Abstract
Background
Initiating varenicline use 4 weeks before the target quit date (TQD) reduces smoking in the run-in phase and increases end-treatment cessation rates; however, the lack of a smoke intake plateau suggests longer preloading periods are required. This study assessed whether varenicline preloading for 6 weeks reduced pre-quit smoke intake and enhanced 6-month abstinence outcomes compared with the standard 1-week preloading.
Methods
In this randomised single-centre controlled trial, (ClinicalTrials.gov identifier: NCT02634281), conducted between February 2016 and July 2018 in Israel, daily smokers (n = 242) aged ≥ 18 years were randomly assigned (1:1) to receive varenicline preloading for 6 weeks (n = 121) or a placebo for 5 weeks followed by varenicline for 1 week (n = 121) before the TQD. Participants and researchers were masked to both group assignment and treatment allocation. Both groups received standard 12-week post-TQD varenicline treatment. The primary outcome was the 24-week biochemically verified continuous abstinence rate (CAR) from weeks 6 (TQD)–30. Secondary outcomes included the 23-week CAR from 1-week post-TQD (week 7) to week 30, and the 7-day point-prevalence (PP) abstinence at week 30. Other measures included pre- and post-quit rewards, smoking urges, nausea, aversion, and markers of cigarette consumption.
Findings
By intention-to-treat, the 24-week CAR, weeks 6–30 with extended preloading was significantly higher than with standard preloading (23·1% vs. 4·1%; risk reduction [RR]: -0·19 [95% confidence interval [CI]:-0·10—0·24]; p < 0·001). Extended preloading also showed better secondary outcomes. Extended preloading significantly decreased pre-quit rewards, urges, and smoke intake, including unsolicited smoking abstinence. Post-quit urges remained remarkably lower with extended preloading. Participants receiving extended preloading reported more nausea at week 4 (39.6% vs 11.5%) and abnormal dreams at week 6 (7.7% vs. 0%). Participants receiving standard preloading reported more constipation at week 7 (7.6% vs. 0%) and dizziness at weeks 7 (12.1% vs. 2.5%) and 12 (10.7% vs 1.4%).
Interpretation
Extended preloading reduced ad lib smoking, enhanced cessation rates at 3 and 6 months, and decreased pre- and post-quit rewards and smoking drive in a pattern compatible with a reinforcement-reduction mechanism. These data substantiate extending the standard pre-treatment period, and suggest that targeting pre-quit smoking sensations should be a treatment priority, although confirmatory evidence is needed from larger clinical trials.
Funding
This study was funded by a 2013 Global Research Award for Nicotine Dependence (GRAND) supported by Pfizer, Inc. (#WI182915).
Keywords: Smoking cessation, Smoking reduction, Varenicline, Extended preloading
Research in context.
Evidence before this study
Using a similar research design, 2 “proof-of-concept” studies found that 4-week varenicline preloading reduced smoke intake and cigarette enjoyment during the pre-quit period or the taste and “buzz” from the first cigarette. Although both studies showed a trend towards improved 12-week quit rates, their sample sizes were not robust enough to detect any medium- to long-term effects on abstinence.
Added value of this study
The present, single-centre study addressed the above limitations by showing, for the first time, that 6-week varenicline preloading reduces ad lib smoking and improves cessation outcomes at 6 months. Furthermore, the data show that the main mechanism of varenicline preloading may be a reduction in the drive to smoke, leading to lower cigarette consumption, thus undermining the learnt drive to smoke. These findings are consistent with those of the largest trial of nicotine preloading published to date and provide evidence for a unified mechanism of preloading, supporting the view that the benefits of pre-treatment are not specific to varenicline.
Implications of all available evidence
Together with data from previous studies, the present findings have several practical implications. First, they strongly support the concept that the contemporary varenicline treatment schedule may lead to sub-optimal results and provide a rationale for extending the current 1-week standard for preloading. Second, a 6-week preloading tactic may be a valid option for smokers willing to reduce the number of cigarettes per day with the goal of quitting in the next few weeks. Targeting the pre-cessation period is a new therapeutic strategy likely to improve abstinence outcomes that should be considered when using existing or new smoking cessation treatments, although confirmatory evidence is needed from larger clinical trials.
Alt-text: Unlabelled box
1. Introduction
Tobacco smoking remains a major cause of health problems in economically developed countries and has a substantial effect on morbidity and mortality [1]. Smoking cessation medications are invaluable tools that help smokers quit smoking [2]. Varenicline is the best single pharmacotherapy for supporting smoking cessation [3]. In clinical practice, people aiming to quit smoking with the aid of varenicline begin taking the medication 1 week before the target quit day (TQD) to reduce the adverse events that might occur with a rapid escalation of the dose. In a “proof-of-concept” study, Hajek and colleagues [4] found that in patients receiving the standard varenicline treatment, increasing the pre-quit period to 4 weeks was associated with reduced ad libitum smoking and increased 12-week abstinence rates. This finding supported their hypothesis that, since varenicline lowers the subjective reward connected to smoking, its use over an extended period helped to weaken the association of smoking with reward, enhancing cessation. Subsequently, using a similar research design Hawk and colleagues [5] observed greater pre-quit reductions in smoking rates in smokers receiving varenicline for a pre-quit period of 4 weeks; however, post-treatment abstinence was enhanced only among women. Although these studies provide useful initial information about extended varenicline preloading, their sample sizes were not powerful enough to estimate the effect of preloading for medium-to-long term follow-up periods. Additionally, as noticed by Hajek and colleagues [4], at week 4 the effects of varenicline on smoke intake did not plateau, suggesting that longer preload periods may be needed.
With the above considerations in mind, this controlled clinical trial was conducted to examine whether, in participants receiving the standard 12-week varenicline treatment, using varenicline for 6 weeks prior to the TQD, as opposed to starting 1 week before the TQD, enhanced cessation outcomes 30 weeks after randomisation. Additionally, adverse events (AEs) and the safety of extended varenicline preloading were investigated.
2. Methods
2.1. Study design
This study was a single-centre, randomised, double blind, placebo-controlled trial for smoking cessation conducted at the Pulmonary Institute of Shaare Zedek Medical Center (SZMC), Jerusalem, Israel, between February 2016 and July 2018. The study consisted of an 18-week treatment period (a 6-week preloading phase followed by a 12-week abstinence phase) and a 12-week non-treatment follow-up phase, for a study duration of 30 weeks; the full protocol is available in the e-Supplement. Daily smokers were randomly allocated to receive varenicline preloading for 6 weeks before the TQD (Extended preloading group) or to receive placebo preloading for 5 weeks followed by standard varenicline preloading for 1 week before the TQD (Standard preloading group). After the TQD, both groups used varenicline for an additional 12 weeks.
The study was conducted in compliance with the recommendations guiding physicians in biomedical research involving humans adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964 and its later revisions. The “Helsinki Committee” of the SZMC approved all study procedures and all participants provided written, informed consent prior to any procedures. The trial was registered on ClinicalTrials.gov (Identifier: NCT02634281).
