Abstract
Follicular thyroid carcinoma (FTC) is a common endocrine malignancy with highly aggressive features. In this study, next-generation sequencing technology was used to identify aberrant expression of sialyltransferase (ST) family members in FTC. Aberrant high expression of alpha-2,6-sialyltransferase 2 (ST6GAL2) was demonstrated to promote tumorigenesis of FTC in vitro and in vivo. Furthermore, ST6GAL2 promoted tumorigenesis by inactivating the Hippo signaling pathway. Resveratrol is a native compound extracted from Vitis species, and many studies have confirmed its protective cardiovascular and antineoplastic effects. Here we found that resveratrol can inhibit the tumorigenesis of FTC by suppressing the expression of ST6GAL2, further activating the Hippo pathway. In summary, this study revealed the role of the ST6GAL2-Hippo signaling pathway in FTC tumorigenesis and indicated that resveratrol, a commonly found antineoplastic compound, could inhibit tumorigenesis of FTC by regulating the abovementioned pathways.
Keywords: follicular thyroid carcinoma, resveratrol, Hippo signaling pathway, sialyltransferase families
Introduction
Thyroid cancer is the most common endocrine malignancy, and it is also the fastest-growing solid tumor.1 During the past few decades, the annual percentage change (APC) in the incidence rate of thyroid cancer has increased substantially worldwide.2 Follicular thyroid carcinoma (FTC) is one of four pathological types of thyroid cancer, with a morbidity rate ranked second only to that of papillary thyroid carcinoma (PTC). In terms of cancer characteristics, FTC is more aggressive and grows faster than PTC, and FTC is difficult to diagnose without paraffin sections.3,4 Because FTC is highly aggressive and readily undergoes hematogenous metastasis, most FTC patients need immediate treatment, such as total thyroidectomy.1 Although most patients’ prognoses are satisfactory because of timely and accurate therapies, postoperative scars and lifelong medication regimens are permanent and unavoidable. In addition, it has been shown that surgical treatment for patients with distant metastasis (including lung, bone, and brain metastases) is ineffective or detrimental.5, 6, 7, 8, 9 In addition it, there is a need for development of alternative therapeutic interventions to treat and control the progression of FTC.
Recently, sialyltransferase (ST) families have been reported to be critical cancer-regulating factors.10 Sialylation is a key process in eukaryotic cells that has been shown to act in cell recognition, cell adhesion, receptor activation, and protein stability.11 Therefore, STs contribute greatly to tumor development by acting as catalysts of sialylation. STs are categorized into four families: ST3GAL, ST6GAL, ST6GALNAC, and ST8SIA.12 As reported, ST6GAL1 and ST3GAL6 regulate tumorigenesis of human colorectal carcinoma,13,14 ST6GALNAC2 regulates breast cancer invasion,15 and ST8SIA2 regulates cell invasion of non-small cell lung cancer.16 Moreover, similar findings were reported in our previous study, which showed that alpha-2,6-sialyltransferase 2 (ST6GAL2) is highly expressed in FTC and regulated by the long noncoding RNA-HLA complex P5 (HCP5).17 However, the downstream regulatory mechanisms of ST6GAL2 in FTC are not clear.
Resveratrol is a polyphenolic phytoalexin extracted primarily from grapes and is well known for its protective cardiovascular effects and antineoplastic activity.18,19 Resveratrol’s antineoplastic effects have been confirmed in hematological and solid tumors during the last decade.20 Regarding thyroid cancer, previous studies have shown that resveratrol has preventive potential in a murine model of carcinogen-induced thyroid cancer.21 Moreover, resveratrol acts as an antineoplastic agent in poorly differentiated anaplastic thyroid carcinoma (ATC) by inducing redifferentiation.22 However, the effects of resveratrol on highly invasive FTC have not been reported. In our preliminary experiment, we measured the effects of various Chinese medicine compounds on FTC cells and finally screened resveratrol for its inhibitory effect on FTC tumorigenesis. However, the mechanism underlying resveratrol’s inhibitory effect on FTC cells is not clear.
The Hippo signaling pathway has been reported to be a key regulator in development of different types of cancer, including thyroid cancer.23, 24, 25, 26, 27, 28 Yes-associated protein (YAP), the key member of the Hippo signaling pathway, has been implicated as an oncogene in the regulation of tumor suppression concerning tissue repair and regeneration.29 When the Hippo signaling pathway is activated, YAP is phosphorylated into p-YAP; p-YAP cannot enter the nucleus and bind to TEA domain transcription factor (TEAD) to exert transcriptional regulation activities, contrary to YAP.30 Therefore, activation of the Hippo signaling pathway inhibits tumor progression.
This study aims to reveal new pathogenic mechanisms of FTC and proposes measures for intervention. Here we reveal a mechanism that involves ST6GAL2 in promoting FTC tumorigenesis. Furthermore, our study of FTC drug interventions revealed that resveratrol can inhibit FTC tumorigenesis via a mechanism possibly related to direct action toward ST6GAL2 and the Hippo signaling pathway.
Results
ST6GAL2 Is Aberrantly Overexpressed in FTC
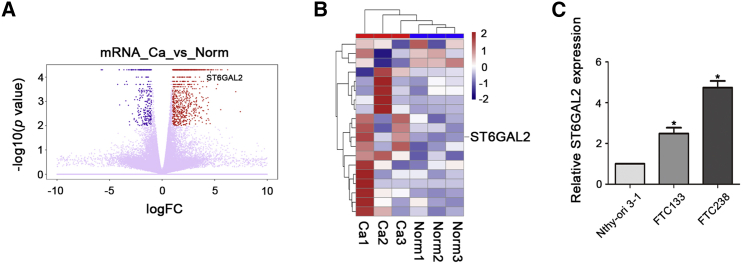
To investigate the role of STs in the development of FTC, we performed next-generation sequencing and compared mRNA expression in FTC patient samples with that in normal adjacent tissue samples obtained from the same patient. Analysis of the sequencing data revealed significant changes in mRNA expression profile in FTC patient samples compared with that in normal samples (Figures 1A and 1B). A fold change of greater than or equal to 2 is considered a significant change, and compared with the mRNAs of other members of the ST family, ST6GAL2 mRNA exhibited dramatically upregulated expression in FTC (fold change = 2.29387, p = 0.00005, q value = 0.005485). In addition, we used qPCR to examine ST6GAL2 expression in three kinds of cells: normal thyroid cells (Nthy-ori 3-1) and two kinds of FTC cells with different degrees of invasiveness (FTC133 and FTC238). According to the qPCR results, ST6GAL2 was overexpressed in FTC cells relative to its expression in Nthy-ori 3-1 cells and was especially overexpressed in highly invasive FTC238 cells compared with minimally invasive FTC133 cells (Figure 1C). The qPCR results were consistent with the sequencing results, showing that ST6GAL2 is aberrantly overexpressed in FTC. Thus, we hypothesized that the aberrantly high expression of ST6GAL2 may contribute to the progression of FTC.
