Background & Aims
Insulin resistance is a core pathophysiological defect underscoring type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD). Both conditions improve with duodenal exclusion surgery. Duodenal mucosal resurfacing (DMR) is an endoscopic intervention developed to treat metabolic disease which has been shown to improve glycaemia in patients with poorly controlled T2DM. Herein, we aimed to further analyse the effects of DMR on hepatic and metabolic parameters in this patient cohort.
Methods
Eighty-five patients with T2DM who received endoscopic DMR treatment were enrolled from 5 centres and followed up for 6 months. We assessed safety in all patients. Efficacy was evaluated in patients who received at least 9 cm of duodenal ablation (n = 67). Endpoints included HbA1c, fasting plasma glucose, weight and aminotransferase levels. Metabolomic analysis was conducted in a subgroup (n = 14). Data were analysed using paired t test or ANOVA for repeated measures with Bonferroni correction and correction for initial weight loss if applicable.
Results
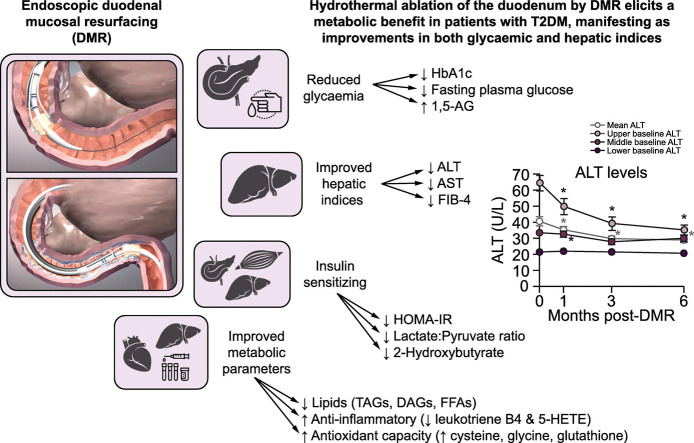
The DMR procedure was completed with no intraprocedural complications in the entire cohort. HbA1c was lower 6 months after DMR than at baseline (7.9 ± 0.2% vs. 9.0 ± 0.2% [mean ± SE], p ≪0.001). Fasting plasma glucose was also significantly lower 6 months after DMR compared to baseline (161 ± 7 mg/dl vs. 189 ± 6 mg/dl, p = 0.005). Body weight decreased slightly. At 6 months, alanine aminotransferase had decreased from 41 ± 3 IU/L to 29 ± 2 IU/L (p ≪0.001) and aspartate aminotransferase had decreased from 30 ± 2 IU/L to 23 ± 1 IU/L (p ≪0.001). Metabolomic analysis demonstrated that DMR had key lipid-lowering, insulin-sensitizing and anti-inflammatory effects, as well as increasing antioxidant capacity. Mean FIB-4 was also markedly decreased.
Conclusion
Hydrothermal ablation of the duodenum by DMR elicits a beneficial metabolic response in patients with T2DM. DMR also improves hepatic indices, potentially through an insulin-sensitizing mechanism. These encouraging data deserve further evaluation in randomized controlled trials.
Lay summary
Hydrothermal duodenal mucosal resurfacing (DMR) is an endoscopic technique designed to treat metabolic disease through ablation of the duodenal mucosa. DMR is a safe procedure which improves glycaemia and hepatic indices in patients with type 2 diabetes mellitus. DMR is an insulin-sensitizing intervention which can be complementary to lifestyle intervention approaches and pharmacological treatments aimed at preserving the pancreas and liver from failure. DMR is a potential therapeutic solution for patients with type 2 diabetes and fatty liver disease.
Keywords: Duodenal Mucosal Resurfacing, therapeutic endoscopy, Type 2 Diabetes Mellitus, T2DM, Metabolic Syndrome, Blood Glucose, Glycated Haemoglobin A1c, NAFLD, Non-Alcoholic Fatty Liver Disease, Aminotransferase levels, Insulin Resistance, Metabolomics
Graphical abstract
Highlights
-
•
Duodenal mucosal resurfacing elicits a metabolic benefit in patients with T2DM.
-
•
At 6 months post-duodenal mucosal resurfacing, HbA1c decreases by 1.0-1.5%.
-
•
In patients with high ALT baseline levels, duodenal mucosal resurfacing elicits an ALT reduction of ~40–50%.
-
•
FIB-4 scores decrease significantly after duodenal mucosal resurfacing.
-
•
Duodenal mucosal resurfacing elicits insulin-sensitizing, lipid-lowering, anti-inflammatory, and antioxidant effects
Introduction
Type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD) are both metabolic disorders1,2 of epidemic scale with a significant overlap between affected populations.3 T2DM has a well understood pathophysiological foundation of insulin resistance leading to pancreatic beta cell failure. Complications may result from the primary effects of insulin resistance, as well as from uncontrolled hyperglycaemia. Despite the availability of over 50 different medicines approved for control of hyperglycaemia, nearly 50% of patients remain poorly controlled4 due to progressive beta cell failure and poor treatment adherence. Insulin resistance also leads to NAFLD, wherein hyperinsulinemia may drive progressive fat accumulation, which in a proinflammatory milieu may foster cell stress, inflammation, and fibrosis in the liver, a condition known as non-alcoholic steatohepatitis (NASH).5 As a consequence of rising prevalence rates, NAFLD/NASH is predicted to become the leading indication for liver transplantation in the United States by 2020.6 The co-existence of T2DM and NAFLD/NASH is associated with a higher incidence of complications of diabetes and liver disease than either alone.7Recent studies have shown that 30% to 70% of patients with T2DM have NAFLD or NASH on biopsy, even in the presence of normal plasma aminotransferase levels.3,8 In contrast to T2DM, there are currently no generally approved pharmacological treatments for NAFLD or NASH. Instead, the mainstay of treatment involves lifestyle changes and weight loss (preferably at least 10% reduction of total body weight) to improve inflammation and fibrosis.9 Although a liver biopsy is still the “gold standard” for the diagnosis and assessment of liver fibrosis, non-invasive measures such as serum markers or elastography are often utilized in clinical practice. Of these, the fibrosis 4 index (FIB-4) shows acceptable diagnostic accuracy in predicting fibrosis stage and is sensitive to improvement or worsening of fibrosis in patients with NAFLD.10,11
Invasive procedures, such as bariatric surgery, have emerged for the treatment of severe dysmetabolic states. Gastric bypass surgery, such as the Roux-en-Y gastric bypass, is the best characterized insulin sensitizing intervention, leading to robust improvements in glycaemic state such that some patients achieve long term disease remission.12,13 Striking improvement in NAFLD/NASH, including reversal of fibrosis,14 has also been observed. Notably, these metabolic improvements are not associated with post-surgery weight loss, occurring independently of body mass index (BMI) and before major weight loss has been obtained.13 However, because of its invasiveness, bariatric surgery is not suitable as a population-wide treatment for metabolic disease.
