Highlights
-
•
Recent approaches for green synthesis of metallic nanoparticles were discussed.
-
•
The antibacterial activities of various metallic nanoparticles were mentioned.
-
•
The different modes and mechanisms of antibacterial property were deciphered.
Keywords: Metallic nanoparticles, Green synthesis, Antibacterial property, Antibiotics resistance
Abstract
Due to development of bacterial resistance to the conventional antibiotics, the treatment of bacterial infections has become a major issue of concern. The unprescribed and uncontrolled use of antibiotics has lead to the rapid development of antibiotic resistance in bacterial strains. Therefore, the development of novel and potent bactericidal agents is of great clinical importance. Interestingly, metallic nanoparticles (NPs) have been proven to be promising alternative to antibiotics. NPs interact with the important cellular organelles and biomolecules like DNA, enzymes, ribosomes, and lysosomes that can affect cell membrane permeability, oxidative stress, gene expression, protein activation, and enzyme activation. Since, NPs target multiple biomolecules concurrently; it becomes very difficult for bacteria to develop resistance against them. Currently, there are different physical and chemical methods utilized for NPs synthesis. However, most of these processes are costly and potentially hazardous for the living organisms and environment. Therefore, there is a need to develop an eco-friendly and cost-effective method of synthesis. Recently, the ‘green synthesis’ approaches are gaining a lot of attention. It is demonstrated that living organisms like bacteria, yeast, fungi, and plant cells can reduce inorganic metal ions into metal NPs by their cellular metabolites. Both the yield and stability of biogenic NPs are quite satisfactory. In the current article, we have addressed the green synthesis of various metal NPs reported till date and highlighted their different modes and mechanisms of antibacterial properties. It is highly anticipated that biogenic metallic NPs could be viable and economical alternatives for treating drug resistant bacterial infections in near future.
1. Introduction
The discovery of antibiotics is one of the great and remarkable achievements in the medical field. They have become indispensible in front line medical procedures like surgery, organ transplantation and treatment of various bacterial infections. Unfortunately the rampant use, particularly the misuse of antibiotics causes the development of antibiotic resistance in bacterial species. Such dramatic increase in bacterial resistance to antibiotics is now threatening the therapeutic achievement. As per World Health Organization report, antibiotic resistance has become one of the leading public health threats of 21st century [1]. Both the inhibition of enzyme activity and efficacy of efflux pumps are the main defensive strategies for bacterial cells to become less susceptible to antibiotics [2]. Since the available antibiotics have become less effective or ineffective against particular bacterial species, it is becoming very tough to treat several bacterial infections.
Nanotechnology has come forth with a fruitful solution for the serious issue of bacterial resistance to antibiotics. NPs can bind efficiently to the bacterial surface and rupture their cell wall that further leads to cell death [2]. It was noticed that NPs with size less than 20 nm can penetrate the bacterial cell wall and in-turn hamper the biochemical pathways through destruction of the cell organelles which ultimately leads to death of bacteria [3]. Biogenic nanoparticles are properly capped with natural flavonoids which inhibit the enzymatic activity that hampers the synthesis of nucleic acids in several micro-organisms [4]. NPs are known to generate reactive oxygen species (ROS) which causes mechanical damage to the bacterial cell membrane [5]. It is reported that NPs can be recycled for repetitive use as antimicrobial agents [6]. It is very difficult for bacteria to develop resistance against NPs, since NPs target several cellular pathways at a time [7].Therefore, NPs can be a great substitute to the conventional antibiotics to treat antibiotic resistant bacterial infections.
The unique properties like large surface area, stability, exceptional mechanical strength and lower melting points make the NPs highly compatible for various clinical applications like drug delivery, cancer therapy, biofilm inhibition and treatment of microbial infections [8]. The property of NPs to hold both the hydrophilic & hydrophobic substances makes them suitable drug carriers [9]. Nanomaterials are fabricated by different physical and chemical methods. However, high energy requirements, complex instrumental design, high expensiveness and low-yield are few crucial shortcomings associated with physical approach [10,11]. The chemical method of synthesis is more economical and provides high yield with simple experimental setup [12]. However, chemical method involves the use of toxic and volatile chemical reactants which are very hazardous and harmful for environment [13]. Due to the high vapour pressure, volatile solvents like aromatic amines and thiols cause air pollution [14]. Uses of reducing agents like sodium borohydrate and hydrazine derivatives are highly noxious for the environment [15]. These facts intrigued the researchers to develop alternative routes which are eco-friendly and sustainable for synthesis of NPs. Therefore, the green protocols for synthetic purposes are gaining significant acceptance from scientific community [16].
Currently, synthesis of biogenic NPs has attracted a great deal of attention for its cost-effectiveness, environmental benignity and relative ease of synthesis [17,18]. In this process, cellular extract of living organisms like bacteria, algae, fungi, actinomycetes, and plants are employed as green reaction media as well as capping agents [19]. Aqueous biological extracts have become suitable media for NPs synthesis due to their non-toxic and nonvolatile nature. Biological extracts are generally mixture of different kinds of active biomolecules like proteins, carbohydrates, vitamins, polymers and natural surfactants that provide high stability and enhanced dispersity to the synthesized NPs [20].
Recently, many review articles dealing with the applications of biogenic NPs for inhibiting pathogenic microorganisms have been published. Duran et al. published a review article which focused on antimicrobial potency of silver NPs [21]. The review article published by Ahmad et al. highlighted the plant extract mediated synthesis of silver NPs and their use in antimicrobial applications [22]. Some other articles were also published to mainly focus on the antimicrobial activity of Ag NPs and Zn NPs [23,24]. In the present review, we have tried our level best to summarize and compile the recent research works dealing with the various modes of green synthesis of NPs. Simultaneously, the applications of various biogenic metallic NPs like silver, gold, zinc, iron, palladium, selenium, cerium and tellurium in the treatment of antibiotics resistant bacterial infections have been critically discussed.
2. Modes of synthesis and purification of biogenic NPs
2.1. Fungi assisted synthesis of nanoparticles (NPs)
Fungi are good sources of secondary metabolites and active biomolecules that are very essential for the synthesis of NPs. Some fungal species like F. oxysporum secrete proteins, polymers and enzymes that voluntarily help in producing metal NPs [25]. These constituents improve the yield and stability of NPs. In other reports, it was found that several fungal species have capability to synthesize NPs using extracellular amino acid residues. For instance, yeast surface contain glutamic acid and aspartic acid that photo reduces silver ions into silver metal in presence of adequate amount of light [26]. Ahmad et al. (2003) noticed that fungal species like F. oxysporum has reductase enzyme in cytosol which reduces silver ions into silver metal in presence of NADH+, a reducing component [27]. Phytochelatin group of compounds which are found mainly in fungus have high capability to reduce silver ion into silver metal [28]. Sanghi and Verma (2009) utilized culture supernatant of fungal species Coriolus versicolor to synthesize silver NPs (Ag NPs). In this study, FTIR data confirmed the occurrence of hydroxyl group in the fungal mycelium which donated electrons to silver ion and reduced it into elemental silver to form Ag NPs. It also confirmed the presence of aliphatic and aromatic amines and some proteins in the fungal extract that acted upon as capping agents to stabilize the formed Ag NPs. Furthermore, it was demonstrated that it stabilized silver metal by binding with protein through amide bond [29]. Tan et al. (2002) also reported the involvement of SH group containing protein from fungal extract in the capping and stabilization of Ag NPs [30].
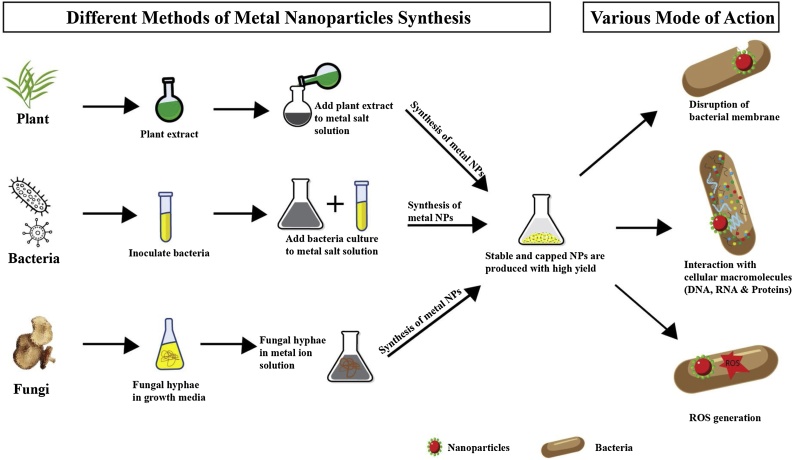
Das et al. (2009) had used mycelia of R. oryzae for synthesis of nano conjugate of gold (Au) NPs through in situ reduction of chloroauric acid (HAuCl4) in acidic medium (pH 3) [31]. Verticillium fungus is also a good mediator for synthesis of silver NPs. Recently, it has been found that biomass of fungi intracellularly synthesized NPs on exposure of AgNO3, in acidic medium (pH 5.5–6) [32]. The pictorial representation for green synthesis of various metallic nanoparticles and the mechanism of their anti-bacterial properties is illustrated in Fig. 1.
Fig. 1.
Pictorial representation for green synthesis of metallic nanoparticles from plant, bacteria and fungi. The various mechanisms of bacterial cell death is also illustrated (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.2. Bacteria assisted synthesis of NPs
Bacteria assisted synthesis of NPs takes place in two ways: extracellular and intracellular approaches. Extracellular synthesis of NPs has advantage over intracellular method in terms of being less time-consuming, since it does not need any downstream process for collection of NPs from the organisms [33].
