Background & Aims
Treatment options remain limited for patients with autoimmune hepatitis (AIH), while there are still concerns over the consequences of long-term corticosteroid use. A few studies have suggested a role for B cell-driven autoimmune liver injury in AIH. This multicentre, international retrospective cohort study from the International Autoimmune Hepatitis Group aims to evaluate the clinical efficacy and safety of rituximab in difficult-to-manage AIH.
Methods
Clinical data from 22 patients who received rituximab between 2007 and 2017 were collected from centres in the United Kingdom, Germany and Canada. Clinical response was assessed using changes in biochemical and immunological parameters up to 24 months post-rituximab infusion. In addition, we compared the doses of prednisolone used 3 months before and 12 months after treatment, and assessed freedom from AIH flares over the post-treatment period.
Results
Twenty-two patients with type-1 AIH were included, with a median age of 40 years at diagnosis (range 19–79); 15/22 (68%) were female and 18/22 (82%) were Caucasian. The median period from diagnosis to the end of follow-up in these patients was 11 years (range 3–28). Values of alanine aminotransferase, aspartate aminotransferase and albumin improved significantly following rituximab therapy, and were sustained for up to 2 years (all p ≪0.001). Prednisolone doses were significantly reduced by 12 months post-treatment (p = 0.003), with 13/21 (62%) patients having a dose reduction. Over a median post-treatment follow-up period of 6 years (range 1–10), 5 patients developed AIH flares at a median of 22 months post-treatment, giving an estimated 71% freedom from AIH flare at 2 years. Four of these patients received a second course of treatment, of whom 2 had subsequent further flares. No serious adverse events attributable to rituximab were recorded.
Conclusion
In patients with difficult-to-manage AIH, rituximab appears to be clinically effective and well tolerated. Rituximab was associated with sustained improvements in serum liver tests, an absence of clinical disease flares, and a reduction in prednisolone dose. Controlled trials are warranted to further evaluate B cell-targeting therapies in patients with AIH.
Lay summary
Autoimmune hepatitis is an autoimmune condition of the liver, usually treated with medications that suppress the immune system, such as steroids. However, some patients do not respond to this treatment. We analysed the safety and efficacy of rituximab in patients who were not responding to first- or second-line therapies. Rituximab was safe and improved liver blood tests in 70% of patients over a 2-year follow-up period, while enabling steroid doses to be reduced in two-thirds of patients, which is a very positive clinical outcome.
Keywords: Autoimmune hepatitis, Prednisolone, Difficult-to-manage, B cell depletion therapy, Rituximab
Graphical abstract
Highlights
-
•
Study of rituximab therapy in 22 patients with autoimmune hepatitis over a follow-up period of 24 months.
-
•
No serious adverse events were noted during follow-up in patients treated with rituximab.
-
•
Rituximab therapy improved liver enzymes significantly during the 2 years of follow-up.
-
•
Prednisolone dose reductions were seen in 62% of patients at 12-month follow-up.
-
•
A total of 71% of patients were free of AIH flares during the 24 months of follow-up.
Introduction
Autoimmune hepatitis (AIH) is a rare, chronic immune-mediated liver disease, with a reported prevalence of 10–17 per 100,000 in Europe.1,2 Due to the lack of a precise understanding of disease pathogenesis, AIH remains a therapeutic challenge. Current treatments are based on broad immunosuppression and associated with significant side effects.3,4 Although AIH is a T cell driven disease,[4], [5], [6] immunoglobulin and antibody producing B cells also play an important role in its pathogenesis.7 The diagnostic criteria proposed by the International Autoimmune Hepatitis Group (IAIHG) include elevated IgG concentrations, the presence of autoantibodies,8,9 and the histological findings of a plasma cell rich interface hepatitis.4,10
Historical data demonstrates that patients with uncontrolled AIH progress to end-stage liver disease, with 5-year mortality of around 50%.11 Progression of liver disease is more common in patients with incomplete treatment response or treatment failure.12,13 A combination of prednisolone and azathioprine is the accepted first-line treatment used for induction and maintenance of remission in AIH.14 Approximately 15% of patients experience insufficient response or intolerance to standard therapy and require alternative agents.15 Mycophenolate mofetil (MMF)11 and calcineurin inhibitors, such as tacrolimus15 are second- and third-line rescue therapies for those who are unable to tolerate azathioprine or mercaptopurine.16 Difficult-to-manage AIH includes patients for whom these therapies are ineffective, not tolerated, and/or associated with an unacceptable long-term side effect burden. Successful treatment with biologics, such as infliximab, an anti-tumour necrosis factor antibody,17 has been reported for such patients. In addition to infliximab, reports have suggested some patients may respond to anti-B cell therapy with rituximab.
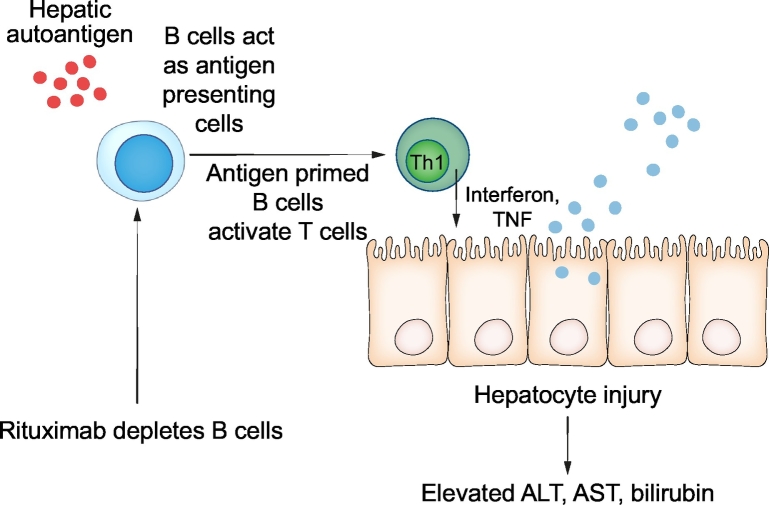
Rituximab is a chimeric human monoclonal anti-CD20 antibody that targets CD20, a cell surface glycoprotein restricted to B cell lineages.4,12 The pathogenesis of AIH is not fully understood, but the current hypothesis is that hepatic autoantigens present to B cells, which triggers a dysregulated T cell response against autoantigens, leading to the release of a cascade of cytokines. Interferon and tumour necrosis factor are produced upon this trigger, contributing to hepatocyte injury, cell death and release of liver enzymes in individuals who are genetically susceptible to AIH.18 The binding of rituximab to CD20 results in apoptotic lysis and depletion of B cells via complement and antibody-mediated pathways.19 The proposed mechanism of action of rituximab is illustrated in Fig. 1.