Figure 1.
ST6GAL2 Expression Is Upregulated in FTC
(A and B) The heatmaps and volcano plot show that ST6GAL2 expression was upregulated in FTC tissues. (C) According to the qPCR results, ST6GAL2 was overexpressed in FTC cells, especially in FTC238 cells. *p < 0.05; scale bars, 20 μm.
ST6GAL2 Can Regulate Tumorigenesis of FTC In Vivo and In Vitro
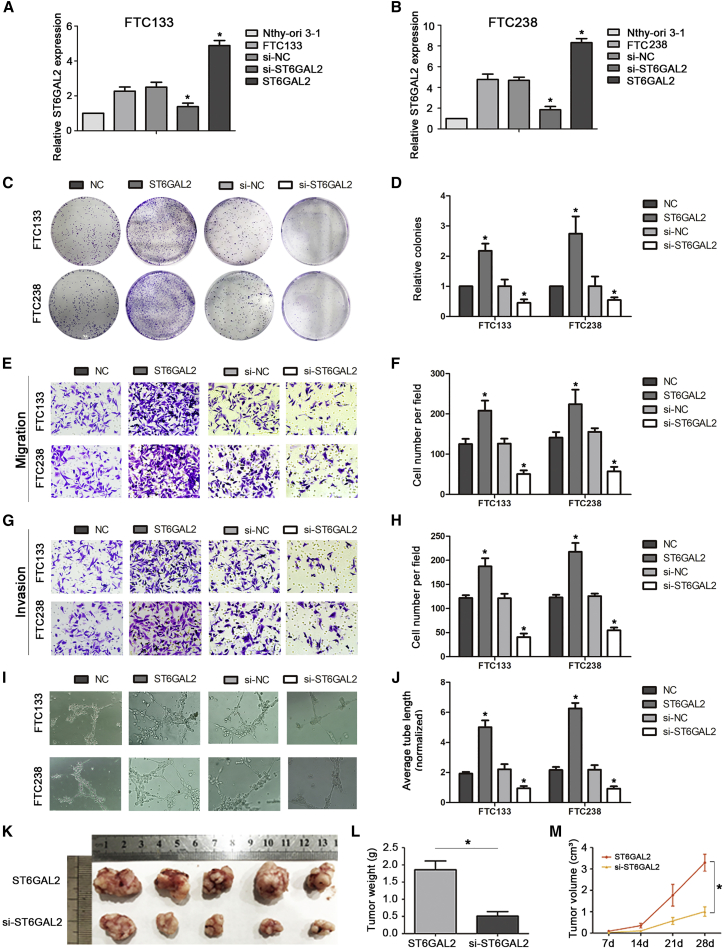
In our previous study, we found that ST6GAL2 is aberrantly highly expressed in FTC and that this expression pattern may be regulated by HCP5 via microRNA (miRNA) sponging.17 However, the regulatory mechanism of ST6GAL2 in FTC has not been studied. To further explore the potential role of ST6GAL2 in FTC, we constructed ST6GAL2-small interfering RNA (siRNA) and ST6GAL2 overexpression plasmids, the efficiency of transfection is ideal (Figures 2A and 2B). The colony formation assay showed that overexpression of ST6GAL2 promoted cell proliferation, whereas inhibition of ST6GAL2 reduced cell proliferation in both FTC133 and FTC238 cells (Figures 2C and 2D). Next we evaluated cancer cell migration and invasion using Transwell-based assays. Cell migration and invasion were augmented in the presence of ST6GAL2 but suppressed in the group with ST6GAL2 inhibition (Figures 2E–2H). Angiogenesis is crucially correlated with tumor growth and metastasis. Thus, a capillary tube formation (CTF) assay was used to assess the effect of ST6GAL2 on tumor growth and metastasis. The length of the tubes was increased by proangiogenic stimuli in the supernatant of ST6GAL2-overexpressing cells, and the opposite results were found in si-ST6GAL2-transfected cells (Figures 2I and 2J). Together, these results indicate that ST6GAL2 promotes tumorigenesis of FTC cells.
Figure 2.
ST6GAL2 Can Regulate the Tumorigenesis of FTC In Vitro and In Vivo
(A and B) ST6GAL2 expression was downregulated and upregulated in the ST6GAL2 and si-ST6GAL2 groups of FTC133 and FTC238 cells, respectively. (C and D) ST6GAL2 expression regulated the proliferation of FTC cells, as evidenced by colony formation assays. (E–J) ST6GAL2 promoted development of FTC via enhancement of the migration, invasion, and angiogenesis capacity of FTC cells, whereas the opposite results were found in si-ST6GAL2-transfected FTC cells. (K and L) Tumor weight was measured after the tumors were removed. (M) Tumor growth curves were measured after injection of FTC238 cells transfected with ST6GAL2 or si-ST6GAL2. *p < 0.05; scale bars, 20 μm.
To further investigate the effect of ST6GAL2 on tumorigenesis of FTC, an athymic nude mouse subcutaneous xenograft model was established. We selected the more aggressive FTC238 cells to establish the models. As presented in Figures 2K–2M, compared with overexpression of ST6GAL2, downregulation of ST6GAL2 significantly decreased the size and inhibited the growth rate of tumors generated by FTC238 cells. Accordingly, the weights and volumes of ST6GAL2-overexpressing tumors were significantly higher than those of si-ST6GAL2-expressing tumors. Collectively, these results suggest that ST6GAL2 significantly regulates the proliferative capacity of FTC cells in vivo and in vitro and controls FTC cell migration, invasiveness, and angiogenesis in vivo.