Bacteria contains reductase enzyme inside the cell that catalyze the reduction of metal ions into metal NPs. Bacterial species like D. radiodurans has great antioxidant activity and is highly resistant to radiation and oxidative stress [34]. It makes it favourable for use in green synthesis of Au NPs from its ionic form. The fabricated Au NPs were stable for a longer time and showed better antimicrobial activity.
In recent study, Kunoh Tatsuki et al. (2017) used Leptothrix bacteria for synthesis of Au NPs by reducing gold salt in aqueous medium [35]. It was noted that that gold salt was reduced by guanine residues of RNA molecules and 2-deoxy guanosine.
2.3. Plant assisted synthesis
Plant assisted synthesis of NPs is more efficient in terms of obtaining a higher yield than the microbial synthesis. Plants have several metabolites and biochemicals (eg. polyphenols) that can function as stabilizing as well as reducing agent in the synthesis of biogenic NPs. Plant mediated synthesis of NPs is eco-friendly (avoiding use of toxic chemicals) and economical. NPs synthesized from plant sources were found to be much more stable than those formed by microbes and fungus [33]. Plant mediated synthesis of NPs can be classified into three groups, viz. extracellular, intracellular and through phytochemicals. Extracellular method is employed when plant extract is used as raw material for NP synthesis. Intracellular synthesis of NPs takes place inside the cells of plant tissue by utilization of cellular enzymes. Post synthesis, the NPs are recovered by rupturing the cell wall of the plant cells. Synthesis of NPs from plant extract is comparatively a cheaper method and it results in higher yield due to presence of larger amount of phytochemicals in plant extract that can either stabilize or reduce the metal ions into metal NPs [36]. Phytochemical mediated synthesis of NPs is not a common process since it requires knowledge of the particular phytochemical needed for synthesis of stabilized NPs [37]. Shakeel Ahmed et al. (2015) synthesized spherical shaped Ag NPs from leaf extract of A. indica. The FTIR study demonstrated that flavonoids and phytochemical compounds of the plant extract work as reducing and stabilizing agent during the synthesis of NPs. These NPs exhibited potent antimicrobial activity [38]. T.Y Suman et al. (2013) extracted
Au NPs from root extract of M. citrifolia which were also found to be antimicrobial in nature [39].
2.4. Purification of biogenic NPs
Upon synthesis, it is very essential to properly purify the biogenic NPs before employing them in any applications. Since long time, researchers have been using the method of centrifugation to purify the nanoparticles due to ease of operation and less time consuming. Thus, repetitive washing and high speed centrifugation are applied for the separation and purification of biologically produced metallic nanoparticles in order to eliminate the unreacted bioactive molecules [37]. However, this method is having several limitations such as centrifugation may cause agglomeration of NPs, destabilization of NPs due to detachment of the surface passivating agents from NPs and change in the intrinsic property of the NPs. Another very simple method of purification is the method of dialysis by using suitable cut off membrane. Small organic molecules present in the plant extracts can be easily pass through the dialysis membrane, whereas organic molecules present as surface passivating agents remain intact and conjugated with NPs inside the dialysis membrane. However, this method of purification is time consuming and it generally takes more than 24 h. However, the technique of diafiltration is not used for biofabricated nanoparticles as they are insoluble in water. Magnetic nanoparticles like Fe2O3 and Fe3O4 are easily separated by applying external magnetic force. However, the removal of tightly held biomolecules over the surface of nanoparticles is still a challenge.
3. Factors influencing the synthesis of various NPs
3.1. Temperature
Significant amount of research works are being undertaken worldwide to study the regulation of temperature over NPs. Temperature is one of the most influencing factors that affect the size and shape (morphology) of the NPs and their rate of synthesis. The various shapes (triangle, octahedral platelets, spherical, and rod like), size and synthesis of NPs are dependent on temperature. As temperature increases, there is an increase in reaction rate and formation of nucleation centres [40]. The research group headed by Sneha et al. (2010) evaluated the effect of temperature on morphology of the Au NPs synthesized from Piper betel leaf extract [40]. It was observed that triangular shaped NPs were formed at 20 °C, whereas octahedral shaped NPs with sizes ranging from 5 to 500 nm were fabricated at temperature ranging from 30 to 40 °C. Further, at higher temperature (50–60 °C), the sizes of the NPs were more consistent and spherical in shape. In another notable work, the biological production of Ag NPs from P. eldarica bark extract at different temperatures has been reported by Iravani and Zolfaghari (2013) [41]. The synthesis was done at various (25, 50, 100 and 150 °C) temperatures. The findings concluded that the size of the NPs decreased with the rise in temperature while their yield was found to be better. This fact was well supported by scanning electron micrographs of NPs. Fleitas-Salazar et al. (2017) studied the impact of oxidizing atmosphere and temperature on the synthesis of Ag NPs using a biocompatible polymer PEG [42]. The study deciphered that the ability of PEG to reduce silver salt was high at 100 °C. They concluded that at 100 °C the functional groups of PEG actively interacted with Ag molecules that resulted in the formation of more stable Ag NPs. At 60 °C, reduction of Ag ions occurred through oxidation of hydroxyl groups present in PEG. Thus, the authors reported that there are different mechanisms for Ag NP synthesis at various temperatures. Islam et al. (2011) standardized the synthesis protocol of Au NPs from silver-ammonia complex in aqueous solution of poly (ethylene oxide)-poly (propylene oxide) at different temperatures. It was inferred that at lower temperature, the size and distribution of NPs is controlled by change in morphology of polymer, but at higher temperature it is controlled by change in polymeric chemical composition [43]. Tan et al. (2015) analyzed the effect of temperature on encapsulation and formation of Au NPs with the use of PNIPAm/PEI as template. The TEM analysis revealed that the most optimum encapsulation of Au NPs and that of formation of stable Au-PNIPAm/PEI composite particles had taken place between 25 °C–30 °C and there was homogeneous distribution of particles around the template. But at lower temperature (15 °C), the encapsulation was very poor [44]. In another research work dealt with Manganese doped cobalt ferrite NPs synthesis [45], it was demonstrated that the size of the NPs increases with increase in temperature.
3.2. pH
The pH of reaction plays a pivotal role in the formation of NPs. Like temperature, pH also regulates the formation of nucleation centres. As the pH increases, number of nucleation centres also increases, leading to enhanced formation of metallic NPs. It has been established that pH takes an important role in formulating the morphology as well as size of the NPs. Armendariz et al. (2004) studied the synthesis of Au NPs from A. sativa at different pH. It was noticed that at lower pH (pH 2), very less amount of NPs were formed but the size of the NPs was considerably larger (25−85 nm). They postulated that at lower pH, Au NPs do not form more nucleation centres, so they aggregate to form larger sized NPs [46]. On the contrary, at slightly higher pH (pH 3–4), small sized NPs were formed. Another work was done by Fan et al. on pH dependent NPs response. They evaluated the release of Camptothecin loaded NIPAm poly-(N-isopropylacrylamide)/ chitosan NPs on tumors. The authors noticed that the loaded NPs show more release to target when NIPAm and chitosan ratio is 4:1(w/w). It was found that the release rate of Camptothecin was optimum at pH 6.8 whereas; it decreased on increasing or decreasing the pH, at 37 °C. Another novel research work on the effects of pH on the size and their average aspect ratio of gold nanorods was carried out by Okitsu et al. (2009) [47]. It was observed that the size and aspect ratio decreases with increase in pH.
3.3. Reaction time
Along with the temperature and pH, reaction time was also found to be one of the major factors that influence the morphology of NPs. A notable research work to study the effect of reaction time on magnetic NPs was performed by Karade et al. (2018) [48]. Fe NPs were synthesized from Ferric nitrate solution using Green tea extract. It was found that the reaction time influences the magnetic as well as structural properties of magnetic NPs. With the increase in reaction time, the particle size increases from 7.5 nm to 12 nm. It was also found that increase in reaction time can improve the saturation magnetization (Ms) of NPs. Simultaneously, the effects of reaction time on the particle size of cadmium selenide NPs was studied [49]. The measured particle sizes were found to be 15.8, 10.5, 6.7 and 111.7 nm at 4, 8, 12 and 16 h of reaction, respectively. Thus, it was concluded that the particle size of cadmium selenide NPs decreases with increase in reaction time. It was proposed that the unusual increase in size at 16 h was due to aggregation of particles. In another work, the effect of reaction time on particle size of ZnO and Cerium doped ZnO was investigated by Flor et al. (2004) [50]. The authors observed the linear increase in particle size with increase in reaction time. It was also noticed that the Cerium doped particle size is bigger as compared to normal ZnO at constant reaction time. Another notable research work was done to investigate and understand the influence of reaction time on size, reduction and stability of Au NPs synthesized from oil palm extract (E. guineensis) [51]. It was found that the rate of reduction for Ag NPs formation was increasing with the increase in reaction time.
4. Antimicrobial activities of different metallic NPs
4.1. Antimicrobial activity of Ag NPs
Silver is being widely used as an antimicrobial agent since ancient times. Presently it is being employed as a potent antibacterial agent for wound dressing. Although antibiotics are extensively used in medical field, their prolonged exposure enables the bacteria to develop resistance. This may be the result of bacteria’s self-defense mechanism due to which mutation in genes might occur. This facilitates the formation of enzymes that inactivate antibiotics [52]. Thus, antibiotic resistance in bacteria has become a major issue of concern. To overcome this challenge, scientists have reported several new approaches; antimicrobial activity of NPs being one of them. Amongst various NPs, silver is the most potent antibacterial agent. Besides, Ag NPs are used as nano carriers for drugs and antibiotics which help in enhancing the activity of antibiotics against resistant microbes [53,54].