Fig. 1.
Proposed mechanism of rituximab therapy in AIH.
The proposed pathogenesis of AIH was that B cells act as antigen presenting cells by presenting autoantigens on hepatocytes to CD4 T- cells (Th1), which leads to the release of a cascade of cytokines (interferon and TNF). TNF and interferon subsequently cause hepatocyte injury, cell death and release of liver enzymes and bilirubin. Rituximab depletes B cells and as a result Th1 activation, which subsequently prevents hepatocyte injury. Th1, T helper 1 cell; TNF, tumour necrosis factor, ALT, alanine aminotransferase, AST, aspartate aminotransferase.
Rituximab has been licenced for clinical use in adults with CD20-positive B cell lymphoma, rheumatoid arthritis, or vasculitis associated with anti-neutrophil cytoplasmic antibodies.20 However, rituximab is not currently a licenced therapy in AIH and representative, multicentre, long-term data on the efficacy of rituximab therapy in AIH is lacking. Herein we report on the efficacy and safety of rituximab in patients with difficult-to-manage AIH.
Patients and methods
Study population
Twenty-two patients with difficult-to-manage type 1 AIH were identified for inclusion in the study. We defined patients with “difficult-to-manage” AIH as those who i) had not achieved normalisation of aminotransferase despite being on first-line therapy (corticosteroid), second-line therapies (either azathioprine or mycophenolate mofetil) or even third-line therapies (tacrolimus or cyclosporine), ii) were compliant with medications and iii) after exclusion of primary sclerosing cholangitis. This is the real-world definition used by hepatologists who are managing difficult AIH cases based on current guidelines.
The patients in this study fulfilled the criteria for a definite diagnosis of AIH, based on international guidelines11 after excluding other causes of hepatitis, and despite being compliant with treatments, these patients continued to have elevated liver enzymes, with median alanine aminotransferase (ALT) of 214 IU/L (interquartile range 115–323 IU/L) and median IgG levels of 18.6 g/dl (interquartile range 12.2–21.9 g/dl) prior to rituximab treatment. The decision to use rituximab in these patients was made by the clinician at each centre.
For each course of treatment, patients received 2 doses of rituximab (1,000 mg in each dose) via intravenous infusion, administered 2 weeks apart, with the exception of a patient from Germany, who received 375 mg/m2 rituximab. Patients received their first dose of rituximab between the years 2007 and 2017, a median of 5 years after AIH diagnosis (range 0–18 years). The data for biochemical parameters and IgG values were collected 1 month before, and at 1, 3, 6, 12 and 24 months after rituximab therapy. Five patients received additional doses of rituximab during the follow-up period, and these patients were censored from the analysis of biochemical parameters and IgG at the time the second dose was administered, with data from later time points excluded from the analysis.
Biochemical assessments included ALT, aspartate aminotransferase (AST), bilirubin and albumin. Data were also collected for haematological measures, platelet count, prothrombin time or international normalised ratio (INR) and immunology parameters (autoantibodies and serial IgG concentrations). In addition, the model for end-stage liver disease (MELD) and Child-Pugh scores, as well as data on prednisolone doses, were retrieved at 3 months before and 12 months after rituximab treatment. A flare of AIH was defined according to IAIHG criteria, as an elevation of ALT (3x upper limit of normal [ULN]).
The study was discussed and approved by IAIHG at a meeting where investigators were present. Each centre obtained its own ethical approval to collect patient data.
Statistical analysis
Initially, the changes in a variety of measures (bilirubin, ALT, AST, IgG, albumin, INR) over the period of follow-up were assessed. As data were not complete for all patients, particularly at later time points, a generalised estimating equation approach was used. This accounted for the non-independence of repeated measures on the same patient, whist allowing for the inclusion of patients who did not have complete data for all of the time points, hence maximising the included sample size. The time point of the measurement was included as a nominal factor in the model, and a first order autoregressive correlation structure was used to account for within-patient correlations. As the biochemical and immunological values analysed all follow skewed distributions, these values were log10-transformed, prior to analysis, to normalise the distributions. As a result, the data were summarised using geometric means and 95% CIs. Any patients that had received a second rituximab treatment were excluded from the analysis of subsequent time points. As a sensitivity analysis, a complete cases analysis was performed for those patients with measurements of each marker pre- and 1-, 3- and 6-months post-treatment. A non-parametric approach was used, with data summarised using medians and IQRs, and comparisons performed using Freidman’s tests, followed by pairwise post hoc tests where the overall effect was significant.
Prednisolone doses and MELD scores were then compared between the pre- and post-treatment assessments using Wilcoxon’s tests. The period of freedom from a flare was estimated using Kaplan-Meier curves. It was assumed that patients would be given rituximab in the presence of a flare. Hence, for each patient, the time from the first treatment of rituximab to any subsequent treatment was calculated, with patients that only received a single rituximab treatment censored at the end of follow-up. A similar analysis was then performed starting with the second dose of rituximab. All analyses were performed using IBM SPSS 22 (IBM Corp. Armonk, NY) and GraphPad Prism version 7.0, with p ≪0.05 deemed to be indicative of statistical significance.
