Highlights
▸ Primiparous mothers were observed during free-play interaction with their 18 month-olds. ▸ During fMRI, mothers were exposed to own and unfamiliar infant's cries and control sound. ▸ Sensitive mothers had greater activation to own infant cry in right frontal regions. ▸ More intrusive mothers had greater activation in the left anterior insula and temporal pole. ▸ Mothers with harmonious interactions had greater activation in left hippocampal regions.
Keywords: Maternal sensitivity, Infant cry, Neural correlates
Abstract
Research on maternal neural response to infant distress highlights circuits that may underlie differences in quality of maternal behavior. However, it is far from clear which circuits are relevant to maternal sensitivity, as opposed to other maternal behavioral dimensions, particularly after the early postpartum. This study examined maternal sensitivity, intrusiveness, and mother–infant dyadic harmony as correlates of mothers’ neural responses to the cries of their own infants. Twenty-two primiparous mothers were observed during an interaction with their infants at 18 months postpartum. In a separate functional neuroimaging session, mothers were exposed to their own infant's cry sound, as well as unfamiliar infant's cry and control sounds. Mothers who displayed more sensitive behaviors with their infant exhibited greater activation to their own infant's cry compared to that of an unfamiliar infant in the right frontal pole and inferior frontal gyrus. Mothers who displayed more intrusive behaviors with their infant showed greater activation in the left anterior insula and temporal pole, while mothers who had more harmonious interactions with their infant displayed greater activation in left hippocampal regions. The roles of these areas in the regulation of maternal emotion and stress, self and other awareness, and empathy are examined.
1. Introduction
To promote optimum development, mothers must detect and respond to their infants’ behavioral and emotional cues, and numerous studies have demonstrated the importance of maternal sensitivity and responsiveness for the socioemotional development of the child (see meta-analysis by DeWolff and van IJzendoorn, 1997). Additionally, previous work has established that maternal insensitivity is a risk factor for a variety of negative behavioral, cognitive, developmental, and relational outcomes among infants and young children (Campbell et al., 1995, Murray et al., 1996a). Understanding the neurobiology of maternal sensitive responding may aid in our understanding of complex and pervasive social problems, such as child maltreatment and neglect, and help to develop more effective interventions for women at risk for maladaptive parenting behaviors.
Maternal sensitivity has been defined as appropriate, contingent, and consistent recognition and responsiveness to infant communicative cues, such as cry (Lohaus et al., 2001). Several studies examining the neural correlates of maternal responses to infant cry point to a network of subcortical and cortical circuits involved in the “maternal brain” (see Swain et al., 2007 for a review). Lorberbaum et al., 1999, Lorberbaum et al., 2002 were the first to compare activations in response to a standardized cry stimulus vs. a control sound among mothers; they found increased cry-related activity in areas associated with animal models of maternal behavior, as well as with human empathy (i.e., anterior and posterior cingulate, thalamus, hypothalamus, dorsal and ventral striatum, medial prefrontal cortex, and right orbitofrontal and insular regions). Additional research in this area has examined neural activation to infant cry among groups with varying levels of parenting experience; parents, but not non-parents, showed increased activation to infant cry compared to infant laughing in the amygdala (Seifritz et al., 2003), suggesting that the emotional and biological response to infant cry evolves with parenting.
Studies have also begun to elucidate individual differences in maternal neural response relevant to mothers’ abilities to connect with and respond appropriately to their infants. Noriuchi et al. (2008) not only identified differential maternal brain activation to video clips related to infant identity (own infant vs. unfamiliar infant) and infant emotional state (distress vs. non-distress), but they also related aspects of these responses to maternal feelings. More intense maternal happy/joyful and anxious feelings toward her infant were associated with stronger response to infant identity in orbitofrontal areas, and more intense excited and loving feelings were associated with stronger response to own infant distress in superior temporal areas. These associations suggest that mothers’ capacity for interpreting and empathizing with their infant's emotional state is tied to specific prefrontal and temporal circuits. Previous research within the current study sample also highlights response differences relevant to the quality of mother–infant interaction; whereas mothers with no history of depression activated to their own infant's cry across a network of prefrontal and limbic/paralimbic regions, mothers who had experienced a major depressive episode during pregnancy and/or postpartum showed no such activation (Laurent and Ablow, 2011). Furthermore, higher levels of concurrent depressive symptoms were associated with diminished response to own infant cry in key emotional response and regulation (orbitofrontal, superior frontal, anterior cingulate) and motivational (ventral striatum) circuits. Disturbance of these neural networks may help explain parenting deficits observed in depressed mothers, but associations with maternal behavior were not directly addressed.
To our knowledge, only one study has examined maternal brain response in relation to behavioral sensitivity (Kim et al., 2011). This study tested breastfeeding and maternal neural response to own infant cry at 2–4 weeks postpartum as predictors of maternal sensitivity at 3–4 months postpartum. Not only did breastfeeding mothers show greater activation to their own infant's cry compared to formula-feeding mothers, but activation in several of these regions (right superior frontal gyrus, amygdala) also related to higher observed sensitivity scores. Conclusions based on this study are limited by several design features. First, both first-time and experienced mothers were included, making it unclear to what extent experience played a role in differential responses. Additionally, the study timing during the first few months postpartum provides limited insight into maternal behavior-relevant response circuits once the infant has developed a more complex interactive repertoire. Furthermore, neural response and sensitivity were examined at different times, limiting conclusions about the role of maternal neural response in sensitive behavior during concurrent mother–infant interactions. Further research on first-time mothers of older infants is needed to more confidently identify sensitivity-related response circuits.
