Summary
Fulminant hepatic failure is an unusual complication of hepatitis A virus infection which, without liver transplantation, is associated with a poor prognosis. We report a case of fulminant hepatitis A complicated by severe cardiac dysfunction, related to Takotsubo syndrome, that was considered a contraindication for transplantation and was successfully managed with standard volume plasma exchange.
Graphical abstract
Introduction
Fulminant hepatic failure is a devastating syndrome characterized by severe coagulopathy (international normalized ratio [INR] ≥1.5) and hepatic encephalopathy in patients without underlying chronic liver disease. Mortality is high in the absence of state-of-the-art intensive care and liver transplantation.1 Takotsubo syndrome, previously known as stress cardiomyopathy, is an acute, transient and reversible syndrome that occurs in the presence of emotional or physical stress triggers and can cause a severe deterioration of cardiac function including cardiogenic shock.2 Only 2 cases of Takotsubo syndrome have been reported in patients with fulminant hepatic failure: a patient with Wilson disease requiring liver transplantation and another patient with acetaminophen-induced acute liver failure (ALF) who recovered with intravenous N-acetyl-cysteine.3,4
We report, for the first time, on a case of hepatitis A-related fulminant hepatic failure, complicated by Takotsubo syndrome, that was successfully treated with standard volume plasma exchange.
Case report
A healthy 61-year-old woman presented in another institution because of asthenia and nausea during the preceding week. Blood analysis showed data compatible with acute liver failure (aspartate aminotransferase [AST]/alanine aminotransferase [ALT] 12,760/11,864 IU/L), hyperbilirubinemia (5.3 mg/dl) and profound coagulopathy (INR 3.5). The patient was immediately transferred to our liver intensive care unit (ICU). At admission she was hemodynamically stable with no hepatic encephalopathy. Physical examination revealed jaundice with no other abnormalities. There was no history of recent travels, sexual risk behavior or drug use. Initial blood analysis disclosed high aminotransferase levels (AST 5,206 IU/L; ALT 7,829 IU/L), mild cholestasis (gamma-glutamyltransferase 256 IU/L; alkaline phosphatase 164 IU/L), severe liver dysfunction (total bilirubin 6.9 mg/dl; INR 3.9) and a moderate increase in serum ammonia levels (86 μmol/L; normal ≪33). Abdominal ultrasound showed no abnormalities. Initial cardiac evaluation disclosed a normal electrocardiogram (ECG) and echocardiogram (ejection fraction [EF] in the lower limit of normality: 52%) with normal serum troponin I levels. A transjugular liver biopsy showed a mixed cholestatic and necroinflammatory pattern with periportal necrosis compatible with acute hepatitis (Fig. 1A). After obtaining the results of the hepatotropic viral serologies, negative for HCV, HVB, HEV and HIV and positive for IgM anti-HAV, severe acute hepatitis A was diagnosed.
Fig. 1.
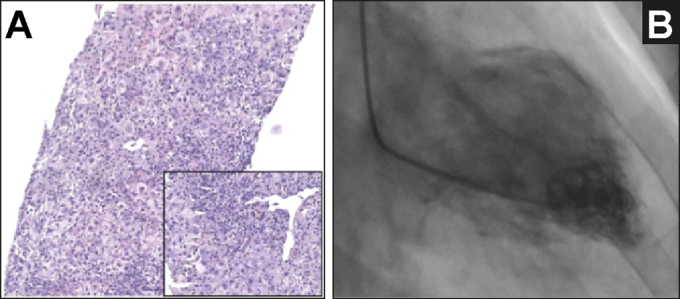
Patient liver biopsy and cardiac ventriculography.
(A) Transjugular liver biopsy showing diffuse acute hepatitis with necroinflammation and cholestasis. Portal tracts were normal in size but showed mild inflammation with a characteristic pattern of periportal necrosis with plasma cells (inset), H&E 20x; (B) Left ventriculography showing severe depression of ventricular function due to a mid-ventricular Takotsubo syndrome. Coronariography showed normal coronary arteries.
