Abstract
Cancer cachexia is a complex metabolic disease so far lacking effective therapy, and it accounts for approximately one third of all cancer-related deaths worldwide. The extracellular ligand Wnt7a has a dual function in skeletal muscle, inducing the anabolic AKT/mammalian target of rapamycin (mTOR) pathway in myofibers and driving muscle stem cell expansion in skeletal muscle, making it a promising candidate for treatment of muscle wasting diseases. In murine and human myotubes, Wnt7a activates the anabolic AKT/mTOR pathway, thereby preventing cachexia-induced atrophy with a single application being sufficient to prevent atrophy independently of the tumor cell type causing cachexia. Addition of Wnt7a also improved activation and differentiation of muscle stem cells in cancer cachexia, a condition under which skeletal muscle regeneration is severely impaired due to stalled muscle stem cell differentiation. Finally, we show that Wnt7a prevents cancer cachexia in an in vivo mouse model based on C26 colon carcinoma cells. Wnt7a has a dual role in cachectic skeletal muscle; that is, it effectively counteracts muscle wasting through activation of the anabolic AKT/mTOR pathway and, furthermore, reverts the loss of muscle stem cell functionality due to cancer cachexia, making Wnt7a a promising candidate for an ameliorative treatment of cancer cachexia.
Keywords: Wnt7a, cancer cachexia, skeletal muscle, muscle stem cell, satellite cell, atrophy, muscle wasting
Introduction
Cancer cachexia is a metabolic wasting syndrome causing a dramatic loss of skeletal muscle and fat tissue. Furthermore, it is associated with a poor prognosis for patients, reduces the quality of life, and is estimated to be the direct cause of death in one third of all cancer patients.1, 2, 3, 4, 5 Of note, most cancer patients will suffer from cancer cachexia in an advanced state.2 Cancer cachexia significantly contributes to cancer-associated co-morbidities by weakening the patients toward the point that neither chemotherapy nor surgery can be tolerated.1,2,6 The mechanisms by which the tumors induce loss of muscle mass and function is multifactorial, mostly caused by pro-inflammatory cytokines, with tumor necrosis factor (TNF)-α, interleukin (IL)-6, and interferon (INF)-γ being important cytokines, which are increased in cancer cachexia.2,7 Importantly, loss of skeletal muscle mass due to cancer cachexia cannot be reversed by conventional nutritional support.8, 9, 10 Despite the widespread need for improved cachexia therapies and immense research on this topic, there remain very few treatments for this disease. Therefore, therapeutic strategies to prevent cachexia remain one of the main promising strategies.8
Myofiber Atrophy as a Cause of Cancer Cachexia
Skeletal muscle of patients suffering from cancer cachexia as well as mouse models of cancer cachexia display severe signs of atrophy, including the loss of muscle mass concomitant with reduced muscle functionality.11 Atrophy of skeletal muscle is mostly caused by aberrant signaling pathways, which perturb the balance between anabolism and catabolism of skeletal muscle proteins. In case of cancer cachexia the balance is tipped toward catabolism; the E3-ubiquitin ligases MuRF-1 and MAFbx/Atrogin-1 are strongly upregulated, leading to protein degradation in skeletal muscle.12 Expression of both E3-ubiquitin ligases, canonical markers for skeletal muscle atrophy, is controlled by transcription factors of the FoxO family.2,11,13,14 In addition, protein synthesis, e.g., driven by the activation of the AKT/mammalian target of rapamycin (mTOR) pathway through insulin-like growth factor-1 (IGF-1), is reduced in tumor-bearing mice suffering from cancer cachexia in various tissues, including adipose tissue and skeletal muscle.2,15,16 This combination of increased catabolism and decreased protein synthesis in skeletal muscle causes muscular atrophy. Interestingly, IGF-1 can dominantly revert AKT inhibition by myostatin in vitro but fails to do so in vivo.17,18 Therefore, potential treatments for cancer cachexia, which are currently being tested, include activation of the AKT/mTOR pathway or inhibition of protein degradation in skeletal muscle. Due to the aforementioned issues regarding efficiency of IGF-1 in vivo, other activators of the AKT/mTOR pathway need to be investigated on their ability to counteract muscle wasting in cancer cachexia in vivo.
Muscle Stem Cells in Cancer Cachexia
In cancer cachexia, myofibers in skeletal muscle become atrophic, a process that is accompanied by a reduced ability to regenerate due to impaired muscle stem cell function.19 Cancer cachexia is also associated with muscle damage resulting in muscle stem cell activation. Although muscle stem cells are activated due to muscle damage in cancer cachexia, they cannot fully differentiate due to the aberrant expression of Pax7, a transcription factor normally responsible for self-renewal of muscle stem cells.19 Regeneration of skeletal muscle is dependent on the presence and full functionality of muscle stem cells, a tightly regulated process that depends on the precise and dynamic integration of multiple signals, allowing the self-renewal and progression of myogenic precursor cells in the myogenic lineage.20 Differentiation of muscle stem cells is impaired, thereby causing a stalling of regeneration and resulting in impairments reminiscent of regeneration in the aged. Geriatric muscle stem cells become overly quiescent, resulting in senescence as well as impaired activation and differentiation.20,21
Wnt7a Signaling in Skeletal Muscle
Wnt signaling plays a crucial role during embryonic development but also in the maintenance of skeletal muscle in the adult.22 Wnt proteins constitute a large family of secreted glycoproteins that are related to the Drosophila wingless gene.23 In mammals, the Wnt family comprises 19 members that share homologies in their amino acid sequence but often have fundamentally distinct signaling properties. Nevertheless, they all share a signal sequence for secretion, several glycosylation sites, and a characteristic distribution of 22 cysteine residues.24 Wnt proteins typically bind to Frizzled (Fzd) receptors located in the plasma membrane of target cells.25 Wnt-receptor interactions can elicit various intracellular responses, with the best understood and most widely studied being the activation of β-catenin/TCF transcriptional complexes, also known as canonical Wnt signaling.26
In skeletal muscle Wnt ligands control the expression of MRFs (myogenic regulatory factors) as well as the differentiation and self-renewal of muscle stem cells.22 The differentiation process of muscle stem cells is mostly regulated by canonical Wnt signaling while self-renewal is controlled by non-canonical Wnt signaling, namely Wnt7a.27, 28, 29 In muscle stem cells Wnt7a has a dual role. On the one hand, it increases the number of symmetric satellite stem cell divisions, a subpopulation of muscle stem cells with high engraftment potential.30 Satellite stem cells can give rise to either daughter satellite stem cells or differentiate into committed progenitor cells, a process that is important for proper regeneration of skeletal muscle. On the other hand, Wnt7a increases the directed migration of muscle stem cells, thereby improving regeneration of skeletal muscle.28,31 Interestingly, in skeletal muscle Wnt7a always signals through the Fzd7 receptor. In muscle stem cells this leads to the activation of the PCP (planar cell polarity) signaling pathway and the activation of Rho/Rac. In myofibers Wnt7a drives the activation of the AKT/mTOR pathway, leading to the induction of myofiber hypertrophy.27,31, 32, 33 Therefore, Wnt7a is a potent new candidate for treatment of skeletal muscle of individuals suffering from cancer cachexia since the binding of one extracellular ligand to one receptor activates three different signaling pathways, thereby enhancing muscle mass and muscle stem cell functionality. This is particularly important since not only muscle mass is severely reduced in patients suffering from cancer cachexia, but also muscle regeneration is impaired. The latter one is especially important in cases when tumors are resected and surrounding skeletal muscles are damaged either due to stretching or even incisions.
