Highlights
-
•
Hyperfractionated RT with augmented dose provides durable enhanced disease control.
-
•
Late toxicity is unchanged with higher optimal doses using smaller fraction sizes.
-
•
Hyperfractionation improves overall survival but mortality profile evolves over time.
-
•
Altered fractionation should avoid unnecessary RT protraction to maximize control.
-
•
Excessive acceleration should be avoided to minimize severe normal tissue sequelae.
Keywords: Head and neck cancer, Radiotherapy, Hyperfractionation, Long-term follow-up, Competing mortality
Abstract
Purpose/objective(s)
To examine the therapeutic ratio and mortality profile over time in a radiotherapy randomized trial in stage III-IV larynx/pharynx cancer with long-term follow-up.
Materials/methods
From 1988 to 1995, 331 cases were randomized to either hyperfractionated (HF) (58 Gy/40 fractions, twice daily) or conventional (CF) (51 Gy/20 fractions, once daily) radiotherapy. Overall survival (OS), locoregional (LRC), distant control (DC), ≥Grade 3 late toxicity (LT), and relative mortality risk profile over time were compared between both arms.
Results
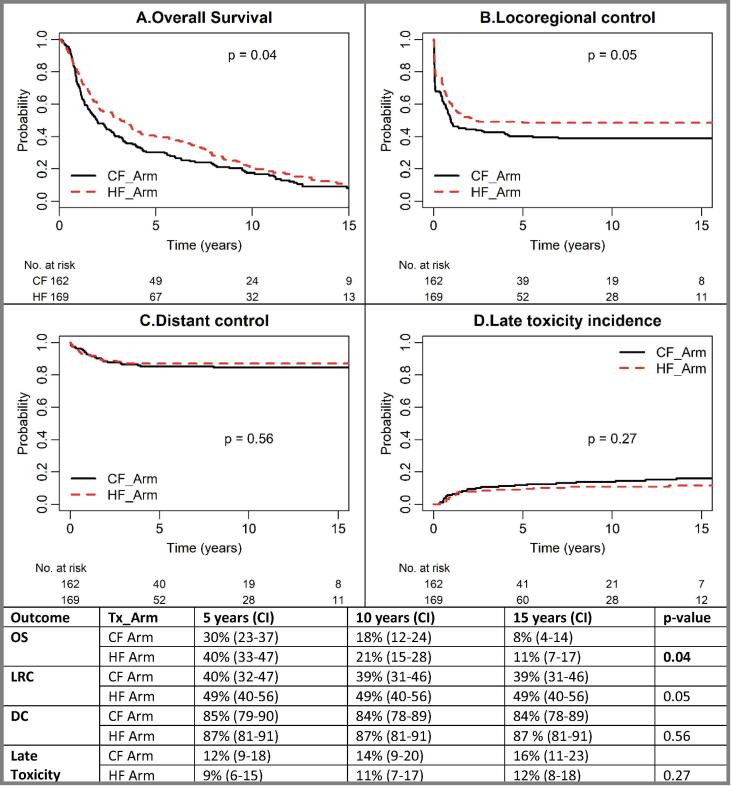
Median follow-up was 13.6 years. HF had a 10% improved OS at 5-years (40% vs 30%, p = 0.04), but the benefit diminished to 3% at 10-years (21% vs 18%). A trend towards higher LRC with HF remained (5-year: 49% vs 40%; 10-year: 49% vs 39%, p = 0.05). DC rates were unchanged (5-year: 87% vs 85%; 10-year: 87 vs 84%, p = 0.56). LT rates were similar (HF vs CF: 5-year: 9% vs 12%; 10-year: 11% vs 14%, p = 0.27). Multivariable analysis confirmed that HF reduced mortality risk by 31% [HR 0.69 (0.55–0.88), p < 0.01] and locoregional failure risk by 35% [HR 0.65 (0.48–0.89), p < 0.01]. Index cancer mortality (5-year: 46% vs 51%; 10-year: 49% vs 55%) was lower in the HF arm. Competing mortality (mostly smoking-related) was also numerically lower with HF at 5-years (14% vs 19%) but became similar at 10-years (30% vs 28%).
Conclusions
This trial confirms that HF with augmented total dose has a durable 10% effect size on LRC with comparable LT. OS benefit is evident at 5-years (10%) but relative mortality risk profile changes in longer follow-up.
1. Introduction
The benefit of hyperfractionation (HF) radiotherapy (RT) as a means of intensified treatment for locally advance head and neck squamous cell carcinoma cancer (LAHNSCC) has been demonstrated in the Meta-Analysis of Radiotherapy in Carcinomas of Head and neck (MARCH) [1]. The rationale for HF is to reduce late toxicity by the use of smaller fraction size and permitting adequate time between fractions for sufficient repair of sub-lethal damage. This strategy permits delivery of a higher total radiation dose to enhance tumor control without a corresponding increase in late toxicity [2], [3]. Previous studies [4], [5], [6] suggested that accelerated tumor clonogen repopulation occurred at 4 (±1 week) following RT initiation, and additional dose (about 0.6 Gy per day) would be needed to compensate for this. Therefore, theoretically, the therapeutic gain from dose augmentation would be optimal if it could be employed within 3–5 weeks. The benefits for accelerated fractionation approaches have been demonstrated in several clinical trials [7], [8], [9]. For RT acceleration, one needs to be mindful to allow sufficient opportunity for normal tissue recovery [10]. Previous trials have shown that an unusually short inter-fraction interval may result in unexpected normal tissue damage, such as spinal cord injury (CHART [11]). Over-acceleration could result in significant mucosal consequential damage due to overly intense dose accumulation throughout the scheduled time-frame (e.g. CAIR [12], [13]; EORTC 22851 [14], BCCA 9113 [15]).
