Abstract
Aims
Guidelines do not recommend to take pattern of atrial fibrillation (AF) into account for the indication of anticoagulation (AC). We assessed AF pattern and the risk of cardiovascular events during 2-years of follow-up.
Methods and results
We categorized AF as paroxysmal, persistent, or permanent in 29 181 patients enrolled (2010–15) in the Global Anticoagulant Registry In the FIELD of AF (GARFIELD-AF). We used multivariable Cox regression to assess the risks of stroke/systemic embolism (SE) and death across patterns of AF, and whether this changed with AC on outcomes. Atrial fibrillation pattern was paroxysmal in 14 344 (49.2%), persistent in 8064 (27.6%), and permanent 6773 (23.2%) patients. Median CHA2DS2-VASc, GARFIELD-AF, and HAS-BLED scores assessing the risk of stroke/SE and/or bleeding were similar across AF patterns, but the risk of death, as assessed by the GARFIELD-AF risk calculator, was higher in non-paroxysmal than in paroxysmal AF patterns. During 2-year follow-up, after adjustment, non-paroxysmal AF patterns were associated with significantly higher rates of all-cause death, stroke/SE, and new/worsening congestive heart failure (CHF) than paroxysmal AF in non-anticoagulated patients only. In anticoagulated patients, a significantly higher risk of death but not of stroke/SE and new/worsening CHF persisted in non-paroxysmal compared with paroxysmal AF patterns.
Conclusion
In non-anticoagulated patients, non-paroxysmal AF patterns were associated with higher risks of stroke/SE, new/worsening HF and death than paroxysmal AF. In anticoagulated patients, the risk of stroke/SE and new/worsening HF was similar across all AF patterns. Thus AF pattern is no longer prognostic for stroke/SE when patients are treated with anticoagulants.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01090362.
Keywords: Atrial fibrillation, Atrial fibrillation type, Stroke prevention, Anticoagulation, Cardiovascular outcome, Registry
Introduction
Patient characteristics included in the CHA2DS2-VASc score are important for risk stratification. Current guidelines in atrial fibrillation (AF) recommend this score when deciding whether anticoagulant therapy should be given for stroke prevention in patients with AF.1,2 The temporal pattern of AF, expressed as type of AF, has shown conflicting results with regard to its impact of stroke risk.3–5
The Global Anticoagulant Registry in the FIELD of AF (GARFIELD-AF) is a multinational prospective registry of more than 50 000 patients with newly diagnosed AF and at least one additional risk factor for stroke.6,7 We used data from GARFIELD-AF to compare the risk of stroke or death in patients with different types of AF, particularly to assess the risk conferred by paroxysmal vs. other patterns, and to evaluate whether the risk differed with the use of anticoagulation (AC). In this report, the patients with at least 2-year follow-up from the first four cohorts of GARFIELD-AF were evaluated
What’s new?
The relationship between atrial fibrillation (AF) pattern and the risk of cardiovascular events is based on the real-world prospective data collected during 2-years of follow-up in newly diagnosed AF patients from the Global Anticoagulant Registry In the FIELD of AF (GARFIELD-AF) registry.
The GARFIELD-AF risk calculator showed a continuum of risk for death as evidenced by a gradual increase in the risk score across all three AF patterns.
The novelty is that in anticoagulated patients, the risk of stroke/systemic embolism was similar across AF patterns.
.
Methods
GARFIELD-AF is a multinational registry of adults aged 18 years or more with non-valvular AF and with at least one additional risk factor for stroke, as judged by the investigator. Atrial fibrillation was diagnosed (according to standard local procedures) within 6 weeks before enrolment. Risk factors were not pre-specified in the protocol and were not limited to the components of existing risk stratification schemes. Patients with a transient and reversible cause of non-valvular AF and those for whom follow-up was not possible were excluded. To minimize recruitment bias, investigator sites were selected randomly from representative care settings in each participating country, and consecutive eligible consenting patients were enrolled.6,7 Informed consent was obtained from all study participants, and the study was approved by research Ethics Committee and Institutional Review Boards.
Collection of follow-up data occurred at 4-month intervals up to 24 months. Outcome measures included clinical events, therapy persistence, and healthcare utilization. The incidences of stroke/systemic embolism (SE), death (cardiovascular and non-cardiovascular), heart failure (HF) (occurrence or worsening), and bleeding (severity and location) were recorded. Data for this report were extracted from the study database in October 2017.
At baseline, investigators collected data on patient demographics, medical history, care setting, type of AF (also collected during follow-up), and antithrombotic treatment [vitamin K antagonists (VKA), non-vitamin K antagonist oral anticoagulants, and antiplatelet (AP) treatment]. Data on components of the CHA2DS2-VASc and HAS-BLED risk stratification schemes were used to assess the risks of stroke and bleeding, retrospectively. HAS-BLED scores were calculated excluding fluctuations in the International Normalized Ratio. In addition, the risks of death, stroke/systemic embolism (SE), and major bleeding were estimated at baseline with the recently described GARFIELD-AF risk calculator.8
For patients with new (unclassified) AF at baseline, the type of AF was assessed by the investigator within 150 days of enrolment. If the AF type could not be assessed at 5 months, the patient was not included in the analysis. The definition of AF types are according to the European Society of Cardiology guidelines.1 Paroxysmal AF lasts no more than 7 days and is self-terminating or is cardioverted within the 7-day window. Persistent AF lasts longer than 7 days and includes episodes that are terminated by drug or direct current cardioversion after 7 days. When no rhythm control strategies are pursued and AF is a continuing condition, the AF is permanent.
