Abstract
Recently, we showed that oral administration of crystallized L-citrulline (L-Cit) caused hypothermia under a control thermoneutral temperature (CT) and provided thermotolerance under high ambient temperature (HT) in chicks. The aim of this study was to clarify whether oral administration of a medium containing L-Cit-producing live bacteria can reduce body temperature in chicks under CT. In Experiment 1, 7-day-old chicks were orally administered either a medium (containing mainly L-Cit-producing live bacteria and 277 mM L-Cit) or an equimolar amount of L-Cit to determine their effects on body temperature (acute treatment). In Experiment 2, chicks were subjected to the same treatment from 7 to 13 days of age (chronic treatment). Rectal and surface body temperatures were recorded daily after 1 h of treatment. Both acute and chronic oral administration of the medium, but not of the equimolar amount of L-Cit, significantly reduced the rectal and surface body temperatures of the chicks. Chronic administration of the medium resulted in consistently low rectal and surface body temperatures during the entire experimental period. In conclusion, acute or chronic administration of the medium containing L-Cit-producing live bacteria, but not of the equimolar amount of L-Cit, reduced the rectal and surface body temperatures of the chicks. Our results suggest that medium containing L-Cit-producing live bacteria can be used as a new feed supplement for lowering the body temperature of chicks.
Keywords: chick, L-citrulline, medium, rectal temperature, surface temperature
Introduction
Chicks are susceptible to high ambient temperature (HT) because of their limited ability to regulate heat loss (Nicol, 2011). Reports show that the body temperature of chicks increases rapidly under HT (Chowdhury et al., 2012a, b; Ito et al., 2014, 2015). Exposure of chicks to extreme HT induces heat stress (Ito et al., 2015), characterized by severe increase in body temperature and heightened stress response, which decreases food intake and feed efficiency, reducing meat product ion in broilers (Howlider and Rose, 1987) and egg production in laying hens (Sterling et al., 2003). Behavioral, physiological, and molecular responses to heat stress have been described previously (Etches et al., 1995; Chowdhury et al., 2012a, b). Recently, alteration in the levels of brain and plasma free amino acids was reported to be one of these responses (Chowdhury et al., 2014; Ito et al., 2014, 2015). For instance, L-citrulline (L-Cit) level was reduced in the plasma of heat-exposed chicks (Chowdhury et al., 2014). Chowdhury et al. (2015, 2017) further showed that oral administration of L-Cit produced hypothermic effects in young chicks. Supplementation of essential amino acids to reduce the negative effects of heat stress and maintain steady production performance has currently attracted the attention of the chicken farming industry. However, utilization of a non-essential amino acid, L-Cit, was a novel approach for attenuating heat stress response in chicks.
L-Cit, a non-protein amino acid, is a physiological amino acid which plays an important role in both cellular metabolism and organ function in most living systems (Curis et al., 2005). It is well-known that L-Cit, a metabolic intermediate in arginine (Arg) metabolism, is produced from L-Arg, an essential amino acid in chicks, by nitric oxide (NO) synthase, along with the production of NO (Palmer et al., 1987). NO is involved in the regulation of body temperature (De Luca et al., 1995; Osaka, 2010). However, L-Cit-dependent hypothermia was not found to be related to the level of plasma NO in chicks, which indicates the involvement of a novel mechanism (Chowdhury et al., 2017). As consumption of crystallized L-Cit has not yet been approved (Food and Agricultural Materials Inspection Center, Japan), its use in poultry ration is a matter of concern. Therefore, studies on the hypothermic functions of non-crystallized L-Cit might provide useful information. Erwan et al. (2014) reported that oral administration of D-aspartate (D-Asp), another hypothermic factor, lowered the body temperature of chicks both under a control thermoneutral temperature (CT) and HT. In addition, the administration of live bacteria into an animal's digestive tract has long been reported to exert beneficial effects on the growth and performance of the host. We recently observed that oral administration of a medium containing D-Asp-producing live bacteria reduced body temperature in chicks (Do et al., 2017). In the present study, we used a medium containing L-Cit-producing live bacteria to examine its effect on body temperature regulation under CT.
