Highlights
-
•
Extremely preterm children are at risk for language impairment.
-
•
Prior studies report structural hypoconnectivity and functional hyperconnectivity.
-
•
We address this incongruency using a data-driven connectometry approach.
-
•
We report interhemispheric hyperconnectivity in an extracallosal pathway.
-
•
These tracks correlate positively with language scores in the preterm group.
Keywords: Prematurity, Language, Diffusion, Connectivity, Development
Abstract
Children born preterm are at increased risk for cognitive impairment, with higher-order functions such as language being especially vulnerable. Previously, we and others have reported increased interhemispheric functional connectivity in children born extremely preterm; the finding appears at odds with literature showing decreased integrity of the corpus callosum, the primary commissural bundle, in preterm children. We address the apparent discrepancy by obtaining advanced measures of structural connectivity in twelve school-aged children born extremely preterm (<28 weeks) and ten term controls. We hypothesize increased extracallosal structural connectivity might support the functional hyperconnectivity we had previously observed. Participants were aged four to six years at time of study and groups did not differ in age, sex, race, ethnicity, or socioeconomic status. Whole-brain and language-network-specific (functionally-constrained) connectometry analyses were performed. At the whole-brain level, preterm children had decreased connectivity in the corpus callosum and increased connectivity in the cerebellum versus controls. Functionally-constrained analyses revealed significantly increased extracallosal connectivity between bilateral temporal regions in preterm children (FDRq <0.05). Connectivity within these extracallosal pathways was positively correlated with performance on standardized language assessments in children born preterm (FDRq <0.001), but unrelated to performance in controls. This is the first study to identify anatomical substrates for increased interhemispheric functional connectivity in children born preterm; increased reliance on an extracallosal pathway may represent a biomarker for resiliency following extremely preterm birth.
1. Introduction
Preterm birth significantly impacts public health. Children born extremely preterm (EPT, <28 weeks gestation) use more healthcare and educational resources compared to term controls (TC) (Johnson et al., 2009). The preterm birth rate is rising, affecting approximately 10% of births in the US (Martin et al., 2017). Increasingly, the smallest and sickest babies – those considered “periviable” – are being resuscitated and surviving (Younge et al., 2017). Advances in clinical practice bring new challenges: children born EPT are at significant risk of cognitive dysfunction, and neurodevelopmental impairments of these children have important public health implications in terms of resource utilization and educational and professional attainments in society (Aarnoudse-Moens et al., 2009; Chau et al., 2013; Hutchinson et al., 2013; Litt et al., 2005; Moore et al., 2013; Taylor et al., 2011).
Despite this, a recent report indicates up to 65% of children born as early as 23 weeks gestation perform within their expected grade level and some are even considered gifted (Garfield et al., 2017). A better understanding of neurodevelopment in EPT children may impact counseling provided to parents in the periviable period and the quality of life of such children and their families. Identification of biomarkers of risk and resiliency in EPT children is paramount.
Children born EPT are vulnerable to impairments in language, a key component of cognition (Barre et al., 2011; van Noort-van der Spek, Franken, and Weisglas-Kuperus, 2012; Vohr, 2014). Conventional assessments, including structural neuroimaging at term and early language testing, leave a large proportion of the variance in later functioning unexplained (Luttikhuizen dos Santos, de Kieviet, Konigs, van Elburg, and Oosterlaan, 2013; Woods et al., 2014). Thus, studying EPT children beyond infancy through school age is paramount to capture a key period of early language development. To optimize language outcomes, we must further elucidate risk factors for impairment and the brain-based mechanisms underlying the insults of prematurity. Imaging studies of well-performing EPT children are relatively scarce, with the majority being conducted in clinically-heterogeneous samples of preterm infants or adolescents and young adults (Aeby et al., 2013; Anderson et al., 2015; Keunen et al., 2017; Northam et al., 2012; Parikh et al., 2013). Most studies on children 4 years or above focus on gross brain morphology, finding smaller brain volumes in EPT versus controls, which correlates with lower IQ, poorer language performance, and executive functioning deficits (Cheong et al., 2013; de Kieviet, Zoetebier, van Elburg, Vermeulen, and Oosterlaan, 2012; Isaacs et al., 2004; Lowe et al., 2011; Omizzolo et al., 2013). In addition to overall brain volume being smaller in EPT children, some structures appear to be specifically impacted, including the corpus callosum, hippocampus, and cerebellum (Counsell and Boardman, 2005; de Kieviet et al., 2012).
Previously, we studied functional networks subserving language in this same sample of 4- to 6-year old children born EPT versus TC using fMRI-constrained magnetoencephalography (MEG) (Barnes-Davis et al., 2018). Language activation maps from the non-invasive gold-standard fMRI did not differ between groups during a stories-listening task. However, MEG revealed significant connectivity differences between groups, with the EPT group showing significantly increased functional connectivity (MEG) between left and right perisylvian cortex despite a typical looking spatial representation in fMRI. Effective connectivity analyses revealed relatively increased right-to-left perisylvian connections in EPT during stories-listening. These findings motivated the current study investigating the white matter substrate for increased connectivity within the language networks of children born extremely preterm.
A handful of prior studies in older preterm children and young adults found similar bitemporal functional hyperconnectivity using fMRI which is similar to our previous report, but they could not conclude if this hyperconnectivity represented an alternate developmental trajectory or simply a delay in the typical left lateralization of language (Gozzo et al., 2009; Mullen et al., 2011; Scheinost et al., 2015). Through the use of MEG, we were able to use the increased temporal resolution to look at the directed flux of information (effective connectivity). This effective connectivity analyses revealed significantly increased right-to-left information flux in EPT. We concluded it is not the precise cortical “real estate,” but instead the connectivity within the language network that is most impacted by prematurity. This conclusion is supported by a fMRI based study of preterm 12-year-old children showing similar patterns of representation, but atypical functional connectivity for the preterm group between canonical language areas such as the left middle temporal gyrus and extracanonical areas such as sensory motor regions (Schafer et al., 2009). In the current study, we investigate alterations in white matter that might support these atypical language network dynamics in children born EPT.
Structural connectivity studies in preterm children and adults are conflicting, but the majority show decreased connectivity overall. Early exposure to the extra-uterine environment is hypothesized to damage oligodendrocytes before myelin forms, causing white matter injury that may be revealed by diffusion tensor imaging (DTI) (Volpe, 2009a). Higher fractional anisotropy (FA) is thought to represent increased white matter integrity as a function of more intact axonal structure constraining water diffusion. Some report lower FA in children born preterm (Eikenes et al., 2011; Mullen et al., 2011; Skranes et al., 2007), but others report no significant difference (Counsell et al., 2006; Feldman et al., 2012, 2012; Li et al., 2015). Still others report complex relationships for white matter development in prematurity, with preterm children exhibiting decreased FA in some tracts (uncinate fasciculus, forceps major) and increased FA in others (inferior fronto-occipital fasciculus) (Dodson et al., 2017). In Feldman et al.’s study utilizing tract-based spatial statistics (TBSS) of DTI data in 23 very preterm children and adolescents and 19 term controls, increased FA of the white matter skeleton in dorsal and ventral tracts in the right hemisphere were positively correlated to improved performance in language for preterm adolescents and adults, but not term controls (Feldman et al., 2012). Increased FA in specific tracts correlated with scores in specific language domains (i.e. the right anterior thalamic radiation correlated with processing speed, the left and right forceps minor contributed to receptive vocabulary, and the right inferior fronto-occipital fasciculus correlated with verbal intelligence). In contrast, for TC, left lateralization of white matter tracts is positively correlated with language skills (Feldman et al., 2012; Lebel and Beaulieu, 2009). The developmental trajectory of white matter in infancy and early childhood is nonlinear, and slower rates of change have been associated with cognitive and language impairment in preterm children (Young et al., 2017). The corpus callosum is the largest commissural bundle of white matter connecting the left and right hemispheres, and is a structure known to be of decreased volume in preterm children (Malavolti et al., 2017; Northam et al., 2012). FA has been reported to be decreased in the corpus callosum in children born preterm as well (Li et al., 2015; Malavolti et al., 2017), and fiber pathways traveling through the corpus callosum have been reported as having decreased FA in language studies of children born preterm (Dodson et al., 2017; Northam et al., 2012).
