Abstract
Lymphorrhea is a rare complication of rectal surgery. Although percutaneous embolization of thoracic or lymphatic ducts is now increasingly being reported for various types of lymphatic leakage, there are only sparse data on lymphatic interventions for lymphorrhea following rectal surgery. A novel balloon-occluded retrograde lymphatic embolization (BRLE) technique can be a simple and effective option for intractable lymphorrhea. We report a case of a man with infected lymphorrhea after rectal resection. Transperineal drainage was performed; however, lymphatic leakage persisted after 1 month of conservative treatment. Lymphangiography revealed multifocal leaks from bilateral iliac lymphatics. The proposed BRLE technique was performed via a balloon catheter inserted through the transperineal drainage. The balloon allowed occlusion of lymphatic outflow and forceful retrograde injection to achieve denser accumulation of n-butyl cyanoacrylate. Tight embolization of bilateral iliac lymphatics and drastic improvement of the leakage was achieved. To the best of our knowledge, this is the first report of percutaneous embolization for lymphorrhea after rectal surgery. This case supports the efficacy of the BRLE as a simple and effective therapeutic option for such persistent multifocal lymphatic leaks.
Keywords: Balloon occlusion, Lymphography, Embolization, Lymphatic vessels, Chylous ascites, Rectal neoplasms
Introduction
Postoperative pelvic lymphatic leakage can occur in the form of lymphatic ascites, lymphocele, or cutaneous lymphatic leakage [1]. Continuous loss of the lymphatic fluid can lead to increased infection rates, delayed wound healing, and prolonged hospital stays [2].
Lymphorrhea from the thoracic duct is now increasingly being treated using thoracic duct embolization. Recently, some authors reported the effectiveness of antegrade lymphatic or lymph node embolization using N-butyl cyanoacrylate (NBCA) for pelvic lymphatic leakage [3,4], but sparse data are available regarding lymphatic intervention after rectal surgery. Besides, when there are multifocal leaks, such percutaneous antegrade embolization technique can be more challenging, necessitating multiple additional puncture and embolization for delicate lymphatics or lymph nodes.
Herein we report a case of intractable lymphorrhea from multiple bilateral pelvic lymphatics successfully treated by a novel balloon-occluded retrograde lymphatic embolization (BRLE) technique via a transperineal approach.
Case report
A 75-year-old man underwent abdominoperineal resection plus D3 and bilateral lateral lymphadenectomy for locally advanced rectal cancer. On postoperative day 25, the patient developed a fever. Contrast-enhanced CT demonstrated presacral fluid accumulation, with features suggesting infection (Fig. 1). Further, complicated lymphoceles communicating with the midline fluid collection were seen bilaterally.
Fig. 1.
Contrast-enhanced CT after transperineal drainage of the infected lymphorrhea. A drainage catheter (arrow) was inserted in the fluid collection in the presacral space. Thin peripheral enhancement indicative of infection is seen. Complicated lymphoceles communicating with the midline fluid collection were also shown bilaterally (asterisks).
A percutaneous transperineal drain (14F SILASCON Duple, Kaneka, Osaka, Japan) was inserted, and the fluid was found to contain milky white chylous ascites. The patient's condition improved after drainage. However, the lymphatic leak from the drainage tube persisted (up to 300 mL/day) even after 1 month of conservative therapy. Besides, persisted leakage from the surgical wound site led to protracted wound healing and decreased patient's quality of life.
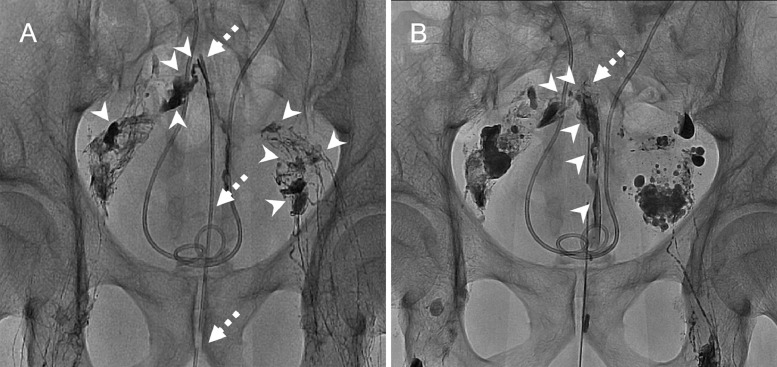
Thus, on postoperative day 64, intranodal lymphangiography was performed for identification of the leakage site and the therapeutic effect of lymphangiography. Lymphangiography with lipiodol (Guerbet, Aulnay-sous-Bois, France) of bilateral inguinal lymph nodes confirmed multifocal bilateral lymphatic injuries and leaks, as well as the lymphorrhea from right iliac lymphatics into the drained cavity (Fig. 2A and B). Communication between the cavity and left iliac lymphatics was not confirmed, despite leaks from bilateral lymphatics were suspected from the preprocedural CT. Continued conservative treatment was administered after lymphangiography; however, the lymphatic leak from the drainage tube persisted with little improvement.
Fig. 2.