Materials and Methods
Animals and Drugs
One-day-old male layer chicks (Julia, Gallus gallus domesticus) were purchased from a local hatchery (Murata Hatchery, Fukuoka, Japan) and housed in groups (15–20 birds per cage) in metal cages (50×35×33 cm) at a constant temperature of 30±1°C under continuous light conditions. Food (Adjust diets; Toyohashi Feed and Mills Co. Ltd., Aichi, Japan; metabolizable energy: > 12.55 MJ/kg, protein > 23%) and water were provided ad libitum. This study was performed in accordance with the guidelines for animal experiments in the Faculty of Agriculture at Kyushu University, and complied with Law No. 105 and Notification No. 6 of the Japanese government (experimental protocol number was A26-074-1). The medium (containing live bacteria [Lactococus lactis subsp. Lactis; the number of live bacteria in the medium was 6.50E+09 cfu/mL] producing L-Cit, supplemented with L-Cit (277 mM), L-ornithine (12.4 mM), L-Arg (2.6 mM), L-lactic acid, and milk peptide (20.3 mM), was provided by Meiji Co., Ltd., Kanagawa, Japan. The components of the medium were decided by the provider. The concentration of L-Cit and other additives in the medium was determined using high-performance liquid chromatography (HPLC).
Experimental Design
Six-day-old chicks were randomly distributed into treatment and control groups based on their body temperature such that the average body temperature between the groups was as uniform as possible. In Experiment 1, 30 chicks (7 days old) were divided into three groups (n=10/group) for different treatments as follows: 1) acute oral administration of 2 mL of the medium (containing 554 µmol L-Cit/chick); 2) acute oral administration of 2 mL solution containing an equimolar amount of crystallized L-Cit/chick; 3) acute oral administration of 2 mL water/chick (control group). Oral administration was performed using an elastic plastic needle on a small syringe. Rectal temperature and body surface temperature were measured 0, 30, 60, and 120 min after oral administration of the medium or the equimolar amount of L-Cit solution.
In Experiment 2, chicks (7 days old) were distributed into three groups (n=10/group) as follows: 1) chronic oral administration of 2 mL of the medium (containing 554 µmol L-Cit/chick); 2) chronic oral administration of 2 mL solution containing an equimolar amount of crystallized L-Cit/chick; 3) chronic oral administration of 2 mL water/chick (control group). The chicks were orally administered in this manner every day from 7 to 13 days. Rectal and body surface temperatures were recorded daily immediately before the oral administration and 60 min after the oral administration during the period of the experiment. The chicks were euthanized following anesthesia with isoflurane (Mylan Inc., Tokyo, Japan) after completion of the experiments.
Measurement of Body Temperature
The rectal temperature of the chicks was measured using a digital thermometer with an accuracy of ±0.1°C (Thermalert TH-5, Physitemp Instruments Inc., Clifton, NJ, USA). The body surface temperature was measured on the earlobe of each chick using a Thermo ShotF30S camera (NEC Avio Infrared Technologies Co., Ltd, Tokyo, Japan) as shown in Fig. 1. The method of measuring surface temperature has been described elsewhere (Giloh et al., 2012).
Fig. 1.
Measurement of body surface temperature using a normal camera (a) and a thermal camera (b). × A indicates the point of the earlobe where measurement of the surface temperature was performed. The temperature range was determined by the color palette as shown in panel (b).
Statistical Analysis
Body temperature data were analyzed using a repeated-measure two-way analysis of variance (ANOVA), and the Tukey-Kramer test was performed as a post-hoc test. P<0.05 indicated statistically significant differences. Values were presented as means±S.E.M. Statistical analysis was performed using the commercially available package Stat View (version 5, SAS Institute, Cary, USA 1998).
Results
Changes in Body Temperature Following Acute Oral Administration of the Medium in Chicks
Changes in the rectal and surface temperatures of the chicks after acute oral administration of the medium containing L-Cit or an equimolar amount of crystallized L-Cit are shown in Fig. 2. The medium significantly (P<0.05) reduced both rectal and surface temperatures in chicks under CT. However, oral administration of crystallized L-Cit at the equimolar dose (554 µmol/2 mL) did not affect the body temperature of the chicks. Time after administration was also significant (P<0.05) in terms of the reduction in rectal temperature (Fig. 2a). Treatment and time interacted significantly (P<0.05) in the reduction of rectal (Fig. 2a) and surface temperatures (Fig. 2b), with pronounced reduction in body temperature occurring 60 min after administration of the medium.
