Abstract
Cordyceps militaris is a well-known Chinese medicinal fungus that has been used as a nutraceutical food in several Asian countries. Cordycepin (3′-deoxyadenosine), a secondary metabolite produced from Cordyceps militaris, has been demonstrated to exert a wide spectrum of pharmacological activities, such as anti-microbial and antitumor activities. However, the effect of cordycepin on immunomodulation in broilers is poorly investigated. In the current study, we investigated the effect of cordycepin (9.69, 19.38, and 38.76 mg) from Cordyceps militaris hot water extract (CMHW) on growth performance and immunocompetence in broilers. Results showed that CMHW significantly decreased inducible nitric oxide synthase (iNOS) mRNA levels in the bursa of Fabricius after 4 weeks of feeding (P<0.05). CMHW treatment reduced cyclooxygenase-2 (COX-2) mRNA levels in the spleen and bursa of Fabricius after 4 weeks of feeding (P<0.05). Supplementation of CMHW for 3 days after vaccination reduced iNOS mRNA level in the spleen of 14 and 28 day-old broilers (P<0.05). Prior to vaccination, CMHW pretreatment significantly down-regulated COX-2 mRNA levels in the spleen and bursa of Fabricius of 14-day-old broilers (P<0.05). Furthermore, CMHW significantly reduced lipopolysaccharide (LPS)-induced iNOS and COX-2 mRNA levels in the spleen and bursa of Fabricius (P<0.05). CMHW treatment attenuated LPS-induced IFN-γ expression in the spleen and bursa of Fabricius, whereas CMHW induced IL-4 expression in these organs in response to LPS challenge (P<0.05). Taken together, these observations demonstrate that CMHW exerts an immunomodulatory role in broilers. CMHW is a potential novel feed additive with applications in inflammation-related diseases and bacterial infection in broilers.
Keywords: broiler, Cordyceps militaris, immunomodulation, lipopolysaccharide
Introduction
Medicinal fungi, such as Cordyceps militaris, has been traditionally used in China since antiquity to treat hyposexuality, hyperlipidemia, asthma, and lung inflammation (Shiao et al., 1994; Huang et al., 2004; Wang et al., 2007; Guo et al., 2010; Das et al., 2010). Studies in the past decade have shown that Cordyceps militaris has multiple pharmacological functions, including anti-oxidant, anti-inflammatory, antimicrobial, anti-tumor, and anti-angiogenic activities and immunopotentiation ability (Ng and Wang, 2005; Das et al., 2010; Shin et al., 2010).
Cordycepin, an analog of 3′-deoxyadenosine, is one of the major bioactive metabolites of Cordyceps militaris. Several studies have demonstrated that cordycepin has various biological activities, such as anti-microbial, anti-fungal, and anti-tumor activities (Sugar and McCaffrey, 1998; Ahn et al., 2000; Nakamura et al., 2006). Cordycepin also exhibits an anti-inflammatory role in LPS-induced murine macrophages and microglia (Kim et al., 2006; Jeong et al., 2010). The chemical structure of cordycepin is similar to that of adenosine. Cordycepin can be used as a nucleotide during RNA synthesis, which leads to premature termination of chain elongation because of the absence of oxygen in the 3′ position of its ribose ring (Siev et al., 1969; Müller et al., 1977). Studies have shown that cordycepin can inhibit the synthesis of Newcastle disease virus and hepatitis C virus (Weiss and Bratt, 1975; King et al., 2002). In addition to cordycepin, the polysaccharides of Cordyceps militaris also possess antiviral and anti-tumor activities (Ohta et al., 2007; Yang et al., 2014).
Previous studies on the pharmacological and biochemical mechanisms underlying the activities of Cordyceps militaris or cordycepin have focused on human or mouse models. Recently, we showed that CMHW inhibits LPS-induced inflammatory response in porcine alveolar macrophages by regulating the p38 mitogen-activated protein kinase (MAPK) signaling pathway (Hsiao et al., 2018). Furthermore, feed supplementation with Cordyceps militaris fermentation products improved growth performance and enhanced cell-mediated immunity in piglets (Cheng et al., 2016).
Prebiotics, herbs, and plant extracts have attracted attention as potential feed additives in poultry (Abudabos et al., 2015a; Abudabos et al., 2015b; Saleh et al., 2018). Cordyceps militaris is widely used as a herb in several Asian countries. However, whether CMHW can improve growth performance and exert immunomodulatory effect in broilers is not known. Therefore, the purpose of this study was to investigate the effects of cordycepin from CMHW on growth performance and immunomodulation in response to vaccination and LPS challenge. These results might provide valuable information regarding the effect of CMHW on immunomodulation, which might promote its development as a feed additive in broilers.
Materials and Methods
Cordyceps Militaris Culture, Extraction, and Analysis
The Cordyceps militaris strain (BCRC® 32219™) was purchased from the Biosource Collection and Research Center (BCRC, Hsinchu, Taiwan). Cordyceps militaris was inoculated into an Erlenmeyer flask containing potato dextrose broth (Difco Laboratories, Detroit, MI, USA) and incubated at 22°C for 5 days with shaking. Wheat-based substrates containing 0.1% CaCO3, 0.05% MgSO4, 0.1% NaH2PO4, 50% H2O, 0.05% KH2PO4, and 1% glucose were inoculated with 10% (v/w) inoculum and mixed carefully under sterile conditions. Cultures were incubated in dark at 22°C for 2 weeks. The fermentation products were baked at 50°C for 24 h and then homogenized by mechanical agitation. The dried fermentation products were heated using distilled deionized water at 100°C for 1 h and then filtered using Whatman No. 2 filter paper. The CMHW filtrate was lyophilized and reconstituted with Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Carlsbad, CA, USA). For quantification of cordycepin from CMHW using high-performance liquid chromatography (HPLC), the SPD-10A system with a programmable UV detector (10A VP, Shimadzu, Columbia, MD, USA) and a reverse phase RP-18 column (LiChrospher® 100 RP-18 end capped, 5 µm) was used throughout the experiments. The mobile phase consisted of methanol: 0.02 mol L−1 KH2PO4 (15:85, v/v). Cordycepin levels were determined at 254 nm using an ultraviolet (UV) detector. The flow rate and recorder were set to 1 mL min−1 and 15 min, respectively. A linear response was obtained over a range of 100–500 µg mL−1 of the cordycepin standard. The standards were analyzed three times (minimum). The concentration of cordycepin was determined based on the slope of the standard curve. The average concentration of cordycepin was 9.69 mg/g of CMHW in the present study.