ST6GAL2 Inactivates the Hippo Signaling Pathway in FTC
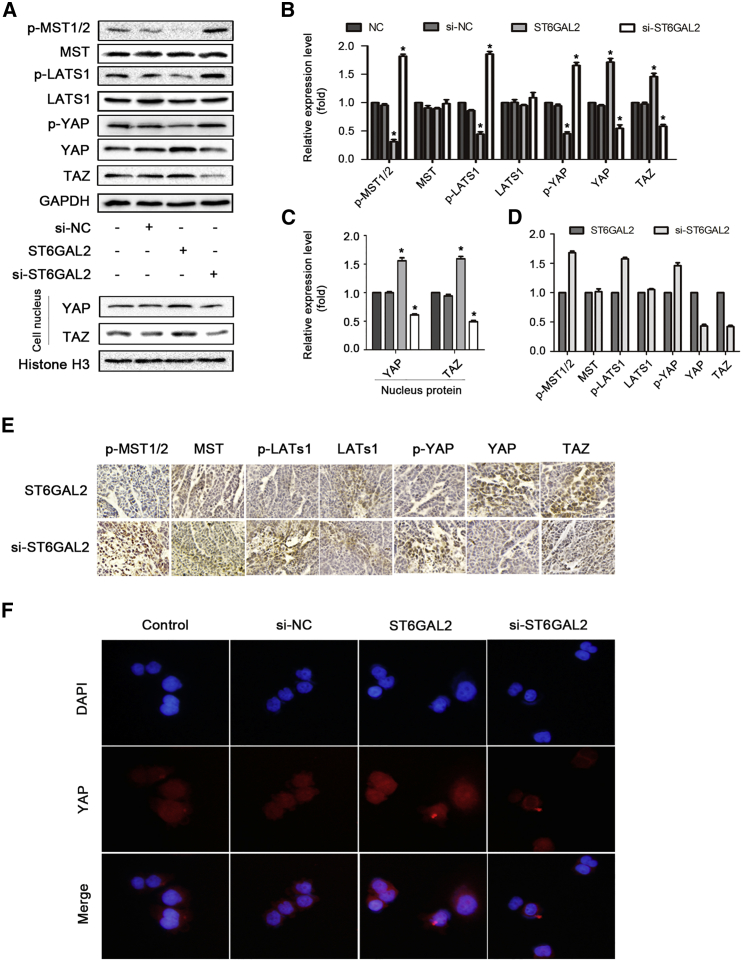
The Hippo signaling pathway is a recognized tumor suppressor pathway.31 Accumulating evidence indicates that this pathway plays important roles in pathogenic mechanisms of cancer, including tumorigenesis and metastasis.32 In this study, the expression of Hippo family members, including p-MST1/2, mamma-lian STE20-like protein kinase 9 MST, p-LATS1, LATS1, p-YAP, YAP, and TAZ, was investigated by western blot analysis. The results revealed that overexpression of ST6GAL2 reduced the p-MST1/2, p-LATS1, and p-YAP levels, increased the YAP and TAZ levels, and did not affect the total expression levels of MST1/2 and LATS1 in FTC cells (Figures 3A and 3B). Moreover, the immunofluorescence (immunohistochemistry [IHC]) staining assay demonstrated that ST6GAL2 upregulation enhanced but ST6GAL2 silencing reduced nuclear translocation of YAP and TAZ (Figure 3F). An IHC staining assay was performed and indicated that the Hippo pathway is inactivated by ST6GAL2 overexpression. However, ST6GAL2 silencing yielded the opposite effect (Figures 3D and 3E). Thus, these results demonstrate that ST6GAL2 inactivates the Hippo signaling pathway in FTC.
Figure 3.
ST6GAL2 Expression Regulates the Hippo Signaling Pathway
(A–C) Expression of the main protein components of the Hippo signaling pathway was investigated in FTC 238 cells by western blotting. Shown is Western blot analysis of nuclear YAP and TAZ expression in the indicated cells. The nuclear protein histone H3 was used as the nuclear protein marker. (D and E) IHC staining was performed to determine the expression levels of Hippo pathway signaling molecules in tumor tissues. (F) Representative immunofluorescence images of FTC238 cells immunostained with an anti-YAP antibody (red). *p < 0.05; scale bars, 20 μm.
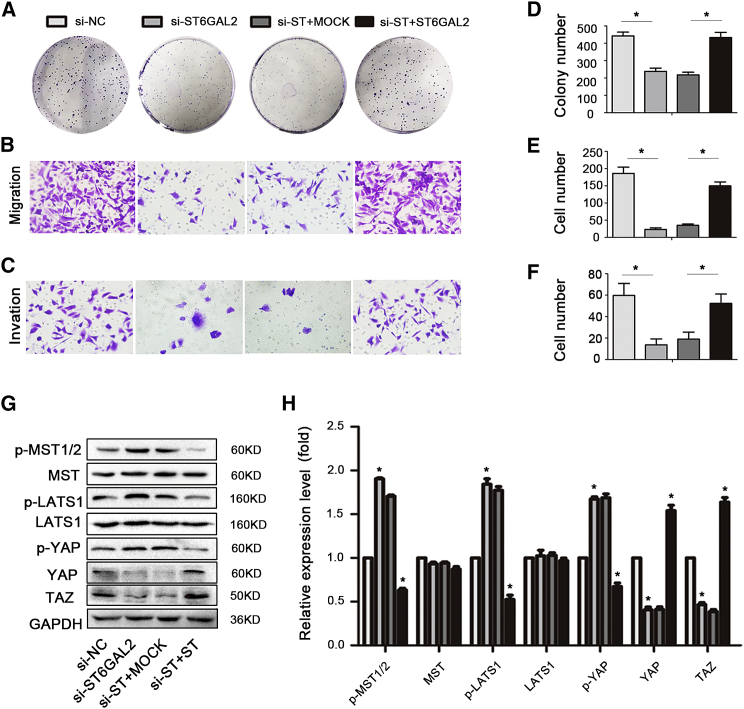
To further validate the regulatory relationship between ST6GAL2 and the Hippo signaling pathway in FTC cell growth and metastasis, ST6GAL2-silenced FTC238 cells were transfected with ST6GAL2 overexpression vectors. Reintroduction of ST6GAL2 into si-ST6GAL2-transfected cells significantly restored the levels of p-MST1/2, MST, p-LATS1, LATS1, p-YAP, YAP, and TAZ (Figures 4G and 4H). The colony formation assay results showed that the colony-forming capacity of ST6GAL2-rescued cells was increased (Figures 4A and 4D). In addition, the migration and invasion abilities of ST6GAL2-rescued cells were enhanced compared with those of si-ST6GAL2-transfected cells (Figures 4B, 4C, 4E, and 4F). Taken together, these findings confirmed that downregulation of ST6GAL2 inactivated the Hippo signaling pathway and reduced the proliferation, migration, and invasion abilities of FTC cells.
Figure 4.
Upregulation of ST6GAL2 Rescues Tumorigenesis of FTC238 Cells and Resuppresses Hippo Signaling Pathway Activity
(A–F) ST6GAL2 knockdown cells were transfected with ST6GAL2 overexpression vectors, and the proliferation, migration, and invasion capacities of FTC cells were enhanced. (G and H) Western blotting was performed to determine the levels of Hippo signaling molecules in FTC cells. *p < 0.05; scale bars, 20 μm.
