Abstract
Background & Aims
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by progressive inflammatory and fibrotic injury to the biliary tree. We sought to further delineate the contribution of macrophage lineages in PSC pathobiology.
Methods
Human liver tissues and/or blood samples from patients with PSC, primary biliary cholangitis, other non-cholestatic/non-autoimmune diseases, including alcohol-related liver disease and non-alcoholic steatohepatitis, as well as normal liver, were sourced from our liver transplantation program. Liver fibrosis was studied using Van Gieson staining, while the frequencies of infiltrating monocyte and macrophage lineages, both in the circulation and the liver, were investigated by flow cytometry, including the expression of TGR-5, a G protein-coupled receptor (GPBAR1/TGR-5).
Results
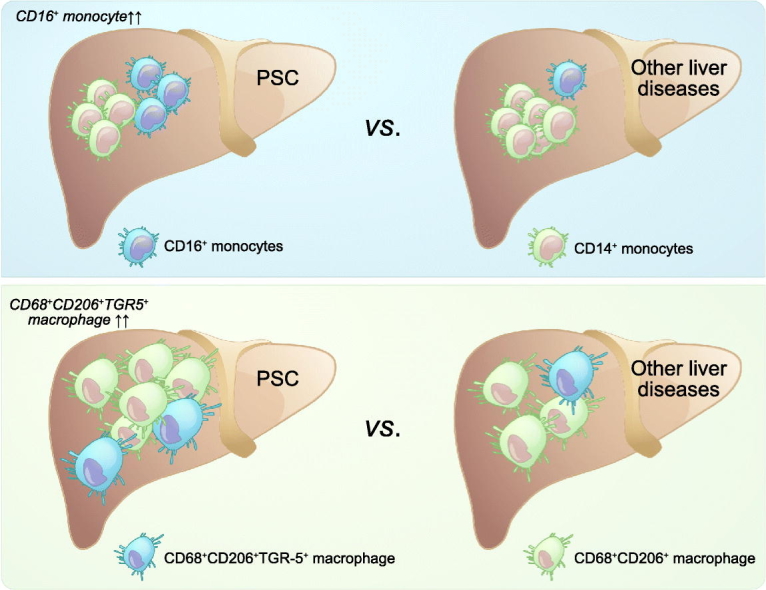
Significantly higher frequencies of CD68+CD206+ macrophages were detected in the livers of patients with PSC (median 19.17%; IQR 7.25–32.8%; n = 15) compared to those of patients with other liver diseases (median 12.05%; IQR 5.61–16.03%; n = 12; p = 0.0373). CD16+ monocytes, including both intermediate (CD14+CD16++) and non-classical (CD14dimCD16++) monocytes, were preferentially recruited into chronically diseased livers, with the highest recruitment ratios in PSC (median 15.83%; IQR 9.66–29.5%; n = 15), compared to other liver diseases (median 6.66%; IQR 2.88–11.64%, n = 14, p = 0.0152). The expression of TGR-5 on CD68+ intrahepatic macrophages was increased in chronic liver disease; TGR-5 expression on intrahepatic macrophages was highest in PSC (median 36.32%; IQR 17.71–63.61%; n = 6) and most TGR-5+ macrophages were CD68+CD206+ macrophages.
Conclusions
Underlying a potential role for macrophages in PSC pathobiology, we demonstrate, using patient-derived tissue, increased CD16+ monocyte recruitment and a higher frequency of CD68+CD206+ macrophages in the livers of patients with PSC; the CD68+CD206+ macrophage subset was associated with significantly higher TGR-5 expression in PSC.
Lay summary
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease associated with progressive inflammation of the bile duct, leading to fibrosis and end-stage liver disease. In this study we explore the role of a type of immune cell, the macrophage, in contributing to PSC as a disease, hoping that our findings direct scientists towards new treatment targets. Our findings based on human liver and blood analyses demonstrate a greater frequency of a particular subset of immune cell, the CD68+CD206+ macrophage, with significantly higher TGR-5 expression on this subset in PSC.
Keywords: Primary sclerosing cholangitis (PSC), macrophage, TGR-5 (G protein-coupled bile acid receptor 1, GPBAR1/TGR-5)
Graphical Abstract
Highlights
-
•
CD68+CD206+ macrophage populations predominate in the liver tissue of patients with primary sclerosing cholangitis.
-
•
Intrahepatic CD16+ monocytes preferentially accumulate in the livers of patients with primary sclerosing cholangitis.
-
•
The expression of TGR-5 is increased in chronic liver disease.
-
•
TGR-5 expression on CD68+CD206+ intrahepatic macrophages is higher in patients with primary sclerosing cholangitis.