As a whole, the studies detailed above suggest aberrant developmental trajectory for white matter in the premature brain into young adulthood. Inconsistent results could reflect the heterogeneity of clinical populations, which included varying ranges of gestational ages and chronological ages. Some studies included children with known neurological deficit or brain injury, such as intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL). These inconsistencies could also result from variability in the years the children were born, as neonatology is a rapidly evolving field and improvements in care have contributed to a reduction in certain patterns of brain injury, such as cystic white matter lesions (Volpe, 2009a). Differences in diffusion imaging analysis could reflect yet another source of variability in reports. Studies have largely relied on DTI. However, DTI estimates are inaccurate in areas of the brain with crossing fibers, present in up to 90% of white matter voxels (Jeurissen et al., 2013). More sophisticated analytical methods have been developed to characterize and quantify white matter in the brain that are well-suited to querying structural connectivity of networks in the preterm brain. These novel methods may help resolve discrepancies in the literature.
Motivated by the literature reviewed above, and our previously-reported functional connectivity findings in this same sample of children, the following research question and hypothesis were generated and guided our experiment. The objective of this current study is to identify the structural correlates of the previously-observed altered functional and effective connectivity in the language networks of children born EPT in order to answer our research question: How can extremely preterm children exhibit increased interhemispheric effective and functional connectivity but decreased structural connectivity? Our specific hypothesis is that extracallosal pathways are needed in EPT children to compensate for white matter injury of prematurity that is known to impact the corpus callosum and periventricular white matter preferentially and that this extracallosal structural connectivity could underlie the interhemispheric functional hyperconnectivity we and others have reported. In order to test this hypothesis, we employ a unique study design involving an a-theoretical approach that does not constrain analyses to classical regions and pathways associated with language in typically developing full-term adolescents and adults. Limiting the analysis to white matter pathways derived from adult language experiments would prevent us from capturing extracallosal pathways which we hypothesize would be adaptive for EPT children and might hinder our ability to address the apparent discrepancy in the literature of preterm language. Our study is unique in its exclusion of known brain injury or neurological disorder, including language impairment, enabling us to attribute group differences to relatively “pure” effects of prematurity. We measure connectivity in white matter pathways (at the whole-brain level and with a focus on regions derived from our fMRI and MEG study in this same population as outlined above) in the EPT brain using Generalized Q-Sampling Imaging (GQI), capable of resolving incoherent fiber orientations (crossing fibers) from conventional diffusion MRI data. Ultimately, we aim to identify biomarkers of resiliency in these children that can be directly related to observable performance and developmental outcomes.
2. Materials and methods
2.1. Experimental design
2.1.1. Participants
A total of 30 children, aged four to less than seven years, participated in this observational study in 2015–2016. Language network functional connectivity in these children, indexed by MEG, has been previously reported (Barnes-Davis et al., 2018). Participants included children born < 28 weeks gestation and followed for a minimum of 2 years at our center (EPT group, n = 15) and typically developing term controls recruited from the community who were born at or beyond 37 weeks of gestation (TC, n = 15). Informed consent was obtained in accordance with the Declaration of Helsinki and the study was approved by our IRB. Inclusion criteria for the EPT group included gestational age < 28 weeks and birth weight less than 1500 g. Exclusion criteria included a history of speech or language disorder, known brain injury such as severe interventricular hemorrhage (IVH) or periventricular leukomalacia (PVL), neurological disorder such as cerebral palsy (CP), seizures, migraines, or autism, or contraindication to MRI or MEG scanning. While excluding children with known brain injury or neurological impairment might limit the generalizability of our findings, it does enable us to investigate a ‘pure’ effect of prematurity in a more homogeneous sample and limit the impact of potential confounding variables such as hemorrhage or clinically significant white matter injury. Demographic data were obtained via parental report to address potential bias/confounders. Brief neuropsychological assessment was carried out prior to MEG and MRI scanning. All testing was completed in a single visit. One EPT participant was excluded due to a quality control failure (criteria outlined below). One EPT participant and five TC participants were excluded due to missing diffusion MRI data (secondary to scheduling constraints or a request to stop scanning after the MEG and fMRI acquisition).
2.1.2. Neuropsychological assessment
Children underwent assessment with the Peabody Picture Vocabulary Test (PPVT4; Dunn et al., 2015), Expressive Vocabulary Test (EVT2; Williams, 2007), and Wechsler Nonverbal Scale of Ability (WNV; Wechsler and Naglieri, 2006). The EVT2 and PPVT4 were used to assess expressive and receptive vocabulary, respectively. Although there are other measures of expressive and receptive language function, these measures can be considered a good assay for gross language skills, and were chosen for their short administration time, validity across all ages (starting at 2.5 years), and their high concordance with verbal intelligence (VIQ), especially in children (Krasileva et al., 2017; Strauss et al., 2006). The EVT2 and PPVT4 are co-normed, and highly-reliable; the composite score based on the arithmetic mean of these two measures provides a coarse but robust assay of gross language ability that is not time- or labor-intensive for young children and that can be used longitudinally along the lifespan. The PPVT, in particular, has been widely utilized in several large studies of outcomes in preterm children, including the TIPP trial of indomethacin in prematurity (Luu et al., 2009; Ment et al., 2006; Mullen et al., 2011; Myers et al., 2010).
2.1.3. Structural MRI acquisition
A T1-weighted structural image was obtained for each subject on a 3.0T Phillips Achieva scanner with a T1 turbo field echo (TFE) sequence (TR/TE = 8.055/3.68 ms, 1 × 1 × 1 mm voxels, 160 slices, Matrix = 256 × 256 × 160). The structural image was used to create an “imitation” T2-weighted image and the original volume was registered to the subject's diffusion data during preprocessing. Matching the contrast of the b0 image by creating an imitation T2 improved the accuracy of the registration and distortion correction of the diffusion data. No quantitative analyses were performed with the imitation volume. All structural preprocessing was performed in AFNI (Cox, 1996).
2.1.4. fMRI activation map
To investigate white matter changes supporting receptive language, we identified cortex preferentially ‘active’ for stories listening in EPT and TC groups. The activation map was obtained from the stories versus noise listening contrast in fMRI as we reported previously (Barnes-Davis et al., 2018). The task and conventional GLM analyses have been used extensively (Holland et al., 2001, 2007; Schapiro et al., 2004). We previously showed that the groups engage the same cortical network during stories listening, thus, a joint activation map was used for subsequent functionally-constrained diffusion connectometry analyses (Figs. 3, 4, 5, 7). The joint activation map was used as a region-of-interest so only tracks containing voxels within these regions were resolved
Fig. 3.
Receptive Language Network Group Comparison, Control > Preterm. Receptive language network connectometry analysis showing the relationship between group (TC vs. EPT) and white matter connectivity. Results show tracks where TC > EPT, which are widely distributed (FDRq < 0.05). The length threshold used to determine FDRq < 0.05 was 30 mm.
Fig. 4.
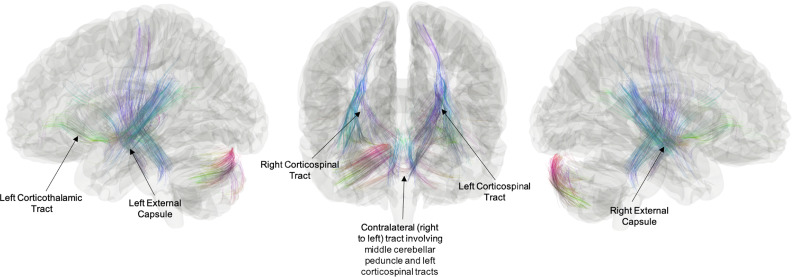
Receptive Language Network Group Comparison, Preterm > Control. Receptive language network connectometry analysis showing the relationship between group (TC vs. EPT) and white matter connectivity. Results show tracks where EPT > TC, including bilateral corticospinal pathways, bilateral external capsules, left corticothalamic pathways, left corticopontine pathways, and bilateral middle cerebellar peduncles. Tract directionality is inferred from known anatomy of the significant tracts resolved. The length threshold used to determine FDRq < 0.05 was 30 mm.
Fig. 5.
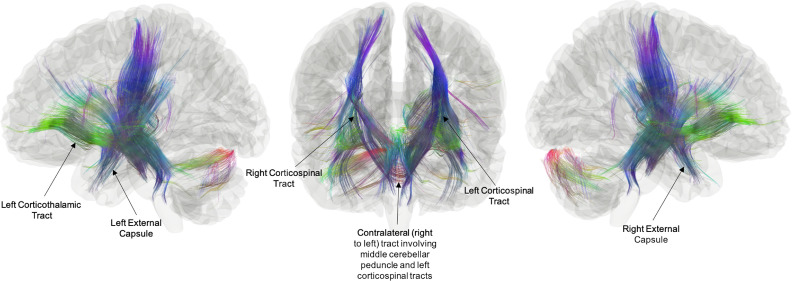
Receptive Language Network Group Comparison while Controlling Group Differences in Performance. Receptive language network connectometry analysis showing the relationship between group (TC vs. EPT) and white matter connectivity while controlling for differences in language performance between groups (FDRq < 0.05). Results show tracks where EPT > TC including bilateral corticospinal pathways, bilateral external capsules, left corticothalamic pathway, bilateral middle cerebellar peduncles, and cerebellar white matter within the right hemisphere. There were no tracks where TC > EPT (FDRq > 0.05). Tract directionality is inferred from known anatomy of the significant tracts resolved. The length threshold used to determine FDRq < 0.05 was 30 mm.