Resveratrol Suppresses the Tumorigenesis of FTC In Vivo and In Vitro
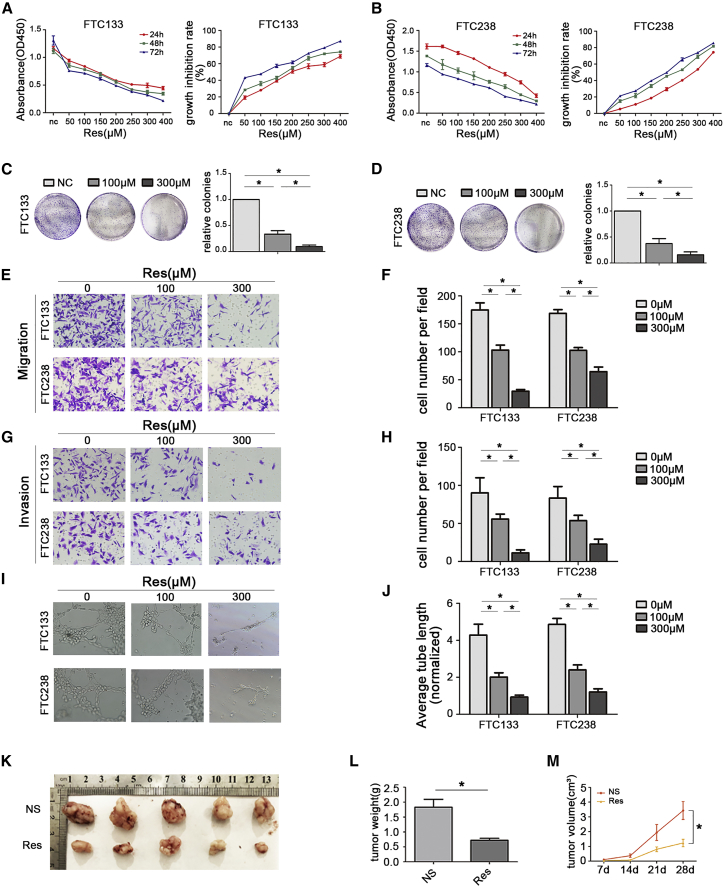
Resveratrol is recognized for its anticancer effects in many kinds of solid tumors.33 Among thyroid cancers, resveratrol has been reported to inhibit ATC22 and to exhibit antineoplastic activity in murine thyroid cancer models.21 Many drug compounds have been reported to have the same antitumor effects as resveratrol. Furthermore, our preliminary experiments showed that, as expected for resveratrol, some of these compounds, such as sinomenine, ginsenoside-Rg1, and ginsenoside-Rb2, did not affect the tumorigenesis of FT (data not shown). To further explore the relationship between resveratrol and FTC cells, the two FTC cell lines FTC133 and FTC238 were treated with different concentrations of resveratrol for 24 h, 48 h, and 72 h. Cell Counting Kit-8 (CCK-8) assays showed that resveratrol treatment significantly reduced the viability of FTC cells compared with that of control cells in a dose-dependent and time-dependent manner (Figures 5A and 5B). The 50% growth-inhibitory concentration (IC50) values for FTC133 and FTC238 cells at 24 h were 199.23 μM and 301.56 μM, respectively. To analyze the long-term effect of resveratrol treatment on the survival of FTC cells, colony formation assays were performed. The results revealed that resveratrol treatment led to a dramatic decrease in the number of colonies formed (Figures 5C and 5D). Together, these results indicate that treatment with resveratrol has an obvious effect on FTC cells and that resveratrol inhibits cell growth in a dose-dependent and time-dependent manner.
Figure 5.
Res Reduces Tumorigenesis of FTC In Vitro and In Vivo
(A and B) CCK-8 viability assays were performed to determine the effective concentration of Res. (C–J) Res suppressed tumorigenesis of FTC by reducing the proliferation, migration, invasion, and angiogenesis capacities of FTC cells. (K and L) Tumor weight was measured after the tumors were harvested. (M) Tumor growth curves were measured after injection of FTC238 cells. Each nude mouse was injected with NS or Res solution once every 2 days. *p < 0.05; scale bars, 20 μm.
Because we observed a significant change in cell proliferation during treatment with resveratrol, we performed a series of assays to address whether resveratrol treatment affected migration, invasiveness, and angiogenesis in FTC cells. Transwell migration assays were performed to evaluate the migration ability of FTC133 and FTC238 cells treated with the indicated concentrations of resveratrol for 24 h. The migration ability of FTC133 and FTC238 cells was significantly reduced after resveratrol gradient treatment (Figures 5E and 5F). Similarly, Transwell assays with Matrigel showed that the invasion capability of FTC133 and FTC238 cells was blocked during resveratrol treatment (Figures 5G and 5H). Moreover, CTF by human umbilical vein endothelial cells (HUVECs) was assessed to determine the angiogenesis ability of FTC133 and FTC238 cells after treatment with different concentrations of resveratrol, and the results indicated that resveratrol treatment inhibited CTF by these cells (Figures 5I and 5J).
To investigate the in vivo activity of resveratrol in FTC, an athymic nude mouse subcutaneous xenograft model was established with the more aggressive FTC238 cells. As shown in Figures 5K–5M, administration of resveratrol to nude mice inhibited the size and growth rate of tumors generated by FTC238 cells in vivo. Accordingly, the weights and volumes of tumors in the normal saline (NS) group were significantly greater than those in the resveratrol (Res) group. Collectively, these data suggest that Res treatment can effectively attenuate the migration, invasion, and angiogenic capacities of FTC cells in a dose-dependent manner in vivo and in vitro.
