Summary
Patients with HCV-related bridging fibrosis or cirrhosis remain at risk of developing life-threatening complications even after achieving a sustained virological response. Although it is reduced, the risk of liver-related events in these patients justifies their inclusion in surveillance programmes dedicated to the early detection of hepatocellular carcinoma and the screening for portal hypertension. Biochemical parameters or non-invasive tests might indicate the potential progression of liver injury despite viral clearance. Specific attention must be focused on the management of comorbidities, while dedicated educational programmes must be encouraged to increase compliance and commitment to surveillance. Better knowledge of the long-term evolution of these patients, who now live longer, is essential to improve risk stratification and refine screening strategies in this growing population.
Keywords: HCV, Hepatocellular carcinoma, Liver failure, Portal hypertension, surveillance, SVR
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; DAAs, direct-acting antivirals; EHC, extrahepatic cancer; FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; HR, hazard ratio; LSM, liver stiffness measurement; MACEs, major adverse cardiovascular events; PHT, portal hypertension; SMR, standardised mortality ratio; SVR, sustained virological response
Key points.
Following SVR, patients with HCV-related bridging fibrosis or cirrhosis must be maintained in surveillance programmes.
This surveillance is similar to that of other patients with extensive fibrosis and is dedicated to the early detection of HCC and the screening for portal hypertension.
The management of comorbidities is also pivotal and must involve dedicated physicians or general practitioners.
There is no recommendation to discontinue surveillance even if non-invasive tests suggest improvement.
Introduction
Antiviral treatments with direct-acting antivirals (DAAs) are associated with a sustained virological response (SVR) in the vast majority of patients infected with HCV, including those with bridging fibrosis or cirrhosis.1 In addition to curing HCV, the treatment shows benefit in terms of the regression of liver fibrosis, the risk of hepatocellular carcinoma (HCC), and the improvement of portal hypertension (PHT) and liver failure. Furthermore, beyond the hepatic outcomes, several extrahepatic benefits may result from sustained HCV eradication.2 The impact of SVR on chronic hepatitis caused by HCV differs widely according to the patient's baseline characteristics, including the presence of pre-therapeutic liver fibrosis and comorbidities. Despite HCV clearance, patients with bridging fibrosis or cirrhosis are still at risk of developing life-threatening conditions due to the persisting risk of liver-related complications and the high rates of comorbidities. Thus it is recommended to include these patients in dedicated surveillance programmes,3 and hepatologists must learn how to manage these individuals, who now survive longer than they did in the past, over the long term.4
This review focuses on the clinical benefits of SVR in patients with advanced pre-therapeutic F3 or F4 stage liver fibrosis according to the METAVIR scoring system (Figs. 1 and 2) and will explore proposed strategies for the surveillance of these patients following viral eradication (Fig. 3). The complex interplay between competing events, which is further influenced by host and environmental factors as well as treatment, highlights the need for the referral of large cohorts of patients with long-term prospective follow-up. Indeed, cirrhosis is a complex chronic disease that may lead to various events over the disease course. In this context, the following considerations will mostly be based on prospective large-scale studies that have examined cirrhosis according to a multi-state disease model5 that utilises accurate competing risk analyses to ensure both the quality and strength of the conclusions drawn.
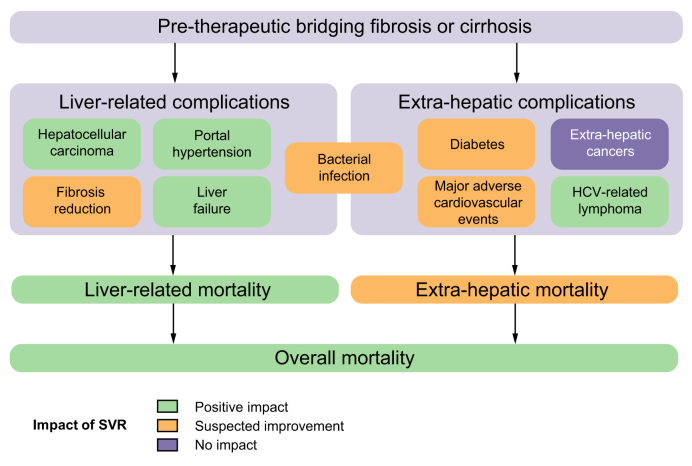
Fig. 1.