Fig. 7.
Language Performance within EPT: Receptive Network. Receptive language network connectometry analysis showing the relationship between language performance and white matter connectivity within the EPT group. Results show widespread positive associations with language performance (FDRq < 0.05). These tracks encompass most of the white matter in the receptive language network with too many pertinent tracks resolved to clearly label, so we focused on the finding most vital to our paper: the track from the right cerebellum to the left corticopontine fiber pathway. There were no connectomes with a negative correlation with language performance. Tract directionality is inferred from known anatomy of the significant tracts resolved. The length threshold used to determine FDRq < 0.05 was 30 mm.
2.1.5. Diffusion acquisition and preprocessing
Diffusion data were acquired on the same scanner with a DWI-SE sequence (b = 800 s/mm2, 32 directions distributed over the full-sphere, 1 b0, TR/TE = 8955/77 ms, 1.875 × 1.875 × 2.37 mm voxels, 55 slices, Matrix = 96 × 96 × 55). The diffusion acquisition takes approximately 5.5 min and occurs approximately 40 min from the beginning of the MRI scanning session. Movement during scanning was minimized by implementation of several child friendly strategies, including a video available before scanning for children to see the MRI scanner and hear scanner noises via an audio file; a detailed explanation of the process prior to scanning that included analogies that have previously been successful (e.g. be like a statue); a selection of age-appropriate videos or shows available for viewing during the diffusion acquisition; close monitoring; and frequent positive reinforcement though praise, sticker charts, and prizes.
Diffusion preprocessing was performed with TORTOISE (Pierpaoli et al., 2010). First, denoising of the diffusion volumes was performed using random matrix theory (Veraart et al., 2016). Then, Gibbs ringing artifact was removed by sub-voxel shift (Kellner et al., 2016). Motion and eddy current correction were then applied using a quadratic eddy current correction with very rigid search space from the b0 volume to the structural image (imitation T2 image). The imitation T2 is used for preprocessing because it more closely matches that contrast of the b0 image, allowing for better registration. However, the imitation volume is not used for any quantification. The b0 image was then nonlinearly aligned to the structural image using the ANTs Symmetric diffeomorphic mapping (ANTSSyN algorithm; Avants et al., 2008). During this alignment, geometric distortions in the b0 volume were corrected by non-uniform B-spline grid interpolation (Irfanoglu et al., 2011). This technique treats the structural image as the “ground truth” for the non-distorted anatomy of the participant, allowing estimation of the geometric distortion in the diffusion acquisition. This transform is then applied to all volumes in the diffusion dataset, resulting in an aligned, denoised dataset that has been corrected for motion, eddy currents, and geometric distortions. At all steps in the pipeline in which the dataset is transformed, the diffusion vectors are rotated accordingly.
Data were then imported and reformatted to be used in diffusion signal reconstruction, tractography, and statistical analyses within DSI Studio (http://dsi-studio.labsolver.org). Before signal reconstruction, all datasets underwent quality control, in which the correlation between neighboring diffusion volumes was calculated to assess any corrupted volumes. All volumes with a correlation r < 0.9 were removed. This is a very conservative threshold for data inclusion, but we felt that it was necessary since our dataset contained so few diffusion directions, meaning that corrupted data could have a large impact on the final results. Participants were excluded from the analysis if more than 10% of volumes were removed from the dataset. The most likely reason for removal of participants at this point in the analysis was intraslice motion, in which the participant has significant movement between diffusion gradient pulses. This causes signal dropout that cannot be recovered by preprocessing. By using motion correction and this conservative threshold for volume removal, all datasets included in the final analysis for both the EPT and TC groups should be comparable in terms of image quality and motion during the scan.
Diffusion data were reconstructed using Q-Space Diffeomorphic Reconstruction (Yeh and Tseng, 2011) to obtain spin distribution functions (SDFs) for each participant. In QSDR, first the participant's diffusion data is reconstructed using generalized Q-sampling imaging (GQI; (Yeh et al., 2010). GQI is a direct approximation of the SDF from the diffusion signal by taking advantage of the Fourier transform relationship between the MR signal and diffusion displacement. Quantitative anisotropy (QA) is derived from these SDFs, which is a quantitative measure of diffusion that incorporates both diffusivity (like FA and generalized FA), but is also weighted by the density of the diffusing spins. Studies have shown that this measure improves the reliability of deterministic tractography compared to diffusivity-specific measures, such as FA or generalized FA (Yeh et al., 2013). GQI was chosen due to its sensitivity to crossing fibers and its flexibility. GQI can be applied to any sequence in which the diffusion scheme is balanced, i.e. the isotropic voxels in the data are reconstructed as an isotropic SDF (Yeh et al., 2010). This is checked during reconstruction, which indicated our sampling scheme is balanced. Previous studies with similar data quality have successfully employed this methodology (Kahn et al., 2017).
The resulting QA map is then diffeomorphically aligned to a template in MNI space, created by averaging 1021 QA maps calculated from Human Connectome Project data (HCP-1021 template). After alignment, the inverse Jacobian from this warp is then applied to the subject space SDF, while adjusting for scaling differences between the subject and the template and conserving the amount of diffusion spins in the original data (Yeh and Tseng, 2011). One limitation of this method is that it assumes that the subject and template have a one-to-one structure correspondence to allow computation of the inverse Jacobian. While the subjects in this study are younger than those used to construct the template, no subject was included with known brain injury so alignment should still be reasonable with the template. Default settings were used for QSDR except for the number of fibers resolved, which was set at 3 due to the low directionality of the data. DSI Studio was used for the reconstruction.
2.2. Statistical analyses
2.2.1. Demographic and behavioral variables
Between group comparisons of continuous variables (age, performance on assessments) were performed using independent samples t-tests. Categorical variables (sex, race, ethnicity, maternal education) were compared between groups using Fisher's exact test.
2.2.2. Group connectometry
Diffusion connectometry analyses (Yeh et al., 2016) were used to study the relationship of white matter connectivity with prematurity and language. First, a study-specific template was created by averaging the SDFs that resulted from the QSDR reconstruction (already in MNI space) for 10 TC and 10 randomly selected EPT participants. Each subject's SDFs are then further aligned to the study-specific template using a similar method to QSDR (diffeomorphic mapping, conservation of spin density). A spatial correlation coefficient was calculated between the subject and template quantitative anisotropy (QA) map, which is derived from the SDF, to assess the quality of the registration (Yeh et al., 2016). Using the SDFs, the local connectome (i.e. the connectivity between adjacent voxels within a white matter bundle based on the density of spins) for each subject is resolved by sampling their SDFs by the local fiber directions in the study-specific template. The standard space template (HCP-1021) was registered to the study-specific template using diffeomorphic mapping to allow consistent identification of anatomical structures.
Each subject's local connectome matrix is shaped into a single row vector and combined into a matrix where the rows are each subject and the columns are SDF values for a local fiber direction. SDF values are then associated with a study variable of interest using a regression model, resulting in a one row vector of beta values for each local fiber direction. Bootstrap resampling with 5000 iterations was used to obtain the empirical distribution of betas. Additionally, 2000 iterations among the row vectors was used to obtain the null distribution of betas. For each of these conditions, only betas that passed 0.6*Otsu's threshold were passed on to subsequent analyses (Otsu, 1979).
After this initial thresholding, an additional t-score threshold can be used to inform fiber tracking. Importantly, statistical significance is not derived from this t-score threshold (from the multiple regression), but from the permutation testing on track length (discussed in the next section). This allows one to adjust the trade-off between specificity and sensitivity by choosing an appropriate t-score threshold for a particular dataset. For our analyses, we chose a t-score threshold of 3.5. This means that we ignored weak to moderate correlations from the regression analysis when calculating the final statistics. Importantly, this threshold is only used to inform tracking and should not be considered in context of statistical significance. These t-scores used for the threshold are not corrected for multiple comparisons, which is accomplished by permutation testing after SDFs are passed to connectometry analyses. While many thresholds were tested, this threshold provided a good balance between specificity and sensitivity.