2.2. Medication
Participants in the Extended preloading group received 0·5 mg of varenicline once daily for 3 days and 0·5 mg twice daily for 4 days (week 1), followed by varenicline 1 mg twice daily for 5 additional weeks. Individuals in the Standard preloading group received matched placebo tablets for 5 weeks, followed by a 1-week titration of varenicline using the same schedule depicted above. Following preloading, both groups received 1 mg varenicline twice daily for 12 weeks. For participants with tolerability problems, the maintenance dose could be reduced temporarily or permanently to 1 mg daily.
2.3. Participants
Daily smokers were recruited through posters placed in the SZMC, flyers, word-of-mouth, community outreach, and social media websites (e.g., Facebook). All participants were screened by telephone or in person. We included participants aged ≥ 18 years who smoked ≥ 10 cigarettes/day, had smoked ≥ 5 pack-years, had a carbon monoxide (CO) level in expired air of ≥ 10 parts per million (ppm), and were willing to stop smoking. Women of childbearing potential were allowed to enrol provided they agreed to avoid pregnancy for 30 days after the last dose of study medication, and to use an effective birth control method.
We excluded people with a history of myocardial infarction within the past 3 months; unstable angina; severe cardiac arrhythmia; psychiatric disorders precluding informed consent and correct use of medication; use of any form of smokeless tobacco, nicotine substitution, or e-cigarettes; and those who were pregnant or breast-feeding. We also excluded people who had participated in a smoking cessation program during the last 3 months, alcoholics, and illegal drug users. All participants received individualised verbal and written instructions regarding the general conduct of the study and the proper use of the study medication.
2.4. Procedures
Participants had 7 visits at baseline and at weeks 4, 6 (TQD), 7, 12, 18 (end-of-treatment), and 30 (end-of-study). Participants were briefly instructed to reduce baseline smoking by 50% or more by week 4, with further reduction thereafter, with the goal of quitting 1 day before the week 6 visit (TQD) [6]. They were not encouraged to make a quit attempt before the TQD, but were free to try if they felt ready. Those who were unable to reduce smoking or make a quit attempt by week 6 were encouraged to continue medication and make quit attempts later. Neither counselling nor financial incentives were provided. Medication was dispensed at baseline and at weeks 4, 6, 7 and 12. The study timeline is shown in e-Table 1.
2.5. Measures
Baseline demographic details, health status, smoking history, and results of the Fagerström Test for Cigarette Dependence (FTCD) were collected [7]. Motivation to quit was assessed using a 10-cm visual analogue scale. Validation of abstinence required an expired CO level of less than 10 ppm during the original study registration. However, due to research indicating that this threshold may misclassify smokers as non-smokers [8] and that a CO ≤ 5 ppm optimally distinguishes smokers from non-smokers [9,10] analyses were conducted using this threshold. Expired CO was measured with a Bedfont monitor (Bedfont, Technical Instruments, Sittingbourne, England) [11]. Additionally, since the biochemically verifiable window for CO is only 1 day, the urinary cotinine was also evaluated at the baseline and at weeks 18 and 30 as the biochemically verifiable window for this measurement is 7 days [10]. The point-of-care SmokeScreenTM test (Smokescreen; GFC Diagnostics, Bicester, UK) was used for this assessment. The SmokeScreenTM test is a disposable colorimetric essay specific to cigarette smoke that measures all the major urinary metabolites of nicotine, including cotinine. A positive test is characterized by a change in urine colour to pink/orange whose degree correlates with the concentration of metabolites present. Subjects were regarded as non-smokers if their urine cotinine equivalent concentration was ≤ 1 mg/mL [12]. Participants who did not attend a validation session or did not pass the validation were classified as non-abstainers. Smoking reduction was defined as a self-reported decrease in the number of smoked cigarettes/day by 50% or more. Participants unavailable for follow-up were assumed to be smokers.
To assess tobacco withdrawal symptoms and the urge to smoke, the Mood and Physical Symptoms Scale (MPSS) was self-administered at the baseline and all subsequent visits by abstinent participants [13]. The MPSS asks individuals to rate how they have been feeling in the past week regarding depression, irritability, restlessness, hunger, poor concentration, and poor sleep at night on a 5-point scale. The items are analysed separately and aggregated to obtain a composite score.
To assess the reinforcing effects of smoking, the modified Cigarette Evaluation Questionnaire (mCEQ) [14] was administered at the baseline and, subsequently, only to those who had smoked since previously completing the questionnaire. The mCEQ is categorised into 5 sub-scales: smoking satisfaction, psychological reward, enjoyment of respiratory tract sensations, craving reduction, and aversion. The questions are organised on a 7-point scale in which higher scores indicate more intense smoking-related symptoms. Aversion to smoke was assessed by the occurrence of nausea and by the aversion subscale of the mCEQ. All reported AEs were documented in case report forms and followed up to resolution or the end of the study. Participants were weighed at all visits.
2.6. Randomisation and masking
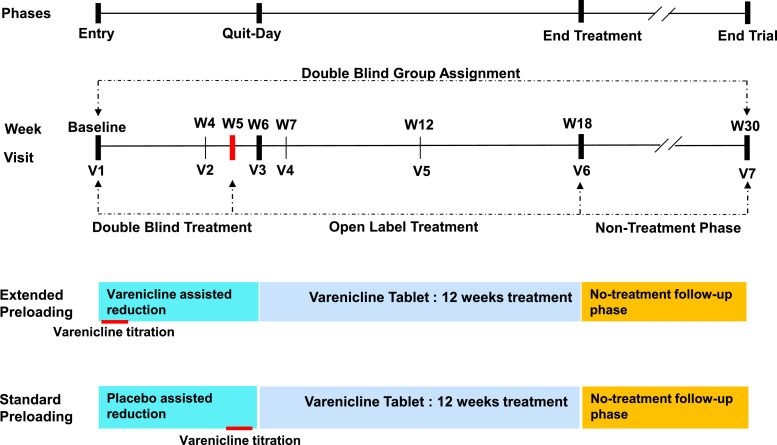
These procedures were performed by the randomisation monitor (VP) in a secure room where the study medication, provided free of charge by Pfizer Inc., NY, was stored in identically packaged, blinded bottles containing varenicline or placebo. Participants were referred to the secure room where they were randomly allocated (in a 1:1 ratio) to the experimental group. Randomisation was accomplished by extracting a systematic sample via a pre-prepared list of alternate allocations. There was no personal judgement and each recruited individual had a 50–50 chance to be in each group. Group assignment and drug allocation were kept secret from participants and the remaining study staff. The randomisation monitor was not involved in follow-up data collection, a task performed by the remaining study staff. Double blinding to group assignment lasted from week 1 (baseline) to week 30 (end-of-study). Double blinding to treatment allocation persisted from visit 1 (baseline) to week 5; from that point onwards, the treatment was open label, as both groups received active treatment (Fig. 1).
Fig. 1.