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease associated with progressive inflammation of the bile duct leading to bile duct strictures, hepatic fibrosis, and end-stage liver disease.1,2 To date, the etiology and the pathogenic mechanisms of PSC remain unclear, with it generally believed to be a multifactorial immune-related illness.3
Hepatic macrophages are a heterogenous population of immune cells that account for the largest non-stromal cell population in the liver; they play a central role in the pathogenesis of chronic liver injury, including inflammation and fibrosis.4 Traditionally, activated macrophages can be classified into 2 major categories: namely the classic, proinflammatory macrophages and the alternatively activated macrophages.5 Proinflammatory macrophages favor the Th1 immune response and are activated through the classical activation pathway by interferon-γ (IFN-γ) combined with lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-α). Upon activation, cytokines such as interleukin (IL)-6, TNF-α, IL-12 are secreted, as well as proinflammatory nitric oxide. In contrast, alternatively activated macrophages are more classically anti-inflammatory and can be induced via alternative activation pathways through IL-4/IL-13. Recent studies have identified the heterogeneity of alternatively activated macrophages and have subdivided them further into different subgroups based on the stimuli involved.5,6 In liver fibrosis the proinflammatory macrophages promote fibrosis in the early stages of the disease via the recruitment of proinflammatory immune cells and the secretion of proinflammatory mediators. For example, transforming growth factor-beta (TGF-β) expression activates myofibroblast and extracellular matrix (ECM) synthesis.7 During remodeling of fibrosis, the alternatively activated macrophages express factors such as matrix metalloproteinases and TNF-related apoptosis-inducing ligands, which contribute to myofibroblast apoptosis, promote ECM degradation and facilitate hepatic resolution.7 Understanding the contribution of these diverse macrophage populations to the dynamic process of fibrosis will provide clues to the establishment and progression of chronic liver diseases. In the setting of PSC, macrophages have been reported to drive disease progression with a threefold increase in relative numbers of Kupffer cells in PSC compared to primary biliary cholangitis (PBC) and healthy livers.8 A recent study by Guicciardi ME et al., demonstrated increased peribiliary recruitment of monocyte-derived macrophages, of both macrophage lineages, as a feature of PSC.9
TGR-5, a G protein-coupled receptor (GPBAR1/TGR-5), is one of the major receptors for bile acids (BAs) along with the farnesoid X receptor (FXR). Whilst expression is widespread, TGR-5 is notably strongly expressed on monocytes and macrophages. When activated, it conveys different effects depending on tissue localization and the signaling cascade it induces.10,11 For example, when TGR-5 is activated on immune cells, it has immunosuppressive effects via the inhibition of the proinflammatory transcription factor NF-κB, which then downregulates the production of proinflammatory cytokines IL-1α, IL-1β, IL-6 and TNF-α.[10], [11], [12], [13], [14] Hov JH et al., have evaluated genetic associations for TGR-5 (at the 2q35 locus) with PSC disease susceptibility; they studied 285 patients with PSC, 882 patients with ulcerative colitis and 2,496 healthy controls. In their work the authors concluded that associations at 2q35 were observed in PSC and patients with ulcerative colitis; however, they were unable to firmly confirm the association hence it remains important that larger studies are conducted to understand if, and how, TGR-5 is associated with PSC.12,15
In this study we sought to delineate the macrophage subsets in PSC and to investigate the association between TGR-5 and macrophage phenotypic changes.
Patients and methods
Human tissue and blood
Fresh human liver tissue was obtained from patients undergoing liver transplantation at the Queen Elizabeth Hospital, Birmingham, (Local Research Ethics Committee Reference Number: 06/Q2702/61, supplementary CTAT table), including PSC (n = 15), PBC (n = 5), alcohol-related liver disease (ALD, n = 9) and non-alcoholic steatohepatitis (NASH, n = 8). Control tissue (normal liver [NL], n = 4) was obtained from donor liver tissue surplus to clinical requirements. Whole blood was obtained from patients attending clinic (Local Research Ethic Committee Reference Numbers: 2003/242, supplementary CTAT table), with PSC (n = 11) or PBC (n = 41). The clinical information for patients is shown in Table 1. Patient consent was obtained according to the ethical guidelines listed.
Table 1.
Clinical information for patient samples. Data for categorical variables expressed as number with percentages in parentheses. Continuous variables expressed as median (IQR).
| Liver explants |
|||
|---|---|---|---|
| PSC (n = 15) | PBC (n = 7) | Others (n = 12) | |
| Age, years | 37 (30–52) | 58 (52–60) | 52.5 (42–67) |
| Male gender, n (%) | 10 (67%) | 2 (29%) | 7 (58%) |
| Serum ALT (IU/L) | 144 (70–295) | 256 (46–773) | 28 (14–45) |
| Serum ALP (IU/L) | 254 (176–539) | 156 (83–265) | 119 (88–145) |
| Bilirubin (μmol/L) | 86 (46–237) | 48 (41–64) | 27.5 (17–44) |
| MELD score | 16 (14–21) | 14 (11–18) | 11 (10–13) |
| Blood samples |
|||
|---|---|---|---|
| PSC (n = 12) | PBC (n = 42) | Others (n.a.) | |
| Age, years | 60.5 (39–66) | 57.5 (51–64) | |
| Male gender, n (%) | 7 (58%) | 3 (7%) | |
| Serum ALT (IU/L) | 39 (29–90) | 38 (23–60) | |
| Serum ALP (IU/L) | 273 (167–311) | 180 (119–322) | |
| Bilirubin (μmol/L) | 20 (12–33) | 10 (6–18) | |
| MELD score | 8 (7–11) | 6.5 (6–8) | |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; MELD, model for end-stage liver disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
Van Gieson staining
Liver fibrosis was evaluated using Van Gieson staining. Briefly, paraffin-embedded liver sections were deparaffinized and hydrated to distilled water first and stained with Wright’s Working Hematoxylin for 10 min and then washed in distilled water. The slides were then stained with Van Gieson solution for 3 min, followed by gradient dehydrating in 95% alcohol, absolute alcohol, and 2 changes in xylene before mounting with DPX. These slides were scanned through slide scanner, Zeiss Axio ScanZ1 (Carl Zeiss Ltd. Cambridge, UK), and the whole field image of each section was processed via ZEN 2010 blue edition software (Carl Zeiss Ltd. Cambridge, UK) and quantified for positive staining for the fibrotic area (pink) using ImageJ analysis software (NIH, USA) and an automated macro. The proportionate area of fibrosis staining (pink) was calculated using: Pixels of area that stained pink / Pixels of the whole image.