Local directions that passed this threshold were mapped using deterministic fiber tracking, which has been shown to be less sensitive to false positives than probabilistic tracking (Maier-Hein et al., 2017). A minimum track length of 30 mm and one iteration of track trimming were used to remove tracks most likely to be false positives. The technique used for trimming was topology-informed pruning (Yeh et al., 2019), which prunes tracts that have an unrealistic topology. A seed density of 20 seeds per mm3 was used because it was identified as an appropriate middle ground for both whole brain and constrained connectometry. A smaller density may lead to results that are too sparse for the constrained analysis while a larger one could lead to an overabundance of less significant results.
Statistical significance–corrected for multiple comparisons using the false discovery rate (FDR)–was calculated by comparing the track length of associations between null local connectomes and the study variable with those from the empirical data. The underlying assumption is that track length along significant associations with the study variable in the non-permuted condition is longer than that of the permuted condition. It is possible for significant tracks to be resolved–especially if they are very short–that are false positives. This necessitates the use of the minimum track length threshold noted previously. As opposed to choosing the FDR threshold, we chose a length threshold (30 mm) for which the FDR was calculated. Results were reported if they passed FDRq < 0.05.
Connectometry was performed as a whole-brain analysis and within a previously identified receptive language network, which was originally in MNI space and registered to the study template using the same transform that was used to register the standard space template to the study-specific template. For a detailed account of the receptive language network identified in these same participants using task-based fMRI and comprised of superior and middle temporal areas, please see our prior report (Barnes-Davis et al., 2018). These areas were utilized as regions of interest (ROIs) for the constrained analysis. The local connectomes generated using these ROIs (and subsequently refined in further analysis, including controlling for multiple comparisons) are detailed in Supplementary Fig. 1. After connectometry and tract identification, all tracts were visually inspected to ensure that they were not labeled as multiple pathways and to ensure that the label was reasonable. All diffusion analyses were performed using DSI Studio (http://dsi-studio.labsolver.org).
2.2.3. Connectometry analyses
Four regression analyses were performed using whole brain and functionally-constrained connectometry. First, group was included in the analysis to observe effects related to prematurity. Second, group and composite language performance were included to observe effects of prematurity while controlling for language ability. Finally, an analysis looking at relationship between white matter and performance was performed within groups: EPT and TC. Language performance for our purposes refers to the composite index derived from the PPVT4 and EVT2 scores. The dependent variable in all models was local connectome connectivity, which measures the density (strength) of diffusing spins in a given direction within a voxel (Yeh et al., 2016). Total white matter volume, determined by structural analysis of the T1 image in FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) did not significantly differ between groups and was not significantly related to performance in either group, justifying its exclusion in statistical modeling (p > 0.05). Collinearity diagnostics were performed to ensure the model could tolerate inclusion of group and performance as unique predictors (VIF < 5).
3. Results
3.1. Demographics and neurobehavioral assessments
One EPT participant was found to be an outlier on analysis of spin distribution functions (SDFs) and was excluded from final analyses. A total of 12 EPT and 10 controls contributed data to the current analyses. There were no significant differences between groups for age, sex, race, or ethnicity. Mean gestational age for the EPT group was 25 weeks 5 days, ranging from 24 weeks 0 days to 26 weeks 6 days. There were four females in the EPT group and five in the TC group. Mean age was 5.3 years in the EPT group and 5.5 years in the TC group. There were eight children in the EPT group whose parent identified as White/Caucasian and seven in the TC group. Demographic data for the sample are available in Table 1. There were no significant differences in maternal income or education level.
Table. 1.
Demographics and neuropsychological data for entire sample.
| Preterm (n = 12) | Term (n = 10) | p Value | ||
|---|---|---|---|---|
| Age (Years, Mean ± SD) | 5.4 ± 0.79 | 5.5 ± 1.06 | 0.705 | |
| Sex | ||||
| Females | 4 | 5 | 0.42 | |
| Males | 8 | 5 | ||
| Race | ||||
| White/Caucasian | 8 | 7 | 0.2 | |
| Black/African American | 3 | 0 | ||
| Other/Multiple | 1 | 1 | ||
| No Response | 0 | 2 | ||
| Hispanic/Latino/Latina | 0 | 1 | 0.078 | |
| Not Hispanic/Latino/Latina | 12 | 7 | ||
| No Response | 0 | 2 | ||
| Receptive language | ||||
| PPVT-4 (Mean ± SD) | 115 ± 11 | 138 ± 9 | <0.001 | |
| Expressive language | ||||
| EVT-2 (Mean ± SD) | 101 ± 11 | 120 ± 12 | <0.001 | |
| General abilities | ||||
| WNV (Mean ± SD) | 106 ± 15 | 120 ± 11 | 0.01 |
Note: Categorical variables were tested using Fisher Exact Test and p values are reported. Continuous variables were tested using t tests and p values are reported. CI = Confidence Interval. SD = Standard Deviation. PPVT-4 = Peabody Picture Vocabulary Test. EVT-2 = Expressive Vocabulary Test. WNV = Wechsler Non-Verbal Scale of Ability.
Analysis of standardized scores on neurobehavioral assessments showed significant group differences (see Table 1). The control group performed better (indicated by higher standardized scores) than the EPT group on all assessments. The mean and standard deviation on the PPVT4 was 115 ± 11 for the EPT group and 138 ± 9 for the TC group (p < 0.001). For the EVT2, the mean scores for the EPT group (101 ± 11) were lower than those of the TC group (120 ± 12, p < 0.001). Similarly, for the WNV, the EPT group mean was (106 ± 15) and the TC group mean was (120 ± 11, p = 0.01). Although the control group performed at a higher level than the EPT group, it is notable that the EPT participants were not impaired, as scores for all three assessments were in the normal range (mean = 100 ± 15).
Because of the small sample size, we performed multiple statistical tests to ensure the validity of the correlation analyses. Normality of language scores was evaluated by Q-Q plot (Supplementary Fig. 2) and formally assessed using the Shapiro–Wilk test (W = 0.921, p = 0.255). To ensure there were no outliers, we used both a boxplot (Supplementary Fig. 3) and a Dixon's Q test for both high (Q = 0.10, p = 0.193) and low values (Q = 0.065, p = 0.08). We also performed a Student's T-test to assess the association between sex and performance (t(11) = −0.074, p = 0.447) and a correlation to assess the association between performance and gestational age (r = 0.069, p = 0.82). We failed to reject the null in all cases, indicating the correlation analysis is appropriate.
3.2. Structural connectivity analyses
3.2.1. Whole-Brain group comparison
Whole-brain analyses showed significantly decreased structural connectivity in distributed tracks, notably in regions of the corpus callosum, for EPT children versus TC (Fig. 1). On whole-brain analysis, there was significantly increased structural connectivity for the EPT group compared to controls in bilateral cerebellar tracks (Fig. 2).
Fig. 1.
Whole-Brain Between-Groups Comparison, Control > Preterm. Tracks in which connectivity was significantly greater for TC children compared to EPT children. Notable tracks include those through the corpus callosum. All connectometry analyses result in subcomponents of pathways that are significantly associated with the study variable, but are not necessarily entire fascicles. Results are color coded for the local directionality of the tracts and colors change along the track as it changes directionality along the path (red = left/right, green = anterior/posterior, blue = inferior/superior). The background image for all figures is a surface rendering of a standard space T1 image from the study specific template warped from MNI space. Results are viewed in a transparent surface rendering, showing tracks from three points of view: left sagittal, front coronal, right sagittal. These views aid visualization because they show the relative depth of the tracks resolved and ensure no tracks are occluded by tracks from the contralateral hemisphere. The length threshold used to determine FDRq < 0.05 was 30 mm.
Fig. 2.
Whole-Brain Between-Groups Comparison, Preterm > Control. Tracks in which connectivity was significantly greater for EPT than TC. Results include white matter within both hemispheres of the cerebellum. The length threshold used to determine FDRq < 0.05 was 30 mm.
3.2.2. Receptive language network group comparison
Within the language network, distributed areas of relative hypoconnectivity for the EPT group, including the corpus callosum, persisted (Fig. 3) However, the EPT group exhibited increased structural connectivity compared to the TC group which may utilize an indirect (extracallosal) pathway (FDRq < 0.05). These local connectomes included bilateral corticospinal pathways, bilateral external capsules, left corticothalamic pathways, left corticopontine pathways, and bilateral middle cerebellar peduncles (Fig. 4). We do hypothesize that this would be a polysynaptic pathway (i.e. right cerebellar white matter to middle cerebellar peduncle to the pons and subsequently the corticospinal pathways). While tracts resulting from this model seem to overlap, they are indeed distinct and seem to suggest different utilization of common pathways. For example, these results suggest EPT may be more reliant on pathways that involve cerebellar tracts and right lateralized tracts (Supplementary Fig 6).
