Abstract
Background
Study populations in clinical research must reflect US changing demographics, especially with the rise of precision medicine. However, racial and ethnic minority groups (REMGs) have low rates of participation in cancer clinical trials.
Methods
Criteria were developed to identify cancer centers able to accrue a higher than average proportion of REMGs into clinical trials. Comprehensive interviews were conducted with leaders of these cancer centers to identify operational strategies contributing to enhanced accrual of REMGs.
Results
Eight US cancer centers reported a REMG accrual rate range in cancer research between 10 and 50% in a 12-month reporting period and met other criteria for inclusion. Fourteen leaders participated in this assessment. Key findings were that centers: had a metric collection and reporting approach; routinely captured race and ethnicity data within databases accessible to research staff; had operational standards to support access and inclusion; developed practices to facilitate sustained patient participation during clinical trials; had strategies to decrease recruitment time and optimize clinical study design; and identified low-resource strategies for REMG accrual. There was also a clear commitment to establish processes that support the patient's provider as the key influencer of patient recruitment into clinical trials.
Conclusion
We have identified operational practices that facilitate increased inclusion of REMGs in cancer trials. In order to establish a sustainable cancer center inclusion research strategy, it is valuable to include an operational framework that is informed by leading US cancer centers of excellence.
Keywords: Cancer research, Clinical trials, Operations, Disparities, Diversity and inclusion, Racial and ethnic minority groups
1. Introduction
There is heightened awareness of disparities in clinical trials across multiple stakeholders [[1], [2], [3], [4]], especially with the accelerating focus of precision medicine across the healthcare continuum. Study populations in clinical research must reflect US changing racial/ethnic demographics of the emerging majority. Adequate representation of patients reflective of those who experience disease in clinical research is imperative as a matter of social justice, economics, and science.
The US Food and Drug Administration (FDA) agrees, “inclusion of US racial/ethnic demographic subgroups in clinical trials in adequate numbers are important to look for differences that impact the safety and efficacy profile of the medical products in US demographic subgroups” [5]. The FDA has responded in multiple ways to the inclusion research challenge, including the development of an extensive action plan, transparency reporting and new results reporting requirements on clinical trials.gov. Congress included Section 907 [6] in the Food and Drug Administration Safety and Innovation Act (FDASIA), giving FDA direction to evaluate and address this issue.
Transparency goals led to the creation of Drug Trials Snapshots [7] that provides public readouts of the demographic profile of clinical trial participants for approved drugs. All new clinical trial results posted on clinicaltrials.gov must include race and ethnicity [8], consistent with scientific interest in the inclusion of minorities in clinical trials and the generalizability of research findings.
Despite widespread, increasing stakeholder commitment and regulatory guidance, inequities continue. These are especially concerning with the advances in science and technology that are driving a paradigm-shift with precision medicine, especially in cancer [[9], [10], [11]].
Currently, adult participation in US cancer clinical trials (CTs) are at less than 10% of cancer patients with even lower rates for racial and ethnic minority groups (REMGs) [12]. For example, African Americans comprise 5% of patients enrolled in CTs that support FDA approval of new drugs, but, represent 13.3% of the general US population [13]. Cancer is the leading cause of death for Asian Americans [14], yet they comprise 3% of cancer CT participants [15]. Hispanics represent less than 3% of cancer CT participants [16], despite accounting for an estimated 17.8% of the US population [17].
REMGs are not benefiting from access to clinical trials which are often standard of care for cancer patients. These same patients could expand the enrollment capability for sponsors. The lower participation rates of REMGs represent missed opportunities for ensuring that new therapies are adequately tested, establishing validated conclusions and generating new hypotheses applicable to broader populations.
Recommendations to address barriers to enrollment in CTs – focusing on people, process and technology practices – have recently been extensively documented [12]. The recommendations are logical, extensively peer-reviewed and form the basis for exploring the degree to which these and other recommendations are actively part of the real-world approach of US leading cancer centers who sustainably recruit and retain diverse populations in CTs. In this paper we provide an operational framework based on recognized practices used by leading cancer centers able to exceed criteria for accrual and retention of REMGs into cancer CTs. Notable practices of US cancer centers in leadership, patient and community engagement have been previously reported [18].
2. Methods
The Diverse Cancer Communities Working Group (CWG) applied co-created selection criteria (Table 1) to identify US cancer centers of excellence able to accrue all major REMGs in cancer clinical research. Ten cancer centers were included in the initial recruitment of centers based on CWG industry sponsor experience with centers able to accrue diverse populations with success. Two centers did not meet the recruitment milestones for the research assessment, therefore did not qualify for the study. Eight centers met all of the selection criteria based on pre-interview survey assessment and also confirmed at the start of the initial interview with each center leader.
Table 1.
