Summary
In the last decade, numerous studies revealed physiologic and pathophysiologic roles of platelets beyond haemostasis, a process to prevent and stop bleeding. These include the activation of the immune system and the promotion of inflammation, infection and cancer. Hence, the emerging view on the role of platelets has shifted – platelets are now seen as alert “sentinels” of the immune compartment, rather than passive bystanders. Herein, we review well-established and newly discovered features of platelets that define their natural role in maintaining blood haemostasis, but also their functional relationship with other cells of the immune system. We focus on recent studies underlining functional involvement of platelets in chronic liver diseases and cancer, as well as the effects of anti-platelet therapy in these contexts. Finally, we illustrate the potential of platelets as possible diagnostic and therapeutic tools in liver disease based on recently developed methodologies.
Keywords: Platelets, cirrhosis, HCC, cancer, immune system, immunology, NASH, NAFLD
Key points
Platelets interact with different resident cellular populations and exert regulatory functions within the immune system in several chronic liver diseases.
Based on experimental data, anti-platelet therapy might become a therapeutic possibility in the treatment of chronic liver diseases characterised by immune-mediated hepatocyte damage.
Platelets might represent a valid therapeutic target even in metabolically driven liver diseases, with the glycoprotein GPIbα seeming to be particularly important.
A better understanding of the cellular and molecular mechanisms of interaction between platelets, cancer cells and immune cells is urgently required to validate the potential efficacy of an anti-platelet approach to liver cancer treatment.
Platelet transcriptome analysis might become a useful tool for the diagnosis of chronic liver diseases.
Alt-text: Unlabelled Box
Introduction
Beyond the well-known classical role of platelets in the regulation of blood haemostasis in physiologic and pathologic conditions, recent studies have shed light on potential roles of platelets that appear to be independent of their main functions, via interactions with the immune system and tumour cells. In the liver, platelets seem to interact with different resident cellular populations and to exert regulatory functions within the immune system in several chronic liver diseases including, for example, viral hepatitis, non-alcoholic steatohepatitis (NASH) and liver cancer. These novel findings prompted the hypothesis that a selective modulation of the activation status of platelets might represent a promising therapeutic approach in the context of several chronic liver diseases and even several liver cancer types.
Considering the active role of the liver in the life cycle of platelets and the complex network of interactions developing within the liver parenchyma, we provide a brief insight into the basic aspects of platelet biology.
The origin of platelets and their life cycle
Two types of blood cells originate from a common hematopoietic progenitor: lymphoid stem cells, from which most of white blood cells originate, and myeloid stem cells, from which red blood cells, platelets and myeloblasts stem. Given their crucial role in blood coagulation and thrombosis, platelets are mainly produced by the liver during foetal life. After birth, the bone marrow becomes the most important source of platelets, where they develop from progenitor cells named megakaryocytes. In this developmental process, a megakaryocyte precursor becomes polyploid by endomitosis and undergoes a maturation phase that results in massive enlargement and elongation of the cytoplasm, from which pro-platelets evaginate and take birth. In detail, mature megakaryocytes lie in proximity to vessels, extend their pseudopods through the vascular endothelium into the lumen of bone marrow vessels and release platelets through a fission-like remodelling of cytoplasm.1 Platelet production, as well as megakaryocyte maturation, are tightly regulated by the action of thrombopoietin (TPO), a hormone produced by the liver and kidney.2
At the end of their lifespan in vessels, or after accomplishing their main function in the bloodstream, they can be removed from the circulation by neutrophils or macrophages and destroyed/phagocytosed in the spleen and liver. Senescent platelets also undergo a process of cell death resembling intrinsic apoptosis.3 During their normal life cycle, platelets not only decrease in size but also undergo a process of progressive de-sialylation that enables their clearance in the liver via the asialoglycoprotein receptor, which is present on the vascular face of hepatocytes and on liver-resident macrophages, Kupffer cells (KCs).4
General functions of platelets – mechanism of platelet aggregation and attachment
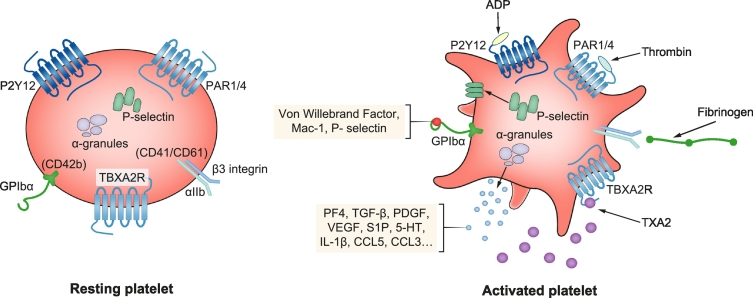
The natural role of platelets in the circulatory tree is to maintain primary haemostasis and blood flow within vessels. The process leading to coagulation is initiated by exposure of the sub-endothelial matrix to platelets, leading to interactions between platelet-receptors and matrix proteins. Hereby, activation of platelets results in release of mediators such as ADP, adrenaline, serotonin (5-HT), thrombin and thromboxane A2 (TXA2), that act in an autocrine and paracrine manner. After this activation process, glycoprotein IIb/IIIa (GPIIb/IIIa) complexes bind fibrinogen enabling platelet aggregation and consequent constitution of the thrombus. Therefore, activation and attachment of platelets is the first step in a closely regulated cascade leading to aggregation (Fig. 1). The understanding of this process led to the development of several drugs that block aggregation, as outlined below.5,6
Fig. 1.
Schematic representation of a platelet in its resting state and upon activation.
Platelets present membrane G-protein coupled receptors on their surface that can bind several ligands resulting in decreased intracellular cAMP, mobilisation of Ca2+ stores and subsequent changes of cell morphology. Upon activation, soluble proteins retained in the granules are released via exocytosis, exerting their biological functions in an autocrine or paracrine manner. Similarly, membrane proteins retained in the granules are mobilised and presented at the cellular surface where they can bind related ligands. 5HT, 5-hydroxytryptamine; CCL2, chemokine ligand 2; CCL5, chemokine ligand 5; GPIbα, glycoprotein Ibα; IL-1β, interleukin-1β; PDGF, platelet-derived growth factor; PF4, platelet factor 4; S1P, sphingosine-1-phosphate; TGF-β, transforming growth factor-β; TXA2, thromboxane A2; VEGF, vascular endothelial growth factor.