Lymphangiography findings of a 75-year-old man with lymphorrhea after rectal resection. (A) Intranodal lymphangiography (obtained 15 minutes following lipiodol injection) via bilateral inguinal lymph nodes demonstrated multifocal lymphatic leaks (arrowheads) draining from multiple bilateral lymphatics. The dotted arrow indicates the transperineal drainage catheter. (B) Intranodal lymphangiography (obtained 30 minutes following lipiodol injection) revealed apparent leakage from the right iliac lymphatics into the drainage catheter (arrowheads), but failed to demonstrate the communication between the drained cavity around the tip of drainage catheter (dotted arrow) and left iliac lymphatics.
Thus, embolization of the pelvic lymphatics was planned, and a balloon catheter (6F Selecon MP; Terumo, Tokyo, Japan) was inserted through the transperineal drainage tube. Retrograde lymphangiography under balloon occlusion revealed leaks from bilateral iliac lymphatics, including the communication between the cavity and left iliac lymphatics, which was not visualized by the intranodal lymphangiography. Therefore, the bilateral lymphatics and the presacral cavity were considered retrogradely embolizable together from the downstream side. Contrarily, antegrade lymphatic embolization can be a more complicated procedure, as the leaks are originated from multiple bilateral delicate lymphatics, requiring additional nodal or lymphatic punctures and embolization.
Retrograde embolization for the bilateral iliac lymphatics was conducted using NBCA via the balloon catheter (Fig. 3A and B). The catheter was flushed with 5% dextrose before and after injection of the glue to prevent premature polymerization of NBCA (ie, catheter blockage and catheter entrapment within polymerized glue). A 6-mL solution of a 1:3 mixture of NBCA and lipiodol was forcefully injected until the feeding lymphatics were filled with the glue. Although adhesion of NBCA to the catheter tip occurred after the administration of the glue, the catheter could be removed without complication.
Fig. 3.
(A) Illustration overlaid on the fluoroscopic image of intranodal lymphangiography elucidating the balloon-occluded retrograde glue embolization technique. Embolization of bilateral iliac lymphatics was performed through injection of glue (dotted arrows, NBCA to lipiodol ratio, 1:3) under balloon occlusion (asterisk). (B) Fluoroscopic image after embolization. Note that communication between the drained cavity and injured left iliac lymphatics (arrow), which was not visualized with intranodal lymphangiography, was clearly shown, and firm embolization of the fistula was achieved. A contralateral outflow lymphatic vessel is also shown and is tightly embolized (arrowheads). (C) Follow-up CT image without contrast enhancement 4 months after embolization showed bilateral iliac lymphatics successfully filled with the glue mixture. NBCA, N-butyl cyanoacrylate.
After the intervention, the output from the drain decreased drastically, and the drain could be removed 3 weeks postprocedure, following 17 days without output. Follow-up CT images 4 months after embolization showed bilateral iliac lymphatics densely filled with the glue mixture (Fig. 3C). There was no recurrence of lymphorrhea and no procedure-related complications.
Discussion
This case provides 2 valuable clinical suggestions: BRLE can be a simple and effective alternative option for complex lymphorrhea from multiple bilateral pelvic lymphatics, and percutaneous lymphatic embolization can be beneficial for successful management of refractory lymphorrhea after rectal surgery.
First, BRLE can be a promising treatment option for such complex lymphorrhea from multiple bilateral pelvic lymphatics. Antegrade coaxial catheterization for leaks occurring along fine lymphatic vessels in the pelvis may be technically demanding. Lymphatic embolization techniques using puncture of “the site of a leak” or “lymphatic inflow” or the “upstream lymph node” have been reported [3,5]. However, these techniques require additional nodal or lymphatic puncture after diagnostic lymphangiography, which can be more challenging if there are multiple leaks involving multiple bilateral lymphatics, as in this case. Recently, nodal embolization by injection of glue through the lymphangiography needle has been reported in a few reports as a less invasive treatment for postoperative lymphorrhea [4,6]. This method seems promising and simple, although reports on the technique are limited to date. Nonetheless, premature polymerization of the NBCA can be a concern, and Gemmete et al reported a case in which glue injection failed to penetrate to the extravasation level [7]. With the BRLE technique, lymphatic leaks from multiple inflow lymphatics could be easily embolized together from the downstream side, without the need for additional puncture or cannulation of fine lymphatics.
Besides, the balloon-occluded NBCA embolization method enables easy control of cast length by increasing the infusion volume of the embolic material, allowing more precise and stronger embolization (forceful injection of glue) of the target vasculature without excessive reflux [8,9]. The present technique may be applicable for lymphatic injury resulting from various procedures involving pelvic lymph node dissection, such as gynecologic and urological tumor resection.
Second, refractory lymphorrhea after rectal surgery was successfully managed with lymphatic embolization. Lymphatic leakage after colorectal surgery is uncommon but cannot always be avoided because of the variable anatomy of lymphatics [10]. Previous studies have reported a 1.0%-6.6% incidence of chylous ascites after colorectal cancer operations [11]. Although most pelvic lymphorrhea cases can be managed conservatively, some leaks require more aggressive treatment [3,10]. To our knowledge, this is the first report of successful treatment with lymphatic embolization for postoperative lymphatic leakage after surgery for rectal cancer.