The antibacterial activity of Ag NPs depends upon their size and shape. Pal et al. (2007) discovered that if size of Ag NPs decreases, their surface area increases that lead to enhanced binding affinity with molecules. It was also noticed that a triangular shaped Ag NP showed a much more pronounced antimicrobial activity in comparison to a spherical or a rod shaped one [55].
Ag NPs generate reactive oxygen species (ROS) that are responsible for oxidation of cellular components i.e. cellular DNA and protein [56]. Upon oxidation of these components, cell becomes unstable at physiological and genetic level causing failure of metabolism and cell division process. Ag NPs affect the signal transduction in microbial cells of E. coli through the alteration of phosphorylation states of tyrosine [57]. Ag NPs also affect biofilm formation by preventing its growth. Ag NPs bind to the membrane of the cells and alter its permeability by changing the membrane potential [57]. Once it enters the cell, these Ag NPs get converted into silver ions and start interacting with cellular biomolecules that cause damage to the cells. It hinders DNA replication by binding with DNA [58]. It interferes with the cell division process by binding with membrane proteins and cellular proteins that assist cell division. [59].
It was found that Ag NPs are more successful against Gram-negative bacteria than Gram-positive ones. This is because the former are capsulated with lipopolysaccharides possessing negative charge which binds with positively charged silver. Contrarily, Gram-positive bacteria are covered by thick layer of peptidoglycans and linear polysaccharides that are cross-linked with each other by embedded proteins, providing rigidity to the cell and thus preventing the binding of NPs to its surface. When silver ions bind with Gram-negative bacteria, holes are created in the cell wall which enables the penetration of Ag NP inside the cell [60].
Kim Soo-Hwan et al. (2011) studied the effect of Ag NPs on S. aureus and E. coli at various temperature and pH. They concluded that change in temperature and pH did not affect its antimicrobial activity; Ag NPs are highly active at long range of temperature and pH. It was found that these NPs affected cellular morphology, membrane proteins and respiratory enzymes [61].
4.2. Antimicrobial activity of Fe NPs
Like Ag and Au NPs, nano-sized biogenic iron was proven to be a potent anti-microbial agent. The semi-crystalline biogenic iron oxide nanoparticles (Fe NPs) of size 80−100 nm, that were developed from T. procumbens leaf extract demonstrated bactericidal potency against a Gram-negative bacteria like P.aeruginosa [62]. Naseem and Farrukh (2015) reported the G. jasminoides and L. inermis extract mediated production of Fe NPs and studied their repressive effect against S. aureus, S. enterica, P. mirabilis and E. coli. Both NPs successfully inhibited the microbial growth under test [63]. Recently, Alam et al. (2019) used Skimmia laureola extract for the synthesis of poly-dispersed Fe NPs which showed remarkable antibacterial activity against a wild pathogen R. solanacearum through disintegration of cell wall [64]. In another effort, Fe NPs synthesized from M. oleifera leaf extract (Aisida et al. 2019) [65] demonstrated antibacterial activity against P. aeruginosa, E. coli, S. aureus, S. typhi and P. multocida. In another praiseworthy attempt, bioreductive preparation of Fe NPs was reported by using root and leaf extracts of A. conyzoides (Madivoli et al.) [66]. Prepared NPs showed astonishing antibacterial potential against both Gram-positive and Gram-negative bacteria. The NPs exhibited maximum activity against P. aeruginosa as compared to E. coli, B. subtilis, S. aureus and C. albicans. It was perceived that the killing of bacterial strains occurred due to the physical interaction between bacterial cells and NPs. The positively charged NPs easily get attached to the surface of negatively charged bacterial cells that result in rupture of cell wall followed by cell death [66]. Another group synthesized biocompatible Fe NPs from the peel extract of P. granatum which exhibited efficient antibacterial effects against P.aeruginosa [67]. Khalil et al. (2017) synthesized Fe NPs using aqueous extract of Sageretia thea and found its applicability to inhibit growth of P. aeruginosa, S. epidermidis, B. subtilis, E. coli, and K. pneumoniae. Among them, P. aeruginosa and S. epidermidis were most susceptible with minimum inhibitory concentration of 7.8 μg/L [68]. Rajiv et al. (2018) biologically produced highly stable Fe NPs from L. camara plant extract for inhibition of some pathogenic bacteria. Antibacterial assay showed that the formed NPs effectively inhibited Pseudomonas species, Klebsiella and Staphylococcus [69]. In another work, E. crassipes leaf extract were utilized for bio-manufacturing rod shaped Fe NPs which showed good inhibitory effect against S. aureus and P. fluorescens [69]. Antibacterial assay depicted that 100 μg/ml aqueous Fe NPs showed maximum efficiency against the bacterial strains under investigation. Groiss et al. (2016) prepared spherical shaped and crystalline Fe NPs through bioreduction induced by C. ramiflora leaf extract and examined its bactericidal potential against E. coli and S. epidermidis. Antibacterial assay demonstrated that both the bacterial strains were considerably inhibited at 37 °C [70]. In another approach, C. guianensis fruit extract mediated Fe NPs were fabricated and efficiently applied as an inhibitor for some pathogenic microorganisms. Dose-dependent disc diffusion experiment elucidated that the bio-modulated Fe NPs exhibit better antibacterial activity against Gram-negative bacteria like K. penumoniae MTCC 530, E. coli MTCC 2939, and S. typhi MTCC 3917 than Gram-positive S. aureus MTCC 96 [71]. Rufuse et al. (2017) synthesized hematite (α-Fe2O3) nanosized materials using A. occidentale leaf extract in order to inhibit Gram-positive Staphylococcus aureus and Gram-negative E.coli. The antibacterial test demonstrated that synthesized NPs were convincingly effective in inhibiting the bacterial strains under trial [72].
Recently, pure hematite phase magnetic Fe NPs were synthesized from floral extract of C. viminalis which was further employed as a novel antibacterial agent against some Gram-negative and Gram-positive bacteria [73]. Antibacterial test concluded that these biologically synthesized Fe NPs proved to be most effective against S. typhi, S. aureus, S. enterica and K. pneumonia while S. dysenteriae was moderately inhibited.
4.3. Bactericidal properties of zinc oxide NPs
Increasing antibiotic resistance and re-emergence of bacterial infections across the world, pose a severe threat to human life. Zinc oxide nanoparticles (ZnO NPs) are widely being explored these days due to its unique antibacterial and antifungal properties. ZnO is known to possess photo-oxidising and photocatalytic effect and is regarded biosafe [74]. ZnO NPs are found to be effective on microorganisms in the size range of nanometers to micrometres.
Due to the nano scaled size of ZnO, it is more convenient for it to interact with bacterial cell by entering inside it. Mostly the antibacterial properties of ZnO NPs are due to their unique physico-chemical properties and high surface area to volume ratios. ZnO NPs were also demonstrated to be biocompatible in human cells in several studies; therefore it is being thoroughly explored by researchers presently [75].
Pathogenic bacteria are known to have cell surface proteins for adhesion and formation of colonies. Polysaccharides and tiechoic acid are also present on the cell wall that protects it from host defence mechanism and environmental conditions. Since, all these are charged macromolecules, the surface modified NPs can be used to induce specific interactions in order to disrupt the cell wall integrity. ZnO NPs directly interact with the cell wall of bacterial cells leading to the disruption of its integrity. ZnO can be used as efficient bactericidal agent for Gram-negative as well as Gram-positive bacteria [76]. Recently, it is reported by Jones et al. that ZnO NPs exhibit higher bactericidal effects on S. aureus in comparison to MgO, TiO2, Al2O3, CuO and CeO2 NPs [77].
ZnO is much more biocompatible than TiO2 and also has very high photocatalytic efficiency as compared to other inorganic photocatalytic materials [78]. ZnO is known to absorb UV light heavily [7]. This property of ZnO is associated with its conductivity and thus an increase in interaction of ZnO NPs with bacteria [79]. Interaction with UV light causes desorption of these oxygen species from the surface [80]. Thus improved photoconductivity is achieved due to reduction in surface electron depletion region. Significant ROS can be observed on treating bacterial broth with ZnO NP and illuminating it with UV light due to its phototoxic effects. These species help the NPs in entering inside the bacteria and further killing them [78,79].
Shape of ZnO NP also plays a crucial role in its antibacterial properties. Studies have proved that toxicity of these NPs depends upon its morphology. The process and conditions of synthesis decide the morphology of the synthesized NPs [81]. Controlling the synthesis parameters like type of precursor being used, solvents used and physical conditions such as temperature and pH, help in attaining desired antibacterial properties in the ZnO NPs. Studies have revealed the enhanced bactericidal effect of flower shaped NPs against S. aureus and E. coli as compared to those that are rod shaped [82]. Also it has been proved experimentally that more exposed Zn terminated polar facets show better antibacterial results [83].
Sirelkhatim et al. (2015) revealed that Oxygen annealing of ZnO increased the amount of oxygen atoms on the surface. This leads to high adsorbance of O2 atoms onto the ZnO surface that causes generation of more ROS leading to more oxidative stress and enhanced antibacterial activity [83]. Another study by Mamat et al. revealed that oxygen annealing caused nanohole formation on the surface, thus increasing the surface area as well as O2 adsorbance on surface that ultimately results in generation of more ROS and enhanced antibacterial effect [84].