Isolation of mononuclear cells from human peripheral blood and human liver tissue
Human peripheral blood mononuclear cells (PBMCs) were isolated and purified from peripheral blood using a Lymphoprep™ (STEMCELL Technologies, UK, Ltd., Cambridge, UK) density gradient step according to the manufacturer’s instructions. Liver-infiltrating mononuclear cells (LIMCs) were isolated from fresh explanted human liver tissue as described previously.16 Briefly, sliced liver tissue was washed in ice-cold PBS to remove residuals of blood. The tissue was then cut into small cubes, followed by mechanical digestion in RPMI-1640 using a Seward Stomacher400 Circulator (Cole-Parmer Instrument Co. Ltd., London, United Kingdom) for 5 min at a paddle speed of 260. The mechanically digested liver was then filtered and purified before density gradient isolation for LIMCs using Lymphoprep™ as described above. The immunophenotype of PBMCs and LIMCs were then analyzed using flow cytometry as described below.
Immunophenotyping using flow cytometry
Freshly prepared PBMCs or LIMCs (0.5–1x106 cells) were resuspended in FACS buffer (PBS supplemented with 2% FBS) and stained with LIVE/DEAD dye Vio-green (Molecular Probes) to exclude non-viable cells, together with optimized panels comprising the following antibodies (Detailed information can be found in the supplementary CTAT table): i) Monocytes: CD14-APC-eFluor780 (clone 61D3, Thermo Fisher Scientific), CD16-PeCF594 (3G8, BD Biosciences), HLA-DR (L243, Thermo Fisher Scientific), CD66b-FITC (G10F5, BD Biosciences), CD3-PE (OKT3, Thermo Fisher Scientific), CD19-PE (HIB19, Thermo Fisher Scientific), CD56-PE (CMSSB, Thermo Fisher Scientific); ii) Macrophage panel: CD68-PE-Cy7 (Y1/82A, Thermo Fisher Scientific), CD163-APC (GHI/61, Thermo Fisher Scientific), CD206-PerCP-eFluor710 (19.2, Thermo Fisher Scientific), TGR-5-PE (R&D systems); in the dark at 4°C for 1 h and then washed once with 2 ml of FACS buffer. The mean fluorescence intensity (%) of each monocyte or macrophage subset marker was normalized by subtracting the percentage of the corresponding fluorescence-minus-one (FMO) control channel. Unstained, single color and FMO control tubes were used to generate compensation matrices that correct for spectral overlap or to assist with gating. Frequencies of circulating or liver-infiltrating monocytes and macrophages, as well as the TGR-5 expression were acquired by flow cytometry (CyAn ADP, Beckman Coulter) and analyzed by FlowJo software (V.10.0.8, supplementary CTAT table).
Immunohistochemistry
The distribution of TGR-5 in the liver was studied using an antibody raised against human TGR-5 (Rabbit polyclonal, 5μg/ml; LifeSpan Biosciences, Inc. Seattle, USA) on paraffin-embedded liver sections. For this, paraffin-embedded liver sections were deparaffinized and hydrated to distilled water first, then endogenous peroxidases were blocked using methanol-based hydrogen peroxide solution (0.3%), followed by antigen retrieval using citric acid-based (pH 6) antigen unmasking solution (Vector Laboratories, Ltd. Peterborough, UK). Slides were then blocked with casein for an additional 30 min before addition of the primary antibody, for 1 h at room temperature. Slides were then washed 3x with PBS with 0.5% Tween (PBST), 5 min each time, and subsequently incubated with ImmPRESS™ HRP Universal antibody (Vector Laboratories, Ltd. Peterborough, UK) for a further 30 min. Finally, the slides were washed 3x with PBST before visualization using Impress-DAB substrate (Vector Laboratories, Ltd. Peterborough, UK). All slides were imaged using a slide scanner, Zeiss Axio ScanZ1 (Carl Zeiss Ltd. Cambridge, UK), and processed and quantified (TGR-5, brown color) as described earlier using ImageJ software (supplementary CTAT table). The proportionate area of TGR-5 staining (brown) was calculated using: Pixels of area that stained brown / Pixels of the whole image.
Statistical analysis
Non-parametric Mann-Whitney U and one-way ANOVA for multiple comparisons tests were used for statistical analysis in this study.
For further details regarding the materials used, please refer to the CTAT table.
Results
Baseline fibrosis in explanted human diseased livers
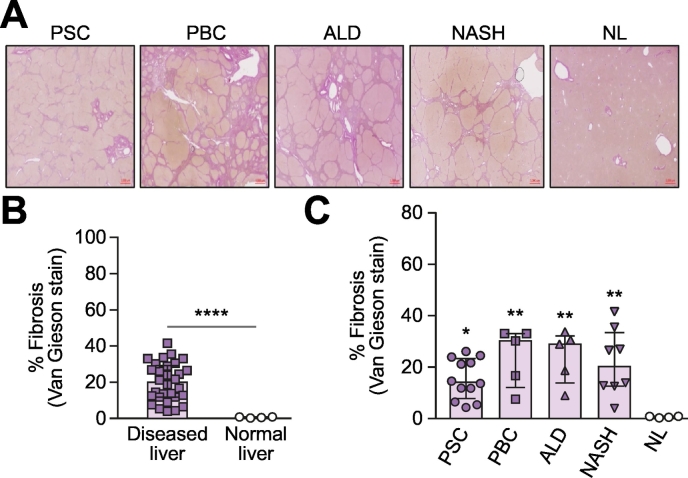
We evaluated the extent of fibrosis in explanted human liver samples used in this study by Van Gieson staining, including PSC (n = 12), PBC (n = 5), ALD (n = 5), NASH (n = 8) and NL (n = 4); (Fig. 1A). Diseased livers exhibited extensive fibrosis, as measured by the proportionate area of Van Gieson positive staining (pink) (median 19.90%; IQR 11.78–29.20%) compared to NL with no fibrosis (median 0.62%; IQR 0.27–0.83%) (Fig. 1B). Among the diseased livers we used in this study, we found the degrees of liver fibrosis varied, with a higher fibrotic burden in PBC (median 30.17%; IQR 12.09–33.01%), ALD (median 28.88%; IQR 13.79–32.08%) and NASH (median 20.17%; IQR 12.57–33.37%); and reduced fibrosis in PSC (median 14.05%; IQR 7.74–23.33%) (Fig. 1C).
