Abstract
Background
Racial/ethnic minority populations in the United States are consistently underrepresented in genetic research. Large‐scale public participation is required to ensure discoveries from precision medicine research are applicable to everyone. To evaluate views toward and facilitators of participation among minority populations in the United States, we conducted a systematic review of literature.
Methods
Six databases were searched for articles published from 2005 to 2018 assessing minority populations’ views and/or willingness to participate in genetic research. A thematic framework was applied to extracted data to synthesize findings, and the Socio‐Ecological Model was used to evaluate papers.
Results
Review of 2,229 titles and abstracts identified 27 papers (n = 8 qualitative, n = 19 quantitative). Themes included knowledge of genetics, engagement in research, facilitators and barriers to participation, and cultural considerations. Understanding of genetics was low, yet the majority of participants were willing to participate in genetic research among all populations included in the literature (range: 57%–97%). Recommendations for research included utilizing community‐based participatory approaches, evaluating participants’ informational needs, incentivizing participation, and providing direct benefits (e.g., genetic test results).
Conclusion
Results could influence future study designs that incorporate all levels of the Socio‐Ecological Model and better meet the needs of underrepresented groups, thereby ensuring precision medicine research findings are applicable to all.
Keywords: genetic research, precision medicine research, racial/ethnic minorities, research participation strategies
This systematic review details the general public's knowledge of genetics, engagement and participation in genetic research, facilitators and barriers to participation in research, and cultural considerations for conducting genetic research with minority populations. We conclude that the majority of participants of all racial and ethnic backgrounds are willing to participate in precision medicine research studies. However, there are many different facilitators and barriers to participation that may not be as simple as previously outlined in the literature and which must be collectively addressed in order to create more inclusive research practices.

1. INTRODUCTION
The era of precision medicine is rapidly approaching, and clinical care plans that are targeted to an individual's unique genetic and environmental information will soon be widely applied in medicine (Adams & Petersen, 2016). Extensive research efforts are ongoing to refine our understanding of the genetic mechanisms of disease, establish methods to target these mechanisms with cutting‐edge treatments, and develop strategies to tailor each therapy to an individual's unique genetic profile and lifestyle (Bentley, Callier, & Rotimi, 2017). In 2007, the Genomics and Personalized Medicine Act was passed by the United States Congress, and research efforts ramped up drastically in January 2015 with the implementation of the Precision Medicine Initiative by President Barack Obama (Adams & Petersen, 2016; Barlas, 2015). However, in order for this innovative movement to become commonplace in modern health care, it is important to consider the general public's understanding and acceptance of precision medicine research. Without large‐scale public participation in research involving genetic testing and precision medicine practices, this new approach to medicine will not be successful.
Wide‐scale public participation in genetic‐based research enables investigators to cultivate databases that capture genetic diversity from a broad range of populations, thereby facilitating the development of effective individualized therapies for people of all racial and ethnic backgrounds (Sirugo, Williams, & Tishkoff, 2019). However, there is a consistent underrepresentation of individuals from racial and ethnic minority groups in the United States in genetic research (Claw et al., 2018; Need & Goldstein, 2009; Popejoy & Fullerton, 2016). A 2009 analysis reported that 96% of genetic studies were conducted on populations of European descent (terminology defined by authors; Need & Goldstein, 2009). Ten years later, Sirugo et al. (2019 reported that the majority (78%) of participants included in genome‐wide association studies are still White. Participation of diverse populations allows researchers to analyze population‐specific sequence variation that is linked to geographic ancestry and can influence disease presentation, medication response, diagnostic accuracy, and response to therapy (Buseh, Underwood, Stevens, Townsend, & Kelber, 2012; Sirugo et al., 2019; Spratt et al., 2016). Lack of inclusion of diverse populations in genetic research will likely lead to the inability to accurately translate findings from precision medicine research from White populations, in which the research was conducted, to racial and ethnic minority populations that are underrepresented in research. This might subsequently lead to disparities in precision medicine‐based clinical care for non‐White communities in the United States.
Mistrust in healthcare providers and systems as a result of historical malpractices and exploitation of racial/ethnic minority groups in medicine and research is well‐documented and has often been generalized as the primary prohibiting factor to participation in research among minority populations (Corbie‐Smith, Thomas, Williams, & Moody‐Ayers, 1999; Keller, 2006; McDonald et al., 2014). It is now recognized that the reasons for lower research participation rates among individuals from racial/ethnic minority groups are multifaceted and cannot be fully explained by medical mistrust (Bentley et al., 2017; Sheppard et al., 2018). Considering the multiple levels of influence in society that impact participation rates, such as those described in the Socio‐Economic Model (intrapersonal, interpersonal, organizational community, and policy), might be important to understand barriers to participation beyond medical mistrust (McLeroy, Bibeau, Steckler, & Glanz, 1988; Richard, Potvin, Kishchuk, Prlic, & Green, 1995). For example, studies have reported that lack of access and awareness, fear of discrimination, concerns about privacy and misuse of information, and differences in cultural beliefs contribute to the lack of diversity in precision medicine research (Bates, Lynch, Bevan, & Condit, 2005; Diaz, Mainous, Gavin, & Wilson, 2014; Glenn, Chawla, & Bastani, 2012; Yancey, Ortega, & Kumanyika, 2006). It is imperative to assess the perspectives and attitudes of individuals from racial/ethnic minority groups in order to provide insights into study design and recruitment strategies that will assist in inclusion of these groups in precision medicine research. Increasing participation of underrepresented groups in genetic research represents a first step toward ensuring that the advancements made by precision medicine are equally beneficial to all racial and ethnic groups, not just individuals from European backgrounds.
This systematic review attempts to fill the existing gap in the literature regarding the current understanding of attitudes and perspectives of racial/ethnic minority populations toward precision medicine research. To address what is already known about the views of racial/ethnic minority populations toward genetic testing and genetic research, we conducted a systematic review of the literature to answer the major research question: How do views and attitudes toward precision medicine research differ between minority groups, including African Americans, Asian Americans, and Hispanic individuals, compared with White individuals in the general population? We aim to bolster understanding and appreciation of minority perspectives toward genetic‐based research, identify areas of research that are currently lacking, and provide recommendations that can be incorporated into future precision medicine research efforts with racial/ethnic minority populations.
2. METHODS
2.1. Editorial policies and ethical considerations
This research did not require approval from an ethics committee.
2.2. Inclusion and exclusion criteria
The protocol for this review was registered in the PROSPERO International Prospective Register of Systematic Reviews from the National Institute for Health Research, protocol number CRD42019119677. Comprehensive search strategies were developed based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines and Cochrane guidelines to retrieve articles relating to minority groups’ and majority groups’ attitudes toward precision medicine research. Studies were limited to those that were conducted in the United States to control for the effect of different healthcare systems, variations in legal protections for genetic testing and research, and sociocultural differences among various populations outside of the United States. Precision medicine research was defined as research involving precision or personalized medicine, genomic‐ and genetic‐based medicine, research use of DNA, or genetic testing that was specifically performed in a research setting. Primary outcomes were defined as the following: study participants’ views, attitudes, beliefs, perspectives, opinions, knowledge, understanding, willingness, and/or likelihood of participating in precision medicine research. Minority groups were defined as Black, African American, African, Hispanic, Latino/a, Asian, Asian American, South Asian, Asian Indian, Native American, American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, immigrant, refugee, mixed race, mixed ancestry, bi‐racial, multiracial, and/or interracial participants. Majority groups were defined as Whites, Caucasians, and/or participants of Northern European descent.
Studies were excluded from analysis if they met any of the following criteria: not written in English language; not based in the United States; published before 2005; animal or in vitro studies; not original research studies (case reports, review articles, meta‐analyses, commentaries, conference proceedings, abstract‐only); participants who were not a member of the general public (such as healthcare providers); abstracts explicitly stating >90% of study participants were from majority groups; studies of direct‐to‐consumer or employment‐related genetic testing; studies in which the majority of outcome measures were associated with genetic counseling rather than genetic testing. Studies were limited to the 2005–2018 timeframe due to the growing initiatives that began in 2005 to increase diversity in genetic research (FDA, 2005, 2013). The decision to exclude studies with sample populations of more than 90% White participants was implemented to avoid including findings that may too heavily represent majority group opinions.
2.3. Systematic literature search
Database searches were performed on 12 July 2018 in six databases: Medline via Ovid, EMBASE via Ovid, PsycINFO via Ovid, CINAHL via EBSCO, Web of Science, and Scopus. Search language was adapted to individual database formats. The complete search strategy for Medline is shown in Appendix A. Two thousand three hundred seven citations were returned by search queries in the six databases. Search results were downloaded into EndNote citation management software for deletion of duplicates. After deduplication, 2,229 articles were loaded into Rayyan QCRI for screening.
2.4. Manuscript selection process
The inclusion and exclusion criteria established before conducting the database searches were applied to the final search yield (n = 2,229 articles). The primary author (E.F.) used the criteria to screen all titles and abstracts in Rayyan QCRI. To ensure general agreement in the approach taken by the primary reviewer, an independent reviewer (R.E.) screened 50% of all articles before making final inclusion/exclusion decisions. Disagreements were resolved through discussion with a third reviewer (H.Z.). Of the 50% of articles that were screened by an independent reviewer, there was a 3.76% conflict rate for inclusion/exclusion decisions (42 of 1,116 articles). The majority of discrepancies between inclusion/exclusion decisions between reviewers stemmed from one of three issues: differing perspectives of whether the article was a review or an original research study; confusion regarding the population of participants; and differentiation between genetic testing versus genetic counseling. There were zero articles that the reviewers (E.F., R.E., H.Z.) were unable to agree upon during the abstraction process.