The production and secretion of soluble ligands is an essential process for the formation of the thrombus, as they trigger signals in an autocrine and paracrine manner to sustain activation and attract further circulating platelets. Three distinct types of granules have been identified in platelets: alpha-granules are the most abundant and contain mainly membrane receptor proteins (integrins and P-selectins), and soluble cargo proteins (fibrinogen, von Willebrand factor (vWF), platelet factor 4 (PF4), chemokines and growth factors).7 Dense granules are less abundant and represent a group of lysosome-related organelles that contain bioactive amines, adenine nucleotides and calcium cations. Platelet-lysosomes are the third group of granules and they contain many proteases like carboxypeptidases that modulate inflammatory processes and tissue reactions.8,9 The most well known and investigated molecular activators are TXA2, ADP, epinephrine and thrombin as they enable full activation and interaction with other extracellular components. They induce increases in intracellular Ca2+ levels resulting in changes to platelet shape, increased protein synthesis and activation of further adhesion molecules (e.g. GPIIb/IIIa).10 Platelets possess 2 ADP receptors (P2Y1 and P2Y12) which are G-coupled protein receptors, contributing to their initial aggregation and triggering a decrease of intracellular concentrations of cAMP, with consequent changes to platelet shape.11 Similarly, thrombin also represents one of the key mediators of the coagulation process. It also binds to a G-protein coupled class of receptors defined as protease-activated receptors, activating a signalling cascade of events that results in decreased cAMP concentration and increased Ca2+ concentration. This intracellular cascade leads to TXA2 production via cyclooxygenase-1 activation (COX-1), ADP release, mobilisation of P-selectin and CD40L, integrin activation and finally platelet aggregation.12
Platelets, dynamic sentinels interacting with immune and non-immune cells
Platelets have been recognised as key players in numerous immunological contexts ranging from inflammation, bacterial and viral immune reaction, to immunity against tumours and tumour metastases. At the platelet surface, several receptors are able to interact with not only leukocytes, but also other immune and non-immune cells such as endothelial cells. Many of these dynamic multiple interactions commonly occur within the hepatic microenvironment in the context of liver injury and repair.13
It was recently shown that platelets express all toll-like receptor (TLR)-family members, enabling them to recognise molecular motifs like, for instance, pathogen-associated molecular patterns. For instance, TLR-2 and TLR-4 were shown to have a functional role in responses to bacterial endotoxins.14,15 This interaction at the site of infection induces the release of microvesicles containing IL-1β from platelets and the organisation of neutrophil extracellular traps, which act as an antibacterial mechanism alongside the inflammatory process taking place in the liver sinusoids.[15], [16], [17] Direct platelet-microbe interactions are described as well, leading to platelet aggregation and sequestration of bacteria, enhancing removal of bacteria by the reticuloendothelial system.18,19 In the liver, platelets seem to adhere to blood pathogens sequestered by KCs, emphasising their supportive role in bacterial clearance.20 Indeed, this interaction mediated by GPIbα on platelets and vWF on KCs was shown to be a very dynamic and continuous “patrolling” process, occurring specifically in the hepatic sinusoids. This is supported by data showing that platelet depletion and Gp1b-/- knockout mice are more prone to develop vascular leak damage during bacterial infection, because of the reduced clearance of bacteria from the liver.21,22 Elegant work by Gaertner et al. illustrated that platelets are able to migrate independently of the blood stream through a process involving morphological changes, adhesion via GPIIb/IIIa and increases in intracellular Ca2+ concentration.23 Fascinatingly, this process allows platelets to behave as mechano-scavengers, facilitating the collection and phagocytosis of bacterial particles by neutrophils along the liver sinusoids. An interesting recent study by Burzynski et al. provided evidence for a direct link between the coagulation and immune systems.24 In this study, the authors showed that thrombin can cleave and activate the production of IL-1α on platelets and macrophages, therefore contributing to the sustainment of inflammation during haemostasis.
Beside interactions with the innate immune system, platelets also participate in the humoral immune response. It was reported that platelets express specific receptors for protein members of the complement system enabling them to trigger the activation of the classical pathway.25 The adherence of platelets to bacteria, via interactions with the opsonising complement factor C3, was shown to enhance the bactericidal activity of CD8α+ dendritic cells.26 In their granules, platelets also contain transforming growth factor (TGF)-β, a molecule promoting the development of regulatory T cells or T-helper 17 cells in the context of viral infections and the antitumour immune response.27 Thus, platelets also interfere with elements of the adaptive immune response. Indeed, activated platelets were shown to contribute to the maturation process of dendritic cells and to enhance CD8+ T cell responses during adenoviral infection.28 Also, in the liver, this process seems to be recapitulated during viral infection, as described in detail later. In fact, platelets’ interaction with cytotoxic T cells was shown to enable the infiltration of these lymphocytes into the hepatic parenchyma in a mouse model of viral hepatitis, a process mediated by hyaluronan-CD44 binding.29
Finally, a clear implication of platelets in tumour progression and metastasis formation was recently corroborated.30 Novel findings illustrate that platelets can aggregate and adhere to tumour cells acting as a “protective shield” from immune-regulated clearance.31 Platelets also favour the adhesion of metastatic cells to the endothelium, entrapping them with other immune cells (leukocytes/monocytes) mainly via interactions mediated by selectins.32 Interestingly, the interaction between platelets and tumour cells was reported to be essential to avoid detachment-induced apoptosis (anoikis), a major feature of metastasis.33 Recently, platelet-derived TXA2 (which is generated by activated COX-1) was reported to promote a pro-metastatic niche involving endothelial cells, myeloid cells and tumour cells.34 In this context, platelets can also produce pro-angiogenic and growth factors that facilitate tumour growth and survival, as well as promoting the metastatic potential of tumour cells. In turn, cancer cells can influence platelet activation and shape by releasing growth factors that bind to specific receptors on their surface (tumour “education”). Moreover, the tight communication between platelets and tumour cells, as well as their physical interactions, indicate a possible role for platelets as promising vectors for targeted drug delivery.35
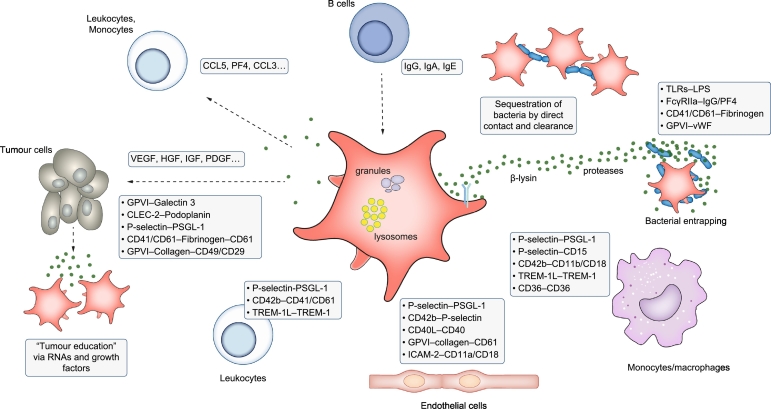
Therefore, platelets take part in an intricate interplay with innate and adaptive immune responses, perpetuating inflammatory and malignant processes via various mechanisms (summarised in Fig. 2).
Fig. 2.
Schematic representation of interactions of platelets with immune and non-immune cells (endothelium, bacteria, tumour cells).
Platelets express a variety of membrane receptors and ligands enabling them to interact with several immune and non-immune cells. Vice versa soluble proteins or other external stimuli activate platelets leading to granule release via exocytosis. Furthermore, direct interactions enable platelets to entrap bacteria and clear them via the reticuloendothelial system. CLEC-2, C-type lectin-like receptor-2; GPIbα, glycoprotein Ibα; HBD-1, human beta defensin 1; HMGB-1, high mobility group box 1; IL-1β, interleukin-1β; miRNA, microRNA; PF4, platelet factor 4; PSGL1, P-selectin glycoprotein ligand 1; TREM-1, triggering receptor expressed on myeloid cells-1; TGF-β, transforming growth factor-β; TLR, toll-like-receptor; Th, T helper; Treg, regulatory T.
This brief description of the life cycle and aggregation of platelets, as well as of their interactions with immune cells, is critical to understanding their interactions with different resident and non-resident liver cells, their roles in different liver diseases, and how their modulation can be applied to therapeutic interventions.
Interaction of platelets with liver cells
Platelets and hepatocytes: liver injury and regeneration
As mentioned, the liver is a central regulator of the number of circulating platelets, through TPO production and clearance of aged platelets. Thrombocytopenia is a common complication of chronic liver disease, characterised by decreased TPO synthesis, reduced haematopoiesis and increased platelet destruction in the spleen. Indeed, a direct correlation between liver functionality and platelet count is often reported in patients with chronic liver disease.36 Conversely, it was also shown that thrombocytopenia could aggravate liver functionality and fibrosis in experimental models of chronic liver injury, as reported here later. Therefore, considering the dynamic interactions with immune cells, the understanding that platelets actively participate in pathophysiologic processes in the liver is clearly strengthening. Upon liver damage, platelets are recruited to the site of injury and contribute to liver repair and regeneration partly through a direct effect on hepatocyte proliferation. Platelet-derived serotonin was shown to initiate and promote liver regeneration in mouse models of partial hepatectomy and ischaemia-reperfusion injury.37 Interestingly, this phenotype was reversed by administration of serotonin agonists, indicating that platelet-derived serotonin plays a central role in the initiation of liver regeneration. A recent elegant work indicates that fibrin contributes to liver regeneration by promoting intrahepatic platelet accumulation.38 In this work, the authors showed that the tissue factor produced by hepatocytes after partial hepatectomy is critical for activating a coagulation cascade that leads to intrahepatic fibrin deposition and platelet accumulation in the remnant liver. Accordingly, thrombocytopenia was shown to impair hepatocyte proliferation in a surgical murine model of liver regeneration and many studies based on therapeutic enrichment of platelets during partial hepatectomy indicate pro-regenerating beneficial effects.39 Furthermore, this pro-regenerative activity on hepatocytes seems to be related to the release of various growth factors and cytokines contained in the platelet granules. In fact, platelets were shown to induce direct hepatocyte proliferation in vitro via secretion of growth factors such as hepatic growth factor (HGF) and insulin-like growth factor (IGF).40
Platelets, liver sinusoidal endothelial cells and Kupffer cells: organisation of a proinflammatory partnership
The interaction of platelets with non-parenchymal cells turned out to modulate the immune response to liver damage differently. Contact with liver sinusoidal endothelial cells (LSECs) represents the first way for platelets to access the liver parenchyma. This interaction was shown to be a priming process for the initiation of liver repair upon injury and the orchestration of liver regeneration. The recruitment of platelets at the site of damage seems to be a very dynamic process that consists of a sudden adhesion of platelets to the altered sinusoidal cells, which delimits the site of injury – these interactions are dependent on GPIIb/IIIa and at a later point GPIb receptors.41 Interestingly, in this specific condition, platelets do not occlude the vessels but rather organise in a paved structure that enables neutrophils to crawl to the site of injury. A detailed molecular analysis of this cellular interaction was reported in the regenerative liver following chemical injury or partial hepatectomy.42 In this study, platelets were shown to release stromal derived factor (SDF)-1 and vascular endothelial growth factor (VEGF)-1 – at the site of injury – that bound to their relative receptors on LSECs and myeloid cells, thereby stimulating a haematopoietic-vascular niche that induces and sustains liver regeneration. Notably, the regeneration process was impaired in mice lacking the CXCR7 receptor on LSECs or VEGFR1 on myeloid cells.