Head and neck cancer (HNC) patients are at high risk of death from competing risks, such as treatment-related sequelae (e.g. aspiration pneumonia, cerebrovascular disease), smoking and/or alcohol-related comorbidities, and second malignancies [16]. Thus, the ability to demonstrate an overall survival advantage from a specific treatment strategy in any one clinical trial in HNC is generally compromised by the unavoidable inclusion of patients who are also susceptible to death from other competing causes beyond their index cancer. Competing cause of death is increasingly relevant in long-term HNC survivors since the profile of cause of death may change over time and treatment efficacy may alter with long-term follow-up. Composite end points, such as overall survival (OS) and disease-free survival, may not distinguish the influence of treatment versus confounding factors on survival. Long-term follow-up of two RTOG trials [17], [18] has shown a different result after long-term follow-up compared to the initial report. This may be relevant in HPV-mediated [HPV(+)] oropharyngeal carcinoma (OPC) trials requiring long follow-up to examine non-inferiority of deintensification strategies.
We conducted a randomized trial in stage III-IV larynx/pharynx cancer between 1988 and 1995 testing the efficacy of dose intensification delivered prior to full onset accelerated clonogenic proliferation (i.e. within 4 weeks) using HF compared to a conventional fractionated (CF) RT regimen in use at the time at our centre and delivered in the same overall treatment time. The trial demonstrated a 10% survival benefit at 5-years with a 13% dose augmentation (58 Gy vs 51 Gy) while retaining iso-effectiveness for late toxicity [8] using smaller fraction sizes (1.45 Gy vs 2.55 Gy). Whether this therapeutic gain would remain with long-term follow-up is unknown. This updated report examines whether a durable therapeutic gain remains in the longer term, and to what degree the mortality profile might change with more than 10 years follow-up.
2. Methods and materials
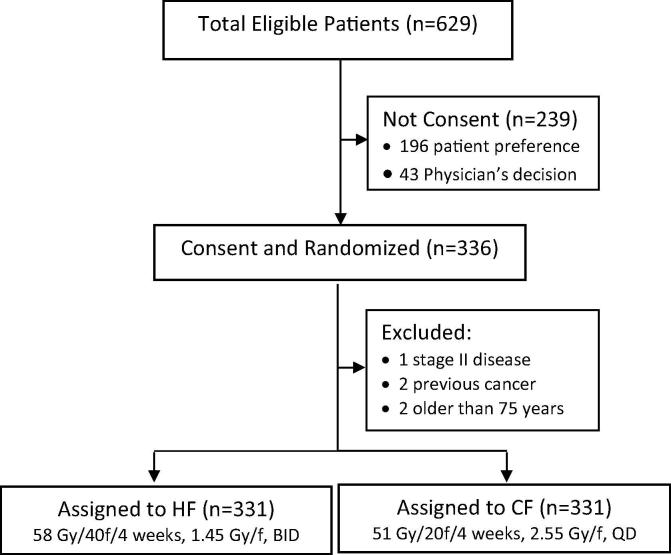
Following institutional Research Ethics Board approval, 331 patients with biopsy-proven stage III-IV laryngeal, oropharyngeal, and hypopharyngeal squamous cell carcinoma provided consent and were randomized to either HF arm (n = 169) (58 Gy in 40 fractions, twice-daily, administered at least 6 hours apart, 10 fractions per week) or conventional daily fraction (CF) arm (n = 162) (51 Gy in 20 fractions, once-daily, 5 fractions per week) in use at the time in our centre. The total dose of 58 Gy in the experimental arm was chosen based on two pilot step-wise studies progressing from 56 to 58 Gy that demonstrated that this RT schedule was tolerable for acute normal tissue effects, although heightened supportive care compared to our usual practice at the time (e.g. feeding gastrostomy tubes and sustained and high doses of analgesics) was often required for many patients. The tumor dose was prescribed with the intent of administering 51 Gy in 20 equal fractions to the ICRU target absorbed dose (TAD) point from ICRU report 29 [19] in an overall time of 28 days. This equated to a prescription of 50 Gy in 20 fractions prescribed at the 98% isodose line traditionally used at the PMH. No chemotherapy was delivered to either arm. The 58 Gy in 40 fraction RT regimen was derived by estimating iso-effectiveness for late responding normal tissues using an α/β ratio of 7 compared to the standard fractionation of 50 Gy in 20 fractions delivered in 4 weeks; these parameters were underpinned by clinical studies of normal tissue toxicity available at the time the trial was designed [20]. With obvious caveats regarding such assumptions, it seemed that late reacting normal tissue toxicity should not increase and might be reduced if these tissues had α/β values less than of 7 Gy and a therapeutic gain could be realized in tumors with α/β values exceeding 7 Gy, which probably represented the majority of HNC. The staging was based on the Union for International Cancer Control (UICC) / American Joint Committee for Cancer (AJCC) 1987 TNM staging system. Patients had to be between age 19 and 75 years and fit enough for definitive RT. The detailed study protocol was described previously [8] and summarized in Fig. 1.
Fig. 1.
CONSORT Diagram Abbreviation: HF: hyperfractionation radiotherapy; CF: conventional radiotherapy; f: fraction; BID: twice-daily, 6 h apart; QD: once-daily.