Cancer center selection criteria.
| Criteria |
|---|
|
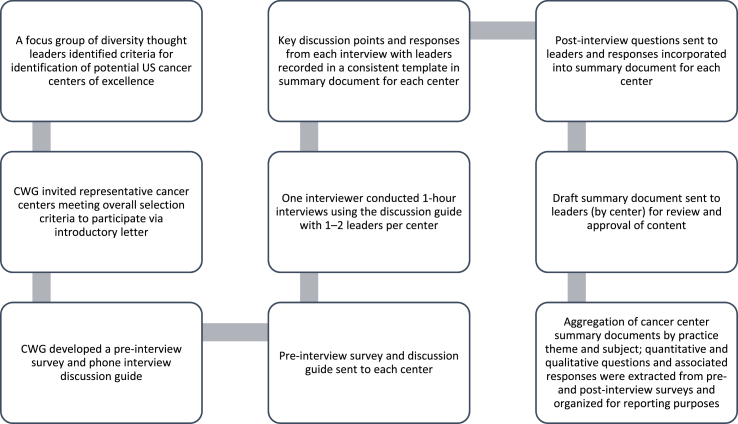
The CWG conducted a review of general best practice publications and outputs were used to inform research methodology. Pre-/post-interview surveys and a discussion guide were developed and sent to each center of excellence prior to interviews. Interviews were undertaken between November 2017–February 2018 with center leaders across selected centers by a single interviewer, using the standardized survey instruments and discussion guide. Full details regarding the actual survey instruments and discussion guide have been previously reported [18]. Center leaders validated the content of the surveys and discussion guide. The discussion guide was used to capture notable practices across several themes: leadership/commitment; operational capabilities; community engagement; patient engagement; investigator training and hiring/mentoring; and recommended sponsor practices for enhanced racial and ethnic minority recruitment. Pre-/post-interview surveys were used to confirm participation eligibility, align on key definitions and explore emergent themes (Fig. 1). A consistent definition of accrual was used for cross-center assessment, based on the number of participants that have completed or are actively in the process of completing the study. This includes dropouts but does not include screen failures. Consistent definitions of other terms were also used during this assessment (Table 2). Center leader agreement was secured by survey instruments and interview summaries were sent to each center leader to validate the accuracy of responses prior to aggregation.
Fig. 1.
Research methodology flow diagram.
Table 2.
Definition of key terms used in the assessments.
| Term | Definition and reference |
| Cancer research | As defined in the NIH statute [1] |
| Minority groups | As defined in the NIH statute [1] |
| Race and ethnicity | As defined in the Office of Minority Health (OMH) 2016 Industry Guidance Document Collection of Race and Ethnicity Data in Clinical Trials [2] |
| Cancer health disparity | The National Cancer Institute defines a cancer health disparity as an adverse difference in cancer incidence (new cases), cancer prevalence (all existing cases), cancer death (mortality), cancer survivorship, and burden of cancer or related health conditions that exist among specific population groups in the US [3] |
| Accrual | Accrual is based on the number of participants that have completed or are actively in the process of completing the study. This includes dropouts but does not include screen failures [4] |
[1] Available at: https://grants.nih.gov/grants/funding/women_min/guidelines.htm (accessed May 01, 2019); [2] Available at: https://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126396.pdf (accessed May 01, 2019); [3] Available at: https://www.cancer.gov/about-nci/organization/crchd/about-health-disparities/definitions (accessed May 01, 2019); [4] Corregano L et al. Clin Transl Sci. 2015; 8(6): 655–661.
3. Results
The CWG selected the following eight centers meeting all selection criteria: Fox Chase Cancer Center/Temple Health (Philadelphia, PA); Harold C. Simmons Comprehensive Cancer Center/UT Southwestern Medical Center (Dallas, TX); Henry Ford Cancer Institute (Detroit, MI); Hollings Cancer Center/MUSC (Charleston, SC); John T. Vucurevich Cancer Institute/Rapid City Regional Hospital (Rapid City, SD); MD Anderson Cancer Center/UT (Houston, TX); UC Davis Comprehensive Cancer Center (Sacramento, CA); Winship Cancer Institute/Emory (Atlanta, GA).