Animal Experiment I
For assessing the immunomodulatory property of CMHW, 48 one-day-old male broilers (Ross 308) were purchased from a local commercial hatchery. All the broilers were randomly divided into four groups with three replicates. Each replicate was assigned to a cage (four chicks per cage, 68×66×33 cm). CMHW powder was supplied in drinking water for 5 days after vaccination on days 4 and 14, respectively. The volume of drinking water in each group was measured based on group body weight. The four groups (n=12 per group) were: (1) basal diet (control), (2) basal diet plus 1 g/L (9.69 mg cordycepin) CMHW in drinking water, (3) basal diet plus 2 g/L (19.38 mg cordycepin) CMHW in drinking water, and (4) basal diet plus 4 g/L (38.76 mg cordycepin) CMHW in drinking water. The basal diets were formulated based on the recommendations of the National Research Council (NRC, Nutrient Requirements for Poultry, 1994) as shown in Table 1. Feed and water were offered ad libitum. The birds were housed in stainless-steel temperature-controlled batteries for four weeks. Broilers were vaccinated by nose-drop administration with combined Newcastle disease (ND)-infectious bronchitis (IB) live vaccines at 4 and 14 days of age. The temperature was set at 32°C on the first day, gradually reduced to 24°C by the third week, and then maintained at 24°C till the completion of the experiment. The lighting schedule was 22 h light: 2 h dark throughout the experiment. The average body weight, daily gain, daily feed intake, and feed conversion ratio (FCR) were recorded every week. For determining the peak of antibody titer response within two weeks after vaccination, blood was collected by cardiac puncture from 14 day-old-birds. For determining immune response-related gene expression, 14 and 28-day-old broilers were sacrificed by cervical dislocation. The spleen and bursa of Fabricius were excised and analyzed, respectively.
Table 1. Composition of basal diets.
| Item | |
|---|---|
| Ingredient, g kg−1 | |
| Corn, yellow | 511.8 |
| Soybean meal, 36.7% CP | 350.0 |
| Fish meal | 100.0 |
| CaCO3, 38% | 20.0 |
| CaHPO4 | 10.0 |
| DL-Methionine, 99.5% | 2.0 |
| Mineral premix1 | 1.0 |
| Vitamin premix2 | 1.0 |
| Choline chloride, 50% | 0.2 |
| Sodium chloride | 4.0 |
| Calculated value, g kg−1 | |
| Crude protein | 230.0 |
| Analyzed calcium | 13.8 |
| Analyzed total phosphorus | 7.0 |
| Lysine | 13.4 |
| Methionine + Cystine | 10.0 |
| ME, kcal/kg | 3411 |
Supplied per kilogram of diet: Cu (CuSO4·5H2O), 20 mg; Zn (ZnO), 100 mg; Fe (FeSO4·H2O), 140 mg; Mn (MnSO4·H2O), 4 mg; Se (Na2SeO3), 0.1 mg and I (ethylenediamine dihydriodide), 0.2 mg
Supplied per kilogram of diet: vitamin A, 6,000 IU; vitamin D3, 900 IU; vitamin E, 30 IU; vitamin K3, 3 mg; riboflavin, 6 mg; niacin, 60 mg; pantothenic acid, 18 mg; and vitamin B12, 30 µg
Animal Experiment II
For evaluating vaccine adjuvant activity of CMHW and optimal timing for CMHW supplementation during vaccination, 48 one-day-old male broilers (Ross 308) were purchased from a local commercial hatchery. All the broilers were randomly divided into four groups with three replicates. Each replicate was assigned to a cage (four chicks per cage, 68×66×33 cm). All broilers were fed basal diet based on the recommendations of the National Research Council (NRC, Nutrient Requirements for Poultry, 1994) as shown in Table 1. The four groups (n=12 per group) were: (1) control group, (2) supplementation of 2 g/L CMHW in drinking water for 3 days before vaccination (A), (3) supplementation of 2 g/L CMHW in drinking water for 3 days before and after vaccination (AP), and (4) supplementation of 2 g/L CMHW in drinking water for 3 days after vaccination (P). Feed and water were offered ad libitum. The birds were housed in stainless-steel temperature-controlled batteries for five weeks. Broilers were vaccinated by nose-drop administration with combined ND-IB live vaccines when they were 4, 14, and 28 days old. The temperature was set at 32°C on the first day, gradually reduced to 24°C by the third week, and then maintained at 24°C till the completion of the experiment. The lighting schedule was 22 h light: 2 h dark throughout the experiment. The average body weight, daily gain, daily feed intake, and FCR were recorded every week. For determining the peak of antibody titer response within two weeks after vaccination, blood was collected from 35-day-old birds by cardiac puncture. For determining immune response-related gene expression, 21 and 35-day-old broilers were sacrificed by cervical dislocation. The spleen and bursa of Fabricius were excised and analyzed, respectively.
Animal Experiment III
For evaluating the anti-inflammatory efficacy of CMHW in response to LPS stimulation, 36 one-day-old male broilers (Ross 308) were purchased from a local commercial hatchery. All the broilers were randomly divided into three groups with three replicates each. Each replicate was assigned to a cage (four chicks per cage, 68×66×33 cm). The broilers were fed basal diet based on the recommendations of the National Research Council (NRC, Nutrient Requirements for Poultry, 1994) as shown in Table 1. CMHW powder was supplied in drinking water and the volume of drinking water in each group was measured based on group body weight. The three groups (n=12 per group) were: (1) basal diet (control), (2) basal diet plus intraperitoneal administration of LPS (1 mg/kg), and (3) basal diet plus intraperitoneal administration of LPS (1 mg/kg) in combination with 2 g/L CMHW in drinking water. Feed and water were offered ad libitum. The birds were housed in stainless-steel temperature-controlled batteries for 12 days. To determine whether pre-treatment of CMHW exerted an immunomodulatory effect after acute LPS stimulation, CMHW powder was supplied in drinking water from 8 day to 10 day of age and LPS was administered intraperitoneally on 11 day of age. The broilers were sacrificed by cervical dislocation 3 h and 24 h post-injection of LPS. The spleen and bursa of Fabricius were excised and analyzed, respectively.
Antibody Analysis
Blood samples were collected by cardiac puncture and separated by centrifugation at 1,500×g for 10 min. Serum was harvested and preserved at −20°C. The antibody response against ND and IB viruses was determined using the enzyme-linked immunosorbent assay (ELISA) commercial diagnostic kits (BioChek, Gouda, The Netherlands).
Quantitative Reverse Transcription-polymerase Chain Reaction (qRT-PCR)
Six birds from each group were randomly selected, sacrificed, and used for gene expression analysis. Total RNA was isolated from the spleen and bursa of Fabricius and homogenized in TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) using a homogenizer (SpeedMill PLUS, Analytik Jena, Jena, Germany). Total RNA was then purified and reverse transcribed using a Transcriptor reverse transcriptase kit (Roche Applied Science, Indianapolis, IN, USA). qRT-PCR was performed using MiniOpticon™ real-time PCR detection system (Bio-Rad, Hercules, CA, USA) and KAPA SYBR® FAST qPCR kit (Kapa Biosystems, Boston, MA, USA). PCR was performed at 95°C for 30 s, 58–60°C for 60 s, and 72°C for 30 s for 40 cycles. The β-actin mRNA was used as the internal control. The sequence of the primers used in qRT-PCR is listed in Table 2. The mRNA level of each gene was normalized to that of β-actin mRNA in the same sample. Threshold cycle (Ct) values were obtained and relative gene expression was calculated using the formula (1/2)Ct target genes−Ct β-actin.