Fig. 2.
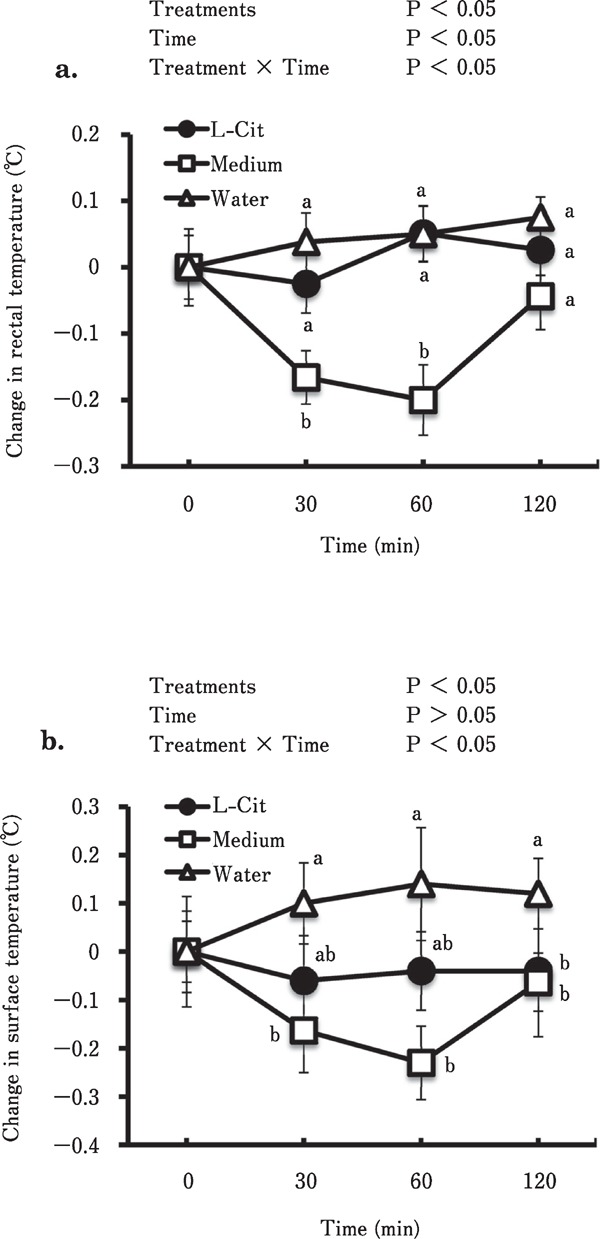
Effects of acute oral administration of the medium containing L-Cit-producing live bacteria on rectal temperature (a) and body surface temperature (b) of chicks. The number of chicks used in each group ranged between 8 and 9. Data are expressed as means±S.E.M. L-Cit, L-citrulline.
Changes in Body Temperature Following Chronic Oral Administration of the Medium in Chicks
Figure 3 shows the changes in body temperature of chicks after chronic oral administration of the medium containing L-Cit or an equimolar amount of crystallized L-Cit. Chronic oral administration of the medium, but not of the equimolar amount of crystallized L-Cit, significantly (P<0.05) reduced both the rectal (Fig. 3a) and surface temperature (Fig. 3b) of the chicks. Time significantly affected the reduction in rectal temperature (P<0.05).
Fig. 3.