In this study, we demonstrate that Wnt7a counteracts cancer cachexia-induced muscle loss through activation of the AKT/mTOR pathway independent of the tumor type causing cachexia. We show that myotube size is increased after addition of Wnt7a, which can be inhibited by addition of rapamycin. Of note, Wnt7a prevents myotube atrophy in murine and human myogenic cells, demonstrating high translational potential for ameliorative treatments of cancer cachexia patients. Furthermore, we show that Wnt7a increases the number of muscle stem cells by driving planar muscle stem cell divisions. Furthermore, the number of muscle stem cells is enhanced after addition of Wnt7a concomitant with an increase in further differentiated cells, suggesting that Wnt7a also improves the differentiation process of muscle stem cells, which is impaired in cancer cachexia. Finally, we demonstrate that Wnt7a prevents myofiber atrophy and loss of muscle stem cells in vivo using a C26 colon cancer mouse model.
Results
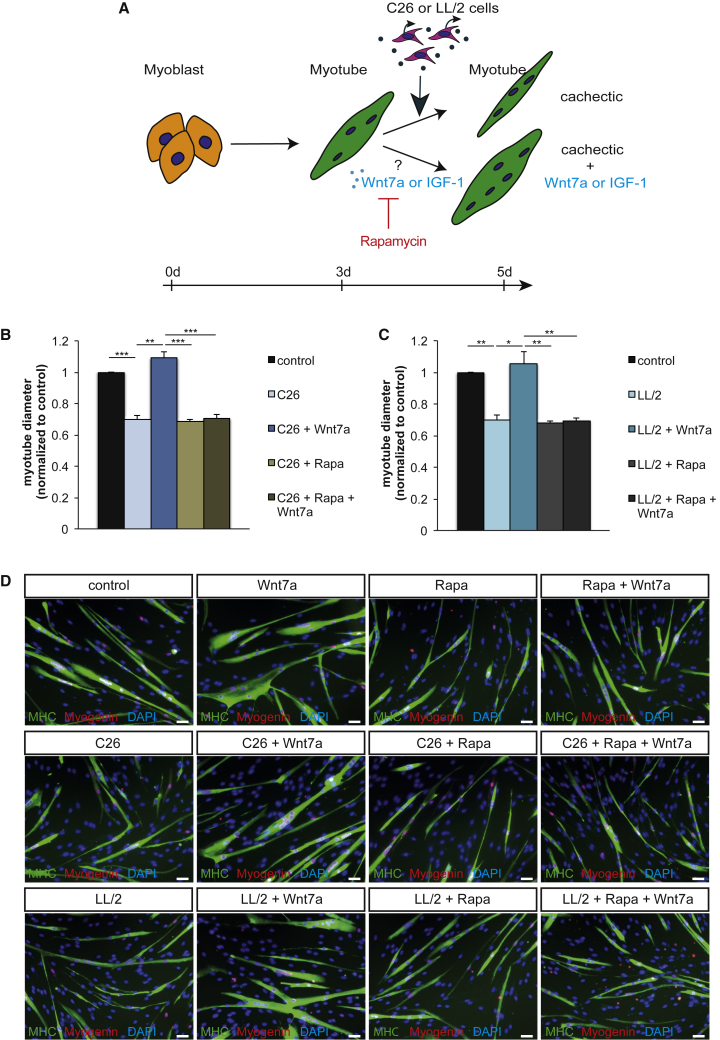
Wnt7a Prevents Myotube Atrophy Caused by Cancer Cachexia
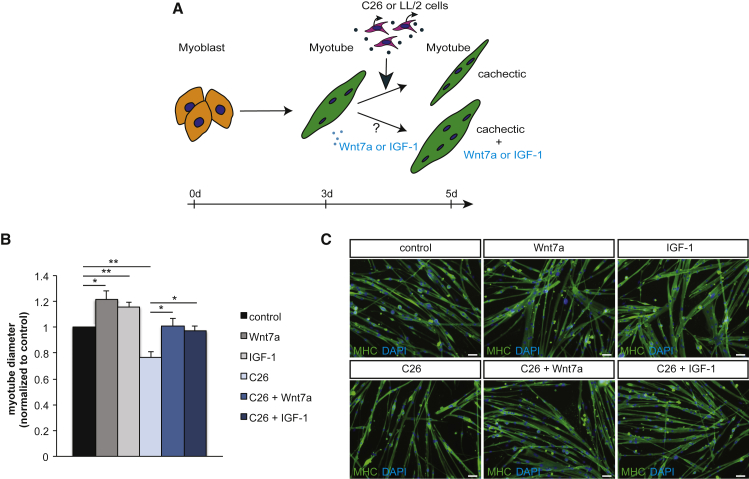
Wnt7a is a known activator of the anabolic AKT/mTOR pathway in skeletal muscle.32 We first asked whether Wnt7a can prevent atrophy of myotubes caused by cancer cachexia (Figure 1A; Figure S1A). Therefore, we used a well-established cell culture system using primary murine myoblasts incubated with supernatant from either C26 colon carcinoma cells or LL2 Lewis lung carcinoma cells, two independent cell lines known to contain a cocktail of cachexia-inducing cytokines.34 As expected, myotubes incubated with C26 or LL2 supernatant developed myotube atrophy, as demonstrated by a decrease in myotube diameter of 24% and 29%, respectively (Figures 1B and 1C; Figures S1B and S1C). Notably, a single treatment with Wnt7a or IGF-1 prevented development of myotube atrophy independent of the cancer type inducing cachexia-dependent atrophy (Figures 1B and 1C; Figures S1B and S1C). Importantly, the diameter of myotubes in cancer cachexia treated with either a single dose of Wnt7a or IGF-1 was similar to control conditions, independently of the tumor cell type causing cachexia.
Figure 1.
Wnt7a Prevents Myotube Atrophy
(A) Experimental schematic outlining induction of myotube atrophy and treatment with Wnt7a or IGF-1 using murine primary myoblasts. (B) Measurement of the maximal myotube diameter demonstrates that myotube atrophy caused by conditioned medium from C26 colon carcinoma cells is prevented by addition of Wnt7a or IGF-1. (C) Representative images of myotubes from control conditions, after induction of myotube atrophy by C26 cell conditioned medium with and without Wnt7a or IGF-1 treatment. Images show myosin heavy chain (in green) and DAPI (in blue). Scale bars, 50 μm. n = 3. Error bars represent SEM. *p < 0.05, **p < 0.01.