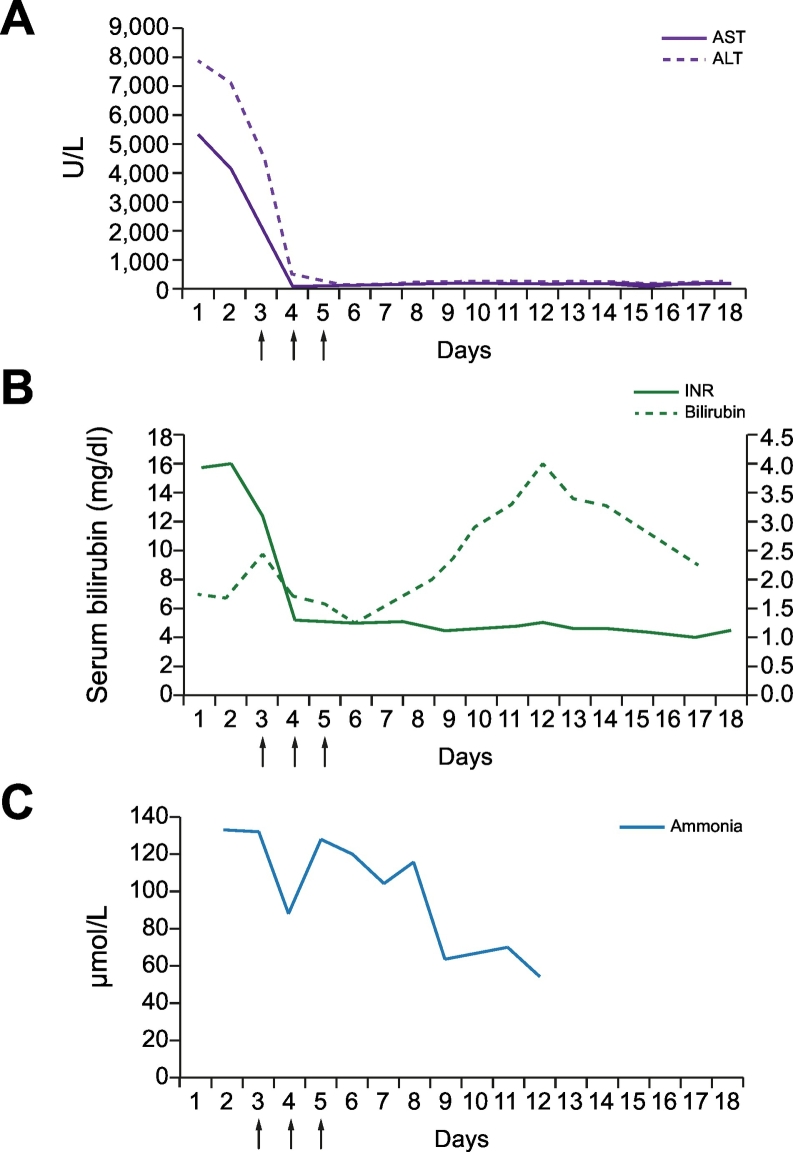
On day 2 after ICU admission, the patient developed progressive hepatic encephalopathy, reaching grade IV in a few hours, and required endotracheal intubation and the initiation of sedation and analgesia. At that time, blood analysis showed a decrease in aminotransferase levels (AST 893 IU/L; ALT 3,922 IU/L), severe liver dysfunction (total bilirubin 14.1 mg/dl; INR 2.7) and a relevant increase in serum ammonia levels (132 μmol/L). Transcranial doppler ultrasonography showed a diffuse increase in the pulsatility index (between 1.5 to 1.8) of the different brain arteries suggestive of intracranial hypertension. In this setting, she was considered for emergent liver transplantation according to the Hospital Clinic and the King’s College criteria and a clinical re-evaluation was performed. A new ECG showed a mild decrease in the ST segment from V1 to V6 that was associated with a moderate increase in serum troponin I levels (11.19 ng/ml; normal ≪0.05). A new echocardiogram was then performed showing marked hypokinesia of the mid-basal segments of the left ventricle and severe myocardial dysfunction (EF 32%); a pattern compatible with the diagnosis of Takotsubo syndrome. This entity was confirmed by cardiac catheterization and ventriculography that showed normal coronary arteries and severe depression of left ventricular function due to mid-ventricular hypokinesia (Fig. 1B). Within the next few hours the patient became hypotensive, requiring the initiation of dobutamine and low doses of noradrenaline. In this setting, liver transplantation was contraindicated due to cardiogenic shock with severe cardiac dysfunction. Plasma exchange was started as salvage therapy. Three consecutive sessions of standard volume plasma exchange (1.5 plasma volumes; 6.5% of the ideal body weight) were performed during the next 3 days using methylene-blue inactivated fresh frozen plasma as replacement solution. Treatment was well tolerated and associated with a progressive clinical improvement, with rapid normalization of cardiac function, marked improvement in liver tests (sustained decreases in aminotransferase levels, ammonia levels and INR), and normalization of pulsatility index in transcranial doppler ultrasonography. This improvement was sustained after plasma exchange, except for serum bilirubin levels, that transitorily increased after stopping the treatment but finally normalized (Fig. 2). Nine days after ICU admission the patient was extubated. On day 14 she was discharged to the regular ward, following resolution of ALF and normalization of cardiac function.
Fig. 2.
Impact of plasma exchange on the evolution of liver function tests.
(A) Aminotransferase levels; (B) INR and serum bilirubin levels; (C) serum ammonia levels. Arrows indicate plasma exchange sessions. ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio.