3. Radiation treatment
All patients underwent clinical, endoscopic and computerized tomography (CT) evaluation to assess primary tumor extent and the presence of regional lymph nodes. The extent of disease was documented by two clinicians in clinical research forms. RT was given with conventional 2D techniques in use at the time. These generally covered the primary tumor area and bilateral levels 2–4 cervical lymph node regions [8]. Unilateral neck irradiation was used for selected cases with small (T1-T2) well-lateralized tonsillar lesions with limited gross lymph node diseases [21]. RT volumes for primary tumor included the gross tumor volume (GTV) with 3 cm field coverage, which approximated a 1.5 cm margin for clinical target volume (CTV) beyond the GTV according to contemporary guidelines. Some cases used smaller field coverage when intervening anatomic barrier protected the tumor pathway. All patients underwent conventional 2-dimentional simulation and were immobilized with vacuum formed masks; verification portal films were taken to verify treatment position on day 1 prior to commencement of RT.
3.1. Patient assessment and Follow-up
Patients were assessed at least weekly during treatment, at the 4th and 12th weeks after RT, at 3-monthly intervals for the first 2 years, 4-monthly in the 3rd year, 6 monthly up to 5 years, and annually thereafter. Acute toxicity scores were documented weekly during the RT course and at the 4th and 12th week following RT. Late toxicity was recorded after 3 months following RT. Acute and late toxicity scores were based on Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) toxicity scales [22].
3.2. Cause of death attribution:
Patient vital status was confirmed with the provincial Cancer Registry. Cause of death attribution was based on clinical trial records and death certificates (from the Province of Ontario Cancer Registry via internal linkage). Unknown cause of death within the first 5 years was attributed to the index cancer; those evident without recurrence more than 5 years following trial enrollment were classified as died of other cause.
3.3. Statistical analysis
Outcomes were compared between HF and CF arms according to intention-to-treat analysis. Outcome endpoints for this study included overall survival (OS), loco-regional control (LRC), distant control (DC), and Grade 3–4 late toxicity (LT). All “time-to-event” analyses were calculated from the date of trial enrollment, except LT which was calculated from the completion of RT. Actuarial rates of OS were estimated using the Kaplan-Meier method with log-rank test for comparison; LRC, DC, and LT rates were calculated using the competing risk method [23] (death without relapse was considered as a competing risk) with Gray’s test for statistical significance. Multivariable analysis (MVA) with the Cox proportional hazard method was used to calculate relative risk of death between HF and CF arms adjusting for other confounders. Relative mortality risk profile for index cancer mortality and non-index cancer competing mortality (including second malignancy and other causes) were calculated and compared between HF and CF arms. All tests were two-tailed with a probability of < 0.05 considered statistically significance.
4. Results
The clinical characteristics were similar between HF (n = 169) and CF (n = 162) arms (Table 1). Ninety-four percent (94%) of patients in each arm were smokers. About 40% of patients in each arm had OPC and 93% of the study population had more than 10 pack-year smoking history. HPV status was not tested due to unavailability of tumor tissue specimens presently and lack of awareness of HPV-mediated oropharyngeal cancer at the time of the trial.
Table 1.
Baseline characteristics for study patients.
| Characteristics | Category | HF Arm (n = 169) | CF Arm (n = 162) | P |
|---|---|---|---|---|
| Age (median) | 60 (31–75) | 60 (35–75) | 0.92 | |
| Gender | Male Female |
141 (83%) 28 (17%) |
130 (80%) 32 (20%) |
0.45 |
| Disease site | Oropharynx Larynx Hypopharynx |
69 (41%) 65 (38%) 35 (21%) |
64 (40%) 67 (41%) 31 (19%) |
0.86 |
| ECOG performance status | 0–1 2–3 |
144 (85%) 25 (15%) |
137 (84%) 25 (15%) |
0.73 |
| T-category | T1-3 T4 |
115 (68%) 54 (32%) |
106 (65%) 56 (35%) |
0.74 |
| N-category | N0-N1 N2-N3 |
98 (58%) 71 (42%) |
99 (61%) 63 (39%) |
0.80 |
| Smoking pack-year | <=10 >10 |
12 (7%) 148 (93%) |
11 (7%) 149 (93%) |
0.83 |
| Smoking Status | Current smoker Ex-smoker Non-smoker Unknown |
122 (72%) 29 (17%) 9 (5%) 9 (5%) |
126 (78%) 25 (15%) 9 (6%) 2 (1%) |
0.84 |
| Local Failure | Number 10-year rates |
69 (41%) 59% (51–66) |
82 (51%) 49% (41–57) |
0.09 |
| Regional Failure | Number 10-year rates |
52 (31%) 69% (61–75) |
49 (30%) 70% (62–76) |
0.90 |
| Distant Failure | Number 10-year rates |
22 (13%) 87% (81–91) |
25 (15%) 84% (78–89) |
0.56 |
| Second Primary | Total Number Site: Lung Head and Neck Esophagus Other |
45 (27%) 20 (44%) 11 (24%) 7 (16%) 7 (16%) |
33 (20%) 15 (45%) 10 (30%) 2 (6%) 6 (18%) |
0.17 |
| Late Toxicity | Total Number 1st Toxicity: Larynx (edema/paralysis) Neck Fibrosis Osteoradionecrosis Esophageal stricture Other |
19 (11%) 6 4 2 3 4 |
25 (15%) 3 7 2 6 7 |
0.26 |
| Deaths | Number | 149 (88%) | 149 (92%) | 0.25 |
| Cause of death | Index Cancer Other Cancer Other Cause Unknown* |
85 (57%) 39 (26%) 18 (12%) 7 (5%) |
91 (61%) 30 (20%) 17 (11%) 11 (7%) |
0.44 |
Abbreviation: HF: hyperfractionation; CF: conventional fractionation
Unknown’ cause of death occurring within first 5 years was attributed to index cancer deaths while those occurring more than 5 years without evidence of recurrence were classified as death of other causes.