Table 2. Primer sequences for quantitative reverse transcription-PCR.
| Gene1 | GenBank accession number | Primer sequences (5′-3′) |
|---|---|---|
| iNOS | NM_204961 | F2: AGGCCAAACATCCTGGAGGTC R: TCATAGAGACGCTGCTGCCAG |
| COX-2 | NM_001167719 | F: AACACAATAGAGTCTGTGACGTCTT R: TATTGAATTCAGCTGCGATTCGG |
| IFN-γ | GQ421600 | F: ACACTGACAAGTCAAAGCCGCACA R: AGTCGTTCATCGGGAGCTTGGC |
| IL-4 | GU119892 | F: TGTGCCCACGCTGTGCTTACA R: CTTGTGGCAGTGCTGGCTCTCC |
| β-actin | NM_205518 | F: CATCACCATTGGCAATGAGAGG R: GGTACATTGTGGTACCACCAGAC |
iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2; IFN-γ: interferon-γ; IL-4: interleukin-4
F, forward primer; R, reverse primer
Ethics Statement
All experiments were performed in accordance with the approved guidelines. The animal protocol was approved by the National Ilan University Institutional Animal Care and Use Committee (IACUC Approval No. 103–05).
Statistical Analysis
The data were analyzed in a completely randomized design using the general linear model (GLM) procedure of the SAS software (SAS Institute, Cary, NC, USA). Each broiler formed an experimental unit. Means were compared using Tukey's test when the probability values were significant (P<0.05).
Results
Effect of CMHW Supplementation on Growth Performance and Vaccine Adjuvant Activity in Broilers
To examine the effect of CMHW supplementation on growth performance, broilers were fed with 1, 2, and 4 g/L CMHW for four weeks. However, there were no statistically confirmed differences between body weight and CMHW supplementation during the entire feeding period (Table 3). Broilers fed 1 g/L CMHW had lower feed intake from day 14 to 28 compared to other treatments (Table 3, P<0.05). Supplementation of high concentration of CMHW had the highest potential to improve FCR in broilers from day 1 to 14, whereas this beneficial effect was reversed from day 14 to 28 (Table 3, P<0.05). Supplementation of CMHW in drinking water 3 days before vaccination increased the body weight at 21 days of age compared to other treatments (Table 4, P<0.05). Similar results were also observed in daily gain and feed intake from day 1 to 21 (Table 4, P<0.05). However, broilers fed CMHW for 3 d after vaccination showed higher FCR from day 1 to 21 (Table 4, P<0.05). CMHW supplementation did not improve the response of antibody titer to ND and IB viruses (Fig. 1a and b). In addition, different CMHW supplementation periods did not affect the antibody titer of ND and IB viruses (Fig. 1c and 1d).
Table 3. Effects of different concentration of CMHW on growthperformance in broilers.
| Control (n=12) |
1 g/L1 (n=12) |
2 g/L (n=12) |
4 g/L (n=12) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p value | ||
| Body weight (g/bird) | ||||||||||
| 1 d | 48.33 | 11.55 | 50.00 | 0.01 | 50.00 | 0.01 | 50.00 | 0.01 | 0.86 | |
| 14 d | 376.67 | 75.72 | 385.00 | 91.65 | 381.67 | 167.73 | 393.33 | 41.61 | 0.98 | |
| 28 d | 1106.67 | 121.66 | 1068.89 | 181.48 | 1113.33 | 260.00 | 1082.22 | 190.09 | 0.94 | |
| Average daily gain (g/d) | ||||||||||
| 1∼14 d | 23.45 | 1.15 | 23.93 | 1.64 | 23.69 | 2.99 | 24.53 | 0.74 | 0.52 | |
| 14∼28 d | 43.18 | 4.16 | 39.68 | 3.61 | 43.17 | 3.17 | 39.84 | 4.42 | 0.06 | |
| Average daily feed intake (g/d) | ||||||||||
| 1∼14 d | 28.92 | 0.38 | 28.92 | 1.04 | 29.00 | 2.82 | 28.83 | 0.80 | 1.00 | |
| 14∼28 d | 82.05a | 3.14 | 76.51b | 1.68 | 83.49a | 7.91 | 82.22a | 4.63 | 0.01 | |
| Feed conversion ratio | ||||||||||
| 1∼14 d | 1.23a | 0.05 | 1.21ab | 0.05 | 1.23a | 0.03 | 1.18b | 0.01 | 0.01 | |
| 14∼28 d | 1.91a | 0.14 | 1.94ab | 0.18 | 1.93ab | 0.12 | 2.07b | 0.13 | 0.04 | |
concentration of CMHW in drinking water
Means within a row with no common superscript are significantly different (p<0.05)
Table 4. Effects of CMHW on growthperformance in broilers during vaccination.
| Control (n=12) |
A1 (n=12) |
AP (n=12) |
P (n=12) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p value | ||
| Body weight (g/bird) | ||||||||||
| 1 d | 47.83 | 0.38 | 47.83 | 0.29 | 47.83 | 0.29 | 47.75 | 0.00 | 0.86 | |
| 21 d | 708.33a | 28.43 | 771.67b | 15.28 | 728.33a | 97.00 | 718.33a | 32.53 | 0.03 | |
| 35 d | 1651.11 | 118.57 | 1741.11 | 62.75 | 1748.89 | 193.95 | 1677.78 | 131.71 | 0.23 | |
| Average daily gain (g/d) | ||||||||||
| 1∼21 d | 31.45a | 1.36 | 34.47b | 0.74 | 33.9ab | 4.21 | 31.93ab | 1.55 | 0.01 | |
| 22∼35 d | 67.34 | 6.65 | 69.25 | 4.49 | 72.90 | 9.52 | 68.53 | 10.37 | 0.38 | |
| Average daily feed intake (g/d) | ||||||||||
| 1∼21 d | 43.65a | 3.28 | 47.22b | 1.72 | 45.48ab | 4.69 | 45.16ab | 0.73 | 0.05 | |
| 22∼35 d | 118.57 | 9.09 | 117.94 | 10.28 | 123.97 | 13.77 | 120.08 | 11.36 | 0.56 | |
| Feed conversion ratio | ||||||||||
| 1∼21 d | 1.39ab | 0.06 | 1.37ab | 0.02 | 1.34a | 0.07 | 1.42b | 0.09 | 0.03 | |
| 22∼35 d | 1.76 | 0.05 | 1.70 | 0.13 | 1.71 | 0.08 | 1.76 | 0.13 | 0.34 | |
A: anterior supplementation of 2 g/L CMHW in drinking water for 3 d before vaccination; AP: anterior and posterior supplementation of 2 g/L CMHW in drinking water for 3 d before and after vaccination; P: posterior supplementation of 2 g/L CMHW in drinking water for 3 d after vaccination
Means within a row with no common superscript are significantly different (p<0.05)
Fig. 1.