Discussion
Hepatitis A, B and E viruses represent the main cause of ALF in developing countries and the second leading cause in Western countries. HAV infection is usually unapparent or subclinical in young children. The ALF presentation is more frequent in adults, especially in older patients, in whom it is associated with a poorer prognosis. HAV causes ALF in less than 1% of the cases and is responsible for around 2 to 4% of all ALF cases reported in Western countries, and for 4% and 6% of those reported in Australia and Japan, respectively.5 Hepatitis A-related ALF has a spontaneous survival rate of 69%; the remaining 31% require emergency liver transplant or die. Around 12% of patients with fulminant hepatitis A listed for emergent liver transplantation show spontaneous improvement and are delisted.6
Takotsubo syndrome, previously known as stress-induced or “broken-heart” cardiomyopathy, is a reversible syndrome characterized by transient hypokinesis, akinesis or dyskinesis of the left ventricular mid-segments with or without apical involvement that mimics the presentation of myocardial infarction. The coronary vasculature is by definition normal, and the proposed mechanism is a catecholamine storm causing reversible myocardial “stunning” during physical or emotional stress. Treatment is predominantly supportive. Hospital mortality is around 15% in cases that cause cardiogenic shock.2
Whether Takotsubo syndrome constitutes a contraindication for major surgery is currently unknown. It has been reported during anesthesia care and after any type of surgery. Takotsubo syndrome is extremely infrequent in patients undergoing liver transplantation. The majority of the cases have been reported in the post-operative period (prevalence of 1.2–1.5%) and the prognosis seems to be good with relatively fast reversibility and recovery.7 Four additional cases have been reported during liver transplantation, 3 of which led to cardiac arrest. The recovery of cardiac function varied from 3 to 16 days and all these patients survived except for 1 who died of unrelated causes.8 Finally, Takotsubo syndrome has also been reported in 2 patients with ALF, one of whom required liver transplantation.3,4
Our case is unique for 2 reasons. First, it is the only report of Takotsubo syndrome complicating the course of a HAV-related fulminant hepatic failure. Second, and more importantly, we show that standard volume plasma exchange can be an effective therapy to allow liver recovery, removing the need for liver transplantation in patients with fulminant hepatic failure and potential contraindications for liver transplantation. A recent study by Larsen et al.,9 showed that high volume plasma exchange (3 plasma volumes; 15% ideal body weight; fresh frozen plasma as replacement fluid) improves transplant-free survival in patients with fulminant hepatic failure and contraindications for liver transplantation, an effect attributable to the attenuation of immune activation and amelioration of organ dysfunction. However, it is unclear whether conventional plasma exchange (replacing 1.0 to 1.5 plasma volumes) is also associated with similar effects on outcome. The evolution observed in our patient and a recently published retrospective cohort study10 support the efficacy of standard volume plasma exchange in patients with acute liver failure. Our case also leads to the hypothesis that recovery of Takotsubo syndrome could be accelerated by plasma exchange, given the rapid hemodynamic and echocardiographic improvement observed in this patient after the intervention. The removal of circulating damage-associated molecular patterns and the subsequent decrease in the production of proinflammatory cytokines could at least partially explain these positive effects, as these mediators have been involved in the pathogenesis of the syndrome.
In summary, in this case of HAV-related fulminant hepatic failure complicated by a Takotsubo syndrome, standard volume plasma exchange was a successful salvage therapy, allowing fast liver and cardiac recovery. Further studies are needed to confirm our findings.
Financial support
The authors received no financial support to produce this manuscript.
Conflict of interest
All authors declare no conflicts of interest regarding this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
BC, JC, MHT, MT, JF participated in the writing group, ML and ER critically revised the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.10.004.
Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Association E EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ. International expert consensus document on Takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adar T, Chen S, Mizrahi M. A heartbreaking case of Wilson’s disease: Takotsubo cardiomyopathy complicating fulminant hepatic failure. Transpl Int. 2014;27:e109–e111. doi: 10.1111/tri.12350. [DOI] [PubMed] [Google Scholar]
- 4.Jophlin L, Koch D. Takotsubo cardiomyopathy following acute liver failure. Hepatology. 2015;61:1430–1431. doi: 10.1002/hep.27247. [DOI] [PubMed] [Google Scholar]
- 5.Manka P, Verheyen J, Gerken G, Canbay A. Liver failure due to acute viral hepatitis (A-E) Visc Med. 2016;32:80–85. doi: 10.1159/000444915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor R, Davern T, Munoz S, Han S, McGuire B, Larson A. Fulminant hepatitis A virus infection in the United States: incidence, prognosis, and outcomes. Hepatology. 2009;377:364–377. doi: 10.1002/hep.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maestas C, Lazkani M, Sultan M, Kolli G, Sheikh M, Cherukuri M. Severe takotsubo cardiomyopathy following orthotopic liver transplantation: A case series. Clin Res Hepatol Gastroenterol. 2019;43:e48–e53. doi: 10.1016/j.clinre.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Vitin A, Azamfirei L, Tomescu D. Perioperative Stress-Induced (Takotsubo) Cardiomyopathy in Liver Transplant Recipients. J Crit Care Med. 2018;4:56–63. doi: 10.2478/jccm-2018-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen F, Schmidt L, Bernsmeier C, Rasmussen A, Isoniemi H, Patel V. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016;64:69–78. doi: 10.1016/j.jhep.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Stahl K, Hadem J, Schneider A, Manns MP, Wiesner O, Schmidt BMW. Therapeutic plasma exchange in acute liver failure. J Clin Apher. 2019;34:589–597. doi: 10.1002/jca.21737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2