Study outcomes and definitions
Clinical endpoints of the study were: (i) stroke/SE, (ii) major bleeding, (iii) all-cause mortality, (iv) cardiovascular mortality, (v) non-cardiovascular mortality, (vi) new acute coronary syndromes (ACS), and (vii) new or worsening HF at 2-year follow-up.
Oral anticoagulants (OAC) included VKAs, direct factor Xa inhibitors, and direct thrombin inhibitors. Antiplatelet therapy included: aspirin, adenosine diphosphate receptor antagonists (P2Y12 inhibitors) or both.
Vascular disease included peripheral artery disease or coronary artery disease with ACS. Chronic kidney disease was classified according to National Kidney Foundation guidelines into two groups: moderate-to-severe (stages 3–5), or mild (stages 1 and 2) or none.9 Heart failure at baseline was defined as current/prior history of congestive heart failure (CHF) or left ventricular ejection fraction (LVEF) of <40%.
Data were collected using an electronic case report form and were examined for completeness and accuracy by the coordinating centre (Thrombosis Research Institute, London, UK). In accordance with the study protocol, 20% of all data submitted electronically were monitored against source documentation.
Ethics
The registry is being conducted in accordance with the principles of the Declaration of Helsinki, local regulatory requirements, and the International Conference on Harmonisation–Good Pharmacoepidemiological and Clinical Practice guidelines.
Statistical analysis
Baseline patient characteristics were presented for the three AF categories (paroxysmal, persistent, or permanent), classified by the investigators within the first 150 days of enrolment. Continuous variables were expressed as median [interquartile range (IQR)] or mean [standard deviation (SD)] and compared across the three AF categories using the Kruskall–Wallis test. Categorical variables were presented as frequencies (percentages) and were compared using the Pearson χ2 test or exact test when appropriate.
Clinical outcomes were compared between patients with each type of AF. Occurrence of major clinical outcomes was expressed as person-time event rates (per 100 person-years) and 95% confidence intervals (CIs). Person-year rates were estimated using a Poisson model with the number of events as the dependent variable and the log of time as an offset, i.e., a covariate with a known coefficient of 1. Only the first occurrence of events was taken into account. Hazard ratios (HRs) were estimated using a proportional hazards Cox model. The proportional hazard assumption was assessed visually using plots of the cumulative hazard function. The following variables were included in the Cox model: age groups (<65, 65–69, 70–74, ≥75 years), gender, race (Caucasian/Hispanic/Latino, Asian, other race—including Afro-Caribbean, mixed/other, and unwilling to declare/not recorded), smoking (no, ex-smoker, current), diabetes mellitus, hypertension, previous stroke/transient ischaemic attack/SE, history of bleeding, HF, vascular disease, moderate-to-severe renal disease, anticoagulant treatment, and heavy alcohol consumption (only in the model for bleeding). Data analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 39 871 patients included in GARFIELD-AF, 22 805 patients had a known type of AF, and 17 786 patients had new AF of unclassified type (Supplementary material online, Figure S1). Of the 17 786 patients with AF of unknown type, 7096 were classified by the investigator within the first 150 days of enrolment (median 35 days, interquartile range 8–90 days), bringing the total number of patients with a known type of AF to 29 181. Of these patients, 14 344 (49.2%) had paroxysmal, 8064 (27.6%) persistent, and 6773 (23.2%) permanent AF.
Compared to patients with other AF types, those with paroxysmal AF had a slightly lower body mass index, were less likely to have HF or a LVEF <40%, but they were as likely to have history of stroke, transient ischaemic attack, carotid artery occlusive disease, or ACS.
Median CHA2DS2-VASc and HAS-BLED scores were similar in all three AF categories, but patients with permanent AF were more likely to be ≥75 years of age (Table 1). The estimated risks of stroke/SE and major bleeding, as assessed with the GARFIELD-AF calculator, were similar in all three AF categories, but the estimated risk of death in patients with persistent and permanent AF patients was numerically higher than in patients with paroxysmal AF.
Table 1.