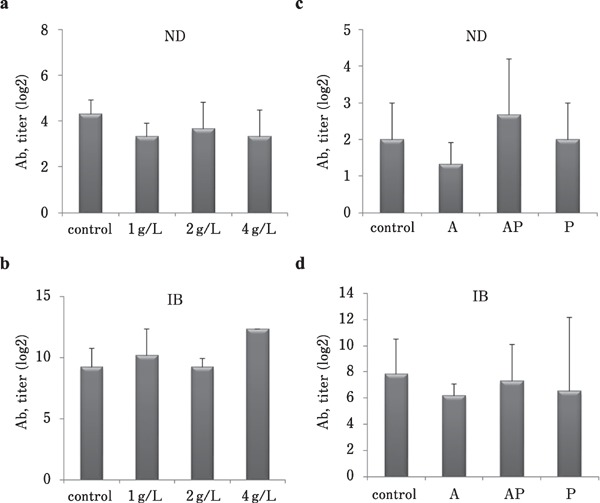
Effects of CMHW supplementation on antibody titer in broilers. (a) Effect of different concentrations of CMHW (1, 2, and 4 g/L in drinking water) on antibody titers against Newcastle disease (ND) in 14-day-old broilers. (b) Effect of different concentrations of CMHW (1, 2, and 4 g/L in drinking water) on antibody titers against infectious bronchitis (IB) in 14-day-old broilers. (c) Effect of different supplementation period (A, anterior only; AP: anterior and posterior; P, posterior only) of CMHW (2 g/L in drinking water) on antibody titers against ND in 35-day-old broilers. (d) Effect of different supplementation period (A, anterior only; AP: anterior and posterior; P, posterior only) of CMHW (2 g/L in drinking water) on antibody titers against IB in 35-day-old broilers. Values represent mean±SD (n=3).
Effect of CMHW Supplementation on Regulation of Immunomodulation-related Gene Expression in Broilers
To examine the effect of different concentrations of CMHW on inflammation-related gene expression in the spleen and bursa of Fabricius, broilers were fed 1, 2, and 4 g/L CMHW. Results showed that CMHW supplementation in drinking water for 14 d had no significant effect on iNOS mRNA level in the spleen (Fig. 2a), whereas it was significantly reduced after 28 day-treatment with 2 g/L of CMHW (Fig. 2b, P<0.05). Similarly, CMHW supplementation did not inhibit COX-2 expression in the spleen of 14-day-old broilers (Fig. 2c). However, COX-2 mRNA level was significantly reduced in the spleen in a dose-dependent manner in 28-day-old broilers (Fig. 2d, P<0.05). CMHW treatment did not affect iNOS mRNA level in the bursa of Fabricius of 14-day-old broilers (Fig. 2e), whereas it efficiently inhibited iNOS expression in 28-day-old broilers (Fig. 2f, P<0.05). The high concentration of CMHW (4 g/L) attenuated COX-2 expression in the bursa of Fabricius of 14-day-old broilers (Fig. 2g, P<0.05). Similar inhibitory effects on COX-2 mRNA levels after CMHW treatment were also observed in 28-day-old broilers (Fig. 2h, P<0.05).
Fig. 2.
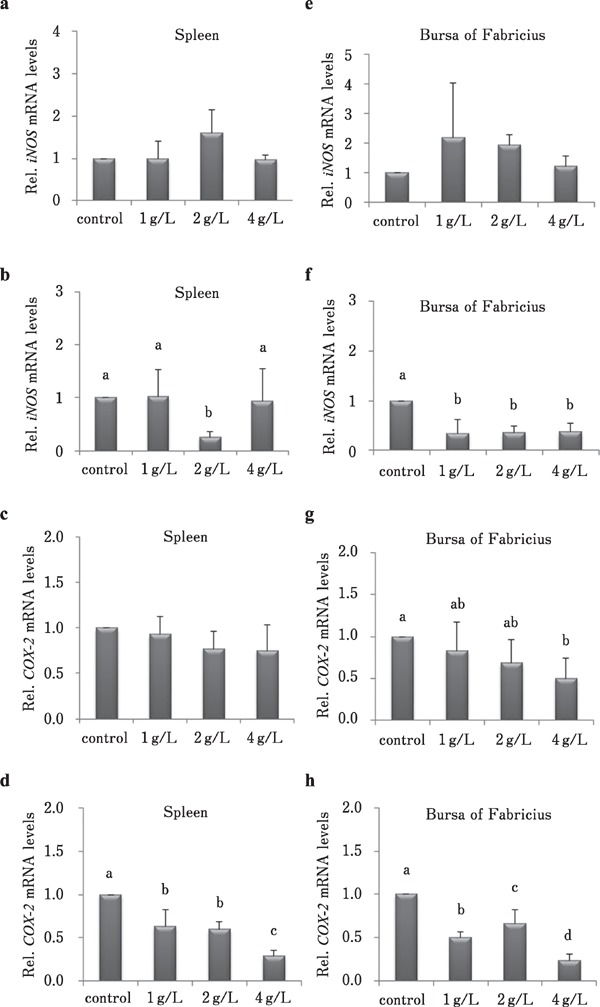
Effects of different concentrations of CMHW on inflammation-related gene expression in broilers. Effect of different concentrations of CMHW (1, 2, and 4 g/L in drinking water) on iNOS mRNA level in the spleen of 14-day-old (a) and 28-day-old broilers (b). COX-2 mRNA level in the spleen of 14-day-old (c) and 28-day-old broilers (d). Effect of different concentrations of CMHW (1, 2, and 4 g/L in drinking water) on iNOS mRNA level in the bursa of Fabricius of 14-day-old (e) and 28-day-old broilers (f). COX-2 mRNA level in the bursa of Fabricius of 14-day-old (g) and 28-day-old broilers (h). Values represent mean±SD (n=6). Means with different letter superscripts are significantly different (P<0.05)
To investigate whether CMHW has an immunomodulatory role in the spleen and bursa of Fabricius, broilers were fed 2 g/L CMHW before or after vaccination. Results showed that compared to other treatments, posterior supplementation of CMHW in drinking water reduced iNOS mRNA level in the spleen of 21-day-old broilers (Fig. 3a, P<0.05). In contrast, iNOS mRNA level in the spleen was induced after anterior and posterior supplementation of CMHW in 35-day-old broilers (Fig. 3b, P<0.05). Compared to other treatments, anterior supplementation of CMHW reduced COX-2 mRNA levels in the spleen of 21- and 35-day-old broilers (Fig. 3c and d, P<0. 05). iNOS expression was attenuated in the bursa of Fabricius of 21-day-old broilers at all CMHW supplementation periods (Fig. 3e, P<0.05). However, CMHW supplementation during vaccination did not significantly affect iNOS expression in the bursa of Fabricius of 35-day-old broilers (Fig. 3f). Anterior only and anterior in combination with posterior supplementation of CMHW inhibited COX-2 expression in the bursa of Fabricius of 21 day-old broilers (Fig. 3g, P<0.05). Similar to iNOS expression in the bursa of Fabricius, CMHW supplementation during vaccination did not significantly affect COX-2 expression in the bursa of Fabricius of 35-day-old broilers (Fig. 3h).