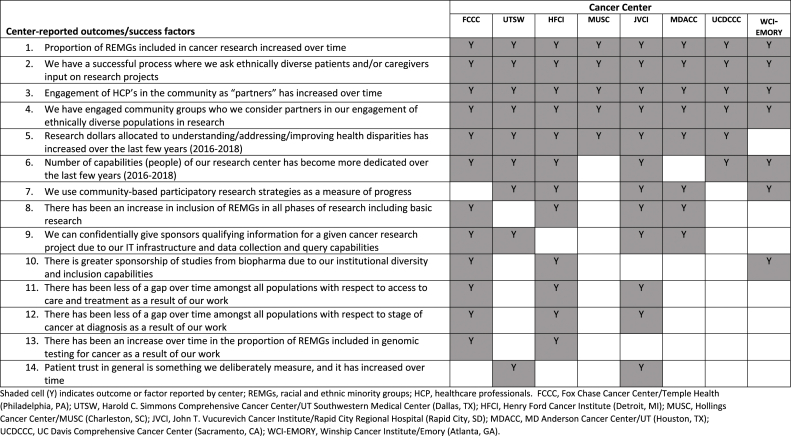
Overall results from the quantitative surveys are summarized in Table 3, Table 4. Centers represented every major REMG, according to the Health and Human Services (HHS), Office of Management and Budget (OMB) race and ethnicity designations [19]. Overall, 14 Leaders representing eight cancer centers participated in this assessment. Eight centers reported a REMG accrual rate range in cancer research between 10 and 50% in a 12-month reporting period (between 2016 and 2018; Table 3). A summary of center-reported outcomes and success factors for recruitment of REMGs in cancer research is presented (Table 4).
Table 3.
Summary of results from quantitative surveys.
| Center | Percentage of REMG participants in cancer clinical trials | REMG populations engaged in cancer clinical trials | Availability of metric scorecard | Where OMH REMG data is captured | How and by whom OMH REMG data is captured | Format of REMG data capture |
|---|---|---|---|---|---|---|
| FCCC | 10–20% | African Americans, Hispanic Americans, Asian Americans | Yes, use NCI grant Criteria* for REMGs | Captured in clinical trial database by research staff and non-research staff throughout continuum | Patient reported (directly) | Written and web-based |
| UTSW | 30–40% (40–50% in population science research) | African Americans, Hispanic Americans | Yes, use NCI grant Criteria* for REMGs | Captured in clinical trial database by research staff and non-research staff throughout continuum | Reported by research staff, informed by direct patient query | Written and web-based |
| HFCI | 30–40% | African Americans | Yes (extensive metrics provided) | Captured in clinical trial database by research staff and non-research staff throughout continuum | Reported by research staff, informed by direct patient query | Written and web-based |
| MUSC | 20–30% (27% in interventional trials & 32% in non-treatment trials) | African Americans, Hispanic Americans | Yes, extensive metrics provided and NCI Grant Criteria* for REMGs | Captured in clinical trial database by research staff and non-research staff throughout continuum | Patient reported (directly) | Written |
| JVCI | 10–20% | American Indians, Alaskan Natives (primarily from Lakota Country) | Yes (extensive metrics provided) | Captured in clinical trial database | Patient reported (directly) | Written and web-based |
| MDACC | 10–20% | African Americans, Hispanic Americans, Asian Americans | Yes, extensive metrics provided and NCI Grant Criteria* for REMGs | Captured in clinical trial database | Patient reported (directly) | Web-based |
| UCDCCC | 30–40% (30–40% Asian Am. participants in non-interventional research) | Asian Americans | Yes, use NCI grant Criteria* for REMGs | Degree/extent of capture in clinical trial database unknown | Reported by research staff, based on visual observation | Written |
| WCI-EMORY | 20–30% | African Americans, Hispanic Americans | Yes (extensive metrics provided) and NCI grant Criteria* for REMGs | Captured in clinical trial database by research staff and partners throughout continuum | Varies by trial | Written and web-based |
FCCC, Fox Chase Cancer Center/Temple Health (Philadelphia, PA); UTSW, Harold C. Simmons Comprehensive Cancer Center/UT Southwestern Medical Center (Dallas, TX); HFCI, Henry Ford Cancer Institute (Detroit, MI); MUSC, Hollings Cancer Center/MUSC (Charleston, SC); JVCI, John T. Vucurevich Cancer Institute/Rapid City Regional Hospital (Rapid City, SD); MDACC, MD Anderson Cancer Center/UT (Houston, TX); UCDCCC, UC Davis Comprehensive Cancer Center (Sacramento, CA); WCI-EMORY, Winship Cancer Institute/Emory (Atlanta, GA); REMGs, racial and ethnic minority groups; OMH, Office of Minority Health. *NCI Comprehensive Cancer Center Research Strategy Expectations for Catchment areas. Available at: https://cancercenters.cancer.gov/documents/CCSGFAQs508C.pdf (accessed May 01, 2019).
Table 4.
Summary of center-reported outcomes and success factors for recruitment of REMGs in cancer research.
