Abstract
The presence of anti-melanoma differentiation-associated gene 5 antibody (anti-MDA5 Ab) is closely associated with rapidly progressive interstitial lung disease (RP-ILD) in patients with clinically amyopathic dermatomyositis. Despite intensive immunosuppressive therapies, some of these patients still have a poor prognosis with few treatment options. Although removal of pathogenic autoantibodies and cytokines by plasma exchange (PE) could be a treatment option, its safety and efficacy have never been determined. We report a patient with anti-MDA5 Ab-positive RP-ILD who was refractory to intensive therapies including steroids, cyclosporine, and intravenous cyclophosphamide, and then treated by PE to prevent the progression of RP-ILD. Shortly after the initiation of PE therapy, however, his respiratory condition suddenly deteriorated due to acute pulmonary edema and the patient died on the following day. Transfusion-related acute lung injury (TRALI) would be the most likely cause of the acute pulmonary edema because there was no sign of circulatory overload. To the best of our knowledge, this is the first report showing a critical adverse event associated with PE therapy for these patients. This case supports the idea that the presence of ILD could increase a risk for TRALI and therefore we should carefully evaluate the eligibility for PE therapy of anti-MDA5 Ab-positive RP-ILD patients given the risk of acute lung injury. Further studies collecting more clinical data are necessary to assess the efficacy, safety, and risk factors of PE therapy for these patients.
Keywords: Acute lung injury, Plasma exchange, Anti-MDA5 antibody, Interstitial pneumonia, Clinically amyopathic dermatomyositis
Abbreviations: RP-ILD, rapidly progressive interstitial lung disease; anti-MDA5 Ab, anti-melanoma differentiation-associated gene 5 antibody; IVCY, intravenous cyclophosphamide; PE, plasma exchange; TRALI, Transfusion-related acute lung injury; CADM, Clinically amyopathic dermatomyositis; EF, Ejection Fraction; CK, creatine phosphokinase; CRP, C-reactive protein; SP-D, surfactant protein D; ANCA, antineutrophil cytoplasmic antibody; ANA, antinuclear antibody; ARS, anti-aminoacyl-tRNA sythetase; GGA, ground-glass attenuation; ALI, acute lung injury; ADAMTS, a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs
1. Introduction
Clinically amyopathic dermatomyositis (CADM) is defined as dermatomyositis that shows typical skin symptoms without obvious myositis [1]. Patients with CADM often develop rapidly progressive interstitial pneumonia (RP-ILD), and the anti-melanoma differentiation-associated gene 5 antibody (anti-MDA5 Ab) is closely associated with the RP-ILD in these patients [2,3] regardless of the typical skin manifestations of dermatomyositis [[4], [5], [6], [7]]. The mortality rate of anti-MDA5 Ab-positive RP-ILD patients is high. Therefore, for these patients, immediate and intensive immunosuppressive therapies are required to avoid a fatal outcome; intravenous administration of cyclophosphamide (IVCY), as well as the concomitant use of calcineurin inhibitors (cyclosporine or tacrolimus) with high-dose steroids, is recommended to prevent the progression of ILD [[8], [9], [10]]. In addition, recent studies have demonstrated that the administration of mycophenolate mofetil or rituximab may be considered for these high-risk RP-ILD patients who are refractory to the combination therapies described above [8,[11], [12], [13]]. However, the efficacy of these treatments has never been fully established.
Plasma exchange (PE) is a therapeutic procedure used to treat a variety of diseases through the bulk removal of pathologic substances such as pathologic antibodies, immune complexes, and cytokines [14]. PE has been recognized as a therapeutic option for refractory or severe autoimmune diseases because it effectively removes pathogenic autoantibodies and cytokines [15]. Recently, some papers have reported that CADM-associated/anti-MDA5 Ab-positive patients with RP-ILD were successfully treated by PE [[16], [17], [18]]. Although these recent reports would support the efficacy of PE therapy even for anti-MDA5 Ab-positive RP-ILD, the data evaluating the efficacy or safety of PE therapies are still limited.