3.2.3. Receptive language network group comparison while controlling group differences in performance
After controlling for group differences in language performance, children born EPT still exhibited increased structural connectivity, with local connectomes including bilateral corticospinal pathways, bilateral external capsules, left corticothalamic pathways, and bilateral middle cerebellar peduncles. Additionally, this included findings within the right cerebellar white matter and the left corticopontine pathway (Fig. 5). Again, the proposed extracallosal pathway would involve the right corticospinal pathway, the white matter in the right cerebellar hemisphere, the middle cerebellar peduncle, and the left corticopontine and corticospinal pathways. There were no tracks in which EPT children had decreased connectivity relative to the TC group after controlling for group differences in performance.
3.2.4. Language performance within EPT group
There were widespread positive correlations between subcomponents of many white matter pathways and language performance in the EPT group in the whole-brain connectometry data (Fig. 6). Similarly, within the receptive language network, there were positive associations between performance and connectivity in most of the network, including bilateral arcuate fasciculi, white matter within the cerebellum, the corpus callosum, bilateral corticospinal pathways, bilateral corticothalamic pathways, bilateral internal and external capsules, and bilateral inferior fronto-occipital fasciculi (Fig. 7). These results suggest that white matter connectivity positively correlates with better language performance in EPT children. When we further corrected analyses at a more conservative threshold (FDRq < 0.001) we resolve the most robust effects related to performance in the EPT group. In this case, language performance was positively associated with local connectomes in the bilateral external capsules, left inferior fronto-occipital fasciculus, left corticothalamic pathway, left corticopontine pathway, bilateral middle cerebellar peduncles, and intrahemispheric white matter bundles within the right hemisphere of the cerebellum. A plot showing the relationship between mean SDF and performance in the EPT group can be found in Supplementary Fig. 4. To ensure that the contralateral tracts resolved in the group analysis in which EPT > TC were the ones also related to performance, the mean SDF of the within stories network group analysis (Fig. 4) were plotted against language performance. Results showed that there was indeed a positive correlation between performance and those tracts that were resolved by the group analysis in the stories network in which EPT > TC (Supplementary Fig 7). Supplementary Fig. 8 has been added to show the t-score threshold distribution of the tracts that passed the initial t-score threshold > 3.5. Additionally, we performed an analysis investigating the relationship between SDF and performance in the EPT group while controlling for sex (Supplementary Fig. 5) that shows results are similar after accounting for sex in the model.
Fig. 6.
Language Performance within EPT: Whole Brain. Whole-brain connectometry analysis showing the relationship between language performance and white matter connectivity within the EPT group. Results show widespread positive associations with language performance (FDRq < 0.05). There were no tracks with a negative correlation with language performance. Tract directionality is inferred from known anatomy of the significant tracts resolved. The length threshold used to determine FDRq < 0.05 was 30 mm.
4. Discussion
4.1. Significance
We hypothesized the increased functional connectivity is supported by an indirect–perhaps compensatory–structural language network in EPT. Our results support our hypothesis that an indirect structural network exists, as we observed increased structural connectivity in an indirect, interhemispheric (i.e. left perisylvian, right cerebellum, and right perisylvian) pathway that may underlie this difference. Local connectomes which may be a part of this pathway include parts of major white matter tracts including the right corticospinal pathways, the middle cerebellar peduncle, white matter within the right cerebellar hemisphere, the left corticopontine pathway, and the left corticospinal pathway. These findings persisted after controlling for group differences in performance, suggesting that they exhibit a true association with prematurity and not simply a performance effect. Increased extracallosal structural connectivity may represent a biomarker for resiliency in preterm children specifically, as it is positively correlated with performance in EPT children.
Our findings–while novel–are consistent with some previous neuroimaging reports. Previous studies assessing functional connectivity of language networks have reported increased bitemporal connectivity in children born preterm versus controls (Gozzo et al., 2009; Schafer et al., 2009; Scheinost et al., 2015; Wilke et al., 2014), apparently contradicting structural imaging studies showing decreased interhemispheric connectivity in children born preterm, reflected by decreased FA diffusely and in the corpus callosum (Constable et al., 2008; Northam et al., 2012) and/or decreased callosal volume (Malavolti et al., 2017; Mullen et al., 2011; Solsnes et al., 2016). The current study provides clear structural evidence addressing this discrepancy, perhaps for the first time.
Our study relies on data acquisition at a single timepoint and is therefore unable to speak to the causality behind this association. However, when taken in the context of the aforementioned literature reporting functional hyperconnectivity in EPT children, it is plausible to speculate that the extent to which a preterm child engages this interhemispheric cortico-cerebellar pathway predicts performance (greater engagement leads to better performance). This would be similar to the phenomenon in which contralateral reserves in the right hemisphere are recruited during a language task in children who suffer early neurological insult or lesions, producing atypical language representation maps that persist into adulthood instead of the typical development of left lateralization (Branch et al., 1964; Gaillard et al., 2003; Kadis et al., 2007, 2011; Rasmussen and Milner, 1977; Satz et al., 1988; Wood et al., 2004). Future longitudinal studies assessing the developmental trajectory of the preterm connectome supporting language should test this hypothesis.
In addition to our novel finding of extracallosal structural hyperconnectivity relating to language performance, we did find evidence of distributed tracts showing structural hypoconnectivity in preterm children versus controls. This included significantly decreased connectivity in the corpus callosum, similar to that reported by another recent paper utilizing deterministic tractography in preterm children (Dodson et al., 2017). Our study differs from theirs in a number of key ways. First, their sample is more heterogenous, including children with a wider range of gestational ages and children who had other neurological issues, such as cerebral palsy. Second, in order to increase their sensitivity, they assessed individual pathways in the native space for each child, and limited group-level analyses to a priori defined tracts selected based on their findings in older children and adolescents. We chose to pursue a more data-driven approach. Third, our study is not directly computing FA or other conventional tensor-based metrics–which can be inaccurate in areas of complex fiber orientation and can fail to capture subtle variations in highly complex fiber orientation such as the lateral fanning aspects of the forceps major or in highly interdigitated areas such as the corpus callosum – so the results of our local connectometry analyses are not immediately comparable. Previous studies from our lab have shown variable developmental trajectories across the structure, so we feel to make statements about the averaged connectivity across the callosum would be to miss out on regional variation throughout this highly interdigitated structure (Williamson et al., 2019). Thus, we feel our findings of simultaneous hyper- and hypo-connectivity in children born extremely preterm are biologically plausible.
The aim of our experiment is not to delineate the initial insults of prematurity that might necessitate the development of alternate networks in the extremely preterm brain, as risk has been a primary interest of other investigators in the realm of perinatal neurology. For excellent reviews of the neuropathology thought to underlie the so-called encephalopathy of prematurity, please see texts by Joseph Volpe in which he elucidates many mechanisms, including ischemia; lack of cerebral autoregulation; focal and diffuse necrosis; damage to premyelinating oligodendrocytes; astrogliosis and microgliosis; axonal degeneration in the corpus callosum and periventricular white matter; and volumetric loss diffusely and preferentially for the corpus callosum, parieto-occipital, temporal, and hippocampal cortices (Volpe, 2009a, Volpe, 2009b; Volpe, 2009c). Of particular interest in the context of our between-groups analysis (increased connectivity in the bilateral cerebellar white matter for EPT children, Fig. 2) is the rapid growth which occurs perinatally in the cerebellum. The cerebellum increases in neuron count, volume, and surface area dramatically in the last trimester of gestation and the first year of life. Peak growth and differentiation at a corrected gestational age of 30–40 weeks results in a 30-fold increase in cerebellar hemispheral surface area (Volpe, 2009b). This period is extremely relevant for development in EPT children, as they are born before the transition between the second and third trimesters; and they experience this maturation–or dysmaturation–outside of the womb. While cerebellar injury and associated decreased size does occur in EPT infants–resulting from hemorrhage, infarction, indirect insult from remote areas of injury (cerebrocerebellar diaschisis) and dysmaturation–it is often in the context of more severe IVH or PVL (Volpe, 2009b). This was intentionally excluded in our sample to try to tease out effects of prematurity from those of frank brain injury and might preclude direct comparison with other preterm neuroimaging studies conducted with a more heterogeneous sample. It should be noted that leukomalacia in the cerebellum is relatively rare, occurring in only 8% of preterm infants on autopsy (Pierson et al., 2007).