Following review of titles and abstracts, 158 publications met inclusion criteria and were assessed for eligibility. Of these publications, 124 studies were excluded because they were conducted in a clinical setting rather than a research setting (i.e., studies that performed genetic testing for clinical management purposes rather than within the context of a voluntary research study). Thirty‐four publications were included for full‐text review by the primary author (E.F.). Of the 34 publications that were eligible for inclusion in this precision medicine research systematic review, seven articles were excluded upon further review of full text due to the demographics of study participants not meeting inclusion criteria (study participants did not consist of underrepresented minority groups) or the study not being conducted within the United States, which was not apparent from the abstracts. Reasons for exclusion at this stage were explicitly noted (Figure 1). In cases of doubt, the decision was discussed with author H.Z. before proceeding with final decisions.
Figure 1.
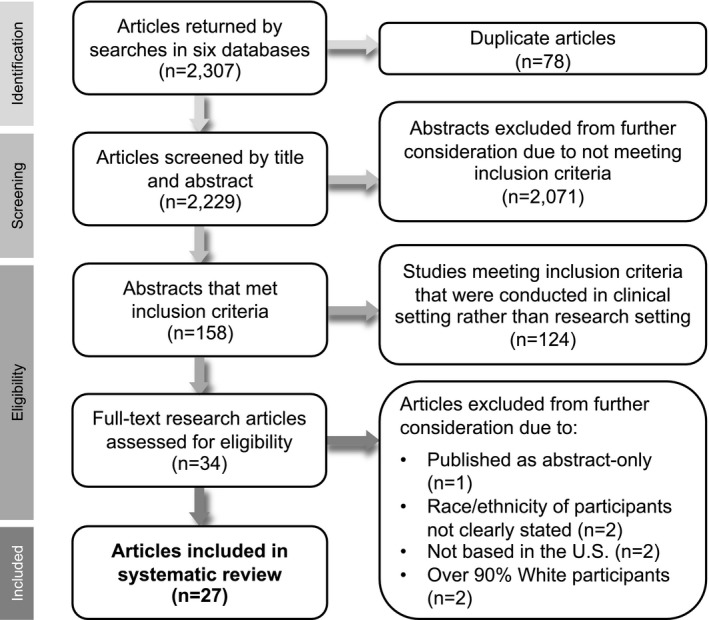
Flowchart of systematic review process. Visual representation of the process of selecting the 27 publications included in this systematic review from the 2,307 abstracts returned by the literature search
2.5. Data extraction and synthesis
Once the final group of publications was established (n = 27), the following data were systematically extracted into tables from each article: study aims, methods, participant demographics, results, themes, discussion, conclusions, and future research/recommendations for practice. Individual study biases were also collected, including those that were explicitly stated by the authors and those that were noted externally by reviewer E.F.
Themes were synthesized from each included paper based on the guidelines described here. First, each manuscript was read in‐depth, noting the major themes and outcomes reported in each paper and developing a thematic framework to encompass all identified outcomes. This thematic framework was then applied to the extracted data and used to interpret and summarize the data. Authors H.Z. and R.P. acted as arbiters throughout the process, providing professional opinion and assisting with consensus regarding extraction, themes, and tables. Areas of disagreement during data synthesis were approached through discussion and, if required, by revisiting the source material until a consensus was achieved.
2.6. Quality assessment and application of theoretical framework
Qualitative papers (n = 8) were assessed using the Critical Appraisal Skills Programme (CASP) for Qualitative Research to examine the reliability and relevance of the studies (CASP, 2018). Quantitative papers (n = 19) were assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tool for Analytical Cross‐Sectional Studies to analyze the methodological quality and potential for bias in the studies (Moola et al., 2017). Two items on the JBI Critical Appraisal Tool, regarding measurement of the exposure and the condition, were not assessed because they were not relevant to this selection of quantitative studies. Author R.E. performed both the CASP and JBI quality assessments for all qualitative and quantitative papers.
Author E.F. applied the Socio‐Ecological Model (SEM) theoretical framework to the introduction, study design and methods, results, and discussion sections of each study in order to characterize the various sociocultural and environmental factors that were addressed by each publication.
3. RESULTS
3.1. Overview
Of the 2,229 abstracts that were screened for inclusion, 34 full‐text articles were assessed for eligibility for this systematic review focused on views of minority populations toward precision medicine research. Of the eligible publications, 27 studies met inclusion criteria and were evaluated for data extraction, quality assessment, and thematic analysis (Figure 1). Out of over 146,000 cumulative individuals included in the 27 studies, there were 102,421 White participants, 15,081 African American participants, 11,877 Asian American participants, approximately 4,500 Hispanic participants, and over 11,500 individuals in “other race” categories.
Five major themes were delineated from the included 27 articles: (a) knowledge and understanding of genetic testing and research; (b) engagement and participation in genetic research; (c) practical considerations that facilitate participation in genetic research; (d) concerns and barriers to participation in genetic testing and research; (e) cultural‐ and community‐specific considerations in genetic research. Four studies addressed all five themes (Frazier, Calvin, Mudd, & Cohen, 2006; Hull et al., 2008; Murphy & Thompson, 2009; Pettey et al., 2015). The objectives, sample demographics, and major findings for all publications included in the systematic review are summarized in Table 1.
Table 1.
Overview of included studies and thematic results
| Study | Design and study goals | Population | THEME 1: Knowledge and understanding of genetics | THEME 2: Engagement and participation in research | THEME 3: Practical considerations that facilitate participation | THEME 4: Concerns and barriers to participation | THEME 5: Cultural‐ and community‐specific considerations | ||
|---|---|---|---|---|---|---|---|---|---|
| Subtheme A: Motivations | Subtheme B: Willingness | Subtheme C: Predictors | |||||||
| Aagaard‐Tillery (2006) |
Design: Quantitative; in‐person questionnaire Goals: To assess whether reproductive‐aged women enrolling in a genetic study would demonstrate a bias in their willingness to participate in a repository for future genetic research |
African Americans (n = 1,727), Hispanics (n = 1,594), Whites (n = 1,576), Asian Americans (n = 40), Native Americans (n = 10), “other” (n = 55) | – | – |
|
|
|
– | – |
| Akinleye (2011) |
Design: Quantitative; telephone and in‐person surveys; randomization into two study arms Goals: To examine differences between African Americans and Whites in knowledge, attitudes, and motivations regarding genetic susceptibility testing for Alzheimer's disease |
Whites (n = 249), African Americans (n = 64) |
|
|
– |
|
– |
|
– |
| Almeling (2014) |
Design: Quantitative; cross‐sectional online survey Goals: To examine public opinion on policy issues in genetics, including federal spending on genetic research, the perceived significance of genetic nondiscrimination laws, and clinicians’ involvement in direct‐to‐consumer genetic testing |
Whites (n = 1,584), African Americans (n = 206), Hispanics (n = 172), “other” (n = 138) | – | – |
|
|
|
– | – |
| Bloss et al. (2018) |
Design: Quantitative; cross‐sectional online survey Goals: To analyze the demographics of a sample of blood bank donors to inform on whether recruitment of blood bank donors for precision medicine research would produce participants representative of the United States. |
Whites (n = 85,952), Asian Americans and/or Pacific Islanders (n = 9,234), African Americans (n = 4,973), Native Americans (n = 561), “other” (n = 9,407) |
|
– | – |
|
– | – | – |
| Buseh et al. (2014) |
Design: Quantitative; cross‐sectional exploratory survey design Goals: To examine the knowledge of medical genetics, group‐based medical mistrust, and future expectations of genetic research and the influence of these measures on perceived disadvantages of genetic testing among Black African immigrants and/or refugees |
Black African immigrants and refugees (n = 212) |
|
|
– |
|
– |
|
|
| Buseh et al. (2012) |
Design: Qualitative; in‐person focus group interviews Goals: To explore perspectives on genomics research and DNA biobanking among Black African immigrant community leaders and to discern how to best invite and sustain engagement of Black African immigrants in research endeavors. |
Black African immigrant community leaders (n = 27) | – |
|
– | – |
|
|
|
| Cox (2007) |
Design: Quantitative; in‐person survey Goals: To evaluate demographic and psychosocial factors associated with consent for genetic testing among a large sample of African Americans entered in a smoking cessation clinical trial |
African Americans (n = 745) | – | – |
|
|
– | – | – |
| Culhane‐Pera et al. (2017) |
Design: Quantitative; in‐person survey Goals: To assess the feasibility of conducting genomic and pharmaco‐genomic‐based research for genetic variants that are relevant to the Hmong community using a community‐based participatory research process |
Hmong individuals (n = 237) | – | – |
|
– | – |
|
– |
| Dye et al. (2016) |
Design: Quantitative; cross‐sectional online survey Goals: To assess attitudes toward genetic testing and genetic research and to compare attitudes by racial group between African Americans and Whites |
Whites (n = 403), African Americans (n = 56) | – | – |
|
|
– | – | – |
| Frazier et al. (2006) |
Design: Qualitative; semi‐structured focus group interviews Goals: To describe and compare the attitudes, knowledge, and beliefs of older adults from three ethnic groups about genetic testing and genetic research and to determine how these attitudes influence informed consent and decision‐making about participation in genetic research |
African Americans (n = 9), Hispanics (n = 8), Whites (n = 6) |
|
|
– | – |
|
|
|
| Freedman et al. (2013) |
Design: Quantitative; exploratory design; in‐person; and telephone surveys Goals: To examine the views of African Americans and European Americans at risk for end‐stage kidney disease on the value and use of genetic testing in research. |
Whites (n = 66), African Americans (n = 64) | – |
|
|
|
– |
|
– |
| Halbert et al. (2006) |
Design: Quantitative; cross‐sectional structured telephone interviews Goals: To describe intentions to participate in smoking and genetics research and to determine factors that are associated with participation intentions among African American smokers |