4. Findings from interviews with cancer center leaders
4.1. Metric collection and reporting
All centers report a metric collection and reporting approach. Metric definition, including a description of cancer research that addresses the areas that a cancer center serves, is standard for NCI-Designated Cancer Centers [20] many of which are included in this assessment [21]. In addition, MD Anderson has a goal to engage two new community partners per year and onboard them as part of their commitment to engage diverse populations for access to health care and potentially relevant clinical research. Rapid City, South Dakota tracks patient trust by patient survey over the course of the care continuum [22]. The Emory Winship clinical trials office captures studies by disease condition/site and by phase of research. They track accrual and inclusion of populations by month. These data are reviewed by leaders for action on an ongoing basis and the clinical trial office holds periodic retreats to bring people together to assess the data. Core metrics from Henry Ford Cancer Institute include accruals per study coordinator, total screen failures, accruals by study, and median time to trial activation (which can impact the capability to recruit diverse populations in an efficient manner). These are closely monitored to allow timely intervention if needed. UC Davis Comprehensive Cancer Center ensures the measurement of integrated and ongoing cancer research programs (e.g. smoking cessation program, biospecimen donation) with results, and enables a tracking mechanism for system-wide health interventions and communications.
4.2. Collection of race and ethnicity data: mechanism and reporting
All centers capture REMGs in databases accessible to the research staff according to HSS/OMB definition [19]. The accessibility of REMG data varies across centers, can be written and or/web based and the data itself is “patient reported or informed directly by patients” except for UC Davis Comprehensive Cancer Center which reports REMG data by research staff based on their visual observation and language fluency. UC Davis chooses to disaggregate data by Asian ethnicity, since in the majority of studies, interventions are linguistically-specific (use Hmong or Vietnamese or Cantonese/Mandarin, rather than English only). Thus, they can report findings more based on ethnic homogeneity and/by language fluency rather than an aggregated Asian American category; this approach goes beyond the OMB definition of Asian Americans when capturing data in their cancer center database. Such granularity allows more precise identification of populations at risk by race/ethnicity, age, gender, socio-economic status, and stage of disease so that approaches and targeted interventions to mitigate disparities can be developed. A caveat to collection of data and use of technology is provided by UT Southwestern leadership:
“… it is always an important and a continual balance to adjust process and capabilities and messaging so that the system does not come across like it's soliciting the patient – the messaging and offerings must be part of the continuum of care and delivery must come from trusted sources with respect for the primary physician role” -UTSW
4.3. Operational practices
High REMG recruiting cancer centers had deliberate operational standards to support access to healthcare innovations and sustainable and productive inclusion standards in cancer research. This included having the right people, processes, and technological capabilities to ensure inclusion of racially, ethnically and otherwise diverse populations in clinical trials. Representative examples include the Best Chance Network (MUSC), precision medicine focused approaches (HFCI) and care continuum system integration (UTSW).
The Best Chance Network [23] demonstrates sustained “people capability” and has contributed to the lack of difference in breast cancer incidence between Whites and Non- Whites in MUSC's catchment area in South Carolina. The success of this program is a result of dedicated volunteer breast cancer patient navigators who work in a mobile health unit and are connected to patients in the community diagnosed with breast cancer. Their aim is to reduce barriers and work closely with the Best Chance Network to optimize care and treatment for vulnerable populations. Many patients entered clinical trials because of the trust developed and nurtured by lay patient navigators.
Evolving molecular profiling and precision diagnostics approaches represent a challenge to maintaining productivity. They are used to identify rarer tumor mutations and targets and are consequently associated with an increased screen failure rate among potential study participants and greater time commitment of research staff: more screens and effort are required to achieve a single clinical trial accrual. To combat this issue, HFCI has partnered with ‘Syapse’ [24] to leverage Henry Ford Center for Precision Diagnostics capabilities which directly correlates, identifies and includes all patients potentially eligible for precision medicine-based clinical trials, thus reducing effort by study teams and reducing the number of screen failures.
UTSW focuses on the capture of race and ethnicity accurately at the clinic level for all patients. These data are entered into the electronic medical record (EMR) which is synced with the clinical trials management system allowing patient demographics to be tracked and reported in conjunction with clinical trials to which they may be enrolled. Front-line staff are encouraged to have a live conversation with new patients to gather this information and enter it during the registration process.
A UTSW campaign – “Count Me In” – allows patients to “opt in” to potential clinical trial participation through the MYCHART application of the EMR. MYCHART links interested patients with clinical research opportunities at UTSW thereby improving efficiency of research recruitment and encouraging clinical research participation by all patients [25].
4.4. Practices to ensure sustained patient participation during clinical trials
Center leaders indicated that sustained participation in clinical trials can be challenging, especially for REMGs more likely to face access barriers and other social determinants of health that negatively impact their health outcomes. As many African Americans and Hispanic patients lack adequate health insurance, ongoing treatment access impacts clinical trial adherence. Linking to service providers in the community, providing charity care, and supporting needs such as transportation, are approaches centers reported implementing. Trial designs that incorporate cumbersome requirements for patients over multiple visits are another barrier to sustained participation. Centers minimize the patient burden with strategies ranging from careful selection of trials that better match the needs of the patient population to engagement of patients and community representatives in designing trials from the start. Effective communication with the REMG communities, providing education about the research process, was also used to build trust and engagement in the overall research enterprise.