Baseline characteristics
| Paroxysmal (N = 14 344) | Persistent (N = 8064) | Permanent (N = 6773) | P-value | |
|---|---|---|---|---|
| Sex, n (%) | <0.0001 | |||
| Male | 7577 (52.8) | 4796 (59.5) | 3833 (56.6) | |
| Female | 6767 (47.2) | 3268 (40.5) | 2940 (43.4) | |
| Age, median (IQR), years | 70.0 (61.0; 77.0) | 70.0 (62.0; 77.0) | 74.0 (66.0; 80.0) | <0.0001 |
| Age group, n (%) | <0.0001 | |||
| <65 years | 4770 (33.3) | 2464 (30.6) | 1438 (21.2) | |
| 65–74 years | 4747 (33.1) | 2754 (34.2) | 2110 (31.2) | |
| ≥75 years | 4827 (33.7) | 2846 (35.3) | 3225 (47.6) | |
| Ethnicity, n (%) | <0.0001 | |||
| Caucasian | 8375 (60.2) | 4830 (61.0) | 4854 (73.0) | |
| Hispanic/Latino | 775 (5.6) | 485 (6.1) | 699 (10.5) | |
| Asian (not Chinese) | 3835 (27.6) | 2244 (28.4) | 742 (11.2) | |
| Chinese | 693 (5.0) | 237 (3.0) | 221 (3.3) | |
| Afro-Caribbean/Mixed/Other | 240 (1.7) | 117 (1.5) | 130 (2.0) | |
| Vital measures | ||||
| Body mass index, median (IQR), kg/m² | 26.0 (24.0–30.0) | 27.0 (24.0–31.0) | 28.0 (24.0–31.0) | <0.0001 |
| Pulse, median (IQR), b.p.m. | 80.0 (68.0–103.0) | 88.0 (74.0–105.0) | 84.0 (72.0–100.0) | <0.0001 |
| Systolic BP, median (IQR), mm Hg | 130.0 (120.0–145.0) | 130.0 (120.0–144.0) | 134.0 (120.0–145.0) | <0.0001 |
| Diastolic BP, median (IQR), mmHg | 80.0 (70.0-86.0) | 80.0 (70.0–90.0) | 80.0 (70.0–89.0) | <0.0001 |
| Left ventricular ejection fraction, n (%) | <0.0001 | |||
| <40% | 498 (5.7) | 645 (12.4) | 445 (13.1) | |
| ≥40% | 8227 (94.3) | 4557 (87.6) | 2943 (86.9) | |
| Care setting specialty at diagnosis, n (%) | <0.0001 | |||
| Cardiology | 9945 (69.3) | 5516 (68.4) | 3521 (52.0) | |
| Geriatrics | 47 (0.3) | 29 (0.4) | 37 (0.6) | |
| Internal medicine | 2411 (16.8) | 1389 (17.2) | 1523 (22.5) | |
| Neurology | 307 (2.1) | 86 (1.1) | 132 (2.0) | |
| Primary care/general practice | 1634 (11.4) | 1044 (13.0) | 1560 (23.0) | |
| Care setting location at diagnosis, n (%) | <0.0001 | |||
| Anticoagulation clinic/thrombosis centre | 68 (0.5) | 56 (0.7) | 96 (1.4) | |
| Emergency room | 1681 (11.7) | 813 (10.1) | 548 (8.1) | |
| Hospital | 8551 (59.6) | 4843 (60.1) | 3447 (50.9) | |
| Office | 4044 (28.2) | 2352 (29.2) | 2682 (39.6) | |
| Medical history, n (%) | ||||
| Congestive heart failure | 2202 (15.4) | 2033 (25.2) | 1649 (24.4) | <0.0001 |
| Coronary artery disease | 2904 (20.3) | 1514 (18.8) | 1469 (21.7) | <0.0001 |
| Acute coronary syndromes | 1328 (9.3) | 669 (8.3) | 663 (9.8) | 0.0048 |
| Carotid occlusive disease | 450 (3.2) | 216 (2.7) | 255 (3.8) | 0.0007 |
| Pulmonary embolism/deep vein thrombosis | 321 (2.2) | 193 (2.4) | 212 (3.1) | 0.0004 |
| Coronary artery bypass graft | 398 (2.8) | 233 (2.9) | 209 (3.1) | 0.4266 |
| History of stroke | 1182 (8.3) | 581 (7.2) | 572 (8.5) | 0.0074 |
| History of transient ischaemic attack | 639 (4.5) | 312 (3.9) | 385 (5.7) | <0.0001 |
| History of systemic embolism | 87 (0.6) | 65 (0.8) | 53 (0.8) | 0.1506 |
| History of bleeding | 363 (2.5) | 204 (2.5) | 211 (3.1) | 0.0318 |
| History of hypertension | 10 819 (75.5) | 6157 (76.5) | 5300 (78.4) | <0.0001 |
| Hypercholesterolaemia | 6019 (43.0) | 3197 (40.9) | 2760 (41.6) | 0.0055 |
| Diabetes mellitus | 2911 (20.3) | 1797 (22.3) | 1574 (23.2) | <0.0001 |
| Hyperthyroidism | 234 (1.7) | 140 (1.8) | 122 (1.8) | 0.6446 |
| Hypothyroidism | 856 (6.1) | 366 (4.6) | 443 (6.6) | <0.0001 |
| Cirrhosis | 59 (0.4) | 58 (0.7) | 40 (0.6) | 0.0081 |
| Vascular disease | 2082 (14.5) | 1058 (13.1) | 1013 (15.0) | 0.0022 |
| Dementia | 173 (1.2) | 111 (1.4) | 142 (2.1) | <0.0001 |
| Moderate-to-severe chronic renal disease | 1347 (10.7) | 809 (11.7) | 901 (15.2) | <0.0001 |
| Smoking status, n (%) | <0.0001 | |||
| Never-smoker | 8742 (67.1) | 4756 (64.2) | 4066 (64.1) | |
| Ex-smoker | 2864 (22.0) | 1839 (24.8) | 1727 (27.2) | |