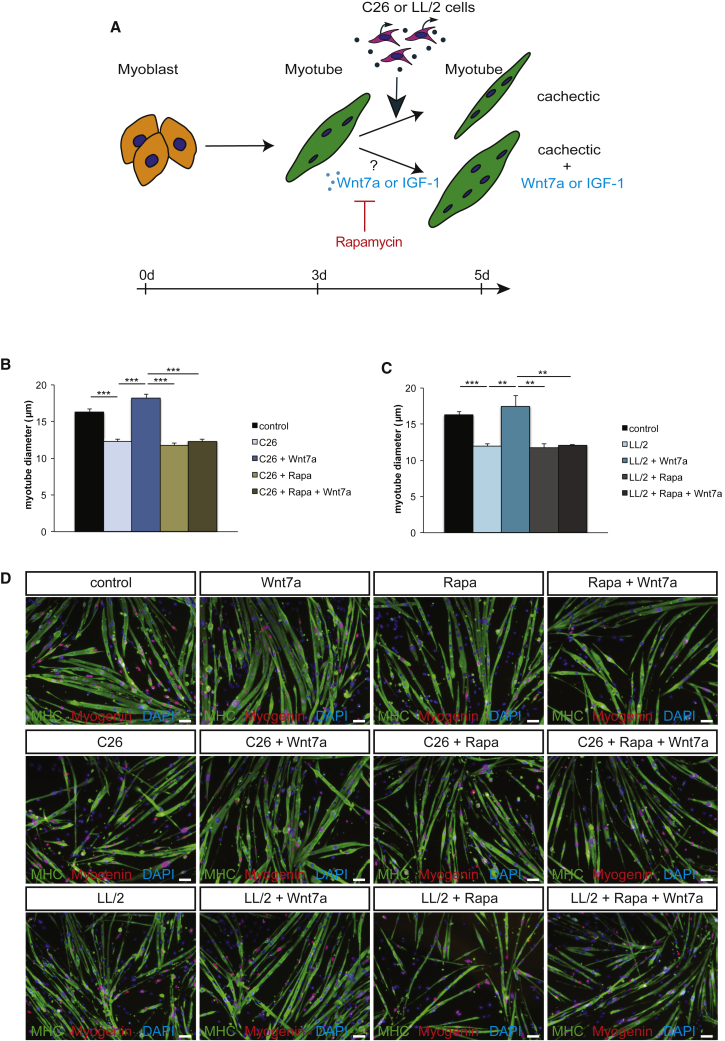
Prevention of Myotube Atrophy by Wnt7a Is Dependent on mTOR Activity
Our previous studies have demonstrated that Wnt7a induces hypertrophy through the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway via the Fzd7 receptor.32 To investigate whether activation of the anabolic AKT/mTOR pathway is the main pathway driving Wnt7a-induced prevention of myotube atrophy in cancer cachexia, we used rapamycin, a well-known inhibitor of mTOR activity.35 Indeed, inhibition of mTOR activity completely blunted the ability of Wnt7a to counteract myotube atrophy in cancer cachexia (Figures 2A–2D), independent of the tumor cell type. This suggests that Wnt7a drives the activation of the anabolic AKT/mTOR pathway in cancer cachexia rather than inhibiting catabolic pathways such as activation of MAFbx/Atrogin-1 and MuRF-1.36 This is further supported by previous reports showing that phosphorylated (p-)AKT and p-mTOR levels are decreased in myotubes cultured in cancer cachexia conditions.37,38 As a control, we used IGF-1, which is known to activate the AKT/mTOR pathway via the IGF receptor.13 As expected, inhibition of mTOR activity by rapamycin also fully blunted the prevention of myotube atrophy (Figures 2A–2D; Figure S2). In conclusion, we demonstrate that Wnt7a counteracts myotube atrophy induced by cancer cachexia through activation of the AKT/mTOR pathway independent of the cachexia-inducing tumor type.
Figure 2.
Wnt7a Counteracts Myotube Atrophy through the AKT/mTOR Pathway
(A) Experimental schematic outlining induction of myotube atrophy and treatment with Wnt7a and rapamycin using murine primary myoblasts. (B) Counteracting myotube atrophy caused by conditioned medium from C26 colon carcinoma cells measured as the maximal myotube diameter is dependent on the AKT/mTOR pathway, as shown by addition of the mTOR inhibitor rapamycin. (C) Counteracting myotube atrophy via Wnt7a application caused by conditioned medium from LL/2 lung carcinoma cells measured as the maximal myotube diameter is dependent on the AKT/mTOR pathway, as shown by addition of the mTOR inhibitor rapamycin. (D) Representative images of myotubes from control conditions, after induction of myotube atrophy by C26 or LL/2 cell conditioned medium with and without Wnt7a treatment and/or addition of rapamycin. Images show myosin heavy chain (in green), myogenin (in red), and DAPI (in blue). Scale bars, 50 μm. n = 3. Error bars represent SEM. *p < 0.05, **p < 0.01.
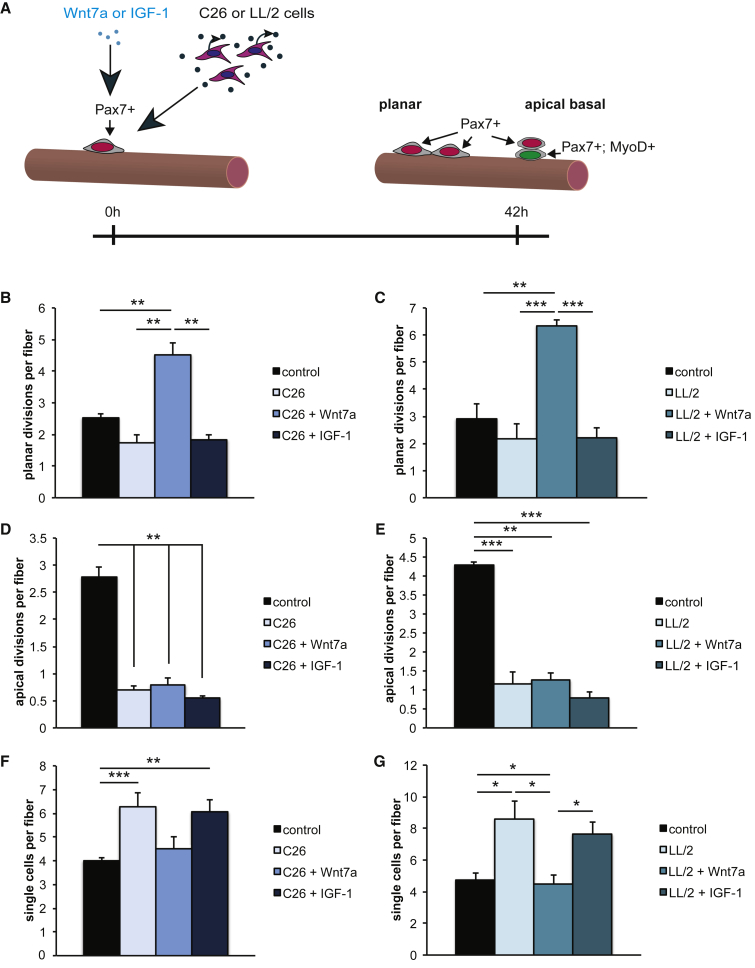
Wnt7a Drives Planar Muscle Stem Cell Divisions in Cancer Cachexia
Cancer cachexia affects myofibers, causing atrophy. Furthermore, cancer cachexia impairs muscle stem cell function, in particular through inhibiting muscle stem cell differentiation.19 Muscle stem cells can undergo symmetric planar and asymmetric apical-basal divisions needed for proper regeneration of skeletal muscle after injury.30 Therefore, we first asked whether the first division of muscle stem cells is affected by cancer cachexia (Figure 3A). Indeed, we found a slight reduction of 31% (C26 cells) and 26% (LL/2 cells) in the number of planar muscle stem cell divisions after 42 h of culture (Figures 3B and 3C) in cancer cachexia independent of the tumor cell type. Strikingly, the amount of apical-basal muscle stem cell divisions was reduced by 75% (C26 cells) and 73% (LL/2 cells) when muscle stem cells were cultured under cancer cachexia conditions (Figures 3D and 3E). Wnt7a was shown to drive planar symmetric satellite stem cell divisions, a subpopulation of muscle stem cells significantly contributing to the muscle stem cell reservoir, through activation of planar cell polarity (PCP) signaling.27 Therefore, we asked whether Wnt7a would also increase the number of planar muscle stem cell divisions in cancer cachexia, thereby boosting the muscle stem cell pool. Indeed, addition of recombinant Wnt7a protein significantly increased the amount of muscle stem cell divisions with a planar orientation of the division axis in cancer cachexia (Figures 3B and 3C; increase by 2.6-fold or 2.9-fold, respectively), while IGF-1 did not have any effect. In agreement with the data from Le Grand et al.,27 we did not find an increase in the number of apical-basal divisions after addition of Wnt7a recombinant protein (Figures 3D and 3E). Of note, also IGF-1 did not affect apical-basal muscle stem cell divisions.