Advances in bariatric science have demonstrated that the exposure of the duodenum to nutrients is associated with systemic insulin resistance in obese patients with T2DM.15 Bypass of the duodenum after bariatric surgery leads to improvements in insulin sensitivity.16,17 Conversely, nutrient re-exposure to the bypassed duodenal mucosa in rodents and humans rapidly restores hepatic insulin resistance.18,19 Furthermore, excessive fat and hexose ingestion has been shown to induce duodenal mucosa hypertrophy, with unusually high densities of enteroendocrine cells in mice and men.2,20 Taken together, these observations suggest that the duodenal mucosa is an important metabolic regulator that determines systemic insulin sensitivity and represents an interesting therapeutic target as a means of modulating insulin resistance.
Hydrothermal duodenal mucosal resurfacing (DMR [Revita™ DMR], Fractyl Laboratories, Inc., Lexington, MA, USA) is an endoscopic technique that has been designed to treat metabolic disease through ablation of the duodenal mucosa. DMR is performed using a trans-oral endoscopic catheter that allows hydrothermal ablation of the duodenal mucosa, all under endoscopic visualization with fluoroscopic support. Clinical data suggest that DMR is well tolerated and elicits a clinically significant improvement in hyperglycaemia at 6 and 12 months in patients with T2DM poorly controlled on oral antidiabetic medications.21,22 In this article, we report the effect of DMR on hepatic and metabolic indices in patients with poorly controlled T2DM followed for 6 months after the procedure. Data were pooled from 2 single-arm, open-label studies: a single-centre first-in human study21 (NCT01927562) and a multicentre study22 (NCT02413567). We also report the effect on glycaemia in this composite cohort.
Patients and methods
Patients
We report composite data extracted from 2 studies: an initial single-centre study21 and a subsequent multicentre study22 conducted in 5 centres (including the centre from the original study). Patients with T2DM were eligible if they were treated with at least 1 oral glucose-lowering drug (with no changes to medication for at least 3 months prior to screening in the multicentre study) and had fasting C-peptide ≫1 ng/ml (indicative of sufficient beta cell reserve). They were adults (age 28–75 years) with T2DM duration ≪10 years, BMI 24–40 kg/m2, and glycosylated haemoglobin 1Ac (HbA1c) of 7.5–12.0% in the single-centre study and 7.5–11.0% in the multicentre study (upper limit of normal: 6.0%). The main exclusion criteria were: diagnosis of type 1 diabetes or history of diabetic ketoacidosis, use of insulin or GLP-1RA, autoimmune disease, history of acute or chronic pancreatitis, known active hepatitis or active liver disease, symptomatic kidney stones or gallstones, use of weight loss medication or anti-inflammatory drugs, anticoagulation therapy, or gastrointestinal surgery or duodenal abnormalities that would impede the DMR procedure.
Sites and study design
The single-centre study was conducted in South America (CCO Clinical Center for Diabetes, Obesity and Reflux, Santiago, Chile). Patients underwent DMR from August 2013 until November 2014. The multicentre study was conducted at 7 sites: The 7 study sites were the Academic Medical Center, Amsterdam, the Netherlands; Erasme University Hospital, Brussels, Belgium; Policlinico Gemelli, Catholic University of Rome, Rome, Italy; University College London Hospital, London, United Kingdom; CCO Clinical Center for Diabetes, Obesity and Reflux, Santiago, Chile; King’s College Hospital, London, United Kingdom; and University Hospital Leuven, Leuven, Belgium. Patients underwent DMR from April 2015 until November 2015. In the single-centre study, patients were screened (including screening endoscopy of the upper gastrointestinal tract) and eligible patients were enrolled, treated with DMR, and seen for follow-up visits at day 7, day 14 and 1, 3, and 6 months after DMR. Patients and study physicians were advised to refrain from altering patients’ oral glucose-lowering medication use except when clinically necessary. The multicentre study included 2 visits before DMR. 1) Screening visit to select eligible patients based on the inclusion-exclusion criteria. Eligible patients underwent a 4-week run-in period during which sulfonylureas or meglitinides were discontinued. 2) Baseline visit after the run-in. The DMR procedure was scheduled no more than 14 days after baseline. Follow-up visits were scheduled at 1, 3, 4.5, and 6 months after the procedure and glucose-lowering medication was kept stable for at least 6 months after the procedure. At 3 (single-centre study) or 6 months (multicentre study) a follow-up endoscopy was performed to assess the duodenum. Screening visits in both studies included completion of informed consent, physical examination and medical history. All patients provided written informed consent. Study protocols were approved by the ethics committees of the respective sites and complied with the recommendations of the Declaration of Helsinki. The effects of DMR on glycaemia have already been reported for the single-centre and multicentre study separately.21,22 In this paper, we additionally report the effect of DMR on hepatic parameters and carry out additional metabolomic analysis.
