Abstract
Background
Oxidative stress and chronic inflammatory states triggered by a single‐nucleotide polymorphism (SNP) in superoxide dismutase manganese‐dependent gene (Val16Ala‐SOD2) have been associated with the risk of developing several chronic, nontransmissible diseases. However, it is still not clear whether the VV‐SOD2 genotype that causes higher basal superoxide anion levels has any impact on the risk for depression and self‐reported psychological stress in elderly people.
Methods
In the present study, we tested this hypothesis using a case‐control study where depression was detected using the Geriatric Depression Scale‐15 (GDS‐15). A total of 612 Brazilian free‐living elderly subjects with a mean age of 67.1 ± 7.1 years old (number of controls, C = 497, and depressive individuals, D = 115) were included in this study. All participants had similar social, health, and lifestyle variables, with the exception of polypharmacy (≥5 medicines daily intake), which was higher in the D group, compared to C subjects.
Results
Our results showed that the VV‐SOD2 genotype significantly increased the risk for depression and psychological stress in the elderly subjects, independently of sex/gender, age, and other prior diseases and health indicators (depression risk = 1.842, 1.109–3.061 95% CI, p = .018). VV‐subjects also had a higher daily intake of antidepressants, anxiolytics, and anti‐inflammatory drugs than A‐allele subjects.
Conclusion
Our findings support the hypothesis that genetically induced oxidative superoxide‐hydrogen peroxide imbalance may be involved in an increased risk for developing depression and psychological stress in free‐living elderly people without other chronic nontransmissible diseases.
Keywords: depression, elderly, psychological stress, superoxide dismutase polymorphism
Our results showed that the VV‐SOD2 genotype significantly increased the risk for depression and psychological stress in the elderly subjects. VV‐subjects also had a higher daily intake of antidepressants, anxiolytics, and anti‐inflammatory drugs. The findings support the hypothesis that genetically induced oxidative superoxide‐hydrogen peroxide imbalance may be involved in an increased risk for developing depression and psychological stress in free‐living elderly people.
1. INTRODUCTION
Depression and psychological stress are highly prevalent in contemporary societies, mainly in elderly people (Demyttenaere et al., 2009; Kok & Reynolds, 2017; Niraula, Sheridan, & Godbout, 2017). Moreover, these conditions can affect the well‐being and increase the risk of certain chronic nontransmissible diseases, such as cancer and cardiovascular morbidities (Bortolato et al., 2017; Zhang, Chen, & Ma, 2018). The convergence of psychological stress, depression, and other chronic nontransmissible diseases may be related to the establishment of inflammatory oxidative states present in all these conditions (Halaris, 2017; Kruse & Strouse, 2015).
Numerous studies have consistently suggested the existence of a bidirectional relationship between oxidative stress inflammation and these conditions (Demyttenaere et al., 2009; Liu et al., 2018; Prenderville, Kennedy, Dinan, & Cryan, 2015; Straub & Cutolo, 2018). Previous investigations have suggested that oxi‐inflammatory states triggered by depression and/or chronic psychological stress appear to be associated with the elevation of reactive oxygen species (ROS) (Lopresti, Maker, Hood, & Drummond, 2014), including superoxide anion levels produced by NADPH oxidase activation or some impairment in superoxide dismutase (SOD) enzymes (Seo et al., 2012; Uchihara, Tanaka, Asano, Tamura, & Mizushima, 2016; Xie et al., 2017).
Genetic studies involving the imbalance of superoxide‐hydrogen peroxide associated with a single‐nucleotide polymorphism (SNP) located in the SOD2 (OMIM: 147460), manganese‐dependent enzyme gene (Val16Ala‐SOD2, rs4880) have suggested an association of both AA‐ and AV‐genotypes of this SNP with some chronic nontransmissible diseases. This association correlates with a superoxide‐hydrogen peroxide imbalance that involves a higher efficiency of SOD2 in the AA‐genotype; this generates elevated basal hydrogen peroxide levels, and a lower efficiency of SOD2 in the VV‐genotype generating elevated basal superoxide levels in carriers (Bresciani, Cruz, & González‐Gallego, 2015; Bresciani, González‐Gallego, da Cruz, de Paz, & Cuevas, 2013). Specifically, the VV‐genotype has been linked to chronic inflammatory states with higher levels of proinflammatory cytokines, such as the interleukins, IL‐1 and IL‐6, tumor necrosis factor‐alpha (TNF‐α), and anti‐inflammatory interleukin IL‐10 (Barbisan, Azzolin, & Ribeiro, 2017; Duarte et al., 2016; Montano et al., 2012). Flores et al. (2017) also suggested that the VV‐genotype could increase the risk for hypercholesterolemia and higher glucose levels in patients in the late phase of stroke (>6 months).
However, studies correlating the Val16Ala‐SOD2 SNP with depression are still inconclusive. While some studies have not found an association between this SNP and depression (Elbozan Cumurcu et al., 2013; Pae et al., 2006), other investigations have described a higher frequency of both VV‐genotypes and depression in female subjects (Gałecki et al., 2010) when examining the severity of depression in patients with chronic obstructive pulmonary disease (Pietras et al., 2010) as well as in adult patients with depression (Wigner et al., 2018).
Considering that oxidative stress and chronic inflammation are two processes that increase during the aging process (Picca et al., 2018), the present study postulated that the risk of both depression and psychological stress in elderly people may be influenced by superoxide‐hydrogen peroxide imbalance triggered by the Val16Ala‐SOD2 SNP.
2. MATERIALS AND METHODS
2.1. General study design
To investigate the possible effects of genetically determined superoxide‐hydrogen peroxide imbalance on depression and self‐reported chronic psychological stress, we performed a case‐control analysis that examined the association between the Val16Ala‐SOD2 SNP and depression, and self‐reported psychological stress in the free‐living elderly and subjects without cognitive impairment. All participants provided written informed consent, and this protocol was approved by the Human Ethics Committee of the Federal University of Santa Maria (number 23081.009087/2008). The work described in the present paper has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.2. Genetic Analyses
The Val16Ala‐SOD2 SNP of all study participants was analyzed from DNA samples using standard polymerase chain reaction and restriction fragment length polymorphism (PCR‐RFLP) techniques, previously described by Montano et al. (2009). Briefly, after obtaining DNA from total peripheral blood leukocyte extraction, SOD2 genotyping was performed following amplification of a 110‐bp fragment of the human SOD2: 5′‐ACCAGCAGGCAGCTGGCGCCGG‐3′ (sense‐strand) and 5′GCGTTGATGTGAGGTTCCAG‐3′ (antisense‐strand). Further, PCR products (10 µl) obtained after amplification were digested with HaeIII (15 U; at 37°C, for 6 hr, Gibco Inc.) and two digested products (23 and 85 bp) were visualized on a 6% agarose gel (Amersham Biosciences Inc.) stained with ethidium bromide.