Another important point to clarify is the distinction between neural correlates of maternal sensitivity, specifically, and those of distinct, but potentially related, maternal behavior qualities. In particular, maternal sensitivity should be separated from more general involvement with her infant and/or the emotional valence of mother–infant interaction. Whereas sensitive maternal behavior, as defined above, has been shown to be central to positive parenting and to support children's physiological, cognitive, and social-emotional development (Feldman, 2007, Feldman et al., 2004), maternal approach orientation may give rise to intrusiveness, which has been associated with unique negative child outcomes (Murray and Cooper, 1997, Murray and Cooper, 2001). Additionally, dyadic relationship harmony may be a better indicator of quality of the mother–infant relationship than maternal behavior alone, as this construct also taps the infant's response to maternal cues and has been uniquely associated with maternal depression and dyadic autonomic regulation (Porges et al., 1991, Porges, 2001). To our knowledge, only one previous study has examined specific neural correlates of maternal behavior. Specifically, Atzil et al. (2011) examined neural correlates of synchrony and intrusiveness in response to video clips of their own infant's behavior as well as videos of the mother's interaction with her own infant. This study showed that synchronous mothers displayed greater activations in the left nucleus accumbens, while intrusive mothers exhibited higher activations in the right amygdala (Atzil et al., 2011). However, it should be noted that this study looked at much younger infants (4–6 months), and this study utilized a composite measure of sensitivity and harmony, synchrony, which may provide a more general understanding of maternal behavior than examining these domains separately (Atzil et al., 2011).
1.1. Hypotheses
This study aims to further understanding of neural circuits underlying maternal behavior by examining maternal sensitivity, intrusiveness, and dyadic harmony as correlates of neural response to own infant cry among first-time mothers. We hypothesized that each of these behaviors – coded during a free play and clean-up task at 18 months postpartum – would be associated with activation to own infant cry (compared to unfamiliar infant's cry) in regions involved in approach motivation, decision making, emotion recognition and regulation, and social cognition. Based on previous maternal neuroimaging research reviewed above, these may include orbitofrontal and other prefrontal areas, the striatum, cingulate cortex, temporal and insular regions, as well as the amygdala. More specifically, mothers, who display more sensitive behaviors with their infant, may exhibit greater activation to their own infant's cry compared to that of an unfamiliar infant in the right frontal regions (Swain et al., 2008, Swain et al., in press), while mothers who had more harmonious interactions with their infant may display greater activation in the left nucleus accumbens and hippocampal regions (Atzil et al., 2011). In contrast, mothers, who display more intrusive behaviors, may show greater activation in the insula and right amygdala (Atzil et al., 2011).
1.2. Method and materials
1.2.1. Participants
Twenty-two primiparous mothers (M age = 24.1 years, SD = 4.1) of 15–18-month-old infants were recruited from the Women Infant Children (WIC) program. Mothers gave written informed consent, were screened for MRI contraindications, and were interviewed with the Structured Clinical Interview for the DSM-IV (SCID) to assess current and past psychopathology as described by Laurent and Ablow (2011). Half of participants met criteria for a major depressive episode during the perinatal period, and half did not meet criteria for any diagnosable psychopathology. At the time of the current study assessments, none of the mothers met DSM-IV criteria for a major depressive episode, though 10 reported elevated depressive symptoms (>17) on the Center for Epidemiological Studies Depression Scale (CESD; Radloff, 1977). This sample comprises a subset of those originally screened into the study (n = 36); reasons for discontinuation included new pregnancy (n = 2) and failure to collect a cry sample (n = 12). Nonsignificant differences were observed between mothers who completed and those who did not complete the study.
Reflective of the community from which mothers were recruited, the majority were Caucasian (77%), with smaller numbers of African Americans (14%), and Latinas (9%). Most women reported household incomes in the low SES range (32% household income <$10,000 per year, 36% $20,000–40,000, 32% >$40,000). While a substantial proportion (64%) had engaged in post-high school education, only 18% completed college. Half (50%) of mothers were in a current relationship with their infant's biological father (32% married). No demographic differences were associated with the behavior variables of maternal depression symptoms or maternal behavioral dimensions.
1.2.2. Procedure
Written informed consent was obtained at the start of each data collection session (laboratory interaction, cry recording, neuroimaging). Mothers completed a home questionnaire booklet, including the CESD, before arriving for the laboratory session. Within one week of the laboratory session, participants attended a neuroimaging session, described below.
1.2.3. Free play and clean-up task
Mothers were videotaped while interacting with their own 18-month-old infants in a laboratory session. These sessions included free play without toys (2 min), free play with toys (4 min), and a clean-up condition (2 min). Mothers and their infants were brought into a large, empty room with a blanket spread on the floor and instructed to play as they would at home. After 2 min of play, the experimenter brought in a basket of age-appropriate toys and spread them on the blanket; again, mothers were instructed to play with their infants as they would at home. Finally, the experimenter brought in a basket and explained it was time to clean up. Mothers were instructed that the infant should be the one to place the toys in the basket, but that she could help or support her infant as needed.
1.2.4. Structured Play Interaction Coding Scheme
Using the Coding Scheme for Structured Mother–Infant Play Interactions, two research assistants rated maternal behavior on the dimensions of sensitivity, intrusive–coercive control, and overall dyadic harmony as an overall score (Murray and Cooper, 2001). According to Murray and Cooper (2001), sensitivity reflects the quality of the mother's attunement and responsiveness to the infant's signals, while intrusiveness taps the forceful positioning or guidance of the infant, and dyadic harmony reflects the overall quality of the interaction and the amount of conflict observed within the dyad. Each two-minute segment of the task was rated on these individual dimensions using a 5-point scale (ranging from highly insensitive to highly sensitive, for example, for sensitivity) and then averaged across task conditions to provide an overall score across task conditions for each dyad. Average scores for the three dimensions (sensitivity, intrusiveness, and harmony) were used in the imaging analyses. Reliability was assessed by checking agreement between the first author and the two research assistants on a sub-sample of 18 mother–infant play sessions. Kappas were .89 for sensitivity, .86 for intrusiveness, and .91 for harmony, which are consistent with previous reports of inter-rate reliability for this measure (Murray et al., 2007). Additionally, this method of coding mother–infant interactions has shown a predictive validity regarding infant and child cognitive outcomes at 5 years of age (Murray et al., 1996a, Murray et al., 1996b), and good discriminant validity for a number of clinical groups, such as those with maternal depression and schizophrenia, and infants at low-risk/high-risk (Murray, 1996, Riordan et al., 1999).