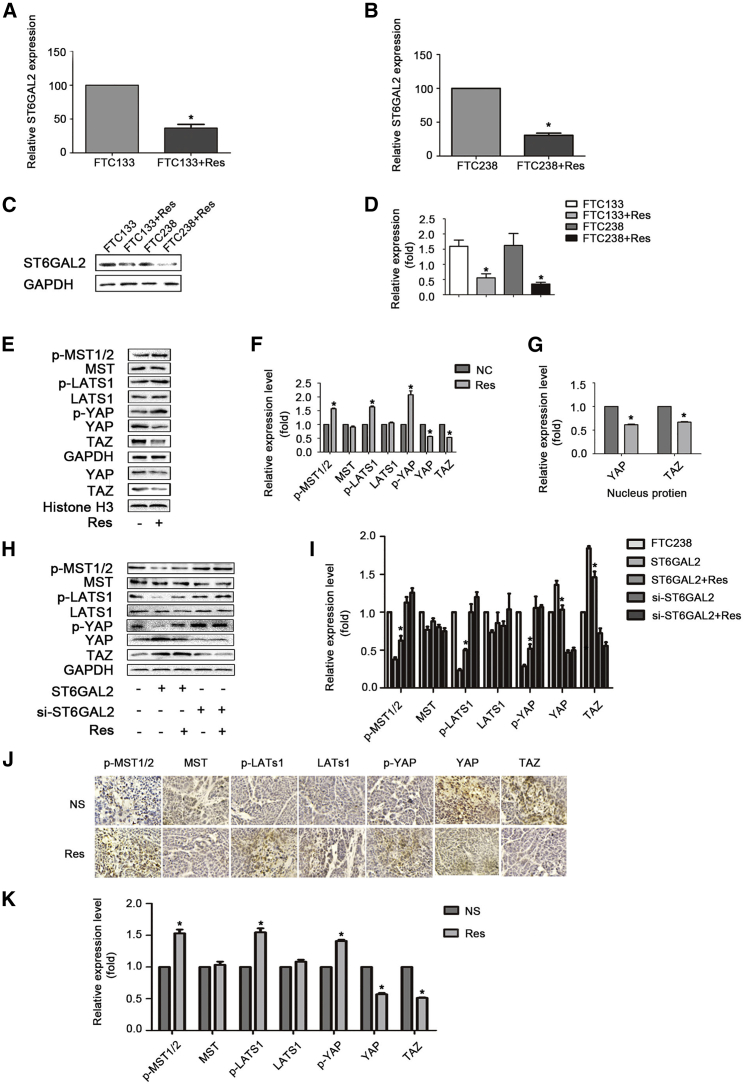
Res Reduces the Expression of ST6GAL2 and Activates the Hippo Signaling Pathway in FTC Cells
Based on the above research results, we hypothesized that the regulatory mechanism of Res is related to the ST6GAL2 pathway. To investigate this relationship, we examined the expression of ST6GAL2 in FTC133 and FTC238 cells treated with Res (300 μM, 24 h). The qPCR and western blot results showed that ST6GAL2 expression was significantly reduced after Res treatment compared with that in control FTC133 and FTC238 cells (Figures 6A–6D), indicating that Res plays a role in downregulating the expression of ST6GAL2 in FTC. To further demonstrate the molecular mechanisms of Res intervention in FTC, western blotting was performed to assess the expression of molecules in the Hippo signaling pathway and showed that Res activated the Hippo signaling pathway in FTC cells (Figures 6E–6G). An IHC staining assay was performed to assess the expression of molecules in the Hippo signaling pathway in the NS group and the Res group. FTC tumor tissues exhibited activation of the Hippo signaling pathway after Res treatment (Figures 6H and 6I). Accordingly, Res treatment can activate the Hippo pathway, most likely because of the reduction in ST6GAL2 expression.
Figure 6.
Res Reduces ST6GAL2 Expression and Activates the Hippo Signaling Pathway in FTC Cells
(A–D) The qPCR and western blotting results indicated that ST6GAL2 expression changes after Res treatment in FTC cells. (E–G) Expression of the main protein components of the Hippo signaling pathway in FTC238 cells was assessed by western blotting. Also shown is western blot analysis of nuclear YAP and TAZ expression in the indicated cells. The nuclear protein histone H3 was used as the nuclear protein marker. (H and I) IHC staining was performed to determine the expression levels of Hippo pathway signaling molecules in tumor tissues. *p < 0.05; scale bars, 20 μm.
Discussion
FTC is a highly aggressive malignant carcinoma of the thyroid.34 In this study, we aim to reveal new pathogenic mechanisms of FTC and propose intervention measures. We provide first evidence for three findings. (1) ST6GAL2 was aberrantly overexpressed in FTC. (2) Overexpression of ST6GAL2 promoted tumorigenesis of FTC in vitro and in vivo, and these effects may be attributed to suppression of the Hippo signaling pathway. (3) Res acted as a ST6GAL2 inhibitor to suppress the tumorigenesis of FTC, and these effects may occur through Res-induced inhibition of ST6GAL2-YAP/TAZ signaling.
Aberrant cell surface glycosylation is prevalent in various cancers and has been demonstrated to play a role in tumorigenesis.11 STs are glycosyltransferases that control the linkage and level of cell-surface sialic acids, playing key roles in many biological processes of human disease, including multiple solid tumors.10 STs are categorized into four families: ST6GAL, ST3GAL, ST6GALNAC, and ST8SIA). Previous studies have reported that ST6GALNAC2 acts as a suppressor of breast cancer metastasis and can also be used for early detection of human colorectal carcinomas. According to our previous reports, ST6GAL1 and ST8SIA2 regulate the chemoresistance of human hepatoma cells, and ST3GAL6 mediates cell growth and invasion in human hepatoma cells. In addition, ST6GALNAC2 regulates breast cancer invasion. In summary, members of ST families are involved in the basic molecular and cellular biological processes that occur in several cancers.14,15,17,35, 36, 37, 38 However, the expression levels and functions of STs in FTC are not clear. Therefore, we used next-generation sequencing of clinical FTC samples to determine the expression levels of ST family members in FTC. The expression of ST6GAL2 was significantly increased in FTC compared with ST family members. Our previous research showed that ST6GAL2 had an obvious regulatory effect during the occurrence and progression of FTC. In the current study, in vitro experiments showed that ST6GAL2 had a regulatory effect on the proliferation, migration, and invasion ability of tumors. To further verify this hypothesis, we performed in vivo experiments and confirmed that upregulation of ST6GAL2 can increase the proliferation of FTC cells.
Hippo signaling pathway dysregulation has been reported in the occurrence and development of multiple human cancers.30 This pathway is activated via YAP and TAZ phosphorylation under normal conditions. However, its dysregulation is an important cause of tumor occurrence, progression, and drug resistance.39 We found that the expression levels of YAP/TAZ increased when ST6GAL2 was overexpressed in FTC cells. Therefore, we hypothesized that the regulatory mechanism of ST6GAL2 in FTC operated via inactivation of the Hippo signaling pathway, and the results of western blotting and IHC staining for Hippo signaling pathway proteins confirmed our hypotheses. YAP/TAZ enter the nucleus and induce the transcriptional activity of TEAD1–TEAD4 as transcriptional coactivators, which further upregulates multiple downstream effectors to play a pleiotropic role in tumor progression and metastasis.40 We performed an immunofluorescence assay to assess the nuclear expression of YAP/TAZ and showed that nuclear expression of YAP/TAZ increased or decreased with increased or decreased expression of ST6GAL2, respectively. Thus, our results reveal a novel mechanism by which ST6GAL2 can inactivate the Hippo pathway and promote tumorigenesis of FTC cells.
Res, a nontoxic compound obtained mostly from grapes, has been proven to have appreciable anticancer effects in diverse cancers.20 This compound has multiple molecular targets, including those involved in proliferation, survival, and death of cancer cells. For instance, Res enhances the rate of 131I-induced death in thyroid cancer cells and suppresses the growth of and overcomes retinoic acid resistance in human anaplastic thyroid cancer cells.22 However, previous studies have not reported the effect of Res on FTC. Our experiment confirmed that Res can inhibit tumorigenesis of FTC in vitro and in vivo. In addition, the expression of ST6GAL2, which was aberrantly high in FTC cells, decreased after treatment with Res. Moreover, the effect of Res was diminished in FTC cells with artificially high expression of ST6GAL2. Therefore, Res may attenuate tumorigenesis of FTC through inhibition of ST6GAL2 expression. In addition, as shown in the Figure S1, the anti-proliferation effect of Res was almost abrogated in ST6GAL2 knockdown cells. Thus, we suggest that the inhibitory pathway of Res is through ST6GAL2, although the ST6GAL2 pathway might not be the only target of Res. Furthermore, the Hippo signaling pathway was activated after treatment with Res in vitro and in vivo. Thus, our results indicate that Res can inhibit FTC tumorigenesis and that this mechanism may be related to regulation of the ST6GAL2-Hippo pathway. Conversely, the ST6GAL2-Hippo pathway may be not the only pathway through which Res works, which should be explored further.