| Current smoker | 1429 (11.0) | 815 (11.0) | 548 (8.6) | |
| Alcohol consumption, n (%) | <0.0001 | |||
| Abstinent | 6710 (55.3) | 3687 (53.1) | 3009 (51.2) | |
| Light | 3971 (32.7) | 2311 (33.3) | 2177 (37.0) | |
| Moderate | 1189 (9.8) | 749 (10.8) | 560 (9.5) | |
| Heavy | 260 (2.1) | 194 (2.8) | 131 (2.2) | |
| CHA2DS2-VASc score, median (IQR) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | <0.0001 |
| CHA2DS2-VASc score, mean (SD) | 3.1 (1.6) | 3.1 (1.6) | 3.5 (1.5) | |
| HAS-BLED score, median (IQR)a | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | <0.0001 |
| HAS-BLED score, mean (SD)a | 1.4 (0.9) | 1.4 (0.9) | 1.5 (0.9) | |
| GARFIELD death score, median (IQR) | 1.8 (1.0; 3.4) | 2.6 (1.4; 5.0) | 3.3 (1.9; 5.8) | <0.0001 |
| GARFIELD death score, mean (SD) | 2.9 (3.6) | 4.3 (5.1) | 4.9 (5.1) | |
| GARFIELD stroke score, median (IQR) | 0.9 (0.6; 1.4) | 0.9 (0.6; 1.4) | 1.0 (0.7; 1.6) | <0.0001 |
| GARFIELD stroke score, mean (SD) | 1.2 (1.0) | 1.2 (1.1) | 1.4 (1.2) | |
| GARFIELD bleeding score, median (IQR) | 0.9 (0.6; 1.3) | 0.9 (0.6; 1.3) | 1.0 (0.8; 1.5) | <0.0001 |
| GARFIELD bleeding score, mean (SD) | 1.0 (0.7) | 1.1 (0.7) | 1.2 (0.7) |
BP, blood pressure; IQR, interquartile range; SD, standard deviation.
The risk factor ‘labile INRs’ is not included in the HAS-BLED score as it is not collected at baseline. As a result, the maximum HAS-BLED score at baseline is 8 points (not 9).
Antithrombotic therapy
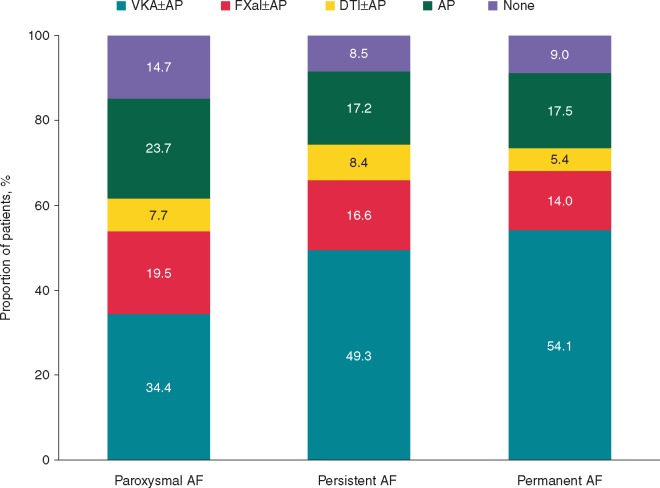
Patients with paroxysmal AF were less likely to receive anticoagulant therapy (with or without AP agents) than those with persistent or permanent AF, and more likely to receive AP agents alone or no antithrombotic treatment (Figure 1). Patients with permanent AF were less likely to be treated by cardiologists and in a hospital than patients in the other two categories of AF.
Figure 1.
Antithrombotic therapy at diagnosis according to type of AF. AF, atrial fibrillation; AP, antiplatelet; DTI, direct thrombin inhibitor; FXa, factor Xa; VKA, vitamin K antagonist.
Among patients without vascular disease, 7463 (29.9%) were prescribed with AP therapy. Among patients with vascular disease, 2462 (59.3%) were prescribed with AP therapy.
Cardiovascular outcomes
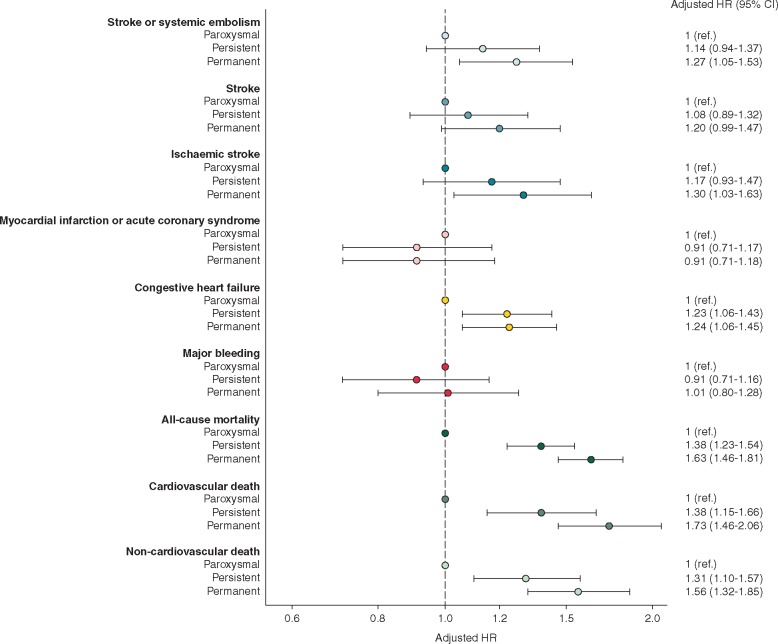
At 2-year follow-up, the rates of death (both cardiovascular and non-cardiovascular mortality), stroke/SE, stroke, and new or worsening HF were higher in patients with persistent and permanent AF than in patients with paroxysmal AF (Table 2). The rates of these endpoints were all significantly higher in patients with permanent AF compared with paroxysmal AF. The same was true for the comparison of persistent AF vs. paroxysmal AF, except for the risk of stroke/SE that was non-significantly higher in persistent AF. Finally, no significant differences were observed for the rates of major bleeding and myocardial infarction/ACS across patients with the different AF types (Table 2). After adjustment for age, gender, race, smoking, diabetes, hypertension, stroke/transient ischaemic attack, history of bleeding, cardiac failure, vascular disease, moderate-to-severe chronic kidney disease, and anticoagulant treatment at baseline, permanent AF was significantly associated with a higher risk of stroke/SE, ischaemic stroke, new or worsening HF, all-cause death, cardiovascular, and non-cardiovascular death compared with paroxysmal AF subgroup. Persistent AF was significantly associated with higher risk of new or worsening HF, all-cause death, cardiovascular, and non-cardiovascular death compared with paroxysmal AF subgroup (Figure 2). Full details of the crude and adjusted rates for all major events, and their components, are provided in Supplementary material online, Table S1.