1.2.5. Stimulus collection and presentation
Researchers attended mothers’ 18-month well-baby visits and recorded infant cry sound following vaccination injections. Twenty-one continuous seconds from the beginning of the first cry expiration were selected for the “own cry” stimulus. Additionally, a cry sound from an infant unfamiliar to all participants was collected using the same procedures (“other cry” stimulus), and a non-cry control sound was developed by editing a rising and falling tone to have a fundamental frequency within the range of normal infant cry (400–600 Hz; Zeskind and Lester, 1978). All sounds were edited to have the same maximum amplitude but otherwise unaltered to retain their natural frequency and temporal characteristics. All own infant cry sound characteristics were unrelated to behavior variables of maternal depression symptoms or maternal behavioral dimensions.
The stimulus protocol consisted of two 9-min runs of a block design presenting own cry, other cry, control sound, and rest periods. Ordering of blocks within runs was counterbalanced within and across participants, and each run contained 6 repetitions of each block (2 s pause + 21 s sound for cry/control sound; 21 s pause for rest). Participants were instructed to listen to the sounds but were given no further task instructions to allow the most natural range of response to cry. Sound was presented via earphones in the scanner, and a sound check was carried out before each scan to ensure audibility.
1.2.6. Scanning
MR imaging was carried out with a 3 T Siemens Allegra 3 scanner. A standard birdcage coil was used to acquire data from the whole brain. Sessions began with a shimming routine to optimize signal-to-noise ratio, followed by a fast localizer scan (FISP) and Siemens Autoalign routine, then the 2 functional runs and anatomical scan.
1.2.7. Functional
T2*-weighted gradient echo sequence, 64 × 64 voxel matrix, TE = 30 ms, TR = 2000 ms, flip angle = 80°, 32 contiguous slices thickness = 4 mm; 273 volumes per run.
1.2.8. Structural
T1-weighted 3D MP-RAGE sequence, TI = 1100 ms, TR = 2500 ms, TE = 4.4 ms, 176 transverse slices 1 mm thick, 256 × 176 matrix FOV = 256 mm.
1.3. Data analysis
Functional imaging data were analyzed with tools from the fMRIB Software Library (FSL v.4.1). Preprocessing steps included motion correction with MCFLIRT, nonbrain structure removal with BET, spatial smoothing using Gaussian kernel 5 mm FWHM, intensity normalization using grand mean scaling, and high-pass temporal filtering (sigma = 65 s). Within-subject time series data were analyzed using FILM with local autocorrelation correction, and boxcar models indicating onset/offset of each sound stimulus were convolved with a double-gamma basis function. Functional data were registered to the participant's own high-resolution structural image (6 DF) and to a standard brain (Montreal Neurological Institute template; 12 DF) using FLIRT. All data were checked for excessive motion (>1 mm) and artifacts.
Within-participant and group-level analyses were carried out using FEAT v.5.98. For each participant, three explanatory variables (EVs) modeled signal associated with own infant cry, other infant cry, and control sound; zero for all 3 stimulus EVs corresponded to rest. Contrasts of parameter estimates (COPEs) for own cry > other cry tested primary hypotheses regarding response specifically to own infant cry. The own cry > other cry contrast was used specifically to examine the main hypotheses of this study. Specifically, it was anticipated that the own cry > other cry contrast would be more specific to the examination of maternal sensitive behaviors (the time-averaged dimension scores for sensitivity, intrusiveness, and harmony) in mothers of 18-month-old infants, as these mothers have had the opportunity to become attuned to the specific acoustics associated with the cries of their own infants. We also examined the own cry > control sound contrast, but found no significant differences related to mother–infant behaviors; thus, those results are not discussed further. The own cry > rest and other cry > rest contrasts were also tested to describe signal change relative to baseline and to create descriptive plots, but not as primary response measures.
First-level COPE images were averaged across runs using fixed-effects analysis. These served as inputs to higher-level group analyses, conducted using FLAME to model random-effects components of mixed-effects variance. AlphaSim was used to determine cluster size needed, in conjunction with intensity threshold p < .005, to achieve a false discovery rate (FDR) of .05 for whole-brain analyses (Cox, 1996). Using these criteria, activation clusters exceeding 16 voxels, or 615 mm3, were considered significant in group analyses.