African Americans (n = 128) | – |
|
|
|
– |
|
|
| Halbert et al. (2016) |
Design: Quantitative; cross‐sectional telephone survey Goals: To assess the willingness of African Americans to participate in a clinical study for precision medicine and to identify variables that have a significant independent association with participation. |
African Americans (n = 510) | – |
|
|
|
|
|
|
| Hensley Alford et al. (2011) |
Design: Quantitative; prospective observational study (online and in‐person survey and consent process) Goals: To evaluate whether gender, race, and education status influences interest and participation in a multiplex genetic susceptibility test using a population‐based sample of healthy adults |
African Americans (n = 3,740), Whites (n = 2,608) | – | – |
|
|
– | – | – |
| Hooper et al. (2013) |
Design: Quantitative; cross‐sectional in‐person survey Goals: To examine aspects of study design that are important to individuals at risk for Alzheimer's disease in determining whether they would be willing to undergo genetic testing, learn the results, and participate in the study. |
Hispanics (n = 26), Whites (n = 8) 10 of 26 Hispanic participants were living in Mexico |
– |
|
|
– | – |
|
|
| Hull et al. (2008) |
Design: Mixed‐methods telephone interviews (quantitative and qualitative data) Goals: To examine patients’ attitudes and preferences regarding use of anonymous and identifiable clinical samples for genetic research |
Whites (76%), African Americans (16%), Asian Americans (2%), Native Americans (2%), “other” (4%) Reported separately: Hispanic (5%), Not Hispanic (95%) N = 1,193 total |
|
|
|
|
|
|
|
| Jazwinski et al. (2013) |
Design: Quantitative; post hoc analysis of a larger study Goals: To characterize groups of patients who accepted or declined pharmaco‐genomic testing as part of a larger treatment study on hepatitis C |
Whites (n = 2,096), African Americans (n = 547), Hispanics (n = 211), Asian Americans (n = 51), “other” (n = 44) | – | – |
|
|
– | – | – |
| Jenkins et al. (2011) |
Design: Qualitative; in‐person focus group interviews Goals: To understand motivations and barriers to participation in studies that use DNA collection. |
African Americans (n = 32), Whites (n = 5), “other” (n = 1) | – |
|
– |
|
|
|
|
| Kinney et al. (2006) |
Design: Quantitative and qualitative methods Goals: To examine predictors of BRCA1 testing decisions, as well as barriers and facilitators to participation, in male and female members of an African American kindred with a BRCA1 mutation |
African Americans (n = 161) | – |
|
|
|
– |
|
|
| Lakes et al. (2013) |
Design: Qualitative; in‐person focus group interviews Goals: To study maternal preferences for the return of their child's genetic results and to describe the experiences, perceptions, attitudes, and values that are considered when individuals from different racial and cultural backgrounds consider participating in genetic research |
Whites (49%), Asian Americans (21%), Pacific Islanders (6%), Iranians (4%), African American and White (2%), Native American/Alaskan and White (2%), no response or “other” (17%) Reported separately: Hispanic (28%), not Hispanic (72%) N = 50 total |
– |
|
– | – |
|
|
|
| Murphy and Thompson (2009) |
Design: Qualitative; in‐person focus group interviews Goals: To explore Black participants’ attitudes toward and willingness to participate in genetic studies of psychiatric disorders |
African Americans (n = 18), Whites (n = 8) |
|
|
|
‐ |
|
|
|
| Nodora et al. (2016) |
Design: Quantitative; in‐person survey, randomized into two study arms Goals: To assess Hispanic individuals’ willingness to donate biospecimens for research and determine whether the type of healthcare provider approaching the participants impacts rates of consent |
Hispanic women (n = 140) |
|
– |
|
|
– | – | – |
| Pettey et al. (2015) |
Design: Qualitative; in‐person semi‐structured individual interviews Goals: To examine the feasibility of developing pedigrees and to explore perceptions of family history and genetic research among African Americans with hypertension |
African Americans (n = 29) |
|
|
|
– |
|
|
|
| Rew et al. (2010) |
Design: Qualitative; semi‐structured individual interviews Goals: To determine levels of knowledge and approaches to decision‐making regarding genetics and genetic testing in adolescents and their parents |
Whites (n = 16), Hispanics (n = 8), Asian Americans (n = 5), African Americans (n = 4) |
|
|
– |
|
|
|
– |
| Sanderson et al. (2017) |
Design: Quantitative; randomized three‐arm mailed survey design Goals: To assess willingness to participate in a biobank using different consent and data sharing models and to examine perceived benefits, concerns, and information needs regarding participation in biobank research |
Whites (51%), Asian Americans (17%), African Americans (12%), Native American or Alaska Natives (5%), Native Hawaiian or Pacific Islanders (1%), “other” (10%), more than one race (3%) Reported separately: Hispanic (18%), not Hispanic (82%) N = 13,000 total |
– |
|
|
|
|
|
|
| Sheppard et al. (2018) |
Design: Quantitative; telephone survey and mailed specimen kit Goals: To understand sociocultural, health care, and clinical factors that impact women's participation in genetic research in Black and White breast cancer survivors |
Whites (n = 391), African Americans (n = 155), Asian Americans and “other subgroups” (n = 23) | – |
|
|
|
– | – | – |
| Simon et al. (2017) |
Design: Qualitative; semi‐structured focus group interviews Goals: To describe attitudes toward, and barriers and facilitators of, participation in biospecimen research among Chinese older women |
Chinese women (n = 47) | – |
|
– | – |
|
|
|
All quantitative findings were significant (*p ≤ .05 and **p ≤ .01) unless otherwise stated. Odds ratio (OR).
3.2. Quality assessment and theoretical framework analysis
All publications had quality assessment scores of at least 6 out of 9 possible points (average: 7.1 points; range: 6 to 8 points) using the Critical Appraisal Skills Programme for qualitative studies (n = 8 papers) and at least 3 out of 6 points possible (average: 4.8 points; range 3 to 6 points) using the Joanna Briggs Institute Critical Appraisal Tool for quantitative studies (n = 19 papers; Supporting Information). Two hundred eighty‐seven participants were included in the qualitative studies, and 146,435 participants were included in the quantitative studies.
When the Socio‐Ecological Model framework was applied to assess the sociocultural and environmental factors addressed in each publication, the vast majority of studies were found to have focused on the organizational/institutional and community influences on participants and study results (Supporting Information). Only 7 of the 27 publications addressed implications of their findings at the policy level (Almeling & Gadarian, 2014; Buseh, Kelber, Millon‐Underwood, Stevens, & Townsend, 2014; Buseh et al., 2012; Dye et al., 2016; Hull et al., 2008; Rew, Mackert, & Bonevac, 2010; Sanderson et al., 2017), which could represent a lack of recognition or focus on the higher‐level changes that are required to increase minority participation in genetic research. Three papers addressed all five levels of influence in the Socio‐Ecological Model, none of which overlapped with the four studies assessing all themes described in this systematic review (Buseh et al., 2014; Dye et al., 2016; Sanderson et al., 2017).
Publications that addressed multiple levels of the SEM model in both study design and in discussion of study findings recognized the various layers of influence in society that could impact an individual's perspectives of and willingness to participate in genetic research. For example, Buseh et al. (2014) trained individuals known and trusted in the community as field interviewers (thereby increasing trust and establishing relationships at the interpersonal/social level), conducted the interviews at a mutually agreed upon place and time with all participants (to increase access to participation and reduce barriers at the organizational/institutional level), partnered with a community‐based organization (CBO) and requested permission from the executive director of the CBO before study initiation (respectful engagement at the community level), and stated that it is important for healthcare professionals to engage with diverse racial/ethnic populations in order to develop culturally relevant policies to address public concerns toward genetics initiatives (thereby calling for changes at the policy level).
3.3. Theme 1: Knowledge and understanding of genetic testing and research
Knowledge and understanding of genetics were typically defined using assessments of health literacy, familiarity with genetics terms, and participants’ interpretations of the definition of genetics. Nine of 27 articles assessed participants’ knowledge and understanding of genetics topics (Akinleye et al., 2011; Bloss et al., 2018; Buseh et al., 2014; Frazier et al., 2006; Hull et al., 2008; Murphy & Thompson, 2009; Nodora et al., 2016; Pettey et al., 2015; Rew et al., 2010). Overall, knowledge and understanding of genetics was reportedly limited among participants of all races and ethnicities, including White participants, in the general population in these nine articles. Many participants had heard of genetic‐related topics such as genetic testing, genetic research, or the Human Genome Project, but few had a comprehensive understanding of these topics. Participants’ definitions of genetics often included concepts of inheritance, family history of disease, susceptibility and risks for developing disease, and beliefs about the origins of disease.