Impact of SVR on life-threatening events and overall/specific mortality in patients with HCV-related cirrhosis or bridging fibrosis. Positive impact: multiple and adequately powered longitudinal observational studies of cirrhotic patients with appropriate adjustment for confounders. Suspected improvement: findings supported by some observational studies but may be limited by insufficient follow-up time, incomplete adjustment for confounders, small sample size or the existence of contradictory reports. SVR, sustained virological response.
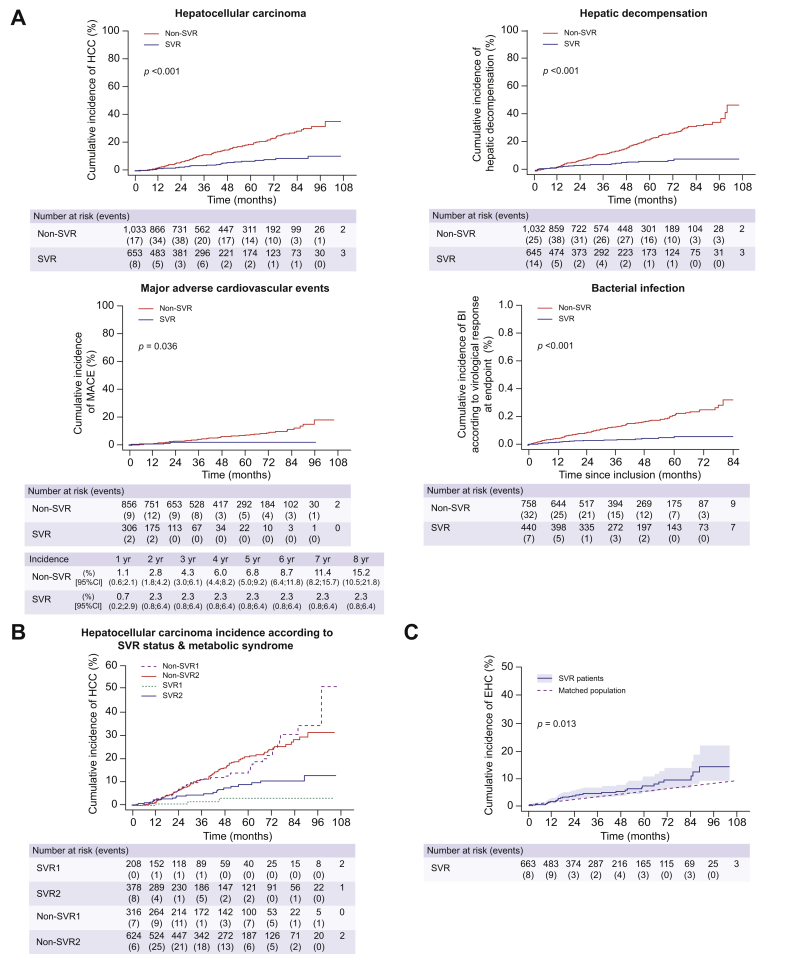
Fig. 2.
Incidences of liver-related and extrahepatic events as a function of SVR status in patients with compensated biopsy-proven cirrhosis included in the ANRS CO12 CirVir cohort. (A) Risks of hepatocellular carcinoma, liver decompensation, major adverse cardiovascular events and bacterial infection are decreased following SVR resulting from treatment with interferon- and DAA-based regimens. (B) Presence of metabolic syndrome (diabetes and/or overweight and/or dyslipidaemia) at the time of SVR is a major contributor to residual HCC risk in cirrhotic patients following SVR. SVR1: SVR and no metabolic syndrome; SVR2: SVR and metabolic syndrome; non-SVR1: no SVR and no metabolic syndrome; non-SVR2: no SVR and metabolic syndrome. (C) Increased incidence of EHCs in HCV-related cirrhosis patients with SVR compared with age-matched and sex-matched controls from the general French population. EHC was the leading cause of death in the CirVir cohort in patients who achieved SVR. Reproduced from Nahon et al.,14,57 Allaire et al.80 and Cacoub et al.75 with permission. BI, bacterial infection; DAA, direct-acting antiviral; EHCs, extrahepatic cancers; SVR, sustained virological response.
