Abstract
Aim
To assess the long-term anatomical and functional outcomes in addition to complications of a new surgical technique of localized intraocular application of mitomycin C (MMC) to prevent proliferative vitreoretinopathy (PVR) in eyes with open globe trauma.
Methods
Prospective non-comparative interventional case series of 16 consecutive eyes with perforating and deep choroidal impact foreign body injuries presenting over a 2-year period. Patients underwent vitrectomy with intraocular application of MMC at the site of the chorioretinal injury and were followed-up for 1 year. The primary outcome measure was the rate of postoperative PVR. Secondary outcome measures were number of vitreoretinal surgeries (VRS) required, best corrected visual acuity (BCVA), final anatomical success rate and globe survival rate (GSR).
Results
Patients underwent VRS at a mean time of 8.5 ± 4.6 days after the injury. Postoperative PVR developed in 2 (13 %) eyes and required only one additional VRS each. One other eye underwent further peeling of an epimacular membrane. BCVA improved from mean LogMAR 3.08 ± 0.72 preoperatively to 0.66 ± 0.79 at 1 year. All 10 eyes without a macular injury had a final BCVA of LogMAR 0.40 or better. The final anatomical success rate was 94% and GSR rate was 100%. There were no complications related to the intraocular use of MMC.
Conclusions
Vitrectomy and intraocular application of Mitomycin C may have a potential role in reducing the rate of post traumatic PVR and improving anatomical and functional outcomes in eyes with perforating and deep choroidal impact foreign body injuries.
Subject terms: Trauma, Retinal diseases
Introduction
Perforating and severe intraocular foreign body (IOFB) injuries remain a major cause of visual loss due to the irreversible damage caused to the macula or optic nerve and to the ensuing proliferative vitreoretinopathy (PVR) response and associated retinal detachment (RD) [1–3].
Post traumatic PVR occurs at a predictably high rate, estimated between 40–74% of cases [1–6] and it usually develops around the impact site or exit wound causing retinal contraction and subsequent retinal folds and RD [7–10].
The pathophysiology of PVR at the site of the chorioretinal wound is thought to represent an abnormal form of wound healing with fibrocellular proliferation and periretinal membrane formation. The cells involved are inflammatory, glial, retinal pigment epithelium (RPE) derived and fibroblastic with a possible episcleral component [11–13].
In order to minimize, control or even prevent the fibrocellular proliferation, these cells may be amenable to pharmacological treatment including antiproliferative agents [14] at the very site of the chorioretinal injury which is typically involved in the proliferative and subsequent contractile response.
The current prospective non-comparative interventional study was designed to evaluate the long-term anatomical and functional outcomes in addition to complications of a new surgical technique of applying intraocular mitomycin C (MMC) at the site of the chorioretinal laceration in eyes with perforating and severe (deep choroidal impact) IOFB injuries and high risk of PVR.
Materials and methods
Seventeen consecutive patients (18 eyes) with perforating or deep choroidal impact IOFB injury involving the posterior segment presenting to the Beirut Eye and ENT Specialist Hospital from January 2015 to December 2016 were recruited and operated on by the same surgeon (AA). An informed consent was obtained from all patients and the study was approved by the Hospital Institutional Review Board and carried out in compliance with the protocol and principles of the Helsinki Declaration.
Injuries were classified in accordance with the Birmingham Eye Trauma Terminology classification [15] and the International Classification System [16] was used to determine the zone of injury.
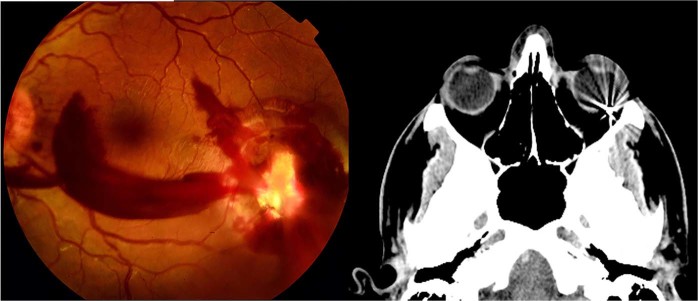
Each patient had a complete preoperative evaluation including cause of injury, measurement of best corrected visual acuity (BCVA), testing of pupillary reflexes, slit lamp examination and fundoscopy. The nature, location and extent of anterior and posterior segment injuries and complications were recorded but more accurate and detailed information of the posterior segment findings and IOFBs was later obtained from the per-operative observations. A B-scan ultrasound and computed tomography (CT) scan was obtained in all cases when a perforating injury was suspected and where media opacities precluded an adequate assessment of the posterior segment (Fig. 1).
Fig. 1.
Case 14, perforating injury temporal to the macula with orbital foreign body on CT scan
The ocular trauma score (OTS) was calculated according to the variables and raw points described by Kuhn [17]. When both eyes were injured the relative afferent pupillary defect (RAPD) was estimated by relying on the visual acuity and severity of posterior segment damage.
Postoperative data were collected at day 1, week 1, month 1 and monthly thereafter for a duration of 1 year. Patients were also evaluated outside the scheduled visits as and when medically required.