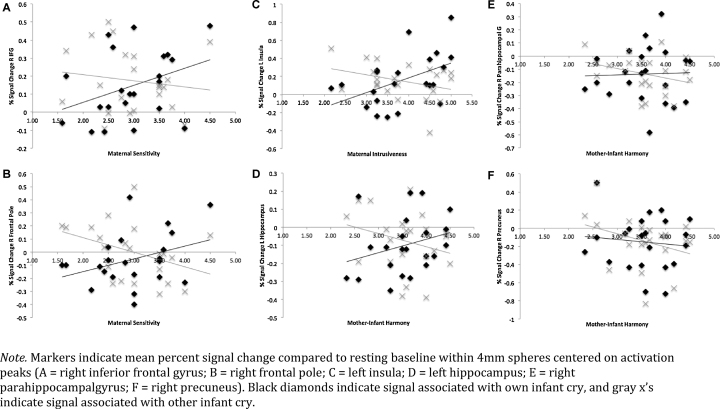
At the group level, mother–infant behavior EVs were tested in relation to mothers’ neural response to own > other infant cry using the General Linear Model. Mean-centered scores for the maternal behaviors of interest (i.e., sensitivity, intrusiveness, and harmony averaged across free-play and cleanup) were entered as a set of simultaneous EVs. Thus, any effects for a specific behavior were produced while controlling for the effects attributable to the other behaviors. Both positive and negative contrast weights were tested for each behavior predictor to determine whether it was related to increased or decreased neural response. Finally, to visualize the data driving continuous behavior effects, but not to perform new statistical tests (Poldrack and Mumford, 2009), spherical ROIs (r = 4 mm) centered on activation peaks were used to compute percent signal change associated with sound stimuli (compared to rest) and generate illustrative figures (Fig. 1, Fig. 2, Fig. 3).
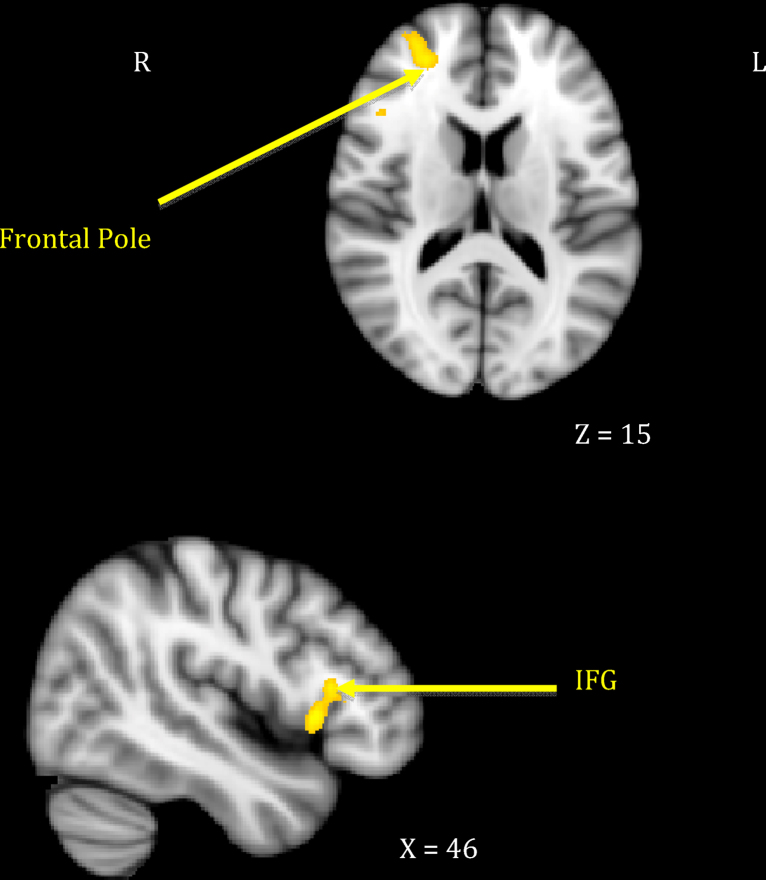
Fig. 1.
Maternal neural response to own > other infant cry related to maternal sensitivity.
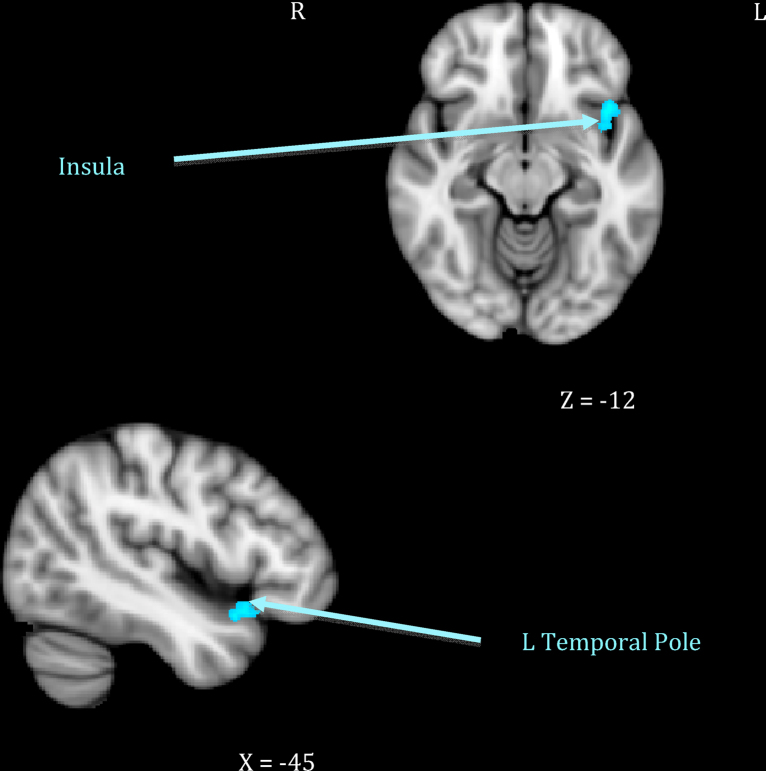
Fig. 2.
Maternal neural response to own > other infant cry related to maternal intrusiveness.