Fig. 1.
Fibrosis (Van Gieson stain) in human explanted diseased livers.
(A) Representative immunohistochemistry staining (Van Gieson staining) of livers from patients with PSC (n = 12), PBC (n = 5), ALD (n = 5), NASH (n = 8), as well as NL (n = 5). (B) Diseased livers, including PSC, PBC, ALD and NASH, are significantly more fibrotic compared to rejected donor livers (NL). (C) Varying degrees of fibrosis in the diseased livers were evaluated. Data are represented as median ± IQR. Mann-Whitney U test was used for statistical analysis. *p ≪0.05, ** p ≪0.05, ****p ≪0.0001 with respect to NL.
ALD, alcohol-related liver disease; NASH, non-alcoholic steatohepatitis; NL, normal liver; PBC, primary biliary cholangitis, PSC, primary sclerosing cholangitis.
CD68+CD206+ macrophage populations predominate in the liver tissue of patients with PSC
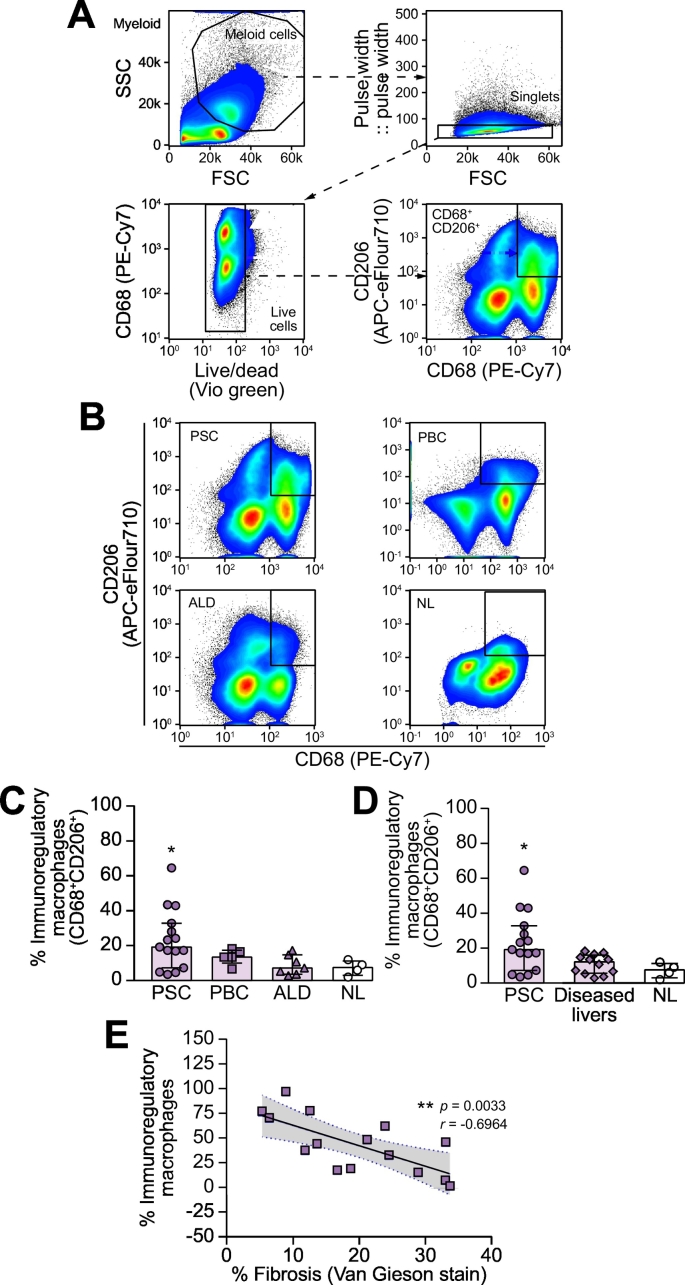
The phenotype of intrahepatic macrophages in PSC was studied using flow cytometry. In this study, LIMCs isolated from freshly explanted end-stage livers included PSC (n = 15), PBC (n = 5), ALD (n = 7) and donor livers (NL, n = 4). Fig. 2A illustrates the gating strategy used to identify intrahepatic CD68+CD206+ macrophages, whilst a representative dot plot for CD68+CD206+ intrahepatic macrophages across different etiologies is shown in Fig. 2B. We identified higher proportions of CD68+CD206+ macrophages in PSC (median 19.17%; IQR 7.25–32.8%), compared to NL (median 7.47%; IQR 2.96–11.21%, p = 0.0236), and other diseased livers (median 12.05%; IQR 5.61–16.03%, p = 0.0373), including ALD (median 7.16%; IQR 3.62–14.71%) and PBC (median 13.43%; IQR 10.01–17.35%) (Fig. 2C,D). Across diseases, the frequency of intrahepatic immune regulatory macrophages was negatively associated (p ≪0.05) with the degree of liver fibrosis (Fig. 2E), possibly reflecting the fact that patients with PSC are often transplanted at an earlier (fibrosis) stage.
Fig. 2.
Higher frequency of CD68+CD206+ macrophages in PSC liver.
(A) Gating strategy for intrahepatic CD68+CD206+ macrophages. (B) Representative flow cytometry dot plots showing the populations of macrophage subsets (CD68+CD206+) from explanted human livers, including, PSC (n = 15), PBC (n = 5), ALD (n = 7) and NL (n = 4); Gated region: CD68+CD206+ populations. (C,D) The percentages of CD68+CD206+ macrophages in PSC, other diseased livers (PBC and ALD, n = 12) and NL were analyzed by flow cytometry. (E) The frequency of anti-inflammatory macrophages was negatively associated with liver fibrosis. Data are represented as median ± IQR. *p ≪0.05 with respect to NL. r = -0.6964. One-Way ANOVA with multiple comparisons was used for statistical analysis.