In this report, the case of an anti-MDA5 Ab-positive RP-ILD patient, who was refractory to intensive immuno-suppressive therapies including high-dose steroid, tacrolimus, and IVCY, is presented. PE was started as an additional therapy to save the patient from severe acute respiratory failure. However, approximately 1 hour after starting PE, his respiratory condition suddenly worsened, and he died of exacerbated respiratory failure possibly due to acute lung injury triggered by the PE therapy. To the best of our knowledge, this is the first report showing a critical adverse event associated with PE therapy performed for anti-MDA5 Ab-positive RP-ILD patients.
2. Case report
A 59-year-old Japanese man with a 3-week history of dry cough, fever, and fatigue visited his primary care doctor. He was diagnosed with pneumonia, and tosufloxacin was given for a week. However, his respiratory symptoms worsened progressively, and he began to feel dyspneic. When he was transferred to our hospital he showed mild hypoxemia.
On admission, his vital signs were: blood pressure, 125/75 mmHg; pulse rate, 113/min; respiratory rate, 20/min; and temperature, 37.5 °C. Percutaneous arterial blood oxygen saturation was 94% (2 L per minute of oxygen through a nasal cannula). Heart sounds were normal. Bilateral fine crackles at the lung bases were present. No superficial lymph node swelling was found. No skin manifestations or arthritis was observed. There was no muscle weakness on manual muscle testing.
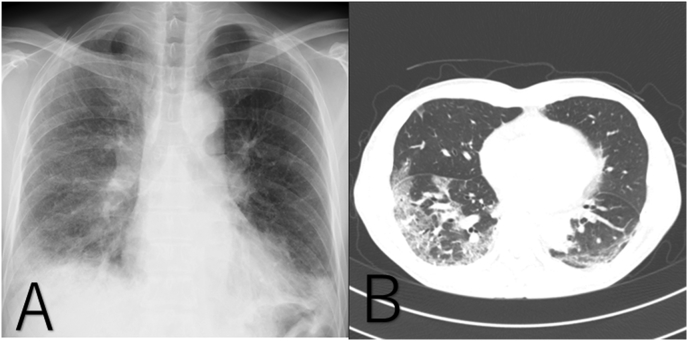
Chest CT showed ground glass opacity in the left upper lobe and sub-pleural consolidation with traction bronchiectasis in both lower lobes (Fig. 1A and B). Echocardiography was normal. No obvious wall motion or valve abnormalities were observed. The ejection fraction (EF) was 70%. Laboratory analyses were as follows: white blood cell (WBC) count, 9380/μl; platelets (Plt), 390,000/μl; creatine phosphokinase (CK), 163 IU/L; lactate dehydrogenase (LDH), 455 IU/L; C-reactive protein (CRP), 3.6 mg/dL; ferritin, 1910 ng/ml; β-D-glucan, 9.0 pg/ml; surfactant protein A, 177 ng/ml; surfactant protein D, 878 ng/ml; and Krebs von den Lungen-6, 646.6 U/mL. The patient's serum was negative for antineutrophil cytoplasmic antibody, antinuclear antibody, and anti-aminoacyl-tRNA synthetase antibody. Although the typical skin manifestations of dermatomyositis were not apparent, the high level of serum ferritin, the clinical features of RP-ILD, and the chest CT findings in this patient were consistent with anti-MDA5 Ab-positive RP-ILD reported in CADM patients [7]. Thereafter, anti-MDA5 Ab was measured and found to be positive (titer index >150).
Fig. 1.
The admission chest X-ray shows ground-glass opacity predominantly in the lower lung field (A), and chest CT shows sub-pleural ground-glass opacity and consolidation with traction bronchiectasis in both lower lung lobes (B).