One of these nine studies specifically reported on differences in knowledge and understanding of genetics between White participants and other racial/ethnic groups (Akinleye et al., 2011). Akinleye et al. reported that African American participants had lower knowledge of Alzheimer's disease and genetic testing compared with White participants in their sample. Two studies examined how participants acquired knowledge of genetics by inquiring about sources of information; the primary resources for genetic information included healthcare providers and organizations, the Internet, and the media (Frazier et al., 2006; Rew et al., 2010).
3.4. Theme 2: Engagement and participation in genetic research
Overall engagement with genetic research was divided into three subthemes and assessed participants’ motivations for participation (Subtheme A), willingness to participate (Subtheme B), and predictors of participation in genetic research (Subtheme C). Motivations (Subtheme A) included attitudes toward research, perceived benefits of participating, and reasons to participate in genetic research. Willingness to participate (Subtheme B) was defined as participants’ reported intentions to participate in research, interest in receiving results from genetic testing, and actual uptake of genetic testing or consent for research. Predictors of participation (Subtheme C) included factors that were either positively or negatively correlated with willingness to participate in genetic research. Null findings were also included under this subtheme, such as variables that were not found to be correlated with participation rates. All 27 articles assessed at least one of these factors associated with engagement and participation.
3.4.1. Subtheme A: Motivations for participation
The majority of participants believed genetic research produces beneficial outcomes to society and that there are personal benefits to individuals who participate in genetic research (Akinleye et al., 2011; Buseh et al., 2014, 2012; Frazier et al., 2006; Freedman et al., 2013; Halbert, Gandy, Collier, & Shaker, 2006; Halbert, McDonald, Vadaparampil, Rice, & Jefferson, 2016; Hooper et al., 2013; Hull et al., 2008; Jenkins et al., 2011; Kinney et al., 2006; Lakes et al., 2013; Murphy & Thompson, 2009; Pettey et al., 2015; Rew et al., 2010; Sanderson et al., 2017). The most often cited reasons for participating in genetic testing and research were to learn more information, to contribute to the development of medical treatments and prevention of disease, and to positively impact future generations. Participants often cited benefits of participating for themselves, such as using the information obtained from testing to improve health, seek treatment, or for future planning. Participants recognized that genetic research is useful for the diagnosis and treatment of disease and felt that their participation could benefit future generations.
3.4.2. Subtheme B: Willingness to participate
Fifteen articles reported that the majority (defined as over 50%; range 57%–97%) of respondents in their sample were willing to participate in genetic testing or research and were willing to receive results from testing. This applied to studies that examined reported interest and intentions to participate (n = 7; Freedman et al., 2013; Halbert et al., 2006; Hooper et al., 2013; Hull et al., 2008; Murphy & Thompson, 2009; Pettey et al., 2015; Sanderson et al., 2017), as well as studies that measured definitive consent for genetic testing and biospecimen collection for research (n = 8; Aagaard‐Tillery et al., 2006; Cox et al., 2007; Culhane‐Pera et al., 2017; Hensley Alford et al., 2011; Jazwinski et al., 2013; Kinney et al., 2006; Nodora et al., 2016; Sheppard et al., 2018), and there were no substantial differences between the two types of studies. Approximately half of the research scenarios in which these high consent rates were found involved consent for unrestricted access to participants’ samples and health information for future use by other researchers (Aagaard‐Tillery et al., 2006; Cox et al., 2007; Culhane‐Pera et al., 2017; Nodora et al., 2016; Pettey et al., 2015; Sanderson et al., 2017; Sheppard et al., 2018).
Three studies reported consent rates lower than 50% in their sample (Halbert et al., 2016; Hensley Alford et al., 2011; Jazwinski et al., 2013). Halbert et al. reported a 31% intention to participate rate among African American participants (n = 150) for a hypothetical government‐sponsored study with open data sharing and no option for participants to receive individual results (Halbert et al., 2016). Jazwinski et al. and Hensley Alford et al. reported lower participation rates among a subset of their participants in their studies measuring actual consent; namely 41% of Asian American participants (n = 51; Jazwinski et al., 2013) and 30% of African American participants (n = 3,740; Hensley Alford et al., 2011) were willing to consent for genetic testing.
3.4.3. Subtheme C: Predictors of participation
Factors that clearly predicted a higher likelihood of participating in research included greater perceived benefits and values to participating in research (Bloss et al., 2018; Halbert et al., 2006; Sanderson et al., 2017), fewer concerns about the limitations and risks of research (Halbert et al., 2006; Sanderson et al., 2017), greater willingness to share personal health information (Bloss et al., 2018), fewer informational needs (Sanderson et al., 2017), and higher satisfaction with healthcare providers (Sheppard et al., 2018). Less favorable views about the value of research predicted a lower willingness to participate in research (Bloss et al., 2018).
Importantly, while some studies reported a correlation between being a member of a racial/ethnic minority group (Hispanic, African American, or Asian American) and decreased willingness to participate (n = 5; Aagaard‐Tillery et al., 2006; Bloss et al., 2018; Dye et al., 2016; Hensley Alford et al., 2011; Sanderson et al., 2017), other studies found that race and ethnicity were not predictors of consent for research (n = 4; Cox et al., 2007; Freedman et al., 2013; Jazwinski et al., 2013; Sheppard et al., 2018). Similarly, the findings were conflicting regarding whether higher mistrust in researchers and healthcare systems was a negative predictor of participation (Halbert et al., 2016; Sanderson et al., 2017) or did not correlate with willingness to participate (Sheppard et al., 2018). Higher levels of genetics knowledge and precision medicine literacy were found to be positively associated with willingness to participate in two studies (Bloss et al., 2018; Kinney et al., 2006), while Sheppard et al. reported that consent for research did not vary according to level of healthcare literacy (Sheppard et al., 2018). Two studies reported that greater concerns about data privacy, control, and ownership were associated with decreased likelihood of participation (Bloss et al., 2018; Sanderson et al., 2017), while a study by Halbert et al. did not uphold this finding (Halbert et al., 2016). Additionally, two publications reported a higher willingness to participate in individuals who were less religious (Sanderson et al., 2017; Sheppard et al., 2018), while another study did not support the association of religiosity with lower participation rates (Kinney et al., 2006).
There were clear associations surrounding participants’ preferences for research communication practices and the use of biospecimens that had already been donated to research. African Americans and Hispanic participants were more likely to prefer to discard their sample after initial study use than White participants (Aagaard‐Tillery et al., 2006). African Americans were less likely to agree to subsequent use of their biospecimens in future research compared with White participants (Aagaard‐Tillery et al., 2006). Participants who were more private and less trusting of researchers were more likely to want to be informed of future research utilizing their sample (Hull et al., 2008). Individuals were more likely to prefer permission to be sought for future research use of an anonymously donated sample if they were African American, less religious, more private, or less trusting of researchers (Hull et al., 2008). However, these findings were typically presented by only one study each and were unreplicated among this selection of articles.
3.5. Theme 3: Practical considerations about studies that facilitate participation in research
Participants stated preferences for practical aspects of a research study that would increase their willingness to participate in the study. Fifteen articles described facilitating factors (Aagaard‐Tillery et al., 2006; Almeling & Gadarian, 2014; Bloss et al., 2018; Buseh et al., 2012; Frazier et al., 2006; Freedman et al., 2013; Halbert et al., 2016; Hull et al., 2008; Jenkins et al., 2011; Lakes et al., 2013; Murphy & Thompson, 2009; Pettey et al., 2015; Rew et al., 2010; Sanderson et al., 2017; Simon, Tom, & Dong, 2017). The primary facilitators of participation were receiving direct benefits including return of individual results to participants (n = 8; Frazier et al., 2006; Halbert et al., 2016; Hull et al., 2008; Jenkins et al., 2011; Lakes et al., 2013; Murphy & Thompson, 2009; Pettey et al., 2015; Simon et al., 2017), fulfillment of information needs (n = 6; Buseh et al., 2012; Hull et al., 2008; Lakes et al., 2013; Pettey et al., 2015; Rew et al., 2010; Sanderson et al., 2017), and upfront assurance of privacy and confidentiality (n = 3; Buseh et al., 2012; Hull et al., 2008; Pettey et al., 2015). Participants desired direct benefits, such as monetary compensation, free healthcare services, or hospitable accommodation while participating, as well as to receive individual results from testing (Frazier et al., 2006; Halbert et al., 2016; Hull et al., 2008; Jenkins et al., 2011; Lakes et al., 2013; Murphy & Thompson, 2009; Pettey et al., 2015; Simon et al., 2017). Common information needs included wanting to know about the logistics of the study, the validity of the test, the context of the disease being studied, whether future research would utilize the samples, and the conduct of the researchers and institutions involved in the study (Buseh et al., 2012; Hull et al., 2008; Lakes et al., 2013; Pettey et al., 2015; Rew et al., 2010; Sanderson et al., 2017).
Other facilitators focused on preferences and expectations about the informed consent process, study materials, or return of results process (Frazier et al., 2006; Jenkins et al., 2011; Lakes et al., 2013; Sanderson et al., 2017). Participants in three studies cited a preference for researchers to ask permission before using their donated sample in future research (Aagaard‐Tillery et al., 2006; Buseh et al., 2012; Hull et al., 2008). Several concrete methods to improve participation rates were also mentioned, such as reminder phone calls to participants, spreading awareness about ongoing studies through word of mouth, allowing alternative specimen types other than blood, and increasing clinician involvement in testing (Almeling & Gadarian, 2014; Jenkins et al., 2011; Murphy & Thompson, 2009; Simon et al., 2017).