Moreover, in vitro, the cross-talk between platelets and endothelial cells in the liver sinusoids, via sphingosine-1-phosphate receptors (S-1-P), results in IL-6 release from endothelial cells, promoting hepatocyte proliferation.43 Whereas during liver regeneration the adhesion to the endothelium represents a rapid and transient event,44 platelet adhesion to the liver sinusoids results in deleterious changes to the microcirculation in the context of hepatic ischaemia-reperfusion injury.45 Furthermore, platelet adhesion to KCs was shown to occur in the early phases of the reperfusion injury, contributing to the recruitment of neutrophils to the liver and to the increased sinusoidal perfusion failure rate after transient ischaemia. Although the effects on hepatocyte proliferation might indicate a role for platelets in promoting liver regeneration, the picture emerging from multiple interactions with different cell populations suggests that caution is warranted in the development of a therapeutic approach for hepatic surgery (e.g. liver resection or transplantation).46
The platelet-KC cooperation is particularly intense in the context of innate immunity. Accumulation of platelets in the liver was reported in the early phases of Klebsiella O3 lipopolysaccharide (LPS) infection which induced anaphylactic shock within minutes of injection.47 Depletion of KCs by clodronate liposomes resulted in reduced platelet accumulation and could prevent LPS-induced shock. Similarly, the contact mediated through the receptor GPIb on platelets and the vWF on the KC surface was shown to be critical for systemic bacterial clearance.20 Therefore, this partnership appears to be mainly responsible for the phenomena of infection-driven thrombosis. Interestingly, a significant increase in aggregates of inflammatory monocytes and platelets was also observed in an experimental model of liver cholestasis, the bile-duct ligation (BDL) model.48 This interaction, apparently occurring via the TLR-4 receptor, was essential for the full activation of macrophages and the establishment of a proinflammatory environment. Cholestatic liver injury was previously shown to induce strong platelet activation via GPVI in the early phases of the mouse BDL model. However, chronic cholestasis induced loss of activation, related to impaired vascularisation and the cytotoxic effects of bile acids on platelets, leading to bleeding complications.49 Accordingly, anti-platelet therapy through administration of anti-GPIb antibodies was shown to improve sinusoidal perfusion and reduce hepatocellular damage in BDL-induced cholestasis.50
Platelets and hepatic stellate cells: role in hepatic fibrosis?
Based on clinical evidence, platelet transfusion and anti-platelet therapy were reported to improve liver functionality and reduce liver fibrosis in patients with chronic liver injury.51 Accordingly, TPO administration revealed beneficial effects in a rat model of dimethylnitrosamine-induced cirrhosis, limiting the progression of liver fibrosis.52 These findings led scientists to investigate the cellular mechanisms that underly the interaction between platelets and HSCs. As mentioned, platelets display a wide range of cellular mediators stored in their granule cargo that can be released upon activation and adhesion. S-1-P is one of these mediators that was shown to induce proliferation and activation of rat HSCs in vitro.53 In another study, accumulation of platelets and the platelet-derived chemokine CXCL4 were detected in close proximity to fibrotic areas in patients with chronic liver disease and in mice subjected to models of liver fibrosis.54 In vitro, stimulation with platelet-derived CXCL4 was able to induce HSC proliferation and chemotaxis, whereas genetic deletion of the chemokine in mice significantly reduced liver damage and fibrosis. In line with these findings, platelet-derived growth factor-β (PDGF-B), directly produced by platelets, was shown to activate HSCs and promote liver fibrosis in 2 models of biliary fibrosis in mice.55 Notably, PDFG-B is well known to be one of the most potent mitogens for HSCs.56 In this experimental setting, anti-platelet therapy through anti-CD41 antibodies or low doses of aspirin was able to reduce the progression of fibrosis. Accordingly, co-culturing platelets with HSCs induced activation of pro-fibrogenic genes. Finally, platelets carry significant amounts of TGF-β1 in their cargo; mice genetically lacking this cytokine specifically in platelets exhibited less fibrosis compared to control mice after chronic CCl4 administration.57 However, on the contrary, other studies indicate that platelets might contribute to limit or suppress hepatic stellate activation via the cAMP pathway, triggered by direct contact with ATP-enriched granules of adhesive platelets.58 Moreover, platelet granules also contain large amounts of HGF – at least in rodent models – that can contribute to inhibit HSC activation.59 In fact, whereas it was reported that platelets might exert an anti-fibrotic effect by inhibiting HSCs through the HGF-c-Met axis, which resulted in reduced expression of type I collagen genes in the same BDL model,60 in another study, treatment with an anti-coagulant was reported to reduce liver necrosis and neutrophil migration in a model of drug-induced liver cholestasis.61
Although growing evidence indicates that platelets can influence the progression of liver fibrosis through cellular interaction with HSCs, the cellular mechanisms and the biologic effects of this interaction are still unclear.
Role of platelets in liver diseases and potential therapeutic interventions
Platelets in chronic viral hepatitis
In relation to the loss of liver functionality, blood platelet counts progressively decrease and thrombocytopenia becomes an important feature of chronic viral hepatitis and cirrhosis. Many factors were reported to contribute to the reduced number of circulating platelets, such as increased splenic clearance (often in association with portal hypertension), reduced production in the bone marrow, sequestration of platelets in the liver and generation of anti-platelet antibodies. In patients with HCV infection, in whom platelet count decreased in relation to the severity of fibrosis, achieving a sustained antiviral response positively correlated with a recover in platelet number and a reduction in spleen size.62 Interestingly, similar findings were confirmed in a recent retrospective analysis of patients with chronic HCV infection treated with antiviral therapy, in which a significant increase in platelet count was observed after viral elimination. However, in this case, changes in platelet count were independent of changes in liver fibrosis.63 A therapeutic approach aimed at stimulating bone marrow production using a thrombopoietin receptor agonist (Eltrombopag) was shown to increase platelet counts in a group of patients with HCV and advanced fibrosis, permitting antiviral pegylated-interferon therapy and reducing the interferon-mediated decrease in platelet count.64 Currently, the use of thrombopoietin receptor agonists is emerging as a common therapeutic strategy in patients with chronic liver disease presenting with thrombocytopenia. In this direction, new drugs like avatrombopag were recently shown to restore platelet count without affecting their activation status.65
An interesting clinical study by Kondo et al.66 performed on liver biopsies from patients with HCV-induced HCC revealed an increase of infiltrating platelets in the peritumoral area of cirrhotic liver tissue compared to healthy tissue, despite a systemic decrease of circulating platelets. Of note, infiltrating platelets were mainly located in necrotic periportal areas of inflammation, along with CD68 positive cells, indicating possible co-localisation with KCs. However, the number of platelets and KCs was significantly decreased in the tumour compared to the peritumoral tissue. Finally, anti-platelet antibodies, most likely targeting antigens like GPIIb/IIIa and GPIIIa, were detected in the circulation of HCV-positive patients.67
A reduction of platelet count is also observed frequently in the blood of patients with chronic HBV infection, whereas increased platelet count seems to be significantly related to restored liver functionality and decreased liver fibrosis upon antiviral therapy.68 Experimentally, it has been shown that platelets play a pivotal role in the pathophysiology of HBV, mainly by enhancing the infiltration of virus-specific T-cells.69 In this specific case, Guidotti et al.70 showed that platelets are essential for arresting CD8+T cells within the liver sinusoids; these CD8+T cells then crawl along the sinusoids before exerting their antiviral activity via antigen recognition. By using genetic and pharmacological lack of function approaches, the authors demonstrated that platelets adhere to hyaluronan on sinusoids via CD44 molecules, favouring lymphocyte arrest and transmigration.
