Highlights
▸ Participants demonstrated a significant behavioral and neural ratio effects. ▸ Behavioral ratio effect negatively correlated with math achievement. ▸ Neural ratio effect in the left IPS positively correlated with math achievement. ▸ Behavioral and IPS ratio effects unrelated to measures of reading achievement and IQ.
Keywords: Symbolic number processing, Children, Arithmetic skills, Numerical ratio effect, Left intraparietal sulcus
Abstract
The neural foundations of arithmetic learning are not well understood. While behavioral studies have revealed relationships between symbolic number processing and individual differences in children's arithmetic performance, the neurocognitive mechanisms that bind symbolic number processing and arithmetic are unknown. The current fMRI study investigated the relationship between children's brain activation during symbolic number comparison (Arabic digits) and individual differences in arithmetic fluency. A significant correlation was found between the numerical ratio effect on reaction times and accuracy and children's arithmetic scores. Furthermore, children with a stronger neural ratio effect in the left intraparietal sulcus (IPS) during symbolic number processing exhibited higher arithmetic scores. Previous research has demonstrated that activation of the IPS during numerical magnitude processing increases over the course of development, and that the left IPS plays an important role in symbolic number processing. The present findings extend this knowledge to show that children with more mature response modulation of the IPS during symbolic number processing exhibit higher arithmetic competence. These results suggest that the left IPS is a key neural substrate for the relationship between the relative of precision of the representation of numerical magnitude and school-level arithmetic competence.
1. Introduction
Acquiring basic arithmetic skills is a crucial prerequisite for successful participation in modern society (Duncan et al., 2007, Romano et al., 2010). However, the neurocognitive predictors of successful arithmetic learning are not well understood. Recent behavioral studies have revealed that children's symbolic and non-symbolic numerical magnitude processing abilities are related to and predictive of their arithmetic achievement level. Specifically, the degree to which the numerical distance between two numbers, or their ratio, affects response time and accuracy when comparing the relative magnitude of those numbers, is correlated with scores on tests of mental arithmetic (Bugden and Ansari, 2011, De Smedt et al., 2009, Halberda et al., 2008, Holloway and Ansari, 2009). The numerical distance (longer response times when the numerical distance and ratio effects between two numbers is small or the ratio of the small: large number is large) have become litmus tests for probing the representation and processing of numerical magnitude (Moyer and Landauer, 1967).
Studies investigating the functional neuroanatomy underlying numerical magnitude processing in adults have found that numerical distance modulates activity in bilateral regions of the intraparietal sulcus (IPS) (Pinel et al., 2001). Furthermore, comparisons of the brain activation underlying numerical magnitude processing in children and adults indicate that activation of the parietal cortex during numerical comparison exhibits age-related changes (Ansari et al., 2005, Cantlon et al., 2006). Specifically, adult participants exhibit a greater effect of numerical distance/ratio on IPS activation than do children, suggesting a process of age-related specialization for the processing of numerical magnitude.
What is currently not well understood is how brain activation during numerical magnitude processing is related to individual differences in arithmetic competence. Understanding how brain responses during numerical magnitude processing relate to behavioral measures of magnitude processing and individual differences in children's arithmetic achievement will provide insights into both neuronal and behavioral predictors of individual differences in children's arithmetic achievement, and the relationships between these different levels of explanation.
In the present study, we used functional Magnetic Resonance Imaging (fMRI) to measure brain activation during symbolic number comparison in a group of seventeen 8–9 year old children who varied in their performance on a standardized test of mathematical fluency but had normal achievement scores on tests of verbal and non-verbal intelligence as well as reading fluency. On the basis of a recent meta-analysis of number processing in children (Houdé et al., 2010), we extracted estimates of the brain activation during symbolic numerical magnitude comparison from three regions of interest (ROIs) that have been reliably associated with numerical magnitude processing in children and correlated these estimates of brain activation with children's arithmetic achievement scores.
We hypothesize that individual differences in the effect of numerical ratio on brain activation, in particular on responses in the parietal cortex, are correlated with between-subjects variability in children's arithmetic skills.
2. Methods
2.1. Participants
Twenty-two children were recruited to participate in the fMRI experiment. Five children were excluded from the study, because their head movements in the scanner were either greater than 3 mm over the entire scan, or they had a greater than 2 mm jump between subsequent volumes of brain images. Following the application of these exclusion criteria, 17 children were included in the final data set [14 female, mean age 105.59 months (8.8 years), standard deviation 6.09 months]. Nine children were in grade three and eight children were in grade four. All children were right handed and native English speakers with normal or corrected to normal vision. The Health Sciences Research Ethics Board at the University of Western Ontario approved all procedures. Written consent and assent was obtained from all children and parents.
The sample of participants had variable performance on a standardized measure of math fluency with their standard scores ranging between 68 and 104 (with a mean score of 89.41, SD = 9.46).
In contrast, all participants obtained average (within one standard deviation above and below the standardization mean of 100) reading fluency (M = 108.94, SD = 11.58) and intelligence standard scores (M = 109.76, SD = 11.96) (Table 1). This distribution of scores, thus, enables an assessment of the degree to which variability in arithmetic performance is related to brain activation in the absence of similar (and thus potentially confounding) variability in non-arithmetic performance, such as reading, verbal and non-verbal intelligence.
Table 1.
The descriptive statistics for the neuropsychological standardized tests.
| Range | Mean | SD | |
|---|---|---|---|
| Age (months) | 95–116 | 105.59 | 6.09 |
| Math Fluency (RS) | 20–55 | 36.41 | 9.25 |
| Math Fluency (SS) | 68–104 | 89.41 | 9.46 |
| Reading Fluency (RS) | 27–64 | 42.71 | 11.24 |
| Reading Fluency (SS) | 93–133 | 108.94 | 11.58 |
| Verbal IQ (RS) | 48–70 | 57.53 | 6.50 |
| Verbal IQ (SS) | 103–121 | 112.41 | 6.30 |
| Non Verbal IQ (RS) | 15–36 | 27.53 | 6.40 |
| Non Verbal IQ (SS) | 68–124 | 103.82 | 17.58 |
| IQ Composite (SS) | 87–126 | 109.76 | 11.96 |
Note: RS, raw score; SS, standard score; SD, standard deviation.
2.2. Behavioral assessment
Each child visited an fMRI simulation facility at The University of Western Ontario for a first testing session. During this session, children were trained in and familiarized with the procedures associated with fMRI research participation. In addition, each child was tested on a battery of standardized tests measuring mathematical and reading achievement, as well as intelligence.
Two subtests from the Woodcock Johnson III subtests of Achievement (Woodcock et al., 2001) were administered to each child. The Math Fluency subtest, which measures the ability to solve simple addition, subtraction and multiplication facts quickly, and the Reading Fluency subtest, which measures the ability to quickly read simple sentences, were administered. In addition, all participants were also tested using the Kaufman Brief Intelligence Test, Second Edition (Kaufman and Kaufman, 1997). This test takes approximately 15–30 min to be administered and measures both verbal and nonverbal intelligence.