2.3. Case‐control protocol
The current protocol included a Brazilian elderly free‐living community with an average age of 67.1 ± 7.1 years, (minimum = 60 years and maximum = 82 years) enrolled in third age‐social groups or in geriatric social support services (Gravataí city, State of Rio Grande do Sul, Brazil). At the time the study was conducted, there were 27,453 elderly people in the city of Gravataí. Sample size calculation considering 95% confidence and a 5% margin of error estimated the inclusion of 379 elderly. However, considering a prevalence of depression estimated to be 5.8% for population, it was decided to include all elderly participants in the study who did not meet any exclusion criteria. We considered that this population does not present any significant ethnic isolation been appropriate for epidemiological studies on aging based in previous investigations performed by Da Cruz et al. (2003) and Parra et al. (2003). We did not include subjects who were bedridden, hospitalized, or who did not participate regularly in social activities outside their homes. Subjects diagnosed with cognitive decline or immobility problems due to chronic morbidities—such as stroke, Parkinson's disease, hypercholesterolemia, morbid obesity, and some types of cancer—were excluded from the study, since these morbidities are confounding factors associated with high rates of depression. Therefore, from the original databank of 1,058 elderly people previously studied by Montano et al. (2009), 612 subjects were included in the present analysis. Notably, as Brazil is a middle‐income country, the World Health Organization considers elderly people to be those ≥60 years old (WHO, 2015).
Depression in the elderly sample was diagnosed using the Geriatric Depression Scale‐15 (GDS‐15), previously validated in Portuguese (Almeida & Almeida, 1999), which is able to achieve early detection of depression in primary care and other health care settings. GDS is an instrument broadly used to diagnose depression in elderly people, and several studies have demonstrated that GDS offers valid and reliable measures for the evaluation of depressive disorders (Dorow et al., 2018). The Portuguese version of the GDS with 15 questions (GDS‐15) offered valid measurements for the diagnosis of major depressive episodes according to the International Classification of Diseases (ICD‐10) and the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) criteria using a 5/6 cutoff point (noncase/case) (Almeida & Almeida, 1999), which was also predicted previously by Hermann et al. (1996). The 5/6 cutoff point (noncase/case) for the GDS‐15 produced sensitivity indices of 85.4% and a specificity of 73.9% for the diagnosis of depressive episodes, according to the ICD‐10 (Almeida & Almeida, 1999). An evaluation of 23 studies performed by Pocklington, Gilbody, Manea, and McMillan (2016) indicated that a cutoff score of 5 points was appropriate for the GDS‐15. However, it is important to be cautious about the classification of elderly depressive episodes using this cutoff point. Some studies, such as the one performed by Sugishita, Sugishita, and Hemmi (2017), suggested that a higher cutoff point (6/7) could be more accurate for diagnosis of a depressive episode in elderly people. Therefore, in the present study, we decided to use the 6/7 cutoff point.
Self‐perception of current psychological stress was also considered in our analysis. Two physicians and one psychologist determined the GDS score and self‐perception of psychological stress, whereas health professionals conducted a structured interview with questions regarding social, health, and lifestyle variables. Subjects with chronic or noncontrolled diseases that could influence the results were excluded. Since the study includes genetic variables, the subjects were recruited based on a random selection of Brazilians of European ancestry from State of Rio Grande do Sul. All subjects provided written informed consent, and our protocol was approved by the Human Ethics Committee of the Federal University of Santa Maria, Santa Maria, RS, Brazil (number 23081.009087/2008).
Demographic and social variables were considered covariates in the study. Moreover, the following co‐variables that could exert some influence on the association of Val16Ala‐SOD2 SNP with depression were considered in the analysis, since previous reports have shown an association between these variables and the polymorphism studied here. These variables include, obesity determined by body mass index (BMI, Kg/m2) (Hernández‐Guerrero et al., 2016; Montano et al., 2009) or obesity‐related metabolic markers (Becer & Çirakoğlu, 2015), hypercholesterolemia (Chen et al., 2012; Duarte et al., 2010; Duarte et al., 2016; Flores et al., 2017), and diabetes mellitus type 2 and its complications (Gottlieb et al., 2005; Huang, Lyu, Liu, Chen, & Wang, 2017; Möllsten, Jorsal, Lajer, Vionnet, & Tarnow, 2009; Li et al., 2015; Pourvali, Abbasi, & Mottaghi, 2016; Rizvi, Raza, & Mahdi, 2014).
Furthermore, the following three co‐variables were also considered despite a lack of specific associative studies between them and Val16Ala‐SOD2 polymorphism: hypertension, smoking, and polypharmacy. Previous reports describe an association between the Val16Ala‐SOD2 SNP and cardiovascular diseases (Flores et al., 2017; Fujimoto, Kobayashi, Ogasawara, Yamakado, & Ohno, 2010; Fujimoto et al., 2008; Kakko et al., 2003; Karahalil, Elkama, & Orhan, 2017; Souiden et al., 2016), for which hypertension and smoking habits are important risk factors. Regarding polypharmacy, consistent evidences exist supporting an association between certain pharmacological drugs and body weight modulation, including antidepressants (Abosi, Lopes, Schmitz, & Fiedorowicz, 2018) and antipsychotic drugs, which are sometimes prescribed to depressive elderly patients (Dayabandara et al., 2017).
2.4. Statistical analyses
Data analysis was performed with SPSS (version 22.0.1; SPSS Inc.). Initially, the Hardy–Weinberg equilibrium was tested using a Chi‐squared test in which observed and expected genotype frequencies were compared. Univariate association of SOD2 genotypes and V‐ or A‐allele frequencies with depression and self‐reported psychological stress was also determined using Chi‐squared or Fisher test. Quantitative variables were compared among genotypes using analysis of variance (ANOVA) followed by a Bonferroni post hoc test. Considering that the elderly can present some differences related to sex/gender, age, or previous disease, and because some chronic diseases have previously been associated with the Val16Ala‐SOD2 SNP, such as obesity and dyslipidemia, a multivariate analysis was performed using a logistic regression (backward stepwise Wald method) to determine the effect of these variables on the association between depression, psychological stress, and the SOD2 SNP. Odds ratio values and 95% confidence intervals were also calculated. The alpha value was set at 0.05, and all p‐values were two‐tailed.
3. RESULTS
Out of the 612 elderly subjects included in the present analysis, 115 (18.8%) were diagnosed with depression (D group), whereas 497 (81.2%) were not and used as controls (C group). The mean age of the two groups was virtually similar (D = 66.9 ± 6.5 and C = 67.2 ± 7.0 years, p = .728). Social, health, and lifestyle variables were also similar between the two groups. However, polypharmacy, that is, daily intake of ≥5 prescribed drugs, was higher in the D subjects, compared to the C group. Furthermore, subjects in group D also presented a higher prevalence of self‐reported psychological stress than the subjects in group C.
Comparison of the genotypic frequency of the Val16Ala‐SOD2 SNP in groups C and D elderly subjects is presented in Table 1, which shows that the subjects in group D had a significantly higher VV‐frequency than the healthy individuals in group C. Further analysis revealed a significant association between the VV‐genotype and depression, compared to A‐allele carriers. Therefore, this association presented a potential recessive pattern in the population studied here. Elderly subjects who self‐reported psychological stress also had a significantly (p = .02) higher frequency of VV‐genotypes (31.3%, n = 47) than controls (19.8%, n = 51). The relative risk (RR) for the D group of presenting VV‐genotypes was 1.564 (range: 1.146 to 2.134) times higher than the RR of C controls. A multivariate analysis showed that the association between depression and the VV‐genotype was independent of sex, age, and several other health variables, as shown in Table 1.
Table 1.