Regarding pharmacological interventions in this context, aspirin, the most common anti-platelet drug used in clinic to prevent cardiovascular events, seems to represent a valid complementary therapy. Aspirin is able to selectively inactivate COX-1 at very low doses, inhibiting platelet aggregation.71 Indeed, repeated low doses result in permanent enzyme inactivation and reduced TXA2 production with consequent anti-aggregation effects. However, indirect effects of aspirin are also known, such as its anti-inflammatory and oxygen radical scavenger properties.72 A more selective drug, clopidrogel, was specifically designed to inhibit aggregation of platelets and to limit unwanted side effects. Clopidogrel is a thienopyridine that irreversibly inhibits the ADP receptor P2Y12.73 In the same murine model of viral hepatitis mentioned earlier, administration of these platelet-activation inhibitors, aspirin and clopidrogel, resulted in a reduction of CD8+T cells and attenuation of viral infection-derived hepatocyte damage, without causing bleeding effects.74 In a similar study, the same research group showed that, whereas the same pharmacological treatment was able to reduce the development of HCC in an HBV murine model, neither aspirin nor clopidrogel revealed antitumour effects in a non-immunological chemically induced model of hepatocarcinogenesis.75
In another study based on an experimental model of viral hepatitis, infection resulted in platelet recruitment to the liver with consequent activation and impairment of the sinusoidal microcirculation. This process turned out to delay clearance of the virus and increased liver damage. Lack of serotonin in tryptophan hydroxilases-1-deficient mice resulted in improvement of the sinusoidal circulation, reduction of CD8+T cell recruitment and acceleration of hepatic viral clearance, highlighting a deleterious effect of platelet-derived serotonin.76
Beyond obvious considerations regarding haemostasis in the establishment of anti-platelet therapies, the studies performed so far in the context of liver injury indicate that platelets interact with different hepatic and immune cell populations at different stages of the disease. Based on experimental data, anti-platelet therapy might become a therapeutic possibility in the treatment of chronic viral hepatitis characterised by immune-mediated liver damage. It is therefore necessary to understand the biological meaning and the dynamics of this intracellular communication in order to offer valid and safe approaches for the development of new therapeutic strategies.
Platelets in alcohol-related and non-alcoholic steatohepatitis
Although recent prospective clinical studies suggest that patients diagnosed with non-alcoholic fatty liver disease (NAFLD) are at an increased risk of developing thrombocytopenia, a real correlation seems to be reproducible only in the advanced fibrotic stages of the disease.77 However, data in this regard are still quite controversial.78 Patients diagnosed with NAFLD/NASH commonly display increased mean platelet volume, an indicator of platelet activation, which was shown to correlate directly with the severity of inflammation and the grade of fibrosis,79 whereas patients with alcohol-related liver disease seem to display reduced platelet activation and aggregation capacity.80 However, in another study, patients with alcohol-related liver cirrhosis were reported to display a decreased platelet count but a significantly increased mean platelet volume compared to control patients or patients with simple alcohol-related fatty liver disease (AFLD).81
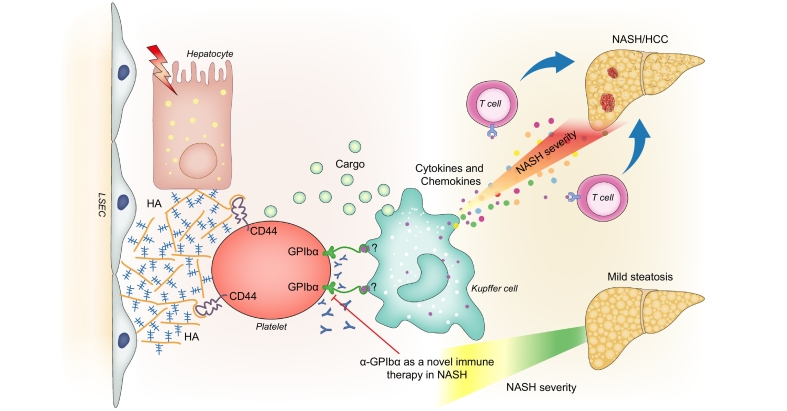
Recent data from our laboratories indicated an increase of infiltrating platelets in hepatic tissue of pre-clinical models of diet-induced NASH and of NASH-diagnosed patients.82 Interestingly, this increase was not observed in livers displaying simple steatosis. However, genetic thrombocytopenia or pharmacological inhibition of platelet activation (aspirin/clopidrogel) not only reduced NAFLD activity score and inflammatory infiltrate but also turned out to improve steatosis, possibly by ameliorating mitochondrial functionality and lipid catabolism. Interestingly, the incidence of NASH-induced hepatocellular carcinoma was dramatically reduced in mice treated with anti-coagulant therapy. Notably, aspirin is considered to be a non-steroidal anti-inflammatory drug and COX-inhibitor rather than an anti-coagulant. In this study, the use of another general non-steroidal anti-inflammatory drug, sulindac, failed to improve the metabolic phenotype and the NAFLD activity score observed after choline-deficient-high-fat diet feeding. Instead, the use of another platelet inhibitor, ticagrelor, a cyclopentyltriazolopyrimidine reversible inhibitor of P2Y12 receptors, with faster onset of platelet inhibition compared to other anti-coagulants, was able to reproduce faithfully the protective effects observed after aspirin/clopidrogel treatment even on NASH-induced HCC. Furthermore, a detailed 3D morphological analysis of cellular localisation revealed a direct interaction of platelets with KCs. More precisely, anchoring of platelets to the extracellular matrix, in particular to hyaluronan via their CD44 receptor, was essential for establishing a direct contact with KCs and triggering the inflammatory response associated with metabolic stress. Indeed, genetic and pharmacologic inhibition of CD44 and hyaluronidase reduced the hepatic accumulation of KCs and platelets, improving the NAFLD activity score and ameliorating inflammation. The precise mechanisms of interaction between CD44 and hyaluronan are not fully understood yet, but their expression could increase in NASH-related liver injury. Hepatic stellate cells are a possible source of hyaluronan that could possibly be activated in this specific context.83 Similarly, liver endothelial cells could actively participate in hyaluronan remodelling and production.84 Finally, genetic impairment of platelets’ ability to release α-granules (in Nbeal2-/- mice) resulted in amelioration of liver damage and inflammation, whereas simple inhibition of platelet aggregation did not reveal any beneficial effect. Interestingly, the receptor responsible for this interaction with the immune system and therefore for platelet activation and granule release turned out to be the glycoprotein GPIb (Fig. 3). This set of data raises hopes for the development of valid therapeutic strategies in the context of NASH-induced HCC which do not alter the haemostatic functionality. Along these lines, preliminary analyses on human biopsies from our laboratories indicate that NASH-diagnosed patients display many more infiltrating platelets in peritumoural areas than patients with other aetiologies of liver disease, like alcohol-related steatohepatitis or chronic hepatitis. However, the number of adhering platelets is always lower in tumoural tissue than in non-tumoural tissue. Interestingly, the number of platelets appears to be associated with the number of CD68+ cells, as observed in the context of HCV.66
Fig. 3.
Schematic cartoon illustrating the proposed cellular mechanisms through which platelets contribute to the activation of the immune responses in NASH.