The raw scores from the standardized tests were used in the correlational analyses presented below because of their higher variability between subjects and thus better estimation of small differences between participants’ ability levels. This is especially true in relatively small samples of young children where only a few items are available to discriminate between children's performance levels and thus, differences between subjects’ standard scores may be less accurately reflective of variability in their performance levels than raw scores (Bracken, 1988). To control for the effects of chronological age, all correlations were partial correlations with age in months partialled out. Since partial correlations were used, all the correlations estimate the relationship between raw scores and behavioral and neural ratio effects independently of chronological age.
2.3. Experimental design
In the second session, each child returned to participate in the fMRI component of the study where they completed a symbolic numerical comparison task while brain function was measured using fMRI. The participants were asked to choose the numerically larger of two presented digits as fast as they could without making any errors by responding with a button press in the scanner. An event-related fMRI design was used to investigate which brain regions were modulated by numerical ratio during the numerical comparison task. Participants were presented with a series of two single digit white Arabic numerals for 1000 ms, with a font size of 60, presented against a black background using E-prime stimulus presentation software (Psychological Software Tools, Pittsburgh, PA). The Arabic numerals presented ranged from one to nine and the ratio between the two numbers was calculated by dividing the small number by the larger number (small/large). Small and large ratio pairs were used with six small ratio pairs ranging from .11 to .22 [the small ratios (with the pair of presented digits in parentheses): .11 (1–9), .13 (1–8), .14 (1–7), .17 (1–6), .20 (1–5) and .22 (2–9)] and six large ratio pairs ranging from .78 to .89 [the large ratios (with the pair of presented digits in parentheses): .78 (9–7), .8 (4–5), .83 (6–5), .86 (6–7), .88 (8–7) and .89 (9–8)]. Each pair was administered four times in two runs for a total of 48 trials. In this design, the frequency of number presentation is confounded with ratio in the small ratio condition given that one is presented more frequently than the other numerals. However, this is an issue characteristic of single-digit number comparison tasks where there are more repetitions in the small compared to the large ratio condition.
After each stimulus presentation, participants were presented with a variable fixation period with either 4000, 6000 or 8000 ms. Each run began and ended with a 16,000 ms fixation period to achieve a better estimation of the baseline. Total running time for the numerical comparison task was approximately 5 min per run.
It should be noted that previous studies investigating the relationship between number comparison and brain activation have frequently used numerical distance (Ansari et al., 2005, Pinel et al., 2001), rather than numerical ratio as an independent variable. However, numerical ratio and distance are highly correlated. Indeed, in the present study, the average numerical distance of the small ratio trials was 1.17, and 6.17 for the large ratio trials. Consistent with this, the correlation between ratio and distance for the trials used was very high [r(46) = −.94, p < 001].
2.4. fMRI data acquisition
Scanning was performed on a 3 T Siemens Tim Trio MRI system with a Siemens 32-channel receive-only head coil (Erlangen, Germany). An anatomical scan was performed encompassing the whole brain after the functional runs were completed. This was achieved by collecting 192 one-mm thick slices using a 3-D T1-weighted acquisition sequence (TI = 900 ms, TE = 4.25 ms, TR = 2300 ms, flip angle = 9°). The in-plane resolution of the anatomical scans was 256 pixels × 256 pixels. To collect functional data, we used a T2*-weighted echo-planar imaging sequence (TE = 30.0 ms, TR = 2000 ms, flip angle = 90°) for BOLD acquisition. The field of view was 21.1 cm × 21.1 cm with an in-plane matrix size of 64 pixels × 64 pixels. Each image consisted of 38 slices (voxel size = 3 mm) with an inter slice time 52 ms. There were no gaps between slices and 160 volumes were collected in a run (no volumes were discarded from the beginning of the run beyond the two removed by the scanner).
2.5. Image pre-processing and statistical analysis
Both structural and functional images were analyzed using Brain Voyager QX 2.1.2 (Brain Innovation, Maastricht, Netherlands). The functional images were corrected for differences in slice time acquisition, head motion, linear trends and low frequency noise. In addition, functional images were spatially smoothed with a 6 mm full width at half maximum Gaussian smoothing kernel. Following initial automatic alignment, the alignment of functional images to the high-resolution T1 structural images was manually fine-tuned. The realigned functional data set was then transformed into Talairach space (Talairach and Tournoux, 1988). A two gamma hemodynamic response function convolved with a 1-volume boxcar function at trial onset was used to model the expected BOLD signal (Friston et al., 1998). Inaccurate responses were modeled as predictors of no interest.
3. Results
3.1. Behavioral data
A paired samples t-test was conducted between small and large ratio conditions for reaction time and mean accuracy respectively. For reaction time (RT), a significant difference was found between small and large ratio conditions [t(16) = −7.24, p < .001], indicating that participants were faster during the small ratio condition (M = 924.89, SD = 175.05) relative to the large ratio condition (M = 1075.18, SD = 192.41). For accuracy (proportion of incorrect responses), a significant difference was also found between large and small ratio conditions [t(16) = −5.03, p < .001], demonstrating that participants were more accurate in the small ratio conditions (M = .02, SD = .04) than the large ratio conditions (M = .18, SD = .13).