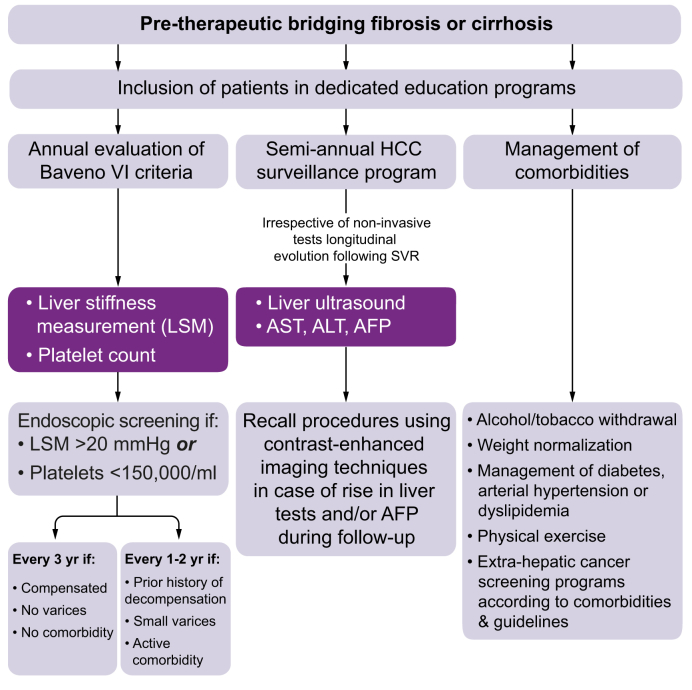
Fig. 3.
Pragmatic management of patients with bridging fibrosis or cirrhosis who achieved HCV eradication according to international guidelines (adapted from the available literature on SVR patients). AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LSM, liver stiffness measurement; SVR, sustained virological response.
Clinical benefits of SVR in patients with advanced liver fibrosis
Hepatocellular carcinoma
Following SVR, the risk of HCC is highest in patients with cirrhosis and is considered negligible in patients with mild or no fibrosis, for whom HCC surveillance is not recommended.6 However, HCC may occur in patients with bridging fibrosis (METAVIR F3)7 due to sampling variation in liver specimens, inaccurate evaluation by non-invasive tests, or the transition to cirrhosis after the F3 stage.8 Whether based on studies conducted in the interferon or DAA treatment eras, the absolute reduction in HCC risk is now well documented, but the probability of primary liver cancer still persists over the long term. In this scenario, all international guidelines endorse indefinite HCC surveillance following SVR in patients with cirrhosis diagnosed before the implementation of antiviral treatment and the development of SVR.6,9,10 The necessity for periodic surveillance of patients with pre-therapeutic bridging fibrosis (METAVIR F3) is more controversial; European guidelines recommend it6,10 while AASLD recommendations remain elusive.9 In addition, recent dedicated analyses suggest that this strategy may not be cost-effective in F3 patients due to the lower HCC incidence observed following SVR than in patients with pre-therapeutic documented cirrhosis.11
HCC incidence in patients with bridging fibrosis or cirrhosis and SVR
After treatment with interferon-based regimens
During the interferon treatment era, numerous studies provided convincing evidence that the risk of HCC decreased after SVR but remained high enough to justify periodic screening. Initially, based on retrospective monocentric studies, a meta-analysis of 6 studies, including 2,649 patients with advanced hepatic fibrosis or cirrhosis, initially reported a 4-fold decrease in the risk of HCC following SVR with a hazard ratio (HR) of 0.23 (95% CI 0.16–0.35).12 Following this report, data obtained from pooled retrospective European cohorts, during prospective follow-up over a median of 8 years, confirmed this benefit: in these 530 patients, the 10-year cumulative HCC incidence rate was 5.1% in patients who achieved an SVR compared with 21.8% in those who failed to clear the HCV infection.13 When restricted to patients unambiguously diagnosed with compensated cirrhosis via biopsy (n = 1,323, median follow-up 58 months), a prospective multicentre study reported a 5-year cumulative incidence of 6.7% (Fig. 2A),14 although the latter comprised a subset of patients treated with protease inhibitors.