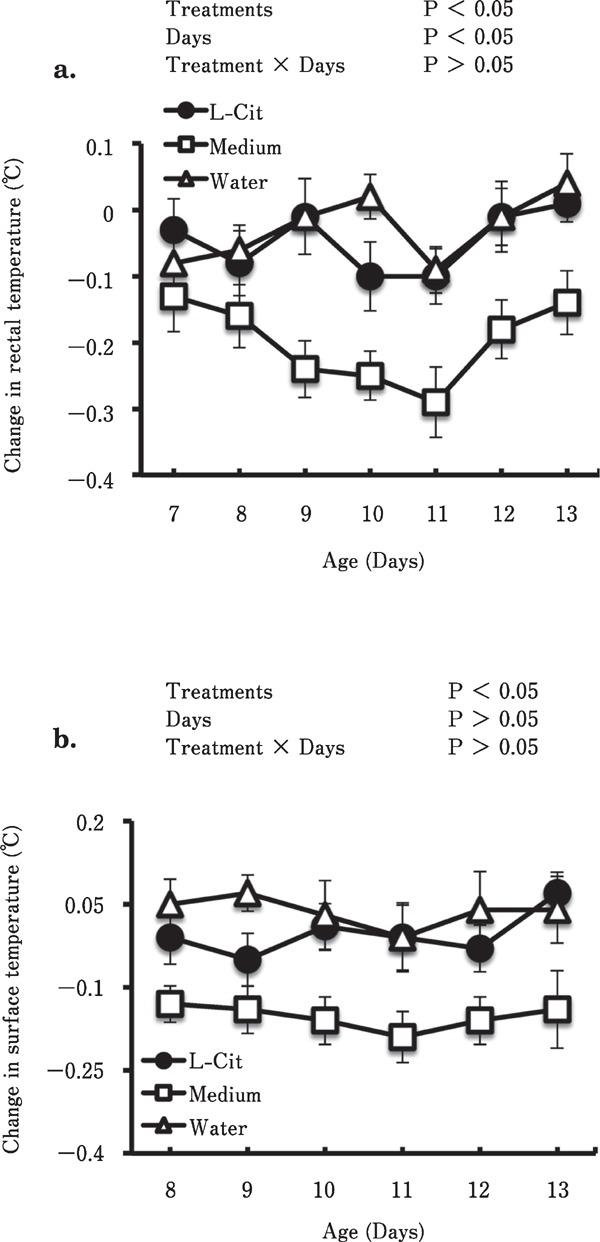
Effects of chronic oral administration of the medium containing L-Cit-producing live bacteria on rectal temperature (a) and body surface temperature (b) of chicks. The number of chicks used in each group ranged between 8 and 9. Data are expressed as means±S.E.M. L-Cit, L-citrulline.
Discussion
The objective of the current study was to examine whether oral administration of a medium containing L-Cit-producing live bacteria can reduce the body temperature of chicks under CT. Interestingly, we observed that both acute and chronic oral administration of a medium containing L-Cit-producing live bacteria reduced the body temperature of chicks. However, oral administration of an equimolar amount of crystallized L-Cit alone was notable to reduce the body temperature of the chicks. Chowdhury et al. (2015) reported that oral administration of 15 mmol/kg body weight, but not of 3.75 or 7.5 mmol L-Cit/kg body weight, reduced the body temperature of 6-day-old chicks. In the present study, the 7-day-old chicks (average body weight 65 g) were administered L-Cit at a dose of 8.52 mmol/kg body weight/day/2 mL and the 13-day-old chicks (average body weight 125 g) were administered L-Cit at a dose of 4.43 mmol/kg body weight/day/2 mL when calculated based on a basal concentration of 277 mM L-Cit in the medium. Therefore, the amount of L-Cit administered to the chicks in this study was lower than the effective dose (15 mmol/kg body weight) required for reducing body temperature as described elsewhere (Chowdhury et al., 2015, 2017). The involvement of several factors should be considered for understanding the hypothermic effect of the treated medium in the present study. First, lactic acid, milk peptide, and other amino acids, the other components of the medium, may contribute to the induction of the main effect of the medium. Second, the survival rate of live bacteria might reduce with the time of preservation and the number of dead and/or damaged bacteria might increase. Dead bacteria have been reported to exert bioactive function in the digestive tract of the host (Yuen and Ausubel, 2018). However, the results of the present study and those of our previous study on the effect of oral administration of a medium containing D-Asp-producing live bacteria (Do et al., 2017) confirm that the introduction of L-Cit-producing live bacteria mainly induced the hypothermic effect of the medium. Further studies should clarify the thermoregulation effect of the medium with and without the presence of other subcomponents and the effect of dead and/or damaged bacteria on the body temperature of neonatal chicks.
