler performance, carcass characteristics and N excretion. International Journal of Poultry Science, 3 (2): 148-152. 2004. [Google Scholar]
- Star L, Rovers M., Corrent E., Klis JDVD. Threonine requirement of broiler chickens during subclinical intestinal Clostridium infection. Poultry Science, 91: 643-652. 2012. [DOI] [PubMed] [Google Scholar]
- Sterling KG, Pesti GM, Bakalli RI. Performance of different broiler genotypes fed diets with varying levels of dietary crude protein and lysine. Poultry Science, 85: 1045-1054. 2006. [DOI] [PubMed] [Google Scholar]
- Sigolo S, Zohrabi Z, Gallo A, Seidavi A., Prandini A. Effect of low crude protein diet supplemented with different levels of threonine on growth performance, carcass traits, blood parameters, and immune responses of growing broilers. Poultry Science, 96: 2751-2760. 2017. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Tazawa M, Sugahara K. Feeding increases 5-hydroxytryptamine and norepinephrine within the hypothalamus of chicks. Comparative Biochemistry and Physiology A 130: 715-722. 2001. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nakano T, Saito R, Akasaka D, Saito K, Ogasawara H, Minashima T, Miyazawa K, Kanaya T, Takakura I, Inoue N, Ikeda I, Chen X, Miyake M, Kitazawa H, Shirakawa H, Sato K, Tahara K, Nagasawa Y, Rose MT, Ohwada S, Watanabe K, Aso H. Serotonin improves high fat diet induced obesity in mice. PLoS ONE 11: e0147143 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Kobayashi H, Shibata M, Kubota M, Kadowaki M, Fujimura S. Reguration of free glutamate content in meat by dietary lysine in broilers. Animal Science Journal, 86: 435-442. 2015. [DOI] [PubMed] [Google Scholar]
- Yue Y, Guo Y, Yang Y. Effect of dietary L-tryptophan supplementation on intestinal response to chronic unpredictable stress in broilers. Amino Acids, 49: 1227-1236. 2017. [DOI] [PubMed] [Google Scholar]
- National Research Council The Nutrient Requirements of Poultry. 9th rev. ed. National Academies. Press, Washington, DC: 1994. [Google Scholar]
- ROSS An Aviagen Brand Web. http://ap.aviagen.com/brands/ross/ Accessed on July 16, 2018