After treatment with DAA-based regimens
Despite a lack of long-term follow-up, early studies conducted in the DAA treatment era reported “unexpectedly high” HCC incidence rates during and after treatment with DAA regimens in cirrhotic patients included in HCC surveillance programmes.[15], [16], [17] Indeed, HCC annual incidence ranged from 3% to 5% during or following DAA therapy in the initial retrospective single-centre studies, which included relatively short follow-up periods following DAA treatment. However, major methodological flaws resulted in these alarming studies, which were mostly based on small uncontrolled samples of patients usually presenting with a more severe phenotype (older age or more advanced cirrhosis) compared to that reported in the interferon era. Indeed, most of the first patients to receive these new drugs in the setting of early-access programmes had previously failed to achieve an SVR on interferon-based therapy, and some of them had already experienced liver failure progression or complications; in other words, they were more prone to develop HCC. However, it has been hypothesised that a rapid reduction in HCV viral load under DAAs may promote an imbalance between pro-tumour and antitumour immune functions,18 and DAA therapy has been associated with increased inflammation19 and angiogenesis.20 Nevertheless, these preliminary reports have not been confirmed. Furthermore, it has been shown that for some of the candidates initially selected, prior HCC surveillance had probably been suboptimal and failed to ensure the adequate detection of an ongoing oncologic process that was perhaps already developing when DAA treatment was started.21,22
The subsequent analyses of numerous large longitudinal cohorts with longer follow-up have demonstrated that DAA-induced SVR is associated with a reduced HCC risk. Initially, the first available multicentre reports were restricted to the retrospective analyses of registries from the Veterans Affairs system, which estimated HCC occurrence in 21,984 patients (all fibrosis stages) treated with DAA-based regimens over a 2-year follow-up period according to the achievement of SVR and reported a 71% lower risk of HCC in patients with viral clearance.[23], [24], [25] This finding has since been prospectively confirmed by an analysis of 9,895 French patients included in the ANRS CO22 Hepather cohort,26 among whom 7,344 were treated with DAAs and followed-up for 33.4 months. After adjustment for various confounders, DAA treatment was associated with decreased HCC risk (adjusted HR 0.66; 95% CI 0.46–0.93). Based on longitudinal cohorts recruited in hepatology units, a recent study performed in Europe reported the annual incidence of HCC as a function of liver function impairment in 2,249 cirrhotic patients following DAA implementation. These analyses confirmed a higher annual incidence of HCC in patients with Child-Pugh Class B than Class A cirrhosis (6.6% vs. 2.1%, respectively).27
Comparisons between therapeutic eras
Several longitudinal cohorts of patients included in HCC surveillance programmes during the interferon or DAA treatment eras were compared in terms of HCC incidence following SVR. For example, meta-regression was performed for a meta-analysis of 9 observational studies of DAA- or interferon-treated patients.28 Although adjustment was restricted to age and the length of follow-up, these analyses reported similar incidences in the 2 groups. Subsequent large-scale studies confirmed this finding. In the Veterans Affairs system, after adjustment for 21 potential confounders among 21,984 patients treated with DAAs and 35,871 patients treated with IFN-based regimens, similar HCC incidences were reported as a function of treatment allocation,[23], [24], [25] leading to a comparable decrease in HCC risk regardless of regimen.29 Finally, robust data were reported by large prospective cohorts of patients followed-up in multiple tertiary hepatology centres and included in HCC surveillance programmes such as the ANRS CO12 CirVir cohort.4 In addition to the rigorous selection of patients with biopsy-proven compensated cirrhosis, the methodical and exhaustive recording of all complications and clinical events occurring during follow-up, liver-related or not, allowed for analyses that accounted for the differing characteristics of patients according to treatment allocation, using the inverse probability of treatment weights method.22
As a whole, the magnitude of the decrease in HCC risk was similar regardless of the antiviral regimen used. However, due to the relatively short follow-up of patients who received DAAs after this treatment was made available, the interpretation of findings based on medium- and long-term follow-up should be undertaken cautiously.