Heat loss is a process via which heat is transferred from the body to the surrounding environment. In humans, the sweat glands of the skin are triggered to produce additional moisture to remove heat as a vapor from the surface of the body when the core body temperature increases (Wilmore et al., 2008). However, chicks cannot dissipate heat via cutaneous evaporation as they lack sweat glands (Marder and Arad., 1989). Therefore, other means of reducing surface temperature is critical for maintaining body temperature homeostasis under heat stress. In the current study, both the core body temperature (rectal temperature) and surface temperature were reduced by the administration of the medium under CT. Reports show that chronic oral administration of a medium containing D-Asp-producing live bacteria has a hypothermic effect in chicks under heat stress (Do et al., 2017). Whether oral administration of the medium containing L-Cit-producing live bacteria can provide thermotolerance in young chicks under heat stress should be investigated in future.
Experiment 1 shows that acute oral administration of the medium containing L-Cit-producing live bacteria reduced the body temperature of chicks. Therefore, L-Cit-producing live bacteria are immediately effective in reducing body temperature. The reduction in body temperature was pronounced 60 min after acute oral administration of the medium, and the effect diminished 120 min after administration. Hence, changes in plasma L-Cit level should be determined in chicks following acute oral administration of the medium compared to that of the control group in future.
Synthesis of endogenous L-Cit, together with the production of NO, has been demonstrated to be a modulator of various behaviors, including thermoregulation (De Luca et al., 1995; Szabo, 1996). However, L-Cit-dependent hypothermia has been reported to be related to hypoglycemia rather than to plasma NO level in chicks (Chowdhury et al., 2017). Reduction in plasma glucose level results in decrease in body temperature in mammals (Branco, 1997). Studies investigating the relationship between hypoglycemia and the hypothermic action of the medium might be useful in this regard.
Nevertheless, chicks can adjust to environmental conditions via a process of non-shivering thermogenesis, which involves the function of mitochondrial uncoupling protein (avUCP) and adenine nucleotide translocator (avANT) (Mozo et al., 2005). Importantly, reduction in body temperature following oral administration of a medium containing D-Asp-producing live bacteria has been observed, together with downregulation of avUCP and upregulation of avANT in the skeletal muscle of the medium-treated chicks (Do et al., 2017). Therefore, avUCP or avANT might have been involved in reducing body temperature in the present study. The involvement of genes associated with mitochondrial biogenesis in the reduction of body temperature following oral administration of medium containing L-Cit-producing live bacteria should be investigated in future.
In conclusion, acute and chronic oral administration of a medium containing L-Cit-producing live bacteria exerted hypothermic effect in young chicks. This medium may be a potential novel feed supplement for inducing hypothermia.
Acknowledgment
This work was partially supported by JSPS KAKENHI Grant Numbers JP15K07694 and JP18K19271 to VSC as well as JP17H01503 to MF.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Branco LGS. Effects of 2-deoxy-D-glucose and insulin on plasma glucose levels and behavioral thermoregulation of toads. American Journal of Physiology, 272: R1-R5. 1997. [DOI] [PubMed] [Google Scholar]
- Chowdhury VS, Tomonaga S, Nishimura S, Tabata S, Cockrem JF, Tsutsui K, Furuse M. Hypothalamic gonadotropin-inhibitory hormone precursor mRNA is increased during depressed food intake in heat-exposed chicks. Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology, 162: 227-233. 2012. a. [DOI] [PubMed] [Google Scholar]
- Chowdhury VS, Tomonaga S, Nishimura S, Tabata S, Furuse M. Physiological and behavioral responses of young chicks to high ambient temperature. Journal of Poultry Science. 49: 212-218. 2012. b. [Google Scholar]