Table 2.
Incidence event rates per 100 person-years and corresponding 95% confidence intervals during 2-year follow-up of patients with different types of AF
| Outcomes | Types of AF |
|||||||
|---|---|---|---|---|---|---|---|---|
| Paroxysmal (N = 14 344) |
Persistent (N = 8064) |
Permanent (N = 6773) |
Overall (N = 29 181) |
|||||
| Events | Rates (95% CI) | Events | Rates (95% CI) | Events | Rates (95% CI) | Events | Rates (95% CI) | |
| Stroke/systemic embolism and its components | ||||||||
| Stroke/systemic embolism | 301 | 1.16 (1.03–1.29) | 184 | 1.29 (1.1–1.49) | 194 | 1.63 (1.42–1.88) | 679 | 1.30 (1.21–1.40) |
| Stroke without systemic embolism | 275 | 1.06 (0.94–1.19) | 161 | 1.12 (0.96–1.31) | 167 | 1.40 (1.20–1.63) | 603 | 1.15 (1.06–1.25) |
| Ischaemic stroke | 191 | 0.73 (0.63–0.84) | 120 | 0.84 (0.70–1.00) | 127 | 1.06 (0.89–1.26) | 438 | 0.84 (0.76–0.92) |
| Ischaemic stroke or unknown type of stroke | 246 | 0.94 (0.83–1.07) | 148 | 1.03 (0.88–1.21) | 149 | 1.25 (1.06–1.46) | 543 | 1.04 (0.95–1.13) |
| Major bleeding and its components | ||||||||
| Major bleeding | 190 | 0.73 (0.63–0.84) | 103 | 0.72 (0.59–0.87) | 116 | 0.97 (0.81–1.16) | 409 | 0.78 (0.71–0.86) |
| Major bleeding other than primary haemorrhagic stroke | 177 | 0.68 (0.58–0.79) | 92 | 0.64 (0.52–0.78) | 100 | 0.84 (0.69–1.02) | 369 | 0.70 (0.63–0.78) |
| Mortality and its components | ||||||||
| All-cause mortality | 716 | 2.72 (2.53–2.93) | 574 | 3.97 (3.65–4.30) | 713 | 5.92 (5.50–6.37) | 2003 | 3.79 (3.63–3.96) |
| Cardiovascular | 259 | 0.99 (0.87–1.11) | 214 | 1.48 (1.29–1.69) | 282 | 2.34 (2.08–2.63) | 755 | 1.43 (1.33–1.54) |
| Non-cardiovascular mortality | 292 | 1.11 (0.99–1.25) | 215 | 1.49 (1.30–1.70) | 275 | 2.29 (2.03–2.57) | 782 | 1.48 (1.38–1.59) |
| Myocardial infraction or acute coronary syndrome | 193 | 0.74 (0.64–0.85) | 95 | 0.66 (0.54–0.81) | 97 | 0.81 (0.66–0.99) | 385 | 0.73 (0.66–0.81) |
| Congestive heart failure | 390 | 1.51 (1.37–1.67) | 331 | 2.35 (2.11–2.62) | 302 | 2.58 (2.31–2.89) | 1023 | 1.98 (1.87–2.11) |
CI, confidence interval.
Figure 2.
Adjusteda hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) during 2-years follow-up according to type of atrial fibrillation. The reference group is patients with paroxysmal AF. aHazard ratios were adjusted for age, gender, race, smoking, diabetes, hypertension, stroke/transient ischaemic attack, history of bleeding, cardiac failure, vascular disease, moderate-to-severe chronic kidney disease, and anticoagulant treatment at baseline. The model for major bleeding was furtherly adjusted for heavy alcohol consumption.
With regard to HF, only few patients had undergone echocardiography. The available data show that 5.7% of the patients in paroxysmal AF had reduced LV function, patients in persistent AF: 12.4%, and patients in permanent AF: 13.1%.