Fibrosis regression and long-term HCC risk
The available data suggest that HCC risk does not decrease with time. Ageing usually triggers the development of various comorbidities known to impact liver-related outcomes, including liver cancer.30 Studies conducted in Japan during the interferon treatment era have reported cumulative incidences of HCC as high as 3.1%, 10.1%, and 15.9% after 5, 10, and 15 years, respectively.31 Similar observations were made in patients with cirrhosis in the West, for whom longitudinal follow-up revealed a 1.39% yearly HCC incidence following SVR in the long term, particularly when SVR was achieved after 55 years of age.32
On the other hand, it is tempting to speculate that HCV eradication might favour fibrosis regression over time and lead to a subsequent decrease in HCC risk.33 Interferon-induced SVR has been shown to decrease the incidence of liver-related outcomes over the long term, which may in part be explained by partial fibrosis regression.8,13,14,34,35 However, studies utilising sequential liver histological examination performed before and after SVR are scarce36 and are usually limited by their retrospective monocentric nature and small sample sizes.37 Fibrosis development following SVR is usually evaluated by non-invasive methods such as transient elastography or the detection of circulating biomarkers.34 Nevertheless, several pitfalls plague the interpretation of these sequential measurements, including i) the need to wait for at least 2–3 years following SVR to avoid misinterpretation,38 ii) the difficulty in correctly interpreting the changes in non-invasive markers.39,40 Most studies published so far have failed to document a decrease in HCC occurrence in patients with “improving” non-invasive “fibrosis” post-SVR.39,41
Recently, the longitudinal assessment of fibrosis-4 score (FIB-4)/aspartate aminotransferase-to-platelet ratio index (APRI) following SVR was performed in more than 6,000 patients from the Veterans Affairs system with pre-therapeutic advanced fibrosis or cirrhosis treated by DAAs.42 Cirrhotic patients who had persistently high FIB-4/APRI during follow-up had the highest HCC incidence (between 3.3 and 6.5 per 100 person-years [PY]), while the risk of HCC decreased in those who experienced a decline in FIB-4/APRI over time (0.6 to 2.8 per 100 PY) but remained above 1.5 per 100 PY for most quarters, thus justifying periodical screening in the latter.
Until further notice, lifelong surveillance for HCC is recommended in patients with documented advanced fibrosis and cirrhosis before SVR, as it seems unlikely that the risk of liver cancer would eventually decline over time to a point at which surveillance becomes unnecessary.
Identification of cirrhotic patients with a higher residual HCC risk following SVR
The goal of HCC surveillance programmes is to detect liver tumours at an early stage when they are eligible for curative therapy, which is known to provide a survival benefit.43 Although abdominal ultrasound is recommended as a standard procedure for HCC surveillance, its sensitivity for the detection of small-size HCC tumours is low.44 Given these concerns, alternative imaging modalities (such as CT or MRI45) and new serum biomarkers46 have been tested, with the aim of improving early HCC detection. However, implementing such costly surveillance programmes may not be cost-effective for all patients eligible for screening, in particular F3/F4 patients who have achieved SVR.11
However, all patients with bridging fibrosis or cirrhosis following SVR do not have the same risk of developing HCC, and it remains difficult to assess the specific risk at an individual level.47 In this scenario, personalised assessment of the individual risk of HCC and reinforcement of screening policies might be utilised for subgroups of patients with a persistent high incidence of liver cancer despite HCV eradication. Until now, various HCC scoring systems have been designed, based on the combination of routine clinical features, to stratify patients into various HCC risk classes, which in most cases do not consider SVR status.48 This field of research highlighted a specific phenotype of patients who are at a higher risk of liver cancer development based on various covariates, including higher rates of comorbidities (in particular metabolic syndrome, Fig. 2B), persistent circulating necroinflammatory markers or impaired liver function despite SVR.14,30,49 It is tempting to speculate that adapting screening strategies to the stratification of patients in groups at a low or high risk of HCC following SVR might optimise both cost-effectiveness and the allocation of limited medical resources.50
Portal hypertension
Evidence from the interferon and DAA treatment eras has revealed that there have been significant decreases in PHT51,52 and life-threatening related events following SVR.14 In this context, the probability of developing de novo endoscopic PHT is low, even over the long term.53 Furthermore, the risk of variceal bleeding appears to be particularly low in these patients, even in those who have pre-existing endoscopic signs of PHT.54 As a whole, compensated cirrhotic patients without a past history of varices and without co-existing causes of liver injury following SVR (in particular metabolic syndrome or excessive alcohol consumption) may not require regular endoscopic follow-up.