In conclusion, ST6GAL2 plays an important role in promoting tumorigenesis of FTC, at least in part by ST6GAL2-regulated inactivation of the Hippo signaling pathway. Res has an effect on the ST6GAL2-Hippo pathway and significantly inhibits tumorigenesis of FTC. Although the specific mechanism of Res in FTC requires further experimentation, our experimental findings might provide a therapeutic pathway toward FTC remission for patients who are intolerant to operation or in whom FTC diagnosis is difficult.
Materials and Methods
Tissue Collection
FTC samples were obtained from 3 patients who provided informed consent in accordance with the ethical standards of the Second Hospital of Dalian Medical University (Dalian, China) review board. Adjacent normal thyroid tissue samples were obtained from the same patients and taken from normal thyroid tissue more than 2 cm away from the tumorous foci. All samples were reviewed by a pathologist and were histologically confirmed as FTC based on histopathological evaluation. No local or systemic treatments were administered to these patients before surgery.
Cell Culture
The human thyroid cell lines Nthy-ori 3-1 and FTC133 were obtained from Jennio Biotech (Guangdong, Guangzhou, China). FTC238 cells were purchased from the European Collection of Authenticated Cell Cultures (ECACC) (Shanghai, China). HUVECs were obtained from the Institute of Biochemistry (Shanghai, China). Nthy-ori 3-1 and FTC133 cells and HUVECs were cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco) and 1% penicillin-streptomycin liquid (Solarbio, Beijing, China). FTC238 cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% FBS and 1% penicillin-streptomycin liquid (Solarbio). All cells were maintained in an incubator at 37°C and 5% CO2.
Animals
Athymic nude mice (aged 6 weeks) were purchased from the specific pathogen free (SPF) Animal Experimental Center of Dalian Medical University. The animal care and experimental procedures were reviewed and approved by the Institutional Animal Care and Ethics Committee of Dalian Medical University. After 1 week of adaptation, 20 nude mice were divided into 4 groups and subcutaneously injected with FTC238 cells (NS group and Res group), FTC238+ST6GAL2 cells (group ST6GAL2), or FTC238+si-ST6GAL2 cells (group si-ST6GAL2) in the right groin (1 × 107 cells in 200 μL of PBS). After 1 week, the tumors had grown to a measurable size. Mice in the NS and Res groups were treated with NS and Res solution (40 mg/kg, intraperitoneal), respectively, at 2-days intervals. Tumor length (L) and width (W) were measured weekly with calipers. Tumor volume (V) was calculated using the equation V = LW2/2. Mice were sacrificed 4 weeks after tumor injection. All tumors were harvested within 15 min after death of the nude mice and placed in liquid nitrogen for rapid freezing.
Next-Generation Sequencing
Next-generation sequencing was performed by Novogene (Beijing, China). The samples we used were obtained from the Second Hospital of Dalian Medical University. A total of 3 μg of RNA per sample was used as input material for the RNA sample preparations. RNA degradation and contamination were monitored on 1% agarose gels. RNA purity was checked using a NanoPhotometer spectrophotometer (Implen, CA, USA). RNA concentration was measured using the Qubit RNA Assay Kit in a Qubit 2.0 fluorometer (Life Technologies, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit and a Bioanalyzer 2100 system (Agilent Technologies, CA, USA). The Ballgown suite includes functions for interactive exploration of the transcriptome assembly and visualization of transcript structures, an abundance of features specific for each locus, and post hoc annotation of assembled features to annotated features. Transcripts with an adjusted p value of less than 0.05 were considered differentially expressed. The sample size consisted of three tissue samples per group.
PCR Analysis
We used the Total RNA Extraction Kit (Solarbio) to isolate total RNA from cell lines and then used the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Otsu, Shiga, Japan) to reverse-transcribe RNA into cDNA. Real-time PCR analysis was performed using SYBR Green (Takara, Otsu, Shiga, Japan), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the quantitative standard. The respective sequences of the upstream and downstream primers were 5′-ACG CTG CTG ATT GAC TCT TCT-3′ and 5′-CAC ATA CTG GCA CTC ATC TAA-3′ for ST6GAL2 and 5′-CTC CTC CAC CTT TGA CGC TG-3′ and 5′-TCC TCT TGT GCT CTT GCT GG-3′ for GAPDH.
IHC Staining Analysis
Tumor tissues from nude mice were fixed, embedded in paraffin, sectioned, and stained using hematoxylin and eosin. Paraffin sections were placed in an oven at 60°C for 30 min. Sections were dewaxed and rehydrated, followed by antigen retrieval with sodium citrate. Sections were incubated with 3% H2O2 and blocked using goat serum. Next, sections were incubated overnight at 4°C with anti-p-MST1/2, anti-MST, anti-p-LATS1, anti-LATS1, anti-p-YAP, anti-YAP, and anti-TAZ antibodies (all at 1:150 dilution). After washing with PBS, sections were incubated with a biotinylated secondary antibody for 30 min at room temperature. Subsequently, sections were treated with 3,3-Diaminobenzidine Tetrahydrochloride (DAB) for cascade amplification and counterstained with hematoxylin for 5 s. Next, sections were dehydrated using a gradient alcohol series and covered with coverslips. Finally, slides were mounted and visualized by microscopy.
Cell Viability Assay
Cells in logarithmic growth phase were digested by trypsin and uniformly seeded in a 96-well plate. Each group contained 5 duplicate wells. The wells on the edges of the 96-well plate were filled with PBS, and the plate was placed in a 37°C, 5% CO2 incubator. A total of 5 × 103 cells were inoculated in each well. The cells were allowed to adhere. The medium was then aspirated, and the cells were treated with medium containing the specified concentrations of the substance for the specified durations. CCK-8 solution was added at 10 μL/well. The 96-well plate was incubated in a 37°C box for 1 h. The optical density (OD) of each well at 490 nm was measured in a microplate reader. The OD values were averaged, and the experiment was repeated at least 3 times.
RNA Interference and Plasmid Constructs
siRNA targeting ST6GAL2, negative control siRNA, and full-length ST6GAL2-pcDNA 3.3 vectors were purchased from GenePharma (Shanghai, China). The sequences of the si-ST6GAL2 constructs were 5′-GCA UCG AGU GUG UCA GUU AUA-3′ and 5′UAA CUG ACA CAC UCG AUG-3′. The transfection procedures were performed according to the manufacturer’s protocols. The cell lines were transfected with specific siRNAs and plasmid vectors using FUGENE HD transfection reagent (Promega, USA).