Study procedure
Hydrothermal DMR is an endoscopic, catheter-based procedure performed under general anaesthesia or sedation with propofol. The procedure is described in detail in previous publications.21,22 Briefly, the procedure comprises catheter-based mucosal lifting with saline, and circumferential mucosal ablation with a hot fluid-filled balloon at the tip of the catheter. Ablation temperature and time are tightly controlled via a console specifically designed for this procedure. The ablated duodenal length was calculated by multiplying the number of duodenal ablations by the length of the ablation balloon. Post-DMR, all patients were to follow a graduated diet for 2 weeks in which clear liquid beverages were gradually expanded to solid food products.
Assessments
For this analysis, a complete DMR procedure was defined as ablated duodenal length of ≥9 cm for both studies. This complete DMR cohort was used for the efficacy analyses in this study. At all study visits, medication use, adverse events, body weight, and blood pressure were determined, and fasting venous blood samples were collected for blood analysis, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), fasting plasma glucose (FPG) and HbA1c. Mean plasma glycaemic and hepatic parameters and body weight were calculated at baseline and 1, 3, and 6 months follow-up. The primary efficacy endpoint was mean reduction in HbA1c from baseline to 6 months after the procedure. Based on baseline ALT and AST levels, patients were divided into 3 tertiles: the lower, middle, and upper tertile. The ALT tertiles were ≤27 IU/L (lower), 28–41 IU/L (middle), and ≥42 IU/L (upper). The AST tertiles were AST ≤22 IU/L (lower), 23–30 IU/L (middle), and ≥31 IU/L (upper). FIB-4 was calculated at baseline and 6 months using the formula: [Age(years)×AST(IU/L)]/[platelet count (×109/L) × ALT(IU/L)1/2]. A FIB-4 score ≪1.30 is associated with a low probability of clinically significant fibrosis.10,11
In a subgroup of patients from the initial single-centre study (n = 14), a mixed meal tolerance test (MMTT) was performed at baseline and 3 months. Patients ingested a standard liquid meal (Fresubin® [200 ml, 2.0 kcal/ml] containing 15.6 g fat, 20 g protein and 45 g carbohydrates per meal). Glucose was measured at fasting and at 15, 30, 45, 60, 90, 120, and 180 minutes postprandially. Metabolomic analysis (Metabolon, Inc., Durham, NC) was conducted on fasting and 60 and 120 min MMTT plasma samples. A non-targeted relative quantitative liquid chromatography–tandem mass spectrometry platform was applied to identify structurally named and unknown molecules (Evans AM, 2009, Evans AM, 2014). All normalized relative ion counts were log-transformed, and the remaining data were imputed with the minimum value on a per metabolite basis. Complex lipids were extracted from samples in the presence of internal standards using a modification of the method of Bligh and Dyer. The extracts were concentrated, reconstituted and transferred to vials for infusion-mass spectrometry analysis. The analysis was performed on a Shimadzu LC with nano PEEK tubing and a Sciex SelexIon equipped-5500 QTRAP. The analysis was performed in DMS-MRM mode with both positive and negative ionization for a total of more than 1,100 MRMs. Individual lipid species were quantified by comparing the intensity ratios of target compounds and their assigned internal standards which were added at known concentrations. Lipid class concentrations were calculated from the sum of all molecular species within a class, and fatty acid compositions were determined by calculating the proportion of each class comprised of individual fatty acids. The false discovery rate for a given set of compounds to correct for multiple testing was estimated using the q-value.23 Repeated measures ANOVA was performed with visit, time, and visit:time as fixed effects and subject as a random effect. The contrasts were calculated as planned contrasts for the ANOVA (Supplementary CTAT Table). During the MMTT, glucose was measured at 0, 15, 30, 45, 60, 90, 120, and 180 min postprandially.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics version 24.0 (SPSS Inc. Chicago, USA) (Supplementary CTAT Table). p values ≪0.05 were considered statistically significant. Data distribution was assessed using histograms and eyeballing. Depending on the data distribution, data are expressed as mean ± SE or median with range. Paired Student’s t tests were used to compare 2 data points before and after the procedure. Differences between repeated measurements of HbA1c, FPG, ALT, and AST during follow-up were tested using repeated measures ANOVA with Bonferroni correction (3 tests, p values ≪0.05/3 were considered statistically significant). Where appropriate (when a linear relationship was found), we corrected for early weight loss (defined as weight loss at post-procedure week 4) or HbA1c change during analysis of the efficacy parameters.
Results
Patients
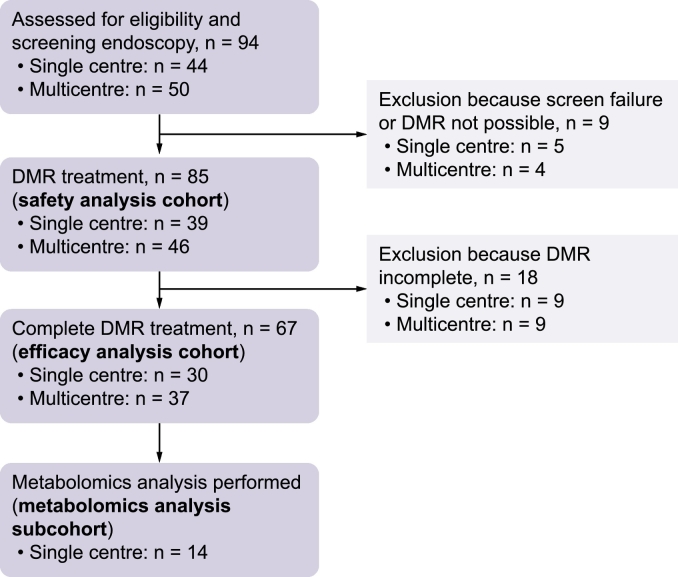
Ninety-four patients (44 single-centre study, 50 multicentre study) underwent the initial endoscopy (Fig. 1) and 85 (39 single-centre study, 46 multicentre study) patients received actual DMR treatment comprising the safety analysis cohort. Reasons to not perform DMR were presence of esophagitis ≥ grade 3 and abnormalities in the gastrointestinal tract preventing endoscopic access to the duodenum or precluding completion of DMR. From the safety analysis cohort, 67 patients (30 single-centre study, 37 multicentre study) received a complete duodenal ablation (minimal duodenal ablation length of 9 cm) comprising the efficacy analysis cohort. The mean length of the ablated segment was 9.4 ± 0.1 cm. Metabolomic analysis was conducted on MMTT plasma samples in a sub-cohort from the single-centre study (n = 14). Table 1 itemizes baseline demographics from the 2 study sources, the efficacy cohort and the metabolomic analysis sub-cohort.