Surgical technique
Following primary repair, the secondary vitreoretinal intervention is performed within 2 weeks of the injury and the exact timing is determined on a case-by-case basis. The operative procedure consists of clearing anterior segment media opacities first. Traumatic cataracts are removed using a pars plana lensectomy approach with sparing of the anterior capsule for placement of a sulcus posterior chamber intraocular lens (PCIOL) as and when required. A three-port 20-gauge pars plana vitrectomy is then performed using the non-contact wide angle viewing system (BIOM; Oculus, Germany). Staining of the posterior cortical vitreous with triamcinolone is performed when practically feasible to ensure its removal as much as possible. Incarcerated vitreous is circumcised down to the chorioretinal wound taking care not to completely remove the tissue plugging the perforation site. IOFBs are removed through a separate pars plana incision and a fluid-air exchange is then performed with drainage of any subretinal fluid and concomitant drying of the edges and site of the chorioretinal laceration. Argon green 532 nm endolaser is next applied in one or two rows around retinal breaks and in a 360-degree band peripherally, just posterior to the vitreous base.
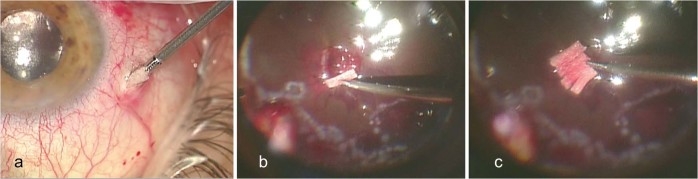
Polyvinyl alcohol (PVA) sponges (Visitec® Eye Wick, Beaver-Visitec, USA) cut in small 4 × 1 mm pieces and soaked in a 0.04% MMC solution are then firmly grasped with 20 G microserrated crocodile forceps and introduced into the air-filled vitreous cavity by gentle squeezing through the 20 G sclerotomy (Fig. 2a). The sponge is then advanced through the vitreous cavity and as it approaches the area of chorioretinal injury, it is released and applied to the surface of the retina at the site of injury (Fig. 2b). Several sponges are applied sequentially in order to cover the whole area of chorioretinal injury (Fig. 2c). After a 5 min application, the sponges are then re-grasped with the same crocodile forceps, lifted off the retinal surface and removed one by one from the vitreous cavity through the same sclerotomy. The sclerotomy and conjunctiva were copiously irrigated with normal saline after insertion and removal of the sponges. Silicone oil is then injected into the vitreous cavity and surgery is completed by closing the sclerotomies with an 8.0 vicryl suture and applying TobraDex ointment (tobramycin 0.3%, dexamethasone 0.1%; Alcon Laboratories Inc, Fort Worth, TX) to the eye.
Fig. 2.
The small MMC soaked sponge is grasped with a microserrated crocodile forceps and introduced into the vitreous cavity through the 20 G sclerotomy (a). Sponges are applied sequentially to the surface of the retina at the site of a 2.4 mm pellet chorioretinal laceration to cover the whole area of chorioretinal injury (b and c)
Postoperatively, patients receive routine topical TobraDex drops and no systemic anti-inflammatory treatment.
Outcome measures
At each postoperative visit, patients underwent measurement of their Snellen BCVA, slit-lamp examination, Goldman applanation tonometry for intraocular pressure (IOP), and fundoscopy. Primary outcome measure was the occurrence of PVR at the site of chorioretinal injury or elsewhere in the eye at any point during the follow up period. PVR was graded as present or absent based on the clinical observation of RD with fixed folds [18]. Retinal incarceration causing full thickness tractional retinal folds defined by Kuhn as “stage 0 PVR” [19] was also noted. Secondary outcome measures included number of vitreoretinal surgeries required, anatomical success rate (defined as complete retinal attachment after removal of silicone oil) at 1 year, globe survival rate and final BCVA. Globe survival rate was defined as complete retinal attachment, normal IOP (≥6 mm Hg) and visual acuity of light perception (LP) or better. Reasons for subnormal visual acuity such as corneal scarring, keratopathy and direct optic nerve or macular damage were documented. The occurrence of any other complications or changes during ocular examination were also noted. Full field electroretinograms (ERG) were performed within 3 months after removing the silicone oil.
Snellen BCVAs were converted to logarithm of the minimum angle of resolution (LogMAR) values for statistical analysis as described by Ferris et al [20]. BCVAs of hand movement (HM) and counting fingers (CF) were assigned a LogMAR value of +3.0 and +2.0 respectively according to methods published by Holladay [21]. In addition, we extrapolated by assigning a LogMAR value of +4.0 for a visual acuity of LP only.
Statistical analyses were primarily descriptive in nature, and summary statistics (mean ± SDs and/or percentages) were calculated for each variable. Spearman’s ρ test was used to assess the statistical correlation between BCVA and OTS.
Results
Pre-operative characteristics
Injuries were caused by gunshot pellets in 11 eyes (69%), hammering metal in 2 eyes (13%), explosive shrapnel in 2 eyes (13%) and accidental metal rod penetration in one eye (6%).
Table 1 represents the demographic and pre-operative characteristics of the study group.
Table 1.