Colony Formation Assay
A total of 1,000 cells were plated in a six-well plate and mixed well with 3 mL of medium containing 10% FBS. Cells were maintained in an incubator at 37°C and 5% CO2 for 1 week. The plate was taken out, the medium was removed, and the residual medium was washed away lightly with flowing water. Cells in the wells were fixed using paraformaldehyde for 30 min and then stained with 0.1% crystal violet. Clusters containing 30 cells or more were counted as a single colony.
Cell Migration and Invasion Assays
Cells were harvested, resuspended in serum-free medium, and seeded in the upper chamber of a Transwell membrane filter (Corning Life Sciences, NY, USA). Medium containing 10% FBS was added to the lower chamber. The cells were incubated for 24 h. After treatment with paraformaldehyde, cells were stained with 0.1% crystal violet. Finally, cells were imaged and counted using an Olympus microscope (Tokyo, Japan).
Endothelial Tube Formation Assay
Matrigel (Corning Life Sciences) (10 μl) was added to each well of a 96-well plate and allowed to solidify at 37°C for 30 min. HUVECs were resuspended in supernatant collected from each group. Then 300 μL of supernatant containing 4 × 104 HUVECs was added to each well and incubated at 37°C. After 8 h, tube formation was observed under a microscope.
Western Blot Analysis
The nuclear protein fraction and total protein were prepared with an isolation kit (KeyGEN Biotech, Nanjing, China). Western blotting was performed with antibodies against ST6GAL2, MST1, TAZ (Abcam), p-MST1/2, p-LATS1, LATS1, p-YAP, YAP (Affinity Biosciences), and GAPDH (ZSGB-BIO, Beijing, China). Protein quantification was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalized to GAPDH values.
Statistical Analysis
The data are presented as the means and standard deviations (SDs) of three replicates per group. Student’s t tests were used to compare values between the tested groups. p < 0.05 indicated statistical significance. Calculations were performed using SPSS software version 13.0.
Author Contributions
G.X. conceived and together with Y.Z. and G.W. designed the study. J.C., G.W. and J.X. were involved in data collection. N.Z. and H.Y. performed the statistical analysis and preparation of figures. G.X. drafted the paper. Y.C., G.W. and G.X. contributed substantially to its revision. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by grants from the Science Research Project of the Department of Education of Liaoning Province (L2016011) and the Doctoral Scientific Research Fund of the Second Hospital of Dalian Medical University (DY2YBSQD201501)
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.12.010.
Contributor Information
Guangzhi Wang, Email: wangguangzhi1986@hotmail.com.
Yongfu Zhao, Email: zyf0386@sina.com.
Supplemental Information
References
- 1.Cabanillas M.E., McFadden D.G., Durante C. Thyroid cancer. Lancet. 2016;388:2783–2795. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 2.James B.C., Aschebrook-Kilfoy B., Cipriani N., Kaplan E.L., Angelos P., Grogan R.H. The Incidence and Survival of Rare Cancers of the Thyroid, Parathyroid, Adrenal, and Pancreas. Ann. Surg. Oncol. 2016;23:424–433. doi: 10.1245/s10434-015-4901-9. [DOI] [PubMed] [Google Scholar]
- 3.Dralle H., Machens A., Basa J., Fatourechi V., Franceschi S., Hay I.D., Nikiforov Y.E., Pacini F., Pasieka J.L., Sherman S.I. Follicular cell-derived thyroid cancer. Nat. Rev. Dis. Primers. 2015;1:15077. doi: 10.1038/nrdp.2015.77. [DOI] [PubMed] [Google Scholar]
- 4.Gillanders S.L., O’Neill J.P. Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC) Eur. J. Surg. Oncol. 2018;44:286–296. doi: 10.1016/j.ejso.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Babala J., Zahradníková P., Béder I., Fedorová L., Lindák M., Košťálová Ľ., Pribilincová Z., Staník J., Králik R. Risk factors of post-surgery complications in children with thyroid cancer. Int. J. Pediatr. Otorhinolaryngol. 2019;127:109673. doi: 10.1016/j.ijporl.2019.109673. [DOI] [PubMed] [Google Scholar]
- 6.Fan D., Ma J., Bell A.C., Groen A.H., Olsen K.S., Lok B.H., Leeman J.E., Anderson E., Riaz N., McBride S. Outcomes of multimodal therapy in a large series of patients with anaplastic thyroid cancer. Cancer. 2019;126:444–452. doi: 10.1002/cncr.32548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraga T.S., Köhler H.F., Chulam T.C., Kowalski L.P. Impact of scalpel type on operative time and acute complications in thyroidectomies. Rev. Bras. Otorrinolaringol. 2019 doi: 10.1016/j.bjorl.2019.08.004. Published online October 3, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin A., Brito J.P., Bahl M., Hoang J.K. Applying Criteria of Active Surveillance to Low-Risk Papillary Thyroid Cancer Over a Decade: How Many Surgeries and Complications Can Be Avoided? Thyroid. 2017;27:518–523. doi: 10.1089/thy.2016.0568. [DOI] [PubMed] [Google Scholar]
- 9.Teshima M., Otsuki N., Morita N., Furukawa T., Shinomiya H., Shinomiya H., Nibu K.I. Postoperative hypoparathyroidism after total thyroidectomy for thyroid cancer. Auris Nasus Larynx. 2018;45:1233–1238. doi: 10.1016/j.anl.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Dall’Olio F., Malagolini N., Trinchera M., Chiricolo M. Sialosignaling: sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta. 2014;1840:2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harduin-Lepers A., Recchi M.A., Delannoy P. 1994, the year of sialyltransferases. Glycobiology. 1995;5:741–758. doi: 10.1093/glycob/5.8.741. [DOI] [PubMed] [Google Scholar]
- 13.Chen W., Xiang J., Chen D.F., Ni B.B., Chen H., Fan X.J., Wang P.N., Song S.X., Fang L.K., Xiao H.Y. Screening for differentially methylated genes among human colorectal cancer tissues and normal mucosa by microarray chip. Mol. Biol. Rep. 2013;40:3457–3464. doi: 10.1007/s11033-012-2338-9. [DOI] [PubMed] [Google Scholar]