Genotype and allele frequencies of Val16Ala‐SOD2 SNP in healthy control (C) and depressive (D) elderly subjects
| Genetic |
C subjects % (n) |
D subjects % (n) |
p |
|---|---|---|---|
| Genotypes | |||
| VV | 21.1 (105) | 33.0 (38) | .014 |
| AV | 62.8 (312) | 49.6 (57) | |
| AA | 16.1 (80) | 17.4 (20) | |
| Allele frequency | |||
| V | 0.525 | 0.665 | |
| A | 0.475 | 0.335 | |
| V‐allele dose effect | |||
| AA + AV | 78.8 (392) | 67.0 (77) | .006 |
| VV | 21.2 (105) | 33.0 (38) | |
Statistical comparisons were performed by Chi‐squared test.
Additional analysis showed that VV‐depressive subjects had a higher frequency of polypharmacy than AA‐ and AV‐depressive subjects (Figure 1a). A complementary analysis was conducted to analyze antidepressants and other drugs used daily by 198 elderly subjects with different Val16Ala‐SOD2 genotypes (Figure 1b). This analysis was performed in 198 subjects who were able to report what drugs they ingested daily. The VV‐carriers reported a significantly higher frequency of daily intake of antidepressants (p = .032) and anti‐inflammatory drugs (p = .011) than A‐allele carriers. The RR of VV‐subjects using antidepressants compared to A‐allele subjects was 3.628 (95% CI = 1.222–10.773) independent of sex, age, BMI, and use of hypolipemic agent. The RR of VV‐subjects using anti‐inflammatory drugs compared to A‐allele subjects was 5.636 (95% CI = 1.638–9.385) independent of sex, age, BMI, and hypolipemic agent use. However, the frequencies of antihypertensive and anxiolytic drugs, including sleeping remedies, were similar among subjects carrying different SOD2 genotypes (see Figure 1b).
Figure 1.
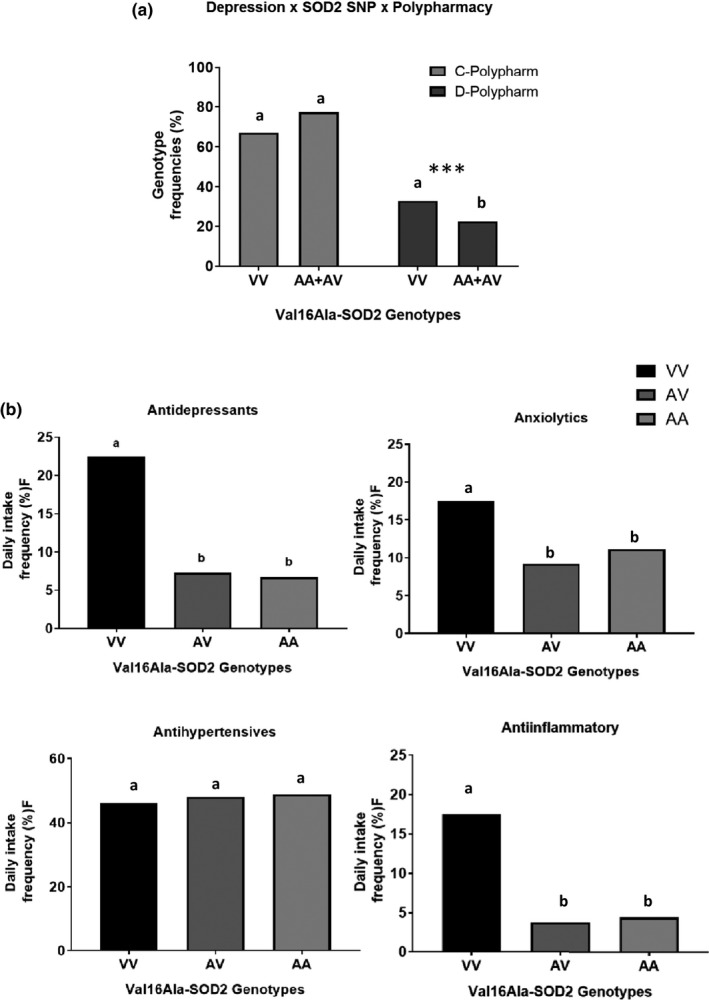
Val16Ala‐SOD2 genotype distribution in control (C) and depressive (D) elderly subjects who presented (a) polypharmacy (daily intake of ≥5 medicines) and (b) hypertension (HAS) disease. The comparison was performed by univariate Chi‐squared analysis. ***p < .01. In logistic regression multivariate analysis, polypharmacy and hypertension were associated with depression diagnosis, but did not significantly influence the association between the VV‐genotype and depression
In this analysis, a total of five VV‐carriers (12.8%) reported the use of statins, whereas in AV‐subjects, the use of this drug was reported by six subjects (5.8%) and by one AA‐subject (2.3%). These results were not significantly different among SOD2 genotype groups (p = .155). The number of elderly subjects reporting the use of hypoglycemic drugs was also similar (p = .748) and restricted (n = 6, 3.1%) in the subsample analyzed here.
4. DISCUSSION
The present investigation found a significant association between depression and self‐reported current psychological stress and the VV‐genotype of the Val16Ala‐SOD2 SNP. These results were independent of sex, age, and several other health variables such as diabetes, dyslipidemia, and obesity (Table 2). However, hypertension and polypharmacy were significantly associated with depression (Table 3). Despite methodological constraints related to case‐control studies, our results suggested a potential association of genetic SNP associated with a lower efficiency in SOD2 enzyme with neuropsychiatric conditions prevalent in elderly subjects. Consequently, some detailed considerations about these results are discussed below.
Table 2.
Characteristics of elderly subjects with (D) and without (C) depression
| Variables |
C subjects % (n) |
D subjects % (n) |
p |
|---|---|---|---|
| Age groups (years) | |||
| 60–64 | 37.6 (187) | 37.4 (43) | .907 |
| 65–69 | 27.4 (136) | 30.4 (35) | |
| 70–74 | 20.9 (104) | 20.9 (24) | |
| 75–79 | 8.9 (44) | 7.8 (09) | |
| >80 | 5.2 (26) | 3.5 (04) | |
| Sex | |||
| Male | 21.7 (108) | 19.1 (22) | .539 |
| Females | 78.3 (389) | 80.9 (93) | |
| Marital status | |||
| Married/partnership | 37.9 (189) | 33.0 (38) | .102 |
| Single | 6.1 (30) | 3.5 (04) | |
| Widow | 46.1 (229) | 58.3 (67) | |
| Divorced | 9.9 (49) | 06 (5.2) | |
| Education (years) | |||
| 0 < 3 | 11.3 (56) | 13.0 (15) | .879 |
| 3 < 8 | 35.3 (175) | 33.9 (39) | |
| >8 | 53.4 (266) | 53.0 (61) | |
| Diabetes mellitus 2 | 11.5 (57) | 15.7 (18) | .348 |
| Hypertension | 56.5 (281) | 66.1 (76) | .061 |
| Dyslipidemia | 47.3 (235) | 49.6 (57) | .659 |
| Obesity | 38.6 (192) | 38.3 (44) | .941 |
| Metabolic syndrome | 12.9 (64) | 13.9 (16) | .767 |
| Polypharmacy | 23.4 (116) | 35.7 (41) | .017* |
| Smoking habit (current/former) | 26.3 (129) | 35.2 (103) | .112 |
| Self‐reported psychological stress | 11.9 (59) | 98.0 (116) | <.0001* |
C = control group; D = group with depression diagnosis; Subject numbers are shown as (n).
Statistical univariate comparison between two elderly groups was performed by Chi‐squared or Fisher's exact test.
Statistically significant difference between the C and D groups.
Table 3.