Phases of the Study and Double Blinding. The study lasted 30 weeks and had 3 phases: preloading, treatment, and follow-up. During the preloading phase, participants randomised to the Extended preloading group received varenicline for 6 weeks, while those randomised to the Standard preloading group received 5 weeks of placebo and 1 week of regular varenicline titration. During the open-label treatment phase, the 2 groups received the 12-week standard varenicline treatment. During the follow-up phase (12 weeks) no treatment was given. Double blinding to group assignment was maintained from week 1 to week 30. Double blinding to treatment was possible only during the first 5 weeks of preloading.
2.7. Abstinence outcomes
End points were selected using recommended outcomes for smoking cessation studies [15]. Since no clear criteria currently exist to judge whether or not a given abstinence measure is better than another, all possible measures of abstinence were calculated. These included continuous abstinence (i.e., abstinence between the TQD and a follow-up time), continuous abstinence after an initial grace period (i.e., prolonged abstinence), and point-prevalence abstinence (PPA; i.e., abstinence during a time window immediately preceding follow-up). The primary outcome was the 24-week biochemically validated continuous abstinence rate (CAR) from weeks 6–30. Secondary outcomes included the: (a) 12-week CAR from weeks 6–18; (b) 11-week CAR from weeks 7–18; (c) 23-week CAR for weeks 7–30; and (d) the 7-day PPA) at all time-points from weeks 4–30. The latter measure was defined as the rate of biochemically validated abstinence from smoking during the 7-day time window immediately preceding follow-up. Finally, data analysis revealed that at week 4 (i.e., 2 weeks prior to the TQD), some participants had stopped smoking spontaneously, without the recommendation to do so. To assess their ability to sustain abstinence, the 14-week CAR from weeks 4–18 and 26-week CAR from weeks 4–30 were examined.
2.8. Possible preloading mechanisms of action
Potential preloading mechanisms were assessed by examining positive and negative reinforcement via items 1, 2, and 12, and items 4–8 of the mCEQ, respectively. The drive to smoke was evaluated using the MPSS-craving subscale (MPSS-C, items 8 and 9), alone or in combination, and the MPSS-mood subscale (MPSS-M, items 1–7). Also, aversion was determined by the occurrence of nausea and through the aversion subscale of the mCEQ [16].
2.9. Adverse events
AE incidents were monitored by counting participants for each AE type every time an event occurred. An AE was considered serious if it was life threatening, resulted in death or hospitalisation, prolonged an existing hospitalisation, caused a persistent or significant incapacity, or substantially disrupted a patient's ability to conduct normal life functions. Finally, a 6-month change in body weight was measured in the participants stratified by smoking status.
2.10. Sample size
The primary outcome concerned continuous smoking cessation. To detect an expected cessation rate difference of 15% between the 2 groups [4], [5], [6] (more specifically, 30% in the Extended preloading group and 15% in the Standard preloading group), a sample size of 121 people per group was deemed necessary (p < 0·05; 2 tailed test; continuity-corrected: 75.4% power).
2.11. Data analysis
Statistical analysis was performed using IBM SPSS statistics software (Version 21·0). The database system included Excel 2010 and SPSS version 21; the data were stored as Excel files and SPSS files. Intent-to-treat analysis was performed and all randomised participants who received at least 1 dose of varenicline or placebo were included in the analysis population. To establish the comparability of the randomised groups, an independent statistician conducted a test for joint orthogonality on the baseline variables [17]. These tests consist of using the treatment group as a dependent variable and using the different variables, for which balance is deemed desirable, as independent variables. Joint orthogonality was tested using all variables known, or suspected, to be related to the outcome. Analysis was performed separately for (a) all variables; (b) socio-demographic and lifestyle variables (i.e. age, sex, height male, height female, body mass index, ethnicity, education, smoking on the Shabbat and beer, wine, and liquor use); (c) clinical variables (i.e. chronic obstructive pulmonary disease, asthma, diabetes, hypertension, dyspnoea, cough, sputum, wheeze, spirometry) and (d) smoking variables (i.e. cigarettes/day, years of smoking, previous quit attempts, FTCD, expired CO, urinary cotinine, partner smokes, and motivation). Cigarette consumption (p.y.) was not included in this assessment as it was derived from cigarettes/day and years of smoking. For testing the primary outcome (24-week CAR for weeks 6–30) and secondary outcomes (12-week CAR for weeks 6–18, 11-week CAR for weeks 7–18, 23-week CAR for weeks 7–30, 14-week CAR for weeks 4–18, 26-week CAR for weeks 4–30, and 7-day PPA at weeks 6, 7, 12, 18, and 30) χ2 (with Yates correction) and Fisher exact tests were used.
2.12. Role of the funding source
Pfizer Inc. had no role in the study design; the collection, analysis, and interpretation of the data; the writing of the report; or the decision to submit the paper for publication.
3. Results
3.1. Participant disposition and baseline characteristics
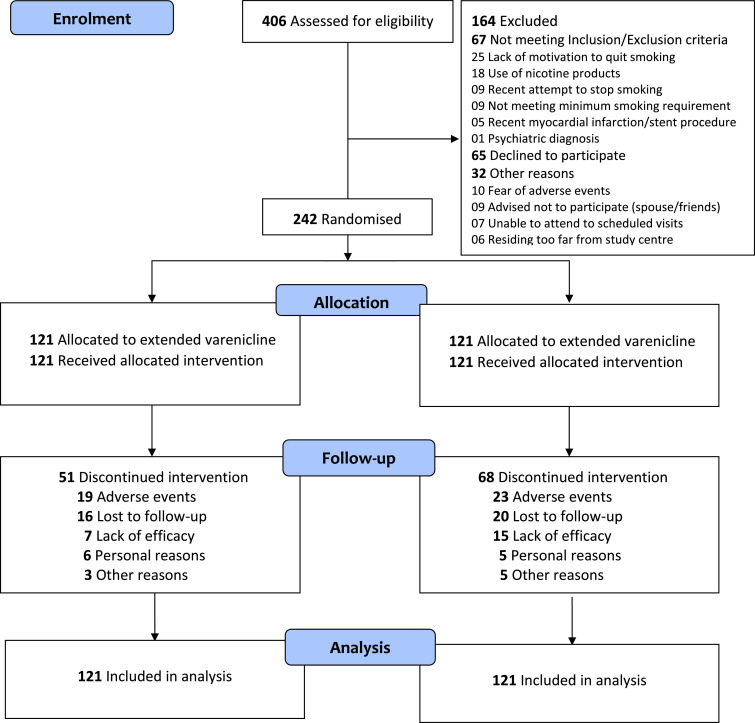
The flow of participants through the study appears in the CONSORT diagram [18] depicted in Fig. 2. Of the 406 smokers who responded to the advertisements, 242 were randomised to receive extended varenicline (n = 121) or standard preloading (n = 121). The 6-month completion rate was 57·9% (70/121) for extended preloading and 43·8% (53/121) for standard preloading. Eighty percent of the discontinuations occurred in the run-in phase and were mainly due to adverse events (37·3% with extended preloading vs. 33·8% with standard preloading) and a perceived lack of treatment efficiency (13·7% with extended preloading vs. 23·5% with standard preloading).
Fig. 2.