Antibacterial activity of ZnO-NPs is also affected by its size and concentration [85]. Larger the surface area and higher the concentration, more is the bactericidal effect. It has also been found out that smaller the size of the NP, easier it is to penetrate inside the bacterial cell membrane. This is due to the larger interfacial area in small sized NPs. It was also demonstrated that higher the surface area of the NP, more are the oxygen species present on the surface and thus higher is the ROS generation, resulting in better bactericidal effect [85].
It is reported that ZnO NPs are very promising agents in the array of development of substitute for overcoming antibiotic resistance, because they demonstrate significant bactericidal activity against Gram-negative strains such as K. aerogenes, P. aeruginosa and E. coli etc as well as Gram-positive strain such as S. aureus [86].
4.4. Bactericidal properties of Au NPs
Au NPs are well known for their biocompatibility and antimicrobial activity. Au NPs cannot act singly on the target and must be tagged with other biomolecules to show effective antimicrobial property. Cross linking of either collagen, chitosan or gelatin with Au NPs enable it to easily bind with the macromolecules as discussed by Rajendran et al. [87]. Au NPs can also be incorporated with other drug molecules to observe a synergistic antimicrobial effect. Gu et al. demonstrated the effect of Vancomycin conjugated Au NPs on vancomysin resistant Enterococci (VRE) and E. coli. The antimicrobial effect of Vancomycin was found to be enhanced by 50 folds [88]. Conjugation of Au NPs with pathogen specific antibodies or photosensitizing molecules for photothermal and photodynamic therapy also helps in increasing its antimicrobial activity [89,90].
Au NPs affect bacterial cells following different courses of action. It may enter the cells and alter their membrane potential by inhibiting the activity of ATP synthase. It leads to depletion in the ATP levels that collapses the energy metabolism leading to cell death [91]. Multiple drug resistant (MDR) bacteria are also killed by this non ROS dependent pathway. According to Guerrini, L 2018, multivalency on NPs is enabled due to the high surface/volume ratio, thus incorporating many functional ligands. This multivalency is a way to enhance interaction of NP surface with target bacteria. These features enable NPs to be coupled with antibiotics for the treatment of multiple drug resistance in bacteria. Antibiotics conjugated to NPs were found to be more effective than antibiotics alone [92]. Li et al. (2014) reported that Au NPs (∼2 nm size) having cationic surface properties can be efficiently employed as antibacterial agent. This research group investigated its efficacy against Gram-negative bacteria like P. aeruginosa (intrinsic resistance to many antibiotics because of low membrane permeability) and E. cloacae complex, Gram-positive bacteria S. aureus (resistant to almost all therapeutic chemo-agents) and methicillin-resistant S. aureus (resistant to most antibiotics used). Effective MIC values for these pathogens were in the range of 8–64 nM and the bacterial cell death was presumed to be due to the loss of membrane integrity [93]. Rajan et al. (2015) demonstrated the effect of size on the antibacterial and anti-cancerous activity of Au NPs. They portrayed that smaller Au NPs would show better bactericidal effect than the larger ones [94].
4.5. Bactericidal activity of other metals
Biogenic palladium, selenium, cerium and tellurium NPs have also shown antimicrobial activity. According to a report, methanolic extract of M. oleifera peel was used to produce Pd NPs and harnessed for antimicrobial property against E. coli and S. aureus [95]. Antimicrobial assay revealed that the NPs effectively inhibited the growth of both bacterial strains. The same group biologically synthesized CeO2 NPs from M. oleifera peel extract and monitored their activity against the above bacterial strains [96]. Antibacterial assay confirmed the successful inhibition of both strains. Another amnestic work reports the bio-fabrication of CeO2 NPs from leaf extract of G. superba and their potential application against a set of Gram-negative and Gram-positive bacteria [97]. The NPs performed well against P. aeruginosa, P. vulgaris, K. pneumonia and S. pneumonia. Simultaneously, the chitosan-cerium oxide hybrid NPs were developed from Sida acuta leaf extracts which manifested good repressive effect against B. subtilis and E. coli [98]. It was anticipated that inhibition occurred due to the nanoparticle induced disintegration of cell membrane. Amarnath et al. (2012) prepared chitosan coated Pd nanocomposites and stabilized them using polyphenols obtained from grape extract and examined their application as a potential inhibitor for E. coli. NPs severely damaged the E. coli cells at an optimized concentration of 50 μg/L. It was concluded that the NPs bound with bacterial membrane via electrostatic interaction lead to the bacterial cell death [99]. Sharmila et al. (2017) synthesized biogenic Pd NPs from F. decipiens leaf extract and reported their high bactericidal activity against both Gram-negative bacteria (S. aureus and B. subtilis) and Gram-positive bacteria (P. aeruginosa and E. coli) [100]. In another work, C. guianensis fruit extract was employed for the bio-manufacturing of Pd NPs that exhibited extraordinary bactericidal activity against some human pathogens like S. typhi MTCC 3917, V. cholerae MTCC 3906, E. coli MTCC 1687 and K. pneumonia MTCC 530 whereas moderately active against M. luteus MTCC 1809, B. cerues MTCC 1272, P. mirabilis MTCC 425, P. aeruginosa MTCC 1688, R. rhodochorous MTCC 265 and S. aureus MTCC 96. Oxidative stress induced by the bio-capped Pd NPs was expected to be responsible for the observed inhibitory effect [101]. Some other workers have convincingly demonstrated the usefulness of biogenic Pd NPs against different microorganisms [102,103].
Sonkusre and Cameotra (2015) produced B. licheniformis JS2 derived selenium NPs (Se NPs) which adequately inhibited the growth of microbial colony of Staphylococcus aureus onto different surfaces like polystyrene, glass, and catheter [104]. Cremonini et al., (2016) synthesized two different Se NPs from culture supernatant of S. maltophilia (Gram-negative bacteria) and B. mycoide (Gram-positive bacteria). Both the NPs efficiently prevented the progression of biofilm of P. aeruginosa and Candida spp [105]. Recently, Se NPs with size of 90−150 nm were prepared by using S. aureus, P. aeruginosa and E. coli which displayed astonishing bactericidal activity against E. coli and S. aureus [106].
Zare et al. (2012) formed rod shaped elemental Te (Tellurium) NPs by using Bacillus sp. BZ. The formed Te NPs showed bactericidal activity against S. aureus, P. aeruginosa, S. typhi and K. pneumonia [107]. Rajakumar et al. (2012) also adopted the environmentally benign approach and utilized culture supernatant of A. flavus (MTCC no. 7369) to prepare Te NPs [108]. Well diffusion test established that the Te NPs significantly killed E. coli at the MIC value of 40 μg/ml. Mohanty et al., (2014) synthesized rod shaped Te NPs through a metal reducing bacteria S. oneidensis and reported their exceptional antibacterial property against P. aeruginosa [109].
Biologically prepared titanium NPs also find utility as an antimicrobial agent. Santosh kumar et al., (2014) investigated the bactericidal properties of TiO2 NPs biosynthesized from aqueous leaf extract of Psidium guajava [110]. These NPs impressively prevented the growth of E. coli and S. aureus. T. foenum-graecum leaf extract was used in order to synthesize TiO2 NPs and their antibacterial properties were studied against Y. enterocolitica, P. vulgaris, E. faecalis, P. aeruginosa, S. faecalis, S. aureus, B. subtilis, E. coli and fungus C. albicans. TiO2 NPs effectively inhibited the test microorganisms [111]. Another important work reports the emphatic bactericidal activity of TiO2 NPs synthesized by Prunus yedoensis leaf extract (Manikandan et al., 2018) against E. coli and S. aureus [103].
The summary of various biogenic metallic NPs with anti-bacterial properties is provided in Table 1.
Table 1.
Summary of various biogenic metallic NPs with anti-bacterial properties.