Multivariate logistic regression analysis to determine the influence of some healthy conditions in the association between VV‐SOD2 genotype and depression in elderly free‐living community
| Variables | Wald | Risk | 95% CI | p |
|---|---|---|---|---|
| VV‐genotype | 5.562 | 1.842 | 1.109–3.061 | 0.018* |
| Sex | 0.150 | 1.119 | 0.635–1.971 | 0.698 |
| Age | 0.756 | 0.385 | 1.015–1.049 | 0.982 |
| Smoking habit | 2.660 | 1.518 | 0.919–2.506 | 0.103 |
| Polypharmacy | 6.932 | 0.517 | 0.316–0.845 | 0.008* |
| Obesity | 0.041 | 0.952 | 0.594–1.527 | 0.839 |
| Dyslipidemia | 1.914 | 1.383 | 0.874–2.189 | 0.167 |
| Diabetes mellitus 2 | 0.814 | 1.359 | 0.698–2.645 | 0.367 |
| Hypertension | 8.756 | 2.019 | 1.268–3.215 | 0.003* |
Multivariate analysis: logistic regression Backward Wald method.
Abbreviation: CI, confident interval.
Statistically significant difference.
Depression is a psychiatric disease that affects a large number of people, especially the elderly, and is highly associated with suicide (Bachmann, 2018). Data from the World Mental Health Survey, conducted in 17 countries, estimated that one in 20 people present at least one episode of depression throughout their lifetime (Wang et al., 2007). However, known cases of depression do not provide sufficient explanation for the underlying pathophysiology. Evidence suggests that chronic psychological stress is an important risk factor associated with the development of depression, since it is implicated in the induction of multiple behavioral, neurochemical, and biological alterations (Tagliari et al., 2010). Therefore, the association between VV‐SOD2 and depression described in previous studies (Gałecki et al., 2010; Pietras et al., 2010) and in the present investigation could be epidemiologically relevant.
The plausible association between SOD2 genetic imbalance and depression is based on well‐documented evidence that chronic psychological stress is associated with ROS overproduction and the pathogenesis of depression (Floyd, Towner, He, Hensley, & Maples, 2011; Michel et al., 2007; Siegrist & Sies, 2017; Wei et al., 2009). Moreover, there are some investigations, such as one performed by Maes, Galecki, and Chang (2011), that reported an association between depression and impairment of antioxidant status. Therefore, the results described here regarding case‐control analysis strongly support the hypothesis that basal oxidative stress conditions associated with the SOD2 SNP may have some major influence on the risk of depression in the elderly. From our results, it is possible to infer that basal oxidative imbalance associated with a genetic polymorphism could increase the susceptibility of some people in developing depressive states during old age. However, in the present investigation, we did not performed complementary investigations on levels of some oxidative blood markers that could corroborate the association between VV‐genotype and depression. In fact, it is not always possible to establish a direct association between serum levels of oxidative markers including lipoperoxidation, protein carbonylation, and DNA damage since the superoxide‐hydrogen peroxide imbalance associated with the SOD2 enzyme occurs within mitochondria. However, in some previous studies conducted by our research group, we have even found some level of association between Val16Ala‐SOD2 genotypes and oxidative markers. This was the case for analyzes related to the association between hypercholesterolemia and polymorphism or the pharmacogenetic effect of this polymorphism on rosuvastatin response (Duarte et al., 2010; Duarte et al., 2016). In both cases, the research volunteers were chosen because they had a very similar lifestyle, health, and dietary profile. However, in studies involving the elderly, this type of selection is very difficult to conduct as a result of the heterogeneity of this population group. For this reason, we chose not to include serum analyzes related to the oxidative profile.
Moreover, despite the potential association between the VV‐genotype, which has higher basal levels of superoxide anion, it is important to point out that investigations involving this SNP in psychiatric and psychological conditions are still unclear and controversial. From the literature, we found three previous investigations that suggested some association between depression and the VV‐genotype (Gałecki et al., 2010; Pietras et al., 2010; Wigner et al., 2018). Other studies involving the potential association between the Val16Ala‐SOD2 SNP and mood disorders failed to show a significant association between this SNP and major depressive and/or bipolar disorders. However, these studies were performed with small sample sizes (Elbozan Cumurcu et al., 2013; Pae et al., 2006).
Moreover, it is not easy to establish association studies involving the genetics of oxidative metabolism, since there are several potential intervening factors that can act as attenuators or enhancers of oxidative stress associated with psychiatric diseases. For this reason, we tried to perform a prescreening of our sample population, excluding elderly subjects who presented cognitive impairment, physical and psychological frailty, and previous chronic or noncontrolled diseases. Under these conditions, we opted to investigate depression in an elderly free‐living community that participated in organized social groups. This selection could be relevant in the observation of a significant association between the SOD2 SNP and depression described in the present report.
There are several biological and psychological theories explaining the causes of depression including the hypothesis of an active inflammatory process associated with oxidative metabolism imbalance (Jeon & Kim, 2018). In relation to inflammatory response, there is consistent number of studies indicating that superoxide anion is important in this process. Superoxide anion mainly produced by NAD(P)H oxidases is present in all cell types participating in inflammation (leukocytes, endothelial, and other vascular cells) (Zeng, Miralda, Armstrong, Uriarte, & Bagaitkar, 2019). However, basal uncontrolled superoxide concentration may lead to toxic effects, when produced at high levels during oxidative burst. This process has been associated with VV‐genotype that is associated with higher risk of chronic inflammatory conditions including hypercholesterolemia, obesity, and cardiovascular diseases, such as stroke (Barbisan et al., 2017; Bresciani et al., 2015; Pascotini et al., 2018) and also depression and other mood disorders (Maes et al., 2019; Valvassori et al., 2018). As pointed out by Kalinichenko's review (2019), investigations described that psychological stress and mood disorders, especially depression, can result in abnormal immune responses accompanied by abnormal levels of ROS in the red blood cells, mononuclear cells, cerebrospinal fluid, and the brain. In this process, superoxide is considered a key molecule of inflammatory activation, and previous investigations suggested that its imbalance could directly affect the oxi‐inflammatory metabolic patterns. In order to test this hypothesis, Barbisan et al. (2017) evaluated in vitro inflammatory response of peripheral blood mononuclear cells carrying different Val16Ala‐SOD2 genotypes. Results showed that VV‐cells were associated with higher proinflammatory cytokine levels indicating a genetic role in chronic oxi‐inflammatory processes. Therefore, basal higher superoxide levels in VV‐subjects could explain the association between this genotype and depression previously described by Gałecki et al. (2010), and in the present study.
Despite difficulties in evaluating the impact of basal oxidative imbalance on depression risk in elderly people, the findings summarized here are consistent with a recent investigation published by Wigner et al. (2018) describing a significant association between the Val16Ala‐SOD2 SNP and depression in adult subjects. Similar to what was observed in the elderly subjects in our study, Wigner et al. (2018) showed that subjects with the VV‐genotype presented a higher risk of depression diagnosis than AA‐ and AV‐subjects. The authors concluded that their results supported the hypothesis that oxidative and nitrosative stress are involved in the pathogenesis of depressive disorders. Basal alteration in oxidative metabolism triggered by the VV‐genotype leads to the increase of superoxide anion levels, a key molecule in inflammatory processes.
The potential association between higher superoxide anion levels and psychological stress and depression is also indirectly supported by a study in humans performed by Zuccarella‐Hackl et al. (2016). These authors described an association between higher superoxide anion levels and pathogenesis of coronary artery disease, especially in patients with Type D personalities, that is, those with a tendency to experience negative emotions and to inhibit their expression in a social context.