“Our population is willing to engage in research as long as they are given the chance and understand. It is a myth that it takes longer to enroll AIs [American Indians] in clinical trials as we have proven it can be done. The principal reason why AI patients participate in clinical trials is to help other patients and their relatives with the cancer experience and improve their outcome”-JVCI
4.5. Mitigating strategies to decrease recruitment time
The cancer center leaders were evenly divided on whether it takes longer to recruit REMGs. Respondents identified factors that can lengthen research recruitment including: limited healthcare access; receiving services from safety net providers uninvolved in research and without the necessary resources to recruit for trials; language barriers requiring translated materials; and patient-level barriers such as transportation costs. However, with prior planning and appropriate communication tools and approaches, many centers observed that no additional time is required because the biggest determinant of patient participation is being invited to do so by their physician.
4.6. Clinical study design considerations
Centers routinely engaged with research sites and patients as key partners. This was paramount to sustainable and meaningful inclusion of REMGs. Leaders recommended that sponsors of clinical trials invest in building trusted relationships with trial sites to demonstrate their commitment to inclusion of REMGs in clinical research programs.
“Sponsors should engage in a dialogue with investigators to ensure that the findings are relevant to the patient population who are most likely to benefit from the medication because it's the right thing to do …” MDACC
Minority patient participation and retention was noted to be contingent upon HCPs, industry, and advocacy groups building a comprehensive understanding of patient barriers. Providing support mechanisms to mitigate known obstacles, and proactively communicating these solutions were seen as fundamental to increasing participation. Transportation, meal vouchers, and childcare support required proactive minor sponsor investment and can have major implications for patient engagement and accrual.
Leaders also noted that patients must understand what to expect during the clinical trial process, as well as the potential benefit/risks associated with a study, in plain language. Addressing patients with cultural competency was necessary to reach diverse patients in the most meaningful manner. Often, minorities were disproportionately excluded based on prior cancers or co-morbidities that may not have clinical implications [26]. Sponsors should carefully evaluate the clinical relevance of exclusion criteria with insights from investigators, patients and care partners.
“The patient burden is not always considered- Trials are becoming more complicated with precision medicine – we must work together to facilitate patient participation …” FCCC
4.7. Low resource strategies
Noting the variable levels of resource available to different cancer centers, leaders identified low-resource strategies which, in their experience, may yield incremental improvements in the accrual of REMG populations in cancer research in the US. These are summarized in Table 5.
Table 5.
Low-resource strategies for REMG accrual in cancer research.
| Strategy | References/examples |
|---|---|
| Launch awareness campaigns to raise awareness of cancer clinical trials to patients and support physician recruitment efforts | Be the Breakthrough (FCCC); Count Me In (UTSW) |
| Share recruitment materials with patients and care partners which are health literate and linguistically accessible (i.e., in plain language, appropriately formatted, and in the languages of desired participant population) | Recommended by all centers [27]. Stand Up To Cancer campaign [28] |
| Ask Patients and community leaders (including Primary Care Physicians) for input on questions to be answered in cancer trials and feasibility of trial implementation | Recommended by all centers |
There are different types of research which can be effectively implemented:
|
Recommended by HFCI |
| Offering of clinical trials focusing on reducing treatment length as American Indian (AI) live a median of 140 miles from the cancer center in Rapid City, SD; recommended as part of the Walking Forward Program experience | Recommended by JVCI [29] |
| Advocate for consolidation of tests required for screening for a trial into a one-day process. The resource required is an individual designated to coordinate scheduling the necessary tests in a thoughtful manner | Recommended by WCI-EMORY |
| Engaging a family member or care partner in addition to the patient to serve as a second ‘set of ears’ and reinforcement regarding the trial process | Recommended by WCI-EMORY |
Timing of clinical research offering to patient and consent:
|
Recommended by all centers [30,31] |
Centers: FCCC, Fox Chase Cancer Center/Temple Health (Philadelphia, PA); UTSW, Harold C. Simmons Comprehensive Cancer Center/UT Southwestern Medical Center (Dallas, TX); HFCI, Henry Ford Cancer Institute (Detroit, MI); MUSC, Hollings Cancer Center/MUSC (Charleston, SC); JVCI, John T. Vucurevich Cancer Institute/Rapid City Regional Hospital (Rapid City, SD); MDACC, MD Anderson Cancer Center/UT (Houston, TX); UCDCCC, UC Davis Comprehensive Cancer Center (Sacramento, CA); WCI-EMORY, Winship Cancer Institute/Emory (Atlanta, GA). Stand Up 2 Cancer (SU2C).