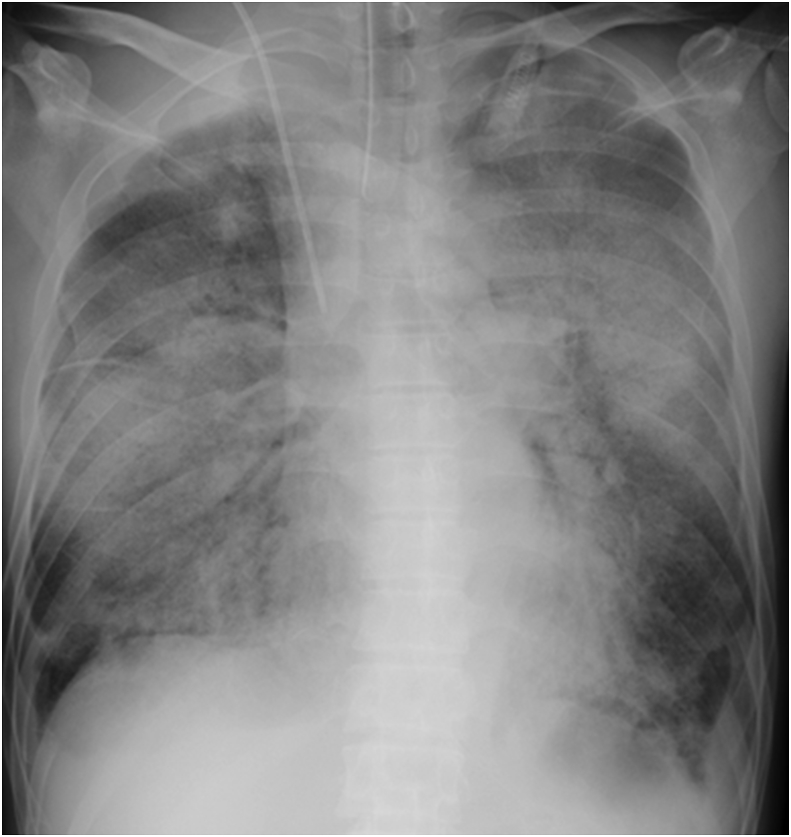
Immediately after the diagnosis of anti-MDA5 Ab-positive RP-ILD, a 3-day course of methylprednisolone (1000 mg/day) was administered intravenously from day 1 to day 3, followed by the daily administration of oral prednisolone (0.8 mg/kg body weight). In addition to steroid therapies, IVCY (500 mg/body) was administered on day 6, and daily oral cyclosporine (100 mg/day) was given concurrently. Despite these intensive immunosuppressive therapies, his respiratory condition worsened, and mechanical ventilation was started on day 8. To prevent progressive respiratory failure, PE was performed as an optional therapy to prevent progressive respiratory failure on day 10. We used 32 units of fresh frozen plasma as the replacement fluid in the PE. However, about 4 hours after the start of the PE therapy, the respiratory failure deteriorated rapidly. Pink frothy fluid was continuously sucked from the endotracheal tube, and the chest X-ray showed a butterfly shadow (Fig. 2), suggesting that acute pulmonary edema was induced by the PE therapy. Extracorporeal ultrafiltration was performed immediately to remove 200 ml of fluid, in addition to the intravenous administration of furosemide, which resulted in urine output of 410 ml over 5 hours. Despite these treatments, his respiratory condition did not improve, and he died on day 11. Transfusion-related acute lung injury (TRALI) was the most likely cause of acute pulmonary edema [19], although this case could not be evaluated by the classical diagnostic criteria of TRALI because the patient already had ALI arising from ILD when the PE therapy was started [20]. There was no sign of circulatory overload, which is the other major cause of pulmonary edema after transfusion [21], since he had a normal cardiac silhouette on chest X-ray, normal cardiac movement and volume on echocardiography, no elevation of Brain Natriuretic Peptide, and no improvement of his respiratory condition after fluid removal.
Fig. 2.
Chest X-ray 4 hours after the start of the plasma exchange shows a butterfly shadow.