Pre-operative characteristics
| Number of patients (eyes) | 15 (16) |
|---|---|
| Age range, years (Mean ± SD) | 8–56 (23.5 ± 14.1) |
| Sex, n (%) | Male 14 (100%) |
| Side, n (%) | |
| Right | 5 (31%) |
| Left | 11 (69%) |
| Cause of injury, n (%) | |
| Gunshot pellet | 11 (69%) |
| Hammering metal | 2 (13%) |
| Explosive shrapnel | 2 (13%) |
| Metal rod | 1 (6%) |
| Type of injury, n (%) | |
| Penetrating + IOFB | 9 (56%) |
| Penetrating no IOFB | 1 (6%) |
| Perforating | 5 (31%) |
| Perforating + penetrating + IOFB | 1 (6%) |
| Entry site, n (%) | |
| Zone 1 | 7 (41%) |
| Zone 2 | 9 (53%) |
| Zone 3 | 1 (6%) |
| Main impact/exit site, n (%)—All zone 3 | |
| Anterior to equator | 4 (24%) |
| Equatorial | 3 (18%) |
| Posterior to equator | 10 (59%) |
| Damage to macula | 6 (38%) |
| Foreign body type, n (%) | |
| Pellet (2.5 mm) | 12 (71%) |
| Metal 3 × 3 mm | 1 (6%) |
| Metal 10 × 3 | 1 (6%) |
| Metal 4 × 2 mm | 1 (6%) |
| Metal 8 × 3 mm | 1 (6%) |
| Non-retained wire | 1 (6%) |
| BCVA, n (%) | |
| LP | 4 (25%) |
| HM | 10 (63%) |
| CF | 1 (6%) |
| 20/400 | 1 (6%) |
| Mean LogMAR ± SD | 3.08 ± 0.72 |
| OTS raw points: range (Mean ± SD) | 35–80 (59.0 + /−12.9) |
| Anterior segment findings, n (%) | |
| Iris trauma | 10 (63%) |
| Hyphaema | 3 (19%) |
| Hypopyon | 1 (6%) |
| Cataract | 5 (31%) |
| Lens status, n (%) | |
| Phakic | 13 (81%) |
| Pseudophakic | 2 (13%) |
| Aphakic | 1 (6%) |
| Vitreous status, n (%) | |
| Mild vitreous haemorrhage | 2 (13%) |
| Moderate vitreous haemorrhage | 3 (19%) |
| Severe vitreous haemorrhage | 10 (63%) |
| Vitritis | 1 (6%) |
| Subretinal haemorrhage, n (%) | |
| Limited around impact / exit site | 5 (31%) |
| Less than 1 quadrant | 6 (38%) |
| 1–2 quadrants | 4 (25%) |
| More than 2 quadrants | 1 (6%) |
| Submacular haemorrhage | 5 (31%) |
| Retinal detachment, n (%) | |
| Total | 1 (6%) |
| Macula-on | 2 (13%) |
| Macula-off | 2 (13%) |
IOFB intraocular foreign body, BCVA best corrected visual acuity, LP light perception, HM hand motion, CF counting fingers, OTS ocular trauma score, SD standard deviation, LogMAR logarithm of the minimum angle of resolution
All patients were male with an average age of 23.5 ± 14.1 years. Of the 10 (63%) eyes with a penetrating eye injury, a retained IOFB was present in 9 (56%) eyes. 5 (31%) other eyes sustained a perforating injury. In addition, one eye (6%) had a double perforating and penetrating injury with both intraocular and orbital foreign bodies.
Entry sites were located in zone 1 and 2 in 15 (94%) eyes and in zone 3 (at 6 mm behind the limbus temporally) in one (6%) eye. Of the 17-impact or exit sites, 10 (59%) were located posterior to the equator and 6 (38%) eyes had associated damage to the macula from a direct or adjacent injury.
Preoperative best corrected visual acuity (BCVA) ranged from LP to 20/400 and the mean OTS was 59.0 ± 12.9. At presentation 5 (31%) eyes had a significant cataract precluding any view of the fundus. 13 (81%) eyes had a moderate to severe intravitreal haemorrhage. Subretinal haemorrhage extending for 1 quadrant or more was present in 5 (31%) eyes and involving the macula in 5 (31%) eyes. A RD was present in 5 (31%) eyes of which three (19%) were macula-off.
All patients had been started on a 10-day course of oral and topical fluoroquinolone antibiotics in addition to topical prednisolone acetate from the date of their injury. One patient with double perforating and penetrating injury with IOFB received intravitreal Amikacin and Vancomycin at the time of the primary repair.
Operative characteristics
All patients underwent vitreoretinal surgery under general anaesthetic at a mean time of 8.5 ± 4.6 days after the initial injury. 5 (31%) eyes required a concurrent lensectomy and a PCIOL was implanted in 2 (13%) eyes. All 10 (100%) IOFBs were removed and 3 (19%) eyes required a limited retinectomy to help reattach the retina. Intraocular silicone oil tamponade was used in 15 (94%) eyes and none of the eyes received a scleral buckle. No intravitreal antibiotics were injected at the end of the vitrectomy and none of the patients had perioperative oral corticosteroids.
Post-operative outcomes
Of the 17 patients (18 eyes) recruited, 15 (88%) patients (16 eyes) completed the 1-year follow-up and were evaluated at each of the scheduled postoperative follow-up visits. Two patients failed to attend after 4 and 7 months respectively and were therefore excluded from the study.
2 (13%) eyes developed PVR after the first vitreoretinal procedure. In both cases, the proliferative process developed at the chorioretinal injury (scar PVR) and away from it (extrascar PVR) leading to a recurrent RD under silicone oil. Both eyes had sustained a penetrating injury with a retained IOFB, severe vitreous haemorrhage, large subretinal haemorrhage and retinal detachment. These two cases underwent further surgery with peeling of epiretinal membranes and additional retinectomy in one eye at 3 and 2 months after the first vitrectomy. 14 of the 15 eyes with silicone oil tamponade underwent removal of silicone oil (ROSO) at an average 5.5 ± 2.5 SD months after the initial vitrectomy. At the time of ROSO one eye had concurrent phacoemulsification and implantation of a PCIOL and one other eye had a secondary IOL sutured to the iris. One (6%) other eye required further peeling of an epimacular membrane 4 months after ROSO. Subsequent cataract surgery was performed in 4 (25%) eyes at various times during the follow-up period. At one year 13 (81%) eyes had required only one vitreoretinal procedure while 3 (19%) other eyes had required two vitreoretinal procedures each excluding ROSO. The final anatomical success rate was 94% and globe survival rate was 100% as all patients had a flat retina with silicone oil present in one eye (6%) due to persistent hypotony (IOP between 6–10 mm Hg).