5. Discussion
All eight centers of excellence intentionally collected data on REMGs with a clear connection to cancer center metrics on diversity and inclusion in cancer research, and roles of research staff, providers and support staff. There was also a clear commitment to: identify culturally-sensitive needs; promote REMG-targeted approaches; and establish processes that support the patient's provider as the key influencer of patient recruitment into clinical trials.
We acknowledge potential limitations in the current study that may impact interpretation of results, including the small sample size, geographical representation and type of Center. Six of eight centers were NCI Designated Cancer Centers with an associated level of NIH funding that may impact ability to establish REMG recruitment initiatives. However, center leaders identified low-cost approaches that could be adopted in lower resource settings. Another potential limitation is the lack of comparison group which may limit the generalizability of our findings. However, we suggest that optimization of identified success factors across other cancer centers would improve REMG recruitment and retention.
Conduct of cancer research requires deliberate coordination of an operational framework that includes the accountability of people, process, and technology governed by metrics in order to sustain a high accrual level of diverse populations in clinical trials. Centers of Excellence have established key operational excellence practices which are critical to ensuring the inclusion of diverse populations in cancer clinical research with sustainability.
There are continued disparities in access to care and standards of care and outcomes in ethnic minorities and vulnerable populations which results in more advanced disease than other communities. Individuals from medically underserved populations are more likely to be diagnosed with late-stage diseases that might have been treated more effectively or cured if diagnosed earlier. This is exacerbated by differences in prevalence, and cancer outcomes by zip code for multiple cancer conditions. This challenge is both notable and critical as the US is last out of 11 countries in health equity [32].
With all these documented trends and disparities during the patient care continuum leading to disparities in cancer outcomes, we are poised at a moment of both challenge and promise. The death rates for many cancers are declining as therapies advance. New, more effective and less toxic immunotherapies are being developed for cancers. However, gender differences already noted with response to innovative anti-cancer therapies [33] highlight the need for evaluation across all populations and subgroups. The substantial and increasing focus on precision medicine, could result in diminishing or expanding disparities. Without a purposeful focus on the former, all historical data would indicate that we will end up with the latter.
The establishment of operational excellence practices within US cancer research centers is critical for the inclusion of diverse populations in cancer research. The need for optimized operational capability, as evidenced by Centers of Excellence, is aligned with a notable commitment from industry sponsors to preferentially partner with US cancer centers able to engage REMGs with sustainability [[34], [35], [36], [37], [38]]. The moment to establish optimal REMG recruitment and retention practices is now.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclaimer
The National Minority Quality Forum (NMQF)'s Center for Sustainable Health Care Quality and Equity (SHC) is the nation's leading healthcare research and education organization whose mission is to strengthen the ability of communities and policy-makers to eliminate the disproportionate burden of premature death and preventable illness in special populations. SHC and NMQF work together to use data to promote equity through research, training and advocacy. The Cancer Communities Working Group (CWG) is a partnership of patient groups, providers and innovators seeking to promote equity in cancer prevention, screening, treatment and inclusion in clinical trials. The CWG is supported by sponsorships and specific programming support from participating for-profit organization members. The views expressed do not necessarily represent the views of participating organizations.
Acknowledgements
The following individuals contributed to the findings and analysis of our findings. Adrienne G. Tilbor, DO, Linda Burhansstipnanov, MSPH, DrPH, CHES, Lisa Newman, MD, MPH, Marvella Ford, PhD, Daniel Petereit, MD, Lorna McNeill, PhD, Alese Olatunji, MD, Keerthi Gogineni, MD, Nestor F. Esnaola, MD, MPH. Editorial and writing services were provided by Ify Sargeant (ISMEDICA Ltd) with support from Center for Sustainable Health Care Quality and Equity (SHC).
Contributor Information
Jeanne M. Regnante, Email: jregnante@lungevity.org.
Nicole Richie, Email: Richie.nicole@gene.com.
Lola Fashoyin-Aje, Email: Ibilola.Fashoyin-Aje@fda.hhs.
Laura Lee Hall, Email: llhall@nmqf.org.
Quita Highsmith, Email: Highsmith.quita@gene.com.
J'Aimee Louis, Email: jlouis@nmqf.org.
Kenneth Turner, Email: kturne29@its.jnj.com.
Spencer Hoover, Email: Shoover1@hfhs.org.
Simon Craddock Lee, Email: simoncraddock.lee@utsouthwestern.edu.
Evelyn González, Email: Evelyn.Gonzalez@fccc.edu.
Erin Williams, Email: Erin.williams@utsouthwestern.edu.
Homer Adams, III, Email: haadamsii@its.jnj.com.