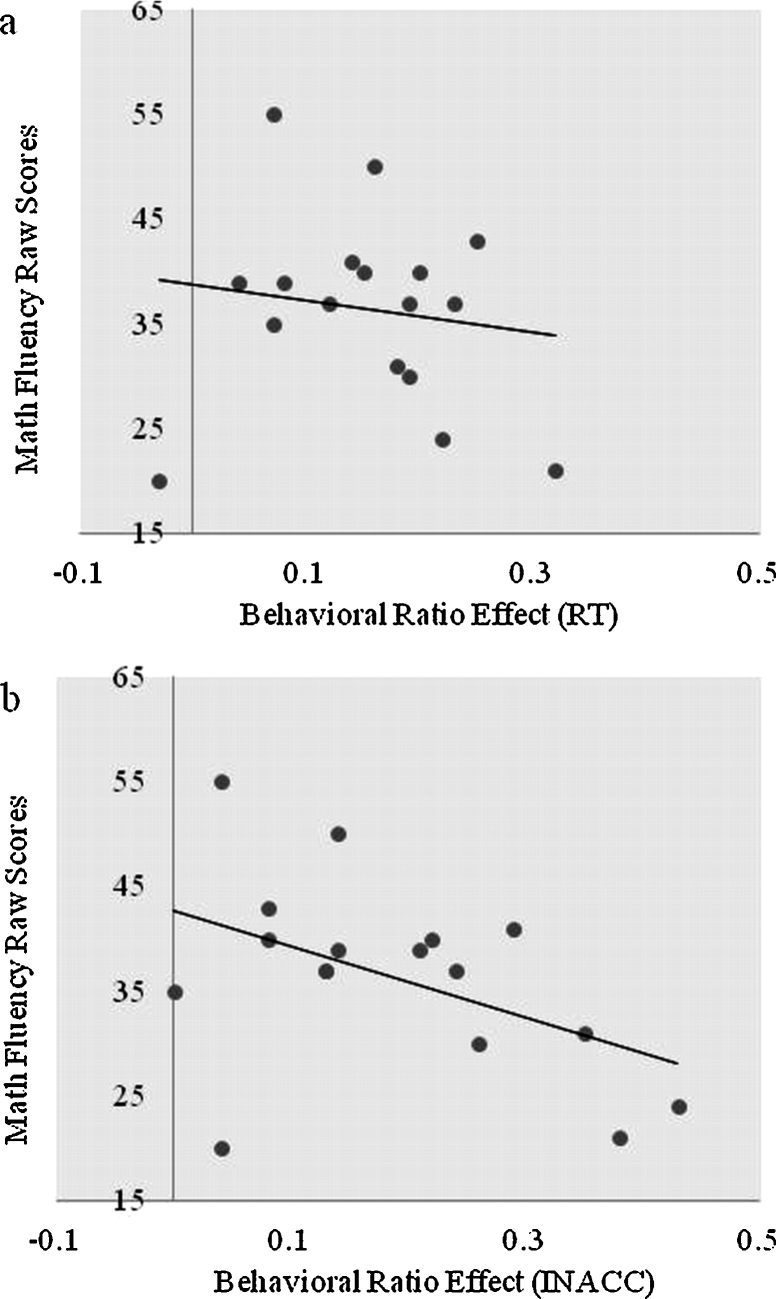
The behavioral ratio effect was calculated by subtracting the mean small ratio condition RT (e.g. .11–.22) from the mean large ratio condition RT (e.g. .78–.89) and dividing the outcome by the mean of the small and large ratio conditions (RT), using the correct responses. This method controls for differences between subjects in general RT by treating the ratio effect as proportional change in RT between the small and the average of the small and large ratio conditions. A significant negative partial correlation was found between the RT ratio effect and math fluency raw scores, after controlling for chronological age [r(14) = −.56, p = .02] (see Fig. 1a). The accuracy ratio effect was calculated using the proportion of incorrect responses (INACC, mean incorrect for large ratio − mean incorrect for small ratio/mean incorrect for small and large ratio). The accuracy ratio effect was also negatively correlated with math fluency scores after controlling for age [r(14) = −.66, p = .002] (see Fig. 1b).
Fig. 1.
(a) Correlation between reaction time behavioral ratio effect and math fluency raw scores. (b) Correlation between accuracy ratio effect and math fluency raw scores. Note: RT, reaction time ratio effect (large ratio RT-small ratio RT/mean of large and small ratio RTs); INACC, accuracy ratio effect (mean incorrect responses for large ratios – mean incorrect responses for small ratios/mean of large and small ratios incorrect responses). Note: two participants had the same data points and are overlapping in (b).
3.2. Neuroimaging data
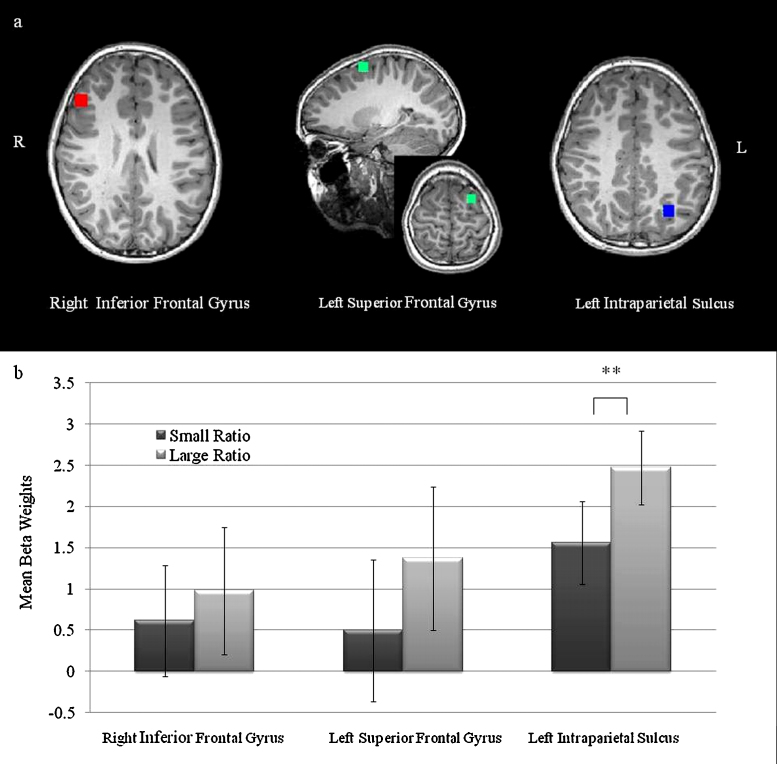
In order to restrict our analyses of the neuroimaging data to brain regions that have previously been reliably associated with numerical processing in children, we carried out a region of interest (ROI) analysis using three brain regions reported in a meta-analysis (Houdé et al., 2010) of neuroimaging studies of numerical processing in children (ages 4–13, with the majority of studies included in this meta-analysis reporting results from children between the ages 8 and 13). These regions were the right inferior frontal gyrus (IFG), left superior frontal gyrus (SFG) and the left intraparietal sulcus (IPS).1 This approach represents a hypothesis driven, independent method for the selection of ROIs for the estimation of brain-behavior correlations (Kriegeskorte et al., 2009, Poldrack, 2007, Vul et al., 2009). The MNI (Montreal Neurological Institute) coordinates from the meta-analysis were converted into Talairach coordinates using a website that converts MNI coordinates into Talairach space (http://www.bioimagesuite.org/Mni2Tal/index.html). Each ROI was defined on the basis of the peak coordinate reported in the meta-analysis by Houdé et al., with a cluster spread for each dimension (x, y and z) of 10 mm. Average fMRI parameter estimates (beta values) for each predictor (small ratio and large ratio conditions) were extracted from the three regions (see Fig. 2a) in order to estimate the effect of numerical ratio on activation in these ROIs, as well as to correlate the neuronal ratio effect with individual differences in children's arithmetic scores.
Fig. 2.
(a) Schematic illustration of the defined ROIs superimposed on a high resolution anatomical image from one of the participants. The size of the square represents the dimensions of the ROI. The Talairach coordinates for the right inferior frontal gyrus, left intraparietal sulcus and the left superior frontal gyrus are the following: x = 43, y = 28, z = 23; x = −26, y = −61, z = 34; x = −22, y = 3, z = 58, respectively. (b) The bar chart illustrates the mean values for large and small ratio conditions during the numerical comparison task in each region. The error bars represent two standard error estimates from the mean. Note: ** denotes a significant difference at p < .01. The error bars represent two standard errors from the mean.