3.6. Theme 4: Concerns and barriers to participation in genetic testing and research
Concerns about participation, reasons not to test or receive results from testing, and factors that presented barriers to participation in research were assessed by 18 publications (Akinleye et al., 2011; Buseh et al., 2014, 2012; Culhane‐Pera et al., 2017; Frazier et al., 2006; Freedman et al., 2013; Halbert et al., 2006, 2016; Hooper et al., 2013; Hull et al., 2008; Jenkins et al., 2011; Kinney et al., 2006; Lakes et al., 2013; Murphy & Thompson, 2009; Pettey et al., 2015; Rew et al., 2010; Sanderson et al., 2017; Simon et al., 2017). The most commonly cited reasons not to participate in genetic testing or research included privacy and confidentiality concerns (n = 7; Buseh et al., 2012; Halbert et al., 2016; Hull et al., 2008; Murphy & Thompson, 2009; Pettey et al., 2015; Sanderson et al., 2017; Simon et al., 2017), use of participants’ genetic information for other research purposes that were not consented for or were undesirable (n = 7; Buseh et al., 2012; Halbert et al., 2006, 2016; Hull et al., 2008; Jenkins et al., 2011; Pettey et al., 2015; Sanderson et al., 2017), concerns about insurance or employment discrimination (n = 5; Akinleye et al., 2011; Buseh et al., 2014, 2012; Frazier et al., 2006; Pettey et al., 2015), concerns about risks or harms of the study procedure (n = 5; Hooper et al., 2013; Jenkins et al., 2011; Murphy & Thompson, 2009; Pettey et al., 2015; Simon et al., 2017), and individual results not being made available to participants (n = 5; Culhane‐Pera et al., 2017; Halbert et al., 2016; Jenkins et al., 2011; Lakes et al., 2013; Simon et al., 2017).
Other barriers to testing included anticipation of negative emotional or interpersonal consequences, doubts about the validity of the testing, and lack of actionable steps to improve health. Three studies reported disapproval of research for profit and patenting of findings as a barrier to participation (Buseh et al., 2012; Halbert et al., 2016; Sanderson et al., 2017).
3.7. Theme 5: Cultural‐ and community‐specific considerations about genetic research
Cultural‐ and community‐specific considerations about genetic research (Theme 5) often involved facilitators (Theme 3) and barriers (Theme 4) to participation but were specifically defined as current beliefs, attitudes, or actions that were likely influenced by historical system‐wide practices affecting certain groups of people (organizational influences) or cultural‐ and community‐specific beliefs (community/group influences) about genetics and research. Fifteen of 27 articles reported results that addressed cultural, community, and organizational/institutional considerations about genetic research (Buseh et al., 2014, 2012; Frazier et al., 2006; Halbert et al., 2006, 2016; Hooper et al., 2013; Hull et al., 2008; Jazwinski et al., 2013; Jenkins et al., 2011; Kinney et al., 2006; Lakes et al., 2013; Murphy & Thompson, 2009; Pettey et al., 2015; Sanderson et al., 2017; Sheppard et al., 2018; Simon et al., 2017).
The majority of participants’ organizational‐ and community‐influenced considerations about research constituted barriers to participation. Overall, in this selection of articles, the barriers included concerns about being viewed negatively or ruining participants’ reputations in the community if test results were perceived as negative, apprehension about sharing health information in cultures where this is discouraged or stigmatized, cultural beliefs that prevented participation such as the desire for the body to remain whole upon death (negating the ability to provide biospecimen), group‐based medical mistrust in providers and healthcare systems, fear of genetics being used to socially oppress certain groups, concerns about government use of participants’ biological material, doubts that research findings would be beneficial to minority communities, and lack of physical access, awareness, and logistical constraints to participation in research (Buseh et al., 2014, 2012; Frazier et al., 2006; Halbert et al., 2006, 2016; Kinney et al., 2006; Lakes et al., 2013; Murphy & Thompson, 2009; Pettey et al., 2015; Simon et al., 2017).
Cultural considerations that facilitated participation in research included trusting a research study if a member of the community was involved in the research team, positive feelings of trust in medical researchers, and beliefs that research findings would benefit minority communities (Buseh et al., 2012; Hull et al., 2008; Sanderson et al., 2017; Simon et al., 2017).
3.8. Population‐specific findings
Ten studies assessed the views and attitudes of participants from racial/ethnic minority populations only (Buseh et al., 2014, 2012; Cox et al., 2007; Culhane‐Pera et al., 2017; Halbert et al., 2006, 2016; Kinney et al., 2006; Nodora et al., 2016; Pettey et al., 2015; Simon et al., 2017), whereas the remainder of the articles (n = 17) included individuals from majority and minority groups.
African American participants were the most studied population among the publications included in this systematic review (n = 22; Buseh et al., 2012; Frazier et al., 2006; Murphy & Thompson, 2009; Sheppard et al., 2018)(Aagaard‐Tillery et al., 2006; Akinleye et al., 2011; Almeling & Gadarian, 2014; Bloss et al., 2018; Buseh et al., 2014; Cox et al., 2007; Dye et al., 2016; Freedman et al., 2013; Halbert et al., 2006, 2016; Hensley Alford et al., 2011; Hull et al., 2008; Jazwinski et al., 2013; Jenkins et al., 2011; Kinney et al., 2006; Pettey et al., 2015; Rew et al., 2010; Sanderson et al., 2017). Five studies reported that African American participants were less likely to participate in genetic testing or research (Aagaard‐Tillery et al., 2006; Bloss et al., 2018; Dye et al., 2016; Hensley Alford et al., 2011; Sanderson et al., 2017), while four studies did not support an association between African American race and likelihood of participating in research (Cox et al., 2007; Freedman et al., 2013; Jazwinski et al., 2013; Sheppard et al., 2018).
Ten studies included Asian Americans in their study populations (Aagaard‐Tillery et al., 2006; Bloss et al., 2018; Culhane‐Pera et al., 2017; Hull et al., 2008; Jazwinski et al., 2013; Lakes et al., 2013; Rew et al., 2010; Sanderson et al., 2017; Sheppard et al., 2018; Simon et al., 2017). High consent rates among Asian American participants were reported by Culhane‐Pera et al., and no difference between participation rates among Asian Americans and participants of other races/ethnicities was reported by Jazwinski et al. and Sheppard et al. However, Bloss et al. reported that Asian American participants were less likely than other groups to indicate interest in a precision medicine research study.
Nine studies included Hispanic individuals in their study populations (Aagaard‐Tillery et al., 2006; Almeling & Gadarian, 2014; Frazier et al., 2006; Hooper et al., 2013; Hull et al., 2008; Jazwinski et al., 2013; Lakes et al., 2013; Nodora et al., 2016; Rew et al., 2010). Two studies reported a lower willingness to participate in genetic testing and research among Hispanic participants compared with other ethnic groups (Aagaard‐Tillery et al., 2006; Bloss et al., 2018). Conversely, Jazwinski et al. described similar rates of participation in research between Hispanic participants and other ethnic groups, and Nodora et al. reported high rates of consent for research among their all‐Hispanic participant population. Of note, three studies did not clearly report the number of Hispanic individuals in their study population (Hull et al., 2008; Lakes et al., 2013; Sanderson et al., 2017).
Nine publications grouped individuals of races/ethnicities other than those specified separately in the study into an “other race” category (Bloss et al., 2018; Hull et al., 2008; Sheppard et al., 2018)(Aagaard‐Tillery et al., 2006; Almeling & Gadarian, 2014; Hull et al., 2008; Jazwinski et al., 2013; Lakes et al., 2013; Sanderson et al., 2017). Overall, details were lacking regarding how this category was defined in each study as well as the specific racial/ethnic composition of the individuals placed within this category. Lakes et al. stated that individuals who did not provide a response for their race/ethnicity constituted a portion of participants included in their “other race” category (Lakes et al., 2013). Only one study specified the race or ethnicity of some participants in the “other race” category (Sheppard et al., 2018).
Five studies included smaller subpopulations of racial or ethnic minority groups, including Native Americans (n = 5 studies; 1,284 cumulative participants; Aagaard‐Tillery et al., 2006; Bloss et al., 2018; Hull et al., 2008; Lakes et al., 2013; Sanderson et al., 2017), Iranians (Lakes et al., 2013), and Native Hawaiians or Pacific Islanders (Sanderson et al., 2017). Two publications included individuals who identified with more than one race/ethnicity (Lakes et al., 2013; Sanderson et al., 2017). It is unknown whether individuals reporting more than one race/ethnicity were included in the “other race” category of the other publications, highlighting the lack of detailed demographic reporting among research studies.