- 14.Venturi G., Gomes Ferreira I., Pucci M., Ferracin M., Malagolini N., Chiricolo M., Dall’Olio F. Impact of sialyltransferase ST6GAL1 overexpression on different colon cancer cell types. Glycobiology. 2019;29:684–695. doi: 10.1093/glycob/cwz053. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer C.M., Reginato M.J. Sticking to sugars at the metastatic site: sialyltransferase ST6GalNAc2 acts as a breast cancer metastasis suppressor. Cancer Discov. 2014;4:275–277. doi: 10.1158/2159-8290.CD-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao J., Zeltz C., Pintilie M., Li Q., Sakashita S., Wang T., Cabanero M., Martins-Filho S.N., Wang D.Y., Pasko E. Characterization of Distinct Populations of Carcinoma-Associated Fibroblasts from Non-Small Cell Lung Carcinoma Reveals a Role for ST8SIA2 in Cancer Cell Invasion. Neoplasia. 2019;21:482–493. doi: 10.1016/j.neo.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang L., Xu J., Wang M., Xu G., Zhang N., Wang G., Zhao Y. LncRNA HCP5 promotes follicular thyroid carcinoma progression via miRNAs sponge. Cell Death Dis. 2018;9:372. doi: 10.1038/s41419-018-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisková T., Kassayová M. Resveratrol Action on Lipid Metabolism in Cancer. Int. J. Mol. Sci. 2019;20:E2704. doi: 10.3390/ijms20112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M., Jiang S., Yu F., Zhou L., Wang K. Noncoding RNAs as Molecular Targets of Resveratrol Underlying Its Anticancer Effects. J. Agric. Food Chem. 2019;67:4709–4719. doi: 10.1021/acs.jafc.9b01667. [DOI] [PubMed] [Google Scholar]
- 20.Asou H., Koshizuka K., Kyo T., Takata N., Kamada N., Koeffier H.P. Resveratrol, a natural product derived from grapes, is a new inducer of differentiation in human myeloid leukemias. Int. J. Hematol. 2002;75:528–533. doi: 10.1007/BF02982118. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X., Jia B., Song X., Kong Q.Y., Wu M.L., Qiu Z.W., Li H., Liu J. Preventive Potential of Resveratrol in Carcinogen-Induced Rat Thyroid Tumorigenesis. Nutrients. 2018;10:E279. doi: 10.3390/nu10030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y.T., Tian X.T., Wu M.L., Zheng X., Kong Q.Y., Cheng X.X., Zhu G.W., Liu J., Li H. Resveratrol Suppresses the Growth and Enhances Retinoic Acid Sensitivity of Anaplastic Thyroid Cancer Cells. Int. J. Mol. Sci. 2018;19:E1030. doi: 10.3390/ijms19041030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goto H., Nishio M., To Y., Oishi T., Miyachi Y., Maehama T., Nishina H., Akiyama H., Mak T.W., Makii Y. Loss of Mob1a/b in mice results in chondrodysplasia due to YAP1/TAZ-TEAD-dependent repression of SOX9. Development. 2018;145 doi: 10.1242/dev.159244. dev159244. [DOI] [PubMed] [Google Scholar]
- 24.Sekido Y. Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma. Cancers (Basel) 2018;10:E90. doi: 10.3390/cancers10040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ugolini C., Borrelli N., Niccoli C., Elisei R., Viola D., Vitti P., Miccoli P., Basolo F. Role of YAP-1 in Thyroid Tumor Progression and Outcome. Appl. Immunohistochem. Mol. Morphol. 2017;25:581–585. doi: 10.1097/PAI.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 26.Wu D.M., Wang S., Wen X., Han X.R., Wang Y.J., Shen M., Fan S.H., Zhang Z.F., Shan Q., Li M.Q. LncRNA SNHG15 acts as a ceRNA to regulate YAP1-Hippo signaling pathway by sponging miR-200a-3p in papillary thyroid carcinoma. Cell Death Dis. 2018;9:947. doi: 10.1038/s41419-018-0975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D.W., Wang Y.C., Wang L., Chen C.Y., Lee H. A low microRNA-630 expression confers resistance to tyrosine kinase inhibitors in EGFR-mutated lung adenocarcinomas via miR-630/YAP1/ERK feedback loop. Theranostics. 2018;8:1256–1269. doi: 10.7150/thno.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L.X., Wu J., Guo M.L., Zhang Y., Ma S.G. Suppression of long non-coding RNA TNRC6C-AS1 protects against thyroid carcinoma through DNA demethylation of STK4 via the Hippo signalling pathway. Cell Prolif. 2019;52:e12564. doi: 10.1111/cpr.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao Y., Li T., Zheng S., Wang H. The Hippo pathway as a drug target in gastric cancer. Cancer Lett. 2018;420:14–25. doi: 10.1016/j.canlet.2018.01.062. [DOI] [PubMed] [Google Scholar]
- 30.Maugeri-Saccà M., De Maria R. The Hippo pathway in normal development and cancer. Pharmacol. Ther. 2018;186:60–72. doi: 10.1016/j.pharmthera.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Misra J.R., Irvine K.D. The Hippo Signaling Network and Its Biological Functions. Annu. Rev. Genet. 2018;52:65–87. doi: 10.1146/annurev-genet-120417-031621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu F.X., Zhao B., Guan K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X., Zhu H.L. Resveratrol and its analogues: promising antitumor agents. Anticancer. Agents Med. Chem. 2011;11:479–490. doi: 10.2174/187152011795677427. [DOI] [PubMed] [Google Scholar]
- 34.Pstrąg N., Ziemnicka K., Bluyssen H., Wesoły J. Thyroid cancers of follicular origin in a genomic light: in-depth overview of common and unique molecular marker candidates. Mol. Cancer. 2018;17:116. doi: 10.1186/s12943-018-0866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedlund M., Ng E., Varki A., Varki N.M. alpha 2-6-Linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer Res. 2008;68:388–394. doi: 10.1158/0008-5472.CAN-07-1340. [DOI] [PubMed] [Google Scholar]
- 36.Lu J., Gu J. Significance of β-Galactoside α2,6 Sialyltranferase 1 in Cancers. Molecules. 2015;20:7509–7527. doi: 10.3390/molecules20057509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murugaesu N., Iravani M., van Weverwijk A., Ivetic A., Johnson D.A., Antonopoulos A., Fearns A., Jamal-Hanjani M., Sims D., Fenwick K. An in vivo functional screen identifies ST6GalNAc2 sialyltransferase as a breast cancer metastasis suppressor. Cancer Discov. 2014;4:304–317. doi: 10.1158/2159-8290.CD-13-0287. [DOI] [PubMed] [Google Scholar]
- 38.Park J.J., Lee M. Increasing the α 2, 6 sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver. 2013;7:629–641. doi: 10.5009/gnl.2013.7.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim M.H., Kim J. Role of YAP/TAZ transcriptional regulators in resistance to anti-cancer therapies. Cell. Mol. Life Sci. 2017;74:1457–1474. doi: 10.1007/s00018-016-2412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi Y., Yu J., Han W., Fan X., Qian H., Wei H., Tsai Y.H., Zhao J., Zhang W., Liu Q. A splicing isoform of TEAD4 attenuates the Hippo-YAP signalling to inhibit tumour proliferation. Nat. Commun. 2016;7 doi: 10.1038/ncomms11840. ncomms11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.