ALD, alcohol-related liver disease; NL, normal liver; PBC, primary biliary cholangitis, PSC, primary sclerosing cholangitis.
Intrahepatic CD16+ monocytes preferentially accumulate in the livers of patients with PSC
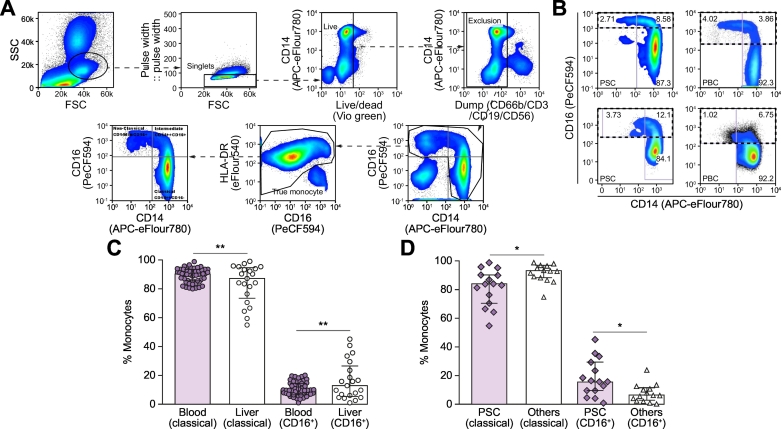
To study the distribution of CD16+ monocytes in PSC, we screened the populations of monocyte subsets (classical, intermediate and non-classical monocytes) in blood (n = 52) from patients with PSC (n = 11) and PBC (n = 41), and in livers from unmatched patients with PSC (n = 15), PBC (n = 5) and ALD (n = 9). The proportion of classical monocytes was higher in blood (median 90.20%; IQR 85.51–93.41%) than in the liver (median 87.10%; IQR 73.45–94.6% p ≪0.01) (Fig. 3). In contrast, the CD16+ monocyte (intermediate and non-classical monocyte) population was significantly increased in the liver (median 12.84%; IQR 5.39–26.5%) compared to the circulation (median 9.66%; IQR 6.50–14.49%; p ≪0.01). Furthermore, we also demonstrate that among diseased livers (PSC, PBC and ALD), the frequency of intrahepatic CD16+ monocytes was significantly higher in PSC (median 15.83%; IQR 9.66–29.5%) compared to other diseases (median 6.66%; IQR 2.88–11.64%, p = 0.0152) (Fig. 3D).
Fig. 3.
Frequencies of monocyte subsets present in the circulation and those isolated from human diseased livers.
Monocytes were divided into classical monocytes (C, CD14++CD16-), intermediate monocytes (I, CD14++CD16+) and non-classical monocytes (N, CD14dim CD16++); (A) Gating strategy for true monocyte subsets. (B) Dot plots showing the CD16+ monocyte populations in circulation (top panels) and liver (bottom panels). PSC (left) and PBC (right). (C) Frequencies of circulatory (n = 52) and intrahepatic (n = 21) monocyte subsets, classical vs. CD16+ (I+N) monocytes, from patients with PSC, PBC and ALD (intrahepatic monocytes); (D) Comparison of the frequencies of intrahepatic classical and CD16+ monocyte populations between PSC (n = 15) and other diseased livers (PBC, n = 5; and ALD, n = 9). Data are represented as median ± IQR. Mann-Whitney U test was used for statistical analysis. *p ≪0.05; **p ≪0.005. (I+N) denotes total CD16+ monocyte population. ALD, alcohol-related liver disease; PBC, primary biliary cholangitis, PSC, primary sclerosing cholangitis.
TGR-5 expression is increased in chronic liver disease and its expression on CD68+CD206+ intrahepatic macrophages is significantly higher in PSC compared to other liver diseases
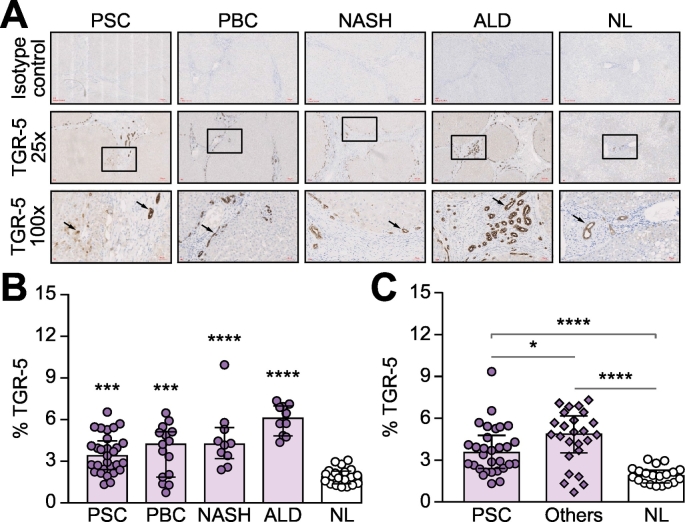
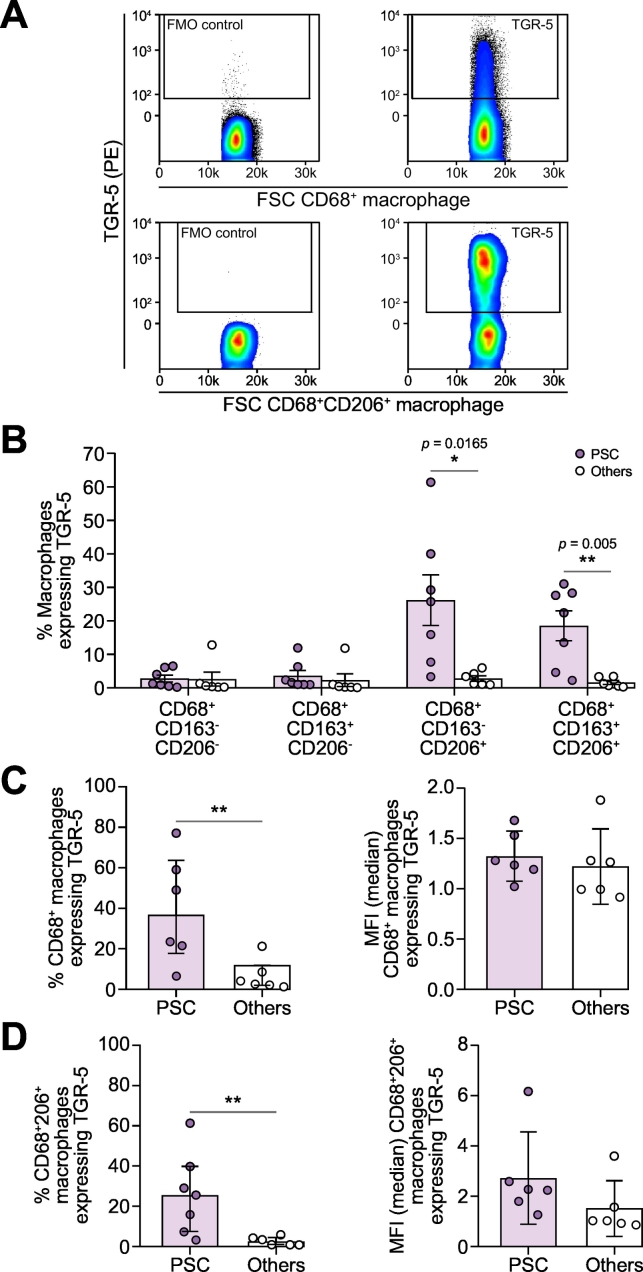
Immunohistochemistical staining was used to investigate the expression pattern of TGR-5 across different livers, including PSC (n = 3), other biliary and parenchymal diseases (n = 4, PBC, ALD and NASH) and NL (n = 2) (Fig. 4A). Using ImageJ analysis, the proportionate area of TGR-5 was calculated as the area of immunopositive cells divided by the whole area of the image taken. The result shows that compared to NL (median 1.88%; IQR 1.36–2.29%), the proportionate area of TGR-5 protein expression was higher in diseased livers, including PSC (median 3.39%; IQR 2.39–4.46%; p = 0.0002), PBC (median 4.21%; IQR 1.85–5.12%; p = 0.0004), ALD (median 6.08%; IQR 4.82–6.97%; p ≪0.0001) and NASH (median 4.22%; IQR 3.19–5.41%; p ≪0.0001) (Fig. 4B). TGR-5 expression was reduced in the livers of patients with PSC (median 3.39%; IQR 2.39–4.46%) compared to the livers of patients with other chronic liver diseases (median 4.87%; IQR 3.52–6.18%, p = 0.0063, Fig. 4C), possibly reflecting a different pattern of expression (Fig. 4A,C). TGR-5 is expressed on multiple cell types in the liver including biliary epithelial cells and macrophages. In tissue taken from patients with PSC we discovered a preponderance of TGR-5 positive cells resembling macrophages, which were lacking in tissue taken from patients with other liver diseases (Fig. 4A, high magnification images). Therefore we evaluated the expression of TGR-5 on isolated intrahepatic macrophages using flow cytometry analysis and found that TGR-5 expression on CD68+ macrophages was significantly higher in PSC (median 36.32%; IQR 17.71–63.61%, p = 0.0087), than in other chronic liver diseases (median 3.32%; IQR 1.90–11.64%), including PBC and ALD (Fig. 5A,C). The increase of TGR-5 expression resulted from increased expression of TGR-5 on the CD68+CD206+macrophage population. The frequency of CD68+CD206+macrophages was highest in PSC (median 25.7%; IQR 7.67–40%, p = 0.0082), compared to other diseases (median 2.27%; IQR 1.03–4.62%) (Fig. 5B,D).
Fig. 4.
The expression of TGR-5 is increased in chronic liver diseases.
The expression of TGR-5 was significantly higher in diseased livers than in NL and the pattern of expression was different within diseases, with PSC liver expressing a moderate level of TGR-5 when compared to other diseases (PBC, ALD and NASH); (A) Representative images for TGR-5 immunohistochemistry staining from different diseased livers and normal control (TGR-5, brown); (B) The pattern of TGR-5 expression in liver tissue from different sources; (C) Tissue taken from patients with PSC expressed lower levels of TGR-5 compared to other diseased livers. Expression was estimated as percent surface area using ImageJ. Data are represented as median ± IQR. One-Way ANOVA with multiple comparison was used for statistical analysis. ****p ≪0.001; ***p ≪0.005; *p ≪0.05.
ALD, alcohol-related liver disease; NASH, non-alcoholic steatohepatitis; NL, normal liver; PBC, primary biliary cholangitis, PSC, primary sclerosing cholangitis.
Fig. 5.
Frequency of TGR-5 expression by intrahepatic macrophages subsets.
(A) Representative FACS dot plot for TGR-5 expression on CD68+ (top panels) and CD68+CD206+ (bottom panels) intrahepatic macrophages. Isotype control (left) and TGR-5+ cells (right); (B) Bar chart representing percentages of TGR-5 expression on intrahepatic macrophage subsets; (C) Bar chart representing percentages (left) and MFI (right) of TGR-5 expression on CD68+ intrahepatic macrophages; (D) Bar chart representing percentages (left) and MFI (right) of TGR-5 expression on CD68+CD206+ intrahepatic macrophages. Data are represented as median ± IQR. Mann-Whitney U test was used for statistical analysis. **p ≪0.01, with respect to PSC. Other livers including PBC and ALD.
ALD, alcohol-related liver disease; MFI, mean fluorescence intensity; PBC, primary biliary cholangitis, PSC, primary sclerosing cholangitis.