Free‐living elderly people as a group probably experience several instances of “loss”, including the death of family members or friends, the loss of social status associated with retirement, and even functional losses. Thus, elderly individuals who do not have some type of depression or more prominent depressive symptoms may be considered more resilient, whereas depressed individuals may be considered more variable. It is important to point out that we concentrated our analysis on this elderly group since elderly people who present devastating chronic, nontransmissible diseases or cognitive dysfunction have a high predisposition for developing depressive disorders as a secondary morbidity. Among these diseases are some neurodegenerative morbidities such as Parkinson's, coronary diseases, including myocardial infarction, and even some types of cancer (Nasca, Davis, Bigio, Sandi, & McEwen, 2017).
Finally, it is important to comment on some aspects of the methodological approaches used in this study, specifically the use of GDS as a tool to evaluate depression. Accurate diagnosis is essential for the management of elderly depression in primary care. For this reason, several scales have been developed, including the GDS‐15, the Hospital Anxiety and Depression Scale, and the Structured Clinical Interview for DSM‐V criteria. However, a recent study showed that there were high levels of inconsistency among depression diagnosis performed by different tools when more than 1,000 75+ years old patients were concomitantly assessed with these measurement scales. Despite these differences, GDS was the tool that achieved results close to those obtained by a general practitioner (GP) based on the DSM‐V. Whereas the GP estimated there to be 24.3% depressive elderly subjects, the GDS‐15 estimated there to be 21.8% (Dorow et al., 2018). Moreover, the GDS was previously validated in Brazil and is broadly used in geriatric anamneses for the screening and diagnosis of depression (Almeida & Almeida, 1999).
However, there are some considerations that need to be commented on regarding the GDS‐15 tool. Many instruments are available to measure depression, including the GDS with 30 questions, first created by Yesavage et al. (1982), which has been tested and used extensively with elderly subjects. A shortened GDS form developed in 1986 contains 15 questions of which 10 questions indicate the presence of depression when answered positively, while five questions indicate depression when answered negatively. When the GDS‐15 was created, older subjects with scores of 0–4 were considered nondepressives, those with a score of 5–8 were considered to have mild depression, 9–11 indicated moderate depression, and 12–15 indicated severe depression. However, authors such as Greenberg (2012) commented that these cutoff points could vary depending on age, education, and complaints. For this reason, studies generally use the GDS‐15 as a screening tool to identify depressive older people, and not to identify the intensity of their psychiatric condition.
Moreover, there are some concerns about the GDS‐15 cutoff points with respect to depression diagnosis. Most studies consider 4/5 points to be an appropriate cutoff (Pocklington et al., 2016), whereas other studies suggest that 6/7 could be a better cutoff (Sugishita et al., 2017). Because of this, we opted to exclude from our main analysis subjects who could present an overlapping diagnosis in relation to noncase and case distributions. Not excluding these subjects could compromise the results and their interpretations, since we would be including individuals with intermediate punctuation. We understand that this strategy is a significant methodological constraint; nonetheless, we have opted to be more cautious regarding this issue in order to accurately test our main hypothesis pertaining to the association between depression and genetic oxidative imbalance.
Another factor that may be considered limiting in the present study is the following question: we found 98% of self‐declaration of psychological stress in depressive subjects, there is really an association of VV‐SOD2 genotype and stress, or there is a bias of the sample selection, since almost all depressive subjects are also stressed subjects? This question is really important to consider. However, we believe the association between the VV‐genotype psychosocial stress and/or depression is plausible and true, and not just a sample selection bias based in the following considerations: Evidences indicate that the hyperactivity of the HPA axis that induces changes in the immune system is responsible for some of the behavioral and biochemical changes that are also typical in depression (review in Kalinichenko, Kornhuber, & Müller, 2019). Therefore, epidemiological and clinical studies have suggested strong association between psychosocial stress and depression. However, previous report estimated that only approximately 3%–5% of human individuals develop depression/anxiety after stressful life events (Wada et al., 2013). Based on these estimative, we find it important to question older people on their self‐perception of chronic stress. In both cases, elderly self‐reported psychosocial stresses and depressed elderly, we found higher frequency of VV‐genotype indicating that this genetic factor could affect the susceptibility for mental conditions.
5. CONCLUSIONS
The results described in the present report, strongly suggest the association between Val16Ala‐SOD2 SNP and risk of depression and self‐reported psychological stress in elderly subjects, independent of other prevalent noncommunicable chronic diseases. Considering previous evidence, this association could be related to the increase of basal oxidative stress and/or inflammatory states prevalent in VV‐subjects. This association also seems to influence the daily intake of prescribed drugs by older subjects.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTION
Ivo Emilio da Cruz Jung—Mainly responsible for execution of study, writing the manuscript. Ivana da Cruz—Co‐responsible for execution of study, data collection, writing the manuscript study design and statistical analysis. Alexis Trott—Standardization of Val16Ala‐SOD2 genotyping, writing the manuscript, corresponding author. Fernanda Barbisan—Data collection. Lucien Houenou—Review of methodological design, statistical analysis, English editing manuscript. Bárbara Osmarin Turra—Genotyping, databank organization. Thiago Duarte—Genotyping, databank organization. Raquel de Souza Praia—Data collection. Ednea Aguiar Maia‐Ribeiro—Data collection. Jaqueline da Costa Escobar Piccoli—Data collection. Claudia Giugliano Bica—Population data collection. Marta Maria Medeiros Frescura Duarte—Scientific coordinator of Project, responsible for study design and statistical analysis.
ACKNOWLEDGMENTS
This work was supported by the Coordenação de Apoio de Pessoal do Ensino Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico (CNPq) through our grant (processes numbers: 302661/2016‐6; 400816/2016‐4) and fellowships.