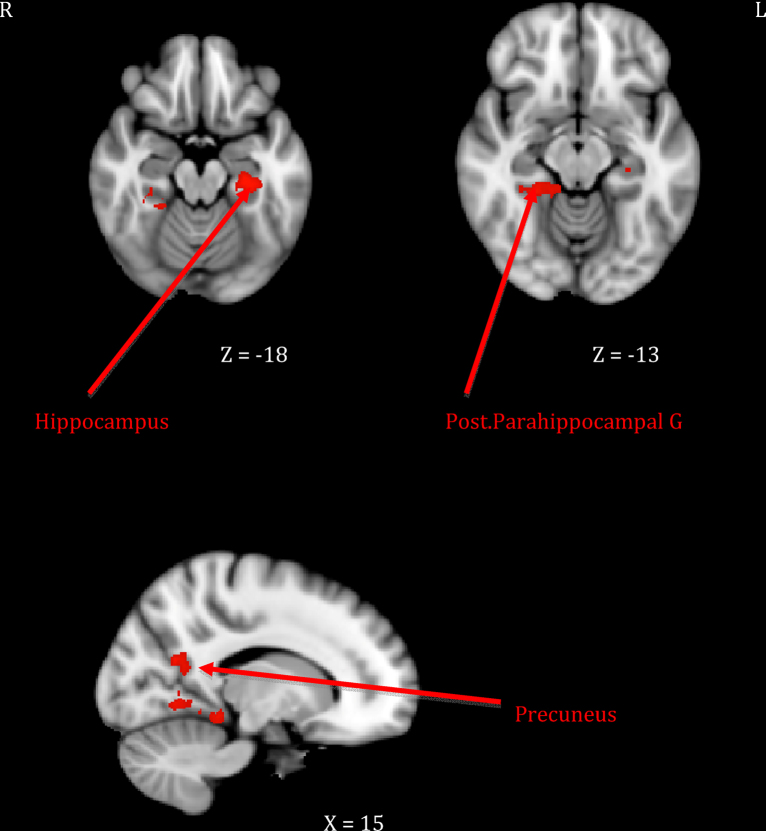
Fig. 3.
Maternal neural response to own > other infant cry related to mother–infant.
2. Results
2.1. Maternal behavior descriptive data and associations with depression
On average, mothers were rated at the midpoint of the 5-point scale on sensitivity (M = 2.97, SD = .74), but above the midpoint on both intrusiveness (M = 3.85, SD = .83) and harmony (M = 3.56, SD = .61). Significant correlations between behavior ratings across free-play and cleanup segments (r = .58–.66) demonstrated behavioral stability, and paired-samples t-tests showed nonsignificant differences in each of the behavior ratings across segments. Behavioral sensitivity and harmony were positively associated with one another, and both were negatively associated with intrusiveness (r = −.66, p < .01for sensitivity and intrusiveness, r = .79, p < .01 for sensitivity and harmony, and r = −.44, p < .01 for intrusiveness and harmony). Such associations confirmed the suspicion that “sensitivity” ratings may overlap with other aspects of maternal behavior, but not be fully captured by other aspects of maternal behavior, underlining the importance of identifying neural correlates distinct from those attributable to mere maternal over-involvement (intrusiveness) and/or the emotional tone of mother–infant interactions (harmony).
As expected, maternal depression was associated with observed maternal behavior, particularly harmony. Mothers with a history of perinatal depression tended to have less harmonious interactions with their infants (M = 3.32 vs. 3.81, t[20] = 2.04, p = .05), and harmony was inversely associated with current self-reported depressive symptoms (r = -.54 with Center for Epidemiologic Studies Depression [CESD] total score). A trend toward lower sensitivity in mothers with a history of depression was detected (M = 2.70 vs. 3.23, t[20] = 1.80, p = .09; r = −.30 with CESD). Although depression-related differences in intrusiveness failed to reach significance, the size of the association with current self-reported symptoms was similar to that for sensitivity (M = 3.62 vs. 4.08, t[20] = 1.30, p = .21; r = −.30 with CESD total score). To better define neural correlates of maternal behavior both overlapping with and distinct from depression, additional predictive models controlling for current symptoms (CESD scores) and separating depressed vs. non-depressed groups were tested.
2.2. Maternal neural response to own–other infant cry associated with behavior
Mothers who showed greater sensitivity1 to infant cues during observed interactions responded more strongly to their own infant's cry in right-sided prefrontal regions including the frontal pole and the inferior frontal gyrus extending to frontal operculum (see Table 1, top section; Fig. 1). This was distinct from neural response related to maternal intrusiveness, which involved the left anterior insula and temporal pole (see Table 1, middle section; Fig. 2). Finally, mothers who engaged in more harmonious interactions with their infants showed increased response to their own infant's cry across several posterior clusters, including the left hippocampus extending to parahippocampal gyrus, the right parahippocampal and lingual gyri, and the right precuneus cortex (see Table 1, bottom section; Fig. 3). There were no significant inverse associations between maternal behaviors and neural response, meaning that higher levels of each behavior always related to increased own – other infant cry response.
Table 1.
Maternal neural response to own–other infant cry related to mother–infant behaviors.
| Predictor | Region | BA | L/R | X | Y | Z | Z max | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|
| 1. Maternal sensitivity | Frontopolar cortex | 10 | R | 30 | 52 | 13 | 3.50 | 1157 |
| Inferior frontal gyrus–operculum | 45 | R | 43 | 21 | 9 | 3.52 | 1238 | |
| 2. Maternal intrusiveness | Anterior insula–temporal pole | 38 | L | −42 | 15 | −12 | 3.42 | 899 |
| 3. Mother–infant harmony | Hippocampus–posterior parahippocampal gyrus | 28, 20 | L | −28 | −24 | −19 | 4.01 | 1624 |
| Posterior parahippocampal gyrus–lingual gyrus | 20, 19 | R | 24 | −38 | −13 | 3.70 | 2543 | |
| Precuneus | 19 | R | 17 | −59 | 21 | 3.82 | 716 | |
Note: Clusters met threshold criteria (>615 mm3, p < .005) based on whole-brain FDR .05. Coordinates based on Montreal Neurological Institute template. BA = putative Brodmann's Area.