Another advantage of the presented technique is that retrograde lymphangiography potentially improves visualization and identification of injured lymphatics. Although visualization of the leak is critical for precise embolization, intranodal lymphangiography failed to identify leaks from left iliac lymphatics into the presacral cavity, which was possible with retrograde lymphangiography under balloon occlusion.
Recently, various types of micro-balloon catheters have been developed, allowing access to more distal and smaller vasculature that cannot be reached by conventional balloon catheters [12]. Future studies will include the application of such micro-balloon catheters for the proposed lymphatic embolization technique.
In conclusion, BRLE through the transperineal approach allowed successful management of refractory multifocal pelvic lymphorrhea. The present case supports the effectiveness of lymphatic embolization as a therapeutic option for lymphorrhea after rectal surgery.
References
- Kim S.W., Hur S., Kim S.Y., Cho J.Y., Kwak C., Kim H.S. The efficacy of lymph node embolization using N-butyl cyanoacrylate compared to ethanol sclerotherapy in the management of symptomatic lymphorrhea after pelvic surgery. J Vasc Interv Radiol. 2019;30(2):195–202. doi: 10.1016/j.jvir.2018.09.038. e1. [DOI] [PubMed] [Google Scholar]
- 2.Orvieto M.A., Coelho R.F., Chauhan S., Palmer K.J., Rocco B., Patel V.R. Incidence of lymphoceles after robot-assisted pelvic lymph node dissection. BJU Int. 2011;108(7):1185–1190. doi: 10.1111/j.1464-410X.2011.10094.x. [DOI] [PubMed] [Google Scholar]
- 3.Baek Y., Won J.H., Kong T-W, Paek J., Chang S-J, Ryu H-S. Lymphatic leak occurring after surgical lymph node dissection: a preliminary study assessing the feasibility and outcome of lymphatic embolization. Cardiovasc Interven Radiol. 2016;39(12):1728–1735. doi: 10.1007/s00270-016-1435-x. [DOI] [PubMed] [Google Scholar]
- 4.Hill H., Srinivasa R.N., Gemmete J.J., Hage A., Bundy J., Chick J.F.B. Endolymphatic ethiodized oil intranodal lymphangiography and cyanoacrylate glue embolization for the treatment of postoperative lymphatic leak after robot-assisted laparoscopic pelvic resection. J Endourol Case Rep. 2018;4(1):66–71. doi: 10.1089/cren.2018.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hur S., Shin J.H., Lee I.J., Min S.K., Min S.I., Ahn S. Early experience in the management of postoperative lymphatic leakage using lipiodol lymphangiography and adjunctive glue embolization. J Vasc Interv Radiol. 2016;27(8):1177–1186. doi: 10.1016/j.jvir.2016.05.011. e1. [DOI] [PubMed] [Google Scholar]
- 6.Majdalany B.S., Khayat M., Downing T., Killoran T.P., El-Haddad G., Khaja M.S. Lymphatic interventions for isolated, iatrogenic chylous ascites: a multi-institution experience. Eur J Radiol. 2018;109:41–47. doi: 10.1016/j.ejrad.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Gemmete J.J., Srinivasa R.N., Chick J.F.B. Treatment of chylous ascites in a child after Wilms tumor resection with intranodal injection of N -butyl cyanoacrylate glue. J Vasc Interv Radiol. 2017;28(7):1067–1069. doi: 10.1016/j.jvir.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi S., Lohman B.D., Ogawa Y., Arai Y., Hashimoto K., Matsumoto J. Preliminary findings of arterial embolization with balloon-occluded and flow-dependent histoacryl glue embolization in a swine model. Jpn J Radiol. 2015;33(6):344–351. doi: 10.1007/s11604-015-0426-1. [DOI] [PubMed] [Google Scholar]
- 9.Soga S., Kuwamura H., Edo H., Suyama Y., Hamabe F., Sugiura H. Double balloon-occluded transarterial chemoembolization (double B-TACE) for hepatocellular carcinomas located in the caudate lobe. Cardiovasc Intervent Radiol. 2020;43(1):162–164. doi: 10.1007/s00270-019-02333-3. [DOI] [PubMed] [Google Scholar]
- 10.Ha G.W., Lee M.R. Surgical repair of intractable chylous ascites following laparoscopic anterior resection. World J Gastroenterol. 2015;21(19):6077–6081. doi: 10.3748/wjg.v21.i19.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek S.J., Kim S.H., Kwak J.M., Kim J. Incidence and risk factors of chylous ascites after colorectal cancer surgery. Am J Surg. 2013;206(4):555–559. doi: 10.1016/j.amjsurg.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T., Tomita K., Suda S., Hashida K., Maegawa S., Hayashi T. Microballoon-related interventions in various endovascular treatments of body trunk lesions. Minim Invasive Ther Allied Technol. 2018;27(1):2–10. doi: 10.1080/13645706.2017.1398174. [DOI] [PubMed] [Google Scholar]