Figure 3.
Wnt7a Drives Planar Muscle Stem Cell Divisions in Cancer Cachexia
(A) Experimental schematic outlining the experimental setup to investigate the first muscle stem cell division under control and in cancer cachexia with or without treatment with Wnt7a or IGF-1. (B) Addition of Wnt7a but not IGF-1 drives planar muscle stem cell divisions in cancer cachexia caused by addition of conditioned medium from C26 colon carcinoma cells. (C) Addition of Wnt7a but not IGF-1 drives planar muscle stem cell divisions in cancer cachexia caused by addition of conditioned medium from LL/2 lung carcinoma cells. (D) Apical-basal muscle stem cell divisions are lost in cancer cachexia caused by addition of conditioned medium from C26 colon carcinoma cells. Neither Wnt7a nor IGF-1 can increase their numbers. (E) Apical-basal muscle stem cell divisions are lost in cancer cachexia caused by addition of conditioned medium from LL/2 lung carcinoma cells. Neither Wnt7a nor IGF-1 can increase their numbers. (F) Wnt7a but not IGF-1 drives activation of muscle stem cells in cancer cachexia caused by addition of conditioned medium from C26 colon carcinoma cells as measured by the reduction in the number of single muscle stem cells per myofiber after 42 h of culture. (G) Wnt7a but not IGF-1 drives activation of muscle stem cells in cancer cachexia caused by addition of conditioned medium from LL/2 lung carcinoma cells as measured by the reduction in the number of single muscle stem cells per myofiber after 42 h of culture. n = 4. Error bars represent SEM. *p < 0.05, **p < 0.01.
It was shown earlier that muscle stem cell activation is impaired in cancer cachexia. We confirmed this finding and found that even the first division of muscle stem cells is impaired due to cancer cachexia. Therefore, we measured the number of single muscle stem cells after 42 h of culture (Figures 3F and 3G), a time point when properly activated muscle stem cells have already undergone their first round of division and can be found as duplexes on their adjacent myofibers. In this study, we found a significant increase in the amount of single muscle stem cells per myofiber of 56% (C26 cells) or 82% (LL/2 cells) when extensor digitorum longus (EDL) myofiber cultures were performed in cancer cachexia conditions, suggesting that activation of muscle stem cells is severely impaired. We then asked whether addition of recombinant Wnt7a protein could counteract this hampered activation. Indeed, the number of single muscle stem cells per myofiber was decreased by 28% (C26 cells) or 48% (LL/2 cells) when a single Wnt7a treatment was applied (Figures 3F and 3G), suggesting that Wnt7a can overcome the impaired activation of muscle stem cells caused by cachexia-inducing factors. Also in this study, IGF-1 did not affect muscle stem cell functionality.
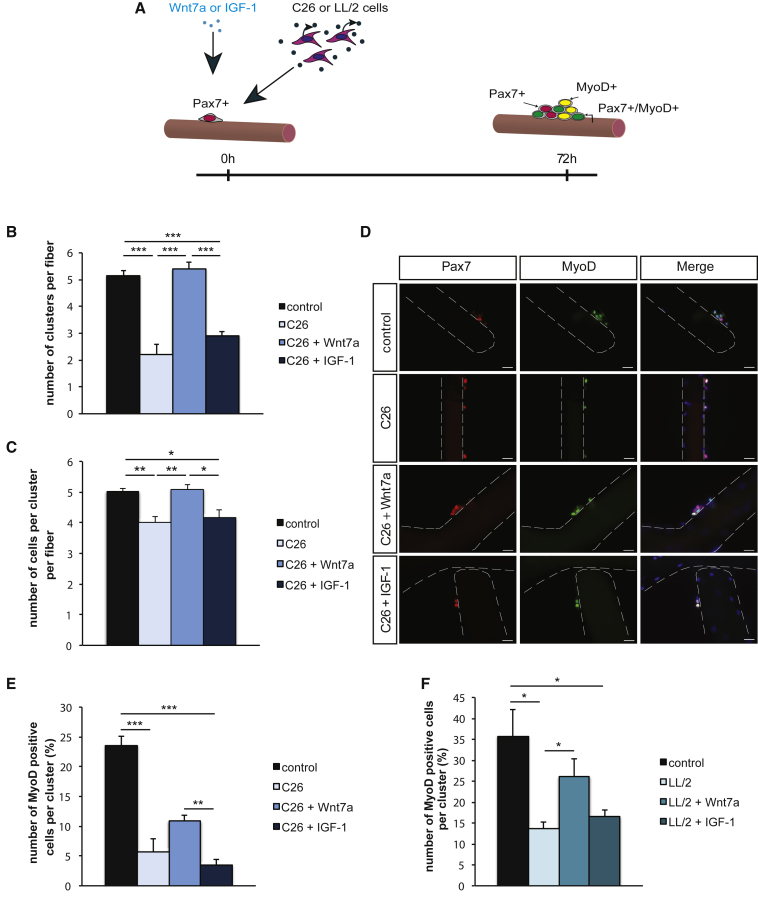
Wnt7a Overcomes the Impairment of Muscle Stem Cell Differentiation Caused by Cancer Cachexia
Cancer cachexia affects muscle stem cell function by impairing activation and differentiation.19 The ability of muscle stem cells to undergo differentiation is a prerequisite for the formation of new myofibers, thereby facilitating regeneration of skeletal muscle.20 Therefore, we investigated whether Wnt7a can improve muscle stem cell differentiation in cancer cachexia (Figure 4A; Figure S3A). First, we confirmed impaired activation and differentiation of muscle cells after 72 h in culture as measured by the decreased number of clusters per myofiber (decrease by 57% and 30%, respectively), a reduced number of cells per cluster (decrease by 20% and 10%, respectively), and reduced numbers of MyoD-only cells per cluster (decrease by 76% or 62% respectively), with the latter ones denominating differentiating muscle stem cells (Figures 4B 4F; Figures S3B and S3C). Importantly, Wnt7a increased the number of clusters per myofiber (Figure 4B; Figure S3B) by 2.5-fold (C26 cells) or 1.6-fold (LL/2 cells), further supporting the notion that addition of Wnt7a recombinant protein improves activation of muscle stem cells in cancer cachexia. Of note, the increase in cluster numbers per myofiber through Wnt7a is independent of the cancer cell type inducing cachexia (Figure 4B; Figure S3B). As observed after 42 h of culture, IGF-1 also did not have any effect on muscle stem cell function after 72 h of culture (Figure 4B; Figure S3B). Furthermore, Wnt7a augmented the loss of proliferative capacity of muscle stem cells in cancer cachexia (Figure 4C; Figure S3C). Application of Wnt7a recombinant protein increased the amount of MyoD-only positive cells per cluster by 1.9-fold, suggesting that Wnt7a drives differentiation of muscle stem cells in cancer cachexia, either directly or indirectly through an overall increase of muscle stem cell numbers per myofiber (Figures 4E and 4F). In summary, we demonstrate that the addition of a single dose of Wnt7a recombinant protein counteracts the impairment of muscle stem cell differentiation caused by cancer cachexia.