Fig. 3.
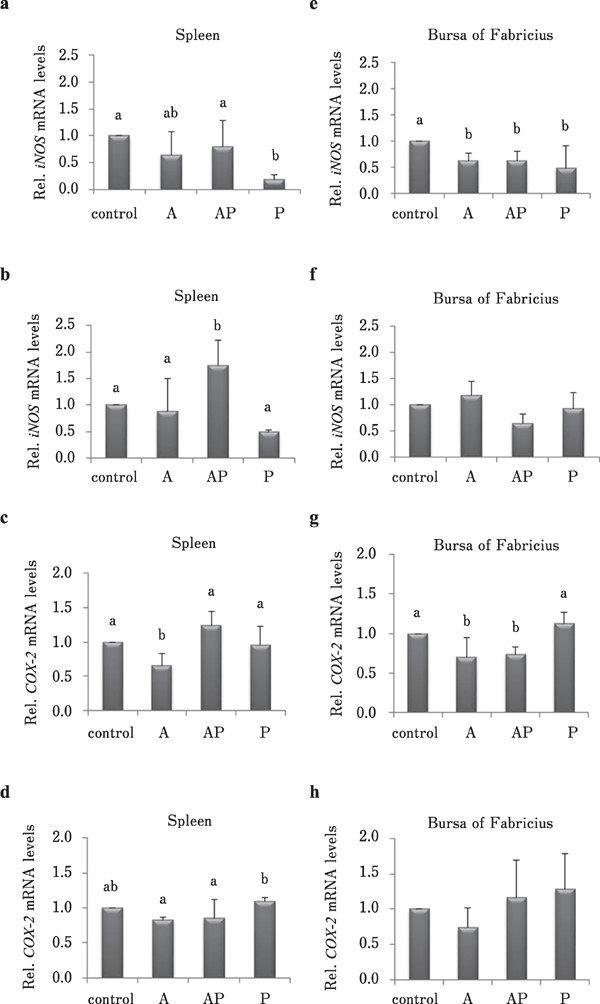
Effects of CMHW supplementation on inflammation-associated gene expression in broilers during vaccination. Effect of different supplementation period (A, anterior only; AP, anterior and posterior; P, posterior only) of CMHW (2 g/L in drinking water) on iNOS mRNA level in the spleen of 21-day-old (a) and 35-day-old broilers (b). COX-2 mRNA level in the spleen of 21-day-old (c) and 35-day-old broilers (d). Effect of different supplementation period (A, anterior only; AP, anterior and posterior; P, posterior only) of CMHW (2 g/L in drinking water) on iNOS mRNA expression in the bursa of Fabricius of 21-day-old (e) and 35-day-old broilers (f). COX-2 mRNA level in the bursa of Fabricius of 21-day-old (g) and 35-day-old broilers (h). Values represent mean±SD (n=6). Means with different letter superscripts are significantly different (P<0.05)
Effect of CMHW Supplementation on Regulation of Immunomodulation-related Gene Expression in LPS-challenged Broilers
CMHW supplementation significantly alleviated LPS-induced iNOS expression in the spleen of broilers 3 h post-injection of LPS (Fig. 4a, P<0.05). No significant difference was observed in iNOS mRNA level in the spleen of broilers 24 h post-injection of LPS in combination with CMHW treatment (Fig. 4b). LPS-induced COX-2 expression in the spleen of broilers was efficiently alleviated by CMHW treatment at 3 and 24 h post-injection (Fig. 4c and d, P<0.05). Interestingly, iNOS mRNA level in the bursa of Fabricius 3 h post-injection of LPS was further reduced in chickens challenged with LPS compared to the control group (Fig. 4e, P<0.05). No significant difference was observed regarding iNOS mRNA level in the bursa of Fabricius of broilers 24 h post-injection of LPS in combination with CMHW treatment (Fig. 4f). Similar to the spleen, LPS-induced COX-2 expression in the bursa of Fabricius of broilers was efficiently alleviated by CMHW treatment 3 and 24 h post-injection (Fig. 4g and h, P<0.05). CMHW supplementation significantly reduced LPS-induced IFN-γ mRNA level in the spleen of broilers 3 and 24 h postinjection of LPS (Fig. 5a and b, P<0.05). IL-4 mRNA levels were increased in the spleen 3 and 24 h post-injection of LPS in combination with CMHW treatment (Fig. 5c and d, P<0.05). LPS-induced IFN-γ expression in the bursa of Fabricius of broilers were efficiently attenuated by CMHW treatment 3 and 24 h post-injection (Fig. 5e and f, P<0.05). Similar to the spleen, IL-4 mRNA levels were significantly increased in the bursa of Fabricius 3 and 24 h post-injection of LPS in combination with CMHW treatment (Fig. 5g and h, P<0.05).
Fig. 4.