Interactions between anticoagulant therapy and cardiovascular outcomes
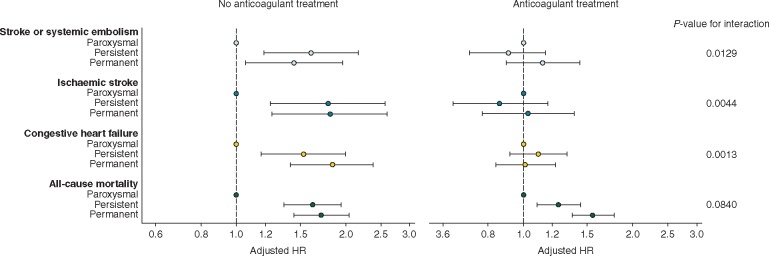
The analysis was repeated to determine whether the observed risks were changed with anticoagulant treatment. There was a significant interaction between type of AF and anticoagulant therapy for the endpoints of stroke/SE, ischaemic stroke and new or worsening HF in the whole population, with higher risks in non-paroxysmal AF in non-anticoagulated patients only. The interaction for death was not statistically significant (Figure 3). In anticoagulated patients, there were no differences in the risks for any event between patients in the paroxysmal and persistent or permanent AF groups, except for the risk of death, which was significantly higher in non-paroxysmal compared with paroxysmal AF.
Figure 3.
Adjusteda hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for stroke or systemic embolism, ischaemic stroke, congestive heart failure or all-cause mortality by type of AF, stratified by anticoagulant treatment at baseline. The reference group is patients with paroxysmal AF. aHazard ratios were adjusted for age, gender, race, smoking, diabetes, hypertension, stroke/transient ischaemic attack, history of bleeding, cardiac failure, vascular disease, moderate-to-severe chronic kidney disease, and anticoagulant treatment at baseline.
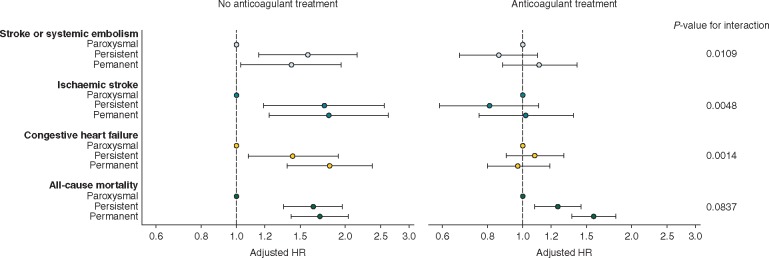
We also performed a sensitivity analysis of the interaction in the population of patients with a CHA2DS2-VASc score ≥2. This analysis confirmed the existence of a significant interaction for stroke/SE, ischaemic stroke and new/worsening HF, with higher risks in non-paroxysmal AF in non-anticoagulated patients only (Figure 4). The interaction for death was not statistically significant irrespective of stroke risk. In anticoagulated patients, there were no differences in the risks for any event between patients in the paroxysmal and persistent or permanent AF groups, except for the risk of death, which was significantly higher in non-paroxysmal compared with paroxysmal AF. In low-risk patients (CHA2DS2-VASc Score 0 or 1), the rate of events was too low to conduct meaningful sensitivity analyses.
Figure 4.
Adjusteda hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for stroke or systemic embolism, ischaemic stroke, congestive heart failure or all-cause mortality by type of AF, stratified by anticoagulant (AC) treatment at baseline in patients with CHA2DS2-VASc score ≥2. The reference group is patients with paroxysmal AF. aHazard ratios were adjusted for age, gender, race, smoking, diabetes, hypertension, stroke/transient ischaemic attack, history of bleeding, cardiac failure, vascular disease, moderate-to-severe chronic kidney disease, and anticoagulant treatment at baseline.
Discussion
Our principal finding is that persistent and permanent AF are associated with a higher risk of stroke/SE, death, and new or worsening HF than paroxysmal AF, even after adjustment for a large variety of clinical features. The second important finding is that the increased risk of all major adverse events was only apparent in the subgroup of patients who was not prescribed anticoagulant therapy. In the anticoagulated subgroup, there was no difference in the risks of stroke/SE and new or worsening HF in paroxysmal compared with non-paroxysmal forms of AF. However, an excess risk of death persisted with AC in non-paroxysmal forms of AF, though at a lower level than observed in the non-anticoagulated subgroup of patients.
This is in line with the findings of most published reports derived from secondary analyses of large-scale clinical trials, registries or meta-analyses, which consistently show that the risk of stroke/SE (and also death in a few studies) was higher in non-paroxysmal forms of AF compared with paroxysmal AF.3,4,10–16 The worse prognosis with non-paroxysmal AF is thought to be linked to the higher risk profile of these patients. In a few reports, the risk of stroke/SE was found to be similar across all patterns of AF, leading the authors to conclude that the decision to anticoagulate should be based on the risk factors rather than the type of AF.3,5,17,18 However, none of these reports analysed the impact of AC on the risk of major adverse events using a large prospective cohort, such as GARFIELD-AF.
This registry provides further confirmation that the AF pattern should not be taken into consideration when deciding on AC. Indeed, there is a continuum of risk for stroke/SE across the different patterns of AF. In patients with paroxysmal AF, the risk of stroke is twice as high as in the general population.17 In paroxysmal AF patients, the burden of AF (as defined by the percentage of time spent in AF during long-term monitoring) is significantly and independently associated with a higher risk of ischaemic stroke as shown by Go et al.19 In other words, what matters is not AF pattern, but the time spent in AF. The most recent Guidelines implicitly suggest that the decision to anticoagulate should be based on the clinical risk profile for stroke as assessed by various risk scoring systems/calculators, such as CHA2DS2-VASc, and not AF pattern.1,3,20 In other words, also paroxysmal AF should be anticoagulated according to the CHA2DS2-VASc assessment.