Fig. 1.
Patient flow in the single-centre and multicentre studies.
DMR, duodenal mucosal resurfacing.
Table 1.
Patient characteristics at baselinea for patients receiving complete DMR.
| Single-centre study complete DMR sub-cohort | Multicentre study complete DMR sub-cohort | Efficacy analysis cohort (pooled complete DMR cohort) | Metabolomic analysis sub-cohort | |
|---|---|---|---|---|
| N | 30 | 37 | 67 | 14 |
| Age, years | 52 ± 1 | 56 ± 1 | 54 ± 1 | 51 ± 2 |
| Male, n (%) | 22 (73) | 23 (62) | 45 (67) | 12 (86) |
| Body weight, kg | 87.5 ± 2.1 | 89.5 ± 2.2 | 88.6 ± 1.5 | 88.6 ± 2.7 |
| Duration of T2DM, years | 5.6 ± 0.4 | 6.1 ± 0.4 | 5.9 ± 0.3 | 6.4 ± 0.6 |
| HbA1c, % (mmol/mol) | 9.7 ± 0.3 (83 ± 3) | 8.4 ± 0.1 (68 ± 1) | 9.0 ± 0.1 (75 ± 1) | 10.2 ± 0.3 (88 ± 4) |
| FPG, mg/dl | 186 ± 11 | 192 ± 7 | 189 ± 6 | 198 ± 14 |
| ALT, U/L | 40 ± 4 | 39 ± 4 | 41 ± 3 | 40 ± 4 |
| ALT, n (%) | ||||
| ≤27, U/L | 10 (33) | 12 (32) | 22 (33) | 2 (14) |
| 28-41, U/L | 9 (30) | 14 (38) | 23 (33) | 6 (43) |
| ≥42, U/L | 11 (37) | 11 (30) | 22 (34) | 6 (43) |
| AST, U/L | 32 ± 3 | 27 ± 2 | 30 ± 2 | 31 ± 3 |
| FIB-4 | 1.13 ± 0.09 | 1.20 ± 0.11 | 1.17 ± 0.07 | 1.10 ± 0.14 |
Data are mean ± SEM unless otherwise indicated. aMeasured at the visit before the endoscopic screening, for single-centre study at screening visit, for multicentre study at baseline visit. ALT, alanine aminotransferase; AST, aspartate aminotransferase; FIB-4, Fibrosis-4 index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin.
Safety
The DMR procedure was completed without any intraprocedural complications in the safety cohort (n = 85) or, as inferred, in the 67 patients in the efficacy cohort. There was no gastrointestinal bleeding, perforation, pancreatitis, severe hypoglycaemia, or evidence of malabsorption, either in the period immediately following the procedure or during later follow-up. Three patients in the single-centre study from the safety cohort experienced duodenal stenosis within 2–6 weeks of the procedure as previously reported.21 These patients developed complaints (difficulties with deglutition, epigastric pain, and/or intermittent vomiting) shortly after DMR. These cases were treated successfully by balloon dilation, following which patients did not develop new or other symptoms indicative of duodenal stenosis during 12–36 months of follow-up (2 patients completed study follow-up, a single patient was lost to follow-up at 12 months). In all other patients, no abnormalities were observed during the follow-up endoscopy after DMR, including no duodenal stenosis.
Efficacy
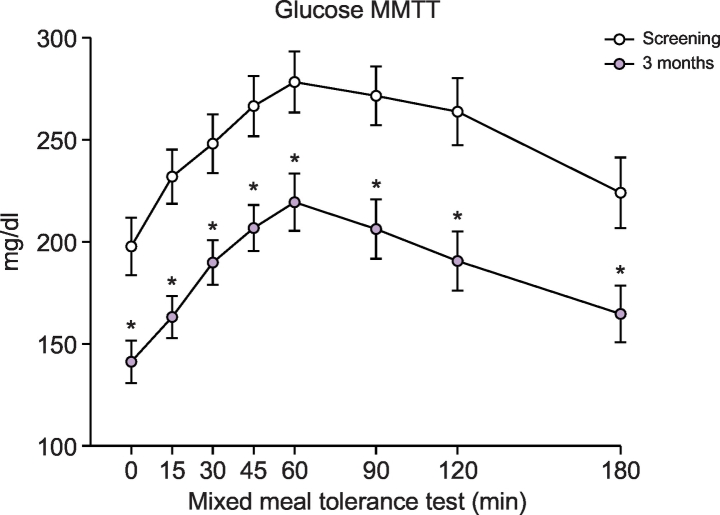
In the composite efficacy cohort, baseline HbA1c was 9.0 ± 0.2%. Single DMR treatment achieved significant HbA1c reductions at 1, 3, and 6 months (8.0 ± 0.1%, 7.6 ± 0.1%, and 7.9 ± 0.2%, respectively) compared with baseline (all p ≪0.001). This was observed despite a net reduction in antidiabetic medication in treated patients at 6 months in the single-centre study21, and withdrawal of sulfonylureas (n = 6) and meglitinides (n = 2) at screening in the multicentre study.22 Other glucose-lowering medication was kept stable during 6 months follow-up in the multicentre study. FPG also decreased significantly post DMR. At 1, 3, and 6 months FPG levels were: 147 ± 5, 151 ± 5, and 161 ± 7 mg/dl, respectively (p ≪0.001, p ≪0.001, and p = 0.005 relative to the baseline of 189 ± 6 mg/dl). Mixed meal challenge (n = 14) showed a reduction in plasma glucose at 3 months (Fig. 2), with most of the overall lowering explained by lower FPG. Body weight post-DMR decreased from 89.4 ± 1.5 kg at baseline to 86.3 ± 1.5, 86.3 ± 1.5, and 87.0 ± 1.4 kg at 1, 3, and 6 months (all p ≪0.001). Importantly, there was no linear relationship between early weight loss and values of HbA1c (p = 0.292) and FPG (p = 0.646).