BCVA improved from mean LogMAR 3.08 ± 0.72 preoperatively to mean LogMAR 0.66 ± 0.79 at 1 year. All 10 eyes (63%) without macular injury had a final BCVA of LogMAR 0.40 or better. 6 other eyes with initial damage to the macula had a final BCVA of LogMAR 0.70 or less. A negative correlation with borderline statistical significance was found between the OTS and final LogMAR visual acuity (rs = −0.49, p = 0.055).
One patient with aphakia and hypotony developed progressive silicone oil induced keratopathy and one other patient required topical treatment for persistent high IOP.
We were able to perform ERG in 14 out of 16 eyes after ROSO as one patient had silicone oil in situ at one year and another patient was uncooperative. There was a reduction in the amplitude of the ERG a and b waves, correlating roughly with the surface area of traumatic chorioretinal damage.
There were no complications such as hypotony, iris atrophy of retinal necrosis related to the intraocular use of MMC. The trauma characteristics and outcomes are summarized in Table 2.
Table 2.
Trauma characteristics and outcomes
| Case | PENET/PERF | Impact/Exit—all zone III | Additional posterior segment findings | Macular injury | Pre op BCVA | OTS- raw points | Injury to VR surgery | Complications | Total No VR surgeries (excluding ROSO) | Anatomical success at 1 y | Final BCVA | Reason for subnormal VA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hammering metal PENET + IOFB (8 × 3 mm) | Equatorial, inferior | Vitritis | No | HM | 53 | 3 days | 1 | Yes | 20/20 | ||
| 2 | Pellet (2.5 mm) PERF | Posterior to equator, temporal | Subretinal haemorrhage 3 quadrants + submacular haemorrhage + RRD macula off | Yes | LP | 35 | 5 days | 1 | SO in situ due to IOP less 10 mm Hg (6–10 mm Hg) | CF | Macular scar, progressive keratopathy | |
| 3 | Explosive shrapnel PENET + IOFB (3 × 3 mm) | Equatorial, superonasal | Subretinal haemorrhage around impact site | No | HM | 70 | 14 days | 1 | Yes | 20/40 | Corneal scar, high astigmatism | |
| 4 | Pellet (2.5 mm) PERF | Posterior to equator, superior | Subretinal haemorrhage around exit site | No | LP | 56 | 15 days | 1 | Yes | 20/20 | ||
| 5 | Pellet (2.5 mm) PENET + IOFB | Posterior to equator, temporal | Subretinal haemorrhage around impact site | No | HM | 70 | 11 days | 1 | Yes | 20/20 | ||
| 6 | Pellet (2.5 mm) PENET + IOFB | Anterior to equator, inferior | Subretinal haemorrhage 1 quadrant + RRD macula-on | No | HM | 59 | 5 days | ERM at macula | ERM peel 8 months post vitrectomy | Yes | 20/50 | IOL haze (Rayner Superflex) |
| 7 | Metal rod PENET | Posterior to equator superior | No | HM | 70 | 5 days | 1 | Yes | 20/20 | |||
| 8 | Pellet (2.5 mm) PENET + IOFB | Posterior to equator, superior | Subretinal haemorrhage 2 quadrants extending to macula + RRD macula-off | Yes | HM | 49 | 6 days | RRD + PVR (CP 5 h) at 6 weeks + ERM and FTMH at 3 months | ERM peel/oil at 3 months post vitrectomy | Yes | 20/100 | FTMH above fovea with atrophy at macula |
| 9 | Pellet (2.5 mm) PERF | Posterior pole macula | Subretinal haemorrhage extending to macula | Yes | HM | 56 | 12 days | 1 | Yes | 20/400 | Macular scar | |
| 10 | Explosive shrapnel PENET + IOFB (10 × 3 mm) | Posterior pole macula | Large submacular haemorrhage + RRD macula-on | Yes | LP | 49 | 7 days | RRD + PVR (CA/CP 5 h) at 1 month | ERM peel/retinectomy/oil at 2 months post vitrectomy | Yes | CF | Macular scar |
| 11 | Hammering metal PENET + IOFB (4 × 2 mm) | Anterior to equator, inferotemporal | Subretinal haemorrhage around impact site | No | 20/400 | 80 | 2 days | 1 | Yes | 20/25 | ||
| 12 | Pellet (2.5mm) PERF | Posterior pole macula | Subretinal haemorrhage 1 quadrant + submacular haemorrhage + RRD total | Yes | LP | 35 | 17 days | 1 | Yes | CF | Macular scar | |
| 13 | Pellet (2.5 mm) PENET + IOFB | Anterior to equator, nasal + macula | Subretinal haemorrhage around impact site | Yes | HM | 70 | 4 days | 1 | Yes | 20/400 | Macular scar, glaucoma | |
| 14 | Pellet (2.5 mm) PERF | Posterior to equator, temporal | Subretinal haemorrhage around exit site | No | CF | 66 | 12 days | 1 | Yes | 20/30 | ||
| 15 | Pellet (2.5 mm) PENET + IOFB | Entry zone III anterior to equator, temporally + impact post to equator, superiorly | Subretinal haemorrhage around entry site | No | HM | 70 | 8 days | 1 | Yes | 20/30 | ||
| 16 | Pellet x2 (2.5mm) PERF + PENET + IOFB | Impact equatorial, inferior + exit anterior to equator, inferotemporal | Subretinal haemorrhage around impact and exit sites | No | HM | 56 | 10 days | 1 | Yes | 20/20 |