4.1. Tumor control
Median follow-up for surviving patients was 13.6 years. A higher LRC (about 10% improvement) with HF was evident and remained over the 15-year period [5-years: 49% (40–56) vs 40% (32–47); 10-years: 49% (40–56) vs 39% (31–46); 15-years: 49% (40–56) vs 39% (31–46), p = 0.05]. DC rates were similar and remained unchanged (5-years: 87% vs 85%; 10-years: 87% vs 84%; 15-years: 87% vs 84%, p = 0.56). LT rates were not different (3–4% non-significantly lower in HF) and also remained unchanged over time (HF vs CF: 5-years: 9 vs 12%; 10-years: 11 vs 14%; 15-years: 12 vs 16%, p = 0.27) (Fig. 2).
Fig. 2.
Oncologic Outcome over Time (A). Overall survival: absolute survival benefit maximized at 5–6 years, but reduced after 9–10 years; (B). Locoregional control: the difference in locoregional control maximized at 5 years and maintained afterwards (p = 0.05); (C). No significant difference between HF and CF arm (p = 0.56); (D). Grade 3–4 late toxicity: No significant difference despite higher total dose in HF arm (p = 0.27). Abbreviation: Tx: treatment; HF: hyperfractionation; CF: conventional fractionation; OS: overall survival; LRC: locoregional control; DC: distant control. p-value in bold indicates statistical significance.
A total of 151 local failures (HF: 69; CF: 82), 101 regional failures (HF: 32; CF: 49), and 47 distant failures (HF: 22; CF: 25) were identified. Salvage surgery was undertaken for 60/99 (61%) cases in the HF and 54/87 (62%) cases in the CF with loco-regional failure (p = 0.84). Grade 3–4 late toxicities were identified in 19/169 (11%) HF versus 25/162 (15%) CF cases.
MVA for LRC confirmed that HF reduced the risk of locoregional failure (LRF) by 35% [Hazard Ratio (HR) 0.65 (0.48–0.89), p = 0.006] after adjusting for age, performance status, disease site, T-category, N-category, and smoking pack-years (Table 2).
Table 2.
Multivariable analysis for overall survival and loco-regional control.
| Outcomes | Variable | Reference | Hazard Ratio (95% Confidence Interval) | p-value |
|---|---|---|---|---|
| Overall Survival | HF | CF | 0.69 (0.55–0.88) | 0.003 |
| Age >60 years | <=60 | 1.36 (1.06–1.73) | 0.014 | |
| Male | Female | 1.22 (0.88–1.67) | 0.228 | |
| ECOG PS 1 ECOG PS 2 ECOG PS 3 |
ECOG PS 0 | 0.98 (0.74–1.30) 1.24 (0.84–1.84) 1.90 (0.97–3.72) |
0.193 | |
| Larynx Oropharynx |
Hypopharynx | 0.55 (0.39–0.78) 0.79 (0.56–1.10) |
0.002 | |
| T2 T3 T4 |
T1 | 2.78 (1.48–5.22) 2.68 (1.42–5.03) 4.03 (2.11–7.72) |
0.002 | |
| N1 N2 N3 |
N0 | 1.47 (1.03–2.09) 1.60 (1.18–2.18) 4.08 (2.25–7.40) |
<0.001 | |
| Smoking pack-years >10 | <=10 | 2.75 (1.55–4.91) | <0.001 | |
| Loco-regional Failure | HF | CF | 0.65 (0.48–0.89) | 0.006 |
| Age (5-year increment) | 1.01 (0.99–1.02) | 0.560 | ||
| Male | Female | 1.66 (1.08–2.55) | 0.020 | |
| ECOG PS 1 ECOG PS 2 ECOG PS 3 |
ECOG PS 0 | 1.29 (0.91–1.83) 1.55 (0.97–2.48) 1.47 (0.67–3.20) |
0.230 | |
| Larynx Oropharynx |
Hypopharynx | 0.57 (0.36-,0.88) 0.72 (0.48–1.09) |
0.043 | |
| T2 T3 T4 |
T1 | 1.98 (0.80–4.90) 2.65 (1.09–6.48) 3.08 (1.21–7.84) |
0.068 | |
| N1 N2 N3 |
N0 | 1.49 (0.94–2.38) 1.80 (1.23–2.62) 3.87 (2.19–6.83) |
<0.001 | |
| Smoking pack-years >10 | <=10 | 2.24 (1.0–5.0) | 0.049 | |
Abbreviation: HF: hyperfractionation; CF: conventional fractionation. p-value in bold indicates statistical significance.