Fig. 2.
Postprandial glucose levels measured during mixed meal tolerance test.
*Indicates a significant (p ≪0.05) change compared to the corresponding time point at screening (paired Student’s t test). Metabolomics analysis sub-cohort (n = 14).
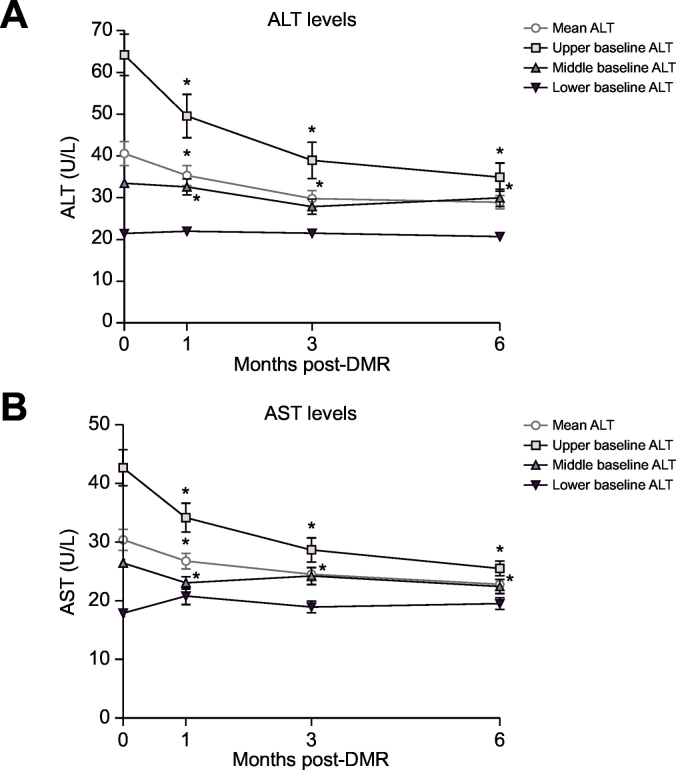
DMR led to a reduction in serum ALT and AST over 6 months (Fig. 3A and 3B black lines) in the composite efficacy cohort. There was a linear relationship between early weight loss and values of ALT (p = 0.039) and AST (p = 0.007) but no linear relationship between HbA1c and values of ALT and AST. Serum ALT decreased from 41 ± 3 to 35 ± 2 IU/L at 1 month post DMR (p = 0.007), continued to decrease to 30 ± 2 IU/L (p ≪0.001) at 3 months, and remained significantly (p ≪0.001) lower at 6 months with a mean value of 29 ± 2 IU/L. A similar reduction was observed in serum AST levels: AST decreased from 30 ± 2 IU/L at baseline to 27 ± 1 U/L at 1 month (p = 0.017), to 25 ± 1 at 3 months after DMR (p ≪0.001) and to 23 ± 1 at 6 months (p ≪0.001). The significance levels were corrected for early weight loss.
Fig. 3.
Effect of single DMR procedure over 6 months on overall mean hepatic transaminases and stratified by baseline levels. (A) Change from baseline in ALT. (B) Change from baseline in AST. Data are mean ± SE. *Indicates a significant (p ≪0.05) change compared to the baseline value (ANOVA repeated measurements with Bonferroni correction). Mean values represent the complete DMR cohort of 67 patients. Upper baseline ALT n = 22; Middle baseline ALT n = 23; Lower baseline ALT n = 22. Upper baseline AST n = 24; Middle baseline AST n = 22; Lower baseline AST n = 21.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DMR, duodenal mucosal resurfacing.
Tertile analysis (Fig. 3A and 3B coloured lines) of ALT and AST showed that the overall fall in aminotransferase levels was mostly driven by patients with higher baseline levels of ALT and AST. Only in the tertile with the highest baseline ALT levels, “high ALT” (63 ± 5 IU/L), was there a linear relationship between values of ALT and early weight loss (p = 0.043). When corrected for this effect, ALT at 6 months (34 ± 3 IU/L) was still lower compared to baseline (p ≪0.001). The “high AST” group (44 ± 3 IU/L) showed a reduction in AST plasma levels across the total follow-up (p ≪0.001) to 26 ± 1 IU/L at 6 months. There was no linear relationship between values of AST and early weight loss in all tertiles.
In the efficacy cohort, mean FIB-4 score decreased from 1.18 to 0.99 (p = 0.001). Eighteen patients (31%) had a baseline FIB-4 score ≫1.30. In 10 of these patients (56%), FIB-4 decreased to ≪1.30. In 1 patient with a FIB-4 score ≪1.30 at baseline, FIB-4 increased to ≫1.30. Nine patients (15%) had a 6-month FIB-4 score ≫1.30.