4. DISCUSSION
This systematic review summarizes 13 years’ worth of literature describing the role of race and ethnicity in views toward and willingness to participate in precision medicine research. Most strikingly, the majority of study participants of all races and ethnicities in these studies were interested in undergoing genetic testing and participating in genetic research. Although understanding of genetics was generally low, participants recognized the value of genetic research and described numerous motivations to participate in genetic testing and research, which commonly involved learning more information, contributing to the development of medical advances, and positively impacting future generations. While a few publications reported lower rates of participation among racial/ethnic minority populations and the range of participation rates in studies reporting majority participation was broad (57%–97%), most studies did not support the association between lower participation rates and being a member of a racial/ethnic minority group. Although the type of genetic research varied and the participant pool likely represents community members who might be more willing to participate in research in general, the overall positive view toward participation in genetic research dispels some previous assumptions in the field that individuals from racial/ethnic minorities in the general population are uninterested in genetic research (Corbie‐Smith et al., 1999). This finding is supported by studies of both hypothetical consent (Freedman et al., 2013; Halbert et al., 2006, 2016; Hull et al., 2008; Murphy & Thompson, 2009; Pettey et al., 2015; Sanderson et al., 2017) and actual consent (Aagaard‐Tillery et al., 2006; Cox et al., 2007; Culhane‐Pera et al., 2017; Hensley Alford et al., 2011; Jazwinski et al., 2013; Kinney et al., 2006; Nodora et al., 2016; Sheppard et al., 2018) for participation in genetic testing and research, further emphasizing the validity of this result.
Participants described many practical factors that increased or decreased their likelihood of participating in genetic research, which have direct implications for future research studies that aim to recruit diverse populations. Many of these practical considerations for study design have been described previously (Catz et al., 2005; Claw et al., 2018; Murphy & Thompson, 2009; Swanson & Ward, 1995; Yancey et al., 2006). Viewing these facilitating factors and obstacles to participation through the lens of the Socio‐Ecological Model enables a more comprehensive understanding of the potential explanations that underlie participants’ preferences for genetic research studies. For example, many reported barriers to participation might reflect broader societal or institutional influences on the public's perspectives of or access to genetic testing and research. Kinney et al. reported that only 18% of participants indicated that they would have undergone genetic testing had it not been accessible and available through the research study (Kinney et al., 2006). Therefore, the commonly cited participation barrier of not receiving individual genetic results might indicate a broader institutional barrier to accessing genetic testing services rather than simply an individual preference for genetic results from research. Recognizing the multiple layers of societal influence on reported barriers and facilitators to participation might lead to a greater willingness among researchers to incorporate participants’ preferences into study design.
Several areas for improvement were consistently noted among the 27 studies included in this systematic review. The most frequent study weaknesses involved lack of clear reporting of participant demographics, ambiguous groupings of participants of different races/ethnicities, and limited inclusion of minority populations other than African Americans, Asian Americans, and Hispanic participants. Lack of demographic details was illustrated in particular by several publications that did not specify the racial identity of their Hispanic participants (Hull et al., 2008; Lakes et al., 2013; Sanderson et al., 2017). Hispanic ethnicity is commonly reported in a separate category from race, and in some cases, there was an inability to form conclusions about study findings that were both racially and ethnically specific and comprehensive. For example, there was an inability to determine the number of participants who were Hispanic and White or Hispanic and another racial group such as African American. Also, placing participants into an “other race” category without defining the demographics of this group reduced the authors’ ability to form racial‐ and ethnicity‐specific conclusions about study findings. Additionally, qualitative studies occasionally grouped White participants and individuals from racial/ethnic minorities into the same focus groups (Hooper et al., 2013; Jenkins et al., 2011; Murphy & Thompson, 2009). For instance, although the explicit research goal of Murphy et al. was to explore Black participants’ attitudes toward genetic studies of psychiatric disorders, the authors included eight White participants in their study population and did not differentiate the data by racial group (Murphy & Thompson, 2009). Alternative focus group designs that distinguish or exclude White participants would have enabled better comparison of views between racial/ethnic groups. Lastly, although African Americans, Asian Americans, and Hispanic individuals constitute the majority of racial/ethnic minority populations in the United States, there is a clear need for further research on other racial/ethnic minority populations, including American Indians, Alaskan Native peoples, and multiracial individuals (File, 2018). All of these limitations persisted even in studies that purposefully recruited underrepresented groups, and ultimately reduced the ability to accurately interpret results from studies that had great potential to provide insight into race‐ and ethnicity‐based differences in views toward precision medicine research. There is an urgent need for better demographic reporting in studies that aim to recruit diverse populations.
The limitations of this systematic review are important to acknowledge. Most notably, there are a number of factors that intersect with race and ethnicity which were not investigated by this systematic review; for example, socioeconomic status, age, gender, sexual orientation, disability status, geographical location, national origin/level of acculturation, and political affiliation intersect with race/ethnicity and might impact attitudes toward precision medicine research and willingness to participate in research endeavors (Andersson, Gadarian, & Almeling, 2017; Crenshaw, 1989; Hamilton et al., 2016; Kolor et al., 2017; Murphy & Thompson, 2009). Although many of the 27 included studies that reported high consent rates involved open data sharing research scenarios, examining whether participation rates varied based on type of consent model and data sharing restrictions was not a primary outcome of this systematic review. The authors recognize that further research assessing the non‐White public's views toward open and closed data sharing models would be a valuable addition to the findings from this synthesis of literature. It is also important to note that the research settings varied (briefly outlined in Table 1); variability in each study's population, recruitment process, and setting could impact overall results, and the socioecological factors of each individual study should be taken into consideration when interpreting findings from this review (outlined in Supporting Information). Because both qualitative and quantitative study designs were incorporated, the findings may be partially skewed due to quantitative study designs that utilized researchers’ preselected response options as compared to the open‐ended questions traditional of qualitative study designs. Additionally, individuals who engage in research studies might be more motivated and willing to become involved in genetic research than individuals who did not participate; thus, it is unknown whether the high participation rates reported across all studies are truly reflective of the general population.
The timeframe of this systematic review was broad, encompassing 13 years of literature on the public's views toward genetic research. While the wide timeframe strengthened the reliability of the findings, the public's attitudes toward and awareness of genetic research are dynamic (Henneman et al., 2013). A systematic literature review restricted to a more recent timeframe might reveal interesting variations in trends due to the implementation of the Genetic Information Nondiscrimination Act in 2008 or the recent rise in popularity and prevalence of direct‐to‐consumer genetic testing, which might influence the public's acceptance of and willingness to participate in precision medicine research (Agurs‐Collins et al., 2015). Additionally, because of the broad timeline of this review and the relatively newer use of the term “precision medicine,” the authors acknowledge that focusing on genetic aspects of precision medicine research does not encompass all components of precision medicine research. This systematic review might also be limited by publication bias in that only original research published in a peer‐reviewed journal was accepted for inclusion, as opposed to unpublished dissertations and scientific conference abstracts. However, this analysis did incorporate null findings with respect to race/ethnicity and willingness to participate in precision medicine research, which might partially mitigate negative publication bias. Finally, the authors acknowledge that not all publications that would have met inclusion criteria were ascertained by the six databases searches. This might be due to inherent weaknesses in search criteria but could also represent broader deficiencies in identifiability of publications, such as lack of descriptive key words, which resulted in a reduced ability to detect all relevant publications.
In light of the results of this systematic review, the authors highlight the following research practices and participant preferences that could be incorporated in future precision medicine research studies, many of which have been noted previously (Catz et al., 2005; Claw et al., 2018; Giuliano et al., 2000; Swanson & Ward, 1995; Yancey et al., 2006). These considerations can be directly applied to recruitment and retention strategies, study design and set up, approach to dissemination of results, and efforts to incorporate cultural adaptations in future studies. First, community‐based participatory research was noted by numerous studies to be an ideal standard to uphold when working with racial/ethnic minority populations. Partnering with a community‐based organization or ensuring known members of the community are in shared positions of power within the research team increases trust in the purpose and conduct of the research study. Second, evaluating and meeting the information needs of participants is a practical and important step, not only for obtaining informed consent, but also to increase comfort and likelihood of participating in the study. Informing potential participants about the purpose of the study, measures taken to ensure privacy and confidentiality, and when and how donated samples would be used in future research might appease the most common information needs of participants. Third, incentivizing study participation or ensuring benefits from research are distributed back to participants or their community was repeatedly noted to be important to participants, especially for precision medicine research studies that are unable to return individual genetic test results. Although many participants might be motivated by an altruistic desire to contribute to research endeavors, direct benefits and incentives remain a practical facilitator to participation particularly for communities that might be disadvantaged or less likely to participate in research due to logistical constraints. Fourth, for studies targeting a particular minority community, researchers should put forth effort to evaluate the cultural facilitators and barriers specific to the target community in order to better understand how to implement modifications in study design that would support and respect these cultural preferences. For example, researchers could consider allowing biospecimens other than blood for communities that are averse to donating blood for cultural reasons, as this might be a major drawback to participation for the community and would only require simple modifications for the research study. Without engaging the community through collaborations with community‐based organizations or by ensuring the research team includes trusted members of the community who are involved in the oversight of study design, these cultural preferences might not be revealed and participation rates might be negatively impacted. Culturally competent research practices may be one explanation for the high rates of participation in the genetic research studies that recruited only racial/ethnic minority populations as described here. However, this approach may not be feasible for studies that attempt to recruit a diverse range of participants with many different cultures. Lastly, this analysis of literature exposed the urgent need for better demographic reporting in research studies, initiation of research on other minority populations as well as individuals who are multiracial, and a renewed focus on the interpersonal and policy levels of influenced as defined by the Socio‐Ecological Model when designing a precision medicine research study.