Extended vs. Standard Varenicline Preloading CONSORT Trial Flow Diagram.
Participants assigned to study groups were similar at baseline (Table 1). The joint test of orthogonality confirmed the clinical impression of balance between the 2 groups by showing p-values > 0·250 for (a) All variables (p = 0·560); (b) Socio-demographic and lifestyle variables; (p = 0·371); (c). Clinical variables (p = 0·407), and (d) Smoking variables (p = 0·438).
Table 1.
Mean (SD) values of baseline characteristics of the intent-to-treat study population (n = 242).
| Characteristic | Extended Preloading n = 121 | Standard Preloading n = 121 |
|---|---|---|
| Age, yr | 48·2 (12·8) | 47·9 (14·4) |
| Male/female, n | 88/33 | 89/32 |
| BMI, kg/m2 | 26·5 (4·9) | 27·7 (5·0) |
| Smoking History | ||
| Cigarette consumption (pack-years) | 31·5 (23·9) | 30·7 (21·0) |
| Cigarettes smoked/day | 24·5 (13·4) | 24·9 (13·4) |
| Years of regular smoking | 28·6 (12·5) | 28·1 (13·4) |
| Previous quit attempts, n | 1·7 (2·6) | 1·5 (2·2) |
| Cigarette dependence, FTCD score | 5·9 (2·4) | 5·7 (2·5) |
| Expired carbon monoxide (ppm) | 16·0 (6·5) | 14·7 (5·9) |
| Urinary cotinine, mg/mL | 9·8 (5·5) | 9·5 (5·1) |
| Partner smokes, n (%) | 44 (36·4) | 31 (25·8) |
| Motivation, pts | 8·9 (1·4) | 8·7 (1·6) |
FTCD: Fagerström Test for Cigarette Dependence ranges from 0 to 10 where a higher score denotes greater dependency; SD: standard deviation.
3.2. Smoking abstinence
The continuous and PPA rates of the 2 groups are shown in Table 2. Participants who received extended varenicline preloading were more likely to achieve continuous abstinence at week 18 (end-of-treatment) and week 30 (end-of-study) whatever the measure, and were more likely to achieve PPA at all time-points than those receiving standard preloading. All participants categorised as abstinent had their expired CO measured at all visits, in combination with urinary cotinine measurements acquired at baseline, week 6 and week 30. The only person considered abstinent at a missed visit was a 71-year-old female receiving standard preloading who reported not smoking when she missed visit 4 and was found biochemically abstinent at visit 3 and at all subsequent visits (visits 5, 6, and 7).
Table 2.
Effect of extended varenicline preloading on biochemically validated abstinence rates intent-to-treat study population (n = 242).
| Intent-to-treat Analysis |
|||||
|---|---|---|---|---|---|
| Abstinence measure | Extended Preloading (n = 121) | Standard Preloading (n = 121) | RR | 95% CI | p value |
| Primary outcome | |||||
| Continuous abstinence | |||||
| Weeks 6 - 30 (24 weeks) n (%) | 28 (23·1) | 5 (4·1) | −0·19 | [−0·10 – −0·24] | < 0·001 |
| Secondary outcomes | |||||
| Continuous abstinence | |||||
| Weeks 4 - 18 (14 weeks) n (%) | 16 (13.2) | 2 (1·7) | −0·12 | [−0·04 – −0·14] | 0·001 |
| Weeks 4 - 30 (26 weeks) n (%) | 14 (11·6) | 2 (1·7) | −0·10 | [−0·03 – −0·13] | 0·003 |
| Weeks 6 - 18 (12 weeks) n (%) | 30 (24·8) | 9 (7·4) | −0·17 | [−0·08 – −0·25] | < 0·001 |
| Weeks 7 - 18 (11 weeks) n (%) | 44 (36·4) | 19 (15·7) | −0·21 | [−0·09 – −0·31] | < 0·001 |
| Weeks 7 - 30 (23 weeks) n (%) | 38 (31·4) | 12 (9·9) | −0·22 | [−0·11 – −0·30] | < 0·001 |
| 7-day point-prevalence abstinence | |||||
| At week 4 (2 weeks pre-TQD) n (%) | 22 (18·2) | 4 (3·3) | −0·15 | [−0·07 – −0·19] | < 0·001 |
| At week 6 (TQD) n (%) | 35 (28·9) | 12 (9·9) | −0·19 | [−0·08 – −0·27] | < 0·001 |
| At week 7 (1-week post-TQD) n (%) | 53 (43·8) | 30 (24·8) | −0.19 | [−0·06 – −0·31] | 0·003 |
| At week 12 (6 weeks post-TQD) n (%) | 52 (43·0) | 30 (24·8) | −0.19 | [−0·06 – −0·31] | 0·004 |
| At week 18 (end-of-treatment) n (%) | 50 (41·3) | 30 (24·8) | −0·17 | [−0·04 – −0·28] | 0·009 |
| At week 30 (end-of-study) n (%) | 45 (37·2) | 19 (15·7) | −0·22 | [−0·10 – −0·32] | < 0·001 |
TQD, target quit date; RR risk reduction; CI, confidence interval.
3.3. Reinforcements, drive to smoke, and smoke aversion
3.3.1. Pre-quit phase
As assessed by mCEQ subscales, extended preloading significantly decreased both positive (3.9 [3.6–4.2] vs. 4.3 [4.1–4.5], p = 0·038) and negative reinforcements (3.8 [3.5–4.1] vs. 4.1 [3.9–4.3], p = 0·050) at week 4, compared with standard preloading (Table 3). As assessed by the MPSS-C subscales, extended preloading significantly decreased the drive to smoke at both weeks 4 and 6 (1.98 [1.8–2.2] vs. 2.64 [2.4–2.9], p < 0·001) and 6 (1.64 [1.4–1.9] vs. 2.37 [2.1–2.6], p < 0·001). Extended preloading significantly increased aversion, measured by nausea (week 4; 42 [30–49] vs. 11 [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], p = 0·001), and by the mCEQ aversion subscale (week 6; 2.6 [2.3–2.9] vs. 2.1 [1.8–2.4], p = 0·029). Extended preloading produced a significant decrease in concomitant smoking intake vs. standard preloading (Table 4).
Table 3.