Interaction of the CD44 receptor on platelets with HA present in the extracellular matrix favours the entrapment and accumulation of platelets in the injured liver. Therefore, KCs bind to the GPIbα receptor on the surface of platelets and become polarised toward a pro-inflammatory phenotype, producing chemokines and cytokines that in turn activate T cells that infiltrate the liver parenchyma. GPIbα, glycoprotein Ibα; HA, hyaluronan; KCs, Kupffer cells; NASH, non-alcoholic steatohepatitis.
Thus, platelets might represent a valid therapeutic target even in metabolically driven liver diseases. An associational study conducted in patients with cardiovascular disease with or without NAFLD, revealed a protective relationship between the use of acetyl salicylic acid (active substance in aspirin) in combination with clopidrogel and the degree of liver fibrosis.85 However, whereas the increase in platelet levels significantly correlated with serum concentrations of PDGF-B in fibrotic patients, anti-platelet therapy did not affect the levels of the growth factor in the blood. A further recent clinical study performed on a cohort of patients diagnosed with NAFLD indicates that aspirin administration is associated with an improvement of NAFLD features and a reduced risk of fibrosis progression.86
Platelets in liver cancer and metastasis
Although data concerning the number of circulating platelets in patients with NASH- induced and virus-induced HCC are still sparse and controversial, an emerging body of evidence supports a pro-carcinogenic environment driven by platelets. However, it is interesting to note that thrombocytopenia is a hallmark of cirrhosis, a condition considered as a risk factor for HCC development. Despite this apparent contradiction, the presence of small HCC, normally in the context of liver cirrhosis, was reported to be associated with reduced platelet counts.87,88 In cirrhotic patients with AFLD or NAFLD-related HCC, a low platelet count was recently included among the parameters considered to be reliable predictors of HCC development.89 Conversely, increased platelet count has often been associated with HCC aggressiveness and size, tumour recurrence and increased metastatic risk.[90], [91], [92] Elevated platelet distribution width, reflecting changes in platelet size and therefore activation status, was shown to be significantly associated with poor prognosis in patients with HCC.93 These observations could indicate different immunological scenarios affecting the carcinogenic process in different ways. The interplay between platelets and the tumour microenvironment certainly deserves deeper investigation, as increased platelet count and/or activation often correlates with a sustained inflammatory response.94 Notably, anti-platelet therapy turned out to repress HCC formation in a mouse model of chronic hepatitis B, through modulation of CD8+T cell-mediated hepatic necro-inflammation.70 Similarly, as described in detail earlier, anti-platelet therapy prevented NASH-induced HCC mainly through modulation of the immune response.82
However, platelet cargo has been shown to contain mediators and growth factors that can directly promote growth and invasion of HCC cell lines.95 Increased levels of serotonin, stored in platelet granules and critical for liver regeneration, were detected in association with early disease recurrence in patients undergoing surgical resection for liver tumours.96 He et al. showed that the release of TGF-β from platelet granules exerts a proliferative effect on HCC by inhibiting the expression of Klf6 on cancer cells.97 Apparently, platelets can directly enhance HCC growth and proliferation mainly through activation of a TGF-β-dependent pathway. Zhang et al. reported that patients with poorly differentiated HCC display increased platelet activation. Local accumulation of platelets was observed in poorly differentiated tissues where they bind to tumour cells mainly via P-selectin interactions. Clopidrogel therapy was able to induce hepatoma cell differentiation, thus limiting tumour progression in a xenograft model of tumour implantation on NOD/SCID mice.98 Accordingly, a very recent study showed that low dose aspirin administration reduces the incidence of HCC and improves survival in patients with hepatitis-related cirrhosis after splenectomy.99
Finally, given their well-known pro-metastatic role, platelets contribute to the spreading of HCC from the site of origin via direct adhesion to cancer cells. Morimoto et al. reported that high platelet count, high tumour number and the presence of high vascularisation were significantly associated with extrahepatic metastasis in patients with liver cancer.90 This process also seems to be regulated by adhesion with endothelial cells and interactions mediated by molecules such as P-selectin or C-type lectin-like receptor 2 (CLEC-2).100,101 It was also proposed that an interaction between TLR-4 on platelets and HMGB-1 released by tumour cells might mediate platelet-tumour cell interactions.102 Using a murine model of melanoma metastasis and a lack of function Tlr4 knockout mouse, the authors showed that the interaction between TLR-4 and HMGB-1 induced the activation of platelets, leading to the production of TGFβ-1 and enhancing metastatic spreading in the lung and liver. In this line, treatment with the platelet aggregation inhibitor ticagrelor led to a reduction in liver metastases in experimental mouse models of cancer.103 In contrast, Kurokawa et al. reported that a TPO receptor agonist could exert antitumour activity independent of restoring platelet counts. In fact, the cytostatic effect of eltrombopag seems to be mediated mainly by an alteration of iron metabolism in cancer cells.104 Regarding patients with cholangiocarcinoma, only a few clinical studies based on the platelet-to-lymphocyte ratio indicate a possible increase in platelet counts related to poor prognosis and survival.105 Therefore, in the context of liver cancer, a better comprehension of the cellular and molecular mechanisms underlying the interactions between platelets, cancer cells and immune cells is urgently required to validate the efficacy of a potential anti-platelet therapeutic approach.
Future perspectives
Whereas the immunomodulatory functions of platelets during chronic liver diseases are starting to be widely recognised, the precise cellular dynamics and the interactions relevant for disease outcomes are still poorly understood. Pre-clinically, more in vivo functional studies are required to understand if the “polarisation/activation” status of platelets might be important to delineate selective or complementary therapeutic strategies. The effects of anti-platelet therapy in relation to the immune system should be carefully analysed, starting from several available models of liver cancer. Data from the clinic are sparse and still quite controversial. Therefore, there is a need for studies with larger and stratified patient cohorts, which will allow researchers to determine the correlation between platelet profile and disease stage, particularly with respect to liver cancer.
Platelets contain all types of RNA molecules, mostly unspliced immature forms of mRNA, which they can translate into proteins or transfer to neighbouring cells, thereby modulating their biological functions.106 Analyses of the processes regulating protein synthesis, storage and release of pre-stored peptides could be the key to understanding platelet functions and the development of selective therapeutic interventions. For many years, the proteomic and transcriptomic arsenal of platelets has been considered static. Instead, it was recently shown that platelets display a functional spliceosome with spliceosome factors enabling signal-dependent splicing, giving birth to mature peptides.107 In this way, external stimuli that activate platelets through contact with surface receptors induce splicing of specific pre-mRNAs in circulating platelets. Considering that platelets can be shaped and re-programmed according to the genetic and immune environment, a detailed analysis of RNA expression patterns (even at the single cell level) might become a valid tool to identify specific biomarkers of disease. The combination of specific splice events in response to external signals and the capacity of platelets to ingest directly (spliced) circulating mRNAs can provide these cells with a highly dynamic mRNA repertoire, with potential applicability to cancer diagnostics. In fact, platelet RNA can be easily isolated from cell pools and subjected to gene-expression analysis. Promisingly, a transcriptomic approach based on next-generation RNA sequencing was shown to be able to offer interesting insight into the platelet transcript profile.108
A transcriptomic/proteomic approach might offer information on how different environmental stimuli or toxicants (e.g. high caloric food, alcohol, exercise, drugs, etc...) affect the platelet mRNA repertoire and thereby influence the responsiveness to therapeutic regimens or immunosuppressants. Finally, it will be important to identify differences in the RNA signature of circulating platelets and activated platelets in the hepatic tissue within the same pathological context. Considering the less invasive and more accessible nature of liquid biopsies, platelet transcriptome analysis might become a useful tool for the diagnosis of chronic liver disease.