Figure 4.
Differentiation of Muscle Stem Cells in Cancer Cachexia Is Improved by Wnt7a
(A) Experimental schematic outlining the experimental setup to investigate muscle stem cell activation and differentiation under control and in cancer cachexia induced by conditioned medium from C26 colon carcinoma cells with or without treatment with Wnt7a or IGF-1. (B) Wnt7a but not IGF-1 drives activation and proliferation of muscle stem cells in cancer cachexia caused by conditioned medium from C26 colon carcinoma cells measured by the number of clusters per myofiber. (C) The total number of cells per cluster is decreased in cancer cachexia induced by conditioned medium from C26 colon carcinoma cells, but can be brought back to control conditions by Wnt7a but not IGF-1. (D) Representative images of clusters of muscle stem cells after 72 h under control and in cancer cachexia induced by conditioned medium from C26 colon carcinoma cells with and without addition of Wnt7a or IGF-1. Images show Pax7 (in red), MyoD (in green), and DAPI (in blue). (E) Differentiation of muscle stem cells is inhibited in cancer cachexia induced by conditioned medium from C26 colon carcinoma cells as measured by the number of MyoD-positive cells per cluster. Addition of Wnt7a but not IGF-1 partially counteracts inhibition of differentiation in cancer cachexia. (F) Differentiation of muscle stem cells is inhibited in cancer cachexia induced by conditioned medium from LL/2 lung carcinoma cells as measured by the number of MyoD-positive cells per cluster. Addition of Wnt7a but not IGF-1 counteracts inhibition of differentiation in cancer cachexia. n = 4. Error bars represent SEM. *p < 0.05, **p < 0.01.
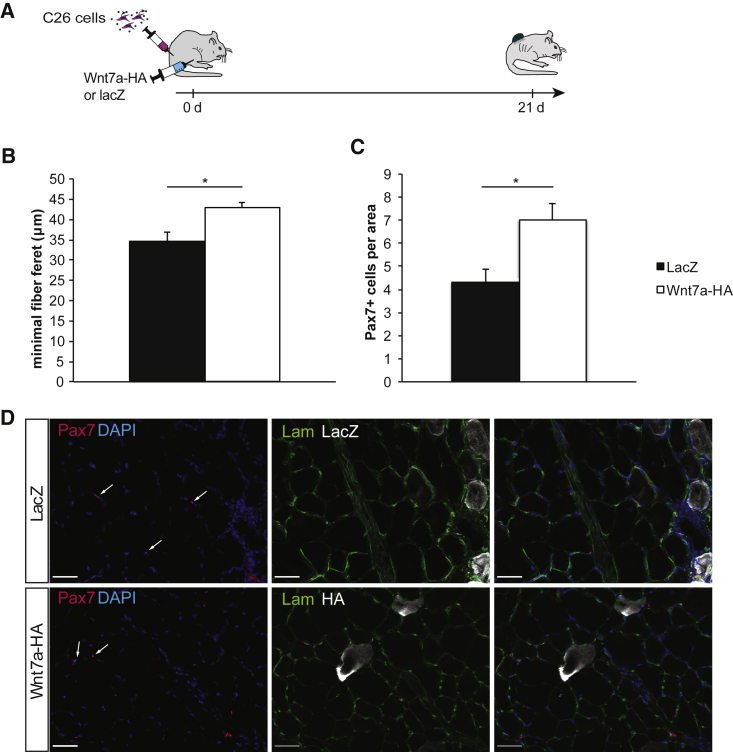
Wnt7a Counteracts Myofiber Atrophy In Vivo
To investigate whether Wnt7a can prevent myofiber atrophy in vivo, we used a mouse model in which cancer cachexia is induced by subcutaneous injection of C26 colon carcinoma cells. In brief, adult BALB/c mice were inoculated with C26 colon carcinoma cells in the flank and one tibialis anterior (TA) muscle was electroporated with a Wnt7a-hemagglutinin (HA) expression plasmid or equal amounts of a lacZ control plasmid (Figure 5A). 3 weeks after tumor cell inoculation, all mice had developed severe signs of cancer cachexia such as a weight loss of ∼20%; at this time point, the TA muscles were harvested and myofiber size was measured as the minimal fiber Feret diameter, and the number of muscle stem cells was evaluated. We found that muscles that had been electroporated with a Wnt7a-HA expression plasmid demonstrated significantly larger myofiber sizes than did muscles with a lacZ control plasmid (Figures 5B and 5D; increase by 1.2-fold), suggesting that Wnt7a also counteracts myofiber atrophy caused by cancer cachexia in vivo. Furthermore, we counted the number of muscle stem cells per area and also found a significant increase (1.6-fold) when we compared muscles electroporated with the Wnt7a-HA expression plasmid with muscles that were electroporated with a control plasmid (Figures 5C and 5D). Electroporation of skeletal muscle always coincides with slight muscle damage also known as electrical damage, followed by activation and differentiation of muscle stem cells to regenerate the damaged myofibers.39 These data suggest that Wnt7a ameliorates myofiber atrophy and improves muscle stem cell functionality in cancer cachexia also in vivo.
Figure 5.