4.2. Survival outcome and mortality profile
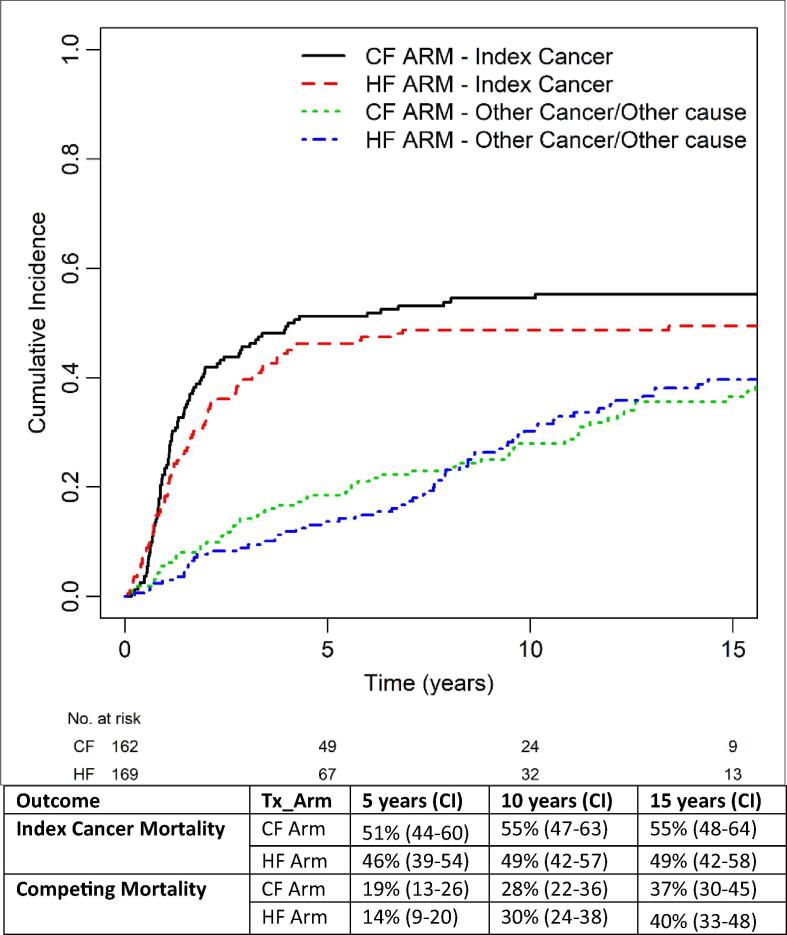
Compared to the CF arm, HF arm had a 10% 5-year improved OS [40% (33–47) vs 30% (23–37)], but the benefit diminished to 3% at 10-years [21% (15–28) vs 18% (12–24)] and 15-years [11% (7–17) vs 8% (4–14)] (p = 0.04). At last follow-up, 149 deaths had occurred in each arm, of which 105 HF and 115 CF deaths manifested within the initial 5 years. Cause of death in this period was mainly from the index cancer (78/105 vs 81/115). After 5-years, the mortality profile evolved (HF vs CF: index cancer: 1 vs 5; other cancer: 23 vs 13; other causes: 18 vs 11, unknown: 6 vs 7). Fig. 3 depicts the changing relative mortality profile over time between CF and HF arms. The cumulative incidence of index cancer death was 5% lower in the HF arm and the magnitude of difference remained over time: 5-year: 46% (39–54) vs 51% (44–60); 10-year: 49% (42–57) vs 55% (47–63); 15-year: 49% (42–58) vs 55% (48–64). Death from competing causes (other cancers or other causes) was also numerically lower in the HF arm at the initial 5-years [14% (9–20) vs 19% (13–26)], but the difference diminished at longer follow-up: 10-year: 30% (24–38) vs 28% (22–36); 15-year: 40% (33–48) vs 37% (30–45). Notably 62/69 (90%) deaths due to second malignancies were potentially related to smoking (i.e. lung, head and neck, esophagus, bladder) while 44/48 (92%) other causes of death were also likely related to smoking: 28/44 died of cardio-vascular diseases; 16/44 died of pulmonary diseases.
Fig. 3.
Evolution of Relative Mortality Profile over Time Note: 90% (62/69) of other cancer deaths were smoking-related Potential for misclassification of 2nd malignancy vs. lung metastasis in the era of the study 92% (44/48) of other cause of death were: “vascular/cardiac” (n = 28) “pulmonary” (n = 16)* * Potentially related to either smoking or treatment toxicity such as silent aspiration ‘Unknown’ cause of death occurrence within first 5 years was attributed to index cancer deaths while those occurring after more than 5 years without evidence of recurrence were classified as deaths from other cause. Abbreviation: Tx: treatment; HF: hyperfractionation; CF: conventional fractionation; OS: overall survival; LRC: locoregional control; DC: distant control.
MVA for OS confirmed that HF reduced the risk of death by 31% [HR 0.69 (0.55–0.88), p = 0.003] after adjusting for age (dichotomized at age 60 years), performance status, disease site, T- and N-category, and smoking pack-years, all of which predicted for survival, with the exception of performance status (likely related to the prominent and confounding influence of age in the model) (Table 2).
5. Discussion
During the 1980′s and 1990′s, no international consensus existed about what should be a “standard” radiotherapy schedule for HNC [24]. HNC was one of the most active disease sites to explore the interaction between tumor and normal tissue in relation to various radiotherapy dose/fractionation regimens, as summarized in the MARCH meta-analysis [1] that included the present trial. Notwithstanding the improved outcome with the HF arm of this study, the current message from the study is not intended to influence practice with a specific RT protocol. Instead this randomized trial is illustrative by remaining the only pure clinical test of the hypotheses of the effects of increased total dose using smaller than conventional doses per fraction that is not also confounded by the influence of accelerated proliferation, since the schedule is completed within 4 weeks, but without excessive dose accumulation that may result in consequential injury with delayed healing of acute toxicity [13], [14], [15].