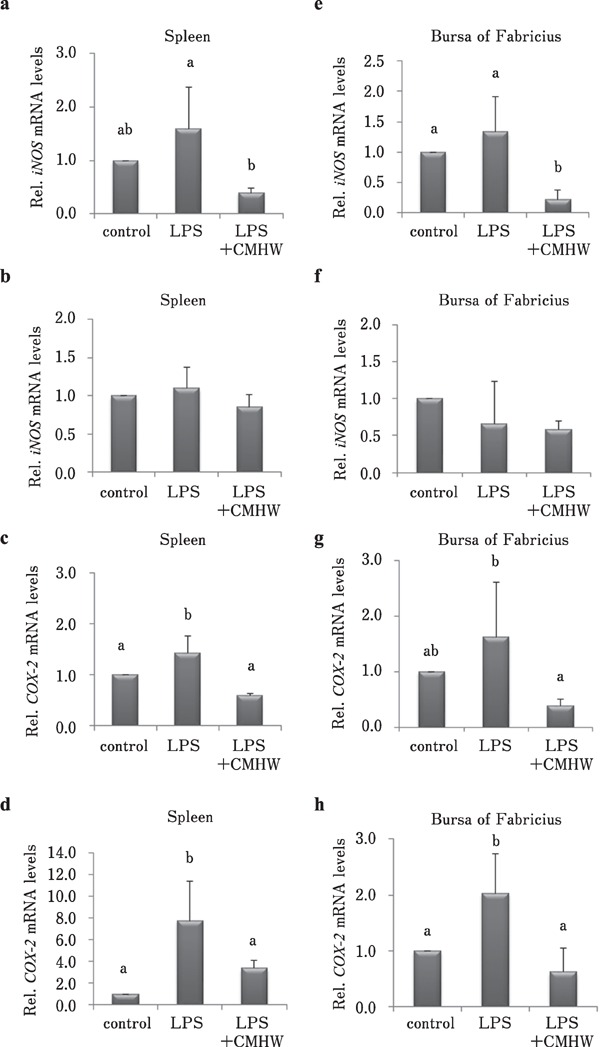
Effects of CMHW supplementation on inflammation-related gene expression in LPS-challenged broilers. Effect of CMHW supplementation on iNOS mRNA level in the spleen of broilers 3 h (a) and 24 h (b) post-injection of LPS. COX-2 mRNA level in the spleen of broilers 3 h (c) and 24 h (d) post-injection of LPS. Effect of CMHW supplementation on iNOS mRNA expression in the bursa of Fabricius of broilers 3 h (e) and 24 h (f) post-injection of LPS. COX-2 mRNA level in the bursa of Fabricius of broilers 3 h (g) and 24 h (h) post-injection of LPS. Values are expressed as mean±SD (n=6). Means with different letter superscripts are significantly different (P<0.05)
Fig. 5.
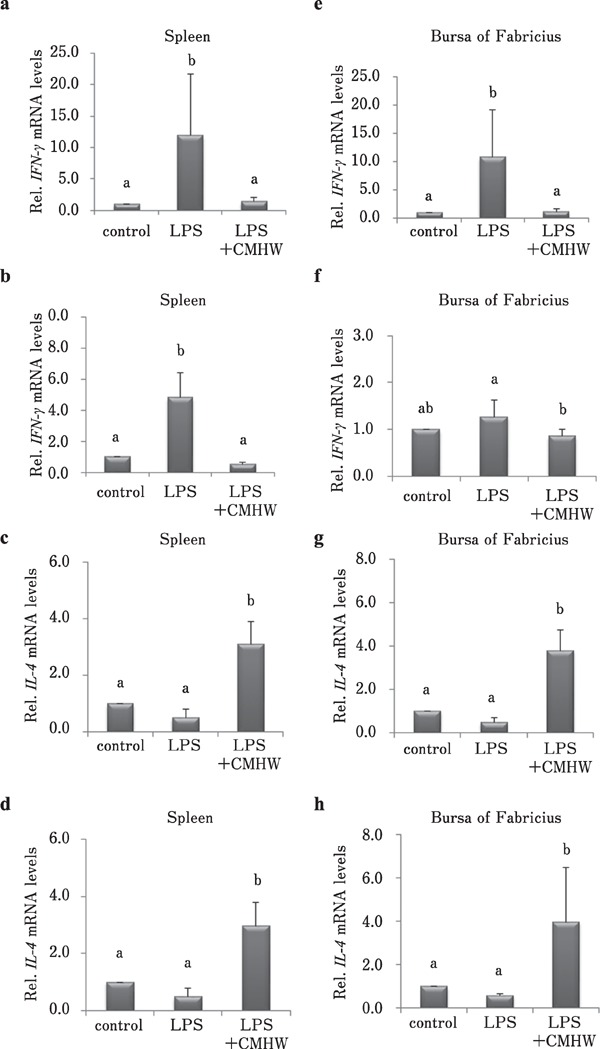
Effects of CMHW supplementation on type 1 helper T-lymphocytes (Th1) /type 2 helper T-lymphocytes (Th2) cytokine expression in LPS-challenged broilers. Effect of CMHW supplementation on IFN-γ mRNA level in the spleen of broilers 3 h (a) and 24 h (b) post-injection of LPS, and IL-4 mRNA level in the spleen of broilers 3 h (c) and 24 h (d) post-injection of LPS. Effect of CMHW supplementation on IFN-γ mRNA level in the bursa of Fabricius of broilers 3 h (e) and 24 h (f) post-injection of LPS. IL-4 mRNA level in the bursa of Fabricius of broilers 3 h (g) and 24 h (h) post-injection of LPS. Values represent mean±SD (n=6). Means with different letter superscripts are significantly different (P<0.05).
Discussion
In this study, we demonstrated that CMHW significantly inhibited iNOS expression in the bursa of Fabricius and COX-2 expression in the spleen and bursa of Fabricius. CMHW pretreatment reduced COX-2 mRNA levels in the spleen and bursa of Fabricius of broilers before vaccination. Furthermore, LPS-induced iNOS and COX-2 mRNA levels in the spleen and bursa of Fabricius were significantly alleviated in CMHW-treated broilers. These results indicated that CMHW has an immunomodulatory function in broilers.
Studies show that oral administration of 600 mg/kg of Cordyceps fermented products in broilers improved growth performance and health index (Koh et al., 2003). Broilers fed 1 and 4 g/kg Cordyceps fermented products have higher body weight (Han et al., 2015). However, no significant difference was observed in growth performance in broilers fed 4 g/L of Cordyceps fermented products in the present study. In addition, supplementation of Cordyceps fermented products in broilers improved the antibody titer of ND virus (Koh et al., 2003). The polysaccharides isolated from Cordyceps militaris also potentiated the antibody titer against ND virus in broilers (Wang et al., 2013). These results imply that Cordyceps fermented products and polysaccharides from Cordyceps militaris may regulate B lymphocyte-mediated humoral immunity in broilers. However, reports show that supplementation of Cordyceps fermented products in broilers did not promote the antibody titer against ND virus (Han et al., 2015). Here, we also demonstrate that CMHW supplementation did not potentiate the antibody titer for ND and IB viruses. These inconsistencies may be attributed to the variations in cordycepin and polysaccharide concentration in the fermented products among these studies. Therefore, the exact concentration of cordycepin and polysaccharides in the fermented products remains to be further determined.
The antibody titer against ND virus is significantly improved in broilers fed Cordyceps fermented products for 5 weeks (Koh et al., 2003). However, the optimal timing for CMHW supplementation during vaccination in broilers remains unclear. In the present study, we observed that different periods of CMHW supplementation did not potentiate the antibody titer against ND and IB viruses in broilers. Interestingly, CMHW supplementation reduced iNOS mRNA levels in the spleen and bursa of Fabricius in 21-day-old broilers after vaccination. However, this effect was not observed in the spleen and bursa of Fabricius in 35-day-old broilers in the posterior supplementation group. In contrast, anterior supplementation of CMHW during vaccination reduced COX-2 mRNA levels in the spleen and bursa of Fabricius in 21-day-old broilers prior to vaccination. Similar phenomenon was not observed post-CMHW supplementation in the spleen and bursa of Fabricius of 35-day-old broilers in the anterior supplementation group. These results demonstrate that differential timing of CMHW supplementation during vaccination did not increase the antibody titer. Furthermore, CMHW differently regulates iNOS and COX-2 expression in the spleen and bursa of Fabricius during vaccination.