Effect of extended preloading on reinforcement, drive to smoke, and aversion: run-in phase intent-to-treat study population (n = 242).
| Variable | Baseline |
Week 4 |
Week 6 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Extended Preloading | Standard Preloading | p value | Extended Preloading | Standard Preloading | p value | Extended Preloading | Standard Preloading | p value | |
| Positive Reinforcement | |||||||||
| mCEQ satisfaction (items 1, 2, 12) | 4·8 (1·3)[4·57– 5·03] | 4·9 (1·4) [4·65 – 5.15] |
0·32 | 3·9 (1·4) [3·63 – 4·17] |
4·3 (1·2) [4·06 – 4·54] |
0·04 | 3·6 (1·4) [3·31 – 3·89] |
3·8 (1·4) [3·50 – 4·11] |
0·24 |
| Negative Reinforcement | |||||||||
| mCEQ psychological reward (item 4– 8) | 4·5 (1·2) [4·29 – 4·71] |
4·7 (1·3) [4·47 – 4·93] |
0·23 | 3·8 (1·4) [3·53 – 4·07] |
4·1 (1·2) [3·86 – 4·34] |
0·05 | 3·5 (1·2) [3·26 – 3·75] |
3·6 (1·3) [3·32 – 3·88] |
0·31 |
| Drive to Smoke | |||||||||
| MPSS-C (items 8– 9) | 3·33 (0·91) [3·17 – 3·49] |
3·33 (1·04) [3·14 – 3·12] |
0·50 | 1·98 (1·13) [1·76 – 2·20] |
2·64 (1·19) [2·40 – 2·88] |
< 0·001 | 1·64 (1·05) [1·43 – 1·85] |
2·37 (1·15) [2·12 – 2·62] |
< 0·001 |
| MPSS-C (item 8) urges | 3·39 (1·16) [3·18 – 3·60] |
3·41 (1·22) [3·19 – 3·63] |
0·44 | 1·96 (1·26) [1·72 – 2·20] |
2·67 (1·30) [2·41 – 2·93] |
< 0·001 | 1·57 (1·07) [1·35 – 1·79] |
2·42 (1·25) [2·15 – 2·69] |
< 0·001 |
| MPSS-C (item 9) strength | 3·26 (1·01) [3·08 – 3·44] |
3·24 (1·06) [3·05 – 3·43] |
0·43 | 1·99 (1·19) [1·76 – 2·22] |
2·61 (1·20) [2·37 – 2·85] |
< 0·001 | 1·72 (1·15) [1·49 – 1·96] |
2·32 (1·19) [2·06 – 2·58] |
< 0·001 |
| MPSS-M (items 1– 7) | 2·02 (0·95) [1·85 – 2·19] |
2·17 (1·02) [1·99 – 2·35] |
0·12 | 1·91 (0·75) [1·77 – 2·05] |
1·95 (0·73) [1·80 – 2·10] |
0·37 | 1·98 (0·75) [1·83 – 2·13] |
1·95 (0·75) [1·79 – 2·11] |
0·39 |
| Aversion | |||||||||
| Nausea n (%) | 0 (0·0) | 0 (0·0) | 1·000 | 42 (39·6) [30·3–48·9] |
11 (11·5) [5·1 – 17·9] |
< 0·001 | 26 (28·6) [19·4 – 37·8] |
19 (23·5) [14·3 – 32·7] |
0·49 |
| mCEQ aversion subscale (items 9– 10) | 2·5 (1·4) [2·25–2·75] |
2·7 (1·5) [2·43–2·97] |
0·11 | 2·8 (1·6) [2·50–3·11] |
2·5 (1·4) [2·22 – 2·78] |
0·10 | 2·6 (1·3) [2·30 – 2·87] |
2·1 (1·2) [1·84 – 2·36] |
0·03 |
The MPSS was administered to all participants. The mCEQ was administered only to participants striving for abstinence. Values are the mean (SD) [95% CI].
MPSS-C, Mood and Physical Symptoms Scale-Craving; MPSS-M, Mood and Physical Symptoms Scale-Mood; mCEQ, modified Cigarette Evaluation Questionnaire; CI, confidence interval; SD, standard deviation.
Table 4.
Change in smoke intake: run-in phase intent-to-treat study population (n = 242).
| Variable | Baseline |
Week 4 |
Week 6 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Extended Preloading | Standard Preloading | p value | Extended Preloading | Standard Preloading | p value | Extended Preloading | Standard Preloading | p value | |
| Number of Participants | 121 | 121 | – | 106 | 96 | 0·119 | 92 | 81 | 0·154 |
| Smoke Intake | |||||||||
| Cigarettes per day | 24·5 (13·4) [22·1 – 26·9] |
24·9 (13·4) [22·5 – 27·3] |
0·80 | 9·7 (10·6) [7·68 – 11·72] |
13·8 (9·2) [11·96 – 15·64] |
0·004 | 5·2 (7·2) [3·73 – 6·77] |
10·8 (9·8) [8·67 – 12·93] |
< 0·001 |
| % fall from baseline | – | – | 60·4 | 44·6 | – | 78·8 | 56·6 | – | |
| Expired CO (ppm) | 16·0 (6·5) [14·8 – 17·2] |
14·7 (5·9) [13·6 – 15·8] |
0·09 | 9·0 (6·2) [7·82 – 10·18] |
12·8 (7·2) [13·36 – 14·24] |
< 0·001 | 6·8 (6·0) [5·57 – 8·03] |
10·1 (6·6) [8·66 – 11·54] |
0·001 |
| % fall from baseline | – | – | – | 43·8 | 12·9 | – | 57·5 | 31·3 | – |
| Urinary cotinine (mg/mL) | 9·8 (5·5) [8·8 – 10·8] |
9·5 (5·1) [8·5 – 10·4] |
0·66 | – | – | – | 3·2 (3·6) [2·46 – 3·94] |
5·3 (4·5) [4·32 – 6·28] |
0·001 |
| % fall from baseline | – | – | – | – | – | – | 67·3 | 44·2 | – |
| Smoking Status | |||||||||
| Abstainers n (%) | – | – | – | 22 (20·8) [13·1 – 28·5] |
4 (4·2) [0·2 – 8·2] |
< 0·001 | 35 (38·0) [28·1 – 47·9] |
12 (14·8) [7·1 – 22·5] |
< 0·001 |
| Reducers n (%) | – | – | – | 53 (50·0) [40·5 – 59·5] |
39 (40·6) [30·8 – 50·4] |
45 (48·9) [38·7 – 59·1] |
28 (34·6) [24·2 – 45·0] |
||
| Non-reducers n (%) | 121 (100) | 121 (100) | 1·00 | 31 (29·2) [20·5 – 37·9] |
53 (55·2) [45·3 – 65·1] |
12 (13·0) [6·1 – 19·9] |
41 (50·6) [39·7 – 61·5] |
||
Values are the mean (SD) [95% CI] SD, standard deviation; CI, confidence interval; CO, carbon dioxide; ppm, parts per million.
3.3.2. Post-quit phase
Table 5 shows that the differences in reinforcements between the 2 groups persisted only until week 12 (3.5 [3.2–3.8] vs. 4.0 [3.7–4.3], p = 0·012 for negative reinforcement) and, in aversion, only until the seventh week (16 [11–29] vs. 24 [25–48], p = 0·048 for nausea). Instead, the drive to smoke assessed by the MPSS-C subscale remained significantly lower for extended preloading vs. standard preloading at all time-points from the TQD onwards (p-values: 0·048–0·009 for the 12 comparisons).
Table 5.