| Element | Biological source | Used component for synthesis | Size (nm) and shape | Affected Bacterial strains | Ref. |
|---|---|---|---|---|---|
| Silver | E. japonica | Leaf extract | 46 to 70 spherical | E. coli and S. aureus | [112] |
| Silver | J. adhatoda L | leaf extract | 5-50 spherical | P. aeruginosa | [113] |
| Silver | A. comosus | Leaf extract | 12.4 spherical | S. aureus, S. pneumoniae, P. mirabilis and E. coli | [114] |
| Silver | M. officinalis | Leaf extract | 12 spherical | S. aureus and E. coli | [115] |
| Silver | Alternaria sp | Fungus | 80 spherical | B. subtilis, S. aureus, E. coli and S. marcescens, | [116] |
| Silver | Penicillium | Fungus | 10-100 spherical | B. cereus, S. aureus, E. coli and P. aeruginosa | [117] |
| Iron | T. procumbens | Plant extract | 80-100 | P. aeruginosa | [62] |
| Iron | G. jasminoides and L. inermis | Plant extract | 21, hexagonal | E. coli, S. enterica, P. mirabilis and S. aureus | [63] |
| Gold | T. harzianum | Fungus | E. coli MTCC 1302 | ||
| Gold | H. Cordata | Plant extract | 30-90 spherical | B. subtillis, S. typhimurium, B. cereus and E. coli | [118] |
| Gold | Streptomyces sp. NH21 | Fungus | 10 spherical | E. coli, K. pneumoniae, P. mirabilisS. infantis, P. aeruginosa and B. subtilis | [119] |
| Gold | C. globosum | Fungus | 12 spherical | E. coli, ATCC 25922; K. pneumoniae, IMS/GN9; P. mirabilis, IMS/GN13 and S. aureus | [120] |
| Gold | A. catechu | Plant extract | 13.7 spherical | E. coli, K. pneumonia, P. aeruginosa, Enterobacter and S. aureus | [94] |
| Gold | C. cujete L (Calabash tree) | Plant extract | 32.89 spherical | E. Coli (MTCC 1687), P. aeruginosa (MTCC 1688), V. cholerae (MTCC 3906), Salmonella typhi (MTCC 531), S. flexneri (MTCC 9543) and B. subtilis (MTCC 441) | [121] |
| Gold | A. terreus | Fungus | 10-19 spherical | Escherichia coli | [122] |
| Zinc oxide | E. faecalis | bacteria | 16 to 96 spherical | E. Coli, K. pneumonia and S. aureus | [123] |
| Zinc oxide | R. graveolens | Herb | 28 wurtzite | K. aerogenes, P. aeruginosa, E. coli, S. aureus | [86] |
| Zinc oxide | E. faecalis | bacteria | 16 to 96 spherical | E. coli 03, K. Pneumonia 125 S. aureus 20 E. coli MTCC 9537, K. pneumonia MTCC 109, S. aureus MTCC 96, P. AeruginosaMTCC 741, S. flexneri MTCC 1457 and E. faecalis NCIM 5025 | [124] |
| Zinc oxide | C. pictus D. Don | Plant extract | 11–25 hexagonal | S. aureus (NCIM 2079), B. subtilis (NCIM 2063) E. coli (NCIM 2065) and S. paratyphi (NCIM 2501) | [125] |
| Zinc oxide | C. halicacabum | Plant extract | 30-80 spherical & rod | E coli and S. aureus | [126] |
5. Mode and mechanism of action of biogenic nanoparticles
5.1. Mechanism of action of metal NPs and metal oxide NPs
The mechanism of action of metal and metal oxide nanoparticles against bacteria is still not very clear and lot of studies is currently going on across the world. Researchers have shown that free metal nanoparticles exert toxic effects in dissolution of the outer membrane of bacterial surface. While oxidative stress induced by reactive oxygen species (ROS) is the principle mechanism in case of metal oxide nanoparticles [127]. Studies have shown that Ag NPs induced formation of pits and gaps on the membrane surface of bacteria by the release of ions which further interact with disulfide or sulfhydryl groups of enzymes causing interruption of metabolic pathway that finally leads to bacterial cell death [128]. Some studies revealed that ZnO NPs augmented the ROS generation on the membrane surface, thereby causing the dysfunction of membrane and death of bacterial cell [129]. Similar mechanism of oxidative stress driven by ROS generation was also observed in case of TiO2 NPs [130]. It was shown that TiO2 NPs can cause lipid peroxidation that affect the fluidity and integrity of the cell wall of bacteria through ROS generation.
5.2. Difference in mode of action of biogenic NPs under aerobic and anaerobic condition
The inhibitory and toxic effect of nanoparticles against bacterial strains depends on the flux of ions released by the nanoparticles. The extent of this ion flux is directly proportional to the amount of oxygen available. It was an interesting observation that Ag NPs showed better toxicity against aerobic E. coli under adequate oxygen supply, while the same nanoparticles exhibit lower toxicity against anaerobic bacteria with low oxygen supply. Accordingly, it was found that the minimum inhibitory concentration (MIC) for Ag NPs against anaerobic bacterial strains was quite high compared to aerobic bacteria [131]. While the MIC values for anaerobic bacteria like F. nucleatum, S. sanguis and S. mutans were 25, 50 and 50 μg/mL, respectively, it was 6 μg/mL in case of aerobic bacterial strain like E. coli. Hence, by manipulating the accessibility of oxygen, we can control the toxicity of nanoparticles.
5.3. Different ways that are targeted by biogenic NPs
The exact pathways that are targeted by nanoparticles for antibacterial activity are still not deciphered. However, biogenic nanoparticles follow two distinct ways to demonstrate their antibacterial potency; either by generating ions (in case of metallic NPs) or by releasing reactive oxygen species (in case of metallic oxide NPs). The released ions and ROS damage the cell wall, genetic material or membrane lipid through oxidation [132]. During the synthesis, biogenic nanoparticles get reduced by bioactive compounds like vitamins, proteins, polyphenols, carbohydrates, polymers etc. that are present in the cellular extract of living plants and microbes [133]. These molecules not only provide high degree of stability to the synthesized NPs but also add different functional groups to the surface of NPs. These functional groups allow the establishment of chemical bonds and offers active sites for physical interaction between nanoparticles and bacterial cell which is necessary for their inhibition [134]. Electrostatic and lipophilic interactions are the principle mechanisms through which biogenic NPs get attached with the bacterial cell. Accordingly, it was noticed that biogenic nanoparticles show better antimicrobial property than chemically synthesized ones. In a study, it was reported that biogenic NPs showed remarkable inhibitory effect against all the bacterial strains studied like. P. vulgaris, K. pneumonia, P. aeruginosa, S. aureus and V. cholera while chemically synthesized nanoparticles were found to be ineffective against P. vulgaris, P. aeruginosa and K. pneumonia [135].
5.4. Effects of biogenic NPs over clinically resistant bacterial strains
Development of antibiotics resistance in bacterial strains has become a major issue of concern. Therefore, a lot of research work is going on in the field of Nanobiotechnology to overcome this health issue. It was reported that biogenic Ag NPs could effectively inhibit the growth of clinical resistant strain of P. aeruginosa isolated from wound site of patient (MIC: 2.0 mg/ml) [136]. In another noticeable approach, it was noticed that biogenic ZnO NPs in combination with antibiotics can effectively kills methicillin resistant S. aureus (MRSA) clinical isolates (MIC: 2000 μg/mL, MBC: 2200 μg/mL) [137]. In addition, it was reported that biogenic nanoparticles can overcome the issues of antibiotics resistance associated with the formation of bacterial biofilm [138].
6. Concluding remarks and future perspectives
In recent times, antibiotic resistance of bacteria has become one of the major concerns in the human healthcare. Most of the antibiotics kill bacteria by disrupting membrane permeability, inhibiting membrane synthesis and modulating enzymatic pathways that are involved in cellular transcription, translation and replication process. It prevents the action of the antibiotics by degrading them or by decreasing their binding affinity towards target enzymes due to mutation in target genes. To overcome this challenge, scientists have focused to develop alternative approaches. The metal nanoparticles have proven to be one of best choices to treat drug resistant bacterial infections. The high surface to volume ratio of NPs increases their affinity towards the cellular membrane. In addition, the positive charge on the NPs can further enhance the affinity for the negatively charged membrane due to the presence of either lipopolysaccharide or techoic acid. It is not easy for bacteria to develop the resistance against NPs, since NPs simultaneously target several pathways to kill bacterial cell.
Scientists have developed several methods for synthesis of metal nanoparticles. The physical method is very expensive and the yield of NPs is also very low. The chemical synthesis methods which were extensively used in the last decades are not eco-friendly and pollutes the environment. Chemical synthesis is indeed costly and time consuming process. In order to overcome these challenges, scientists have recently developed a method of green synthesis which is environmental friendly and cost effective. In green synthesis, plants, bacteria and fungi are extensively used. These organisms are the major sources of metabolites that can work as stabilizing and reducing agent during NPs synthesis. NPs can be synthesized both extracellularly and intracellularly. Extracellular method is more preferred over intracellular method, since it does not need any downstream process for harvest of NPs from the organisms. Plant assisted synthesis is cheaper and more proficient in getting higher yields of NPs than the microbes. Plants have numerous metabolites and biochemicals that can function as stabilizing and/or reducing agent in green synthesis of NPs.
Among the various reaction parameters, temperature, pH and reaction time greatly influence the morphology (size and shape), chemical properties and rate of synthesis of the NPs by monitoring the formation of nucleation centres. The antibacterial property of the NPs changes on altering the size, shape and oxidation state of NPs. It is reported that antibacterial activity is greater with smaller size and decrease with increase in size of the NPs. When we talk about shape, triangular and spherical shaped nanoparticles have great antibacterial property, but the reason behind is not yet well understood. Most of the metal nanoparticles belonging to d-block element are reported as potent antibacterial agents. This is due to the fact that they contain high oxidation number and get easily reduced and oxidized in presence of reducing and oxidizing agent, respectively.