Abbreviations
5HT, serotonin; AFLD, alcohol-related fatty liver disease; BDL, bile-duct ligation; CCL2, chemokine ligand 2; CCL5, chemokine ligand 5; CLEC-2, C-type lectin-like receptor-2; COX-1, cyclooxygenase-1; GPIbα, glycoprotein Ibα; HA, hyaluronan; HBD-1, human beta defensin 1; HGF, hepatic growth factor; HMGB-1, high mobility group box 1; HSCs, hepatic stellate cells; IL-1β, interleukin-1β; KCs, Kupffer cells; miRNA, microRNA; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PDGF, platelet-derived growth factor; PF4, platelet factor 4; PSGL1, P-selectin glycoprotein ligand 1; S1P, sphingosine-1-phosphate; SDF, stromal derived factor; TGF, transforming growth factor; Th, T helper; TLR, toll-like receptor; TPO, thrombopoietin; Treg, regulatory T; TREM-1, triggering receptor expressed on myeloid cells-1; TXA2, thromboxane A2; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor.
Financial support
M.H. was supported by an ERC Consolidator grant (HepatoMetaboPath), the SFBTR 209 and SFBTR179 and the Helmholtz-Gemeinschaft, Zukunftsthema "Immunology and Inflammation" (ZT-0027). This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 667273 and the FWO/FNRS, EOS grant No 30826052 (MODEL-IDI) (Belgium).
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
P.R: conceptualization, writing of the original draft. T.K.: writing of the original draft. N.P.M.: visualization, writing review and editing. M.H.: conceptualization, writing review and editing, funding acquisition.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.10.001.
Contributor Information
Nisar Peter Malek, Email: Nisar.Malek@med.uni-tuebingen.de.
Mathias Heikenwalder, Email: m.heikenwaelder@dkfz-heidelberg.de.
Supplementary data
Supplementary material
References
- 1.Machlus KR, Italiano JE. The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sungaran R, Markovic B, Chong BH. Localization and Regulation of Thrombopoietin mRNA Expression in Human Kidney, Liver, Bone Marrow, and Spleen Using In Situ Hybridization. Blood. 1997;89:101–107. [PubMed] [Google Scholar]
- 3.Lebois M, Josefsson EC. Regulation of platelet lifespan by apoptosis. Platelets. 2016;27:497–504. doi: 10.3109/09537104.2016.1161739. [DOI] [PubMed] [Google Scholar]
- 4.Sørensen AL, Rumjantseva V, Nayeb-Hashemi S, Clausen H, Hartwig JH, Wandall HH. Role of sialic acid for platelet life span: exposure of β-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor–expressing liver macrophages and hepatocytes. Blood. 2009;114:1645–1654. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36:195–198. doi: 10.1007/s10555-017-9677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagel O, Walter E, Jurk K, Zahedi RP. Taking the stock of granule cargo: Platelet releasate proteomics. Platelets. 2017;28:119–128. doi: 10.1080/09537104.2016.1254762. [DOI] [PubMed] [Google Scholar]
- 8.Rendu F, Brohard-Bohn B. The platelet release reaction: granules' constituents, secretion and functions. Platelets. 2001;12:261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 9.Weissmann G. The Role of Lysosomes in Inflammation and Disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]
- 10.Mammadova-Bach E, Mauler M, Braun A, Duerschmied D. Autocrine and paracrine regulatory functions of platelet serotonin. Platelets. 2018;29:541–548. doi: 10.1080/09537104.2018.1478072. [DOI] [PubMed] [Google Scholar]
- 11.Murugappan S, Shankar H, Kunapuli SP. Platelet Receptors for Adenine Nucleotides and Thromboxane A2. Semin Thromb Hemost. 2004;30:411–418. doi: 10.1055/s-2004-833476. [DOI] [PubMed] [Google Scholar]
- 12.Andersen H, Greenberg DL, Fujikawa K, Xu W, Chung DW, Davie EW. Protease-activated receptor 1 is the primary mediator of thrombin-stimulated platelet procoagulant activity. Proc Natl Acad Sci U S A. 1999;96:11189–11193. doi: 10.1073/pnas.96.20.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan A, Lalor P, Watson S, Adams D. Role of CLEC-2-driven platelet activation in the pathogenesis of toxic liver damage. Lancet. 2017;389:S33. [Google Scholar]
- 14.Keane C., Tilley D., Cunningham A., Smolenski A., Kadioglu A., Cox D. Invasive Streptococcus pneumoniae trigger platelet activation via Toll-like receptor 2. J Thromb Haemost. 2010;8:2757–2765. doi: 10.1111/j.1538-7836.2010.04093.x. [DOI] [PubMed] [Google Scholar]
- 15.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 16.Brown GT, McIntyre TM. Lipopolysaccharide Signaling without a Nucleus: Kinase Cascades Stimulate Platelet Shedding of Proinflammatory IL-1β–Rich Microparticles. J Immunol. 2011;186:5489–5496. doi: 10.4049/jimmunol.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark RJ, Aghakasiri N, Rumbaut RE. Platelet-Derived Toll-Like Receptor 4 (Tlr-4) Is Sufficient to Promote Microvascular Thrombosis in Endotoxemia. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam FW, Vijayan KV, Rumbaut RE. Platelets and Their Interactions with Other Immune Cells. Compr Physiol. 2015;5:1265–1280. doi: 10.1002/cphy.c140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youssefian T, Drouin A, Massé J-M, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 20.Wong CHY, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. 2013;14:785. doi: 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PATEL KN, SOUBRA SH, LAM FW, RODRIGUEZ MA, RUMBAUT RE. Polymicrobial sepsis and endotoxemia promote microvascular thrombosis via distinct mechanisms. J Thromb Haemost. 2010;8:1403–1409. doi: 10.1111/j.1538-7836.2010.03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B. Migrating Platelets Are Mechano-scavengers that Collect and Bundle Bacteria. Cell. 2017;171:1368–1382. doi: 10.1016/j.cell.2017.11.001. e1323. [DOI] [PubMed] [Google Scholar]
- 24.Burzynski LC, Humphry M, Pyrillou K, Wiggins KA, Chan JNE, Figg N. The Coagulation and Immune Systems Are Directly Linked through the Activation of Interleukin-1α; by Thrombin. Immunity. 2019;50:1033–1042.e1036. doi: 10.1016/j.immuni.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PEERSCHKE EIB, YIN W, GRIGG SE, GHEBREHIWET B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- 26.Verschoor A, Neuenhahn M, Navarini AA, Graef P, Plaumann A, Seidlmeier A. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol. 2011;12:1194. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 27.Labelle M, Begum S, Hynes Richard O. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR. Platelet-Mediated Modulation of Adaptive Immunity: A Communication Link between Innate and Adaptive Immune Compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 29.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR. Platelets mediate cytotoxic T lymphocyte–induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell–mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 32.Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8:310. doi: 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucotti S, Cerutti C, Soyer M, Gil-Bernabé AM, Gomes AL, Allen PD. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J Clin Invest. 2019;129:1845–1862. doi: 10.1172/JCI121985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyslop SR, Josefsson EC. Undercover Agents: Targeting Tumours with Modified Platelets. Trends Cancer. 2017;3:235–246. doi: 10.1016/j.trecan.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Pradella P, Bonetto S, Turchetto S, Uxa L, Comar C, Zorat F. Platelet production and destruction in liver cirrhosis. J Hepatol. 2011;54:894–900. doi: 10.1016/j.jhep.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W. Platelet-Derived Serotonin Mediates Liver Regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 38.Groeneveld D, Pereyra D, Veldhuis Z, Adelmeijer J, Ottens P, Kopec AK. Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans. Blood. 2019;133:1245–1256. doi: 10.1182/blood-2018-08-869057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimabukuro R, Kawanaka H, Tomikawa M, Akahoshi T, Konishi K, Yoshida D. Effect of thrombopoietin on platelet counts and liver regeneration after partial hepatectomy in a rat model. Surg Today. 2009;39:1054–1059. doi: 10.1007/s00595-008-4054-6. [DOI] [PubMed] [Google Scholar]