PENET penetrating, PERF perforating, Pre op preoperative, OTS ocular trauma score, VR vitreoretinal, ROSO removal of silicone oil, BCVA best corrected visual acuity, VA visual acuity, LP light perception, HM hand movement, CF counting fingers, RD retinal detachment, RRD rhegmatogenous retinal detachment, SO silicone oil, IOFB intraocular foreign body, FTMH full thickness macular hole, ERM epiretinal membrane, PVR proliferative vitreoretinopathy, CA grade C proliferative vitreoretinopathy anterior to the equator, CP grade C proliferative vitreoretinopathy posterior to the equator, h clock hours of proliferative vitreoretinopathy, IOL intraocular lens
Discussion
Recent studies of eyes with penetrating and perforating injuries and have reported PVR rates up to 62%−89% when standard techniques of PPV with endolaser are used [2, 3, 22]. However, only a few techniques have been described to specifically attempt in preventing the occurrence of PVR. Zivojnovic advocated excising the incarcerated retina and scar tissue within the perforation site [23] and more recently Kuhn introduced a technique called prophylactic chorioretinectomy [24] that seemed to yield lower PVR rates (varying between 15–20%) and better visual outcomes according to some retrospective studies [5, 25, 26].
Adjunctive pharmacologic therapy to prevent PVR has been tried is likely to be most effective in high risk patients and when the disease is in its early subclinical stages [1]. In one animal study, pirfenidone was shown to play a potential role in the prevention of fibrotic changes involved in post-traumatic PVR [27]. A number of clinical studies have also shown the potential benefit of different anti-inflammatory and antiproliferative agents during vitrectomy but none of these are implemented in routine clinical use [28–33]. Their failure to treat of prevent PVR could be due to a number of factors including low concentration, mode and site of application and exposure time inside the eye. Moreover, none of the studies has used MMC which is known to be the most powerful antiproliferative agent in cell culture [34, 35].
Our study was designed to evaluate a new surgical technique of applying MMC to the site of the chorioretinal injury in order to target and abort the fibrocellular proliferation and prevent the occurrence of PVR. MMC is a powerful non-cell specific antiproliferative alkylating agent with long-term effect after a single dose application [36, 37]. It has become increasingly used as an adjuvant in glaucoma and corneal surgery because of its properties as a modulator of wound-healing. MMC inhibits RPE, fibroblast and human retina glial cell proliferation in vitro and is cytotoxic in a dose dependent manner [34, 38, 39]. It also appears to be the most powerful in cell culture compared to other antiproliferative agents [34]. On the basis of these in vitro studies, a single application of MMC may be suitable to induce apoptosis, prevent cellular proliferation and abort the process of PVR.
In the natural course of the chorioretinal wound healing response, cellular proliferation and migration is most prominent during the first 1–2 weeks after injury. The application of MMC should therefore not be delayed beyond the second week after which a substantial amount of extracellular matrix including collagen is progressively laid down [7, 11]. Vitrectomy allows the removal of intraocular blood and inflammatory cells that incite the proliferative process and the ensuing application of MMC aborts the proliferation of cells and ensuing deposition of extracellular matrix at the site of injury.
There is still controversy regarding the ideal timing of vitrectomy in open globe injuries [40]. Despite several technical difficulties encountered in freshly injured eyes, several authors have advocated early intervention within a few hours to days of the injury in order to minimize the PVR process [6, 41, 42]. We advocate vitreoretinal surgery around 7–10 days after injury and our deferred timing option offers the surgeon a compromise: it eliminates the potential risks associated with very early intervention namely hazy anterior ocular media, leaky anterior segment wounds, risk of bleeding and risk reopening of the posterior chorioretinal wound, while still retaining the ability to target and treat the proliferating cells involved in the PVR process and arrest it before it becomes established.
Different aspects of adjuvant antiproliferative application including dosage, exposure time and extent of treated surface can determine the successful outcome of treatment. We used a concentration of 0.04% and an application time of 5 min similar to what is generally advocated for glaucoma filtration surgery [43].