3. Discussion
The prognosis of anti-MDA5 Ab-positive RP-ILD is extremely poor [22]. Therefore, the diagnosis and identification of these high-risk patients in the early stage are indispensable when considering earlier initiation of intensive immunosuppressive therapies. Recently, some prognostic factors for ILD patients with dermatomyositis/polymyositis have been reported [8,22]. Measurement of anti-MDA5 Ab is particularly important because its presence is closely associated with RP-ILD with a poor prognosis. Additionally, ferritin levels, anti-MDA5 antibody titers, and high pre-treatment serum IL-6 levels are reported to be useful markers for a poor prognosis and disease severity in dermatomyositis/polymyositis patients. HRCT evaluation is also reported to be useful to detect early minimal changes in the RP-ILD lungs; lower consolidation or a ground-glass attenuation (GGA) pattern, a random GGA pattern, and the absence of intralobular reticular opacities are characteristic imaging features of RP-ILD lungs in CADM patients [23]. On physical examination, the presence of typical skin symptoms (e.g. heliotrope rash and the Gottron sign) supports the diagnosis of dermatomyositis [24]. Recently, however, some anti-MDA5 Ab-positive RP-ILD patients who did not show any apparent skin manifestations but had refractory RP-ILD similar to that in CADM patients were reported [[4], [5], [6], [7]]. Therefore, in a case with hyperferritinemia, RP-ILD development, and typical HRCT findings, it would be necessary to assume that the patient has anti-MDA5 Ab-positive RP-ILD, even if the dermatomyositis-associated skin signs are not observed [4]. In the present case, similarly, no apparent skin symptoms or myositis were observed. However, typical CT findings and the clinical features of RP-ILD prompted the measurement of anti-MDA5 Ab, which led to the early diagnosis and initiation of intensive immunosuppressive therapies. The combination of anti-MDA5 Ab, other serum biomarkers, and HRCT findings would be beneficial for the early selection of therapeutic strategies in RP-ILD patients.
In daily clinical practice when dealing with interstitial pneumonia of unknown etiology, serum autoantibodies have been measured with different aims. Some autoantibodies, which are specifically associated with each connective tissue disease, are measured to estimate the likelihood of connective tissue-associated interstitial lung disease [25]. In clinical studies, some autoantibodies were advocated as biomarkers that can cluster a sub-group of patients within the spectrum of heterogeneous idiopathic interstitial pneumonias [26,27]. The concentration of serum anti-MDA5 Ab reflected the severity of co-morbid interstitial pneumonia in CADM patients, and decreased anti-MDA5 Abs are associated with successful treatment [28,29]. These results suggest that anti-MDA5 autoantibody is associated with the pathogenesis of CADM, although how it affects the etiology of CADM has never been directly proven.
The elimination of autoantibodies is the appropriate treatment for diseases caused by a pathogenic autoantibody such as anti-a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 13 autoantibody in thrombotic thrombocytopenic purpura [30]. Plasma exchange (PE) is often performed to remove both pathogenic autoantibodies and over-produced cytokines in the blood. The addition of PE to immunosuppressive drugs is expected to improve the poor outcome in anti-MDA5 Ab-positive patients with RP-ILD, although the significance of decreasing anti-MDA5 antibody titer by PE has not been determined [31]. Currently, there have been a limited number of case reports of these patients who were successfully treated by PE [[16], [17], [18]]. However, if PE is effective for these patients, it in turn could support the idea that anti-MDA5 antibody is associated with the pathogenesis of RP-ILD.