Since the 1990s, the non-motor significance of the cerebellum has been an area of increasing interest, with domains such as social cognition, visuospatial processing, and language being very applicable to neurocognitive deficits reported in extremely preterm children (Argyropoulos, 2016; Fernandez et al., 2016; Fiez, 2016). The role of the cerebellum as a mediator in the preterm language network is a particularly relevant emerging area of interest, with connectivity analyses of resting state fMRI suggesting it might be important in preterm-born adults (Constable et al., 2013). In term preschoolers, increased activation in the right cerebellum on fMRI was associated with greater interest in shared story reading (Hutton et al., 2017). Additionally, a recent study looking at term born children who were diagnosed with dyslexia and their well-reading controls found that children in the dyslexia group had increased FA in cerebellocerebral pathways from the right cerebellum to the left temporoparietal region. The authors concluded that this projection from the right anterior cerebellum seemed to have a regulatory effect which could help compensate for the weak cortical reading network (Fernandez et al., 2016). We feel it is feasible that a similar mechanism is taking place in our sample of relatively healthy, well-performing extremely preterm children. Corticopontine fibers serve as a line of communication between the hemispheres of the cerebral cortex and the opposite cerebellum to allow coordination of planned motor functions (Rea, 2015). We report significant between-groups differences in the cerebellum, and the extracallosal, interhemispheric tracks we report that have a positive association with performance for the EPT group specifically involve the right cerebellum in particular. Thus, results of the current study suggest more utilization of this pathway in EPT children to compensate for dysmaturation or white matter injury associated with prematurity, which would be in accordance with similar proposed mechanisms in motor networks and with the diffuse white matter hypoconnectivity exhibited in our EPT group (Figs. 1 and 3). This is consistent with the idea that the cerebellum is heavily involved in both adaptive motor control and language and reading performance, adjusting neural pathways after perturbation (Popa and Ebner, 2019). In the EPT brain, white matter injury may be continuously perturbing the process of receptive and expressive language function, necessitating increased input from the cerebellum to achieve typical functioning.
4.2. Strengths
Our study is unique in its exclusion of known brain injury or neurological disorder, including language impairment. While this precludes generalizability to all EPT children, it does enable us to attribute group differences to relatively “pure” effects of prematurity. The diffusion analysis improves upon prior methods in several ways. First, the diffusion profile of each voxel is reconstructed using a modified Q-Ball imaging approach, which is sensitive to crossing fibers known to occupy the medullary center diffusely (Jeurissen et al., 2013; Yeh et al., 2010). Second, connectometry uses the “local connectome” for statistical comparisons which does not rely on whole track averages used in some forms of conventional analysis. This increases sensitivity to local changes that are lost in whole brain analysis (Yeh et al., 2016). Third, we employ an a-theoretical approach that does not constrain analyses to classical regions and pathways associated with language in typically developing full-term adolescents and adults.
4.3. Limitations
Weaknesses of our study include the small number of participants, the use of receptive and expressive language tasks that are highly dependent on vocabulary and semantic knowledge as a gross assay of language abilities, and the low b-value, single shell diffusion acquisition. Future studies will certainly benefit from accelerated multishell diffusion acquisitions currently available on most MR platforms. Furthermore, as these children continue to grow and are studied at more advanced ages, more complex and comprehensive measures of language–including sentence production, syntax, prosody, morphology, comprehension, and semantics–will become more feasible, enabling us to fully parse expressive and receptive language abilities. Additionally, because the groups differed in WNV scores–which can be used as an estimate of nonverbal intelligence–it must be considered that the observed differences could be due to IQ differences. Although there were significant differences between groups on the WNV assessment of nonverbal abilities, when we tested this hypothesis, there were no statistically significant correlations between these scores and our connectometry analysis between or within groups. We utilize a novel analysis pathway, including connectometry. One limitation of connectometry is its reliance on registration and sampling in a template space to obtain results. Misalignment may lead to errors in directional sampling that could influence results. To minimize this limitation, we used a study-specific template and ensured adequate alignment of each subject to this template, hopefully mitigating any erroneous results. Another possible limitation is the necessity of continuity of tracks to pass the minimum length threshold. If there is a track that has a nearly significant middle portion and has significant ends, it would be broken up into smaller, insignificant tracks that would be eliminated by the threshold. While this may lead to false negatives, we feel the conservativeness of this approach leads to results that are highly confirmative. Because connectometry does not take into account the width of the tract or effect size, long tracts (represented by long tracks) are more likely to be significant. This limitation might be the reason that inter-hemispheric tracks are more likely to survive strict corrections for multiple comparisons (as they are usually longer than intra-hemispheric tracks). In that sense, our findings might have been biased by our analysis technique. We acknowledge that the t-score threshold used to inform fiber tracking–while selected to provide a good balance between specificity and sensitivity–is a subjective threshold and is a limitation to this method. Automated selection techniques will be explored in future studies.
Despite these limitations, we feel our results are clinically and scientifically significant. The structural connectometry methods chosen are amenable to smaller sample sizes and are appropriately conservative. The results we report are likely the most robust effects between EPT and TC children in our sample. Future studies with larger samples and relatively rich diffusion acquisitions will enable better characterization of subtle changes, providing a more comprehensive understanding of biomarkers for risk and resiliency. Finally, it should be noted that – while our data may support our hypothesis that the increased connectivity in the EPT group represents an extracallosal pathway supporting language performance – we cannot prove this with the existing data. It is also possible that observable behavior is correlated with these different tracks included in our connectometry results, but that these tracks do not form a continuous pathway.
5. Conclusion
The reported experiments confirmed our initial hypothesis that EPT children who are functioning within normal limits have alternate extracallosal networks positively associated with language performance. Children who are born EPT are at risk for neurocognitive deficits and language impairment. Despite this risk, some children overcome the neurological insults of prematurity and function normally. The current report suggests that some of this resiliency resides in white matter plasticity (both cerebral and cerebellar) that allows for functional and effective hyperconnectivity between canonical language areas bilaterally. Research into the causality and developmental trajectory of this hypothesized relationship between brain structure and observable function specifically–and into factors that confer resiliency generally–should be prioritized alongside research examining those conferring risk. This will allow providers and caregivers to target interventions and counseling in the perinatal period and beyond.
Funding
This work was funded by an award from the National Institute of Child Health and Human Development (K12 HD028827 for MEB-D); an award from the National Center for Advancing Translational Sciences (KL2 TR001426 for SLM); and by a Trustee Award Grant (DSK), a Shared Facilities Discovery Award (SLM), and an Arnold W. Strauss Fellowship Award for Excellence in Research (MEB-D) from the Cincinnati Children's Hospital Medical Center.
Declaration of Competing Interest
None.
Acknowledgements
We would like to thank the participants and their families. We acknowledge Cameron Laue Evans and Claudio Toro-Serey for their study coordination.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102194.