da Cruz Jung IE, da Cruz IBM, Barbisan F, et al. Superoxide imbalance triggered by Val16Ala‐SOD2 polymorphism increases the risk of depression and self‐reported psychological stress in free‐living elderly people. Mol Genet Genomic Med. 2020;8:e1080 10.1002/mgg3.1080
REFERENCES
- Abosi, O. , Lopes, S. , Schmitz, S. , & Fiedorowicz, J. G. (2018). Cardiometabolic effects of psychotropic medications. Hormone Molecular Biology and Clinical Investigation, 36, 1–15. 10.1515/hmbci-2017-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, O. P. , & Almeida, A. S. (1999). Reliability of the Brazilian version of the ++abbreviated form of geriatric depression scale (GDS) short form. Arquivos De Neuro‐Psiquiatria, 57, 421–426. 10.1590/S0004-282X1999000300013 [DOI] [PubMed] [Google Scholar]
- Bachmann, S. (2018). Epidemiology of suicide and the psychiatric perspective. International Journal of Environmental Research and Public Health, 15, 1425 10.3390/ijerph15071425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbisan, F. , Azzolin, V. F. , Ribeiro, E. E. , Duarte, M. M. M. F. , & da Cruz, I. B. M. (2017). The in vitro influence of a genetic superoxide‐hydrogen peroxide imbalance on immunosenescence. Rejuvenation Research, 20, 334–345. 10.1089/rej.2016.1892 [DOI] [PubMed] [Google Scholar]
- Becer, E. , & Çirakoğlu, A. (2015). Association of the Ala16Val MnSOD gene polymorphism with plasma leptin levels and oxidative stress biomarkers in obese patients. Gene, 568, 35–39. 10.1016/j.gene.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Bortolato, B. , Hyphantis, T. N. , Valpione, S. , Perini, G. , Maes, M. , Morris, G. , … Carvalho, A. F. (2017). Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treatment Reviews, 52, 58–70. 10.1016/j.ctrv.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Bresciani, G. , da Cruz, I. B. M. , & González‐Gallego, J. (2015). Manganese superoxide dismutase and oxidative stress modulation. Advances in Clinical Chemistry, 10.1016/bs.acc.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Bresciani, G. , González‐Gallego, J. , da Cruz, I. B. , de Paz, J. A. , & Cuevas, M. J. (2013). The Ala16Val MnSOD gene polymorphism modulates oxidative response to exercise. Clinical Biochemistry, 46, 335–340. 10.1016/j.clinbiochem.2012.11.020 [DOI] [PubMed] [Google Scholar]
- Chen, H. , Yu, M. , Li, M. , Zhao, R. , Zhu, Q. , Zhou, W. , … Liu, L. (2012). Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase‐1 (GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Molecular and Cellular Biochemistry, 363, 85–91. 10.1007/s11010-011-1160-3 [DOI] [PubMed] [Google Scholar]
- Da Cruz, I. B. M. , Oliveira, G. , Taufer, M. , Leal, N. F. , Schwanke, C. H. , Glock, L. , … Moriguchi, E. H. (2003). Angiotensin I‐converting enzyme gene polymorphism in two ethnic groups living in Brazil's southern region: Association with age. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 58, M851–M856. 10.1093/gerona/58.9.M851 [DOI] [PubMed] [Google Scholar]
- Dayabandara, M. , Hanwella, R. , Ratnatunga, S. , Seneviratne, S. , Suraweera, C. , & de Silva, V. A. (2017). Antipsychotic‐associated weight gain: Management strategies and impact on treatment adherence. Neuropsychiatric Disease and Treatment, 13, 2231–2241. 10.2147/NDT.S113099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere, K. , Verhaeghen, A. , Dantchev, N. , Grassi, L. , Montejo, A. L. , Perahia, D. G. , … Bauer, M. (2009). "Caseness" for depression and anxiety in a depressed outpatient population: Symptomatic outcome as a function of baseline diagnostic categories. Primary Care Companion to The Journal of Clinical Psychiatry, 11, 307–315. 10.4088/PCC.08m00748blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorow, M. , Stein, J. , Pabst, A. , Weyerer, S. , Werle, J. , Maier, W. , … Riedel‐Heller, S. G. (2018). Categorical and dimensional perspectives on depression in elderly primary care patients – Results of the AgeMooDe study. International Journal of Methods in Psychiatric Research, 27, e1577 10.1002/mpr.1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. M. M. F. , Moresco, R. N. , Duarte, T. , Santi, A. , Bagatini, M. D. , Da Cruz, I. B. M. , … Loro, V. L. (2010). Oxidative stress in hypercholesterolemia and its association with Ala16Val superoxide dismutase gene polymorphism. Clinical Biochemistry, 43, 1118–1123. 10.1016/j.clinbiochem.2010.07.002 [DOI] [PubMed] [Google Scholar]
- Duarte, T. , da Cruz, I. B. M. , Barbisan, F. , Capelleto, D. , Moresco, R. N. , & Duarte, M. M. M. F. (2016). The effects of rosuvastatin on lipid‐lowering, inflammatory, antioxidant and fibrinolytics blood biomarkers are influenced by Val16Ala superoxide dismutase manganese‐dependent gene polymorphism. The Pharmacogenomics Journal, 16, 501–506. 10.1038/tpj.2015.91 [DOI] [PubMed] [Google Scholar]
- Elbozan Cumurcu, B. , Ozyurt, H. , Ates, O. , Gogcegoz Gul, I. , Demir, S. , & Karlıdag, R. (2013). Analysis of manganese superoxide dismutase (MnSOD: Ala‐9Val) and glutathione peroxidase (GSH‐Px: Pro 197 Leu) gene polymorphisms in mood disorders. Bosnian Journal of Basic Medical Sciences, 13, 109–113. 10.17305/bjbms.2013.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores, A. E. , Pascotini, E. T. , Kegler, A. , Gabbi, P. , Bochi, G. V. , Barbisan, F. , … Fighera, M. R. (2017). ALA16VAL‐MnSOD gene polymorphism and stroke: Association with dyslipidemia and glucose levels. Gene, 627, 57–62. 10.1016/j.gene.2017.05.055 [DOI] [PubMed] [Google Scholar]
- Floyd, R. A. , Towner, R. A. , He, T. , Hensley, K. , & Maples, K. R. (2011). Translational research involving oxidative stress and diseases of aging. Free Radical Biology and Medicine, 51, 931–941. 10.1016/j.freeradbiomed.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto, H. , Kobayashi, H. , Ogasawara, K. , Yamakado, M. , & Ohno, M. (2010). Association of the manganese superoxide dismutase polymorphism with vasospastic angina pectoris. Journal of Cardiology, 55, 205–210. 10.1016/j.jjcc.2009.10.011 [DOI] [PubMed] [Google Scholar]
- Fujimoto, H. , Taguchi, J.‐I. , Imai, Y. , Ayabe, S. , Hashimoto, H. , Kobayashi, H. , … Ohno, M. (2008). Manganese superoxide dismutase polymorphism affects the oxidized low‐density lipoprotein‐induced apoptosis of macrophages and coronary artery disease. European Heart Journal, 29, 1267–1274. 10.1093/eurheartj/ehm500 [DOI] [PubMed] [Google Scholar]
- Gałecki, P. , Śmigielski, J. , Florkowski, A. , Bobińska, K. , Pietras, T. , & Szemraj, J. (2010). Analysis of two polymorphisms of the manganese superoxide dismutase gene (Ile‐58Thr and Ala‐9Val) in patients with recurrent depressive disorder. Psychiatry Research, 179, 43–46. 10.1016/j.psychres.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Gottlieb, M. G. , Schwanke, C. H. , Santos, A. F. , Jobim, P. F. , Müssel, D. P. , & da Cruz, I. B. (2005). Association among oxidized LDL levels, MnSOD, apolipoprotein E polymorphisms, and cardiovascular risk factors in a south Brazilian region population. Genetics and Molecular Research, 4, 691–703. https://doi.org/0143[pii] [PubMed] [Google Scholar]