As with other transfusion therapy, PE also has the risk of TRALI [32]. TRALI is a rare but serious syndrome defined as new acute lung injury (ALI) that occurs during or within 6 h after blood product administration, not explained by another ALI risk factor [19]. TRALI is the leading cause of transfusion-related death [33], and the mortality rate is reported to be 5–8% or 35–58% in critically ill populations [34]. All the blood products have been implicated in TRALI, and it is known that TRALI is also caused by PE [32,35,36]. Although the mechanisms are still unclear, TRALI is thought to be caused by activation of recipient neutrophils by donor-derived antibodies targeting human leukocyte antigen or human neutrophil antigen in most cases [37]. Therefore, in patients with RP-ILD, activated neutrophils in the alveolar capillary bed might increase the risk for TRALI [21]. A recent prospective cohort study revealed that interstitial lung abnormalities on chest CT were associated with an increased risk of acute respiratory distress syndrome with high mortality [38]. In line with this, a previous paper reported that a patient underlying subclinical pulmonary fibrosis experienced acute exacerbation after red cell transfusion [39]. These findings support the idea that patients with interstitial lung disease are susceptible and more likely to develop acute lung injury by an additional risk factor such as transfusion. It is quite likely that patients’ underlying risk factors enhance the risk of TRALI reactions. Liver transplantation surgery, chronic alcohol abuse, shock, higher peak airway pressure while being mechanically ventilated, current smoking, higher interleukin-8 level, and positive fluid balance are identified as host risk factors for TRALI [33]. Interstitial lung disease on mechanical ventilation is associated with higher peak airway pressure because of its low lung compliance. Furthermore, IL-8 is frequently high in patients with interstitial pneumonia [[40], [41], [42]]. Therefore, interstitial lung disease, especially when it exacerbates and needs positive airway pressure ventilation, should be regarded as a risk factor for TRALI. Although PE might be a reasonable treatment for anti-MDA5 Ab-positive RP-ILD patients, its application and the timing when it is performed should be judged carefully, taking into account the risk of TRALI. Because TRALI risk is also associated with the types of blood components independently of the patient clinical conditions described above, both patient-specific and transfusion-specific factors need to be considered when determining the patient-specific risk versus benefit for PE therapies [43]. Regarding transfusion-specific factors, plasma-containing blood is reported to be the most frequent cause of TRALI [44]. For anti-MDA5 Ab-positive RP-ILD patients, therefore, choosing non-plasma-containing products (e.g. purified albumin) as the replacement solution would be one option to decrease the risk of TRALI. Further studies gathering more clinical data are necessary to assess the efficacy, safety, and risk factors of PE therapy for these patients.
In conclusion, anti-MDA5 Ab-positive RP-ILD patients have a poor prognosis with few effective treatment options. PE is a promising option for these patients, but currently there is little evidence regarding its efficacy and safety. This case suggests that we should carefully evaluate RP-ILD patients’ eligibility for PE therapy because of the risk of acute lung injury. More clinical data regarding the efficacy, safety, and the risk factors of PE therapy for anti-MDA5 Ab-positive RP-ILD patients are indispensable.
Funding
Article processing was funded by JSPS KAKENHI Grant Number 16K09537 to HK.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
References
- 1.Sontheimer R.D. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J. Am. Acad. Dermatol. 2002;46(4):626–636. doi: 10.1067/mjd.2002.120621. [DOI] [PubMed] [Google Scholar]