This systematic review revealed high interest in genetic research among all racial/ethnic populations included in the synthesized literature, which might dispute the conception that individuals from minority populations are much less willing to participate in genetic testing or research. While participants expressed specific concerns and preferences for study design and conduct, there is a general recognition of the value and benefits of precision medicine research in the public. These findings expand upon prior research by summarizing additional factors that enable or prohibit participation in genetic research beyond simply medical mistrust and characterize various cultural considerations that should be considered when working with specific populations. Results from this systematic review could be applied to future genetic research studies in order to enhance participation of diverse populations and ultimately ensure that results from precision medicine research are applicable to individuals of all racial and ethnic backgrounds.
Supporting information
ACKNOWLEDGMENTS
Dr. Heather Zierhut's work, although unrelated to this manuscript, has been funded by the National Society of Genetic Counselors. Dr. Zierhut received compensation as a member of the advisory board of GeneMatters, LLC, and owns stock in the company. Riley Esch's work is under the advisement of Dr. Eric A. Hendrickson at the University of Minnesota, who receives funding from the National Institutes of Health, though unrelated to this manuscript. Elena Fisher, Dr. Rebekah Pratt, Megan Kocher, Katie Wilson, and Whiwon Lee declare no potential conflicts of interest. The authors wish to thank Bonnie S. LeRoy, M.S., L.G.C., for her contributions to the review of this manuscript.
Fisher ER, Pratt R, Esch R, et al. The role of race and ethnicity in views toward and participation in genetic studies and precision medicine research in the United States: A systematic review of qualitative and quantitative studies. Mol Genet Genomic Med. 2020;8:e1099 10.1002/mgg3.1099
REFERENCES
- Aagaard‐Tillery, K. , Sibai, B. , Spong, C. Y. , Momirova, V. , Wendel, G. , Wenstrom, K. , … Wapner, R. J. (2006). Sample bias among women with retained DNA samples for future genetic studies. Obstetrics & Gynecology, 108, 1115–1120. 10.1097/01.AOG.0000241536.19539.14 [DOI] [PubMed] [Google Scholar]
- Adams, S. A. , & Petersen, C. (2016). Precision medicine: Opportunities, possibilities, and challenges for patients and providers. Journal of the American Medical Informatics Association, 23, 787–790. 10.1093/jamia/ocv215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agurs‐Collins, T. , Ferrer, R. , Ottenbacher, A. , Waters, E. A. , O’Connell, M. E. , & Hamilton, J. G. (2015). Public awareness of direct‐to‐consumer genetic tests: findings from the 2013 U.S. Health Information National Trends Survey. Journal of Cancer Education, 30, 799–807. 10.1007/s13187-014-0784-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinleye, I. , Roberts, J. S. , Royal, C. D. M. , Linnenbringer, E. , Obisesan, T. O. , Fasaye, G.‐A. , & Green, R. C. (2011). Differences between African American and White research volunteers in their attitudes, beliefs and knowledge regarding genetic testing for Alzheimer’s disease. Journal of Genetic Counseling, 20, 650–659. 10.1007/s10897-011-9377-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeling, R. , & Gadarian, S. K. (2014). Public opinion on policy issues in genetics and genomics. Genetics in Medicine, 16, 491–494. 10.1038/gim.2013.175 [DOI] [PubMed] [Google Scholar]
- Andersson, M. A. , Gadarian, S. K. , & Almeling, R. (2017). Does educational attainment shape reactions to genetic risk for Alzheimer’s disease? Results from a national survey experiment. Social Science & Medicine, 180, 101–105. 10.1016/j.socscimed.2017.03.031 [DOI] [PubMed] [Google Scholar]
- Barlas, S. (2015). Precision medicine initiative aims for a new generation of diagnostics and treatments: But is the promise of genetic targeting overinflated? Pharmacy and Therapeutics, 40, 340–352. [PMC free article] [PubMed] [Google Scholar]
- Bates, B. R. , Lynch, J. A. , Bevan, J. L. , & Condit, C. M. (2005). Warranted concerns, warranted outlooks: A focus group study of public understandings of genetic research. Social Science & Medicine, 60, 331–344. 10.1016/J.SOCSCIMED.2004.05.012 [DOI] [PubMed] [Google Scholar]
- Bentley, A. R. , Callier, S. , & Rotimi, C. N. (2017). Diversity and inclusion in genomic research: Why the uneven progress? Journal of Community Genetics, 8, 255–266. 10.1007/s12687-017-0316-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss, C. S. , Stoler, J. , Schairer, C. E. , Rosenthal, S. B. , Cheung, C. , Rus, H. M. , … Wellis, D. (2018). Characteristics of likely precision medicine initiative participants drawn from a large blood donor population. Health Affairs, 37, 786–792. 10.1377/hlthaff.2017.1591 [DOI] [PubMed] [Google Scholar]
- Buseh, A. , Kelber, S. , Millon‐Underwood, S. , Stevens, P. , & Townsend, L. (2014). Knowledge, group‐based medical mistrust, future expectations, and perceived disadvantages of medical genetic testing: perspectives of Black African immigrants/refugees. Public Health Genomics, 17, 33–42. 10.1159/000356013 [DOI] [PubMed] [Google Scholar]
- Buseh, A. G. , Underwood, S. M. , Stevens, P. E. , Townsend, L. , & Kelber, S. T. (2012). Black African immigrant community leaders’ views on participation in genomics research and DNA biobanking. Nursing Outlook, 61, 196–204. 10.1016/J.OUTLOOK.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Catz, D. S. , Green, N. S. , Tobin, J. N. , Lloyd‐Puryear, M. A. , Kyler, P. , Umemoto, A. , … Wolman, F. (2005). Attitudes about genetics in underserved, culturally diverse populations. Public Health Genomics, 8, 161–172. 10.1159/000086759 [DOI] [PubMed] [Google Scholar]
- Claw, K. G. , Anderson, M. Z. , Begay, R. L. , Tsosie, K. S. , Fox, K. , & Garrison, N. A. (2018). A framework for enhancing ethical genomic research with Indigenous communities. Nature Communications, 9, 2957 10.1038/s41467-018-05188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie‐Smith, G. , Thomas, S. B. , Williams, M. V. , & Moody‐Ayers, S. (1999). Attitudes and beliefs of African Americans toward participation in medical research. Journal of General Internal Medicine, 14, 537–546. 10.1046/J.1525-1497.1999.07048.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, L. S. , Bronars, C. A. , Thomas, J. L. , Okuyemi, K. S. , King, G. , Mayo, M. S. , & Ahluwalia, J. S. (2007). Achieving high rates of consent for genetic testing among African American smokers. Nicotine & Tobacco Research, 9, 711–716. 10.1080/14622200701365228 [DOI] [PubMed] [Google Scholar]
- Crenshaw, K. (1989). Demarginalizing the intersection of race and sex: A black feminist critique of antidiscrimination doctrine, feminist theory and antiracist politics Vol. 1989, (pp. 57–80). Routledge, UK: University of Chicago Legal Forum. [Google Scholar]
- Critical Appraisal Skills Programme (2018). CASP qualitative checklist. Retrieved from https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf [Google Scholar]
- Culhane‐Pera, K. A. , Straka, R. J. , Moua, M. , Roman, Y. , Vue, P. , Xiaaj, K. , … Lor, M. (2017). Engaging Hmong adults in genomic and pharmacogenomic research: Toward reducing health disparities in genomic knowledge using a community‐based participatory research approach. Journal of Community Genetics, 8, 117–125. 10.1007/s12687-017-0292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, V. A. , Mainous, A. G. III , Gavin, J. K. , & Wilson, D. (2014). Racial differences in attitudes toward personalized medicine. Public Health Genomics, 17(1), 1–6. 10.1159/000354785 [DOI] [PubMed] [Google Scholar]
- Dye, T. , Li, D. , Demment, M. , Groth, S. , Fernandez, D. , Dozier, A. , & Chang, J. (2016). Sociocultural variation in attitudes toward use of genetic information and participation in genetic research by race in the United States: Implications for precision medicine. Journal of the American Medical Informatics Association, 23, 782–786. 10.1093/jamia/ocv214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2005). Guidance for industry: Pharmacogenomics data submissions. Silver Spring, MA: United States Food and Drug Administration Procedural Report; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacogenomic-data-submissions [Google Scholar]
- FDA (2013). Paving the way for personalized medicine: FDA’s role in a new era of medical product development. Silver Spring, MA: United States Food and Drug Administration Procedural Report; https://www.fdanews.com/ext/resources/files/10/10-28-13-Personalized-Medicine.pdf [Google Scholar]
- File, T. (2018). Characteristics of Voters in the Presidential Election of 2016 Current Population Survey Reports. United States Census Bureau, P20‐582. [Google Scholar]
- Frazier, L. , Calvin, A. O. , Mudd, G. T. , & Cohen, M. Z. (2006). Understanding of genetics among older adults. Journal of Nursing Scholarship, 38, 126–132. 10.1111/j.1547-5069.2006.00089.x [DOI] [PubMed] [Google Scholar]