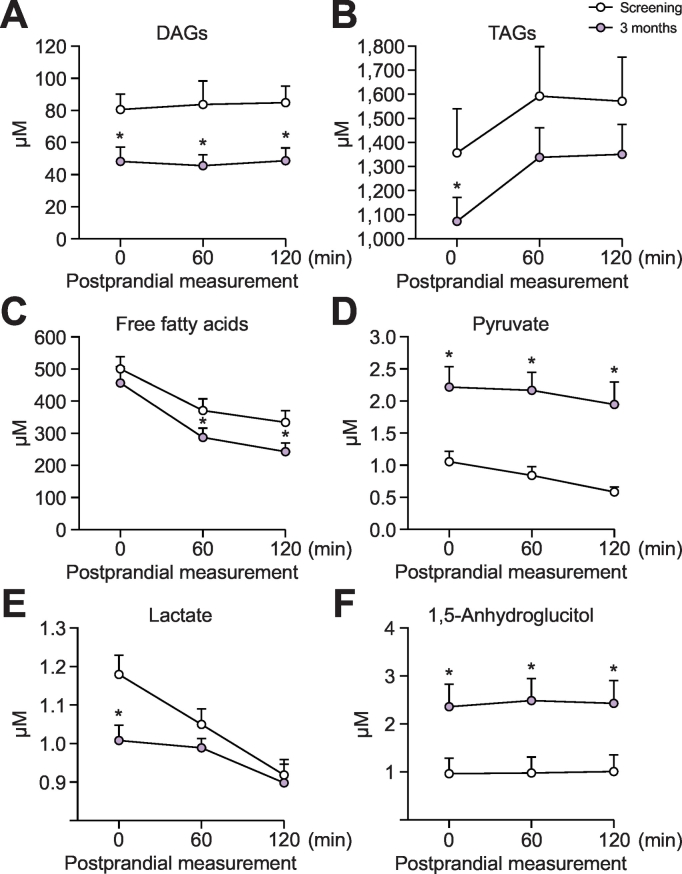
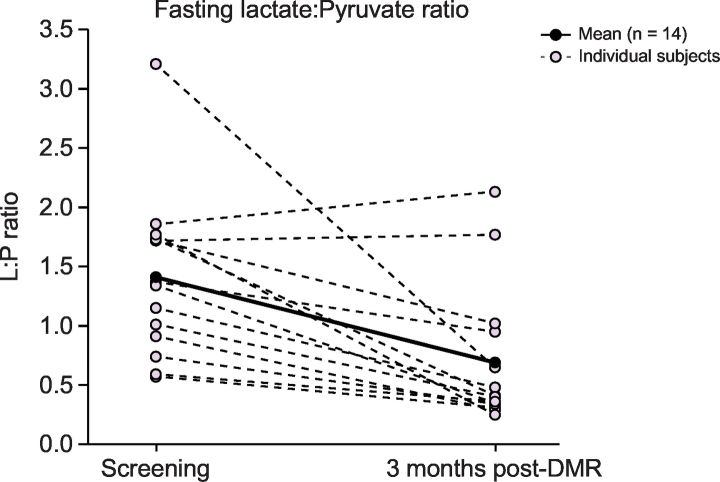
Metabolomic analysis showed combined changes in fasting carbohydrate and lipid metabolism analytes (Fig. 4): (A) lowering of diacylglycerides (DAGs), (B) triacylglycerides (TAGs) and (C) free fatty acids (FFAs); (D) an increase in pyruvate; (E) lowering of lactate; and (F) an increase of 1,5-anhydroglucitol (1,5-AG) levels (overall q = 0.115). Mean fasting lactate to pyruvate (L:P) ratio (of log-transformed lactate and pyruvate measurements) decreased from 1.41 ± 0.18 to 0.69 ± 0.16 (p = 0.003) (Fig. 5). At 60 and 120 min postprandially, L:P ratio decreased from 1.53 ± 0.17 to 0.59 ± 0.09 (p ≪0.001) and from 1.85 ± 0.22 to 0.73 ± 0.15 (p ≪0.001), respectively. The broader metabolomic panel is provided in Table 2 and highlights further significant changes in key metabolites (overall q = 0.117).
Fig. 4.
Change in fasting and postprandial metabolite levels.
*Indicates a significant (p ≪0.05) change at 3 months compared to the corresponding time point at screening (paired Student’s t test). Metabolomics analysis sub-cohort (n = 14). DAGs, diacylglycerides; TAGs, triacylglycerides.
Fig. 5.
Per-patient change in lactate:pyruvate* ratio.
*Lactate and pyruvate measurements were log-transferred before calculating the lactate:pyruvate ratio. Metabolomics analysis sub-cohort (n = 14). DMR, duodenal mucosal resurfacing; L:P, lactate to pyruvate.
Table 2.
Additional metabolomics panel.
| Metabolite | Marker | Effect | 3-month concentration relative to baseline |
||
|---|---|---|---|---|---|
| 0 min | 60 min | 120 min | |||
| 2-hydroxybutyrate | Hepatic insulin resistance | Reduction | 0.79 (p ≤0.05) | 0.85 (p ≤0.05) | 0.86 (p ≤0.05) |
| 13-HODE + 9-HODE | Oxidative stress and inflammation, involved in NAFLD linked pathways | Reduction | 0.48 (p ≤0.05) | 0.51 (p ≤0.05) | 0.49 (p ≤0.05) |
| 4-hydroxynonenal | Reduction | 0.45 (p ≤0.05) | 0.43 (p ≤0.05) | 0.35 (p ≤0.05) | |
| Leukotriene B4 | Inflammation and correlation with NAFLD progression | Reduction | 0.68 (p ≤0.05) | 0.61 (p ≤0.05) | 0.79 (p ≤0.05) |
| 5-HETE | Reduction | 0.58 (p ≤0.05) | 0.67 (p ≤0.05) | 0.61 (p ≤0.05) | |
| Cysteine | Antioxidant capacity | Increase | 1.20 (p ≤0.05) | 1.43 (p ≤0.05) | 1.47 (p ≤0.05) |
| Glycine | Increase | 1.05 (p = n.s.) | 1.09 (p ≤0.05) | 1.07 (p ≤0.05) | |
| 5-oxoproline | Degradation product of the antioxidant molecule glutathione | Reduction | 0.88 (p = n.s.) | 0.86 (p ≤0.05) | 0.82 (p ≤0.05) |
| γ-glutamylglutamate | Reduction | 0.71 (p ≤0.05) | 0.66 (p ≤0.05) | 0.69 (p ≤0.05) | |
Metabolomics analysis sub-cohort (n = 14). 13-HODE, 13-hydroxyoctadecadienoic acid; 9-HODE, 9-hydroxyoctadecadienoic acid; 5-HETE, 5-hydroxyeicosatetraenoic acid.