Wnt7a Counteracts Myofiber Atrophy and Loss of Muscle Stem Cells In Vivo
(A) Experimental schematic outlining the experimental setup to investigate the in vivo effect of Wnt7a in cancer cachexia by injection of C26 colon carcinoma cells in BALB/c mice. (B) Wnt7a counteracts atrophy of skeletal muscle (tibialis anterior) in mice suffering from cancer cachexia induced by subcutaneously injected C26 colon carcinoma cells measured by the minimal fiber Feret diameter. (C) Wnt7a increases the number of muscle stem cells in skeletal muscle (tibialis anterior) in mice suffering from cancer cachexia induced by subcutaneously injected C26 colon carcinoma cells. (D) Representative images of tibialis anterior muscles from cachectic mice, which were electroporated with either a lacZ control plasmid or a Wnt7a-HA expression plasmid. Images show Pax7 (in red), laminin (in green), DAPI (in blue), and β-galactosidase or HA (in white). n = 4 (lacZ). n = 5 (Wnt7a-HA). Error bars represent SEM. *p < 0.05.
Wnt7a Prevents Cachexia-Induced Myotube Atrophy in Human Myotubes
Wnt7a shows a very high homology between mice and humans (more than 99% protein identity). To test whether Wnt7a can prevent myotube atrophy due to cancer cachexia also in the human context through activation of the AKT/mTOR anabolic pathway, we analyzed cachectic human primary myotubes for responsiveness to Wnt7a (Figure 6A). Notably, a single application of Wnt7a recombinant protein resulted in a 1.6-fold (C26 cells) or 1.5-fold (LL/2 cells) increase in myotube diameters independently of the tumor type causing cachexia (Figures 6B–6D). IGF-1 was used as a control (Figure S4). Furthermore, we could also demonstrate that activation of the anabolic AKT/mTOR pathway by Wnt7a is a prerequisite for counteracting myotube atrophy in human myotubes (Figures 6B–6D). Thus, we conclude that the mechanism by which Wnt7a counteracts atrophy in cachectic skeletal muscle is conserved between humans and mice and that Wnt7a might be a promising therapeutic target for ameliorative treatment for patients suffering from cancer cachexia.
Figure 6.
Wnt7a Counteracts Myotube Atrophy through the AKT/mTOR Pathway in Human Primary Myotubes
(A) Experimental schematic outlining induction of myotube atrophy and treatment with Wnt7a and rapamycin using human primary myoblasts. (B) Counteracting myotube atrophy caused by conditioned medium from C26 colon carcinoma cells measured as the maximal myotube diameter is dependent on the AKT/mTOR pathway, as shown by addition of the mTOR inhibitor rapamycin. (C) Counteracting myotube atrophy caused by conditioned medium from LL/2 lung carcinoma cells measured as the maximal myotube diameter is dependent on the AKT/mTOR pathway, as shown by addition of the mTOR inhibitor rapamycin. (D) Representative images of myotubes from control conditions, after induction of myotube atrophy by C26 or LL/2 cell conditioned medium with and without Wnt7a treatment and/or addition of rapamycin. Images show myosin heavy chain (in green), myogenin (in red), and DAPI (in blue). Scale bars, 50 μm. n = 3. Error bars represent SEM. *p < 0.05, **p < 0.01.
Discussion
Cancer cachexia is accompanied by severe muscle wasting caused by aberrant activation of signaling pathways, which perturbs the fine balance between anabolism and catabolism in skeletal muscle.1,2 Furthermore, cancer cachexia severely affects the general health of patients and tolerance versus tumor therapies such as chemotherapy or radiation therapy.1,2,6 Importantly, muscle wasting cannot be counteracted by increased nutritional intake.8,18 Therefore, activation of anabolic signaling pathways independently of nutritional intake is a promising strategy for the ameliorative treatment of cancer cachexia. We have previously demonstrated that Wnt7a activates the anabolic AKT/mTOR pathway even in diseased states such as muscular dystrophy.32,40 Furthermore, Wnt7a was shown to increase regeneration of skeletal muscle, a process severely impaired in cancer cachexia.19,27
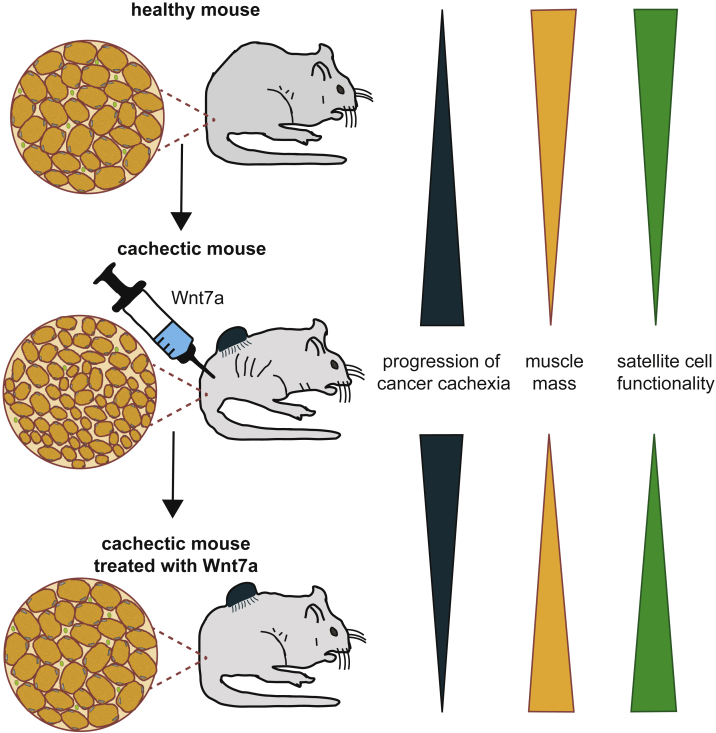
In the present study we investigated whether Wnt7a can fulfil its dual function also in cancer cachexia. Indeed, we demonstrate that Wnt7a can activate the anabolic AKT/mTOR signaling pathway and improve muscle stem cell function in cancer cachexia (Figure 7). Notably, we found that Wnt7a counteracts atrophy in vitro in murine and human myotubes, showing the translational potential of Wnt7a treatment for patients. Furthermore, Wnt7a reduced atrophy in vivo in a C26 colon cancer cell-based mouse cachexia model, in contrast to IGF-1, which was shown to be ineffective in counteracting muscle wasting in vivo.17
Figure 7.
Wnt7a Prevents Cancer Cachexia
Wnt7a has a dual function in counteracting cancer cachexia. On the one hand, it increases muscle mass; on the other hand, it improves muscle stem cell functionality.
Patients suffering from cancer cachexia have a severely reduced ability to withstand cancer treatments such as chemotherapy or radiation.2,15 Current treatment includes pharmacological, nutritional, and exercise treatments, none of which is successful.6 Signaling pathways that have been investigated as targets for treatment of muscle wasting following cancer cachexia are the anabolic AKT/mTOR pathway or negative regulators of muscle growth and development such as myostatin.2 IGF-1 was demonstrated to dominantly revert the inhibition of AKT by myostatin in cell culture but was shown to be ineffective in vivo.17,18 Wnt7a activates the AKT/mTOR pathway through its receptor Fzd7 independently of the IGF receptor, thereby providing an anabolic pathway that is independent of insulin resistance and IGF-1 levels in the blood.32 This is one of the main factors making Wnt7a successful in ameliorating cancer cachexia in vivo (Figures 5 and 7).