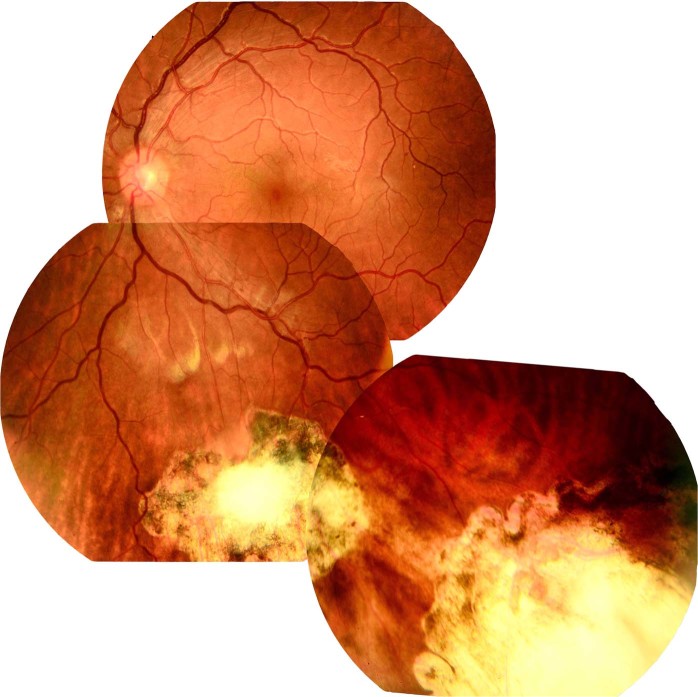
Our rate of PVR (13%) is much lower than that of other studies using traditional surgery [1–6] or chorioretinal techniques [6, 25] In our series, the PVR observed in 2 eyes came from the site of the injury and elsewhere in the inferior retina. Both of these patients had sustained a deep choroidal impact injury with a large subretinal haemorrhage and retinal detachment. It is possible that the intraocular application of MMC was ineffective and failed to reach the target proliferating cells due to the thickness and hardness of the surrounding blood at the site of injury. All 14 eyes that did not develop PVR showed evidence of a flat chorioretinal scar with clear-cut edges and surrounding pigmentation (Fig. 3). None of the scars had signs of significant fibrous proliferation associated with traction or fold formation (stage 0 PVR) suggesting that MMC application may abort the proliferation process at the site of the injury.
Fig. 3.
Case 16, postoperative flat chorioretinal scars with clear-cut edges and surrounding pigmentation in a patient with a double penetrating (inferior equatorial) and perforating injuries (inferotemporal periphery)
In terms of visual acuity, the mean LogMAR BCVA improved from 3.08 ± 0.72 SD preoperatively to 0.66 ± 0.79 SD postoperatively (p < 0.005). All 10 eyes (62.5%) without initial macular injury achieved a final BCVA of 20/50 or better at one year. These results compare very favourably to those of other studies reporting a final BCVA worse than 20/200 in 25–89% of cases (overall average of 72%) [5, 6, 25, 44]. However, such comparisons can be inherently flawed due to the lack of standardized method and timing of visual acuity measurement. Moreover, the groups compared are not always matched at baseline in terms of preoperative characteristics and in particular regarding the nature, site and severity of the injury.
We encountered no apparent clinical toxicity related to the localized topical intraocular application of the drug. All patients with injuries outside the posterior pole regained good visual acuity at last follow-up and there were no cases of unexplained visual loss. MMC was applied to areas of choroid and retina already damaged by the trauma and at high risk of developing PVR. The MMC soaked micro sponges did not come into contact with any other structures inside the air-filled vitreous cavity and therefore MMC would not have been able to diffuse into tissues away from the site of application. The literature reported ocular toxicity such as ciliary body shutdown, iris atrophy and retinal necrosis only in cases where MMC was injected and diffused into the vitreous cavity [45, 46] and none of our patients developed similar signs.
The reduction in the amplitude of ERG waves correlated roughly with the surface area of traumatic chorioretinal damage. Therefore, it was difficult to ascertain whether localized application of MMC had additional toxic effect away from the site of injury but there did not appear to be any. We only performed postoperative optical coherence tomography when clinically indicated and none of our patients had a fluorescein angiogram as we deemed it difficult to interpret in severely traumatized eyes.
The strength of our study lies in the prospective collection of data and in the long one year follow up of all patients. However, our study has two main limitations: its relatively small number of eyes and the absence of a randomized control group. In addition, the calculation of the OTS may have been inaccurate in some patients owing to the difficulty in checking for a RAPD in bilateral injuries. Moreover, the preoperative APD may have also been confounded by dense vitreous haemorrhage or total RD.
Our results appear to suggest that localized application of intraocular MMC may have a potential role in reducing rate of post traumatic PVR and its ensuing complications in patients with perforating and deep choroidal impact foreign body injuries. Further randomized controlled studies are required to corroborate these results and establish the optimal delivery method and concentration of MMC. An animal model may also be needed to gain cellular insight into what is exactly happening both prior to and following intraocular application of MMC.