Plots of signal change associated with behavior scores showed that differences in maternal response could generally be attributed to increasing activation to the own cry stimulus, often paired with decreasing activation to the other cry stimulus (see Fig. 4); the exception was the right-sided harmony-related clusters, which showed differential activation to the other infant cry stimulus only. This means that whereas individual differences in mothers’ sensitivity and intrusiveness related to greater activation (and/or lesser deactivation) to their own infant's cry, at least some of the differences in mother–infant harmony had more to do with selective deactivation to an unfamiliar cry. Examining separate predictive models for behavior during free-play vs. cleanup interaction conditions (rather than averaged behavior scores) revealed that the above associations were largely driven by maternal behavior during free-play observations. This suggests that mothers’ brain responses are more reliably predictive of their behavior when they are unconstrained by task demands.
Fig. 4.
Signal change associated with own and other infant cry.
To evaluate neural responses related to maternal behavior separate from those associated with mood difficulties, the above model was also tested with each behavior orthogonalized with respect to current depressive symptom scores. Maternal sensitivity was still found to relate to the prefrontal activations identified above, and harmony still related to left-sided hippocampal activity. However, intrusiveness was no longer associated with maternal neural response, and the right-sided harmony-related activations were no longer evident. This suggests that although mothers’ current mood states were relevant to behavior with their infants, they did not explain neural responsiveness related to sensitivity, and only partially explained responsiveness related to harmony. Finally, behavioral covariates were tested separately for depressed and non-depressed groups; specifically, separate sensitivity, harmony, and intrusiveness EVs were created for depressed and non-depressed mothers using FSL's multiple group analysis setup. Although there appeared to be stronger evidence for reported activations among the latter, between-group differences in the strength of covariates—tested by a direct contrast of depressed and non-depressed behavior EVs—were nonsignificant. Therefore, we could not conclude that brain–behavior relations differed according to mothers’ history of perinatal depression.
3. Discussion
Results suggest individual differences in the neural processing of infant distress cues are related to differences in maternal behavioral sensitivity, intrusiveness, and mother–infant harmony during mother–infant interactions. As hypothesized the maternal behaviors of interest were associated with activation to one's own infant's cry (compared to unfamiliar infant's cry) in regions involved in approach motivation, decision making, emotion recognition and regulation, and social cognition. Specifically, mothers displaying more sensitive behaviors during a free-play interaction with their infant at 18 months postpartum exhibited greater own vs. other infant cry-related activation in the right frontal pole and inferior frontal gyrus. Mothers displaying a more harmonious interaction with their child showed greater activation in the left hippocampal regions, and mothers displaying more intrusive behaviors showed greater activation in the left anterior insula and temporal pole. Each of these regions has been implicated previously in maternal response. However, this study is the first to show that they play a distinct role in complex maternal behaviors. These results provide further evidence that maternal behavior is not a unitary construct, but rather, composed of specific sub-domains that should be differentiated to fully appreciate the basis of sensitivity, as opposed to more general involvement and/or positivity with one's infant. Thus, this study provides a unique window into the neural networks involved in human attachment.
As hypothesized, activation to mothers’ own infant's cry in the right lateral frontal pole and inferior frontal gyrus differentiated more sensitive from less sensitive mothers at 18 months postpartum, which is consistent with previous studies (Swain et al., 2008). The ventrolateral prefrontal cortex is known to be involved in emotion regulation, especially overriding automatic emotional responses (Roelofs et al., 2008, Wager et al., 2008), with more anterior regions playing a crucial role in integrating and interpreting multiple sources of information to pursue a higher goal (Ramnani and Owen, 2004). Thus, it may be that anterolateral prefrontal activations serve to override negative emotions a mother may associate with infant cry as she assesses and integrates information that will allow her to engage with her infant and reduce her infant's distress. The right inferior frontal gyrus has been implicated in response inhibition and regulatory behaviors, as well as processing others’ emotions (Horton et al., 1996, Noriuchi et al., 2008, Swain et al., 2007, Swain et al., 2008, Vollm et al., 2006). Activation differences in this region further suggest that sensitive mothers are better able to regulate their initial response to their infants’ cues while recognizing their infants’ emotional states in the service of sensitive responding. Our findings are consistent with previous proposals that these prefrontal networks are important in higher order dimensions of maternal attachment behavior (Bartels and Zeki, 2004, Nitschke et al., 2004, Swain et al., 2008).
In contrast, it was hypothesized that mothers with intrusive behavior would show greater activation in the insula and right amygdala (Atzil et al., 2011). Regions of activation found in this study to relate to maternal intrusiveness, the left insular cortex and temporal pole, have been associated with integrating sensory-emotional information, emotion recognition, the experience of empathy when witnessing the pain of a loved one, and actively attempting to understand and make meaning of stimuli (Olson et al., 2007, Singer et al., 2004). Additionally, the insula has been implicated in affiliative behaviors (Olausson et al., 2002). As such, it may be that more intrusive mothers are more reactive to their infant's distress signals and experience an empathic pain response, which causes them to approach their infants in an attempt to soothe them, but this leads to becoming overly involved. While preliminary, these results suggest neurobiologically distinct patterns of processing infant distress that may explain differences in first-time mothers’ ability to respond appropriately and sensitively, compared to overly intrusively, with their infants.