Coleman Obasaju, Email: obasaju_coleman@lilly.com.
Ify Sargeant, Email: ify@ismedica.com.
Jovonni Spinner, Email: Jovonni.spinner@hhs.fda.gov.
Christopher Reddick, Email: Chris.reddick@takeda.com.
Marianne Gandee, Email: mgandee@accc-cancer.org.
Madeline Geday, Email: Madeline_geday@merck.com.
Julie Dang, Email: jtdang@ucdavis.edu.
Rayneisha Watson, Email: rawatson@deloitte.com.
Moon S. Chen, Jr., Email: mschenjr@ucdavis.edu.
References
- 1.Colon‐Otero G., Smallridge R.C., Solberg L.A., Keith T.D., Woodward T.A., Willis F.B., Dunn A.N. Disparities in participation in cancer clinical trials in the United States. Cancer. 2008;112:447–454. doi: 10.1002/cncr.23201. [DOI] [PubMed] [Google Scholar]
- 2.FDA 2017 An FDA perspective on patient diversity in clinical trials. https://www.clinicalleader.com/doc/an-fda-perspective-on-patient-diversity-in-clinical-trials-0001 Available at: (accessed May 01, 2019)
- 3.AAMC 2016 Association of American medical colleges news. More minorities needed in clinical trials to make research relevant to all. https://news.aamc.org/diversity/article/more-minorities-needed-clinical-trials-research/ Available at: accessed May 01, 2019.
- 4.Oh S.S., Galanter J., Thakur N. Diversity in clinical and biomedical research: a promise yet to Be fulfilled. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA Office of minority health progress update. 2016. https://www.fda.gov/downloads/RegulatoryInformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/FDASIA/UCM491486.pdf Available at: February29, 2016 accessed May 01, 2019.
- 6.FDA FDASIA section 907: inclusion of demographic subgroups in clinical trials. 2018. https://www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/FDASIA/ucm389100.htm Available at: accessed May 01, 2019.
- 7.FDA Drug trials Snapshots. 2018. https://www.fda.gov/Drugs/InformationOnDrugs/ucm412998.htm Available at: accessed May 01, 2019.
- 8.Zarin D.A., Tse T., Williams R.J., Carr S. Trial reporting in ClinicalTrials.gov - the final rule. N. Engl. J. Med. 2016;375(20):1998–2004. doi: 10.1056/NEJMsr1611785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCI National cancer Institute: precision medicine in cancer treatment. 2017. https://www.cancer.gov/about-cancer/treatment/types/precision-medicine Available at: accessed May 01, 2019.
- 10.Bode A.M., Dong Z. Precision oncology- the future of personalized cancer medicine? NPJ Precis. Oncol. 2017;1(1):2. doi: 10.1038/s41698-017-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin L., Andersen J.N., Futreal P.A. Cancer genomics: from discovery science to personalized medicine. Nat. Med. 2011;17(3):297–303. doi: 10.1038/nm.2323. [DOI] [PubMed] [Google Scholar]
- 12.Barriers to patient enrollment in therapeutic clinical trials for cancer - a landscape report. April 2018. https://www.acscan.org/sites/default/files/National%20Documents/Clinical-Trials-Landscape-Report.pdf Available at: Accessed May 01.
- 13.Fashoyin-Aje L.A., Fernandes L.L., Lemery S. Racial composition in trials supporting the U.S. approval of anti-cancer new molecular entities (NMEs): 2011-2016. J. Clin. Oncol. 2017;35(15):6518. [Google Scholar]
- 14.Torre L.A., Sauer A.M., Chen M.S., Jr., Kagawa-Singer M., Jemal A.4, Siegel R.L. Cancer statistics for Asian Americans, native Hawaiians, and Pacific islanders, 2016: converging incidence in males and females. CA A Cancer J. Clin. 2016;66(3):182–202. doi: 10.3322/caac.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fashoyin, Aje L.A., Fernandes L.L., Lemery S. Asian representation in clinical trials of new drugs for the treatment of cancer. J. Clin. Oncol. 2017;35 suppl; abstr 6564. [Google Scholar]
- 16.Fashoyin-Aje L.A., Fernandes L.L., Keegan P., Sridhara R., Pazdur R. Enrollment of Hispanics in cancer clinical trials: an FDA analysis. J. Clin. Oncol. 2018;36 suppl; abstr e18670. [Google Scholar]
- 17.Vintage Population estimates. 2016. https://www.census.gov/newsroom/facts-for-features/2017/hispanic-heritage.html Available at: Accessed May 01, 2019.