- Halaris, A. (2017). Inflammation-associated co-morbidity between depression and cardiovascular disease. Current Topics in Behavioral Neurosciences, 31, 45–70. 10.1007/7854_2016_28 [DOI] [PubMed] [Google Scholar]
- Hermann, B. P. , Trenerry, M. R. , & Colligan, R. C. (1996). Learned helplessness, attributional style, and depression in epilepsy. Bozeman Epilepsy Surgery Consortium. Epilepsia, 37(7), 680–686. [DOI] [PubMed] [Google Scholar]
- Hernández‐Guerrero, C. , Hernández‐Chávez, P. , Romo‐Palafox, I. , Blanco‐Melo, G. , Parra‐Carriedo, A. , & Pérez‐Lizaur, A. (2016). Genetic polymorphisms in SOD (rs2070424, rs7880) and CAT (rs7943316, rs1001179) enzymes are associated with increased body fat percentage and visceral fat in an obese population from Central Mexico. Archives of Medical Research, 47, 331–339. 10.1016/j.arcmed.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Huang, L. , Lyu, J. , Liu, Q. P. , Chen, C. , & Wang, T. (2017). MnSOD Val16Ala polymorphism associated with retinopathy risk in diabetes: A PRISMA‐compliant Meta‐analysis of case‐control studies. International Journal of Ophthalmology, 10, 639–645. 10.18240/ijo.2017.04.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, S. W. , & Kim, Y. K. (2018). The role of neuroinflammation and neurovascular dysfunction in major depressive disorder. Journal of Inflammation Research, 11, 179–192. 10.2147/JIR.S141033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakko, S. , Päivänsalo, M. , Koistinen, P. , Kesäniemi, Y. A. , Kinnula, V. L. , & Savolainen, M. J. (2003). The signal sequence polymorphism of the MnSOD gene is associated with the degree of carotid atherosclerosis. Atherosclerosis, 168, 147–152. 10.1016/S0021-9150(03)00091-1 [DOI] [PubMed] [Google Scholar]
- Kalinichenko, L. S. , Kornhuber, J. , & Müller, C. P. (2019). Individual differences in inflammatory and oxidative mechanisms of stress‐related mood disorders. Frontiers in Neuroendocrinology, 55, 100783 10.1016/j.yfrne.2019.100783 [DOI] [PubMed] [Google Scholar]
- Karahalil, B. , Elkama, A. , & Orhan, G. (2017). Oxidative stress gene polymorphisms may have an impact in the development of ischemic stroke. The Journal of Gene Medicine, 19, e2947 10.1002/jgm.2947 [DOI] [PubMed] [Google Scholar]
- Kok, R. M. , & Reynolds, C. F. (2017). Management of depression in older adults: A review. Journal of the American Medical Association, 317, 2114 10.1001/jama.2017.5706 [DOI] [PubMed] [Google Scholar]
- Kruse, J. L. , & Strouse, T. B. (2015). Sick and tired: Mood, fatigue, and inflammation in cancer. Current Psychiatry Reports, 17(3), 555 10.1007/s11920-015-0555-3 [DOI] [PubMed] [Google Scholar]
- Li, J. Y. , Tao, F. , Wu, X. X. , Tan, Y. Z. , He, L. , & Lu, H. (2015). Polymorphic variations in manganese superoxide dismutase (MnSOD) and endothelial nitric oxide synthase (eNOS) genes contribute to the development of type 2 diabetes mellitus in the Chinese Han population. Genetics and Molecular Research, 14, 12993–13002. 10.4238/2015.October.21.20 [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Ren, Z. , Zhang, J. , Chuang, C. C. , Kandaswamy, E. , Zhou, T. , & Zuo, L. (2018). Role of ROS and nutritional antioxidants in human diseases. Frontiers in Physiology, 9, 477 10.3389/fphys.2018.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti, A. L. , Maker, G. L. , Hood, S. D. , & Drummond, P. D. (2014). A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 48, 102–111. 10.1016/j.pnpbp.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Maes, M. , Galecki, P. , Chang, Y. S. , & Berk, M. (2011). A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 35, 676–692. 10.1016/j.pnpbp.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Maes, M. , Landucci Bonifacio, K. , Morelli, N. R. , Vargas, H. O. , Barbosa, D. S. , Carvalho, A. F. , & Nunes, S. O. V. (2019). Major differences in neurooxidative and neuronitrosative stress pathways between major depressive disorder and types I and II bipolar disorder. Molecular Neurobiology, 56, 141–156. 10.1007/s12035-018-1051-7 [DOI] [PubMed] [Google Scholar]
- Michel, T. M. , Frangou, S. , Thiemeyer, D. , Camara, S. , Jecel, J. , Nara, K. , … Riederer, P. (2007). Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder‐a postmortem study. Psychiatry Research, 151, 145–150. 10.1016/j.psychres.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Möllsten, A. , Jorsal, A. , Lajer, M. , Vionnet, N. , & Tarnow, L. (2009). The V16A polymorphism in SOD2 is associated with increased risk of diabetic nephropathy and cardiovascular disease in type 1 diabetes. Diabetologia, 52, 2590–2593. 10.1007/s00125-009-1550-1 [DOI] [PubMed] [Google Scholar]
- Montano, M. A. E. , Barrio Lera, J. P. , Gottlieb, M. G. V. , Schwanke, C. H. A. , da Rocha, M. I. U. M. , Manica‐Cattani, M. F. , … da Cruz, I. B. M. (2009). Association between manganese superoxide dismutase (MnSOD) gene polymorphism and elderly obesity. Molecular and Cellular Biochemistry, 328, 33–40. 10.1007/s11010-009-0071-z [DOI] [PubMed] [Google Scholar]
- Montano, M. A. , da Cruz, I. B. , Duarte, M. M. , Krewer Cda, C. , da Rocha, M. I. , Mânica-Cattani, M. F. , … Lera, J. P. (2012). Inflammatory cytokines in vitro production are associated with Ala16Val superoxide dismutase gene polymorphism of peripheral blood mononuclear cells. Cytokine, 60(1), 30–33. 10.1016/j.cyto.2012.05.022 [DOI] [PubMed] [Google Scholar]
- Nasca, C. , Davis, E. , Bigio, B. , Sandi, C. , & McEwen, B. S. ( 2017). Effects of stress throughout the lifespan on the brain and behavior In Hormones, brain and behavior, 3rd edn (pp. 443–463). Elsevier; 10.1016/B978-0-12-803592-4.00111-5 [DOI] [Google Scholar]
- Niraula, A. , Sheridan, J. F. , & Godbout, J. P. (2017). Microglia priming with aging and stress. Neuropsychopharmacology, 42(1), 318–333. 10.1038/npp.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae, C.‐U. , Yoon, S.‐J. , Patkar, A. , Kim, J.‐J. , Jun, T.‐Y. , Lee, C. , & Paik, I.‐H. (2006). Manganese superoxide dismutase (MnSOD: Ala‐9Val) gene polymorphism and mood disorders: A preliminary study. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 30, 1326–1329. 10.1016/j.pnpbp.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Parra, F. C. , Amado, R. C. , Lambertucci, J. R. , Rocha, J. , Antunes, C. M. , & Pena, S. D. J. (2003). Color and genomic ancestry in Brazilians. Proceedings of the National Academy of Sciences of the United States of America, 100, 177–182. 10.1073/pnas.0126614100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascotini, M. E. T. , Flores, D. A. E. , Kegler, M. A. , Konzen, M. V. , Fornari, M. A. L. , Arend, M. J. , … Fighera, D. M. R. (2018). Brain‐derived neurotrophic factor levels are lower in chronic stroke patients: A relation with manganese‐dependent superoxide dismutase ALA16VAL single nucleotide polymorphism through tumor necrosis factor‐α and caspases pathways. Journal of Stroke and Cerebrovascular Diseases, 27, 3020–3029. 10.1016/j.jstrokecerebrovasdis.2018.06.032 [DOI] [PubMed] [Google Scholar]