- Freedman, B. I. , Fletcher, A. J. , Sanghani, V. R. , Spainhour, M. , Graham, A. W. , Russell, G. B. , … King, N. M. P. (2013). Perceptions regarding genetic testing in populations at risk for nephropathy. American Journal of Nephrology, 38, 453–457. 10.1159/000356244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano, A. R. , Mokuau, N. , Hughes, C. , Tortolero‐Luna, G. , Risendal, B. , Ho, R. C. S. , … McCaskill‐Stevens, W. J. (2000). Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Annals of Epidemiology, 10(8 Suppl), S22–34. 10.1016/S1047-2797(00)00195-2 [DOI] [PubMed] [Google Scholar]
- Glenn, B. A. , Chawla, N. , & Bastani, R. (2012). Barriers to genetic testing for breast cancer risk among ethnic minority women: An exploratory study. Ethnicity & Disease, 22, 267–273. [PubMed] [Google Scholar]
- Halbert, C. H. , Gandy, O. H. , Collier, A. , & Shaker, L. (2006). Intentions to participate in genetics research among African American smokers. Cancer Epidemiology, Biomarkers & Prevention, 15, 150–153. 10.1158/1055-9965.EPI-05-0437 [DOI] [PubMed] [Google Scholar]
- Halbert, C. H. , McDonald, J. , Vadaparampil, S. , Rice, L. , & Jefferson, M. (2016). Conducting precision medicine research with African Americans. PLoS ONE, 11, e0154850 10.1371/journal.pone.0154850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, J. G. , Shuk, E. , Arniella, G. , González, C. J. , Gold, G. S. , Gany, F. , … Hay, J. L. (2016). Genetic testing awareness and attitudes among latinos: Exploring shared perceptions and gender‐based differences. Public Health Genomics, 19, 34–46. 10.1159/000441552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman, L. , Vermeulen, E. , van El, C. G. , Claassen, L. , Timmermans, D. R. M. , & Cornel, M. C. (2013). Public attitudes towards genetic testing revisited: Comparing opinions between 2002 and 2010. European Journal of Human Genetics, 21, 793–799. 10.1038/ejhg.2012.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley Alford, S. , McBride, C. M. , Reid, R. J. , Larson, E. B. , Baxevanis, A. D. , & Brody, L. C. (2011). Participation in genetic testing research varies by social group. Public Health Genomics, 14, 85–93. 10.1159/000294277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper, M. , Grill, J. D. , Rodriguez‐Agudelo, Y. , Medina, L. D. , Fox, M. , Alvarez‐Retuerto, A. I. , … Ringman, J. M. (2013). The impact of the availability of prevention studies on the desire to undergo predictive testing in persons at risk for autosomal dominant Alzheimer’s disease. Contemporary Clinical Trials, 36, 256–262. 10.1016/J.CCT.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull, S. C. , Sharp, R. R. , Botkin, J. R. , Brown, M. , Hughes, M. , Sugarman, J. , … Wilfond, B. S. (2008). Patients’ views on identifiability of samples and informed consent for genetic research. The American Journal of Bioethics, 8, 62–70. 10.1080/15265160802478404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski, A. B. , Clark, P. J. , Thompson, A. J. , Gordon, S. C. , Lawitz, E. J. , Noviello, S. , … Muir, A. J. (2013). Predictors of consent to pharmacogenomics testing in the IDEAL study. Pharmacogenetics and Genomics, 23, 619 10.1097/FPC.0000000000000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, M. M. , Reed‐Gross, E. , Barfield, W. D. , Prue, C. E. , Gallagher, M. L. , Rasmussen, S. A. , & Honein, M. A. (2011). Qualitative assessment of study materials and communication strategies used in studies that include DNA collection. American Journal of Medical Genetics Part A, 155, 2721–2731. 10.1002/ajmg.a.34263 [DOI] [PubMed] [Google Scholar]
- Keller, R. C. (2006). Geographies of power, legacies of mistrust: Colonial medicine in the global present. Historical Geography , 34, 26–48 . [Google Scholar]
- Kinney, A. Y. , Simonsen, S. E. , Baty, B. J. , Mandal, D. , Neuhausen, S. L. , Seggar, K. , … Smith, K. (2006). Acceptance of genetic testing for hereditary breast ovarian cancer among study enrollees from an African American kindred. American Journal of Medical Genetics Part A, 140A, 813–826. 10.1002/ajmg.a.31162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolor, K. , Chen, Z. , Grosse, S. D. , Rodriguez, J. L. , Green, R. F. , Dotson, W. D. , … Khoury, M. J. (2017). BRCA genetic testing and receipt of preventive interventions among women aged 18–64 years with employer‐sponsored health insurance in nonmetropolitan and metropolitan areas — United States, 2009–2014. Morbidity and Mortality Weekly Report Surveillance Summaries, 66(15), 1–11. 10.15585/mmwr.ss6615a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakes, K. D. , Vaughan, E. , Lemke, A. , Jones, M. , Wigal, T. , Baker, D. , … Burke, W. (2013). Maternal perspectives on the return of genetic results: Context matters. American Journal of Medical Genetics Part A, 161, 38–47. 10.1002/ajmg.a.35673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. A. , Vadaparampil, S. , Bowen, D. , Magwood, G. , Obeid, J. S. , Jefferson, M. , … Hughes Halbert, C. (2014). Intentions to donate to a biobank in a national sample of African Americans. Public Health Genomics, 17, 173–182. 10.1159/000360472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeroy, K. R. , Bibeau, D. , Steckler, A. , & Glanz, K. (1988). An ecological perspective on health promotion programs. Health Education Quarterly, 15, 351–377. 10.1177/109019818801500401 [DOI] [PubMed] [Google Scholar]
- Moola, S. , Munn, Z. , Tufanaru, C. , Aromataris, E. , Sears, K. , Sfetcu, R. , … Mu, P. (2017). Chapter 7: Systematic reviews of etiology and risk In Aromataris E., & Munn Z. (Eds.), Joanna Briggs Institute Reviewer’s Manual. Adelaide: The Joanna Briggs Institute. [Google Scholar]
- Murphy, E. , & Thompson, A. (2009). An exploration of attitudes among black Americans towards psychiatric genetic research. Psychiatry: Interpersonal and Biological Processes, 72, 177–194. 10.1521/psyc.2009.72.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need, A. C. , & Goldstein, D. B. (2009). Next generation disparities in human genomics: Concerns and remedies. Trends in Genetics, 25, 489–494. 10.1016/j.tig.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Nodora, J. N. , Komenaka, I. K. , Bouton, M. E. , Ohno‐Machado, L. , Schwab, R. , Kim, H.‐E. , … Martinez, M. E. (2016). Biospecimen sharing among Hispanic women in a safety‐net clinic: Implications for the precision medicine initiative. Journal of the National Cancer Institute, 109, djw201 10.1093/jnci/djw201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettey, C. M. , McSweeney, J. C. , Stewart, K. E. , Price, E. T. , Cleves, M. A. , Heo, S. , & Souder, E. (2015). Perceptions of family history and genetic testing and feasibility of pedigree development among African Americans with hypertension. European Journal of Cardiovascular Nursing, 14, 8–15. 10.1177/1474515114556198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popejoy, A. B. , & Fullerton, S. M. (2016). Genomics is failing on diversity. Nature, 538, 161–164. 10.1038/538161a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rew, L. , Mackert, M. , & Bonevac, D. (2010). Cool, but is it credible? Adolescents’ and Parents’ approaches to genetic testing. Western Journal of Nursing Research, 32, 610–627. 10.1177/0193945909360781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, L. , Potvin, L. , Kishchuk, N. , Prlic, H. , & Green, L. W. (1995). Assessment of the integration of the ecological approach in health promotion programs. American Journal of Health Promotion, 10(4), 318–328. 10.4278/0890-1171-10.4.318 [DOI] [PubMed] [Google Scholar]
- Sanderson, S. C. , Brothers, K. B. , Mercaldo, N. D. , Clayton, E. W. , Antommaria, A. H. M. , Aufox, S. A. , … Holm, I. A. (2017). Public attitudes toward consent and data sharing in biobank research: A large multi‐site experimental survey in the US. The American Journal of Human Genetics, 100, 414–427. 10.1016/J.AJHG.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard, V. B. , Hurtado‐de‐Mendoza, A. , Zheng, Y.‐L. , Wang, Y. , Graves, K. D. , Lobo, T. , … Tadesse, M. (2018). Biospecimen donation among black and white breast cancer survivors: Opportunities to promote precision medicine. Journal of Cancer Survivorship, 12, 74–81. 10.1007/s11764-017-0646-8 [DOI] [PubMed] [Google Scholar]
- Simon, M. A. , Tom, L. S. , & Dong, X. (2017). Knowledge and beliefs about biospecimen research among Chinese older women in Chicago’s Chinatown. The Journals of Gerontology: Series A, 72(suppl 1), S41–S49. 10.1093/gerona/glw333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirugo, G. , Williams, S. M. , & Tishkoff, S. A. (2019). The missing diversity in human genetic studies. Cell, 177, 26–31. 10.1016/j.cell.2019.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt, D. E. , Chan, T. , Waldron, L. , Speers, C. , Feng, F. Y. , Ogunwobi, O. O. , & Osborne, J. R. (2016). Racial/ethnic disparities in genomic sequencing. JAMA Oncology, 2, 1070 10.1001/jamaoncol.2016.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, G. M. , & Ward, A. J. (1995). Recruiting minorities into clinical trials: Toward a participant‐friendly system. Journal of the National Cancer Institute, 87, 1747–1759. 10.1093/jnci/87.23.1747 [DOI] [PubMed] [Google Scholar]
- Yancey, A. K. , Ortega, A. N. , & Kumanyika, S. K. (2006). Effective recruitment and retention of minority research participants. Annual Review of Public Health, 27, 1–28. 10.1146/annurev.publhealth.27.021405.102113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