LPS induces the production of inflammatory mediators, such as NO and prostaglandin E (Milano et al., 1995). COX-2 is a prostanoid-synthesizing enzyme in the prostaglandin E biosynthesis pathway during inflammation (Ricciotti and FitzGerald, 2011). Reports show that iNOS and COX-2 are highly expressed in macrophages in response to inflammation (Kerwin et al., 1995; Mitchell et al., 1995). Macrophages play an important role as the first line of defense against pathogen infections. Studies show that CMHW exerts an inhibitory effect on NO production, and TNF-α and IL-6 secretion in LPS-stimulated murine macrophages (Jo et al., 2010). Cordycepin, the bioactive component isolated from Cordyceps militaris, exhibits anti-inflammatory activity in LPS-stimulated macrophage cells by attenuating iNOS and COX-2 expression via inhibition of NF-κB activation and phosphorylation of Akt and p38 (Kim et al., 2006, Jeong et al., 2010, Choi et al., 2014, Zhang et al., 2014). Furthermore, our previous study showed that CMHW alleviates LPS-induced inflammatory response in porcine alveolar macrophages by regulating the p38 mitogen-activated protein kinase (MAPK) signaling pathway (Hsiao et al., 2018). Here, we demonstrate that CMHW reduces the LPS-induced expression of inflammation-related genes in the spleen and bursa of Fabricius of broilers. These results indicate that cordycepin from CMHW can alleviate production of pro-inflammatory mediators and cytokines in macrophages at the transcriptional level by suppressing the expression of upstream signaling cascades.
The balance between the macrophage-regulated type 1 helper T-lymphocytes (Th1) and type 2 helper T-lymphocytes (Th2) cytokines are important for providing and maintaining adequate protective immunity in broilers (He et al., 2010). IFN-γ is mainly produced from Th1 T-lymphocytes under IL-12 stimulation, which is involved in cell-mediated immunity. IFN-γ expression is associated with pathogen infection in broilers (Janardhana et al., 2007, Kano et al., 2009) as it induces NO production in avian macrophages (He et al., 2010). Studies show that cordycepin purified from Cordyceps militaris can regulate the Th1 and Th2 cytokine secretion in LPS-treated mouse splenocytes (Jeong et al., 2012, Seo et al., 2013). Here, we observed that IFN-γ expression was increased in the spleen and bursa of Fabricius of broilers in response to LPS stimulation, whereas CMHW inhibited these pro-inflammatory effects. This result is consistent with previous observations that LPS-induced Th1 cytokine secretion is suppressed by cordycepin in spleen cells (Seo et al., 2013). IL-4 is mainly produced from Th2 in response to IL-6 stimulation and is involved in the humoral immune response. In addition, IL-4 suppresses the production of pro-inflammatory mediators in macrophages (He et al., 2010). A previous study showed that feed supplementation with Cordyceps militaris fermentation products reduced IL-4 mRNA levels in the spleen of weaning piglets (Cheng et al., 2016). However, high doses of Cordyceps militaris treatment induced IL-4 secretion in humans (Sun et al., 2014). Furthermore, cordycepin purified from Cordyceps militaris also induces IL-4 secretion in LPS-treated mouse splenocytes (Jeong et al., 2012). Similarly, we demonstrated that CMHW induces IL-4 expression in the spleen and bursa of Fabricius of broilers upon LPS challenge. In addition, broilers fed polysaccharides isolated from Cordyceps militaris increased humoral immunity by elevating antibody titer and IL-4 secretion (Wang et al., 2013). IFN-γ and IL-4 are the markers of Th1 and Th2 cytokines, respectively. A slight difference in Th1/Th2 cytokine production was observed when pure cordycepin or CMHW (containing cordycepin and polysaccharide) was supplied to broilers. Based on our observations, we hypothesize that CMHW supplementation in broilers elicits humoral immune response in response to LPS challenge. However, the precise mechanism via which CMHW regulates differential Th1/Th2 cytokine production in broilers remains to be elucidated.
In conclusion, CMHW exerts an immunomodulatory role and inhibits inflammation-related gene expression in response to vaccination and LPS stimulation in broilers. Therefore, CMHW containing cordycepin has high potential for development as a feed additive that may act as an alternative source for immunomodulation in farm animals.
References
- Abudabos AM, Al-Batshan HA, Murshed MA. Effects of prebiotics and probiotics on the performance and bacterial colonization of broiler chickens. South African Journal of Animal Science, 45: 419-428. 2015. a. [Google Scholar]
- Abudabos AM, Alyemni AH, Dafalla YM, Khan RU. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environmental Science and Pollution Research, 23: 24151-24157. 2015. b. [DOI] [PubMed] [Google Scholar]
- Ahn YJ, Park SJ, Lee SG, Shin SC, Choi DH. Cordycepin: selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. Journal of Agricultural and Food Chemistry, 48: 2744-2748. 2000. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Wen CM, Dybus A, Proskura WS. Fermentation products of Cordyceps militaris enhance performance and modulate immune response of weaned piglets. South African Journal of Animal Science, 46: 121-128. 2016. [Google Scholar]
- Choi YH, Kim GY, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Design, Development and Therapy, 16: 1941-1953. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia, 81: 961-968. 2010. [DOI] [PubMed] [Google Scholar]
- Guo P, Kai Q, Gao J, Lian ZQ, Wu CM, Wu CA, Zhu HB. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. Journal of Pharmacological Sciences, 113: 395-403. 2010. [DOI] [PubMed] [Google Scholar]
- Han JC, Qua HX, Wanga JG, Yana YF, Zhanga JL, Yanga L, Zhanga M, Cheng YH. Effects of fermentation products of Cordyceps militaris on growth performance and bone mineralization of broiler chicks. Journal of Applied Animal Research, 43: 236-241. 2015. [Google Scholar]
- He H, Kenneth JG, Michael HK. Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine, 53: 363-369. 2010. [DOI] [PubMed] [Google Scholar]
- Hsiao FSH, Cheng YH, Wang SK, Yu YH. Cordyceps militaris hot water extract inhibits lipopolysaccharide-induced inflammatory response in porcine alveolar macrophages by regulation of mitogen-activated protein kinase signaling pathway. Canadian Journal of Animal Science, 98: 44-52. 2018. [Google Scholar]
- Huang YL, Leu SF, Liu BC, Sheu CC, Huang BM. In vivo stimulatory effect of Cordyceps sinensis mycelium and its fractions on reproductive functions in male mouse. Life Sciences, 75: 1051-1062. 2004. [DOI] [PubMed] [Google Scholar]