Cancer treatment often involves the resection of the tumor, a surgery that mostly coincides with stretching or even cutting of skeletal muscles. Muscles then need to regenerate, a process heavily affected by cancer cachexia due to an impaired muscle stem cell differentiation.19 Wnt7a was previously described to improve regeneration of skeletal muscle, increase the number of symmetric planar satellite stem cell divisions, and to increase proliferation of muscle stem and progenitor cells, all processes improving the regenerative outcome.27,31 Importantly, a single application of Wnt7a recombinant protein increased the number of Pax7-positive muscle stem cells per myofiber in cancer cachexia. Of note, the number of muscle stem cells was similar to the ones in healthy conditions, suggesting that Wnt7a is counteracting the impairments in proliferation and differentiation without causing aberrant proliferation (Figure 4C; Figure S3B). Furthermore, Wnt7a improved activation of muscle stem cells and improved the otherwise hampered differentiation in cancer cachexia, providing sufficient numbers of stem cells for muscle growth after regeneration.
One of the main goals of treatment of cancer cachexia is the preservation of muscle mass and physical performance.6 Furthermore, improving muscle stem function in patients suffering from cancer cachexia is beneficial since it was shown that impaired differentiation of muscle stem cells contributes to muscle wasting, and resection of tumors often coincides with damage of surrounding skeletal muscles.19 Our experiments provide compelling evidence that Wnt7a treatment counteracts muscle wasting and impaired muscle stem cell differentiation due to cancer cachexia, making Wnt7a a promising candidate for development as an ameliorative treatment for cancer cachexia.
Materials and Methods
Cell Culture and Conditioned Medium
For myoblast culture, muscle stem cells were purified by fluorescence-activated cell sorting (FACS) as described earlier28 and plated on collagen-coated dishes (Corning) in Ham’s F10 medium (Thermo Scientific) supplemented with 20% fetal bovine serum (FBS) (Thermo Scientific) and 5 ng/mL basic fibroblast growth factor (FGF) (Thermo Scientific). Human myoblasts were cultured as previously described.40 Differentiation was carried out in DMEM supplemented with 5% horse serum (HS).
C26 and LL/2 cells were cultured in growth media (DMEM, 10% FBS) in a tissue culture incubator at 37°C, 5% CO2, and 95% humidity. C26 and LL/2 cells were cultured in differentiation media of primary myoblasts for approximately 3 days. Media were filtered with a 0.2-μm syringe filter, and 80% of total volume of primary myotube differentiation media was replaced with C26 or LL/2 media at day 3 of the differentiation assay. In addition, recombinant Wnt7a (50 μg/mL; R&D Systems, #3008-WN-025), recombinant IGF-1 (50 μg/mL, Sigma-Aldrich, #I1271-.1MG), or rapamycin (20 μg/mL, Cell Signaling, #9904) was added to the cells.
EDL Single-Fiber Cultures
Preparation and cultivation of EDL fibers was performed as described previously.41 In brief, EDL muscles were taken out under sterile conditions and incubated in DMEM with 0.2% collagenase (from Clostridium histolyticum, Sigma) at 37°C in a water bath for 1 h. Fibers were gently dissociated by using a glass pipette. Single fibers were incubated in fiber culture medium (DMEM, 20% FBS, 1% chicken embryo extract; United States Biological) in a tissue culture incubator at 37°C, 5% CO2, and 95% humidity for 42 or 72 h. For mimicking cancer cachexia in vitro, myofibers were incubated with supernatant of C26 or LL/2 cultured cells in fiber culture medium. Wnt7a (50 μg/mL, R&D Systems, #3008-WN-025) or IGF-1 (50 μg/mL, Sigma-Aldrich, #I1271-.1MG) was added to the culture medium. Myofibers were fixed with 2% paraformaldehyde (PFA) for 5 min and washed twice with PBS (pH 7.4). Fibers were permeabilized (PBS, 0.1% Triton X-100, 0.1 M glycine [pH 7.4]) for 10 min, blocked with 5% HS in PBS (pH 7.4), and incubated with primary antibodies directed against Pax7 (undiluted, PAX7; Developmental Studies Hybridoma Bank [DSHB]) and MyoD (1:100, Invitrogen, #PA5-23078) at 4°C overnight. Fibers were washed three times with PBS (pH 7.4) and incubated with secondary antibodies for 45 min at room temperature (RT). Afterward, fibers were washed with PBS (pH 7.4) three times and nuclei were counterstained with DAPI (50 μg/mL) for 5 min, followed by two washing steps, and fibers were mounted with Permafluor mounting medium on glass microscope slides. Analysis was carried out using the Axio Observer.D1 (Carl Zeiss).
Electroporation of TA Muscles and Injection of C26 Cells
Adult (10 weeks of age) female BALB/c mice were obtained from Janvier. All animal procedures were in accordance with the European Union (EU) directive 2010/63/EU and approved by the Animal Welfare Committee of the Thüringer Landesamt für Lebensmittelsicherheit und Verbraucherschutz (TLV; 03-011/14; Bad Langensalza, Germany) or the University of Ottawa Animal Care Committee.
For in vivo electroporation, 40 μg of plasmid DNA (pHan-Wnt7a-HA or pSport-lacZ as control) in 0.9% NaCl was injected directly into the right TA muscle of anesthetized BALB/c mice (10 weeks of age). Immediately after plasmid injection, electrical stimulation was applied directly to the TA muscle using a pulse generator (ECM 830, BTX) of 100 V for six pulses, with a fixed duration of 20 ms and an interval of 200 ms between the pulses equipped with a 5-mm needle electrode (BTX).
C26 colon carcinoma cells (100,000 cells in 0.9% NaCl) were injected subcutaneously into the flank of female BALB/c mice. Mice were sacrificed 2 weeks after electroporation.
Experimental and contralateral muscles were isolated and embedded in OCT-10% sucrose (Tissue-Tek NEG 50), cooled with liquid nitrogen. Cross-sections (14 μm) of the muscle were obtained using a cryostat (Leica). To ensure that the same areas of the muscles were compared, we cut the TA muscle in the middle of the belly and counted the sections, which were used for immunostainings.
Immunostaining and Antibodies
Muscles frozen in liquid nitrogen were cut into 14-μm cross-sections. Cross-sections and cells were fixed with 2% PFA for 5 min, permeabilized with 0.1% Triton X-100, 0.1 M glycine in PBS (pH 7.4) for 10 min, blocked with 5% horse serum in PBS (pH 7.4) for 1 h, and incubated with specific primary antibody in blocking buffer overnight at 4°C. Samples were subsequently washed with PBS (pH 7.4) and stained with appropriate fluorescently labeled secondary antibodies in blocking buffer for 1 h at room temperature. After washing with PBS (pH 7.4), nuclei were counterstained with DAPI (50 μg/mL) for 5 min, followed by two washing steps with PBS (pH 7.4). Samples were mounted with Permafluor (Thermo Fisher Scientific, #TA-030-FM). Antibodies were as follows: mouse anti-Pax7 (undiluted, PAX7; DSHB), chicken anti-laminin (1:500, LSBio, #LS-C96142), rabbit anti-HA (1:1,000, Cell Signaling Technology, #3724), rabbit anti-LacZ (1:200, AbCam, #ab4671), and mouse anti-myosin (undiluted, MF20; DSHB).