A significant ratio effect was found in the left IPS, t(16) = 3.80, p = .002, Bonferroni corrected. No significant ratio effect was observed in either of the prefrontal regions. [SFG, t(16) = 1.66, p = .12; IFG, t(16) = 1.08, p = .30] (Fig. 2b).
3.3. Brain-behavior correlations
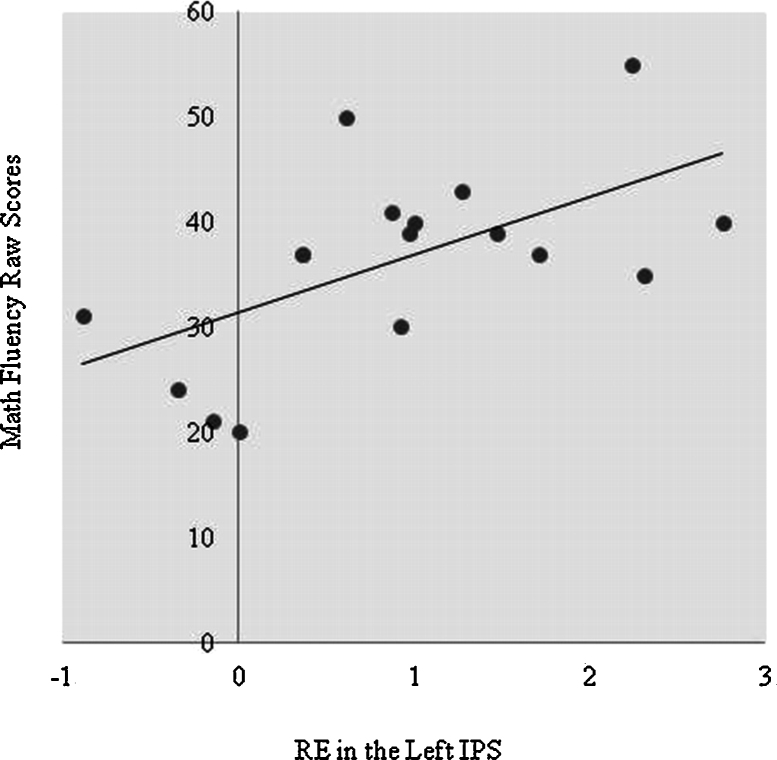
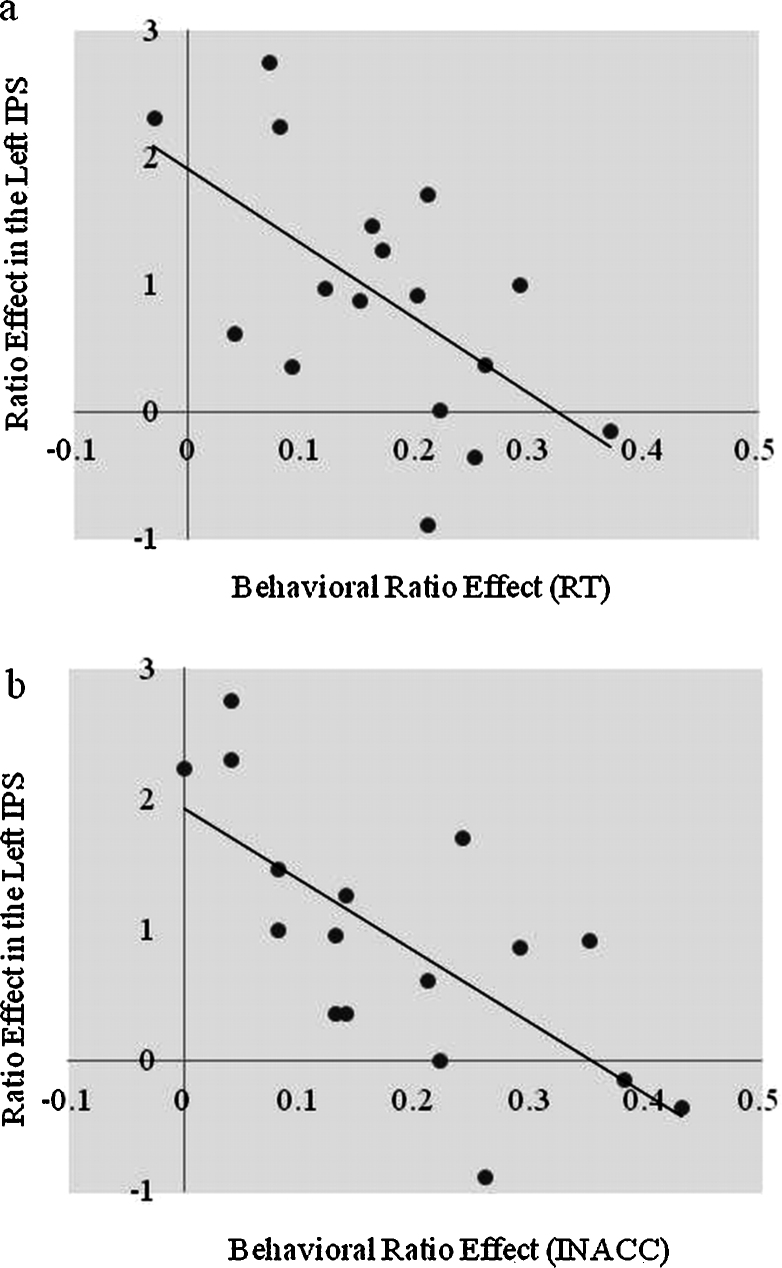
Correlations between the behavioral and neuroimaging data were conducted using beta weights from each of the regions of interest. The neural ratio effect was calculated by subtracting the small ratio mean beta weight values from the large ratio mean beta weight values. A significant partial correlation (removing the variance explained by differences in children's chronological age) was found between children's math fluency raw scores and the neural ratio effect in the left IPS [r(14) = .58, p = .02], indicating that children who exhibited a larger ratio effect in the left IPS had higher math fluency scores (see Fig. 3). Furthermore, a significant negative correlation was found between the neural ratio effect in the IPS and behavioral ratio effects (RT [r(14) = −.58, p = .02] (see Fig. 4a); Accuracy [r(14) = −.69, p = .001]) while controlling for chronological age (Fig. 4b). These data show that children who exhibited a larger neural ratio effect showed a smaller behavioral ratio effect.
Fig. 3.
Correlation between math fluency raw scores and the neural ratio effect in the left IPS.
Fig. 4.
(a) Correlation between the neural ratio effect in the left IPS and the reaction time behavioral ratio effect. (b) Correlation between the neural ratio effect in the left IPS and the accuracy behavioral ratio effect.