The long-term outcome analysis of this RCT has confirmed the therapeutic gain with hyper-fractionation radiotherapy: a 13% higher RT dose delivered with smaller fraction size over the same treatment time to permit acute mucosal healing (4 weeks) results in durable improvement in LRF without increasing late toxicity compared to conventional RT. The improvement in LRF also translated into a survival benefit (10% at 5-years) but the magnitude of the survival benefit with HF is reduced to 3% at 10-years and 15-years. This is not surprising given the uniform influence of other co-morbidities and adverse risk factors for long-term survival such as smoking and alcohol use between the two study arms.
Detailed analysis of mortality profile shows that the leading cause of death at the initial 5 years for HNC patient is index cancer death. However, the relative mortality risk profile between both arms continues to evolve. Competing mortality related to smoking and toxicity seems to gradually trump the survival effect in longer follow-up which is consistent with other reports [25], [26]. Shen et al analysed 23,494 HNC patients, where there were 8,454 deaths, but 34.5% of which were due to causes other than index cancer [27]. The same authors estimated cumulative incident functions for cancer-specific mortality and competing mortality in HNC patients and constructed competing risk nomograms. This emphasizes the need for accurate attribution of cause of death to be applied prospectively and consistently over the time-frame of trials for these patients to take account of cancer-specific death. It may be especially relevant to clinical trials, such as HPV-positive non-inferiority deintensification trials which require long-term follow-up, since more than 50% of these patients remain smokers. A recent report suggests a higher non-cancer mortality in patients with HPV-positive tumours with smoking and alcohol use compared to those without such use [28]; these patients also have non-cancer related mortality causes similar to HPV-negative cases.
The benefit of hyperfractionation for LAHNSCC is an example of adapting therapeutic gains based on radiobiological principles [29]. Late responding normal tissues have lower α/β values (typically ≤ 3) compared to HNC [30], and are consequently more fraction size dependent. Therefore, smaller dose fractions should reduce late toxicity to allow higher total RT doses without increasing late toxicity. Several HF schedules have been tested in the clinical trial setting [14], [26], [31], [32], [33]. The present trial schedule, a hyperfractionated accelerated RT schedule with augmented RT dose, is based on Withers et al’s [4] initial observation that head and neck tumor clonogen repopulation accelerates at 4 (±1) weeks after the start of RT, and the benefit of RT dose augmentation is theoretically maximized if it can be delivered safely within the framework of the 4-week schedule. The durable benefit of HF (about 10%) on LRC with comparable late toxicity has been confirmed in this long-term follow-up report. This trial provides compelling clinical evidence of therapeutic gain from hyperfractionated accelerated schedules within the parameters of respecting principles of safe total dose administration, avoidance of unusually short inter-fraction intervals, and minimizing excessive acceleration that can especially injury acutely responding mucosal tissues.
Cure of head and neck cancer is challenged by susceptibility to competing mortality risk since long-term survivors remain vulnerable to smoking-related second primary malignancies and comorbidities, as well as treatment-related toxicities (e.g. aspiration pneumonia, carotid stenosis) [16], [25], [26], [27], [28], [34], [35], [36], [37]. Toxicity related mortality is often difficult to differentiate from mortality related to smoking and challenging to capture and categorize reliably. In this study, we used multiple sources (prospectively completed clinical trial forms, clinical charts, and death certification) to determine the cause of death, but there remain 18 cases (5%) with undetermined mortality cause. For “cause of mortality” ascertained cases, misclassification is still possible during the study period with the interplay of disease recurrence, emergence of second primaries, treatment related toxicity, and other intercurrent diseases relevant to such patients. Bellera et al [38] recommended classifying cause of death into 5 categories in clinical trials according to: a). primary cancer/to progression, b). second cancer, c). protocol treatment, d). other cause, and e). unknown cause of death. The most reliable determination of cause of death is obviously through autopsy, which is not feasible to undertake for all study patients. One study of 53 autopsies of HNSCC patients reported misdiagnosis or underdiagnoses of 34% LRF and 36% distant metastases [39]. This highlights the importance of compiling cause of death prospectively and attributed accurately over the time-frame. If consent can be sought, autopsy could provide additional and more accurate cause of death attribution [40]. Better methods to reflect burden of toxicity, such as the TAME method [41], [42], would also be informative. This is especially important for clinical trials requiring long-term follow up, such as non-inferiority trials for HPV + OPC, which generally require more than 10 years follow up.
One of the limitations of this study is the inability to ascertain tumor HPV status for the OPC patients (40% of entire cohort) enrolled in this trial. Therefore, we are not able to further analyze whether there is imbalance in HPV-related cancer between two arms. However, smoking pack-year data seems balanced between both arms and the majority (93%) patients were heavy smokers. As well, this trial accrued patients from 1988 to 1995 before the dramatically increased incidence of HPV + OPC which now amounts to 4.62 per 100,000 persons in the U.S. and represents the sixth most common incident non-cutaneous cancer in white males <65 years [43]. The chance of HPV status confounding the oncologic outcomes in this trial is therefore low. However, it is conceivable that the mortality profile of HPV-related LAHNSCC might be different from smoking-related LAHNSCC due to differences in risk factors and lifestyle. In fact, recent studies [28] shows that minimal smoking HPV + OPC had a lower competing mortality compared to those with a heavy smoking history. The latter also had non-cancer related mortality causes similar to HPV-negative cases [28]. Therefore, we strongly advocate smoking cessation as a component of the holistic care of head and neck cancer patients to both minimize long term smoking related co-morbidity and mortality as well as to potentially reduce the impact of smoking during radiotherapy which has been prospectively demonstrated to meaningfully influence hypoxia and disease control [44], [45]. Another important element to be considered in the changing profile of HNC concerns increasing age in our populations, which was significant for survival in MVA as has been shown by others [27].