Discussion
PSC is a chronic inflammatory disease of the biliary tree, with multiple potential pathophysiologic contributors. We sought to understand how macrophages were represented in the liver and blood of patients with PSC, when compared to control tissue. In demonstrably fibrotic livers we show that patients with PSC have increased frequencies of CD68+CD206+ macrophages compared to other explanted livers. Further, we find an increased proportion of CD16+ monocytes in diseased liver relative to blood, suggesting increased infiltration of CD16+ cells from the blood into the liver. A higher frequency of CD16+ monocytes was observed in the livers of patients with PSC, than in those of patients with other liver diseases. These CD16+ monocytes may further differentiate into alternatively activated macrophages and this may relate to the observation that the frequency of CD68+CD206+ macrophages was higher in PSC. Finally, we show that the expression of TGR-5, a bile acid receptor, and PSC-associated genetic risk factor, is increased in chronic liver diseases. In particular, TGR-5 expression was increased on CD68+CD206+ intrahepatic macrophages in patients with PSC.
Monocytes are bone marrow-derived circulating cells that selectively traffic to the site of injury and differentiate locally into diverse myeloid cell populations, manifesting many functions spanning phagocytosis, antigen presentation and tissue repair. Together with liver-resident cells, monocytes and monocyte-derived macrophages participate in both disease progression and resolution.17,18 Based on the surface marker CD14 and CD16 expression, monocytes can be divided into 3 subsets, with classical monocytes, expressing high CD14 but no CD16 expression (CD14++CD16-) comprising 90% of the total monocyte population. The remaining 10% of the monocyte population are positive for CD16 and can be further divided into intermediate monocytes with low CD16 and high CD14 expression (CD14++CD16+) and non-classical monocytes with high CD16 but relatively lower CD14 expression (CD14dimCD16++).19 The functional properties of these monocytes can be identified based on the expression of chemokine receptors, C-C motif chemokine receptor 2 (CCR2) and C-X3-C motif chemokine receptor 1 (CX3CR1). CCR2 is highly expressed on classical monocyte subset and responds to the stimulation from its ligand monocyte chemoattractant protein-1 (MCP-1/CCL2) and promotes monocyte recruitment to the site of inflammation. On the other hand, CX3CR1 is predominantly expressed on CD16+ monocytes, both intermediate and non-classical monocytes. This receptor responds to its ligand CX3CL1 (fractalkine) resulting in a prolonged crawling activity of these monocytes along the vascular endothelial wall, thereby enhancing the binding of monocytes to endothelial cells and promoting extravasation.20 Additional studies suggested that these CX3CR1-expressing monocytes further differentiate into alternatively activated macrophages responsible for immune regulation.21,22 In the case of chronic liver disease, previous studies by Liaskou E. et al., suggested that CD16+ (intermediate monocyte and non-classical) monocytes preferentially accumulate in chronically inflamed human livers and that the accumulation of CD14++CD16+ intermediate monocytes is the result of increased infiltration from the blood to the liver and local differentiation from classical CD14++CD16− monocytes.16 Coincidentally with these observations, our current data also suggest that the CD16+ monocytes preferentially accumulate in the liver compared to the blood. Since CX3CR1 is mainly expressed on CD16+ monocytes that can differentiate into alternatively activated macrophages, the increased frequency of CD16+ monocytes in PSC may imply a potential increased frequency of alternatively activated macrophages in the liver. Macrophages are involved in all stages of liver fibrosis. Our data demonstrated that CD68+CD206+ macrophage populations predominate in the liver tissue of patients with PSC, with our findings further validating previous studies by Cameron RG et al., and Guicciardi ME et al., wherein the investigators suggest that the relative number of intrahepatic macrophages in PSC is increased compared to PBC and NL (with increased peribiliary macrophage recruitment, both of the proinflammatory and alternatively activated monocyte-derived macrophages).8,9 In our study we used flow cytometry to study cell marker expression. Looking forward, opportunities exist to extend our data using methods such as high-density mass spectrometry. Given the precious and limited nature of samples available for this study, we were not able to extend our data at this time, but we recognize based on a number of single-cell RNA sequencing studies that there are clearly more than 2 macrophage populations in the human liver. This has been highlighted in a recent study by Ramachandran P et al. that identified a number of macrophage populations associated with fibrotic liver disease using single-cell RNA sequencing approaches.23 Future studies may reveal disease-specific macrophage subpopulations, and provide an insight into the cellular and molecular pathways that drive disease progression.
In Kupffer cells and other intrahepatic macrophages, TGR-5 stimulation inhibits the release of cytokines, such as TNF-α and IL-1, after LPS stimulation, highlighting an important role for BA-mediated immunoregulation via TGR-5. Despite the protective role of TGR-5 in liver inflammation, this receptor has also been associated with cholangiocarcinoma progression in response to increased concentrations of BAs during prolonged cholestasis.18 Our data confirms that the expression of TGR-5 is increased in chronic liver disease and its expression was significantly higher on CD68+CD206+ intrahepatic macrophages from patients with PSC, highlighting the potential role of this CD68+CD206+ population in PSC pathology. To further investigate the role of TGR-5 on PSC, we attempted some in vitro functional studies to assess the role of TGR-5 on macrophage polarization in the PSC liver – based on observations that TGR-5 signaling has been shown to inhibit the production of proinflammatory cytokines by macrophages in patients with Crohn’s disease,24 that TGR-5 activation can also induce monocyte differentiation towards an IL-12 hypo-producing dendritic cell phenotype,25 and that TGR-5 has been shown to both regulate the intestinal macrophage phenotypes and rescue mice from murine colitis.26 We studied the role of TGR-5 in macrophage polarization using selective bile acid agonists including INT777 (selective agonist for TGR-5), INT747 (selective agonist for FXR), as well as conditioned media prepared from PSC explanted livers. We found monocytes co-cultured with PSC liver conditioned media alone were polarized towards a proinflammatory phenotype. On the contrary, the provision of BA agonists promoted monocyte polarization towards an anti-inflammatory (CD68+CD163+CD206+) phenotype. However, these agonists had little effect on monocyte polarization when added in combination with the PSC conditioned media. Furthermore, the addition of the TGR-5 agonists has little effects in macrophage polarization (Fig. S1). Although we saw differential regulation of this protein in PSC, we did not observe a direct effect on macrophage polarization.