Although the ratio effect was not significant in these regions, partial correlation analyses were also conducted using the neural ratio effect in right IFG and the left SFG and math fluency raw scores while controlling for chronological age. A significant positive correlation was found between the right IFG and math fluency [r(14) = .58, p = .02] and a marginally significant correlation was found between the left SFG and math fluency [r(14) = .48, p = .06], indicating that a larger ratio effect in the prefrontal regions are associated with relatively higher math fluency scores. The right IFG and left SFG showed marginally significant negative correlations with the behavioral ratio effect using accuracy [IFG, r(14) = −.50, p = .05; SFG, r(14) = −.47, p = .07], but did not correlate with the behavioral ratio effect using reaction time (see Table 2 for correlation matrix).
Table 2.
Correlation matrix, showing partial and bivariate correlations between the neural ratio effect in regions of interest and behavioral variables of interest.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Math Fluency (RS) | −.56* | −.66** | .58* | .58* | .48 | .16 | .37 | −.02 | .001 | |
| 2. Ratio Effect (RT) | .54* | −.58* | −.35 | −.36 | −.06 | −.34 | .26 | .27 | ||
| 3. Ratio Effect (INACC) | −.69** | −.50* | −.47 | −.18 | −.39 | −.08 | −.02 | |||
| 4. Left IPS Ratio Effect (ROI) | .60* | .72** | .50* | .20 | −.01 | .29 | ||||
| 5. Right IFG Ratio Effect (ROI) | .44 | .53* | .09 | .38 | −.10 | |||||
| 6. Left SFG Ratio Effect (ROI) | .37 | .15 | .07 | .01 | ||||||
| 7. Right IPS (ROI) | −.07 | .14 | −.08 | |||||||
| 8. Reading Fluency RS | .30 | .53* | ||||||||
| 9. Nonverbal IQ RS | .29 | |||||||||
| 10. Verbal IQ RS | ||||||||||
| 11. IQ Composite SS | −.002 | .28 | −.08 | −.07 | .29 | .06 | .14 | .39 | .94** | .40 |
Note: RS, raw score; RT, reaction time; INACC, percent incorrect; IPS, intraparietal sulcus; IFG, inferior frontal gyrus; SFG, superior frontal gyrus; ROI, region of interest.
Partial correlations controlling for age are reported above the diagonal and bivariate correlations are reported below the diagonal.
p < .05.
p < .01.
Importantly, both the behavioral and the IPS ratio effects were found to be unrelated to measures of reading achievement. Moreover, measures of verbal and non-verbal IQ did not significantly correlate with the ratio effect on behavioral and brain imaging measures, suggesting that the brain-behavior correlations reported above are specific to individual differences in mathematical achievement (see Table 2 for a correlation matrix).
In addition, to ascertain that the key correlations between both behavioral and brain-imaging measures of the ratio effect were significant after controlling for intelligence, partial correlations (controlling for the average of the verbal and non-verbal IQ scores) were run. These revealed a significant correlation between (a) the behavioral ratio effect on reaction time and math fluency [r(14) = −.60, p = .01] and (b) the ratio effect on the left IPS and math fluency scores [r(14) = .59, p = .02] and (c) the ratio effect on reaction time and the ratio effect on left IPS activation [r(14) = −.60, p = .01]. Thus all the key correlations (displayed in Fig. 1, Fig. 3, Fig. 4) remain significant after controlling for individual differences in measures of verbal and non-verbal intelligence.
In addition to the above correlation analyses, a hierarchal regression analysis was conducted to investigate the extent to which the neural ratio effect in the left intraparietal sulcus (IPS) predicted unique variance in math fluency over and above age (step 1), reading fluency (step 2) and nonverbal IQ (step 3). The results demonstrated that after controlling for these factors, the neural ratio effect predicted a significant amount of unique variance in the math fluency [ΔR2 = .25, F(1,12) = 5.23, p = .04]. A second regression analysis was conducted to explore the extent to which the neural ratio effect explained unique variance in the math fluency when verbal IQ was substituted for non verbal IQ in step 3. We found that the neural ratio effect explained a marginally significant amount of unique variance in math fluency [ΔR2 = .21, F(1,12) = 4.30, p = .06]. A third regression analysis investigating the extent to which the neural ratio effect explains unique variance in math fluency when IQ composite standard scores were substituted in step 3. The neural ratio effect was found to explain a significant amount of unique variance in math fluency [ΔR2 = .24, F(1,12) = 4.95, p = .046] (see Table 3).
Table 3.
Hierarchical regression analyses predicting math fluency raw scores.
| Step | Math Fluency |
|||
|---|---|---|---|---|
| Predictor | β | R2 | ΔR2 | |
| 1 | Age (months) | −.08 | .01 | .01 |
| 2 | Reading Fluency (RS) | .31 | .15 | .14 |
| 3 | Nonverbal IQ (RS) | −.10 | .17 | .02 |
| 4 | Ratio Effect IPS | .53* | .42 | .25* |
| 1 | Age (months) | −.12 | .01 | .01 |
| 2 | Reading Fluency (RS) | .26 | .15 | .14 |
| 3 | Verbal IQ (RS) | .04 | .20 | .05 |
| 4 | Ratio Effect IPS | .55† | .41 | .21† |
| 1 | Age (months) | −.11 | .01 | .01 |
| 2 | Reading Fluency (RS) | .32 | .15 | .14 |
| 3 | IQ Composite (SS) | −.09 | .18 | .03 |
| 4 | Ratio Effect IPS | .52* | .42 | .24* |
Note: RS, raw score; SS, standard score; IPS, intraparietal sulcus.
p = .06.
p < .05.
3.4. Brain behavioral correlations in the right IPS
It has been frequently shown that the right IPS is involved in numerical magnitude processing (Piazza et al., 2004, Piazza et al., 2007); therefore, in order to explore any hemispheric differences in the right and left IPS, beta weights were extracted from the right IPS (Talairach coordinates: x = 26, y = −61, z = 34), which were obtained by flipping the x axis from the Houdé et al. (2010) left IPS region from negative to positive. No significant ratio effect was found in this region [t(16) = 1.02, p = .32]. Furthermore, the ratio effect in the right IPS did not correlate with math fluency [r(14) = .16, p = .54]. The results demonstrate that the right IPS region of interest defined on the basis of Houdé et al's meta-analysis was not significantly modulated by ratio during a symbolic numerical comparison task. Furthermore, the ratio effect in this region was not associated with math fluency performance.