Discussion
This report combines data from 2 open-label, single-arm studies of the endoscopic DMR procedure that were primarily designed to ascertain safety and efficacy of DMR as a procedure to improve glycaemic endpoints in patients with T2DM. We found that DMR is a safe procedure which improves glycaemia, hepatic aminotransferases, and insulin sensitivity in patients with T2DM.
The safety profile from this early clinical experience of endoscopic DMR is encouraging. Patients who underwent the procedure experienced minimal intolerance and few gastrointestinal symptoms after the procedure. During the initial development of the procedure, isolated cases of duodenal stenosis were observed and treated by endoscopic balloon dilation without further sequelae.21 In the follow-up period of 12–36 months (2 patients completed study follow-up, a single patient was lost to follow-up at 12 months), no additional symptoms indicative of duodenal stenosis were reported. It is unlikely that additional sequelae would develop later than 6 months after successful balloon dilatation since regeneration of gastrointestinal mucosa occurs within 6–8 weeks. Following these 3 cases, this risk appears to have been mitigated as overlapping ablation is avoided and more extensive mucosal lifting is performed during the subsequent DMR procedures. No further cases of duodenal stenosis are reported in 48 additional cases in these 2 studies.
The data summarizes the improvement in glycaemia, and points at an additional improvement in hepatic aminotransferases and FIB-4 scores, with a metabolomic signature that suggests an insulin sensitizing mechanism. A broader array of inflammatory changes, oxidative stress, and lipid changes suggest that DMR has favourable effects on liver metabolic fitness. Notably, the L:P ratio in blood decreased substantially, indicating a decreased NAD+/NADH ratio in the cytosol of hepatocytes and decreased mitochondrial stress, which are in turn features of improved glucose homeostasis.24 These improvements in hepatic measures cannot be easily explained as either a consequence of improved glycaemia or observed weight loss after the procedure. This therefore implies that alternate independent mechanisms could be responsible for this metabolic change and that DMR possibly alters local duodenal signalling in a manner favourably affecting liver metabolic health.
DMR elicited an approximate 1.0–1.5% reduction in HbA1c 6 months post-DMR. This finding was coupled with a glycaemic lowering effect observed in the patients who underwent a meal challenge test. This effect on glycemia is impressive and comparable with most pharmacological interventions despite net medication reductions in the cohort. DMR also elicited a robust lowering (~40–50%) of ALT and AST in the higher baseline patients with no sign of erosion of effect through 6 months. Active hepatitis, active liver disease and active alcoholism were exclusion criteria for study participation. However, it cannot be ruled out completely that these conditions were possible confounders in this analysis. The metabolomic signature reported key lipid lowering (TAGs, DAGs, FFAs),25 insulin sensitizing (lowering of lactate and increase in pyruvate,26 reduction of 2-hydroxybutyrate27), and anti-inflammatory effects (reduction of leukotriene B4 and 5-HETE),28 as well as increased antioxidant capacity (increase in cysteine and glycine and reduction in degradation products indicate increased availability of glutathione, although not directly measured).29 Improved FIB-4 scores point at improvement of fibrosis in NAFLD. These effects suggest a likely beneficial effect of DMR in fatty liver disease. An increase in 1,5-AG is indicative of an improvement in hyperglycaemia, since it is markedly decreased by inhibition of tubular reabsorption during periods of hyperglycaemia.30,31
A shortcoming of this report is the lack of an appropriate control which would have enabled us to better measure the magnitude of DMR’s effect and the pattern of change over time. That said, the magnitude of change of both glycaemic and hepatic indices and the intriguing metabolomic signature would suggest that the procedure has indeed exerted favourable metabolic change. All patients underwent a 2-week period of graduated diet immediately after the procedure that was not intended to be hypocaloric, but caloric restriction cannot be ruled out and a small but significant reduction in body weight was observed (~2–3%). This weight loss did have an interaction with the decrease in aminotransferase levels, but aminotransferase levels were still significantly lower post-DMR when this effect was taken into account. Additional shortcomings include no accounting for other hepatic confounders (e.g. alcohol consumption), no liver imaging or histological data, and no additional MMTT and metabolomic data at 6 months follow-up. These factors will be better addressed in future studies.
To conclude, hydrothermal ablation of the duodenum by DMR elicits a metabolic benefit in patients with T2DM, manifesting with improvement in both glycaemic and hepatic indices. Achievement of such metabolic benefit through a minimally invasive endoscopic treatment offers a potentially new therapeutic solution for patients with T2DM and fatty liver disease. Given that adherence and persistence to diets and medicines represent important and unmet clinical challenges, a safe and scalable procedural intervention that does not require daily behavioural change could provide meaningful benefit to patients who otherwise struggle to achieve adequate disease control. This effect appears to be elicited through an insulin sensitizing mechanism, in keeping with evidence from the bariatric literature in animal and human studies that highlight the duodenum as a key metabolic signalling organ. Such an insulin sensitizing intervention could be complementary to lifestyle intervention approaches and pharmacological treatments aimed at preserving the pancreas and liver from failure.
At this time, it is unclear what actual signals emanating from the duodenum contribute to the apparent untreated insulin resistant state, and how these are modulated by local DMR. Additional research to elucidate this gut-borne mechanism is underway. Further clinical study is also required to better understand and confirm the clinical utility of DMR as an actual disease intervention for both T2DM and fatty liver disease and their significant overlap that exists between these 2 metabolic conditions. Key clinical questions will focus on establishing efficacy and the magnitude and durability of effect for both conditions. Further clinical investigation will include appropriately controlled prospective study conditions in addition to more detailed clinical and metabolic measures, liver imaging (e.g. MRS-FF) and histology.