- 2.Satoh M., Tanaka S., Ceribelli A., Calise S.J., Chan E.K. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin. Rev. Allergy Immunol. 2017;52(1):1–19. doi: 10.1007/s12016-015-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato S., Murakami A., Kuwajima A., Takehara K., Mimori T., Kawakami A., Mishima M., Suda T., Seishima M., Fujimoto M., Kuwana M. Clinical utility of an enzyme-linked immunosorbent assay for detecting anti-melanoma differentiation-associated gene 5 autoantibodies. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0154285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyama J., Hayashi H., Yajima C., Takoi H., Tanaka T., Kashiwada T., Kokuho N., Terasaki Y., Nishikawa A., Gono T., Kuwana M., Saito Y., Abe S., Seike M., Gemma A. Anti-MDA5 antibody-positive rapidly progressive interstitial pneumonia without cutaneous manifestations. Respir. Med. Case Rep. 2019;26:193–196. doi: 10.1016/j.rmcr.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Moreno J., Raya-Cruz M., Losada-Lopez I., Cacheda A.P., Oliver C., Colom B. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol. Int. 2018;38(7):1293–1296. doi: 10.1007/s00296-018-3991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamai K., Tachikawa R., Otsuka K., Ueda H., Hosono Y., Tomii K. Early pulmonary involvement of anti-CADM-140 autoantibody-positive rapidly progressive interstitial lung disease preceding typical cutaneous symptoms. Intern. Med. (Tokyo, Japan) 2014;53(21):2515–2519. doi: 10.2169/internalmedicine.53.2769. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto N., Ishimoto H., Nakashima S., Yura H., Miyamura T., Okuno D., Hara A., Kitazaki T., Kakugawa T., Ishimatsu Y., Satoh M., Mukae H. Clinical features of anti-MDA5 antibody-positive rapidly progressive interstitial lung disease without signs of dermatomyositis. Intern. Med. (Tokyo, Japan) 2019;58(6):837–841. doi: 10.2169/internalmedicine.1516-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasumi H., Gono T., Kawaguchi Y., Yamanaka H. Recent treatment of interstitial lung disease with idiopathic inflammatory myopathies, clinical medicine insights. Circ., Respir. Pulm. Med. 2015;9(Suppl 1):9–17. doi: 10.4137/CCRPM.S23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakashima R., Hosono Y., Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus. 2016;25(8):925–933. doi: 10.1177/0961203316651748. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima R., Mimori T. [Anti-MDA5 (melanoma differentiation-associated gene 5) antibody and dermatomyositis with rapidly progressive interstitial pneumonia] Nihon Rinsho Men'eki Gakkai kaishi = Jpn. J. Clin. Immunol. 2013;36(2):71–76. doi: 10.2177/jsci.36.71. [DOI] [PubMed] [Google Scholar]
- 11.A. Iwai, K. Tsujino, Y. Yamamoto, T. Kuge, R. Hara, H. Kida, Effective administration of mycophenolate mofetil in anti-MDA5 antibody-positive, rapidly progressive, interstitial lung disease: a case report and literature review, Ann. Jpn. Respir. Soc. in press.
- 12.Tsuchiya H., Tsuno H., Inoue M., Takahashi Y., Yamashita H., Kaneko H., Kano T., Mimori A. Mycophenolate mofetil therapy for rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis. Mod. Rheumatol. 2014;24(4):694–696. doi: 10.3109/14397595.2013.874762. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa Y., Kishida D., Shimojima Y., Hayashi K., Sekijima Y. Effective administration of rituximab in anti-MDA5 antibody-positive dermatomyositis with rapidly progressive interstitial lung disease and refractory cutaneous involvement: a case report and literature review. Case Rep. Rheumatol. 2017:5386797. doi: 10.1155/2017/5386797. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winters J.L. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines, Hematology. Am. Soc. Hematol. Educ. Progr. 2012:7–12. doi: 10.1182/asheducation-2012.1.7. 2012. [DOI] [PubMed] [Google Scholar]