Human-based studies in PSC are challenging and limitations of our study are inevitable and relate to limited access to tissue and a need to study patients with end-stage liver disease, given standard of care in PSC is not to perform liver biopsies. Furthermore, in the context of PSC, transplantation can be indicated for complications of cholangitis, as well as end-stage liver failure, and in that regard, it was notable that our cohort of patients with PSC had less fibrosis in comparison to other patients.
In conclusion, based on human liver and blood analyses, we have demonstrated increased recruitment of CD16+ monocytes and higher frequencies of CD68+CD206+ macrophages in the livers of patients with PSC; this was associated with significantly higher TGR-5 expression on this CD68+CD206+ macrophage subset in PSC. The macrophage-TGR-5 axis is worthy of further investigation, in order to identify novel therapeutic approaches for PSC.
Abbreviations
ALD, alcohol-related liver disease; BA, bile acids; CCR2; C-C motif chemokine receptor 2; CX3CR1, C-X3-C motif chemokine receptor 1; ECM, extracellular matrix; FMO, fluorescence-minus-one; FXR, farnesoid X receptor; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; MFI, mean fluorescence intensity; ASH, non-alcoholic steatohepatitis; NL, normal livers; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; TGF-β, transforming growth factor- β; TNF-α, tumor necrosis factor-α
Financial support
This report presents independent research partly funded by the National Institute for Health Research (NIHR). CJW was supported by funding from the BBSRC (BB/N018869/1). The views expressed are those of the authors(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Y.-Y.C.: Conceptualisation, Formal analysis, Investigation, Methodology, Project administration, Writing-original draft, Writing-review & editing. K.A., G.W., M.C. and S.A.: Investigation. E.L.: Investigation, Writing-review & editing. P.W.: Investigation. D.H.A.: Funding acquisition. C.J.W.: Conceptualisation, Formal analysis, Funding acquisition, Methodology, Supervision, Validation, Writing –review & editing. G.M.H.: Conceptualisation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing –review & editing.
Acknowledgements
This paper presents independent research funded (or supported) by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (Grant Reference Number BRC-1215-20009). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. CJW was supported by funding from the BBSRC (BB/N018869/1), and additional funding was provided by an NIHR Senior Investigator award to DHA (NF-SI-0616-10012) and an NIHR EME grant to GMH (12/165/31/VAP-1).
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.10.003.
Supplementary data
Supplementary material
Supplementary material
Supplementary material
References
- 1.Hirschfield GM. Primary sclerosing cholangitis. Lancet. 2013;382:1587–1599. doi: 10.1016/S0140-6736(13)60096-3. [DOI] [PubMed] [Google Scholar]
- 2.Eaton JE. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67(6):1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Li H. Hepatic macrophages in liver fibrosis: pathogenesis and potential therapeutic targets. BMJ Open Gastroenterol. 2016;3 doi: 10.1136/bmjgast-2016-000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rőszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat Inflamm. 2015;2015:16. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellicoro A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 8.Cameron RG, Blendis LM, Neuman MG. Accumulation of macrophages in primary sclerosing cholangitis. Clin Biochem. 2001;34:195–201. doi: 10.1016/s0009-9120(01)00215-6. [DOI] [PubMed] [Google Scholar]
- 9.Guicciardi ME. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol. 2018;69(3):676–686. doi: 10.1016/j.jhep.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keitel V, Reich M, Haussinger D. TGR5: pathogenetic role and/or therapeutic target in fibrosing cholangitis? Clin Rev Allergy Immunol. 2015;48:218–225. doi: 10.1007/s12016-014-8443-x. [DOI] [PubMed] [Google Scholar]
- 12.Hov JR, Keitel V, Laerdahl JK, Spomer L, Ellinghaus E, Elsharawy A. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Jadhav K, Zhang Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochem Pharmacol. 2013;86:1517–1524. doi: 10.1016/j.bcp.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo C, Chen WD, Wang YD. TGR5, Not Only a Metabolic Regulator. Front Physiol. 2016;7:646. doi: 10.3389/fphys.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hov JR. TGR5 sequence variation in primary sclerosing cholangitis. Dig Dis. 2011;29:78–84. doi: 10.1159/000324138. [DOI] [PubMed] [Google Scholar]
- 16.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brempelis KJ, Crispe IN. Infiltrating monocytes in liver injury and repair. Clin Transl Immunol. 2016;5:e113. doi: 10.1038/cti.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Huang L, Sung SSJ, Vergis AL, Rosin DL, Rose CE., Jr The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CWE Cornwell, V Kim, X Fan, ME Vega, FV Ramsey, GJ Criner, et al., Monocyte Populations Which Participate in Chronic Lung Inflammation. Smoking and Lung Inflammation pp 29-58.
- 22.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019 doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoneno K, Hisamatsu T, Shimamura K, Kamada N, Ichikawa R, Kitazume MT. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn's disease. Immunology. 2013;139:19–29. doi: 10.1111/imm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa R, Takayama T, Yoneno K, Kamada N, Kitazume MT, Higuchi H. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology. 2012;136:153–162. doi: 10.1111/j.1365-2567.2012.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biagioli M, Carino A, Cipriani S, Francisci D, Marchiano S, Scarpelli P. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J Immunol. 2017;199:718–733. doi: 10.4049/jimmunol.1700183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material