- Chowdhury VS, Tomonaga S, Ikegami T, Erwan E, Ito K, Cockrem JF, Furuse M. Oxidative damage and brain concentrations of free amino acid in chicks exposed to high ambient temperature. Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology, 169: 70-76. 2014. [DOI] [PubMed] [Google Scholar]
- Chowdhury VS, Shigemura A, Erwan E, Ito K, Bahry MA, Tran PV, Furuse M. Oral administration of L-citruline, but not Larginine or L-ornithine, acts as a hypothermic agent in chicks. Journal of Poultry Science, 52: 331-335. 2015. [Google Scholar]
- Chowdhury VS, Han G, Bahry MA, Tran PV, Do PH, Yang H, Furuse M. L-Citrulline acts as potential hypothermic agent to afford thermotolerance in chicks. Journal of Thermal Biology, 69: 163-170. 2017. [DOI] [PubMed] [Google Scholar]
- Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S. Almost all about citrulline in mammals. Amino Acids, 29: 177-205. 2005. [DOI] [PubMed] [Google Scholar]
- De Luca B, Monda M, Sulla A. Changes in eating behavior and thermogenic activity following inhibition of nitric oxide formation. American Journal of Physiology, 268: 1533-1538. 1995. [DOI] [PubMed] [Google Scholar]
- Do PH, Tran PV, Bahry MA, Yang H, Han G, Tsuchiya A, Asami Y, Furuse M, Chowdhury VS. Oral administration of a medium containing both D-Aspartate-producing live bacteria and D-aspartate reduces rectal temperature in chicks. British Poultry Science, 58: 569-577. 2017. [DOI] [PubMed] [Google Scholar]
- Erwan E, Chowdhury VS, Nagasawa M, Goda R, Otsuka T, Yasuo S, Furuse M. Oral administration of D-aspartate, but not L-aspartate, depresses rectal temperature an alters plasma metabolites in chicks. Life Sciences, 109: 65-71. 2014. [DOI] [PubMed] [Google Scholar]
- Etches R, John JM, Gibbins AMV. Behavioural, physiological, endocrine and molecular responses to heat stress. In: Poultry production in hot climates (Daghir NJ ed.). pp. 31-65. Editor, Daghir N.J, CAB International, Wallingford: 1995. [Google Scholar]
- Giloh M, Shinder D, Yahav S. Skin surface temperature of broiler chickens is correlated to body core temperature and is indicative of their thermoregulatory status. Poultry Science, 91: 175-188. 2012. [DOI] [PubMed] [Google Scholar]
- Ito K, Erwan E, Nagasawa M, Furuse M, Chowdhury VS. Changes in free amino acid concentrations in the blood, brain and muscle of heat-exposed chicks. British Poultry Science, 55: 644-652. 2014. [DOI] [PubMed] [Google Scholar]
- Ito K, Bahry MA, Hui Y, Furuse M, Chowdhury VS. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acid. Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology, 187: 13-19. 2015. [DOI] [PubMed] [Google Scholar]
- Howlider MAR, Rose SP. Temperature and the growth of broilers. World's Poultry Science Journal, 43: 228-237. 1987. [Google Scholar]
- Marder J, Arad Z. Panting and acid-base regulation in heat stressed birds. Comparative Biochemistry and Physiology Part A, Comparative Physiology, 94: 395-400. 1989. [DOI] [PubMed] [Google Scholar]
- Mozo J, Emre Y, Bouillaud F, Ricquier D, Criscuolo F. Thermoregulation: what role for UCPs in mammals and birds? Bioscience Reports, 25: 227-249. 2005. [DOI] [PubMed] [Google Scholar]
- Nicol C. Management and welfare of farm animals. The UFAW farm handbook: UFAW farm handbook. Editor, Webster John, 5th ed. John Wiley -Blackwell, West Sussex, UK, pp. 25-30. 2011. [Google Scholar]
- Osaka T. Niric oxide mediates noradrenaline-induced hypothermic responses and opposes prostaglandin E2-induced fever in the rostromedial preoptic area. Neuroscience, 165: 976-983. 2010. [DOI] [PubMed] [Google Scholar]
- Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature, 237: 524-526. 1987. [DOI] [PubMed] [Google Scholar]
- Sterling KG, Bell DD, Pesti GM, Aggrey SE. Relationships among strain, performance, and environmental, temperature in commercial laying hens. Journal of Applied Poultry Research, 12: 85-91. 2003. [Google Scholar]
- Szabo C. Physiological and pathophysiological roles of nitric oxide in the central nervous system. Brain Research Bulletin, 41: 131-141. 1996. [DOI] [PubMed] [Google Scholar]
- Wilmore JH, Costill DL, Kenney L. Physiology of sport and exercise. 4th ed. Human Kinetics. 2008. [Google Scholar]
- Yuen GJ, Ausubel FM. Both live and dead Enterococci activate Caenorhabditis elegans host defense via immune and stress pathways. Virulence, 9: 683-699. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