Results
Demographics
Data were collected retrospectively from 22 patients with difficult-to-manage type 1 AIH, who had initial diagnoses of AIH between 1989 and 2012. Of those patients, the majority were recruited from the UK (Birmingham, Leeds, Cardiff; n = 13), with the remainder from Canada (n = 6) and Germany (n = 3). We included previously published data of 6 patients from Canadian Liver Unit [55], as additional long-term follow-up data were now available for these patients beyond 72 weeks follow-up. Most patients were female (n = 15, 68%) and of Caucasian ethnicity (n = 18, 82%), with a median age at diagnosis of 40 years (range 19–79). The median period from diagnosis to the end of follow-up in these patients was 11 years (range 3–28). At initial diagnosis, 10/17 (59%) were positive for antinuclear antibodies and 13/16 (81%) tested positive for anti-smooth muscle antibodies. A total of 18 patients had liver biopsies at the time of initial diagnosis, of whom 3 (17%) were Metavir liver fibrosis stage 4. Of these 3 patients, 2 were Child-Pugh A and 1 was Child-Pugh C. The clinical presentations that led to the diagnosis of AIH were abnormal liver blood tests without any symptoms (n = 9), arthralgia (n = 5), jaundice (n = 4), lethargy (n = 3) and abdominal pain (n = 1). Further demographic data are reported in Table 1.
Table 1.
Demographic and diagnostic factors for patients at diagnosis.
| Median (range)/n (%) | |
|---|---|
| Patient demographics | |
| Age (years) | 40 (19–79) |
| Gender (% female) | 15 (68%) |
| Ethnicity (% caucasian) | 18 (82%) |
| Biochemical parameters at diagnosis | |
| Platelets (x109/L) | 323 (138–582) |
| Alanine aminotransferase (U/L) | 137 (47–518) |
| Aspartate aminotransferase (U/L) | 175 (19–1,208) |
| Bilirubin (μmol/L) | 15 (6–476) |
| Albumin (g/L) | 40 (23–46) |
| International normalised ratio | 1.0 (0.9–1.7) |
| Immunological parameters at diagnosis | |
| IgG (g/L) | 23 (10–45) |
| Antinuclear antibody positivity | 10/17 (59%) |
| Anti-smooth muscle antibody positivity | 13/16 (81%) |
| Prognostic scoring at diagnosis | |
| Simplified autoimmune hepatitis score | 6 (2–8) |
| Model for end-stage liver disease score | 8 (5–25) |
| Metavir fibrosis score | n = 18 |
| Stage 0 | 4 (22%) |
| Stage 1 | 2 (11%) |
| Stage 2 | 3 (17%) |
| Stage 3 | 6 (33%) |
| Stage 4 | 3 (17%) |
| Child-Pugh score for stage 4 fibrosis | n = 3 |
| A | 2 (67%) |
| B | 0 (0%) |
| C | 1 (33%) |
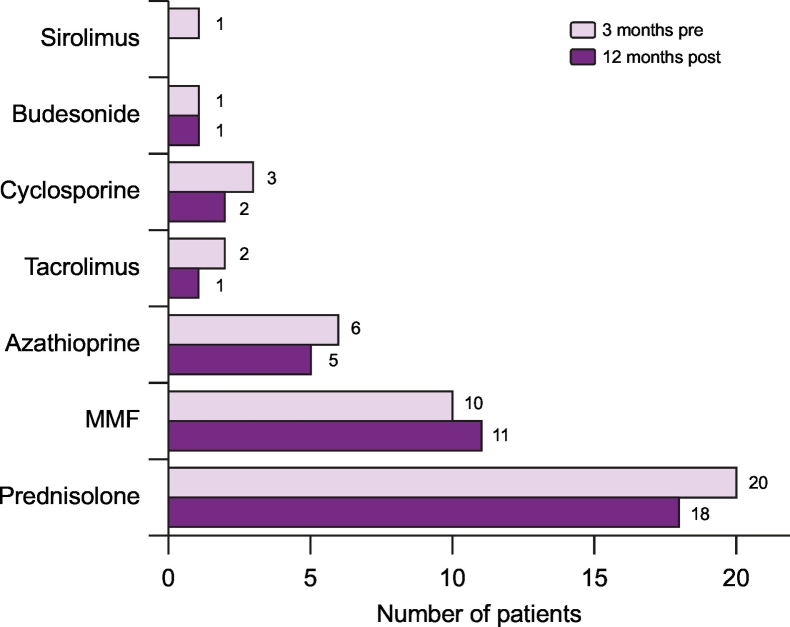
The details of all the immunosuppressive medications that each patient had received at any time point prior to rituximab therapy since diagnosis are shown in Fig. 2. The most commonly used immunosuppressant medications used were prednisolone (n = 22, 100%), azathioprine (n = 18, 82%) and MMF (n = 15, 68%). The immunosuppressive medications that were used within 3 months prior to initiating rituximab and 12 months post therapy are reported in Fig. 3 and Table S4.
Fig. 2.
Medications used by individual patients before rituximab treatment (at any time point).
MMF, mycophenolate mofetil.
Fig. 3.
Medications used 3 months before and 12 months after rituximab therapy in patients with autoimmune hepatitis.
One patient had a previous HBV infection (they were positive for HBV core antibody) and received prophylactic anti-HBV treatment with lamivudine 100 mg per day prior to starting rituximab. This patient did not develop a hepatitis B flare during treatment. Two patients (9%) from the UK (Birmingham centre) underwent liver transplantation at 2 and 8 years, respectively, after their first dose of rituximab. Both patients received a second course of rituximab treatment prior to ultimate liver transplantation, due to AIH flares that occurred at 4 and 23 months after their first rituximab treatments, and both are still alive after liver transplantation at the time of writing. Only 1 patient from the cohort died, due to Staphylococcus aureus bacteraemia, with this occurring 12 years after their AIH diagnosis, and 6 years from the last dose of rituximab infusion. We concluded that the death was unrelated to the rituximab therapy.
Coexisting autoimmune diseases
Thirteen patients (59%) had associated autoimmune conditions, with the most common being systemic connective tissue disorders, followed by endocrine-related conditions. Further details of the associated coexisting autoimmune conditions in the cohort are described in Table 2.