- 40.Matsuo R, Ohkohchi N, Murata S, Ikeda O, Nakano Y, Watanabe M. Platelets Strongly Induce Hepatocyte Proliferation with IGF-1 and HGF In Vitro. J Surg Res. 2008;145:279–286. doi: 10.1016/j.jss.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Slaba I, Wang J, Kolaczkowska E, McDonald B, Lee W-Y, Kubes P. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology. 2015;62:1593–1605. doi: 10.1002/hep.28003. [DOI] [PubMed] [Google Scholar]
- 42.Shido K, Chavez D, Cao Z, Ko J, Rafii S, Ding B-S. Platelets prime hematopoietic and vascular niche to drive angiocrine-mediated liver regeneration. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki T, Murata S, Takahashi K, Nozaki R, Ohshiro Y, Ikeda N. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J Hepatol. 2010;53:648–654. doi: 10.1016/j.jhep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Kirschbaum M, Jenne CN, Veldhuis ZJ, Sjollema KA, Lenting PJ, Giepmans BNG. Transient von Willebrand factor-mediated platelet influx stimulates liver regeneration after partial hepatectomy in mice. Liver Int. 2017;37:1731–1737. doi: 10.1111/liv.13386. [DOI] [PubMed] [Google Scholar]
- 45.Kotecha R, Toledo-Pereyra LH. Kupffer cell and platelet interactions in hepatic ischemia reperfusion. J Surg Res. 2013;181:219–221. doi: 10.1016/j.jss.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 46.Laffi G, Tarquini R, Marra F. Thrombocytopenia in chronic liver disease: lessons from transplanted patients. J Hepatol. 2007;47:625–629. doi: 10.1016/j.jhep.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi K, Yu Z, Kumamoto H, Sugawara Y, Kawamura H, Takada H. Involvement of Kupffer cells in lipopolysaccharide-induced rapid accumulation of platelets in the liver and the ensuing anaphylaxis-like shock in mice. Biochim Biophys Acta. 2006;1762:269–275. doi: 10.1016/j.bbadis.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 48.D'Mello C, Almishri W, Liu H, Swain MG. Interactions Between Platelets and Inflammatory Monocytes Affect Sickness Behavior in Mice With Liver Inflammation. Gastroenterology. 2017;153:1416–1428.e1412. doi: 10.1053/j.gastro.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Gowert NS, Klier M, Reich M, Reusswig F, Donner L, Keitel V. Defective Platelet Activation and Bleeding Complications upon Cholestasis in Mice. Cell Physiol Biochem. 2017;41:2133–2149. doi: 10.1159/000475566. [DOI] [PubMed] [Google Scholar]
- 50.Laschke MW, Dold S, Menger MD, Jeppsson B, Thorlacius H. Platelet-dependent accumulation of leukocytes in sinusoids mediates hepatocellular damage in bile duct ligation-induced cholestasis. Br J Pharmacol. 2008;153:148–156. doi: 10.1038/sj.bjp.0707578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maruyama T, Murata S, Takahashi K, Tamura T, Nozaki R, Ikeda N. Platelet Transfusion Improves Liver Function in Patients with Chronic Liver Disease and Cirrhosis. Tohoku J Exp Med. 2013;229:213–220. doi: 10.1620/tjem.229.213. [DOI] [PubMed] [Google Scholar]
- 52.Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg. 2008;248:821–828. doi: 10.1097/SLA.0b013e31818584c7. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda H, Yatomi Y, Yanase M, Satoh H, Maekawa H, Ogata I. Biological activities of novel lipid mediator sphingosine 1-phosphate in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G304–G310. doi: 10.1152/ajpgi.2000.279.2.G304. [DOI] [PubMed] [Google Scholar]
- 54.Zaldivar MM, Pauels K, von Hundelshausen P, Berres M-L, Schmitz P, Bornemann J. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010;51:1345–1353. doi: 10.1002/hep.23435. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida S, Ikenaga N, Liu SB, Peng Z-W, Chung J, Sverdlov DY. Extrahepatic Platelet-Derived Growth Factor-β, Delivered by Platelets, Promotes Activation of Hepatic Stellate Cells and Biliary Fibrosis in Mice. Gastroenterology. 2014;147:1378–1392. doi: 10.1053/j.gastro.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 56.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghafoory S, Varshney R, Robison T, Kouzbari K, Woolington S, Murphy B. Platelet TGF-β1 deficiency decreases liver fibrosis in a mouse model of liver injury. Blood Adv. 2018;2:470–480. doi: 10.1182/bloodadvances.2017010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ikeda N, Murata S, Maruyama T, Tamura T, Nozaki R, Kawasaki T. Platelet-derived adenosine 5′-triphosphate suppresses activation of human hepatic stellate cell: In vitro study. Hepatol Res. 2012;42:91–102. doi: 10.1111/j.1872-034X.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 59.Kim W-H, Matsumoto K, Bessho K, Nakamura T. Growth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am J Pathol. 2005;166:1017–1028. doi: 10.1016/S0002-9440(10)62323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kodama T, Takehara T, Hikita H, Shimizu S, Li W, Miyagi T. Thrombocytopenia Exacerbates Cholestasis-Induced Liver Fibrosis in Mice. Gastroenterology. 2010;138:2487–2498.e2487. doi: 10.1053/j.gastro.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan BP, Wang R, Tawfik O, Luyendyk JP. Protective and Damaging Effects of Platelets in Acute Cholestatic Liver Injury Revealed by Depletion and Inhibition Strategies. Toxicol Sci. 2009;115:286–294. doi: 10.1093/toxsci/kfq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Meer AJ, Maan R, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F. Improvement of platelets after SVR among patients with chronic HCV infection and advanced hepatic fibrosis. J Gastroenterol Hepatol. 2016;31:1168–1176. doi: 10.1111/jgh.13252. [DOI] [PubMed] [Google Scholar]
- 63.Sayyar M., Saidi M., Zapatka S., Deng Y., Ciarleglio M., Garcia-Tsao G. Platelet count increases after viral elimination in chronic HCV, independent of the presence or absence of cirrhosis. Liver Int. 2019;39:2061–2065. doi: 10.1111/liv.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afdhal NH, Dusheiko GM, Giannini EG, Chen PJ, Han KH, Mohsin A. Eltrombopag Increases Platelet Numbers in Thrombocytopenic Patients With HCV Infection and Cirrhosis, Allowing for Effective Antiviral Therapy. Gastroenterology. 2014;146:442–452.e441. doi: 10.1053/j.gastro.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Frelinger AL, Smolensky Koganov E, Forde EE, Carmichael SL, Michelson AD. Avatrombopag, a Novel Thrombopoietin Receptor Agonist, Increases Platelet Counts without Increasing Platelet Activation in Patients with Thrombocytopenia Due to Chronic Liver Disease. Blood. 2017;130:290. [Google Scholar]
- 66.Kondo R, Yano H, Nakashima O, Tanikawa K, Nomura Y, Kage M. Accumulation of platelets in the liver may be an important contributory factor to thrombocytopenia and liver fibrosis in chronic hepatitis C. J Gastroenterol. 2013;48:526–534. doi: 10.1007/s00535-012-0656-2. [DOI] [PubMed] [Google Scholar]
- 67.Aref S, Sleem T, El Menshawy N, Ebrahiem L, Abdella D, Fouda M. Antiplatelet antibodies contribute to thrombocytopenia associated with chronic hepatitis C virus infection. Hematology. 2009;14:277–281. doi: 10.1179/102453309X439818. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Wang B, You H, Wu X, Zhou J, Ou X. Platelets’ increase is associated with improvement of liver fibrosis in entecavir-treated chronic hepatitis B patients with significant liver fibrosis. Hepatol Int. 2018;12:237–243. doi: 10.1007/s12072-018-9864-z. [DOI] [PubMed] [Google Scholar]