It was also hypothesized that mothers who had more harmonious interactions with their infant would display greater activation in the left nucleus accumbens and hippocampal regions (Atzil et al., 2011). This study found that activations in left hippocampal regions were indeed associated with mother–infant harmony during the interaction task. The hippocampus has been shown to be associated with memory and with stress management via regulation of hypothalamic-pituitary-adrenal output (Dedovic et al., 2009). Thus, it may be that mothers who displayed more harmonious interactions with their infants were better able to recall memories of previous interactions to guide their behavior and/or that they were better equipped to manage their stress responses when exposed to their infants’ cries. As suggested by previous work relating hippocampal integrity to a variety of resiliency measures (i.e., self-esteem, internal locus of control; Pruessner et al., 2010), mothers who can call on strong hippocampal activation to potentially stressful cues may be better able to repair negative emotion and restore positivity, both alone and with their infants.
Together, these patterns delineate distinct neural circuits involved in maternal behavior, some of which supported our hypotheses and overlap with findings highlighted in previous maternal neuroimaging research and/or with those compromised by depression. Although the specific areas of activation differed somewhat, our findings converge with prior studies showing heightened prefrontal and/or temporal activation to their infant's distress in mothers more closely bonded to their infants (Kim et al., 2011, Noriuchi et al., 2008). Differences between our findings and previous studies of maternal response to younger infants (i.e., less prominent role of amygdala) may speak to developmental differences in the mother–infant relationship, with basic emotional reactivity becoming less important and cognitive elaboration more important over time. That is, one reason our hypotheses regarding differences in amygdala activity may not have been supported is the age of infants in this study (i.e., the duration of the post-partum period of the mothers). Specifically, most of the research showing amygdala activation has been among mothers of younger infants (well below 1 year of age. Additionally, Swain et al. (2007) reported decreased maternal amygdala activation from 2–4 weeks to 3–4 months post-partum. Thus, the amygdala may be less important to maternal behaviors at later stages of the post-partum period, while the prefrontal and hippocampal circuits begin to become more important at this more advanced stage of mother–infant relationship development.
Concurrent maternal depressive symptoms were most closely linked to lower levels of harmony in this study, consistent with previous research, though they also related to lower sensitivity and intrusiveness with infants. When the contribution of depressive symptoms to behavior was removed, sensitivity-related activation remained unchanged, but intrusiveness-related activation disappeared. This suggests that whereas blunted response to her infant's cry in insular/temporal regions helped explain a lack of involvement among more depressed mothers, prefrontal response deficits relating to depression were separate from those explaining insensitivity. The lack of depression group differences in behavioral predictors further suggests that perinatal depression history does not alter maternal brain–behavior relations, at least at 18 months postnatal. More work is needed to define mechanisms through which maternal depression and other conditions impact sensitivity with their infants, but these findings underline the fact that “sensitivity” represents a complex program of behaviors related to but not fully captured by approach–avoidance, situational emotional valence, or even maternal mood regulation.
3.1. Limitations and future directions
While this study advances the understanding of neural pathways associated with maternal and attachment behaviors, it is not without limitations. One limitation is a modest sample size. A larger sample of first-time mothers should be recruited to increase power and allow examination of both main and interaction effects of postnatal depression and maternal sensitivity on neural responding, as maternal depression may alter brain–behavior associations in ways we were unable to detect in our limited sample. Additionally, including multiple neurobiological methods to assess first-time mothers’ response to infant distress may allow for greater specificity and convergent validity regarding the motivational profiles underlying these behaviors. Specifically, previous studies have demonstrated that heart rate variability, respiratory sinus arrhythmia, galvanic skin conductance, and other autonomic indices shape both approach/avoidance behaviors and sensitive parenting (Ablow et al., 2009, Musser et al., 2012); by combining these techniques with imaging methods, a fuller picture of the neurobiological matrix shaping sensitive parenting may emerge.
This study, which measured maternal behaviors and neural response at 18 months, also raises questions about temporal precedence. Future studies should examine both concurrent and prospective effects of maternal neural responses on sensitive behavior across early and later postnatal periods, as well as effects of maternal neurobehavioral responses on infant developmental outcomes such as attachment style. Longitudinal designs are needed to determine predictors of sensitive parenting and risk prior to parenthood, as well as neural changes associated with sensitivity during the transition to parenthood and the development of specific infant outcomes as the infant ages and the mother gains experience.
Despite these limitations, the current study adds to understanding the neurobiology of attachment and maternal sensitive responding behaviors, which may in turn allow for the development of improved prevention and intervention methods to alleviate child risk. In particular, this study shows that maternal behaviors can best be understood according to several related but distinct dimensions with different neural substrates. These results suggest mothers struggling with sensitive responding may be helped to regulate their own responses to infant information and be receptive to, but not overly distressed by, infant communicative cues. Additionally, mothers may be coached to develop more harmonious interactions with their infants by helping them recall memories of previous positive interactions and build stress regulation resources. These and future insights into the underlying biology of both constructive and destructive mother–infant interactions add to the scaffolding on which better parenting experiences can be built.
Footnotes
Maternal sensitivity was also tested on its own (not controlling for harmony and intrusiveness) as a predictor of neural response. In addition to the activations reported here, sensitivity related to increased own > other infant cry response in medial prefrontal and striatal regions, suggesting that these represent common areas serving multiple dimensions of maternal behavior. No additional harmony- or intrusiveness-related activations were found when these were tested separately.