Summary
What was known before
Perforating and deep impact intraocular intraocular foreign body injuries carry poor anatomical and functional outcomes due to the high rate of post traumatic PVR—The fibrocellular proliferation response usually develops around the impact site or exit wound leading to retinal detachment
What this study adds
Intraocular application of the antiproliferative mitomycin C at the site of chorioretinal injury may have a potential effect in reducing fibrocellular proliferation and the rate of PVR—The application of MMC should be done during the early cellular proliferation stage and not be delayed beyond the second week after which a substantial amount of extracellular matrix and collagen is progressively laid down
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cardillo JA, Stout JT, LaBree L, et al. Post-traumatic proliferative vitreoretinopathy. The epidemiologic profile, onset, risk factors, and visual outcome. Ophthalmology. 1997;104:1166–73. doi: 10.1016/S0161-6420(97)30167-5. [DOI] [PubMed] [Google Scholar]
- 2.Colyer MH, Weber ED, Weichel ED, et al. Delayed intraocular foreign body removal without endophthalmitis during Operations Iraqi Freedom and Enduring Freedom. Ophthalmology. 2007;114:1439–47. doi: 10.1016/j.ophtha.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 3.Colyer MH, Chun DW, Bower KS, Dick JSB, Weichel ED. Perforating globe injuries during operation Iraqi Freedom. Ophthalmology. 2008;115:2087–93. doi: 10.1016/j.ophtha.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Szurman P, Roters S, Grisanti S, et al. Primary silicone oil tamponade in the management of severe intraocular foreign body injuries: an 8-year follow-up. Retina Phila Pa. 2007;27:304–11. doi: 10.1097/01.iae.0000237685.99713.83. [DOI] [PubMed] [Google Scholar]
- 5.Weichel ED, Bower KS, Colyer MH. Chorioretinectomy for perforating or severe intraocular foreign body injuries. Graefes Arch Clin Exp Ophthalmol. 2010;248:319–30. doi: 10.1007/s00417-009-1236-x. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn F, Schrader W. Prophylactic chorioretinectomy for eye injuries with high proliferative-vitreoretinopathy risk. Clin Anat. 2018;31:28–38. doi: 10.1002/ca.22906. [DOI] [PubMed] [Google Scholar]
- 7.Cleary PE, Ryan SJ. Histology of wound, vitreous, and retina in experimental posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol. 1979;88:221–31. doi: 10.1016/0002-9394(79)90469-0. [DOI] [PubMed] [Google Scholar]
- 8.Cleary PE, Ryan SJ. Method of production and natural history of experimental posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol. 1979;88:212–20. doi: 10.1016/0002-9394(79)90468-9. [DOI] [PubMed] [Google Scholar]
- 9.Cleary PE, Minckler DS, Ryan SJ. Ultrastructure of traction retinal detachment in rhesus monkey eyes after a posterior penetrating ocular injury. Am J Ophthalmol. 1980;90:829–45. doi: 10.1016/S0002-9394(14)75198-0. [DOI] [PubMed] [Google Scholar]
- 10.Punnonen E. Pathological findings in eyes enucleated because of perforating injury. Acta Ophthalmol. 1990;68:265–9. doi: 10.1111/j.1755-3768.1990.tb01920.x. [DOI] [PubMed] [Google Scholar]
- 11.Winthrop SR, Cleary PE, Minckler DS, Ryan SJ. Penetrating eye injuries: a histopathological review. Br J Ophthalmol. 1980;64:809–17. doi: 10.1136/bjo.64.11.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charteris DG, Downie J, Aylward GW, Sethi C, Luthert P. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:93–100. doi: 10.1007/s00417-006-0323-5. [DOI] [PubMed] [Google Scholar]
- 13.Morescalchi F, Duse S, Gambicorti E, Romano MR, Costagliola C, Semeraro F. Proliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloid. Mediators Inflamm. 2013;2013:269787. doi: 10.1155/2013/269787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee PJ, Woodcock MG, Bunce C, Scott R, Charteris DG. A pilot study of intraocular use of intensive anti-inflammatory; triamcinolone acetonide to prevent proliferative vitreoretinopathy in eyes undergoing vitreoretinal surgery for open globe trauma; the Adjuncts in Ocular Trauma (AOT) Trial: study protocol for a randomised controlled trial. Trials. 2013;14:42. doi: 10.1186/1745-6215-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn F, Morris R, Witherspoon CD. Birmingham Eye Trauma Terminology (BETT): terminology and classification of mechanical eye injuries. Ophthalmol Clin N Am. 2002;15:139–43. doi: 10.1016/S0896-1549(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 16.Pieramici DJ, Sternberg P, Aaberg TM, et al. A system for classifying mechanical injuries of the eye (globe). The Ocular Trauma Classification Group. Am J Ophthalmol. 1997;123:820–31. doi: 10.1016/S0002-9394(14)71132-8. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn F, Maisiak R, Mann L, Mester V, Morris R, Witherspoon CD. The Ocular Trauma Score (OTS) Ophthalmol Clin N Am. 2002;15:163–5. doi: 10.1016/S0896-1549(02)00007-X. [DOI] [PubMed] [Google Scholar]
- 18.Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112:159–65. doi: 10.1016/S0002-9394(14)76695-4. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn F, Teixeira S, Pelayes DE. Late versus prophylactic chorioretinectomy for the prevention of trauma-related proliferative vitreoretinopathy. Ophthalmic Res. 2012;48(Suppl 1):32–37. doi: 10.1159/000339846. [DOI] [PubMed] [Google Scholar]
- 20.Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. doi: 10.1016/0002-9394(82)90197-0. [DOI] [PubMed] [Google Scholar]
- 21.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–91. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 22.Szijártó Z, Gaál V, Kovács B, Kuhn F. Prognosis of penetrating eye injuries with posterior segment intraocular foreign body. Graefes Arch Clin Exp Ophthalmol. 2008;246:161–5. doi: 10.1007/s00417-007-0650-1. [DOI] [PubMed] [Google Scholar]
- 23.Zivojnovic R. Silicone oil in vitreoretinal surgery. Vol 12. Springer Science & Business Media, AG Switzerlandd. 2012.