Effect of extended preloading on reinforcements, drive to smoke, and aversion: post-quit phase intent-to-treat study population (n = 242).
| Variable | Week 7 |
Week 12 |
Week 18 |
Week 30 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extended Preloading 95% CI | Standard Preloading 95% CI | p value | Extended Preloading 95% CI | Standard Preloading 95% CI | p value | Extended Preloading 95% CI | Standard Preloading 95% CI | p value | Extended Preloading 95% CI | Standard Preloading 95% CI | p value | |
| Positive Reinforcement | ||||||||||||
| mCEQ satisfaction (items 1, 2, 12) | 3·4 (1·5) [3.70–3.73] |
3·5 (1·7) [3.09–3.91] |
0·44 | 3·5 (1·4) [3.18–3.82] |
4·0 (1·3) [3.66–4.34] |
0·12 | 3·6 (1·3) [3.30–3.90] |
4·0 (1·3) [3.65–4.35] |
0·22 | 4·0 (1·3) [3.70–4.30] |
4·1 (1·3) [3.75–4.45] |
0·34 |
| Negative Reinforcement | ||||||||||||
| mCEQ psychological reward (items 4–8) | 3·3 (1·2) [3.04–3.57] |
3·3 (1·2) [3.01–3.59] |
0·48 | 3·3 (1·3) [3.00–3.60] |
4·2 (1·1) [3.91–4.49] |
0·01 | 3·6 (1·5) [3.25–3.95] |
3·9 (1·3) [3.55–4.25] |
0·26 | 4·0 (1·2) [3.72–4.28] |
4·0 (1·4) [3.62–4.38] |
0·26 |
| Drive to Smoke | ||||||||||||
| MPSS-C (items 8–9) | 1·53 (1·07) [1.29–1.77] |
1·99 (1·36) [1.66–2.32] |
0·01 | 1·27 (1·07) [1.02–1.52] |
1·73 (1·52) [1.33–2.13] |
0·03 | 1·29 (1·26) [1.00–1.58] |
1·76 (1·46) [1.37–2.15] |
0·03 | 1·33 (1·38) [1.01–1.65] |
1·88 (1·56) [1.46–2.30] |
0·02 |
| MPSS-C (item 8) | 1·47 (1·15) [1.22–1.72] |
1·97 (1·39) [1.64–2.30] |
0·01 | 1·19 (1·11) [0.94–1.44] |
1·70 (1·62) [1.28–2.12] |
0·02 | 1·29 (1·41) [0.96–1.62] |
1·81 (1·64) [1.37–2.25] |
0·03 | 1·36 (1·45) [1.02–1.70] |
1·98 (1·72) [1.52–2.44] |
0·02 |
| MPSS-C (item 9) | 1·59 (1·12) [1.34–1.84] |
2·02 (1·46) [1.67–2.37] |
0·03 | 1·34 (1·17) [1.07–1.61] |
1·75 (1·50) [1.36–2.14] |
0·05 | 1·28 (1·18) [1.00–1.55] |
1·72 (1·43) [1.34–2.11] |
0·03 | 1·31 (1·41) [0.98–1.64] |
1·77 (1·48) [1.37–2.17] |
0·04 |
| MPSS-M (items 1–7) | 1·99 (0·84) [1.80–2.18] |
2·00 (0·79) [1.81–2.19] |
0·48 | 2·01 (0·84) [1.82–2.20] |
1·95 (0·85) [1.73–2.17] |
0·36 | 1·92 (0·86) [1.72–2.12] |
1·82 (0·74) [1.62–2.02] |
0·25 | 1·79 (0·79) [1.60–1.98] |
1·96 (0·78) [1.75–2.17] |
0·11 |
| Aversion | ||||||||||||
| Nausea n (%) | 16 (20·3) [11.4–29.2] |
24 (36·4) [24.9–47.9] |
0·05 | 12 (16·4) [7.9–24.9] |
13 (23·2) [12.1–34.3] |
0·46 | 10 (14·1) [6.0–22.2] |
5 (9·4) [1.5–17.3] |
0·61 | 1 (1·4) [0.0–4.2] |
2 (3·8) [0.0–8.9] |
0·58 |
| mCEQ aversion subscale (items 9–10) | 2·6 (1·4) [2.29–2.91] |
2·5 (1·4) [2.17–2.84] |
0·37 | 2·5 (1·4) [2.18–2.82] |
2·1 (1·2) [1.79–2.41] |
0·17 | 2·6 (1·5) [2.25–2.95] |
2·0 (1·1) [2.70–2.30] |
0·08 | 2·0 (1·3) [1.070–2.30] |
2·1 (1·0) [1.83–2.37] |
0·41 |
mCEQ, modified Cigarette Evaluation Questionnaire; MPSS-C, Mood and Physical Symptoms Scale-Craving; MPSS-M, Mood and Physical Symptoms Scale-Mood; CI, confidence interval.
3.4. Adverse events
AEs reported at each time-point by ≥ 5% of participants in at least 1 study group are summarized in Table 6. Nausea was the most common AE in both groups, being more pronounced in participants receiving extended preloading at week 4 (39.6% vs 11.5) and less in week 7 (20.3% vs 36.4%). Vomiting was more common with extended preloading at week 4 (5.7% vs 0.0%), while constipation was more common with standard preloading at week 7 (0.0% vs 7.6%). Dizziness was more common with standard preloading at weeks 7 (2.5 vs 12.1%) and 12 (1.4% vs 10.7%), while abnormal dreams were more common with extended preloading only at week 6 (7.7% vs 0.0%). Extended preloading generated a greater number of participants presenting more than 1 AE at week 7, but this did not produce an excess in the number of participants discontinuing study participation because of AEs. There was 1 serious AE, at the seventh week, reported by a 59-year-old man receiving standard preloading, who sustained a musculoskeletal injury during do-it-yourself work at home. The patient recovered uneventfully, and the serious AE was not adjudicated to be related to the study medication.
Table 6.
Adverse events reported in at least 1 study group (Safety population).