Table 2.
Associated autoimmune conditions.
| Patient ID | Associated autoimmune conditions |
|---|---|
| 1 | Colitis |
| 2 | Multiple sclerosis |
| 3 | Hypothyroidism |
| 4 | Diabetes mellitus, diabetes insipidus Addison's disease, hypopitutarism, hypoadrenalism Arthritis Alopecia |
| 5 | Lupus nephritis Systemic lupus erythematosus |
| 6 | Rheumatoid arthritis |
| 7 | Diabetes mellitus Systemic lupus erythematosus |
| 8 | Wegner's vasculitis |
| 9 | Coeliac Type 1 diabetes |
| 10 | Psoriasis |
| 11 | Primary biliary cholangitis |
| 12 | Colitis Systemic lupus erythematosus, Vasculitis |
| 13 | Membranous glomerulonephritis Systemic lupus erythematosus |
Only those patients with associated autoimmune conditions are included in the table. The remainder of the cohort (n = 9) had no associated autoimmune conditions.
Rituximab treatment leads to a significant improvement in AIH disease activity
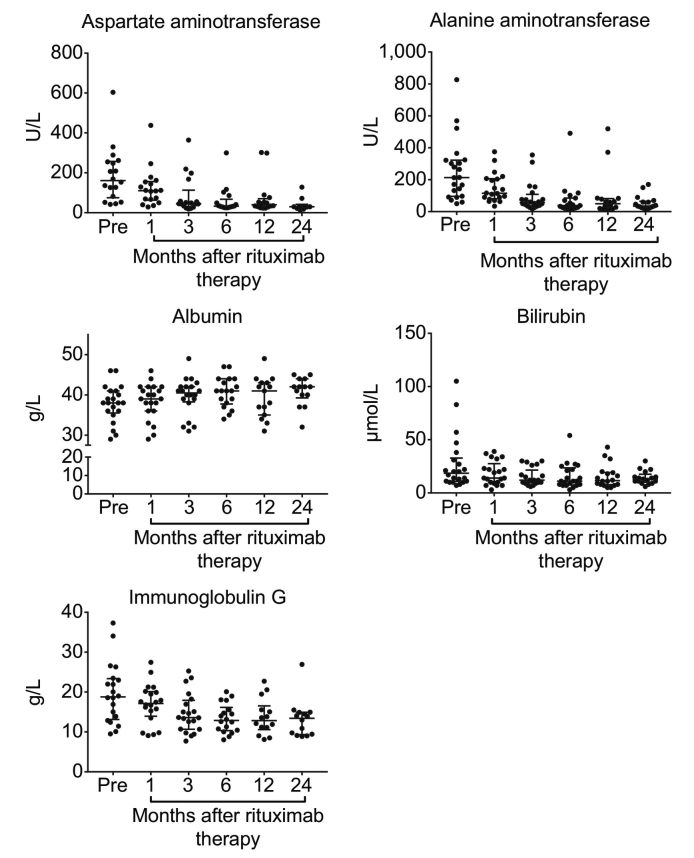
Analyses of biochemical and haematological markers in the period after rituximab therapy are reported in Fig. 4 and Table S2, with the normal ranges of the blood tests shown in Table S1. The primary analysis found the average ALT and AST levels were significantly improved 1 month after the first dose of rituximab, with this improvement sustained up until the end of follow-up at 24 months (p ≪0.001). Albumin levels were significantly improved 3 months after treatment (p = 0.024), with this sustained until 24 months post-treatment (p ≪0.001). No significant changes were seen in either platelet count (p = 0.517), INR (p = 0.093) or bilirubin (p = 0.097) during the period of follow-up. IgG concentrations declined significantly 1-month after treatment (p = 0.006) and were sustained until the end of follow-up at 24 months (p ≪0.001).
Fig. 4.
Changes in aspartate aminotransferase, alanine aminotransferase, bilirubin, albumin and IgG after rituximab treatment.
In addition, sensitivity analyses were performed for each of the markers using a complete cases approach over the first 6 months post-treatment (Table S3). This returned consistent results to those above, with significant improvements in ALT, AST, albumin and IgG observed post-treatment. This analysis also identified a significant change in bilirubin, with median levels declining from 17 to 11 umol/L by 6 months post-treatment (p = 0.007).
MELD scores were recorded at both pre-treatment and 12 months post-treatment in 18 patients, and were not found to change significantly, with medians of 8 (IQR 6–9) and 7 (6–12), respectively (p = 0.821).
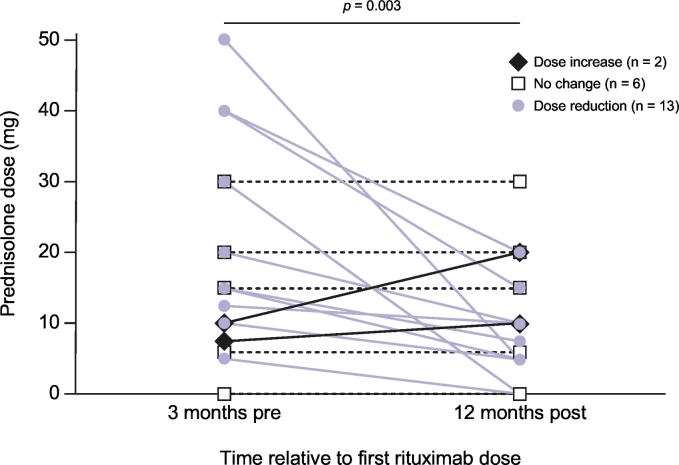
Rituximab therapy leads to a lower burden of corticosteroid use
Changes in prednisolone doses between 3 months pre- and 12 months post-rituximab therapy are shown in Fig. 5. One patient was excluded from this analysis, as they were treated with budesonide (at a dose of 3 mg at both time points). For the remaining 21 patients, a significant reduction in prednisolone doses was detected, from a median of 15 mg to 10 mg (p = 0.003), with a total of 13 (62%) patients having a dose reduction.
Fig. 5.