6. Conclusions
This RCT, which has one of the longest follow-up assessment periods in MARCH, confirms that HF with higher dose has a 10% effect size in LRC and OS at 5-years with comparable late toxicity, but non-index cancer death or smoking morbidity eventually trump the survival effect in longer follow-up. Accurate attribution of cause of death should be applied prospectively and consistently over the time-frame of RCTs to take account of cancer specific death. It may be especially relevant to RCTs requiring long-term follow-up in the current milieu of changing HNC demographics including HPV + disease where more than 50% remain smokers and are still vulnerable to smoking or toxicity-related mortality; similarly elderly patients have commenced to comprise a greater proportion of the incident population with HNC and are particularly vulnerable to competing mortality risk from current treatment approaches due to late toxicities or comorbidities. Analysis of long-term survival in the proportion of the HPV cancer population not exposed to other risk factors will have to take into account the potential for increased detrimental survival effects related to the treatment delivered.
Funding statement
This study was supported in part from 1988 to 1991 by a grant from the National Cancer Institute of Canada (NCIC).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the Bartley-Smith/Wharton, the Gordon Tozer, the Wharton Head and Neck Translational, Dr. Mariano Elia, and Petersen-Turofsky Funds, “The Joe & Cara Finley Center for Head & Neck Cancer Research”, and the “Discovery Fund” at the Princess Margaret Cancer Foundation for supporting the authors’ (SHH, JNW, JS, WX, LT, BOS) academic activities. We also acknowledge the O. Harold Warwick Prize of the Canadian Cancer Society for supporting the author’s (BOS) academic activities.
References
- 1.Bourhis J., Overgaard J., Audry H. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 2.Cummings B.J. Benefits of accelerated hyperfractionation for head and neck cancer. Acta Oncol. 1999;38:131–136. doi: 10.1080/028418699431528. [DOI] [PubMed] [Google Scholar]
- 3.Stuschke M., Thames H.D. Fractionation sensitivities and dose-control relations of head and neck carcinomas: analysis of the randomized hyperfractionation trials. Radiother Oncol. 1999;51:113–121. doi: 10.1016/s0167-8140(99)00042-0. [DOI] [PubMed] [Google Scholar]
- 4.Withers H.R., Taylor J.M., Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 5.Slevin N.J., Hendry J.H., Roberts S.A. The effect of increasing the treatment time beyond three weeks on the control of T2 and T3 laryngeal cancer using radiotherapy. Radiother Oncol. 1992;24:215–220. doi: 10.1016/0167-8140(92)90226-k. [DOI] [PubMed] [Google Scholar]
- 6.Maciejewski B., Preuss-Bayer G., Trott K.R. The influence of the number of fractions and of overall treatment time on local control and late complication rate in squamous cell carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1983;9:321–328. doi: 10.1016/0360-3016(83)90290-0. [DOI] [PubMed] [Google Scholar]
- 7.Overgaard J., Hansen H.S., Specht L. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 8.Cummings B., Keane T., Pintilie M. Five year results of a randomized trial comparing hyperfractionated to conventional radiotherapy over four weeks in locally advanced head and neck cancer. Radiother Oncol. 2007;85:7–16. doi: 10.1016/j.radonc.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Waldron J., Warde P., Irish J. A dose escalation study of hyperfractionated accelerated radiation delivered with integrated neck surgery (HARDWINS) for the management of advanced head and neck cancer. Radiother Oncol. 2008;87:173–180. doi: 10.1016/j.radonc.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Ang K.K. Accelerated fractionation: what is the price for speeding? Radiother Oncol. 1997;44:97–99. doi: 10.1016/s0167-8140(97)00117-5. [DOI] [PubMed] [Google Scholar]
- 11.Saunders M.I., Dische S., Grosch E.J. Experience with CHART. Int J Radiat Oncol Biol Phys. 1991;21:871–878. doi: 10.1016/0360-3016(91)90722-g. [DOI] [PubMed] [Google Scholar]
- 12.Skladowski K., Maciejewski B., Golen M. Randomized clinical trial on 7-day-continuous accelerated irradiation (CAIR) of head and neck cancer - report on 3-year tumour control and normal tissue toxicity. Radiother Oncol. 2000;55:101–110. doi: 10.1016/s0167-8140(00)00139-0. [DOI] [PubMed] [Google Scholar]
- 13.Skladowski K., Maciejewski B., Golen M. Continuous accelerated 7-days-a-week radiotherapy for head-and-neck cancer: long-term results of phase III clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:706–713. doi: 10.1016/j.ijrobp.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Horiot J.C., Bontemps P., van den Bogaert W. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol. 1997;44:111–121. doi: 10.1016/s0167-8140(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 15.Jackson S.M., Weir L.M., Hay J.H. A randomised trial of accelerated versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;43:39–46. doi: 10.1016/s0167-8140(97)01944-0. [DOI] [PubMed] [Google Scholar]
- 16.Baxi S.S., Pinheiro L.C., Patil S.M. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507–1513. doi: 10.1002/cncr.28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forastiere A.A., Zhang Q., Weber R.S. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper J.S., Zhang Q., Pajak T.F. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84:1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICRU: Dose, specification for reporting external beam therapy with photons and electrons ICRU Report 29 1978 International Commission on Radiation Units and Measurement Bethesda, MD.
- 20.H. Thames J. Hendry Fractionation in Radiotherapy 1987 Taylor and Francis.