- 18.Regnante J.M., Richie N.A., Fashoyin-Aje L. US Cancer Centers of Excellence strategies for increased inclusion of racial and ethnic minorities in clinical trials. J. Oncol. Pract. 2019;15(4):e289–e299. doi: 10.1200/JOP.18.00638. [DOI] [PubMed] [Google Scholar]
- 19.HHS . U.S. Department Of Health And Human Services; 2011. Implementation Guidance on Data Collection Standards for Race, Ethnicity, Sex, Primary Language, and Disability Status, October, 2011.https://aspe.hhs.gov/basic-report/hhs-implementation-guidance-data-collection-standards-race-ethnicity-sex-primary-language-and-disability-status Available at: (accessed May 01, 2019) [Google Scholar]
- 20.NCI Comprehensive cancer center research strategy Expectations for catchment areas. 2017. https://cancercenters.cancer.gov/documents/CCSGFAQs508C.pdf Available at: accessed May 01, 2019.
- 21.NCI-Designated Cancer Centers https://www.cancer.gov/research/nci-role/cancer-centers/find Available at: (accessed May 01, 2019)
- 22.Petereit D.G., Burhansstipanov L. Establishing trusting partnerships for successful recruitment of American Indians to clinical trials. Canc. Contr. 2008;15(3):260–268. doi: 10.1177/107327480801500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BCN Best chance network. 2018. http://www.scdhec.gov/Health/DiseasesandConditions/Cancer/FreeCancerScreenings/ Available at: accessed May 01, 2019.
- 24.Syapse 2018. https://www.syapse.com/ Available at: accessed May 01, 2019.
- 25.MyChart https://mychart.utsouthwestern.edu/mychart/ Available at: accessed May 01, 2019.
- 26.Laccetti A.L., Pruitt S.L., Xuan L., Halm E.A., Gerber D.E. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J. Natl. Cancer Inst. 2015;107(4) doi: 10.1093/jnci/djv002. pii: djv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith A., Agar M., Delaney G. Lower trial participation by culturally and linguistically diverse (CALD) cancer patients is largely due to language barriers. Asia Pac. J. Clin. Oncol. 2018;14(1):52–60. doi: 10.1111/ajco.12818. [DOI] [PubMed] [Google Scholar]
- 28.Stand Up To Cancer https://progress.standuptocancer.org/clinicaltrials Available at: accessed July 29, 2019.
- 29.Petereit D.G., Guadagnolo B.A., Wong R., Coleman C.N. Addressing cancer disparities among American Indians through innovative technologies and patient navigation: the walking forward experience. Front. Oncol. 2011;1:11. doi: 10.3389/fonc.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerber D.E., Rasco D.W., Skinner C.S., Dowell J.E., Yan J., Sayne J.R., Xie Y. Consent timing and experience: modifiable factors that may influence interest in clinical research. J. Oncol. Pract. 2012;8(2):91–96. doi: 10.1200/JOP.2011.000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasco D.W., Xie Y., Yan J., Sayne J.R., Skinner C.S., Dowell J.E., Gerber D.E. The impact of consenter characteristics and experience on patient interest in clinical research. Oncol. 2009;14(5):468–475. doi: 10.1634/theoncologist.2008-0268. [DOI] [PubMed] [Google Scholar]
- 32.Commonwealth Report . July 14, 2017. Mirror, Mirror 2017: International Comparison Reflects Flaws and Opportunities for Better U.S. Health Care.https://www.commonwealthfund.org/publications/fund-reports/2017/jul/mirror-mirror-2017-international-comparison-reflects-flaws-and Available at: accessed May 01, 2019. [Google Scholar]
- 33.Capone I., Marchetti P., Ascierto P.A., Malorni W., Gabriele L. Sexual dimorphism of immune responses: a new perspective in cancer immunotherapy. Front. Immunol. 2018;9:552. doi: 10.3389/fimmu.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gertz B.J. Increasing minority representation in clinical trials. Profiles Divers. J. 2013 http://www.diversityjournal.com/10917-increasing-minority-representation-in-clinical-trials/ Available at: accessed May 01, 2019. [Google Scholar]
- 35.Eyeforpharma Bridging the diversity gap in clinical trials. 2015. https://social.eyeforpharma.com/clinical/bridging-diversity-gap-clinical-trials Available at: accessed May 01, 2019.
- 36.America's biopharmaceutical companies. http://innovation.org/about-us/commitment/clinical-trials/recognizing-diversity-in-clinical-trials Recognizing Diversity In Clinical Trials, October 26, 2017. Available at: (accessed May 01, 2019)
- 37.Eli Lilly . 2019. Diversity in Clinical Trials.https://www.lilly.com/who-we-are/diversity-and-inclusion/diversity-in-clinical-trials Available at: accessed May 01, 2019. [Google Scholar]
- 38.Genentech Inclusion research. 2019. https://www.gene.com/patients/advancing-inclusive-research Available at: (assessed July 02, 2019)