Changes in prednisolone dose following rituximab therapy.
Rituximab treatment leads to freedom from AIH flares
A total of 5 patients (23%) developed flares of AIH during the follow-up period. The earliest flare occurred 135 days (4 months) after the first dose of rituximab, with the other 4 cases occurring at 21, 22, 23 and 24 months. One additional patient had a second course of rituximab 9 months after their first dose for the treatment of rheumatoid arthritis rather than AIH, and hence was censored from analysis at this point.
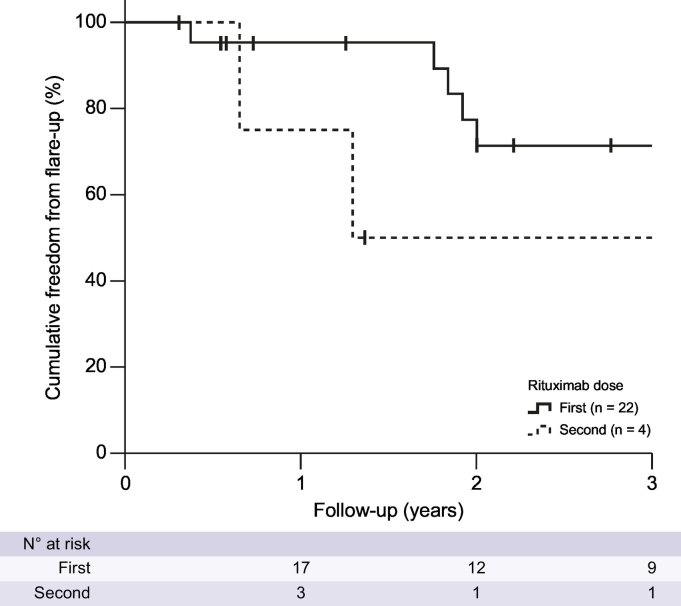
Kaplan-Meier analysis estimated the rates of freedom from flare to be 95% and 71% at 1 and 2 years, respectively, after the first dose of rituximab (Fig. 6). Of the 5 patients who developed AIH flares, 4 (80%) were treated with a second dose of rituximab. Two of these 4 patients subsequently developed recurrence of AIH activity at 236 days (8 months) and 471 days (15 months) after their second rituximab infusions.
Fig. 6.
Kaplan-Meier curve of freedom from autoimmune hepatitis flares following rituximab therapy.
Safety profile
Rituximab was well tolerated in this cohort of patients. One patient suffered with recurrent urinary tract infection, and a second patient developed human papilloma virus-related tongue cancer. Although unlikely, a causative relationship with rituximab use could not be excluded in these cases. One patient died 6 years post-rituximab infusion from Staphylococcus infection, which was thought to be unrelated to the infusion. No other significant side effects with treatment were reported by the investigators.
Discussion
AIH is an immune-mediated liver disease with many unanswered questions relating to its pathogenesis. In most patients, AIH can be controlled with inexpensive, non-specific immunosuppression using prednisolone and azathioprine, which have limited the need to test more targeted immunotherapies for this disease.21 However, a significant minority of patients have AIH that is difficult to control with conventional therapies, and there is no clear evidence relating to which second- or third-line treatments should be used in such patients. We report the results of an international multicentre study of anti-B cell therapy with rituximab for difficult-to-manage AIH. Our demonstration of efficacy and acceptable safety highlights the potential of B cell-targeting therapies for patients with AIH.
The aim of treatment in AIH is to achieve biochemical, immunological and histological remission to prevent the development of cirrhosis and liver-related death.22,23 Corticosteroid therapy followed by the addition of azathioprine has been used as the first-line treatment to induce and then maintain remission on the lowest possible dose of corticosteroid.[24], [25], [26], [27], [28], [29], [30] Mercaptopurine can be used in patients who are intolerant to azathioprine.16 For those who are intolerant to azathioprine and mercaptopurine, MMF[31], [32], [33] is recommended as second-line treatment. However, in some patients, neither the first- or second-line therapies can be tolerated, due to side effects or contraindications associated with the therapies. For example, azathioprine is contraindicated in those with a previous history of squamous cell carcinoma of the skin34 and MMF should be avoided in young women who intend to have a family, because of the reported risk of teratogenicity.35 In these circumstances, calcineurin inhibitors such as tacrolimus15,31,36 or cyclosporine37,38 can be used as third-line treatment. However, the current approach to management of AIH is only effective in 80% of patients,39 and a subgroup of patients with AIH continue to have elevated liver enzyme activities and IgG, despite being adherent to current treatments. This group of patients are known to clinicians as difficult-to-manage AIH cases. However, it is necessary to exclude other causes of hepatitis and evaluate treatment compliance before definitively diagnosing these patients with “difficult-to-manage” AIH.[40], [41], [42], [43], [44], [45]
AIH is an immune-mediated liver disease, thus biological agents, such as infliximab (anti-tumour necrosis factor [TNF] therapy), rituximab (anti-B cell therapy) or even tocilizumab (anti-IL-6 therapy) may have a potential role as disease-modifying agents and salvage therapies.17,46,47 Anti-TNF treatment (infliximab) has been applied successfully as a rescue therapy in AIH.17 Adult patients with AIH who have not responded sufficiently or are intolerant to standard of care are currently being recruited for the first randomised placebo-controlled trial of the B cell-depleting anti-BAFF receptor antibody (VAY736) (NCT03217422).
However, there is currently no consensus or guideline on the optimal timing to introduce biologics. In addition, the paucity of data on treatment response and safety of biologics in AIH does not help clinicians justify what are comparatively expensive therapies. Rituximab binds to the CD20 glycoprotein expressed on the surface of most B cell lineages (although not plasma cells), with little expression found on other cell types. When administered, it leads to a depletion of B cells, thereby reducing the ability of B cells to present antigen to T cells.48 Rituximab has been used for several years in the treatment of various autoimmune conditions, including systemic lupus erythematosus, rheumatoid arthritis and autoimmune haemolytic anaemia.47,49,50 Despite its potential efficacy, it is not currently approved for the treatment of AIH. Although there are case reports of rituximab treatment of AIH,19,[51], [52], [53], [54], [55] cumulative multicentre data are lacking. Published data from other autoimmune diseases suggest that rituximab therapy is safe,19,49,50,55 with the most common side effect being an allergic reaction related to the infusion.19 All the patients in the current study tolerated rituximab well.