The differences in the risks of stroke/SE at 2-year follow-up, though substantial across the AF patterns, were not captured by the current scoring systems as in this population, the median values of CHA2DS2-VASc score (and also HAS-BLED score) were similar irrespective of AF patterns. As recently proposed, employing biomarker measurements, in addition to the clinical risk profile, may further refine the predictive value of such risk calculators.21,22
Though the ability of CHA2DS2-VASc score to assess the risk of stroke/SE is well established in this context, it was suggested that it might benefit from the inclusion of other factors, including the type of AF. As suggested previously, taking AF pattern into consideration could aid the decision to anticoagulate, particularly in patients with a low stroke risk, i.e. a CHA2DS2-VASc score of 2 or less.13 This was not confirmed in this report as the rate of events was too low to conduct meaningful sensitivity analyses in these patients.
GARFIELD-AF risk calculator, derived from GARFIELD-AF cohort and externally validated on ORBIT-AF cohort, was shown to be a better predictor of the risk of stroke/SE than CHA2DS2-VASc score in patients with a high, intermediate, or low stroke risk.8 Using the GARFIELD-AF risk calculator, which incorporates AF patterns in its model, we were not able to show that type of AF was associated with a higher estimated risk of stroke/SE or bleeding. The GARFIELD-AF risk calculator showed a continuum of risk for death as evidenced by a gradual increase in the risk score across all three AF patterns. The risk of death is undoubtedly an important incentive for ensuring the comprehensive management of patients, including the prescription of anticoagulants and the optimal management of comorbidities that have a major impact on outcome, which is chiefly, but not limited to, HF. In evaluations of all patients, regardless of risk, AC was associated with a >30% risk reduction in death rates.23,24
Limitations
The current study has several limitations. The event rates are low in this study, both ischaemic stroke and major bleeding. This raises the concern that not all events have been identified.
The classification of AF at a single time point can be misleading as AF patterns often change over time. Hence the one-time rhythm assessment is a limitation. Furthermore, there was no type of AF determination during the 2-year follow-up period.
This is an observational database. Oral anticoagulant treatments were not randomized. Although adjustments were made for confounding, one cannot make conclusive statements about causation for AF type or treatment with outcomes.
The type of AF classification is a relatively poor surrogate measure for the burden of AF (proportion of time spent in AF). Another difficulty is that patients with paroxysmal AF, in general, are healthier than those with the non-self-limiting types of AF, although our statistical methods have attempted to correct for such differences. Finally, the reasons why individual patients/investigators choose not to use anticoagulants are complex and incompletely understood.24
A further limitation pertains to the question whether results from this registry are generalizable. Precautions have been made that patients opting to be included in the GARFIELD-AF registry are as representable as possible of a general AF population, yet there remains an underlying selection that might introduce a bias. For example, it is conceivable that patients agreeing to be followed in a registry have a different level of interest in the disease studied, which in turn might lead to certain more conscientious treatment decisions.
In a sensitivity analysis comparing the excluded patients with unavailable (new/unclassified) type of AF and the selected patients with known type of AF, some differences emerged both in terms of baseline characteristics and with regard to the event rates (Supplementary material online, Tables S2 and S3).
Lastly, loss to follow-up could potentially be different across exposure groups, since permanent AF patients may be associated with a worse prognosis in general, irrespective of the outcomes investigated, which in turn may lead to a higher drop-out of the registry. Our analysis provided clear evidence that there was no significant difference in drop-out rates or lost-to-follow-up between the type of AF groups (data not shown).
Conclusion
In non-anticoagulated patients, non-paroxysmal AF patterns were associated with significantly higher risks of death, stroke/SE, and new/worsening CHF than paroxysmal AF pattern. In anticoagulated patients, the risks of stroke/SE and new/worsening HF were similar across all AF patterns, but non-paroxysmal AF patterns remained associated with a significantly higher risk of death than paroxysmal AF pattern. A continuum in the risk of death across all AF patterns was shown by GARFIELD-AF risk score. Thus, AF pattern is no longer prognostic for stroke/SE when patients are treated with anticoagulants.’
Supplementary Material
Acknowledgements
We thank the physicians, nurses, and patients involved in GARFIELD-AF. Editorial support was provided by Surekha Damineni (TRI, London, UK). SAS programming support was provided by Madhusudana Rao (TRI, London, UK).
Funding
This work was supported by an unrestricted research grant from Bayer AG (Berlin, Germany) to the Thrombosis Research Institute (London, UK). The funding source had no involvement in the data collection, data analysis, or data interpretation.