- Janardhana V, Ford ME, Bruce MP, Broadway MM, O'Neil TE, Karpala AJ, Asif M, Browning GF, Tivendale KA, Noormohammadi AH, Lowenthal JW, Bean AG. IFN-gamma enhances immune responses to E. coli infection in the chicken. Journal of Interferon & Cytokine Research, 27: 937-946. 2007. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Jin CY, Kim GY, Lee JD, Park C, Kim GD, Kim WJ, Jung WK, Seo SK, Choi IW, Choi YH. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. International Immunopharmacology, 10: 1580-1586. 2010. [DOI] [PubMed] [Google Scholar]
- Jeong MH, Seo MJ, Park JU, Kang BW, Kim KS, Lee JY, Kim GY, Kim JI, Choi YH, Kim KH, Jeong YK. Effect of cordycepin purified from Cordyceps militaris on Th1 and Th2 cytokines in mouse splenocytes. Journal of Microbiology and Biotechnology, 22: 1161-1164. 2012. [DOI] [PubMed] [Google Scholar]
- Jo WS, Choi YJ, Kim HJ, Lee JY, Nam BH, Lee JD, Lee SW, Seo SY, Jeong MH. The anti-inflammatory effects of water extract from Cordyceps militaris in murine macrophage. Mycobiology, 38: 46-51. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano R, Konnai S, Onuma M, Ohashi K. Cytokine profiles in chickens infected with virulent and avirulent Marek's disease viruses : interferon-gamma is a key factor in the protection of Marek's disease by vaccination. Microbiology and Immunology, 53: 224-232. 2009. [DOI] [PubMed] [Google Scholar]
- Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, Sung JM, Jang Y, Chung N, Hwang KC, Kim TW. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. European Journal of Pharmacology, 545: 192-199. 2006. [DOI] [PubMed] [Google Scholar]
- King RW, Zecher M, Jefferies MW. Inhibition of the replication of a hepatitis C virus-like RNA template by interferon and 3′-deoxycytidine. Antiviral Chemistry and Chemotherapy, 13: 363-370. 2002. [DOI] [PubMed] [Google Scholar]
- Kerwin JF, Jr, Lancaster JR, Jr, Feldman PL. Nitric oxide: a new paradigm for second messengers. Journal of Medicinal Chemistry, 38: 4343-4362. 1995. [DOI] [PubMed] [Google Scholar]
- Koh JH, Suh HJ, Ahn TS. Hot-water extract from mycelia of Cordyceps sinensis as a substitute for antibiotic growth promoters. Biotechnology Letters, 25: 585-590. 2003. [DOI] [PubMed] [Google Scholar]
- Milano S, Arcoleo F, Dieli M, D'Agostino R, D'Agostino P, De Nucci G, Cillari E. Prostaglandin E2 regulates inducible nitric oxide synthase in the murine macrophage cell line J774. Prostaglandins, 49: 105-115. 1995. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Larkin S, Williams TJ. Cyclooxygenase-2: regulation and relevance in inflammation. Biochemical Pharmacology, 50: 1535-1542. 1995. [DOI] [PubMed] [Google Scholar]
- Müller WE, Seibert G, Beyer R, Breter HJ, Maidhof A, Zahn RK. Effect of cordycepin on nucleic acid metabolism in L5178Y cells and on nucleic acid-synthesizing enzyme systems. Cancer Research, 37: 3824-3833. 1977. [PubMed] [Google Scholar]
- Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumor effect of cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Research, 26: 43-47. 2006. [PubMed] [Google Scholar]
- Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. Journal of Pharmacy and Pharmacology, 57: 1509-1519. 2005. [DOI] [PubMed] [Google Scholar]
- NRC-National Research Council. Nutritional requirements of poultry (9th ed.). Washington, DC: National Academy Press; 1994 [Google Scholar]
- Ohta Y, Lee JM, Hayashi K, Fujita A, Park DK, Hayashi T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. Journal of Agricultural and Food Chemistry, 55: 10194-10199. 2007. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31: 986-1000. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh AA, Ebeid TA, Abudabos AM. Effect of dietary phytogenics (herbal mixture) supplementation on growth performance, nutrient utilization, antioxidative properties, and immune response in broilers. Environmental Science and Pollution Research, 25: 14606-14613. 2018. [DOI] [PubMed] [Google Scholar]
- Seo MJ, Kim MJ, Lee HH, Park JU, Kang BW, Kim GY, Rhu EJ, Kim JI, Kim KH, Jeong YK. Effect of cordycepin on the expression of the inflammatory cytokines TNF-alpha, IL-6, and IL-17A in C57BL/6 mice. Journal of Microbiology and Biotechnology, 23: 156-160. 2013. [DOI] [PubMed] [Google Scholar]
- Shiao MS, Wang ZN, Lin LJ, Lien JY, Wang JJ. Profiles of nucleosides and nitrogen bases in Chinese medicinal fungus Cordyceps sinensis and related species. Botanical Bulletin-Academia Sinica Taipei, 35: 261-267. 1994. [Google Scholar]
- Shin S, Kwon J, Lee S, Kong H, Lee S, Lee CK, Cho K, Ha NJ, Kim K. Immunostimulatory effects of Cordyceps militaris on macrophages through the enhanced production of cytokines via the activation of NF-kappaB. Immune Network, 10: 55-63. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siev M, Weinberg R, Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. Journal of Cell Biology, 41: 510-520. 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar AM, McCaffrey RP. Antifungal activity of 3′-deoxy adenosine (cordycepin). Antimicrobial Agents and Chemotherapy, 42: 1424-1427. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Shao Y, Zhang Z, Wang L, Mariga AM, Pang G, Geng C, Ho CT, Hu Q, Zhao L. Regulation of human cytokines by Cordyceps militaris. Journal of Food and Drug Analysis, 22: 463-467. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Meng X, Yang R, Qin T, Li Y, Zhang L, Fei C, Zhen W, Zhang K, Wang X, Hu Y, Xue F. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. International Journal of Biological Macromolecules, 59: 178-183. 2013. [DOI] [PubMed] [Google Scholar]
- Wang NQ, Jiang LD, Zhang XM, Li ZX. Effect of dongchong xiacao capsule on airway inflammation of asthmatic patients. Zhongguo Zhong Yao Za Zhi, 32: 1566-1568. 2007. [PubMed] [Google Scholar]
- Weiss SR, Bratt MA. Effect of cordycepin (3'-Deoxyadenosine) on virus-specific RNA species synthesized in Newcastle disease virus-infected cells. Journal of Virology, 16: 1575-1583. 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Jin L, Ren X, Lu J, Meng Q. Optimization of fermentation process of Cordyceps militaris and antitumor activities of polysaccharides in vitro. Journal of Food and Drug Analysis, 22: 468-476. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Xu Y, Shen J. Cordycepin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production via activating amp-activated protein kinase (AMPK) signaling. International Journal of Molecular Sciences, 15: 12119-12134. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