Statistical Analyses
Experiments were done with a minimum of three biological replicates. For statistical comparison of two conditions, the Student’s t test was used. The level of significance is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Author Contributions
M.S. and C.P. designed and performed most experiments, analyzed data, and interpreted results. M.S. and J.v.M. analyzed data, interpreted results, and wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to J.v.M. (MA-3975/2-1) and the Deutsche Krebshilfe (DKH-JvM-861005). The authors would like to thank the animal facility (Claudia Maisch) and the imaging facility of the Leibniz Institute on Aging.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.12.011.
Supplemental Information
References
- 1.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Jatoi A., Loprinzi C., MacDonald N., Mantovani G. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K.C., Glass D.J., Guttridge D.C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 3.von Haehling S., Anker M.S., Anker S.D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J. Cachexia Sarcopenia Muscle. 2016;7:507–509. doi: 10.1002/jcsm.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315:1219–1222. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers C.N. Cancer Cytopathology enters its third decade. Cancer Cytopathol. 2017;125:9–10. doi: 10.1002/cncy.21810. [DOI] [PubMed] [Google Scholar]
- 6.Sadeghi M., Keshavarz-Fathi M., Baracos V., Arends J., Mahmoudi M., Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 2018;127:91–104. doi: 10.1016/j.critrevonc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Mueller T.C., Bachmann J., Prokopchuk O., Friess H., Martignoni M.E. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia--can findings from animal models be translated to humans? BMC Cancer. 2016;16:75. doi: 10.1186/s12885-016-2121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fearon K. Cachexia: treat wasting illness on multiple fronts. Nature. 2016;529:156. doi: 10.1038/529156b. [DOI] [PubMed] [Google Scholar]
- 9.Tisdale M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 10.Bossola M., Marzetti E., Rosa F., Pacelli F. Skeletal muscle regeneration in cancer cachexia. Clin. Exp. Pharmacol. Physiol. 2016;43:522–527. doi: 10.1111/1440-1681.12559. [DOI] [PubMed] [Google Scholar]
- 11.Egerman M.A., Glass D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodine S.C., Baehr L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014;307:E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010;346:267–278. doi: 10.1007/82_2010_78. [DOI] [PubMed] [Google Scholar]
- 14.Jagoe R.T., Goldberg A.L. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr. Opin. Clin. Nutr. Metab. Care. 2001;4:183–190. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Fearon K., Arends J., Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013;10:90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 16.Argilés J.M., Busquets S., Stemmler B., López-Soriano F.J. Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer. 2014;14:754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 17.Penna F., Bonetto A., Muscaritoli M., Costamagna D., Minero V.G., Bonelli G., Rossi Fanelli F., Baccino F.M., Costelli P. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved? Int. J. Cancer. 2010;127:1706–1717. doi: 10.1002/ijc.25146. [DOI] [PubMed] [Google Scholar]
- 18.Trendelenburg A.U., Meyer A., Rohner D., Boyle J., Hatakeyama S., Glass D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 19.He W.A., Berardi E., Cardillo V.M., Acharyya S., Aulino P., Thomas-Ahner J., Wang J., Bloomston M., Muscarella P., Nau P. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Invest. 2013;123:4821–4835. doi: 10.1172/JCI68523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt M., Schüler S.C., Hüttner S.S., von Eyss B., von Maltzahn J. Adult stem cells at work: regenerating skeletal muscle. Cell. Mol. Life Sci. 2019;76:2559–2570. doi: 10.1007/s00018-019-03093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sousa-Victor P., Gutarra S., García-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V., Jardí M., Ballestar E., González S., Serrano A.L. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 22.von Maltzahn J., Chang N.C., Bentzinger C.F., Rudnicki M.A. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi J.K., Vidal-Puig A. Wnt signalling and the control of cellular metabolism. Biochem. J. 2010;427:1–17. doi: 10.1042/BJ20091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 25.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 26.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Le Grand F., Jones A.E., Seale V., Scimè A., Rudnicki M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bentzinger C.F., Wang Y.X., von Maltzahn J., Soleimani V.D., Yin H., Rudnicki M.A. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones A.E., Price F.D., Le Grand F., Soleimani V.D., Dick S.A., Megeney L.A., Rudnicki M.A. Wnt/β-catenin controls follistatin signalling to regulate satellite cell myogenic potential. Skelet. Muscle. 2015;5:14. doi: 10.1186/s13395-015-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentzinger C.F., von Maltzahn J., Dumont N.A., Stark D.A., Wang Y.X., Nhan K., Frenette J., Cornelison D.D., Rudnicki M.A. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. J. Cell Biol. 2014;205:97–111. doi: 10.1083/jcb.201310035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Maltzahn J., Bentzinger C.F., Rudnicki M.A. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat. Cell Biol. 2011;14:186–191. doi: 10.1038/ncb2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Maltzahn J., Zinoviev R., Chang N.C., Bentzinger C.F., Rudnicki M.A. A truncated Wnt7a retains full biological activity in skeletal muscle. Nat. Commun. 2013;4:2869. doi: 10.1038/ncomms3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seto D.N., Kandarian S.C., Jackman R.W. A key role for leukemia inhibitory factor in C26 cancer cachexia. J. Biol. Chem. 2015;290:19976–19986. doi: 10.1074/jbc.M115.638411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballou L.M., Lin R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 2008;1:27–36. doi: 10.1007/s12154-008-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foletta V.C., White L.J., Larsen A.E., Léger B., Russell A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch. 2011;461:325–335. doi: 10.1007/s00424-010-0919-9. [DOI] [PubMed] [Google Scholar]
- 37.Liu D., Qiao X., Ge Z., Shang Y., Li Y., Wang W., Chen M., Si S., Chen S.Z. IMB0901 inhibits muscle atrophy induced by cancer cachexia through MSTN signaling pathway. Skelet. Muscle. 2019;9:8. doi: 10.1186/s13395-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lokireddy S., Wijesoma I.W., Bonala S., Wei M., Sze S.K., McFarlane C., Kambadur R., Sharma M. Retraction. Biochem. J. 2016;473:1111. doi: 10.1042/BJ4731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche J.A., Ford-Speelman D.L., Ru L.W., Densmore A.L., Roche R., Reed P.W., Bloch R.J. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am. J. Physiol. Cell Physiol. 2011;301:C1239–C1250. doi: 10.1152/ajpcell.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Maltzahn J., Renaud J.M., Parise G., Rudnicki M.A. Wnt7a treatment ameliorates muscular dystrophy. Proc. Natl. Acad. Sci. USA. 2012;109:20614–20619. doi: 10.1073/pnas.1215765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hüttner S.S., Ahrens H.E., Schmidt M., Henze H., Jung M.J., Schüler S.C., von Maltzahn J. Isolation and culture of individual myofibers and their adjacent muscle stem cells from aged and adult skeletal muscle. Methods Mol. Biol. 2019;2045:25–36. doi: 10.1007/7651_2019_209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.