We report clinically meaningful reductions in liver enzyme values following rituximab treatment which, in most patients, were maintained over 24 months. This translated into freedom from further AIH flares in 95% and 71% of patients receiving rituximab at 1 and 2 years, respectively. This is an important clinical outcome, suggesting that rituximab may have the potential to settle biochemical response in patients with difficult-to-manage AIH for up to 18 months, allowing the physician to reformulate the next treatment strategy to maintain remission.
Only 2 of the 4 patients who received a second course of infusions for a subsequent AIH relapse were flare-free 2 years following therapy. This could potentially be related to the emergence of anti-rituximab antibodies,56,57 although this was not tested in our study. A significant improvement in IgG was also observed following rituximab therapy. However, longitudinal IgG level assessment may not be a reliable clinical indicator of treatment response in this cohort, since rituximab depletes B cells and, as a consequence, can lead to a reduction in immunoglobulins. There was no evidence of significant changes in INR with rituximab treatment, reflecting the fact that the majority of patients in the study were non-cirrhotic.
The reduction in prednisolone dose following rituximab is a further clinically relevant observation. Prednisolone doses declined significantly by 12 months after treatment, from a median of 15 mg to 10 mg, with almost two-thirds of patients having a dose reduction following rituximab infusion. As most patients with difficult-to-manage AIH have been exposed to a protracted course of corticosteroid treatment, reducing prednisolone dosage is important to prevent patients from developing the serious side effects of corticosteroids. Furthermore, corticosteroid-free therapy in AIH has the potential to substantially enhance patients’ quality of life44,58
Our study is a multicentre collaboration within the IAIHG arena, which sought to use retrospective data to determine whether rituximab could be used as a treatment in patients with difficult-to-manage AIH. AIH is a rare disease, and access to rituximab for non-approved indications is difficult, which explains the relatively small sample size of the study. Our study also suffers from all the limitations associated with a retrospective study design, especially relating to the nature of data collection, the potential for ascertainment bias, cohort heterogeneity and the lack of formal safety monitoring. Furthermore, reimbursement constraints bring in potential bias from non-medical factors that may impact on rituximab use. One of the patients developed human papillomavirus-related tongue cancer and it was difficult to know for definite whether this particular complication was related to rituximab therapy. We acknowledge that some side effects may have been underestimated due to the retrospective study design, which is also regarded as an important limitation of the study.
However, this study provides cumulative data from several centres, providing useful information for the design of future clinical trials in this area. We previously reported the immunological profile of treatment-naïve patients with AIH from the UK, which demonstrated that there is a subgroup of patients with elevated B cells59 before any exposure to immunosuppression. This suggests that rituximab could be useful, even as first-line therapy for selected patients with AIH.
In conclusion, our data clearly demonstrate a temporal association between rituximab therapy in patients with AIH and significant clinically meaningful impacts on serum liver tests, immunoglobulin concentrations, corticosteroid burden, and the subsequent rate of AIH flares. These findings, together with a satisfactory safety profile, provide compelling evidence that further controlled, licencing studies of rituximab and/or other B cell-targeting therapies should be considered for patients with AIH.
Abbreviations
AIH, autoimmune hepatitis; anti-TNF, anti-tumour necrosis factor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IAIHG, International Autoimmune Hepatitis Group; INR, international normalised ratio; MELD, model for end-stage liver disease; MMF, mycophenolate mofetil; TNF, tumour necrosis factor.
Financial support
No grant was received to conduct this study. Dr Ye Htun Oo has received the following financial support: Medical Research Council (G1002552); Sir Jules Thorn Trust Biomedical Research Award; National Institute of Health Research Birmingham Biomedical Research Centre; Queen Elizabeth Hospital Birmingham Charity
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
All co-authors contributed clinical data from each centre. NNT, MB and REW collated the data from other units. JH performed statistical analysis. NNT, YHO and JH interpreted the data and wrote the manuscript. All co-authors involved in the process of reviewing the drafts in various stages giving their expertise suggestions and critical comments to achieve the final version of manuscript. The final version of the manuscript had been approved by all co-authors.
Acknowledgements
We thank patients and participating teams from each liver unit for their contribution with clinical data. We did not receive any external funding for this project.
Ethical statement
Data were collected according to the guidance and permission from the local audit and research departments within each centre. This study was approved by IAIHG annual meeting in Hamburg.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.10.005.
Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary tables
References
- 1.Lohse AW, Dalekos G, Drenth J, Heneghan M, Hofer H, Lammert F. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Than NN, Ching DK, Hodson J, McDowell P, Mann J, Gupta R. Difference in clinical presentation, immunology profile and treatment response of type 1 autoimmune hepatitis between United Kingdom and Singapore patients. Hepatol Int. 2016;10:673–679. doi: 10.1007/s12072-016-9727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffery HC, Jeffery LE, Lutz P, Corrigan M, Webb GJ, Hirschfield GM. Low-dose interleukin-2 promotes STAT-5 phosphorylation, Treg survival and CTLA-4-dependent function in autoimmune liver diseases. Clin Exp Immunol. 2017;188:394–411. doi: 10.1111/cei.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Than NN, Jeffery HC, Oo YH. Autoimmune Hepatitis: Progress from Global Immunosuppression to Personalised Regulatory T Cell Therapy. Can J Gastroenterol Hepatol. 2016;2016:7181685. doi: 10.1155/2016/7181685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberal R, Grant CR, Yuksel M, Graham J, Kalbasi A, Ma Y. Treg Conditioning Endows Activated Teff with Suppressor Function in Autoimmune Hepatitis/Autoimmune Sclerosing Cholangitis. Hepatology. 2017 Nov;66:1570–1584. doi: 10.1002/hep.29307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mix H, Weiler-Normann C, Thimme R, Ahlenstiel G, Shin EC, Herkel J. Identification of CD4 T-cell epitopes in soluble liver antigen/liver pancreas autoantigen in autoimmune hepatitis. Gastroenterology. 2008;135:2107–2118. doi: 10.1053/j.gastro.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiler-Normann C, Sebode M, Lohse AW. Autoimmune hepatitis 2013 and beyond. Minerva Gastroenterol Dietol. 2013;59:133–141. [PubMed] [Google Scholar]
- 8.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology (Baltimore, Md) 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 10.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D. Diagnosis and management of autoimmune hepatitis. Hepatology (Baltimore, Md) 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 11.Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology (Baltimore, Md) 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 12.Selvarajah V, Montano-Loza AJ, Czaja AJ. Systematic review: managing suboptimal treatment responses in autoimmune hepatitis with conventional and nonstandard drugs. Aliment Pharmacol Ther. 2012;36:691–707. doi: 10.1111/apt.12042. [DOI] [PubMed] [Google Scholar]
- 13.Montano-Loza AJ, Carpenter HA, Czaja AJ. Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatology (Baltimore, Md) 2007;46:1138–1145. doi: 10.1002/hep.21787. [DOI] [PubMed] [Google Scholar]
- 14.Malnick S, Duek G, Melzer E, Basevitz A. The treatment of autoimmune hepatitis. Curr Clin Pharmacol. 2012;7:318–327. doi: 10.2174/157488412803305812. [DOI] [PubMed] [Google Scholar]
- 15.Than NN, Wiegard C, Weiler-Normann C, Fussel K, Mann J, Hodson J. Long-term follow-up of patients with difficult to treat type 1 autoimmune hepatitis on Tacrolimus therapy. Scand J Gastroenterol. 2016;51:329–336. doi: 10.3109/00365521.2015.1095351. [DOI] [PubMed] [Google Scholar]
- 16.Hubener S, Oo YH, Than NN, Hubener P, Weiler-Normann C, Lohse AW. Efficacy of 6-Mercaptopurine as Second-Line Treatment for Patients With Autoimmune Hepatitis and Azathioprine Intolerance. Clin Gastroenterol Hepatol. 2016;14:445–453. doi: 10.1016/j.cgh.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529–534. doi: 10.1016/j.jhep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Cropley A, Weltman M. The use of immunosuppression in autoimmune hepatitis: A current literature review. Clin Mol Hepatol. 2017;23:22–26. doi: 10.3350/cmh.2016.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey EJ, Somaratne K, Rakela J. Successful rituximab therapy in refractory autoimmune hepatitis and Evans syndrome. Rev Med Chil. 2011;139:1484–1487. [PubMed] [Google Scholar]
- 20.Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A. Review article: autoimmune hepatitis -- current management and challenges. Aliment Pharmacol Ther. 2013;38:887–913. doi: 10.1111/apt.12470. [DOI] [PubMed] [Google Scholar]
- 21.Sebode M, Hartl J, Vergani D, Lohse AW. Autoimmune hepatitis: From current knowledge and clinical practice to future research agenda. Liver Int. 2018 Jan;38:15–22. doi: 10.1111/liv.13458. [DOI] [PubMed] [Google Scholar]
- 22.Montano-Loza AJ, Carpenter HA, Czaja AJ. Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int. 2007;27:507–515. doi: 10.1111/j.1478-3231.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- 23.Liberal R, de Boer YS, Andrade RJ, Bouma G, Dalekos GN, Floreani A. Expert clinical management of autoimmune hepatitis in the real world. Aliment Pharmacol Ther. 2017;45:723–732. doi: 10.1111/apt.13907. [DOI] [PubMed] [Google Scholar]
- 24.Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 25.Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159–185. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 26.Czaja AJ. Global Disparities and Their Implications in the Occurrence and Outcome of Autoimmune Hepatitis. Dig Dis Sci. 2017 Sep;62:2277–2292. doi: 10.1007/s10620-017-4675-y. [DOI] [PubMed] [Google Scholar]
- 27.Kirk AP, Jain S, Pocock S, Thomas HC, Sherlock S. Late results of the Royal Free Hospital prospective controlled trial of prednisolone therapy in hepatitis B surface antigen negative chronic active hepatitis. Gut. 1980;21:78–83. doi: 10.1136/gut.21.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamers MM, van Oijen MG, Pronk M, Drenth JP. Treatment options for autoimmune hepatitis: a systematic review of randomized controlled trials. J Hepatol. 2010;53:191–198. doi: 10.1016/j.jhep.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet (London, England) 1973;1:735–737. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- 30.Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnick GL, Elveback IR. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–833. [PubMed] [Google Scholar]