Abbreviations
1,5-AG, 1,5-anhydroglucitol; 13-HODE, 13-hydroxyoctadecadienoic acid; 5-HETE, 5-hydroxyeicosatetraenoic acid; 9-HODE, 9-hydroxyoctadecadienoic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DAG, diacylglyceride; DMR, duodenal mucosal resurfacing; FFAs, free fatty acids; FIB-4, fibrosis 4 index; FPG, fasting plasma glucose; GLP-1, glucagon like peptide-1 receptor agonist; HbA1c, glycated haemoglobin A1c; L:P, lactate to pyruvate ratio; MMTT, mixed meal tolerance test; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; T2DM, type 2 diabetes mellitus; TAG, triacylglyceride.
Financial support
Funding for this study was provided by Fractyl Laboratories, Inc.
Conflict of interest
RH, GC, JD, AH, LR, MGN, and JB have received research support from Fractyl Laboratories Inc. for the IRB approved study. KW is employee of Metabolon Inc. SG is a consultant for Fractyl Laboratories, Inc. and Novo Nordisk. JLT is employee of Fractyl Laboratories, Inc. MGN receives personal fees for consulting and proctoring from Fractyl Laboratories, Inc., GI dynamics, GI Windows, Apollo EndoSurgery, and Ethicon Endosurgery. JB received consultancy fee for a single advisory board meeting of Fractyl in September 2019. No other potential conflicts of interest relevant to this article were reported.
Please refer to the accompanying ICMJE disclosure forms for further details.
Funding information
Funding for this study was provided by Fractyl Laboratories, Inc.
Authors’ contributions
AB and UB conceived this article. AB and UB wrote the first drafts, with further contributions from JB and JLT. AB did the statistical analysis, with guidance from UB and JB. KW performed the metabolomics analysis. KW, SG and AS assisted in interpretation of the results of the metabolomics analysis. RH, GC, JD, LR, MGN and JB performed the study procedures. AS and KW performed critical revision of the manuscript for important intellectual content. RH, GC, AH, JD, SG, LR, MGN critically revised the manuscript. All authors had access to the data, interpreted data, reviewed successive drafts, and approved the final version of the article.
Footnotes
Clinical trial numbers: NCT01927562 and NCT02413567.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2019.10.006.
Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.NCD Risk Factor Collaboration Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diab Rep. 2015;15:607. doi: 10.1007/s11892-015-0607-4. [DOI] [PubMed] [Google Scholar]
- 3.Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139–1144. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 5.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Lallukka S, Yki-Jarvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2016;30:385–395. doi: 10.1016/j.beem.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100:2231–2238. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Joo SK, Kim W, Kim D, Kim JH, Oh S, Lee KL. Steatosis severity affects the diagnostic performances of noninvasive fibrosis tests in nonalcoholic fatty liver disease. Liver Int. 2017 doi: 10.1111/liv.13549. [DOI] [PubMed] [Google Scholar]
- 11.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mingrone G, Cummings DE. Changes of insulin sensitivity and secretion after bariatric/metabolic surgery. Surg Obes Relat Dis. 2016;12:1199–1205. doi: 10.1016/j.soard.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Verdam FJ, Greve JW, Roosta S, van Eijk H, Bouvy N, Buurman WA. Small intestinal alterations in severely obese hyperglycemic subjects. J Clin Endocrinol Metab. 2011;96:E379–E383. doi: 10.1210/jc.2010-1333. [DOI] [PubMed] [Google Scholar]
- 16.Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care. 2009;32:514–520. doi: 10.2337/dc08-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salinari S, Carr RD, Guidone C, Bertuzzi A, Cercone S, Riccioni ME. Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am J Physiol Endocrinol Metab. 2013;305:E59–E66. doi: 10.1152/ajpendo.00559.2012. [DOI] [PubMed] [Google Scholar]
- 18.Dirksen C, Hansen DL, Madsbad S, Hvolris LE, Naver LS, Holst JJ. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33:375–377. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu H, Eldar S, Heneghan HM, Schauer PR, Kirwan JP, Brethauer SA. The effect of selective gut stimulation on glucose metabolism after gastric bypass in the Zucker diabetic fatty rat model. Surg Obes Relat Dis. 2014;10:29–35. doi: 10.1016/j.soard.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Gniuli D, Calcagno A, Dalla Libera L, Calvani R, Leccesi L, Caristo ME. High-fat feeding stimulates endocrine, glucose-dependent insulinotropic polypeptide (GIP)-expressing cell hyperplasia in the duodenum of Wistar rats. Diabetologia. 2010;53:2233–2240. doi: 10.1007/s00125-010-1830-9. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan H, Cherrington AD, Thompson CC, Kaplan LM, Rubino F, Mingrone G. Endoscopic Duodenal Mucosal Resurfacing for the Treatment of Type 2 Diabetes: 6-Month Interim Analysis From the First-in-Human Proof-of-Concept Study. Diabetes Care. 2016 doi: 10.2337/dc16-0383. [DOI] [PubMed] [Google Scholar]
- 22.van Baar ACG, Holleman F, Crenier L, Haidry R, Magee C, Hopkins D. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut. 2019 doi: 10.1136/gutjnl-2019-318349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nocito L, Kleckner AS, Yoo EJ, Jones Iv AR, Liesa M, Corkey BE. The extracellular redox state modulates mitochondrial function, gluconeogenesis, and glycogen synthesis in murine hepatocytes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonks KT, Coster AC, Christopher MJ, Chaudhuri R, Xu A, Gagnon-Bartsch J. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity (Silver Spring) 2016;24:908–916. doi: 10.1002/oby.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeva-Andany M, Lopez-Ojen M, Funcasta-Calderon R, Ameneiros-Rodriguez E, Donapetry-Garcia C, Vila-Altesor M. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014;17:76–100. doi: 10.1016/j.mito.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narasimhan S, Gokulakrishnan K, Sampathkumar R, Farooq S, Ravikumar R, Mohan V. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem. 2010;43:815–821. doi: 10.1016/j.clinbiochem.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 31.Nerby CL, Stickle DF. 1,5-anhydroglucitol monitoring in diabetes: a mass balance perspective. Clin Biochem. 2009;42:158–167. doi: 10.1016/j.clinbiochem.2008.08.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2