- 15.Bambauer R., Latza R., Bambauer C., Burgard D., Schiel R. Therapeutic apheresis in autoimmune diseases. Open Access Rheumatol. Res. Rev. 2013;5:93–103. doi: 10.2147/OARRR.S34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo Y., Koga T., Suzuki T., Hara K., Ishida M., Fujita Y., Tsuji S., Takatani A., Shimizu T., Sumiyoshi R., Igawa T., Umeda M., Fukui S., Nishino A., Kawashiri S.Y., Iwamoto N., Ichinose K., Tamai M., Nakamura H., Origuchi T., Kuwana M., Kawakami A. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis: a case report. Medicine. 2018;97(15) doi: 10.1097/MD.0000000000010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagishita M., Kondo Y., Terasaki T., Terasaki M., Shimizu M., Honda F., Oyama A., Takahashi H., Yokosawa M., Asashima H., Hagiwara S., Tsuboi H., Matsumoto I., Sumida T. Clinically amyopathic dermatomyositis with interstitial pneumonia that was successfully treated with plasma exchange. Intern. Med. (Tokyo, Japan) 2018;57(13):1935–1938. doi: 10.2169/internalmedicine.0297-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silveira M.G., Selva-O'Callaghan A., Ramos-Terrades N., Arredondo-Agudelo K.V., Labrador-Horrillo M., Bravo-Masgoret C. Anti-MDA5 dermatomyositis and progressive interstitial pneumonia. QJM : Mon. J. Assoc. Phys. 2016;109(1):49–50. doi: 10.1093/qjmed/hcv068. [DOI] [PubMed] [Google Scholar]
- 19.Toy P., Lowell C. TRALI--definition, mechanisms, incidence and clinical relevance, Best practice & research. Clin. Anaesthesiol. 2007;21(2):183–193. doi: 10.1016/j.bpa.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinman S., Caulfield T., Chan P., Davenport R., McFarland J., McPhedran S., Meade M., Morrison D., Pinsent T., Robillard P., Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44(12):1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 21.Bux J., Sachs U.J. The pathogenesis of transfusion-related acute lung injury (TRALI) Br. J. Haematol. 2007;136(6):788–799. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa T., Hozumi H., Kono M., Enomoto N., Hashimoto D., Nakamura Y., Inui N., Yokomura K., Koshimizu N., Toyoshima M., Shirai T., Yasuda K., Hayakawa H., Suda T. Prognostic factors for myositis-associated interstitial lung disease. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0098824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanizawa K., Handa T., Nakashima R., Kubo T., Hosono Y., Watanabe K., Aihara K., Oga T., Chin K., Nagai S., Mimori T., Mishima M. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir. Med. 2011;105(9):1380–1387. doi: 10.1016/j.rmed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Muro Y., Sugiura K., Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features-a comprehensive review. Clin. Rev. Allergy Immunol. 2016;51(3):293–302. doi: 10.1007/s12016-015-8496-5. [DOI] [PubMed] [Google Scholar]
- 25.Fischer A., Antoniou K.M., Brown K.K., Cadranel J., Corte T.J., du Bois R.M., Lee J.S., Leslie K.O., Lynch D.A., Matteson E.L., Mosca M., Noth I., Richeldi L., Strek M.E., Swigris J.J., Wells A.U., West S.G., Collard H.R., Cottin V. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015;46(4):976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 26.Hamano Y., Kida H., Ihara S., Murakami A., Yanagawa M., Ueda K., Honda O., Tripathi L.P., Arai T., Hirose M., Hamasaki T., Yano Y., Kimura T., Kato Y., Takamatsu H., Otsuka T., Minami T., Hirata H., Inoue K., Nagatomo I., Takeda Y., Mori M., Nishikawa H., Mizuguchi K., Kijima T., Kitaichi M., Tomiyama N., Inoue Y., Kumanogoh A. Classification of idiopathic interstitial pneumonias using anti-myxovirus resistance-protein 1 autoantibody. Sci. Rep. 2017;7:43201. doi: 10.1038/srep43201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshifuji H., Fujii T., Kobayashi S., Imura Y., Fujita Y., Kawabata D., Usui T., Tanaka M., Nagai S., Umehara H., Mimori T. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006;39(3):233–241. doi: 10.1080/08916930600622884. [DOI] [PubMed] [Google Scholar]
- 28.Sato S., Kuwana M., Fujita T., Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod. Rheumatol. 2013;23(3):496–502. doi: 10.1007/s10165-012-0663-4. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita T., Mizumaki K., Kano M., Yagi N., Tennichi M., Takeuchi A., Okamoto Y., Hamaguchi Y., Murakami A., Hasegawa M., Kuwana M., Fujimoto M., Takehara K. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br. J. Dermatol. 2017;176(2):395–402. doi: 10.1111/bjd.14882. [DOI] [PubMed] [Google Scholar]