Appendix. Supplementary materials
References
- Aarnoudse-Moens C.S., Weisglas-Kuperus N., van Goudoever J.B., Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Aeby A., De Tiege X., Creuzil M., David P., Baleriaux D., Van Overmeire B., Van Bogaert P. Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. NeuroImage. 2013;78:145–151. doi: 10.1016/j.neuroimage.2013.03.076. [DOI] [PubMed] [Google Scholar]
- Anderson P.J., Cheong J.L., Thompson D.K. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol. 2015;39(2):147–158. doi: 10.1053/j.semperi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Argyropoulos G.P.D. The cerebellum, internal models and prediction in ‘non-motor’ aspects of language: a critical review. Brain Lang. 2016;161:4–17. doi: 10.1016/j.bandl.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davis M.E., Merhar S.L., Holland S.K., Kadis D.S. Extremely preterm children exhibit increased interhemispheric connectivity for language: findings from fMRI-constrained MEG analysis. Dev. Sci. 2018;21(6):e12669. doi: 10.1111/desc.12669. Epub 2018 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre N., Morgan A., Doyle L.W., Anderson P.J. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J. Pediatr. 2011;158(5) doi: 10.1016/j.jpeds.2010.10.032. 766-774 e761. [DOI] [PubMed] [Google Scholar]
- Branch C., Milner B., Rasmussen T. Intracarotid sodium amytal for the lateralization of cerebral speech dominance; observations in 123 patients. J. Neurosurg. 1964;21:399–405. doi: 10.3171/jns.1964.21.5.0399. [DOI] [PubMed] [Google Scholar]
- Chau V., Synnes A., Grunau R.E., Poskitt K.J., Brant R., Miller S.P. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;81(24):2082–2089. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J.L., Anderson P.J., Roberts G., Burnett A.C., Lee K.J., Thompson D.K., Doyle L.W. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PLoS One. 2013;8(10):e77475. doi: 10.1371/journal.pone.0077475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable R.T., Ment L.R., Vohr B.R., Kesler S.R., Fulbright R.K., Lacadie C., Reiss A.R. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Constable R.T., Vohr B.R., Scheinost D., Benjamin J.R., Fulbright R.K., Lacadie C., Ment L.R. A left cerebellar pathway mediates language in prematurely-born young adults. Neuroimage. 2013;64:371–378. doi: 10.1016/j.neuroimage.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell S.J., Boardman J.P. Differential brain growth in the infant born preterm: current knowledge and future developments from brain imaging. Semin. Fetal Neonatal. Med. 2005;10(5):403–410. doi: 10.1016/j.siny.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Counsell S.J., Shen Y., Boardman J.P., Larkman D.J., Kapellou O., Ward P., Rutherford M.A. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117(2):376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Kieviet J.F., Zoetebier L., van Elburg R.M., Vermeulen R.J., Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev. Med. Child Neurol. 2012;54(4):313–323. doi: 10.1111/j.1469-8749.2011.04216.x. [DOI] [PubMed] [Google Scholar]
- Dodson C.K., Travis K.E., Ben-Shachar M., Feldman H.M. White matter microstructure of 6-year old children born preterm and full term. Neuroimage Clin. 2017;16:268–275. doi: 10.1016/j.nicl.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L.M., Dunn D.M., Lenhard A. Vol. 4. Pearson; 2015. (Peabody Picture Vocabulary Test: PPVT). [Google Scholar]
- Eikenes L., Lohaugen G.C., Brubakk A.M., Skranes J., Haberg A.K. Young adults born preterm with very low birth weight demonstrate widespread white matter alterations on brain DTI. Neuroimage. 2011;54(3):1774–1785. doi: 10.1016/j.neuroimage.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Feldman H.M., Lee E.S., Loe I.M., Yeom K.W., Grill-Spector K., Luna B. White matter microstructure on diffusion tensor imaging is associated with conventional magnetic resonance imaging findings and cognitive function in adolescents born preterm. Dev. Med. Child. Neurol. 2012;54(9):809–814. doi: 10.1111/j.1469-8749.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H.M., Lee E.S., Yeatman J.D., Yeom K.W. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez V.G., Juranek J., Romanowska-Pawliczek A., Stuebing K., Williams V.J., Fletcher J.M. White matter integrity of cerebellar-cortical tracts in reading impaired children: a probabilistic tractography study. Brain Lang. 2016;161:45–56. doi: 10.1016/j.bandl.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez J.A. The cerebellum and language: persistent themes and findings. Brain Lang. 2016;161:1–3. doi: 10.1016/j.bandl.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Sachs B.C., Whitnah J.R., Ahmad Z., Balsamo L.M., Petrella J.R., Grandin C.B. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum. Brain Mapp. 2003;18(3):176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield C.F., Karbownik K., Murthy K. Educational performance of children born prematurely. JAMA Pediatr. 2017;171(8):764–770. doi: 10.1001/jamapediatrics.2017.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo Y., Vohr B., Lacadie C., Hampson M., Katz K.H., Maller-Kesselman J., Ment L.R. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48(2):458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.K., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holland S.K., Vannest J., Mecoli M., Jacola L.M., Tillema J.M., Karunanayaka P.R., Byars A.W. Functional MRI of language lateralization during development in children. Int. J. Audiol. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E.A., De Luca C.R., Doyle L.W., Roberts G., Anderson P.J. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131(4):e1053–e1061. doi: 10.1542/peds.2012-2311. Epub 2013 Mar 18. Erratum in: Pediatrics. 2013 Oct;132(4):780.PMID: 23509167. [DOI] [PubMed] [Google Scholar]
- Hutton J.S., Phelan K., Horowitz-Kraus T., Dudley J., Altaye M., DeWitt T., Holland S.K. Story time turbocharger? Child engagement during shared reading and cerebellar activation and connectivity in preschool-age children listening to stories. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfanoglu M.O., Walker L., Sammet S., Pierpaoli C., Machiraju R. Susceptibility distortion correction for echo planar images with non-uniform B-spline grid sampling: a diffusion tensor image study. Med. Image Comput. Comput. Assist. Interv. 2011;14(Pt 2):174–181. doi: 10.1007/978-3-642-23629-7_22. [DOI] [PubMed] [Google Scholar]
- Isaacs E.B., Edmonds C.J., Chong W.K., Lucas A., Morley R., Gadian D.G. Brain morphometry and IQ measurements in preterm children. Brain: J. Neurol. 2004;127(Pt 12):2595–2607. doi: 10.1093/brain/awh300. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J.D., Jones D.K., Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 2013;34(11):2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Hennessy E., Smith R., Trikic R., Wolke D., Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch. Dis. Child Fetal Neonatal Ed. 2009;94(4):F283–F289. doi: 10.1136/adc.2008.152793. [DOI] [PubMed] [Google Scholar]
- Kadis D.S., Iida K., Kerr E.N., Logan W.J., McAndrews M.P., Ochi A., Smith M.L. Intrahemispheric reorganization of language in children with medically intractable epilepsy of the left hemisphere. J. Int. Neuropsychol. Soc. 2007;13(3):505–516. doi: 10.1017/S1355617707070397. [DOI] [PubMed] [Google Scholar]
- Kadis D.S., Pang E.W., Mills T., Taylor M.J., McAndrews M.P., Smith M.L. Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. J. Int. Neuropsychol. Soc. 2011;17(5):896–904. doi: 10.1017/S1355617711000932. [DOI] [PubMed] [Google Scholar]
- Kahn A.E., Mattar M.G., Vettel J.M., Wymbs N.F., Grafton S.T., Bassett D.S. Structural pathways supporting swift acquisition of new visuomotor skills. Cereb. Cortex. 2017;27(1):173–184. doi: 10.1093/cercor/bhw335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner E., Dhital B., Kiselev V.G., Reisert M. Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magn. Reson. Med. 2016;76(5):1574–1581. doi: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- Keunen K., Benders M.J., Leemans A., Fieret-Van Stam P.C., Scholtens L.H., Viergever M.A., van den Heuvel M.P. White matter maturation in the neonatal brain is predictive of school age cognitive capacities in children born very preterm. Dev. Med. Child Neurol. 2017;59(9):939–946. doi: 10.1111/dmcn.13487. [DOI] [PubMed] [Google Scholar]
- Krasileva K.E., Sanders S.J., Bal V.H. Peabody picture vocabulary test: proxy for verbal IQ in genetic studies of autism spectrum disorder. J. Autism Dev. Disord. 2017;47(4):1073–1085. doi: 10.1007/s10803-017-3030-7. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30(11):3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Sun Z., Han Y., Gao L., Yuan L., Zeng D. Fractional anisotropy alterations in individuals born preterm: a diffusion tensor imaging meta-analysis. Dev. Med. Child Neurol. 2015;57(4):328–338. doi: 10.1111/dmcn.12618. [DOI] [PubMed] [Google Scholar]
- Litt J., Taylor H.G., Klein N., Hack M. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J. Learn. Disabil. 2005;38(2):130–141. doi: 10.1177/00222194050380020301. [DOI] [PubMed] [Google Scholar]
- Lowe J., Duvall S.W., MacLean P.C., Caprihan A., Ohls R., Qualls C., Phillips J. Comparison of structural magnetic resonance imaging and development in toddlers born very low birth weight and full-term. J. Child Neurol. 2011;26(5):586–592. doi: 10.1177/0883073810388418. [DOI] [PubMed] [Google Scholar]