| Week 4 |
Week 6 |
Week 7 |
Week 12 |
Week 18 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extended Preloading | Standard Preloading | Extended Preloading | Standard Preloading | Extended Preloading | Standard Preloading | Extended Preloading | Standard Preloading | Extended Preloading | Standard Preloading | |
| Participants with > 1 AE | 27 (25·5) | 16 (16·7) | 22 (24·2) | 19 (23·5) | 13 (16·4) | 24 (35·8) | 10 (13·7) | 12 (21·4) | 9 (12·7) | 5 (9·4) |
| Risk difference | −0.09 (−0.20 - 0.02) | −0.01 (−0.13 - 0.12) | 0.19 (0.05 – 0.33) | 0.08 (−0.06 – 0.21) | −0.03 (−0.14 – 0.08) | |||||
| Participants Who Withdrew from the Trial Due to AEs | 5 (4·7) | 6 (6·3) | 6 (6·6) | 6 (7·4) | 5 (6·3) | 0 (0·0) | 3 (4·1) | 4 (9·1) | 0 (0·0) | 0 (0·0) |
| Risk difference | 0.02 (−0.05 – 0.08) | 0.01 (−0.07 – 0.08) | 0.04 (−0.05 – 0.13) | 0.05 (−0.05 – 0.15) | 0 (0–0) | |||||
| Serious AEs | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 1 (1·5) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Risk difference | 0 (0–0) | 0 (0–0) | 0.01 (−0.01 – 0.04) | 0 (0–0) | 0 (0–0) | |||||
| Gastrointestinal Disorders | ||||||||||
| Nausea | 42 (39·6) | 11 (11·5) | 26 (28·6) | 19 (23·5) | 16 (20·3) | 24 (36·4) | 12 (16·4) | 13 (23·2) | 10 (14·1) | 5 (9·4) |
| Abdominal pain | 5 (4·7) | 2 (2·1) | 1 (1·1) | 4 (4·9) | 1 (1·3) | 5 (7·6) | 1 (1·4) | 3 (5·4) | 0 (0·0) | 0 (0·0) |
| Vomiting | 6 (5·7) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Constipation | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 5 (7·6) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Nervous System | ||||||||||
| Poor-quality sleep | 5 (4·7) | 6 (6·3) | 11 (11·9) | 10 (12·3) | 9 (11·3) | 14 (20·9) | 8 (11·0) | 6 (10·7) | 5 (7·0) | 4 (7·5) |
| Dizziness | 8 (7·5) | 7 (7·3) | 2 (2·2) | 4 (4·9) | 2 (2·5) | 8 (12·1) | 1 (1·4) | 6 (10·7) | 0 (0·0) | 0 (0·0) |
| Headache | 7 (6·6) | 10 (10·4) | 11 (12·1) | 7 (8·6) | 8 (10·1) | 7 (10·6) | 1 (1·4) | 3 (5·4) | 0 (0·0) | 0 (0·0) |
| Abnormal dreams | 5 (4·7) | 4 (4·2) | 7 (7·7) | 0 (0·0) | 1 (1·3) | 3 (4·5) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
AE, adverse event.
3.5. Weight gain
Weight gain from the baseline to the end of the study was analysed separately for participants classified as abstainers, reducers, or continuing smokers at week 30. No between-group differences were observed for any category. Abstainers receiving extended preloading gained 3·8 ± 4·0 kg, while those receiving standard preloading gained 2·9 ± 3·3 kg (p = 0·391). For reducers, individuals receiving extended vs. standard preloading gained 2·8 ± 2·6 kg vs. 1·7 ± 3·6 kg, respectively (p = 0·351). Finally, smokers receiving extended preloading gained an average of 0·5 ± 3·1 kg compared with −0·3 kg ± 1·5 kg for those receiving standard preloading (p = 0·380).
4. Discussion
Among cigarette smokers motivated to quit, using varenicline for 6 weeks before the TQD, as opposed to starting 1 week before the TQD, significantly reduced smoke intake during the run-in period and produced validated continuous abstinence rates 3·3 (week 18) to 5·6 (week 30) times that obtained with standard preloading. The treatment effect between extended and standard preloading, expressed as differences between the quit rates, varied from 14·9% at week 4, to 21·5% at week 30. The robust and consistent superiority of 6-week preloading was notable, especially considering that standard preloading is the most efficient treatment currently available for smoking cessation.
There is scarce research similar to this study that can provide data for comparison. Two earlier studies found that, compared with the 1-week standard for preloading, 4 weeks of varenicline preloading reduced smoke intake in the run-in period, associated with decreases in smoking urges and cigarette enjoyment [4] or in the taste and “buzz” of the first cigarette [5]. This study builds on these data and strengthens the evidence for the efficacy of extended varenicline preloading in both the run-in and the post-quit phase. In the run-in phase, extended preloading produced a significant reduction in smoking rewards and in the drive to smoke. These changes translated into a marked decrease in smoking intake with, incidentally, a significant minority of participants in the Extended preloading group reporting “early”, unsolicited smoking cessation (i.e., cessation prior to the TQD). Remarkably, most of these individuals sustained abstinence continuously, without a single puff, from 2 weeks prior to the TQD through 6 months. Second, the findings show, for the first time, a significant decrease in the drive to smoke produced by extended preloading that persisted after preloading had finished through 6 months; this provides a plausible explanation for the sustained enhanced cessation outcomes observed concomitantly.
The present findings considerably strengthen the existing evidence for a unified mechanism of preloading and support the view that the benefits of pre-treatment are not specific to varenicline. In the largest trial of nicotine preloading published to date, Aveyard and colleagues [16] found an increase in long-term abstinence after 4 weeks of preloading with a nicotine patch followed by standard cessation therapy. Interestingly, in the initial analysis, the nicotine preloading benefit was undermined because it reduced the use of varenicline in the post-quit treatment; however, when the results were adjusted for the use of post-quit varenicline, the effect of nicotine preloading was larger than the unadjusted value and was statistically significant.
The pattern of the preloading-related reduction in smoke intake was consistent with a reinforcement-reduction mechanism [4,5,19]. Conceptually, a behaviour that has been previously reinforced but no longer produces reinforcing consequences tends to gradually extinguish itself. The cyclic repetition of this mechanism during a 6-week period could have weakened the learnt association between reinforcement and smoking, facilitating cessation. This analysis is supported by the above study of Aveyard and colleagues [16], showing that changes in abstinence were mediated mainly by a reduction in the drive to smoke before and after smoking cessation, and by a reduction in smoke intake. The present findings also support previous suggestions that the 1-week preloading, recommended per the current varenicline labelling, appears too short to elicit the preloading effect of varenicline [4,5,19]. The observation that many participants had stopped spontaneously by week 4 builds on the earlier studies by raising the possibility that, beyond week 1, the optimal period of preloading varies among individuals, a hypothesis that warrants further investigation.
The cessation rates of participants receiving standard varenicline preloading were lower than those reported in the literature [3]. However, participants receiving standard varenicline treatment in this study cannot be compared to participants receiving standard treatment in regular trials of varenicline. First, in this study, varenicline titration was performed after 5 weeks of placebo, instead of conducting the immediate titration of the standard varenicline treatment. Due to this long pre-titration period, many participants may have assumed they were assigned to the placebo group and lost motivation. In the absence of counselling (and reimbursement), this negative feeling can produce lapses and relapses, leading to the abstinence violation effect, which manifests as uncontrolled smoking. Secondly, since plans to quit smoking may change very quickly, deferring the TQD for 5 weeks may have also reduced motivation to pursue the quit attempt. Incidentally, it is possible that many participants classified as abstinent in previous studies would have been classified as non-abstinent in the present study. Indeed, our definition of abstinence was based on an expired carbon monoxide (CO) cut off value of ≤ 5 ppm (corroborated by urinary cotinine measure), a value 50% lower than the usual 10 ppm cut-off value in cessation trials. Finally, abstinence was measured continuously (not a single puff) over the whole 24-week study duration, a period several times longer than the usual assessment during the last 4 weeks of treatment found in the literature.
Extended varenicline preloading was safe, with a tolerability profile similar to that of standard preloading and AE incidence rates similar to those found in other preloading studies [4,5]. No new, unwanted effects were observed, and the 2 groups did not differ in the number of participants withdrawing from the trial because of AEs. The serious AE reported in this study was not considered to be caused by the treatment. Finally, the longer period of abstinence among abstainers receiving extended varenicline preloading, compared with those receiving standard preloading, could explain the ~ 1 kg excess weight gain observed in the former group.