- 30.Rock G.A., Shumak K.H., Buskard N.A., Blanchette V.S., Kelton J.G., Nair R.C., Spasoff R.A. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Can. Apher. Study Group, New Engl. J. Med. 1991;325(6):393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 31.Abe Y., Matsushita M., Tada K., Yamaji K., Takasaki Y., Tamura N. Clinical characteristics and change in the antibody titres of patients with anti-MDA5 antibody-positive inflammatory myositis. Rheumatology. 2017;56(9):1492–1497. doi: 10.1093/rheumatology/kex188. [DOI] [PubMed] [Google Scholar]
- 32.Mateen F.J., Gastineau D. Transfusion related acute lung injury (TRALI) after plasma exchange in myasthenic crisis. Neurocritical Care. 2008;8(2):280–282. doi: 10.1007/s12028-007-9000-8. [DOI] [PubMed] [Google Scholar]
- 33.Toy P., Gajic O., Bacchetti P., Looney M.R., Gropper M.A., Hubmayr R., Lowell C.A., Norris P.J., Murphy E.L., Weiskopf R.B., Wilson G., Koenigsberg M., Lee D., Schuller R., Wu P., Grimes B., Gandhi M.J., Winters J.L., Mair D., Hirschler N., Sanchez Rosen R., Matthay M.A. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlaar A.P., Juffermans N.P. Transfusion-related acute lung injury: a clinical review. Lancet. 2013;382(9896):984–994. doi: 10.1016/S0140-6736(12)62197-7. [DOI] [PubMed] [Google Scholar]
- 35.Askari S., Nollet K., Debol S.M., Brunstein C.G., Eastlund T. Transfusion-related acute lung injury during plasma exchange: suspecting the unsuspected. J. Clin. Apher. 2002;17(2):93–96. doi: 10.1002/jca.10013. [DOI] [PubMed] [Google Scholar]
- 36.P'Ng S S., Hughes A.S., Cooney J.P. A case report of transfusion-related acute lung injury during plasma exchange therapy for thrombotic thrombocytopenia purpura. Ther. Apher. Dial. : Off. Peer Rev. J. Int. Soc. Apher., Jpn. Soc Apher., Jpn. Soc. Dial. Ther. 2008;12(1):78–81. doi: 10.1111/j.1744-9987.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 37.Petraszko T. Professional Education Canadian Blood Services; 2019. Transfusion-related Acute Lung Injury (TRALI) [DOI] [PubMed] [Google Scholar]
- 38.Putman R.K., Hunninghake G.M., Dieffenbach P.B., Barragan-Bradford D., Serhan K., Adams U., Hatabu H., Nishino M., Padera R.F., Fredenburgh L.E., Baron R.M., Englert J.A. Interstitial lung abnormalities are associated with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;195(1):138–141. doi: 10.1164/rccm.201604-0818LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodske M., Donahoe M.P., Yazer M., Lee J.S. Acute exacerbation of subclinical pulmonary fibrosis after red blood cell transfusion: a case report. Transfusion. 2012;52(3):589–594. doi: 10.1111/j.1537-2995.2011.03296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou J., Chen J., Yan Q., Guo Q., Bao C. Serum IL8 and mRNA level of CD11b in circulating neutrophils are increased in clinically amyopathic dermatomyositis with active interstitial lung disease. Clin. Rheumatol. 2016;35(1):117–125. doi: 10.1007/s10067-015-3080-1. [DOI] [PubMed] [Google Scholar]
- 41.Papiris S.A., Tomos I.P., Karakatsani A., Spathis A., Korbila I., Analitis A., Kolilekas L., Kagouridis K., Loukides S., Karakitsos P., Manali E.D. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine. 2018;102:168–172. doi: 10.1016/j.cyto.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Kawasumi H., Gono T., Kawaguchi Y., Kaneko H., Katsumata Y., Hanaoka M., Kataoka S., Yamanaka H. IL-6, IL-8, and IL-10 are associated with hyperferritinemia in rapidly progressive interstitial lung disease with polymyositis/dermatomyositis. BioMed Res. Int. 2014;2014:815245. doi: 10.1155/2014/815245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A.B. Benson, Pulmonary Complications of Transfused Blood Components, (1558-3481 (Electronic)). [DOI] [PMC free article] [PubMed]
- 44.A.F. Eder, A. Herron R Fau - Strupp, B. Strupp A Fau - Dy, E.P. Dy B Fau - Notari, L.A. Notari Ep Fau - Chambers, R.Y. Chambers La Fau - Dodd, R.J. Dodd Ry Fau - Benjamin, R.J. Benjamin, Transfusion-related Acute Lung Injury Surveillance (2003-2005) and the Potential Impact of the Selective Use of Plasma from Male Donors in the American Red Cross, (0041-1132 (Print)). [DOI] [PubMed]