- Luttikhuizen dos Santos E.S., de Kieviet J.F., Konigs M., van Elburg R.M., Oosterlaan J. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: a meta-analysis. Early Hum. Dev. 2013;89(7):487–496. doi: 10.1016/j.earlhumdev.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Luu T.M., Vohr B.R., Schneider K.C., Katz K.H., Tucker R., Allan W.C., Ment L.R. Trajectories of receptive language development from 3 to 12 years of age for very preterm children. Pediatrics. 2009;124(1):333–341. doi: 10.1542/peds.2008-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein K.H., Neher P.F., Houde J.-.C., Côté M.-.A., Garyfallidis E., Zhong J., Descoteaux M. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017;8(1):1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavolti A.M., Chau V., Brown-Lum M., Poskitt K.J., Brant R., Synnes A., Miller S.P. Association between corpus callosum development on magnetic resonance imaging and diffusion tensor imaging, and neurodevelopmental outcome in neonates born very preterm. Dev. Med. Child Neurol. 2017;59(4):433–440. doi: 10.1111/dmcn.13364. [DOI] [PubMed] [Google Scholar]
- Martin J.A., Hamilton B.E., Osterman M.J.K. Births in the United States. NCHS Data Brief. 2017;(287):1–8. 2016. [PubMed] [Google Scholar]
- Ment L.R., Peterson B.S., Meltzer J.A., Vohr B., Allan W., Katz K.H., Constable R.T. A functional magnetic resonance imaging study of the long-term influences of early indomethacin exposure on language processing in the brains of prematurely born children. Pediatrics. 2006;118(3):961–970. doi: 10.1542/peds.2005-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G.P., Lemyre B., Barrowman N., Daboval T. Neurodevelopmental outcomes at 4 to 8 years of children born at 22 to 25 weeks' gestational age: a meta-analysis. JAMA Pediatr. 2013;167(10):967–974. doi: 10.1001/jamapediatrics.2013.2395. [DOI] [PubMed] [Google Scholar]
- Mullen K.M., Vohr B.R., Katz K.H., Schneider K.C., Lacadie C., Hampson M., Ment L.R. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54(4):2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E.H., Hampson M., Vohr B., Lacadie C., Frost S.J., Pugh K.R., Ment L.R. Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage. 2010;51(4):1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam G.B., Liegeois F., Tournier J.D., Croft L.J., Johns P.N., Chong W.K., Baldeweg T. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain: J. Neurol. 2012;135(Pt 12):3781–3798. doi: 10.1093/brain/aws276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omizzolo C., Thompson D.K., Scratch S.E., Stargatt R., Lee K.J., Cheong J., Anderson P.J. Hippocampal volume and memory and learning outcomes at 7 years in children born very preterm. J. Int. Neuropsychol. Soc. 2013;19(10):1065–1075. doi: 10.1017/S1355617713000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cybern. 1979;9(1):62–66. [Google Scholar]
- Parikh N.A., He L., Bonfante-Mejia E., Hochhauser L., Wilder P.E., Burson K., Kaur S. Automatically quantified diffuse excessive high signal intensity on MRI predicts cognitive development in preterm infants. Pediatr. Neurol. 2013;49(6):424–430. doi: 10.1016/j.pediatrneurol.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Walker L., Irfanoglu M., Barnett A., Basser P., Chang L.-.C., Wu M. TORTOISE: An Integrated Software Package for Processing of Diffusion MRI Data. 2010 [Google Scholar]
- Pierson C.R., Folkerth R.D., Billiards S.S., Trachtenberg F.L., Drinkwater M.E., Volpe J.J., Kinney H.C. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114(6):619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa L.S., Ebner T.J. Cerebellum, predictions and errors. Front. Cell Neurosci. 2019;12 doi: 10.3389/fncel.2018.00524. 1–13 pp. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T., Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann. N Y Acad. Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Rea P. Chapter 5 – Hindbrain (rhombencephalon) In: Rea P., editor. Essential Clinical Anatomy of the Nervous System. Academic Press; San Diego: 2015. pp. 91–98. [Google Scholar]
- Satz P., Strauss E., Wada J., Orsini D.L. Some correlates of intra- and interhemispheric speech organization after left focal brain injury. Neuropsychologia. 1988;26(2):345–350. doi: 10.1016/0028-3932(88)90087-5. [DOI] [PubMed] [Google Scholar]
- Schafer R.J., Lacadie C., Vohr B., Kesler S.R., Katz K.H., Schneider K.C., Ment L.R. Alterations in functional connectivity for language in prematurely born adolescents. Brain: J. Neurol. 2009;132(Pt 3):661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro M.B., Schmithorst V.J., Wilke M., Byars A.W., Strawsburg R.H., Holland S.K. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15(17):2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Lacadie C., Vohr B.R., Schneider K.C., Papademetris X., Constable R.T., Ment L.R. Cerebral lateralization is protective in the very prematurely born. Cereb. Cortex. 2015;25(7):1858–1866. doi: 10.1093/cercor/bht430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J., Vangberg T.R., Kulseng S., Indredavik M.S., Evensen K.A., Martinussen M., Brubakk A.M. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain: J. Neurol. 2007;130(Pt 3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Solsnes A.E., Sripada K., Yendiki A., Bjuland K.J., Ostgard H.F., Aanes S., Skranes J. Limited microstructural and connectivity deficits despite subcortical volume reductions in school-aged children born preterm with very low birth weight. NeuroImage. 2016;130:24–34. doi: 10.1016/j.neuroimage.2015.12.029. [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E.M.S., Spreen O. Oxford University Press; New York, NY, US: 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed. [Google Scholar]
- Taylor H., Klein N., Anselmo M.G., Minich N., Espy K.A., Hack M. Learning problems in kindergarten students with extremely preterm birth. Arch. Pediatr. Adolesc. Med. 2011;165(9):819–825. doi: 10.1001/archpediatrics.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort-van der Spek I.L., Franken M.C., Weisglas-Kuperus N. Language functions in preterm-born children: a systematic review and meta-analysis. Pediatrics. 2012;129(4):745–754. doi: 10.1542/peds.2011-1728. [DOI] [PubMed] [Google Scholar]
- Veraart J., Fieremans E., Novikov D.S. Diffusion MRI noise mapping using random matrix theory. Magn. Reson. Med. 2016;76(5):1582–1593. doi: 10.1002/mrm.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohr B. Speech and language outcomes of very preterm infants. Semin. Fetal Neonatal Med. 2014;19(2):78–83. doi: 10.1016/j.siny.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. The encephalopathy of prematurity–brain injury and impaired brain development inextricably intertwined. Semin. Pediatr. Neurol. 2009;16(4):167–178. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol. 2009;24(9):1085–1104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D., Naglieri J. Harcourt Assessments; San Antonio, TX: 2006. Wechsler Nonverbal Scale of Ability. [Google Scholar]
- Wilke M., Hauser T.K., Krageloh-Mann I., Lidzba K. Specific impairment of functional connectivity between language regions in former early preterms. Hum. Brain Mapp. 2014;35(7):3372–3384. doi: 10.1002/hbm.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Pearson; 2007. Expressive Vocabulary Test. 2nd ed. [Google Scholar]
- Williamson B.J., Altaye M., Kadis D.S. Detrended connectometry analysis to assess white matter correlates of performance in childhood. NeuroImage. 2019;186:637–646. doi: 10.1016/j.neuroimage.2018.11.043. [DOI] [PubMed] [Google Scholar]
- Wood A.G., Harvey A.S., Wellard R.M., Abbott D.F., Anderson V., Kean M., Jackson G.D. Language cortex activation in normal children. Neurology. 2004;63(6):1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Woods P.L., Rieger I., Wocadlo C., Gordon A. Predicting the outcome of specific language impairment at five years of age through early developmental assessment in preterm infants. Early Hum. Dev. 2014;90(10):613–619. doi: 10.1016/j.earlhumdev.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Yeh F., Badre D., Verstynen T. Connectometry: a statistical approach harnessing the analytical potential of the local connectome. NeuroImage. 2016;125:162–171. doi: 10.1016/j.neuroimage.2015.10.053. [DOI] [PubMed] [Google Scholar]
- Yeh F., Panesar S., Barrios J., Fernandes D., Abhinav K., Meola A., Fernandez-Miranda J. Automatic removal of false connections in diffusion MRI tractography using topology-informed pruning (TIP) Neurotherapeutics. 2019;16(1):52–58. doi: 10.1007/s13311-018-0663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F., Tseng W. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage. 2011;58(1):91–99. doi: 10.1016/j.neuroimage.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Yeh F., Verstynen T., Wang Y., Fernández-Miranda J., Tseng W. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8(11):e80713. doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F., Wedeen V., Tseng W. Generalized q-sampling imaging. IEEE Trans. Med. Imaging. 2010;29(9):1626–1635. doi: 10.1109/TMI.2010.2045126. [DOI] [PubMed] [Google Scholar]
- Young J.M., Morgan B.R., Whyte H.E.A., Lee W., Smith M.L., Raybaud C., Taylor M.J. Longitudinal study of white matter development and outcomes in children born very preterm. Cereb. Cortex. 2017;27(8):4094–4105. doi: 10.1093/cercor/bhw221. [DOI] [PubMed] [Google Scholar]
- Younge N., Goldstein R.F., Bann C.M., Hintz S.R., Patel R.M., Smith P.B., Cotten C.M. Survival and neurodevelopmental outcomes among periviable infants. N. Engl. J. Med. 2017;376(7):617–628. doi: 10.1056/NEJMoa1605566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.