3.5. Whole brain analyses and correlations
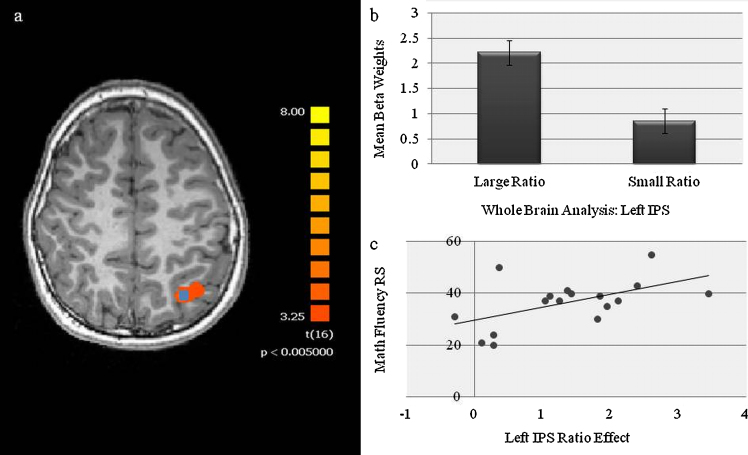
To address whether (a) the effect of ratio on left IPS activation was significant at the whole brain level and whether (b) individual differences in any such whole brain ratio effect were correlated with individual differences in children's arithmetic achievement scores, a whole brain general linear model (GLM) analysis of the ratio effect was conducted to identify regions that showed a statistical difference between large and small ratio conditions. The resulting statistical maps were corrected for multiple comparisons using a cluster thresholding method (Forman et al., 1995, Goebel et al., 2006). For this analysis, the initial random-effects threshold was set at p < 005, uncorrected, and the resulting map was submitted to different correction criterion based on the estimates of the map's smoothness and on an iterative procedure for estimating cluster-level false positive rates. After 1000 iterations, the minimum cluster size that yielded a cluster-level false positive rate of .05 was 648 structural voxels per cluster. Therefore, only activations whose cluster size met or exceeded the cluster threshold of 648 remained on the statistical maps.
As can be seen from Fig. 5, a significant effect of ratio at the whole brain level in the left IPS was found (x = −29, y = −67, z = 34; number of voxels = 2563) (Fig. 5b). Furthermore, as indicated by the super-imposed blue square, this activation overlapped precisely with the region determined by the Houdé et al.’s (2010) meta-analysis to be consistently associated with number processing in children and subsequently used for the above reported ROI (Fig. 5a). To assess the degree to which individual differences in the ratio effect on this left parietal brain region were correlated with variability in children's math achievement, the beta values for small and large ratio conditions were extracted from the left IPS region and individual differences in the brain ratio effect (large–small) were correlated with math fluency raw scores. Consistent with the ROI analysis, the neural ratio effect in the left IPS resulting from the whole brain analysis significantly correlated with math fluency raw scores after partialling out the variance due to chronological age [r(14) = .54, p = .03] (Fig. 5c).
Fig. 5.
(a) An illustration of the whole brain analysis revealing an effect of numerical ratio on the left IPS. The superimposed blue area represents the region from the Houdé meta-analysis used in the main results. (b) Parameter estimates for small and large ratios obtained from the left IPS cluster revealed by the whole brain analysis. (c) Partial correlation (controlling for chronological age) of the ratio effect in the left IPS (extracted from the regions identified through the whole brain analysis) and math fluency raw scores. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Greater modulation of ratio was also found in the following clusters with the Talairach coordinates and number of voxels in parentheses: the left parahippocampal gyrus (x = −14, y = −5, z = −12; number of voxels = 772); the medial occipitotemporal gyrus (x = 12, y = −38, z = −4; number of voxels = 881); the left lingual gyrus (x = −14, y = −45, z = −1; number of voxels = 1413); the cerebellum (x = 6, y = −68, z = −22, voxels = 1302), and a region extending from the medial occipitotemporal gyrus to the cuneus (x = −1, y = −77, z = 4; voxels = 7897). The whole brain analysis did not reveal a significant ratio effect in the prefrontal cortex or in the right IPS. Furthermore, the ratio effect did not significantly correlate with mathematical fluency in any of these regions (all p's > .05) (see Table 4).
Table 4.
Correlation matrix showing partial and bivariate correlations between neural ratio effects found in the whole brain analysis and behavioral variables of interest.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Math Fluency (RS) | −.56* | −.66** | .09 | .03 | .25 | .54* | .19 | .08 | .37 | −.02 | .001 | |
| 2. Behavioral Ratio Effect (RT) | .54* | −.17 | −.26 | −.15 | −.41 | −.23 | .29 | −.36 | .26 | .27 | ||
| 3. Behavioral Ratio Effect (INACC) | −.09 | .16 | .13 | −.62** | .02 | .03 | −.39 | −.08 | −.02 | |||
| 4. Left Parahippocampal Gyrus | .66** | .23 | .26 | .61* | .49 | −.21 | .03 | −.35 | ||||
| 5. Medial Occipitotemporal Gyrus | .32 | .28 | .60* | .16 | −.42 | −.39 | −.57* | |||||
| 6. Medial Occipitotemporal Gyrus to Cuneus | .10 | .47 | .12 | −.06 | −.03 | −.11 | ||||||
| 7. Left IPS | .11 | −.05 | .03 | −.11 | −.30 | |||||||
| 8. Left Lingual Gyrus | .43 | −.15 | −.27 | −.26 | ||||||||
| 9. Cerebellum | −.54* | .08 | −.09 | |||||||||
| 10. Reading Fluency (RS) | .30 | .53* | ||||||||||
| 11. Nonverbal IQ (RS) | .29 | |||||||||||
| 12. Verbal IQ (RS) | ||||||||||||
| 13. IQ Composite (SS) | −.002 | .28 | −.08 | −.06 | −.46 | −.08 | .14 | −.34 | .02 | .39 | .94** | .40 |
Note: RS, raw score; RT, reaction time; INACC, percent incorrect; IPS, intraparietal sulcus; SS, standard score.
Partial correlations controlling for age are reported above the diagonal and bivariate correlations are reported below the diagonal.
p < .05.
p < .01.
4. Discussion
The acquisition of arithmetic competence is a crucial predictor of school and life success (Duncan et al., 2007, Romano et al., 2010). Recent evidence has demonstrated that the representation and processing of numerical magnitude relates to individual differences in math achievement (Bugden and Ansari, 2011, De Smedt et al., 2009, Holloway and Ansari, 2009). The roles of neuronal mechanisms subserving basic numerical magnitude processing in explaining individual differences in arithmetic achievement, however, are poorly understood. The results of the present study provide the first demonstration that individual differences in the engagement of the left parietal cortex during numerical magnitude processing are related to variability in children's arithmetic achievement performance. Moreover, this relationship between parietal brain activation during symbolic number processing and arithmetic competence was found to be domain specific, as no relationships between brain activation and measures of reading achievement or intelligence were observed.