- Picca, A. , Lezza, A. M. S. , Leeuwenburgh, C. , Pesce, V. , Calvani, R. , Bossola, M. , … Marzetti, E. (2018). Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and Inflammation During Aging and Muscle Wasting Disorders. Rejuvenation Research, 21(4), 350–359. 10.1089/rej.2017.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras, T. , Witusik, A. , Panek, M. , Gałecki, P. , Szemraj, J. , & Górski, P. (2010). Anxiety, depression and polymorphism of the gene encoding superoxide dismutase in patients with chronic obstructive pulmonary disease. Polski Merkuriusz Lekarski, 29, 165–168. [PubMed] [Google Scholar]
- Pocklington, C. , Gilbody, S. , Manea, L. , & McMillan, D. (2016). The diagnostic accuracy of brief versions of the Geriatric Depression Scale: A systematic review and meta‐analysis. International Journal of Geriatric Psychiatry, 31, 837–857. 10.1002/gps.4407 [DOI] [PubMed] [Google Scholar]
- Pourvali, K. , Abbasi, M. , & Mottaghi, A. (2016). Role of superoxide dismutase 2 gene ala16Val polymorphism and total antioxidant capacity in diabetes and its complications. Avicenna Journal of Medical Biotechnology, 8(2), 48–56. [PMC free article] [PubMed] [Google Scholar]
- Prenderville, J. A. , Kennedy, P. J. , Dinan, T. G. , & Cryan, J. F. (2015). Adding fuel to the fire: The impact of stress on the ageing brain. Trends in Neurosciences, 38, 13–25. 10.1016/j.tins.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Rizvi, S. , Raza, S. T., & Mahdi, F. (2014). Association of genetic variants with diabetic nephropathy. World Journal of Diabetes, 5, 809–816. 10.4239/wjd.v5.i6.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, J.‐S. , Park, J.‐Y. , Choi, J. , Kim, T.‐K. , Shin, J.‐H. , Lee, J.‐K. , & Han, P.‐L. (2012). NADPH oxidase mediates depressive behavior induced by chronic stress in mice. Journal of Neuroscience, 32, 9690–9699. 10.1523/JNEUROSCI.0794-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist, J. , & Sies, H. (2017). Disturbed redox homeostasis in oxidative distress: A molecular link from chronic psychosocial work stress to coronary heart disease? Circulation Research, 121(2), 103–105. 10.1161/CIRCRESAHA.117.311182 [DOI] [PubMed] [Google Scholar]
- Souiden, Y. , Mallouli, H. , Meskhi, S. , Chaabouni, Y. , Rebai, A. , Chéour, F. , & Mahdouani, K. (2016). MnSOD and GPx1 polymorphism relationship with coronary heart disease risk and severity. Biological Research, 49, 22 10.1186/s40659-016-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, R. H. , & Cutolo, M. (2018). Psychoneuroimmunology-developments in stress research. Wiener Medizinische Wochenschrift, 168(3–4), 76–84. 10.1007/s10354-017-0574-2 [DOI] [PubMed] [Google Scholar]
- Sugishita, K. , Sugishita, M. , Hemmi, I. , Asada, T. , & Tanigawa, T. (2017). A validity and reliability study of the Japanese version of the geriatric depression scale 15 (GDS‐15‐J). Clinical Gerontologist, 40, 233–240. 10.1080/07317115.2016.1199452 [DOI] [PubMed] [Google Scholar]
- Tagliari, B. , Noschang, C. G. , Ferreira, A. G. , Ferrari, O. A. , Feksa, L. R. , Wannmacher, C. M. , … Wyse, A. T. (2010). Chronic variable stress impairs energy metabolism in prefrontal cortex and hippocampus of rats: Prevention by chronic antioxidant treatment. Metabolic Brain Disease, 25(2), 169–176. 10.1007/s11011-010-9194-x [DOI] [PubMed] [Google Scholar]
- Uchihara, Y. , Tanaka, K.‐I. , Asano, T. , Tamura, F. , & Mizushima, T. (2016). Superoxide dismutase overexpression protects against glucocorticoid‐induced depressive‐like behavioral phenotypes in mice. Biochemical and Biophysical Research Communications, 469, 873–877. 10.1016/j.bbrc.2015.12.085 [DOI] [PubMed] [Google Scholar]
- Valvassori, S. S. , Bavaresco, D. V. , Feier, G. , Cechinel‐Recco, K. , Steckert, A. V. , Varela, R. B. , … Quevedo, J. (2018). Increased oxidative stress in the mitochondria isolated from lymphocytes of bipolar disorder patients during depressive episodes. Psychiatry Research, 264, 192–201. 10.1016/j.psychres.2018.03.089 [DOI] [PubMed] [Google Scholar]
- Wada, K. , Sairenchi, T. , Haruyama, Y. , Taneichi, H. , Ishikawa, Y. , & Muto, T. (2013). Relationship between the onset of depression and stress response measured by the Brief Job Stress Questionnaire among Japanese employees: A cohort study. PLoS ONE, 8, e56319 10.1371/journal.pone.0056319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. S. , Aguilar-Gaxiola, S. , Alonso, J. , Angermeyer, M. C. , Borges, G. , Bromet, E. J. , … Wells, J. E. (2007). Worldwide use of mental health services for anxiety, mood, and substance disorders: Results from 17 Countries in the WHO world mental health (WMH) surveys. Lancet, 370(9590), 841–850. 10.1016/S0140-6736(07)61414-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y.‐C. , Zhou, F.‐L. , He, D.‐L. , Bai, J.‐R. , Ding, H. , Wang, X.‐Y. , & Nan, K.‐J. (2009). Oxidative stress in depressive patients with gastric adenocarcinoma. International Journal of Neuropsychopharmacology, 12, 1089–1096. 10.1017/S1461145709000091 [DOI] [PubMed] [Google Scholar]
- Wigner, P. , Czarny, P. , Synowiec, E. , Bijak, M. , Białek, K. , Talarowska, M. , … Sliwinski, T. (2018). Variation of genes involved in oxidative and nitrosative stresses in depression. European Psychiatry, 48, 38–48. 10.1016/j.eurpsy.2017.10.012 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2015). Sixty‐fifth world health assembly 2015. Retrieved from http://www.who.int/mediacentre/events/2012/wha65/journal/en/index4.html. Accessed in October 10, 2017.
- Xie, X. , Chen, Y. , Ma, L. , Shen, Q. , Huang, L. , Zhao, B. , … Fu, Z. (2017). Major depressive disorder mediates accelerated aging in rats subjected to chronic mild stress. Behavioral Brain Research, 329, 96–103. 10.1016/j.bbr.2017.04.022 [DOI] [PubMed] [Google Scholar]
- Yesavage, J. A. , Brink, T. L. , Rose, T. L. , Lum, O. , Huang, V. , Adey, M. , & Leirer, V. O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Zeng, M. Y. , Miralda, I. , Armstrong, C. L. , Uriarte, S. M. , & Bagaitkar, J. (2019). The roles of NADPH oxidase in modulating neutrophil effector responses. Molecular Oral Microbiology, 34, 27–38. 10.1111/omi.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Chen, Y. , & Ma, L. (2018). Depression and cardiovascular disease in elderly: Current understanding. Journal of Clinical Neuroscience, 47, 1–5. 10.1016/j.jocn.2017.09.022 [DOI] [PubMed] [Google Scholar]
- Zuccarella‐Hackl, C. , von Känel, R. , Thomas, L. , Kuebler, P. , Schmid, J.‐P. , Mattle, H. P. , … Wirtz, P. H. (2016). Higher macrophage superoxide anion production in coronary artery disease (CAD) patients with Type D personality. Psychoneuroendocrinology, 68, 186–193. 10.1016/j.psyneuen.2016.02.031 [DOI] [PubMed] [Google Scholar]