- 69.Iannacone M, Sitia G, Ruggeri ZM, Guidotti LG. HBV pathogenesis in animal models: Recent advances on the role of platelets. J Hepatol. 2007;46:719–726. doi: 10.1016/j.jhep.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guidotti Luca G, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L. Immunosurveillance of the Liver by Intravascular Effector CD8+ T Cells. Cell. 2015;161:486–500. doi: 10.1016/j.cell.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Born G, Patrono C. Antiplatelet drugs. Br J Pharmacol. 2006;147:S241–S251. doi: 10.1038/sj.bjp.0706401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-Dose Aspirin for the Prevention of Atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 73.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP. Identification and Biological Activity of the Active Metabolite of Clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 74.Iannacone M, Sitia G, Narvaiza I, Ruggeri ZM, Guidotti LG. Antiplatelet Drug Therapy Moderates Immune-Mediated Liver Disease and Inhibits Viral Clearance in Mice Infected with a Replication-Deficient Adenovirus. Clin Vaccine Immunol. 2007;14:1532–1535. doi: 10.1128/CVI.00298-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci. 2012;109:E2165–E2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 77.Liu F, Zhou H, Cao L, Guo Z, Dong C, Yu L. Risk of reduced platelet counts in patients with nonalcoholic fatty liver disease (NAFLD): a prospective cohort study. Lipids Health Dis. 2018;17:221. doi: 10.1186/s12944-018-0865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Potze W, Siddiqui MS, Boyett SL, Adelmeijer J, Daita K, Sanyal AJ. Preserved hemostatic status in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;65:980–987. doi: 10.1016/j.jhep.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Alkhouri N, Kistangari G, Campbell C, Lopez R, Zein NN, Feldstein AE. Mean platelet volume as a marker of increased cardiovascular risk in patients with nonalcoholic steatohepatitis. Hepatology. 2012;55:331. doi: 10.1002/hep.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinholt PJ, Hvas A-M, Nielsen C, Söderström AC, Sprogøe U, Fialla AD. Reduced platelet activation and platelet aggregation in patients with alcoholic liver cirrhosis. Platelets. 2018;29:520–527. doi: 10.1080/09537104.2017.1349308. [DOI] [PubMed] [Google Scholar]
- 81.Lee WS, Kim TY. Alcoholic fatty liver disease and alcoholic liver cirrhosis may be differentiated with mean platelet volume and platelet distribution width. Platelets. 2010;21:584–585. doi: 10.3109/09537104.2010.500423. [DOI] [PubMed] [Google Scholar]
- 82.Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641–655. doi: 10.1038/s41591-019-0379-5. [DOI] [PubMed] [Google Scholar]
- 83.Yang YM, Noureddin M, Liu C, Ohashi K, Kim SY, Ramnath D. Hyaluronan synthase 2–mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nandi A, Estess P, Siegelman MH. Hyaluronan Anchoring and Regulation on the Surface of Vascular Endothelial Cells Is Mediated through the Functionally Active Form of CD44. J Biol Chem. 2000;275:14939–14948. doi: 10.1074/jbc.275.20.14939. [DOI] [PubMed] [Google Scholar]
- 85.Schwarzkopf K, Bojunga J, Rüschenbaum S, Martinez Y, Mücke MM, Seeger F. Use of Antiplatelet Agents Is Inversely Associated With Liver Fibrosis in Patients With Cardiovascular Disease. Hepatol Commun. 2018;2:1601–1609. doi: 10.1002/hep4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(13):2776–2784.e4. doi: 10.1016/j.cgh.2019.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carr BI, Lin C-Y, Lu S-N. Platelet-Related Phenotypic Patterns in Hepatocellular Carcinoma Patients. Semin Oncol. 2014;41:415–421. doi: 10.1053/j.seminoncol.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carr BI, Guerra V, De Giorgio M, Fagiuoli S, Pancoska P. Small Hepatocellular Carcinomas and Thrombocytopenia. Oncology. 2012;83:331–338. doi: 10.1159/000341533. [DOI] [PubMed] [Google Scholar]
- 89.Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71(3):523–533. doi: 10.1016/j.jhep.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morimoto Y, Nouso K, Wada N, Takeuchi Y, Kinugasa H, Miyahara K. Involvement of platelets in extrahepatic metastasis of hepatocellular carcinoma. Hepatol Res. 2014;44:E353–E359. doi: 10.1111/hepr.12315. [DOI] [PubMed] [Google Scholar]
- 91.Xue T-C, Ge N-L, Xu X, Le F, Zhang B-H, Wang Y-H. High platelet counts increase metastatic risk in huge hepatocellular carcinoma undergoing transarterial chemoembolization. Hepatol Res. 2016;46:1028–1036. doi: 10.1111/hepr.12651. [DOI] [PubMed] [Google Scholar]
- 92.Scheiner B, Kirstein M, Popp S, Hucke F, Bota S, Rohr-Udilova N. Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma. Liver Cancer. 2019;8:203–217. doi: 10.1159/000489833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuo X, Kong W, Feng L, Zhang H, Meng X, Chen W. Elevated platelet distribution width predicts poor prognosis in hepatocellular carcinoma. Cancer Biomark. 2019;24:307–313. doi: 10.3233/CBM-182076. [DOI] [PubMed] [Google Scholar]
- 94.Margetts J, Ogle LF, Chan SL, Chan AWH, Chan KCA, Jamieson D. Neutrophils: driving progression and poor prognosis in hepatocellular carcinoma? Br J Cancer. 2017;118:248. doi: 10.1038/bjc.2017.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carr BI, Cavallini A, D’Alessandro R, Refolo MG, Lippolis C, Mazzocca A. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer. 2014;14:43. doi: 10.1186/1471-2407-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Padickakudy R, Pereyra D, Offensperger F, Jonas P, Oehlberger L, Schwarz C. Bivalent role of intra-platelet serotonin in liver regeneration and tumor recurrence in humans. J Hepatol. 2017;67:1243–1252. doi: 10.1016/j.jhep.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 97.He A-D, Xie W, Song W, Ma Y-Y, Liu G, Liang M-L. Platelet releasates promote the proliferation of hepatocellular carcinoma cells by suppressing the expression of KLF6. Sci Rep. 2017;7:3989. doi: 10.1038/s41598-017-02801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang R., Guo H., Xu J., Li B., Liu Y.J., Cheng C. Activated platelets inhibit hepatocellular carcinoma cell differentiation and promote tumor progression via platelet-tumor cell binding. Oncotarget. 2016;7:60609–60622. doi: 10.18632/oncotarget.11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Du Z-Q, Zhao J-Z, Dong J, Bi J-B, Ren Y-F, Zhang J. Effect of low-dose aspirin administration on long-term survival of cirrhotic patients after splenectomy: A retrospective single-center study. World J Gastroenterol. 2019;25:3798–3807. doi: 10.3748/wjg.v25.i28.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki-Inoue K. Roles of the CLEC-2–podoplanin interaction in tumor progression. Platelets. 2018;29:786–792. doi: 10.1080/09537104.2018.1478401. [DOI] [PubMed] [Google Scholar]
- 101.Coupland LA, Chong BH, Parish CR. Platelets and P-Selectin Control Tumor Cell Metastasis in an Organ-Specific Manner and Independently of NK Cells. Cancer Res. 2012;72:4662–4671. doi: 10.1158/0008-5472.CAN-11-4010. [DOI] [PubMed] [Google Scholar]
- 102.Yu L-X, Yan L, Yang W, Wu F-Q, Ling Y, Chen S-Z. Platelets promote tumour metastasis via interaction between TLR4 and tumour cell-released high-mobility group box1 protein. Nat Commun. 2014;5:5256. doi: 10.1038/ncomms6256. [DOI] [PubMed] [Google Scholar]
- 103.Gebremeskel S, LeVatte T, Liwski RS, Johnston B, Bezuhly M. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int J Cancer. 2015;136:234–240. doi: 10.1002/ijc.28947. [DOI] [PubMed] [Google Scholar]
- 104.Kurokawa T, Murata S, Zheng YW, Iwasaki K, Kohno K, Fukunaga K. The Eltrombopag antitumor effect on hepatocellular carcinoma. Int J Oncol. 2015;47:1696–1702. doi: 10.3892/ijo.2015.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu G, Liu Q, Ma JY, Liu CY. Prognostic Significance of Platelet-to-Lymphocyte Ratio in Cholangiocarcinoma: A Meta-Analysis. Biomed Res Int. 2018;2018:7375169. doi: 10.1155/2018/7375169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nassa G, Giurato G, Cimmino G, Rizzo F, Ravo M, Salvati A. Splicing of platelet resident pre-mRNAs upon activation by physiological stimuli results in functionally relevant proteome modifications. Sci Rep. 2018;8:498. doi: 10.1038/s41598-017-18985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Best MG, In 't Veld S, Sol N, Wurdinger T. RNA sequencing and swarm intelligence-enhanced classification algorithm development for blood-based disease diagnostics using spliced blood platelet RNA. Nat Protoc. 2019;14:1206–1234. doi: 10.1038/s41596-019-0139-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material