Using an independent, region of interest (ROI) approach, we investigated the ratio effect in three cortical regions that have been consistently implicated in numerical processing in children (Houdé et al., 2010). Correlation analyses revealed that the degree to which the left IPS is modulated by numerical ratio is related to individual differences in children's scores on a standardized test of math achievement. Specifically, children who exhibited a larger ratio effect in the left IPS had relatively higher math fluency scores. This evidence points to a relationship between neural processing of numerical magnitude and individual differences in children's arithmetic achievement. The ROI analyses were also confirmed by a whole-brain analysis (see Fig. 5) that demonstrated a significant effect of ratio on a region of the left IPS that closely overlaps with the ROI from the Houdé et al. meta-analysis. Moreover, individual differences in the ratio effect on activation in this region were found to positively correlate with children's math fluency scores.
At the behavioral level, the results of the present study show a negative correlation between the behavioral ratio effect (RT and Accuracy) and math fluency scores. These results support previous findings of a relationship between individual differences in the magnitude of children's ratio/distance effect and their math achievement (Bugden and Ansari, 2011, De Smedt et al., 2009, Holloway and Ansari, 2009). Interestingly, the relationship between behavioral measures of the ratio effect and math fluency is in the opposite direction to those between the neuronal ratio effect and arithmetic achievement measures. Specifically, a greater neural ratio effect was found to be associated with higher math fluency scores, whereas a greater behavioral ratio effect was associated with lower math fluency scores. A possible explanation for these differences in the direction of the relationships is as follows: children who exhibit a smaller neural ratio effect have a noisier representation of numerical magnitude leading to greater overlap (similarity) of representations in the IPS and thus, similar levels of engagement of this brain region. Put differently, the neuronal processes underlying the discrimination of small and large ratios are more similar in children with a less mature representation of numerical magnitude (Ashkenazi et al., 2012). Therefore, similar processes are being engaged during the processing of different ratios leading to the absence of a clear differentiation between activation correlated with the processing of relatively small and large ratios. Support for this interpretation comes from the study of numerical magnitude processing in children with developmental dyscalculia (DD). In these studies, children with DD, in contrast to typically developing children, were found to not exhibit a significant distance effect on IPS activation (Mussolin et al., 2010, Price et al., 2007). In other words, while children with DD activated the IPS during the comparison of both small and large distance conditions, no activation differences between these conditions were observed. Furthermore, developmental literature has consistently demonstrated that there are larger effects of distance on the IPS in the brains of adults compared to children (Ansari and Dhital, 2006, Ansari et al., 2005). Taken together, these results suggest that greater similarity in the activation of the IPS for small and large distance conditions is associated with immature and atypical processing of numerical magnitude.
Therefore, a smaller ratio effect in the IPS may reflect a less refined representation and processing of numerical magnitude in this region. Consequently, greater effort is required to discriminate between numerical magnitudes, which, in turn, results in a larger behavioral ratio effect. As children develop more precise and less overlapping representations of numerical magnitude in the IPS, a greater difference between the processing of small and large ratios can be detected. Thus, while a small behavioral ratio effect may be indicative of greater precision in the processing of numerical magnitude, the reverse is true for neuronal measures of the ratio effect, where a greater neural ratio effect is associated with more efficient processing and higher arithmetic achievement. The fact that the relationships between math fluency and the neural and behavioral ratio effects go in opposing directions, highlights the unique contribution that neuroimaging research can make to understanding cognitive phenomena, when both behavioral and neuroimaging methods are combined.
The above interpretation is also consistent with the present finding of a negative correlation between the magnitude of the behavioral ratio effect and the neuronal (fMRI) ratio effect. In other words, children with a greater effect of ratio on brain activation were also those that had a comparatively small effect of ratio on response times and accuracy, suggesting that an increased neural ratio effect supports improved behavioral performance indexed by a smaller behavioral ratio effect. These findings suggest that the neural ratio effect in the left IPS is not simply a correlate of increased task difficulty and increased reaction time, but that the neural processing of symbolic numerical magnitude plays an important role in the relationship between basic number processing and arithmetic skills.
Interestingly, both the ROI and whole-brain analysis revealed that ratio-related activation in the left, but not right IPS is associated with individual differences in math achievement. These findings are consistent with a growing body of evidence suggesting that the left IPS is engaged during the processing of symbolic numerical magnitude (Ansari, 2007, Butterworth, 1999, Cohen Kadosh et al., 2010, Notebaert et al., 2011, Pesenti et al., 2000, Piazza et al., 2007). In contrast, the right IPS appears to be associated with the non-symbolic processing of numerical magnitude as well as non-numerical magnitude processing (Cohen Kadosh and Walsh, 2009, Notebaert et al., 2011, Piazza et al., 2007). The present findings extend this body of evidence by revealing that symbolic numerical magnitude processing in the left but not right IPS is related to individual differences in children's arithmetic competence.
It is important to point out that the ROI analysis also revealed significant relationships between the ratio effects in the right inferior frontal gyrus (IFG) and left superior frontal gyrus (SFG) and children's math fluency scores. However, in the present manuscript, we focus the discussion on the relationship between the ratio effect in the left intraparietal sulcus (IPS) and math achievement. We do so because both the region of interest and whole brain analyses did not demonstrate these regions to be significantly modulated by ratio. Moreover, only the ratio effect on the left IPS was correlated with both measures of children's arithmetic achievement, as well as the behavioral ratio effect on reaction times. Finally, none of the regions, that were modulated by ratio at the whole brain level, other than the left IPS, significantly correlated with math fluency.
Notwithstanding, we recognize that studies of numerical magnitude processing frequently reveal the involvement of other brain regions and that our findings are consistent with the notion that the IPS is part of a wider network of brain regions that mediates numerical magnitude processing. It is also important to acknowledge that working memory processes play an important role in arithmetic performance (Dumontheil and Klingberg, 2011); however, the current study focused on the relationship between symbolic numerical processing skills and arithmetic achievement. The numerical comparison task in the current study presented Arabic stimuli simultaneously on the screen, and both digits were visible during the comparison judgment; therefore, this task contained no working memory demands. Thus, it is unlikely that the relationship between symbolic magnitude processing skills and mathematical achievement is explained by working memory. However, given the findings presented by Dumontheil and Klingberg (2011), future research should investigate the relative contributions of activation of the IPS during working memory as well as basic number processing tasks to individual differences in children's math achievement. Such research will help to further our understanding of how the similarities and differences in the neural correlates of these two predictors of variability in children's math achievement.
The present findings provide a deeper characterization of the relationship between numerical magnitude processing and individual differences in children's math achievement, a relationship that has been consistently found in the behavioral literature (Bugden and Ansari, 2011, De Smedt et al., 2009, Holloway and Ansari, 2009). In addition, the results of the present study elucidate the neural and cognitive processes involved in driving this relationship. Consistent with previous findings (Notebaert et al., 2011, Piazza et al., 2007) and hypotheses (Ansari, 2007), these data suggest that the left IPS plays a critical role in the developmental scaffolding of number processing that is the consequence of enculturation, such as symbolic number processing and arithmetic. In contrast, the right IPS has been implicated in non-symbolic numerical magnitude processing (Piazza et al., 2004, Piazza et al., 2007) and is activated in early development (Cantlon et al., 2006, Hyde et al., 2010). Thus while the foundations of numerical magnitude processing may be supported by the right IPS, the left IPS is involved in the symbolic number representations and their use during mental arithmetic.