- 31.Efe C, Hagstrom H, Ytting H, Bhanji RA, Muller NF, Wang Q. Efficacy and Safety of Mycophenolate Mofetil and Tacrolimus as Second-line Therapy for Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2017 Dec;15:1950–1956. doi: 10.1016/j.cgh.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naive patients. J Hepatol. 2011;55:636–646. doi: 10.1016/j.jhep.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103:3063–3070. doi: 10.1111/j.1572-0241.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 34.Harrison L, Gleeson D. Review article: stopping immunosuppressive treatment in autoimmune hepatitis (AIH): is it justified (and in whom and when) Liver Int. 2019 Apr;39:610–620. doi: 10.1111/liv.14051. [DOI] [PubMed] [Google Scholar]
- 35.Coscia LA, Armenti DP, King RW, Sifontis NM, Constantinescu S, Moritz MJ. Update on the Teratogenicity of Maternal Mycophenolate Mofetil. J Pediatr Genet. 2015;4:42–55. doi: 10.1055/s-0035-1556743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805–809. doi: 10.1097/01.mcg.0000139050.67178.be. [DOI] [PubMed] [Google Scholar]
- 37.Malekzadeh R, Nasseri-Moghaddam S, Kaviani MJ, Taheri H, Kamalian N, Sotoudeh M. Cyclosporin A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321–1327. doi: 10.1023/a:1010683817344. [DOI] [PubMed] [Google Scholar]
- 38.Nasseri-Moghaddam S, Nikfam S, Karimian S, Khashayar P, Malekzadeh R. Cyclosporine-A Versus Prednisolone for Induction of Remission in Auto-immune Hepatitis: Interim Analysis Report of a Randomized Controlled Trial. Middle East J Dig Dis. 2013;5:193–200. [PMC free article] [PubMed] [Google Scholar]
- 39.Jothimani D, Cramp ME, Mitchell JD, Cross TJ. Treatment of autoimmune hepatitis: a review of current and evolving therapies. J Gastroenterol Hepatol. 2011;26:619–627. doi: 10.1111/j.1440-1746.2010.06579.x. [DOI] [PubMed] [Google Scholar]
- 40.van Gerven NM, van der Eijk AA, Pas SD, Zaaijer HL, de Boer YS, Witte BI. Seroprevalence of Hepatitis E Virus in Autoimmune Hepatitis Patients in the Netherlands. J Gastrointestin Liver Dis. 2016;25:9–13. doi: 10.15403/jgld.2014.1121.251.hpe. [DOI] [PubMed] [Google Scholar]
- 41.Taubert R, Diestelhorst J, Junge N, Kirstein MM, Pischke S, Vogel A. Increased seroprevalence of HAV and parvovirus B19 in children and of HEV in adults at diagnosis of autoimmune hepatitis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-35882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zenouzi R, Lohse AW. Long-term outcome in PSC/AIH "overlap syndrome": does immunosuppression also treat the PSC component? J Hepatol. 2014;61:1189–1191. doi: 10.1016/j.jhep.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Kerkar N, Annunziato RA, Foley L, Schmeidler J, Rumbo C, Emre S. Prospective analysis of nonadherence in autoimmune hepatitis: a common problem. J Pediatr Gastroenterol Nutr. 2006;43:629–634. doi: 10.1097/01.mpg.0000239735.87111.ba. [DOI] [PubMed] [Google Scholar]
- 44.Schramm C, Wahl I, Weiler-Normann C, Voigt K, Wiegard C, Glaubke C. Health-related quality of life, depression, and anxiety in patients with autoimmune hepatitis. J Hepatol. 2014;60:618–624. doi: 10.1016/j.jhep.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Sockalingam S, Blank D, Abdelhamid N, Abbey SE, Hirschfield GM. Identifying opportunities to improve management of autoimmune hepatitis: evaluation of drug adherence and psychosocial factors. J Hepatol. 2012;57:1299–1304. doi: 10.1016/j.jhep.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 46.Muratori L, Longhi MS. The interplay between regulatory and effector T cells in autoimmune hepatitis: Implications for innovative treatment strategies. J Autoimmun. 2013;46:74–80. doi: 10.1016/j.jaut.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Gottenberg JE, Morel J, Perrodeau E, Bardin T, Combe B, Dougados M. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ (Clinical research ed) 2019;l67:364. doi: 10.1136/bmj.l67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol (Orlando, Fla) 2008;127:280–285. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Michel M, Terriou L, Roudot-Thoraval F, Hamidou M, Ebbo M, Le Guenno G. A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study) Am J Hematol. 2017;92:23–27. doi: 10.1002/ajh.24570. [DOI] [PubMed] [Google Scholar]
- 50.Cobo-Ibanez T, Loza-Santamaria E, Pego-Reigosa JM, Marques AO, Rua-Figueroa I, Fernandez-Nebro A. Efficacy and safety of rituximab in the treatment of non-renal systemic lupus erythematosus: a systematic review. Semin Arthritis Rheum. 2014;44:175–185. doi: 10.1016/j.semarthrit.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Barth E, Clawson J. A Case of Autoimmune Hepatitis Treated with Rituximab. Case Rep Gastroenterol. 2010;4:502–509. doi: 10.1159/000322693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Agostino D, Costaguta A, Alvarez F. Successful treatment of refractory autoimmune hepatitis with rituximab. Pediatrics. 2013;132:e526–e530. doi: 10.1542/peds.2011-1900. [DOI] [PubMed] [Google Scholar]
- 53.Al-Busafi SA, Michel RP, Deschenes M. Rituximab for refractory autoimmune hepatitis: a case report. Arab J Gastroenterol. 2013;14:135–138. doi: 10.1016/j.ajg.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Burak KW, Swain MG, Santodomino-Garzon T, Lee SS, Urbanski SJ, Aspinall AI. Rituximab for the Treatment of Patients with Autoimmune Hepatitis Who are Refractory or Intolerant to Standard Therapy. Can J Gastroenterol. 2013;27 doi: 10.1155/2013/512624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos ES, Arosemena LR, Raez LE, O'Brien C, Regev A. Successful treatment of autoimmune hepatitis and idiopathic thrombocytopenic purpura with the monoclonal antibody, rituximab: case report and review of literature. Liver Int. 2006;26:625–629. doi: 10.1111/j.1478-3231.2006.01262.x. [DOI] [PubMed] [Google Scholar]
- 56.Li T, Zhang LJ, Zhang QX, Yang CS, Zhang C, Li YJ. Anti-Rituximab antibody in patients with NMOSDs treated with low dose Rituximab. J Neuroimmunol. 2018;316:107–111. doi: 10.1016/j.jneuroim.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Randall KL. Rituximab in autoimmune diseases. Aust Prescr. 2016;39:131–134. doi: 10.18773/austprescr.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi A, Moriya K, Ohira H, Arinaga-Hino T, Zeniya M, Torimura T. Health-related quality of life in patients with autoimmune hepatitis: A questionnaire survey. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeffery HC, Braitch MK, Bagnall C, Hodson J, Jeffery LE, Wawman RE. Changes in natural killer cells and exhausted memory regulatory T Cells with corticosteroid therapy in acute autoimmune hepatitis. Hepatol Commun. 2018;2:421–436. doi: 10.1002/hep4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary tables