During the study, we observed that the formation of biogenic metallic nano-materials with antimicrobial property is quite simple and economical. Many biologically produced NPs showed excellent inhibition against several pathogenic microorganisms. Some of the nano-materials remarkably killed the various microbial species which developed high resistance to available drugs. Moreover, very small-sized nano-materials can even act as a carrier to deliver the antimicrobial drugs to the internal organs of the body which got affected by microbes. It is highly anticipated that these biogenic NPs will be able to successfully replace the available drugs against which the bacteria have developed resistance. Apart from biomedical applications, these ecofriendly NPs can even solve the problem of microbial contamination of potable water. Water contamination by bacteria causes millions of death every year worldwide, particularly in third world countries. In this regard, these NPs may be harnessed as an effective water disinfectant in order to provide contamination-free drinking water which is safe for human consumption.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The authors AS, AV, V, PMS and SS are thankful to Ministry of Human Resource and Development, Govt. of India for JRF and SRF fellowship. PKG is grateful to Science and Engineering Research Board, Govt. of India for NPDF scheme (Grant No. PDF/2016/002910). We would like to acknowledge Indian Institute of Information Technology, Allahabad for financial assistance by providing Institutional Seed grant project.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00427.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.W.H. Organization . World Health Organization; 2014. Antimicrobial Resistance: Global Report on Surveillance. [Google Scholar]
- 2.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomedicine. 2017;12:1227. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakha M., Pal S., Samantarrai D., Panigrahi T.K., Mallick B.C., Pramanik K., Mallick B., Jha S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015;5:14813. doi: 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fayaz A.M., Balaji K., Girilal M., Yadav R., Kalaichelvan P.T., Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010;6(1):103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Li B., Webster T.J. Bacteria antibiotic resistance: new challenges and opportunities for implant‐associated orthopedic infections. J. Orthop. Res. 2018;36(1):22–32. doi: 10.1002/jor.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajipour M.J., Fromm K.M., Ashkarran A.A., de Aberasturi D.J., de Larramendi I.R., Rojo T., Serpooshan V., Parak W.J., Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A., Mumtaz S., Li C.-H., Hussain I., Rotello V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019;48(2):415–427. doi: 10.1039/c7cs00748e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thanh N.T., Green L.A. Functionalisation of nanoparticles for biomedical applications. Nano Today. 2010;5(3):213–230. [Google Scholar]
- 9.Raghupathi K.R., Koodali R.T., Manna A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 10.Rajput N. Methods of preparation of nanoparticles - a review. Int. J. Adv. Eng. Technol. 2015;7(6):1806. [Google Scholar]
- 11.Swihart M.T. Vapor-phase synthesis of nanoparticles. Curr. Opin. Colloid Interface Sci. 2003;8(1):127–133. [Google Scholar]
- 12.Schmidt H. Nanoparticles by chemical synthesis, processing to materials and innovative applications. Appl. Organomet. Chem. 2001;15(5):331–343. [Google Scholar]
- 13.Abbasi A., Khojasteh H., Hamadanian M., Salavati-Niasari M. Synthesis of CoFe 2 O 4 nanoparticles and investigation of the temperature, surfactant, capping agent and time effects on the size and magnetic properties. J. Mater. Sci. Mater. Electron. 2016;27(5):4972–4980. [Google Scholar]
- 14.Ju-Nam Y., Lead J.R. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008;400(1-3):396–414. doi: 10.1016/j.scitotenv.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 15.Fabiano B., Reverberi A.P., Varbanov P.S. Safety opportunities for the synthesis of metal nanoparticles and short-cut approach to workplace risk evaluation. J. Clean. Prod. 2019;209:297–308. [Google Scholar]
- 16.Willard M., Kurihara L., Carpenter E., Calvin S., Harris V. Chemically prepared magnetic nanoparticles. Int. Mater. Rev. 2004;49(3–4):125–170. [Google Scholar]
- 17.Duan H., Wang D., Li Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015;44(16):5778–5792. doi: 10.1039/c4cs00363b. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed S., Chaudhry S.A., Ikram S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: a prospect towards green chemistry. J. Photochem. Photobiol. B, Biol. 2017;166:272–284. doi: 10.1016/j.jphotobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar M.S., Panwar J., Yun Y.-S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013;1(6):591–602. [Google Scholar]
- 20.Sharma D., Kanchi S., Bisetty K. Biogenic synthesis of nanoparticles: a review. Arab. J. Chem. 2015 [Google Scholar]
- 21.Kannan N., Subbalaxmi S., Durán N., Nakazato G., Seabra A.B. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: an overview and comments. Appl. Microbiol. Biotechnol. 2016;100(15):6555–6570. doi: 10.1007/s00253-016-7657-7. [DOI] [PubMed] [Google Scholar]
- 22.Durán N., Nakazato G., Seabra A.B. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: an overview and comments. Appl. Microbiol. Biotechnol. 2016;100(15):6555–6570. doi: 10.1007/s00253-016-7657-7. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rai M., Deshmukh S., Ingle A., Gade A. Silver nanoparticles: the powerful nanoweapon against multidrug‐resistant bacteria. J. Appl. Microbiol. 2012;112(5):841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- 25.Riddin T., Gericke M., Whiteley C. Analysis of the inter-and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology. 2006;17(14):3482. doi: 10.1088/0957-4484/17/14/021. [DOI] [PubMed] [Google Scholar]
- 26.Nam K.T., Lee Y.J., Krauland E.M., Kottmann S.T., Belcher A.M. Peptide-mediated reduction of silver ions on engineered biological scaffolds. ACS Nano. 2008;2(7):1480–1486. doi: 10.1021/nn800018n. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum, Colloids Surf. B. Biointerfaces. 2003;28(4):313–318. [Google Scholar]
- 28.Lee S.H., Jun B.-H. Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019;20(4):865. doi: 10.3390/ijms20040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanghi R., Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009;100(1):501–504. doi: 10.1016/j.biortech.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Tan Y., Wang Y., Jiang L., Zhu D. Thiosalicylic acid-functionalized silver nanoparticles synthesized in one-phase system. J. Colloid Interface Sci. 2002;249(2):336–345. doi: 10.1006/jcis.2001.8166. [DOI] [PubMed] [Google Scholar]
- 31.Das S.K., Das A.R., Guha A.K. Gold nanoparticles: microbial synthesis and application in water hygiene management. Langmuir. 2009;25(14):8192–8199. doi: 10.1021/la900585p. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Parishcha R., Ajaykumar P., Alam M., Kumar R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1(10):515–519. [Google Scholar]
- 33.Singh P., Kim Y.-J., Zhang D., Yang D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34(7):588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Li J., Li Q., Ma X., Tian B., Li T., Yu J., Dai S., Weng Y., Hua Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomedicine. 2016;11:5931. doi: 10.2147/IJN.S119618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunoh T., Takeda M., Matsumoto S., Suzuki I., Takano M., Kunoh H., Takada J. Green synthesis of gold nanoparticles coupled with nucleic acid oxidation. ACS Sustain. Chem. Eng. 2017;6(1):364–373. [Google Scholar]
- 36.Mohammadinejad R., Shavandi A., Raie D.S., Sangeetha J., Soleimani M., Hajibehzad S.S., Thangadurai D., Hospet R., Popoola J.O., Arzani A. Plant molecular farming: production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019;21(8):1845–1865. [Google Scholar]
- 37.Dauthal P., Mukhopadhyay M. Noble metal nanoparticles: plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind. Eng. Chem. Res. 2016;55(36):9557–9577. [Google Scholar]
- 38.Ahmed S., Saifullah M., Ahmad B.L., Swami S. Ikram, Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016;9(1):1–7. [Google Scholar]
- 39.Suman T., Rajasree S.R., Ramkumar R., Rajthilak C., Perumal P. The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2014;118:11–16. doi: 10.1016/j.saa.2013.08.066. [DOI] [PubMed] [Google Scholar]
- 40.Sneha K., Sathishkumar M., Kim S., Yun Y.-S. Counter ions and temperature incorporated tailoring of biogenic gold nanoparticles. Process Biochem. 2010;45(9):1450–1458. [Google Scholar]
- 41.Iravani S., Zolfaghari B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res. Int. 2013;2013 doi: 10.1155/2013/639725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleitas-Salazar N., Silva-Campa E., Pedroso-Santana S., Tanori J., Pedroza-Montero M.R., Riera R. Effect of temperature on the synthesis of silver nanoparticles with polyethylene glycol: new insights into the reduction mechanism. J. Nanoparticle Res. 2017;19(3):113. [Google Scholar]
- 43.Islam A.M., Mukherjee M. Effect of temperature in synthesis of silver nanoparticles in triblock copolymer micellar solution. J. Exp. Nanosci. 2011;6(6):596–611. [Google Scholar]
- 44.Tan N.P.B., Lee C.H., Li P. Influence of temperature on the formation and encapsulation of gold nanoparticles using a temperature-sensitive template. Data Brief. 2015;5:434–438. doi: 10.1016/j.dib.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar E.R., Jayaprakash R., ArunKumar T., Kumar S. Effect of reaction time on particle size and dielectric properties of manganese substituted CoFe2O4 nanoparticles. J. Phys. Chem. Solids. 2013;74(1):110–114. [Google Scholar]