In summary, of three brain regions reliably associated with numerical processing in children, only the left IPS demonstrated a neural ratio effect. Brain-behavior correlations revealed that a larger ratio effect in the left IPS was associated with higher math achievement scores. This result was also substantiated by a whole-brain analysis. Future research is necessary in order to understand whether this relationship between numerical magnitude processing and tests of math achievement holds true in groups of atypically developing participants, such as children with dyscalculia.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by funding from the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant), an Operating Grant from the Canadian Institutes of Health Research (CIHR Operating Grant), an Infrastructure Grant from the Canada Foundation for Innovation and the Canada Research Chairs Program to DA.
Footnotes
Houdé et al. (2010) refer to this region as the Left Middle Occipital Gyrus, however, after detailed inspection of multiple stereotactic atlases, we believe that this region is more accurately characterized as the left IPS.
References
- Ansari D. Does the parietal cortex distinguish between 10, ten, and ten dots? Neuron. 2007;53:165–167. doi: 10.1016/j.neuron.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Ansari D., Dhital B. Age-related changes in the activation of the intraparietal sulcus during nonsymbolic magnitude processing: an event-related functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2006;18:1820–1828. doi: 10.1162/jocn.2006.18.11.1820. [DOI] [PubMed] [Google Scholar]
- Ansari D., Garcia N., Lucas E., Hamon K., Dhital B. Neural correlates of symbolic number processing in children and adults. Neuroreport. 2005;16:1769–1773. doi: 10.1097/01.wnr.0000183905.23396.f1. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Rosenberg-Lee M., Tenison C., Menon V. Weak task-related modulation and stimulus representations during arithmetic problem solving in children with developmental dyscalculia. Developmental Cognitive Neuroscience. 2012;2:S152–S166. doi: 10.1016/j.dcn.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken B.A. Ten psychometric reasons why similar tests produce dissimilar results. Journal of School Psychology. 1988;26:155–166. [Google Scholar]
- Bugden S., Ansari D. Individual differences in children's mathematical competence are related to the intentional but not automatic processing of Arabic numerals. Cognition. 2011;118:32–44. doi: 10.1016/j.cognition.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Butterworth B. Macmillan; London: 1999. The Mathematical Brain. [Google Scholar]
- Cantlon J.F., Brannon E.M., Carter E.J., Pelphrey K.A. Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biology. 2006;4:e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R.C., Walsh V. Numerical representation in the parietal lobes: abstract or not abstract? Behavioral and Brain Sciences. 2009;32:313–328. doi: 10.1017/S0140525X09990938. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R.C., Muggleton N., Silvanto J., Walsh V. Double dissociation of format-dependent and number-specific neurons in human parietal cortex. Cerebral Cortex. 2010;20:2166–2171. doi: 10.1093/cercor/bhp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt B., Verschaffel L., Ghesquiere P. The predictive value of numerical magnitude comparison for individual differences in mathematics achievement. Journal of Experimental Child Psychology. 2009;103:469–479. doi: 10.1016/j.jecp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr175. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Dowsett C.J., Claessens A., Magnuson K., Huston A.C., Klebanov P. School readiness and later achievement. Developmental Psychology. 2007;43:1428–1446. doi: 10.1037/0012-1649.43.6.1428. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Josephs O., Rees G., Turner R. Nonlinear event-related responses in fMRI. Magnetic Resonance in Medicine. 1998;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberda J., Mazzocco M.M., Feigenson L. Individual differences in non-verbal number acuity correlate with maths achievement. Nature. 2008;455:665–668. doi: 10.1038/nature07246. [DOI] [PubMed] [Google Scholar]
- Holloway I.D., Ansari D. Mapping numerical magnitudes onto symbols: the numerical distance effect and individual differences in children's mathematics achievement. Journal of Experimental Child Psychology. 2009;103:17–29. doi: 10.1016/j.jecp.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Houdé O., Rossi S., Lubin A., Joliot M. Mapping numerical processing, reading and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Developmental Science. 2010:1–10. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Hyde D.C., Boas D.A., Blair C., Carey S. Near-infrared spectroscopy shows right parietal specialization for number in pre-verbal infants. Neuroimage. 2010;53:647–652. doi: 10.1016/j.neuroimage.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman C.L. 2nd ed. Pearson; Minneapolis: 1997. Kaufman Brief Intelligence Test. [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S., Baker C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer R.S., Landauer T.K. Time required for judgements of numerical inequality. Nature. 1967;215:1519–1520. doi: 10.1038/2151519a0. [DOI] [PubMed] [Google Scholar]
- Mussolin C., De Volder A., Grandin C., Schlogel X., Nassogne M.C., Noel M.P. Neural correlates of symbolic number comparison in developmental dyscalculia. Journal of Cognitive Neuroscience. 2010;22:860–874. doi: 10.1162/jocn.2009.21237. [DOI] [PubMed] [Google Scholar]
- Notebaert K., Nelis S., Reynvoet B. The magnitude representation of small and large symbolic numbers in the left and right hemisphere: an event-related fMRI study. Journal of Cognitive Neuroscience. 2011;23:622–630. doi: 10.1162/jocn.2010.21445. [DOI] [PubMed] [Google Scholar]
- Pesenti M., Thioux M., Seron X., De Volder A. Neuroanatomical substrates of Arabic number processing, numerical comparison, and simple addition: a PET study. Journal of Cognitive Neuroscience. 2000;12:461–479. doi: 10.1162/089892900562273. [DOI] [PubMed] [Google Scholar]
- Piazza M., Izard V., Pinel P., Le Bihan D., Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Piazza M., Pinel P., Le Bihan D., Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53:293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Pinel P., Dehaene S., Riviere D., LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G.R., Holloway I., Rasanen P., Vesterinen M., Ansari D. Impaired parietal magnitude processing in developmental dyscalculia. Current Biology. 2007;17:R1042–R1043. doi: 10.1016/j.cub.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Romano E., Babchishin L., Pagani L.S., Kohen D. School readiness and later achievement: replication and extension using a nationwide Canadian survey. Developmental Psychology. 2010;46:995–1007. doi: 10.1037/a0018880. [DOI] [PubMed] [Google Scholar]
- Talairach P., Tournoux J. Thieme; Stuttgart, Germany: 1988. A Stereotactic Coplanar Atlas of the Human Brain. [Google Scholar]
- Vul E., Harris C., Winkielman P., Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Woodcock R.W., McGrew K.S., Mather N. Riverside Publishing; Itasca, IL: 2001. Woodcock-Johnson III Tests of Achievement. [Google Scholar]