- 21.O'Sullivan B., Warde P., Grice B. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys. 2001;51:332–343. doi: 10.1016/s0360-3016(01)01613-3. [DOI] [PubMed] [Google Scholar]
- 22.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 23.Pintilie M. Analysing and interpreting competing risk data. Stat Med. 2007;26:1360–1367. doi: 10.1002/sim.2655. [DOI] [PubMed] [Google Scholar]
- 24.Withers H.R., Peters L.J., Taylor J.M. Local control of carcinoma of the tonsil by radiation therapy: an analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys. 1995;33:549–562. doi: 10.1016/0360-3016(95)00228-Q. [DOI] [PubMed] [Google Scholar]
- 25.Tiwana M.S., Wu J., Hay J. 25 year survival outcomes for squamous cell carcinomas of the head and neck: population-based outcomes from a Canadian province. Oral Oncol. 2014;50:651–656. doi: 10.1016/j.oraloncology.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Beitler J.J., Zhang Q., Fu K.K. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89:13–20. doi: 10.1016/j.ijrobp.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen W., Sakamoto N., Yang L. Cancer-specific mortality and competing mortality in patients with head and neck squamous cell carcinoma: a competing risk analysis. Ann Surg Oncol. 2015;22:264–271. doi: 10.1245/s10434-014-3951-8. [DOI] [PubMed] [Google Scholar]
- 28.Lop J., Garcia J., Lopez M. Competing mortality in oropharyngeal carcinoma according to human papillomavirus status. Head Neck. 2019;41:1328–1334. doi: 10.1002/hed.25559. [DOI] [PubMed] [Google Scholar]
- 29.Withers H.R. Biological basis of radiation therapy for cancer. Lancet. 1992;339:156–159. doi: 10.1016/0140-6736(92)90218-r. [DOI] [PubMed] [Google Scholar]
- 30.van Leeuwen C.M., Oei A.L., Crezee J. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol. 2018;13:96. doi: 10.1186/s13014-018-1040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dische S., Saunders M., Barrett A. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;44:123–136. doi: 10.1016/s0167-8140(97)00094-7. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen M.G., Denham J.W., Peters L.J. A randomised trial of accelerated and conventional radiotherapy for stage III and IV squamous carcinoma of the head and neck: a Trans-Tasman Radiation Oncology Group Study. Radiother Oncol. 2001;60:113–122. doi: 10.1016/s0167-8140(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 33.Horiot J.C., Le Fur R., N'Guyen T. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25:231–241. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 34.Kwon M., Roh J.L., Song J. Noncancer health events as a leading cause of competing mortality in advanced head and neck cancer. Ann Oncol. 2014;25:1208–1214. doi: 10.1093/annonc/mdu128. [DOI] [PubMed] [Google Scholar]
- 35.Mell L.K., Dignam J.J., Salama J.K. Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol. 2010;28:15–20. doi: 10.1200/JCO.2008.20.9288. [DOI] [PubMed] [Google Scholar]
- 36.Montero-Miranda P.H., Ganly I. Survivorship–competing mortalities, morbidities, and second malignancies. Otolaryngol Clin North Am. 2013;46:681–710. doi: 10.1016/j.otc.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Rose B.S., Jeong J.H., Nath S.K. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;29:3503–3509. doi: 10.1200/JCO.2011.35.7301. [DOI] [PubMed] [Google Scholar]
- 38.Bellera C.A., Penel N., Ouali M. Guidelines for time-to-event end point definitions in sarcomas and gastrointestinal stromal tumors (GIST) trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials)dagger. Ann Oncol. 2015;26:865–872. doi: 10.1093/annonc/mdu360. [DOI] [PubMed] [Google Scholar]
- 39.Jennings C.R., Bradley P.J. Are autopsies useful? Do premorbid findings predict postmortem results in head and neck cancer patients? Ann R Coll Surg Engl. 2002;84:133–136. [PMC free article] [PubMed] [Google Scholar]
- 40.Sano Hajime, Nitta Mitsukuni, A Meijin Nakayam, Yao Kazuo, Nagai Hirom I, Makoshi Tomohiro, Inagi Katsuhide, Takahashi Hiroomi, Okamoto Makito. Clinical review of autopsy cases that succumbed to head and neck malignancies. Acta Otolaryngol. 2002;122(4):64–66. doi: 10.1080/000164802760057608. [DOI] [PubMed] [Google Scholar]
- 41.Bentzen S.M., Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25:4096–4103. doi: 10.1200/JCO.2007.13.3983. [DOI] [PubMed] [Google Scholar]
- 42.Trotti A., Pajak T.F., Gwede C.K. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol. 2007;8:613–624. doi: 10.1016/S1470-2045(07)70144-4. [DOI] [PubMed] [Google Scholar]
- 43.Mahal B.A., Catalano P.J., Haddad R.I. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28:1660–1667. doi: 10.1158/1055-9965.EPI-19-0038. [DOI] [PubMed] [Google Scholar]
- 44.Hoff C.M., Grau C., Overgaard J. Effect of smoking on oxygen delivery and outcome in patients treated with radiotherapy for head and neck squamous cell carcinoma–a prospective study. Radiother Oncol. 2012;103:38–44. doi: 10.1016/j.radonc.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Smith J., Nastasi D., Tso R. The effects of continued smoking in head and neck cancer patients treated with radiotherapy: A systematic review and meta-analysis. Radiother Oncol. 2019;135:51–57. doi: 10.1016/j.radonc.2019.02.021. [DOI] [PubMed] [Google Scholar]