Apart from being conducted with rigor, according to a pre-specified published protocol, this study has additional strengths such as the randomised, double-blind, controlled design and an appropriate sample size to assess the effects of preloading. Also, the use of a CO cut off ≤ 5 ppm is important in trials like this one where individuals may reduce—but not completely quit—smoking, or for heavy smokers who reduce but remain light smokers with CO values below 10 ppm.
This study is not without limitations. First, for safety concerns with a long period of preloading, we excluded participants with severe diseases, illicit drug users, and individuals with psychiatric disorders (i.e., populations that might benefit from the extended preloading approach). Second, despite the efforts to retain and follow all participants throughout the 30-week study period, the attrition rate was higher than observed in other varenicline studies [20], [21], [22], [23], albeit in the middle of the 10–77% range reported in smoking cessation studies [24]. While counselling and financial incentives could have improved retention, these elements would have increased the cost of the study; also, counselling could have diluted the effects of the pharmacotherapy.
Finally, it must be stressed that data from single-centre trials need confirmation by larger trials before they can be used in decision-making. However, it should be kept in mind that although data produced by multi-centre trials have the potential for increased generalisability, these trials are more expensive to run and more complex to coordinate than single-centre studies.
In summary, using varenicline for 6 weeks before a quit attempt effectively reduced smoking rates before cessation, facilitating and enhancing abstinence at 6 months. The pattern of decreased smoking with varenicline preloading was consistent with a reinforcement-reduction mechanism. Therefore, our data support the view that the current 1-week varenicline use prior to treatment should be extended to enhance the pre-treatment effect of the drug. The present trial also supports the notion that interventions targeting the pre-quit period should be a crucial component of smoking cessation strategies.
Data sharing
Data are available upon request from the corresponding author.
Data types: Deidentified participant data.
How to access data: abohadana@szmc.org.il.
When available: Three months after publication.
Supporting documents
Document types: None.
Additional information
Who can access the data: Researchers whose proposed use of the data has been approved.
Types of analyses: For any legitimate scientific purpose.
Mechanisms of data availability: After approval of a proposal by the authors and the SZMC Ethics Committee.
Authors and contributors
Bohadana, Freier-Dror, Peles, Babai and Izbicki had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study was funded by a 2013 Global Research Award for Nicotine Dependence (GRAND), a competitive, independent investigator-initiated research program supported by Pfizer, Inc. However, Pfizer had no further role in the study design; collection, analysis and interpretation of the data; writing of the report; or in the decision to submit the paper for publication.
Contributions
Study concept and design: Bohadana
Acquisition, analysis and interpretation of data: Bohadana, Peles, Peker, Freier-Dror, Babai, and Izbicki
Randomisation monitoring: Peles
Collection of follow-up data: Bohadana, Peker, Shabtay and Izbicki.
Writing – original draft: Bohadana
Writing – review & editing: Bohadana, Freier-Dror and Izbicki
Clinical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Freier-Dror and Babai
Administrative, technical, or material support: Peles, Izbicki, Peker and Bohadana
Study supervision: Bohadana, Izbicki and Peles
Other contributions
Editorial support in the form of editing, proofing and formatting the text and preparing the manuscript for submission was provided by Elsevier Editing Services and funded by the Pulmonary Institute of Shaare Zedek Medical Center.
Trial registration
Clinicaltrials.gov identifier: NCT01544153
Declaration of competing interest
None.
Acknowledgments
The authors thank Dr. Pascal Wild, PhD from the Institut National de Recherche et de Sécurité, INRS in Nancy, France for performing the joint orthogonality analysis on the baseline variables. The authors appreciate the technical assistance of Mrs. Bela Peker and Mrs. Ayelet Shabtay and are grateful to all participants as well as the Israel Electric Corporation and the Jerusalem Municipality for informing their employees about this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.11.021.
Appendix. Supplementary materials
References
- 1.Report of the Surgeon General: How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease, 2010: The Report Available at: http://www.surgeongeneral.gov/library/reports/tobaccosmoke/index.html. [PubMed]
- 2.Fiore MC, Jaén CR, Baker TB. Treating tobacco use and dependence: 2008 update. US Depart Health Hum Serv. 2008 [Google Scholar]
- 3.Cahill K., Stevens S., Perera R., Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajek P., McRobbie H.J., Myers K. Use of varenicline for 4 weeks before quitting smoking. Arch Intern Med. 2011;171:770–777. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- 5.Hawk LH, Jr, Ashare RL, Lohnes SF. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther. 2012;91(2):172–180. doi: 10.1038/clpt.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation. A randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2000;160:3128–3134. doi: 10.1001/archinte.160.20.3128. [DOI] [PubMed] [Google Scholar]
- 7.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 8.Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res. 2014;16(10):1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrone GF, Shakleya DM, Scheidweiler KB, Singleton EG, Huestis MA, Heishman SJ. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction. 2011;106(7):1325–1334. doi: 10.1111/j.1360-0443.2011.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benowitz NL. Biochemical verification of tobacco use and cessation: an update. SRNT Webinar. 2017 October 13. [Google Scholar]
- 11.Jarvis M.J., Russell M.A., Saloojee Y. Expired air carbon monoxide: a simple breath test of tobacco smoke intake. Br Med J. 1980;281:484–485. doi: 10.1136/bmj.281.6238.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spears M., Donnelly I., Jolly L. Effect of low-dose theophylline plus beclomethasone on lung function in smokers with asthma: a pilot study. Eur Respir J. 2009;33:1010–1017. doi: 10.1183/09031936.00158208. [DOI] [PubMed] [Google Scholar]
- 13.West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology (Berl) 2004;177(1–2):195–199. doi: 10.1007/s00213-004-1923-6. [DOI] [PubMed] [Google Scholar]
- 14.Cappelleri J, Bushmakin A, Baker C, Merikle E, Olufade A, Gilbert D. Confirmatory factor analysis and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 16.Aveyard P, Lindson N, Tearne S. Nicotine preloading for smoking cessation: the preloading RCT. Health Technol Assess. 2018;22(41):1–84. doi: 10.3310/hta22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruhn M., McKenzie D. In pursuit of balance: randomization in practice in development field experiments. Am Econ J Appl Econ. 2009;1(4):200–232. [Google Scholar]
- 18.Moher D, Hopewell S, Schulz KF. CONSORT 2010 Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashare R.L., Tang Z.K., Mesaros A.C., Blair I.A., Leone F., Strasser A.A. Effects of 21 of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26(10):1383–1390. doi: 10.1177/0269881112449397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koegelenberg CF, Noor F, Bateman ED. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155–161. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- 21.Ebbert JO, Hatsukami DK, Croghan IT. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311(2):155–163. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzales D, Rennard SI, Nides M. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 23.Jorenby DE, Hays JT, Rigotti NA. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Belita E., Sidani S. Attrition in smoking cessation intervention studies: a systematic review. Can J Nurs Res. 2015;47:21–40. doi: 10.1177/084456211504700402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.