- 46.Armendariz V., Herrera I., Jose-yacaman M., Troiani H., Santiago P., Gardea-Torresdey J.L. Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J. Nanoparticle Res. 2004;6(4):377–382. [Google Scholar]
- 47.Okitsu K., Sharyo K., Nishimura R. One-pot synthesis of gold nanorods by ultrasonic irradiation: the effect of pH on the shape of the gold nanorods and nanoparticles. Langmuir. 2009;25(14):7786–7790. doi: 10.1021/la9017739. [DOI] [PubMed] [Google Scholar]
- 48.Karade V., Dongale T., Sahoo S.C., Kollu P., Chougale A., Patil P., Patil P. Effect of reaction time on structural and magnetic properties of green-synthesized magnetic nanoparticles. J. Phys. Chem. Solids. 2018;120:161–166. [Google Scholar]
- 49.Rose I.C., Sathish R., Rajendran A.J., Sagayaraj P. Effect of reaction time on the synthesis of cadmium selenide nanoparticles and the efficiency of solar cell. J. Mater. Environ. Sci. 2016;7:1589–1596. [Google Scholar]
- 50.Flor J., de Lima S.M., Davolos M.R. Springer; 2004. Effect of Reaction Time on the Particle Size of ZnO and ZnO: Ce Obtained by a Sol–gel Method, Surface and Colloid Science; pp. 239–243. [Google Scholar]
- 51.Ahmad T., Irfan M., Bustam M.A., Bhattacharjee S. Effect of reaction time on green synthesis of gold nanoparticles by using aqueous extract of Elaise guineensis (oil palm leaves) Procedia Eng. 2016;148:467–472. [Google Scholar]
- 52.Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002;92:55S–64S. [PubMed] [Google Scholar]
- 53.Yount N.Y., Yeaman M.R. Emerging themes and therapeutic prospects for anti-infective peptides. Annu. Rev. Pharmacol. Toxicol. 2012;52:337–360. doi: 10.1146/annurev-pharmtox-010611-134535. [DOI] [PubMed] [Google Scholar]
- 54.Singh R., Smitha M., Singh S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014;14(7):4745–4756. doi: 10.1166/jnn.2014.9527. [DOI] [PubMed] [Google Scholar]
- 55.Pal S., Tak Y.K., Song J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chatzimitakos T.G., Stalikas C.D. Qualitative alterations of bacterial metabolome after exposure to metal nanoparticles with bactericidal properties: a comprehensive workflow based on 1H NMR, UHPLC-HRMS, and metabolic databases. J. Proteome Res. 2016;15(9):3322–3330. doi: 10.1021/acs.jproteome.6b00489. [DOI] [PubMed] [Google Scholar]
- 57.Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markowska K., Grudniak A.M., Wolska K.I. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Pol. 2013;60(4) [PubMed] [Google Scholar]
- 59.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. Coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Rai M., Deshmukh S., Ingle A., Gade A. Silver nanoparticles: the powerful nanoweapon against multidrug‐resistant bacteria. J. Appl. Microbiol. 2012;112(5):841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim S.-H., Lee H.-S., Ryu D.-S., Choi S.-J., Lee D.-S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011;39(1):77–85. [Google Scholar]
- 62.Senthil M., Ramesh C. Biogenic synthesis of Fe3O4 nanoparticles using tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Digest Journal of Nanomaterials & Biostructures (DJNB) 2012;7(4) [Google Scholar]
- 63.Naseem T., Farrukh M.A. Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. J. Chem. 2015;2015 [Google Scholar]
- 64.Alam T., Khan R.A.A., Ali A., Sher H., Ullah Z., Ali M. Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Mater. Sci. Eng. C. 2019;98:101–108. doi: 10.1016/j.msec.2018.12.117. [DOI] [PubMed] [Google Scholar]
- 65.Aisida S.O., Madubuonu N., Alnasir M.H., Ahmad I., Botha S., Maaza M., Ezema F.I. Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Appl. Nanosci. 2019:1–11. [Google Scholar]
- 66.Madivoli E.S., Kareru P.G., Maina E.G., Nyabola A.O., Wanakai S.I., Nyang’au J.O. Biosynthesis of iron nanoparticles using Ageratum conyzoides extracts, their antimicrobial and photocatalytic activity. SN Applied Sciences. 2019;1(5):500. [Google Scholar]
- 67.Irshad R., Tahir K., Li B., Ahmad A., Siddiqui A.R., Nazir S. Antibacterial activity of biochemically capped iron oxide nanoparticles: a view towards green chemistry. J. Photochem. Photobiol. B, Biol. 2017;170:241–246. doi: 10.1016/j.jphotobiol.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 68.Khalil A.T., Ovais M., Ullah I., Ali M., Shinwari Z.K., Maaza M. Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 2017;10(4):186–201. [Google Scholar]
- 69.Jagathesan G., Rajiv P. Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatal. Agric. Biotechnol. 2018;13:90–94. [Google Scholar]
- 70.Groiss S., Selvaraj R., Varadavenkatesan T., Vinayagam R. Structural characterization, antibacterial and catalytic effect of iron oxide nanoparticles synthesised using the leaf extract of Cynometra ramiflora. J. Mol. Struct. 2017;1128:572–578. [Google Scholar]
- 71.Sathishkumar G., Logeshwaran V., Sarathbabu S., Jha P.K., Jeyaraj M., Rajkuberan C., Senthilkumar N., Sivaramakrishnan S. Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities, Artificial cells, nanomedicine. and biotechnology. 2018;46(3):589–598. doi: 10.1080/21691401.2017.1332635. [DOI] [PubMed] [Google Scholar]
- 72.Rufus A., Sreeju N., Vilas V., Philip D. Biosynthesis of hematite (α-Fe2O3) nanostructures: size effects on applications in thermal conductivity, catalysis, and antibacterial activity. J. Mol. Liq. 2017;242:537–549. [Google Scholar]
- 73.Hassan D., Khalil A.T., Saleem J., Diallo A., Khamlich S., Shinwari Z.K., Maaza M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018;46(sup1):693–707. doi: 10.1080/21691401.2018.1434534. [DOI] [PubMed] [Google Scholar]
- 74.Sur D.H., Mukhopadhyay M. Role of zinc oxide nanoparticles for effluent treatment using Pseudomonas putida and Pseudomonas aureofaciens. Bioprocess Biosystems Eng. 2019;42(2):187–198. doi: 10.1007/s00449-018-2024-y. [DOI] [PubMed] [Google Scholar]
- 75.Jones N., Ray B., Ranjit K.T., Manna A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008;279(1):71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 76.Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seil J.T., Webster T.J. Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomedicine. 2012;7:2767. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raghupathi K.R., Koodali R.T., Manna A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L., Ding Y., Povey M., York D. ZnO nanofluids–a potential antibacterial agent. Prog. Nat. Sci. 2008;18(8):939–944. [Google Scholar]
- 80.Wang Z.L. Zinc oxide nanostructures: growth, properties and applications. J. Phys. Condens. Matter. 2004;16(25):R829. [Google Scholar]
- 81.Palanikumar L., Ramasamy S.N., Balachandran C. Size-dependent antimicrobial response of zinc oxide nanoparticles. IET Nanobiotechnol. 2014;8(2):111–117. doi: 10.1049/iet-nbt.2012.0008. [DOI] [PubMed] [Google Scholar]
- 82.Talebian N., Amininezhad S.M., Doudi M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B, Biol. 2013;120:66–73. doi: 10.1016/j.jphotobiol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mamat M.H., Khusaimi Z., Zahidi M.M., Mahmood M.R. Performance of an ultraviolet photoconductive sensor using well-aligned aluminium-doped zinc-oxide nanorod arrays annealed in an air and oxygen environment. J. Appl. Phys. 2011;50(6S) [Google Scholar]
- 85.Padmavathy N., Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Sci. Technol. Adv. Mater. 2008;9(3) doi: 10.1088/1468-6996/9/3/035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lingaraju K., Naika H.R., Manjunath K., Basavaraj R., Nagabhushana H., Nagaraju G., Suresh D. Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl. Nanosci. 2016;6(5):703–710. [Google Scholar]
- 87.Rajendran N.K., Kumar S.S.D., Houreld N.N., Abrahamse H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018;44:421–430. [Google Scholar]
- 88.Gu H., Ho P., Tong E., Wang L., Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003;3(9):1261–1263. [Google Scholar]
- 89.Savas S., Ersoy A., Gulmez Y., Kilic S., Levent B., Altintas Z. Nanoparticle enhanced antibody and DNA biosensors for sensitive detection of Salmonella. Materials. 2018;11(9):1541. doi: 10.3390/ma11091541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.EZorkany H.E., Youssef T., Mohamed M.B., Amin R.M. Photothermal versus photodynamic treatment for the inactivation of the bacteria Escherichia coli and Bacillus cereus: an in vitro study. Photodiagnosis Photodyn. Ther. 2019 doi: 10.1016/j.pdpdt.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 91.Cui Y., Zhao Y., Tian Y., Zhang W., Lü X., Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33(7):2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 92.Guerrini L., Alvarez-Puebla R., Pazos-Perez N. Surface modifications of nanoparticles for stability in biological fluids. Materials. 2018;11(7):1154. doi: 10.3390/ma11071154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X., Robinson S.M., Gupta A., Saha K., Jiang Z., Moyano D.F., Sahar A., Riley M.A., Rotello V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8(10):10682–10686. doi: 10.1021/nn5042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajan A., Vilas V., Philip D. Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles. J. Mol. Liq. 2015;212:331–339. [Google Scholar]
- 95.Surendra T., Roopan S.M., Arasu M.V., Al-Dhabi N.A., Rayalu G.M. RSM optimized Moringa oleifera peel extract for green synthesis of M. Oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol. B, Biol. 2016;162:550–557. doi: 10.1016/j.jphotobiol.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 96.Surendra T., Roopan S.M. Photocatalytic and antibacterial properties of phytosynthesized CeO2 NPs using Moringa oleifera peel extract. J. Photochem. Photobiol. B, Biol. 2016;161:122–128. doi: 10.1016/j.jphotobiol.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 97.Arumugam A., Karthikeyan C., Hameed A.S.H., Gopinath K., Gowri S., Karthika V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater. Sci. Eng. C. 2015;49:408–415. doi: 10.1016/j.msec.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 98.Senthilkumar R., Bhuvaneshwari V., Ranjithkumar R., Sathiyavimal S., Malayaman V., Chandarshekar B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: as a bionanomaterials. Int. J. Biol. Macromol. 2017;104:1746–1752. doi: 10.1016/j.ijbiomac.2017.03.139. [DOI] [PubMed] [Google Scholar]