References
- Ablow J.C., Measelle J.R., Cowan P.A., Cowan C.P. Linking marital conflict and children's adjustment: the role of young children's perceptions. Journal of Family Psychology. 2009;23:485–499. doi: 10.1037/a0015894. [DOI] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A., Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Campbell S.B., Cohn J.F., Meyers T. Depression in first-time mothers: mother–infant interaction and depression chronicity. Developmental Psychology. 1995;31:349–357. [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Rexroth M., Wolff E., Duchesne A., Scherling C., Beaudry T., Lue S.D., Lord C., Engert V., Pruessner J.C. Neural correlates of processing stressful information: an event-related fMRI study. Brain Research. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- DeWolff M.S., van IJzendoorn Sensitivity and attachment: a meta-analysis on parental antecedents of infant attachment. Child Development. 1997;68:571–591. [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony: biological foundations and developmental outcomes. Current Directions in Psychological Science. 2007;16:340–346. [Google Scholar]
- Feldman R., Eidelman A.I., Rotenberg N. Parenting stress, infant emotion regulation, maternal sensitivity and the cognitive development of triplets: a model for parent and child influences in a unique ecology. Child Development. 2004;75:1774–1791. doi: 10.1111/j.1467-8624.2004.00816.x. [DOI] [PubMed] [Google Scholar]
- Horton S., Sanghvi T., Philips M., Fiedler J., Perez-Escamilla R., Lutter C., Rivera A., Segall-Correa A.M. Breastfeeding promotion and priority setting in health. Health Policy and Planning. 1996;11:156–168. doi: 10.1093/heapol/11.2.156. [DOI] [PubMed] [Google Scholar]
- Kim P., Feldman R., Mayes L.C., Eicher V., Thompson N., Leckman J.F., Swain J.E. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry. 2011;52:907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent, H.K., Ablow, J.C., 2011. A cry in the dark: depressed mothers show reduced neural activation to their own infant's cry. Social Cognitive Affective Neuroscience, http://dx.doi.org/10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed]
- Lohaus A., Keller H., Ball J., Elben C., Voelker S. Maternal sensitivity: components and relations to warmth and contingency. Parenting: Science and Practice. 2001;1:267–284. [Google Scholar]
- Lorberbaum J.P., Newman J.D., Dubno J.R., Horwitz A.R., Nahas Z., George M.S. Feasibility of using fMRI to study mothers responding to infant cries. Depression and Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lorberbaum J.P., Newman J.D., Horwitz A.R., Dubno J.R., Lydiard R.B., Hamner M.B., Bohning D.E., George M.S. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Musser E.D., Measelle J.R., Ablow J.C. Predicting maternal insensitivity: the roles of postnatal depressive symptoms and parasympathetic dysregulation. Infant Mental Health Journal. 2012;34 doi: 10.1002/imhj.21310. Available online. [DOI] [PubMed] [Google Scholar]
- Murray L., Fiori-Cowley A., Hooper R., Cooper P. The impact of postnatal depression and associated adversity on early mother–infant interactions and later infant outcomes. Child Development. 1996;67:2512–2526. [PubMed] [Google Scholar]
- Murray L., Hipwell A., Hooper R., Stein A., Cooper P. The Cognitive Development of 5-Year-Old Children of Postnatally Depressed Mothers. Journal of Child Psychology and Psychiatry. 1996;37:927–935. doi: 10.1111/j.1469-7610.1996.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Murray L. The impact of postpartum depression on child development. International Review Of Psychiatry. 1996;8(1):55. [serial online] [Google Scholar]
- Murray L., Cooper P.J. Effects of postnatal depression on infant development. Achrives of Disease in Childhood. 1997;77:99–101. doi: 10.1136/adc.77.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, L., Cooper, P., 2001. Global Coding Scheme of Mother–Infant Interaction. Reading, England, unpublished manuscript.
- Nitschke J.B., Nelson E.E., Rusch B.D., Fox A.S., Oakes T.R., Davidson R.J. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Noriuchi M., Kikuchi Y., Senoo A. The functional neuroanatomy of maternal love: mother's responses to infant's attachment behavior. Biological Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., Morin C., Wallin B.G., Starck G., Ekholm S., Strigo I., Worsley K., Vallbo A.B., Bushnell M.C. Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience. 2002;5:900–905. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Mumford J.A. Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W., Simons R.F., Haynes O.M., Hyde C., Parisi M., Cohen B. Infant cardiac activity: developmental changes and relations with attachment. Developmental Psychology. 1991;27:432–439. [Google Scholar]
- Porges S.W. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Pruessner M., Lord C., Buss C., Collins L., Dagher A., Lupien S.J. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramnani N., Owen A.M. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Riordan D., Appleby L., Faragher B. Mother–infant interaction in post-partum women with schizophrenia and affective disorders. Psychological Medicine. 1999;29:991–995. doi: 10.1017/s0033291798007727. [DOI] [PubMed] [Google Scholar]
- Roelofs K., Minelli A., Mars R.B., van Peer J., Toni I. On the neural control of social emotional behavior. Social Cognitive and Affective Neuroscience. 2008;4:50–58. doi: 10.1093/scan/nsn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E., Esposito F., Neuhoff J.G., Luthi A., Mustovic H., Damman G., von Bardeleben U., Radue E.W., Cirillo S., Tedeschi G., Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J., Kaube H., Dolan R.J., Frith C.D. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Swain J.E., Lorberbaum J.P., Kose S., Strathearn L. Brain basis of early parent–infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain J.E., Tasgin E., Mayes L.C., Feldman R., Constable R.T., Leckman J.F. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain, J.E., Leckman, J.F., Mayes, L.C., Feldman, R., Hoyt, E., Kang, H., Kim, P., Dayton, C.J., Constable, R.T., Schultz, R.T., in press. Functional brain activations of parents listening to their own baby-cry that change over the early postpartum. Developmental Psychobiology.
- Vollm B.A., Taylor A.N., Richardson P., Corcoran R., Stirling J., McKie S., Deakin J.F., Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron. 2008;25:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeskind P.S., Lester B.M. Acoustic features and auditory perceptions of the cries of newborns with prenatal and perinatal complications. Child Development. 1978:49. [PubMed] [Google Scholar]