- 24.Kuhn F, Mester V, Morris R. A proactive treatment approach for eyes with perforating injury. Klin Monatsbl Augenheilkd. 2004;221:622–8. doi: 10.1055/s-2004-813535. [DOI] [PubMed] [Google Scholar]
- 25.Ozdek S, Hasanreisoglu M, Yuksel E. Chorioretinectomy for perforating eye injuries. Eye Lond Engl. 2013;27:722–7. doi: 10.1038/eye.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira N, Monteiro S, Meireles A, Kuhn F. Outcome of vitrectomy and chorioretinectomy in perforating eye injuries. Ophthalmic Res. 2015;53:200–6. doi: 10.1159/000371494. [DOI] [PubMed] [Google Scholar]
- 27.BNMK Khanum, Guha R, Sur VP, et al. Pirfenidone inhibits post-traumatic proliferative vitreoretinopathy. Eye Lond Engl. 2017;31:1317–28. doi: 10.1038/eye.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumenkranz M, Hernandez E, Ophir A, Norton EW. 5-fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology. 1984;91:122–30. doi: 10.1016/S0161-6420(84)34318-4. [DOI] [PubMed] [Google Scholar]
- 29.Blankenship GW. Evaluation of a single intravitreal injection of dexamethasone phosphate in vitrectomy surgery for diabetic retinopathy complications. Graefes Arch Clin Exp Ophthalmol. 1991;229:62–65. doi: 10.1007/BF00172263. [DOI] [PubMed] [Google Scholar]
- 30.Wiedemann P, Hilgers RD, Bauer P, Heimann K. Adjunctive daunorubicin in the treatment of proliferative vitreoretinopathy: results of a multicenter clinical trial. Daunomycin Study Group. Am J Ophthalmol. 1998;126:550–9. doi: 10.1016/S0002-9394(98)00115-9. [DOI] [PubMed] [Google Scholar]
- 31.Asaria RH, Kon CH, Bunce C, et al. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: results from a randomized, double-blind, controlled clinical trial. Ophthalmology. 2001;108:1179–83. doi: 10.1016/S0161-6420(01)00589-9. [DOI] [PubMed] [Google Scholar]
- 32.Kirchhof B. Strategies to influence PVR development. Graefes Arch Clin Exp Ophthalmol. 2004;242:699–703. doi: 10.1007/s00417-004-0978-8. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee PJ, Quartilho A, Bunce C, et al. Slow-release dexamethasone in proliferative vitreoretinopathy: a prospective, randomized controlled clinical trial. Ophthalmology. 2017;124:757–67. doi: 10.1016/j.ophtha.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Wu WC, Kao YH, Hu DN. A comparative study of effects of antiproliferative drugs on human retinal pigment epithelial cells in vitro. J Ocul Pharmacol Ther. 2002;18:251–64. doi: 10.1089/108076802760116179. [DOI] [PubMed] [Google Scholar]
- 35.Wu WC, Kao YH, Tseng HY. The cell cycle distribution of cultured human retinal pigmented epithelial cells under exposure of anti-proliferative drugs. J Ocul Pharmacol Ther. 2003;19:83–90. doi: 10.1089/108076803762718141. [DOI] [PubMed] [Google Scholar]
- 36.Erlichman C, Kerr IG. Antineoplastic drugs. Princ Med Pharmacol. 1989;8:604–14.
- 37.Kang SG, Chung H, Yoo YD, Lee JG, Choi YI, Yu YS. Mechanism of growth inhibitory effect of Mitomycin-C on cultured human retinal pigment epithelial cells: apoptosis and cell cycle arrest. Curr Eye Res. 2001;22:174–81. doi: 10.1076/ceyr.22.3.174.5513. [DOI] [PubMed] [Google Scholar]
- 38.Khaw PT, Sherwood MB, MacKay SL, Rossi MJ, Schultz G. Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon’s capsule fibroblasts. Arch Ophthalmol Chic Ill 1960. 1992;110:1150–4. doi: 10.1001/archopht.1992.01080200130040. [DOI] [PubMed] [Google Scholar]
- 39.Cai J, Wei R, Ma X, Zhu H, Li Y. Cytotoxic effects of antiproliferative agents on human retinal glial cells in vitro. Int Ophthalmol. 2001;24:225–31. doi: 10.1023/A:1022509614815. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal R, Shah M, Mireskandari K, Yong GK. Controversies in ocular trauma classification and management: review. Int Ophthalmol. 2013;33:435–45. doi: 10.1007/s10792-012-9698-y. [DOI] [PubMed] [Google Scholar]
- 41.Kuhn F, Morris R, Witherspoon CD, Mann L. Blunt-force injuries involving the posterior segment. Retin Physician. 2007;4:20–24. [Google Scholar]
- 42.Schrader WF. Head, vitreoretinal department, maximilians eye clinic, nürnberg. innovative treatment for severe ocular trauma. Eur Ophthalmic Rev. 2009;02:32. doi: 10.17925/EOR.2009.02.01.32. [DOI] [Google Scholar]
- 43.Bindlish R, Condon GP, Schlosser JD, D’Antonio J, Lauer KB, Lehrer R. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002;109:1336–41. doi: 10.1016/S0161-6420(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn F, Morris R, Witherspoon CD, Mann LR. Epidemiology of blinding trauma in the United States eye injury registry. Ophthalmic Epidemiol. 2006;13:209–16. doi: 10.1080/09286580600665886. [DOI] [PubMed] [Google Scholar]
- 45.Ryoo NK, Kim MK, Wee WR. Consequences of accidental mitomycin C intraocular injection. JAMA Ophthalmol. 2013;131:1197. doi: 10.1001/jamaophthalmol.2013.347. [DOI] [PubMed] [Google Scholar]
- 46.Mirshahi A, Lashay A, Mehrabi Bahar MR, Abrishami M. Consequences of inadvertent intravitreal Mitomycin C injection. Int J Retina Vitr. 2018;4:7. doi: 10.1186/s40942-018-0110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]