- 99.Amarnath K., Kumar J., Reddy T., Mahesh V., Ayyappan S.R., Nellore J. Synthesis and characterization of chitosan and grape polyphenols stabilized palladium nanoparticles and their antibacterial activity. Colloids Surf. B Biointerfaces. 2012;92:254–261. doi: 10.1016/j.colsurfb.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 100.Sharmila G., Fathima M.F., Haries S., Geetha S., Kumar N.M., Muthukumaran C. Green synthesis, characterization and antibacterial efficacy of palladium nanoparticles synthesized using Filicium decipiens leaf extract. J. Mol. Struct. 2017;1138:35–40. [Google Scholar]
- 101.Gnanasekar S., Murugaraj J., Dhivyabharathi B., Krishnamoorthy V., Jha P.K., Seetharaman P., Vilwanathan R., Sivaperumal S. Antibacterial and cytotoxicity effects of biogenic palladium nanoparticles synthesized using fruit extract of Couroupita guianensis Aubl. J. Appl. Biomed. 2018;16(1):59–65. [Google Scholar]
- 102.Mallikarjuna K., Sushma N.J., Reddy B.S., Narasimha G., Raju B.D.P. Palladium nanoparticles: single-step plant-mediated green chemical procedure using Piper betle leaves broth and their anti-fungal studies. Int. J. Chem. Anal. Sci. 2013;4(1):14–18. [Google Scholar]
- 103.Manikandan V., Velmurugan P., Jayanthi P., Park J.-H., Chang W.-S., Park Y.-J., Cho M., Oh B.-T. Biogenic synthesis from Prunus× yedoensis leaf extract, characterization, and photocatalytic and antibacterial activity of TiO 2 nanoparticles. Res. Chem. Intermed. 2018;44(4):2489–2502. [Google Scholar]
- 104.Sonkusre P., Cameotra S.S. Biogenic selenium nanoparticles inhibit Staphylococcus aureus adherence on different surfaces. Colloids Surf. B Biointerfaces. 2015;136:1051–1057. doi: 10.1016/j.colsurfb.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 105.Cremonini E., Zonaro E., Donini M., Lampis S., Boaretti M., Dusi S., Melotti P., Lleo M.M., Vallini G. Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016;9(6):758–771. doi: 10.1111/1751-7915.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Medina Cruz D., Mi G., Webster T.J. Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin‐resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa. J. Biomed. Mater. Res. A. 2018;106(5):1400–1412. doi: 10.1002/jbm.a.36347. [DOI] [PubMed] [Google Scholar]
- 107.Zare B., Faramarzi M.A., Sepehrizadeh Z., Shakibaie M., Rezaie S., Shahverdi A.R. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012;47(11):3719–3725. [Google Scholar]
- 108.Rajakumar G., Rahuman A.A., Roopan S.M., Khanna V.G., Elango G., Kamaraj C., Zahir A.A., Velayutham K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2012;91:23–29. doi: 10.1016/j.saa.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 109.Mohanty A., Kathawala M.H., Zhang J., Chen W.N., Loo J.S.C., Kjelleberg S., Yang L., Cao B. Biogenic tellurium nanorods as a novel antivirulence agent inhibiting pyoverdine production in Pseudomonas aeruginosa. Biotechnol. Bioeng. 2014;111(5):858–865. doi: 10.1002/bit.25147. [DOI] [PubMed] [Google Scholar]
- 110.Santhoshkumar T., Rahuman A.A., Jayaseelan C., Rajakumar G., Marimuthu S., Kirthi A.V., Velayutham K., Thomas J., Venkatesan J., Kim S.-K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014;7(12):968–976. doi: 10.1016/S1995-7645(14)60171-1. [DOI] [PubMed] [Google Scholar]
- 111.Subhapriya S., Gomathipriya P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018;116:215–220. doi: 10.1016/j.micpath.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 112.Rao B., Tang R.-C. Green synthesis of silver nanoparticles with antibacterial activities using aqueous Eriobotrya japonica leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017;8(1) [Google Scholar]
- 113.Bose D., Chatterjee S. Antibacterial activity of green synthesized silver nanoparticles using Vasaka (Justicia adhatoda L.) leaf extract. Indian J. Microbiol. 2015;55(2):163–167. doi: 10.1007/s12088-015-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Emeka E.E., Ojiefoh O.C., Aleruchi C., Hassan L.A., Christiana O.M., Rebecca M., Dare E.O., Temitope A.E. Evaluation of antibacterial activities of silver nanoparticles green-synthesized using pineapple leaf (Ananas comosus) Micron. 2014;57:1–5. doi: 10.1016/j.micron.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 115.de Jesús Ruíz-Baltazar Á., Reyes-López S.Y., Larrañaga D., Estévez M., Pérez R. Green synthesis of silver nanoparticles using a Melissa officinalis leaf extract with antibacterial properties. Results Phys. 2017;7:2639–2643. [Google Scholar]
- 116.Singh T., Jyoti K., Patnaik A., Singh A., Chauhan R., Chandel S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. 2017;15(1):31–39. doi: 10.1016/j.jgeb.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maliszewska I., Sadowski Z. Synthesis and Antibacterial Activity of of Silver Nanoparticles, Journal of Physics: Conference Series; IOP Publishing; 2009. p. 012024. [Google Scholar]
- 118.Sreekanth T., Eom I.Y. Biogenic gold nanoparticles and its antibacterial activities: Houttuynia Cordata leaf extract, advanced materials research. Trans Tech Publ. 2014:392–397. [Google Scholar]
- 119.Składanowski M., Wypij M., Laskowski D., Golińska P., Dahm H., Rai M. Silver and gold nanoparticles synthesized from Streptomyces sp. Isolated from acid forest soil with special reference to its antibacterial activity against pathogens. J. Clust. Sci. 2017;28(1):59–79. [Google Scholar]
- 120.Singh D.K., Kumar J., Sharma V.K., Verma S.K., Singh A., Kumari P., Kharwar R.N. Mycosynthesis of bactericidal silver and polymorphic gold nanoparticles: physicochemical variation effects and mechanism. Nanomedicine. 2018;13(2):191–207. doi: 10.2217/nnm-2017-0235. [DOI] [PubMed] [Google Scholar]
- 121.Seetharaman P., Chandrasekaran R., Gnanasekar S., Mani I., Sivaperumal S. Biogenic gold nanoparticles synthesized using Crescentia cujete L. and evaluation of their different biological activities. Biocatal. Agric. Biotechnol. 2017;11:75–82. [Google Scholar]
- 122.Priyadarshini E., Pradhan N., Sukla L.B., Panda P.K. Controlled synthesis of gold nanoparticles using Aspergillus terreus IF0 and its antibacterial potential against Gram negative pathogenic bacteria. J. Nanotechnol. 2014;2014 [Google Scholar]
- 123.Kelmani Chandrakanth R. 2020. Antibacterial Activity of Biogenic Zinc Oxide Nanoparticals Synthesised From Enterococcus faecalis. [Google Scholar]
- 124.Ashajyothi C., Harish K.H., Dubey N., Chandrakanth R.K. Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: a nanoscale approach. J. Nanostructure Chem. 2016;6(4):329–341. [Google Scholar]
- 125.Suresh J., Pradheesh G., Alexramani V., Sundrarajan M., Hong S.I. Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018;9(1) [Google Scholar]
- 126.Das M.P., Rebecca L.J. Evaluation of antibacterial efficacy of biogenic zinc oxide nanoparticles on cotton fabrics. Journal of Pharmaceutical Sciences and Research. 2017;9(12):2553–2557. [Google Scholar]
- 127.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 128.Kailasa S.K., Park T.-J., Rohit J.V., Koduru J.R. Antimicrobial activity of silver nanoparticles. Nanoparticles in Pharmacotherapy. 2019:461–484. [Google Scholar]
- 129.Santhoshkumar J., Kumar S.V., Rajeshkumar S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Technol. 2017;3(4):459–465. [Google Scholar]
- 130.Allahverdiyev A.M., Abamor E.S., Bagirova M., Rafailovich M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011;6:933–940. doi: 10.2217/fmb.11.78. [DOI] [PubMed] [Google Scholar]
- 131.Lu Z., Rong K., Li J., Yang H., Chen R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013;24(6):1465–1471. doi: 10.1007/s10856-013-4894-5. [DOI] [PubMed] [Google Scholar]
- 132.Sintubin L., Gusseme B., Van der Meeren P., Pycke B.F., Verstraete W., Boon N. The antibacterial activity of biogenic silver and its mode of action. Appl. Microbiol. Biotechnol. 2011;91(1):153–162. doi: 10.1007/s00253-011-3225-3. [DOI] [PubMed] [Google Scholar]
- 133.Gautam P.K., Singh A., Misra K., Sahoo A.K., Samanta S.K. Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. J. Environ. Manage. 2019;231:734–748. doi: 10.1016/j.jenvman.2018.10.104. [DOI] [PubMed] [Google Scholar]
- 134.Baker S., Pasha A., Satish S. Biogenic nanoparticles bearing antibacterial activity and their synergistic effect with broad spectrum antibiotics: emerging strategy to combat drug resistant pathogens. J. Saudi Pharm. Soc. 2017;25(1):44–51. doi: 10.1016/j.jsps.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sudhasree S., Shakila Banu A., Brindha P., Kurian G.A. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol. Environ. Chem. 2014;96(5):743–754. [Google Scholar]
- 136.Ma Y., Liu C., Qu D., Chen Y., Huang M., Liu Y. Antibacterial evaluation of silver nanoparticles synthesized by polysaccharides from Astragalus membranaceus roots. Biomed. Pharmacother. 2017;89:351–357. doi: 10.1016/j.biopha.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 137.Ali K., Dwivedi S., Azam A., Saquib Q., Al-Said M.S., Alkhedhairy A.A., Musarrat J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolate. J. Colloid Interface Sci. 2016;472:145–156. doi: 10.1016/j.jcis.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 138.Banerjee D., Shivapriya P.M., Gautam P.K., Misra K., Sahoo A.K., Samanta S.K. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches. Proc. Acad. Sci. India Sect. B. 2018:1–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.