Conflict of interest: D.A.: Grant support to institution from Medtronic and BMS/Pfizer Personal fees from Bayer Healthcare, BMS/Pfizer, Boehringer-Ingelheim, MSD; E.B.: None; J.-Y.L.H.: Personal fees from Boehringer-Ingelheim, Bayer, BMS/Pfizer, Daiichi Sankyo, Servier, Meda; S.V.: None; A.J.C.: Institutional grants and personal fees from Bayer, Boehringer Ingelheim, BMS/Pfizer and Daichi Sankyo; J.S.: Consultant and/or speaker fees from Abbott, Amgen, Astra-Zeneca, Atricure, Bayer, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis, WebMD, and Zoll. Ownership of CorXL. Grant support through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic; HG.: Personal fees from Bayer AG, Pfizer Australia, BMS Australia; S.Z.G.: Grants from Boehringer-Ingelheim, Bristol Meyers Squibb, BTG EKOS, Daiichi Sankyo, National Heart Lung and Blood Institute of the National Institutes of Health, Janssen, Thrombosis Research Group, personal fees from Bayer, Boehringer-Ingelheim, Bristol Meyers Squibb, Daiichi Sankyo, Janssen; S.G.: Personal fees from Thrombosis Research Institute and the American Heart Association, grants from Sanofi, Pfizer, Ono, Bristol Myer Squibb, the Vehicle Racing Commemorative Foundation and Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering. G.K.: None; F.M.: employee of Bayer AG, the funding source of this investigation, significant shareholder of Bayer AG shares; J.S.: Grants from Bayer, personal fees from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, BMS/Pfizer, Novartis, Sanofi, Servier, expert witness for Boehringer Ingelheim; A.G.G.T.: Personal fees from Bayer Healthcare, Janssen Pharmaceutical Research & Development LLC, Portola; J.-P.B.: None; A.K.K.: Research grants from Bayer AG, personal fees from Bayer AG, Boehringer-Ingelheim Pharma, Daiichi Sankyo Europe, Pfizer, Janssen Pharma, Verson, Sanofi SA.
References
- 1.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 3. Banerjee A, Taillandier S, Olesen JB, Lane DA, Lallemand B, Lip GY. et al. Pattern of atrial fibrillation and risk of outcomes: the Loire Valley Atrial Fibrillation Project. Int J Cardiol 2013;167:2682–7. [DOI] [PubMed] [Google Scholar]
- 4. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P. et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–602. [DOI] [PubMed] [Google Scholar]
- 5. Nieuwlaat R, Dinh T, Olsson SB, Camm AJ, Capucci A, Tieleman RG. et al. Should we abandon the common practice of withholding oral anticoagulation in paroxysmal atrial fibrillation? Eur Heart J 2008;29:915–22. [DOI] [PubMed] [Google Scholar]
- 6. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S. et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One 2013;8:e63479.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S. et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:13–9 e11. [DOI] [PubMed] [Google Scholar]
- 8. Fox KAA, Lucas JE, Pieper KS, Bassand JP, Camm AJ, Fitzmaurice DA. et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open 2017;7:e017157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens PE, Levin A.. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. [DOI] [PubMed] [Google Scholar]
- 10. Link MS, Giugliano RP, Ruff CT, Scirica BM, Huikuri H, Oto A. et al. Stroke and mortality risk in patients with various patterns of atrial fibrillation: results from the ENGAGE AF-TIMI 48 trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol 2017;10: [DOI] [PubMed] [Google Scholar]
- 11. Senoo K, Lip GY, Lane DA, Buller HR, Kotecha D.. Residual risk of stroke and death in anticoagulated patients according to the type of atrial fibrillation: AMADEUS trial. Stroke 2015;46:2523–8. [DOI] [PubMed] [Google Scholar]
- 12. Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ. et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J 2015;36:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M. et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J 2015;36:281–287a. [DOI] [PubMed] [Google Scholar]
- 14. Hohnloser SH, Vamos M.. Not all types of atrial fibrillation carry the same stroke risk, but most benefit from oral anticoagulation. Circ Arrhythm Electrophysiol 2017;10: [DOI] [PubMed] [Google Scholar]
- 15. Koga M, Yoshimura S, Hasegawa Y, Shibuya S, Ito Y, Matsuoka H. et al. Higher risk of ischemic events in secondary prevention for patients with persistent than those with paroxysmal atrial fibrillation. Stroke 2016;47:2582–8. [DOI] [PubMed] [Google Scholar]
- 16. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH. et al. ‘Real-world’ management and outcomes of patients with paroxysmal vs. non-paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme-Atrial Fibrillation (EORP-AF) General Pilot Registry. Europace 2016;18:648.1–57. [DOI] [PubMed] [Google Scholar]
- 17. Friberg L, Hammar N, Rosenqvist M.. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J 2010;31:967–75. [DOI] [PubMed] [Google Scholar]
- 18. Lip GY, Frison L, Grind M.. Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med 2008;264:50–61. [DOI] [PubMed] [Google Scholar]
- 19. Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SH. et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol 2018;3:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- 21. Hall A, Simpson RFG, Mitchell A.. Biomarker assays for personalised stroke risk assessment in atrial fibrillation. Cardiovasc Hematol Disord Drug Targets 2017;17:58–63. [DOI] [PubMed] [Google Scholar]
- 22. Hijazi Z, Lindback J, Alexander JH, Hanna M, Held C, Hylek EM. et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bassand JP, Accetta G, Al Mahmeed W, Corbalan R, Eikelboom J, Fitzmaurice DA. et al. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: rationale for comprehensive management of atrial fibrillation. PLoS One 2018;13:e0191592.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bassand JP, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KA. et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J 2016;37:2882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.