In recent years, the guidelines of the Baveno VI Consensus Workshop55 have endorsed risk stratification based on a transient elastography cut-off of <20 kPa and a platelet count >150,000/ml to identify patients for whom screening for varices might be unnecessary because of the low risk of clinically significant endoscopic PHT. However, the extent to which such a recommendation holds true once the aetiological agent has been cured had not been adequately assessed at the time of this workshop. Recently, a dedicated analysis conducted in the ANRS CO12 CirVir cohort established the validity of these non-invasive indices in patients with compensated viral cirrhosis who achieved SVR.56 Based on the sequential evaluation of the Baveno VI criteria, endoscopic surveillance no longer appears to be necessary in this subgroup of low-risk patients, in whom PHT progression is a rare event and 1-year overall survival is higher than 90%. These are important messages for both patients and physicians in clinical practice, as reducing unnecessary endoscopies in selected patients will enable the restriction of efficient screening to those who require close surveillance.
Liver failure
SVR is a major milestone in the management of cirrhosis, but the magnitude of the benefit widely differs according to baseline liver function impairment.
Patients with baseline compensated cirrhosis
The clearance of HCV clearly improves outcomes in patients with compensated cirrhosis, as extensively demonstrated by the ANRS CO12 CirVir prospective cohort (Fig. 2A).14 Among 1,323 patients with biopsy-proven uncomplicated HCV-related Child-Pugh Class A cirrhosis who were followed-up for almost 5 years, a dramatic decrease in hepatic decompensation was observed along with a significantly reduced 5-year cumulative incidence in patients who achieved SVR (HR 0.26; 95% CI 0.17–0.39).14 A dramatic reduction in bacterial infection, which is known to trigger hepatic decompensation, was also reported in this population over the long term in patients who achieved SVR (Fig. 2A).57 These observations were, however, obtained from patients treated with either interferon-based therapies or DAAs, and the specific evaluation of the effect of the latter regimen on the risk of decompensation, which tends to occur over the long term, was hampered by the short-term follow-up of patients. In this context, although a study of the ANRS CO22 Hepather cohort reported a decrease in liver-related mortality in more than 3,000 patients with extensive fibrosis, this large-scale study failed to demonstrate lower rates of decompensation in patients exposed to DAAs (HR 0.95; 95% CI, 0.48–1.89) after adjustment for numerous variables including various comorbidities (alcohol consumption, diabetes, and arterial hypertension).26
Patients with baseline decompensated cirrhosis
In patients with baseline decompensated cirrhosis, unlike interferon-based regimens, non-protease inhibitor containing DAAs were shown to be safe and effective therapies that resulted in an SVR rate of at least 78% in those with the most pronounced liver failure.[58], [59], [60] However, despite SVR, the outcomes of patients with decompensated cirrhosis due to HCV remain problematic, as clinical deterioration and the need for liver transplantation may still arise.
The benefits of the achievement of SVR due to DAA treatment in patients with Child-Pugh Class B cirrhosis were demonstrated in at least 3 longitudinal studies showing short-term improvements in liver function. In a retrospective UK study of 409 patients, among whom 381 achieved SVR, treatment with DAAs led to a significant decrease in model for end-stage liver disease (MELD) scores (mean change −0.85) within 6 months compared to untreated patients, for whom the MELD scores increased (mean +0.75, p <0.0001);61 patients with a baseline serum albumin level <35 g/L, a low serum sodium level (<135 mmol/L) and aged >65 years were the least likely to benefit from DAAs.62 In a prospective Italian multicentre HCV cohort (LINA cohort), 89 patients who received treatment with DAAs showed a significant increase in the rate of re-compensation (62% switched to Child-Pugh Class A) between baseline and 12 weeks after the end of treatment.63 At 24 weeks post-SVR, the MELD score decreased from baseline by at least 1 point in 61% of patients. Finally, in the ASTRAL-4 randomised controlled trial, among 250 patients treated with sofosbuvir/velpatasvir, 47% and 51% of them showed improvement compared to baseline at post-treatment week 12 according to the Child-Pugh score and the MELD score, respectively. The MELD score improved in 81% of patients with a baseline MELD score of 15 or more.58 In more advanced cirrhosis (Child-Pugh Class C, MELD ≥20), a “point-of-no-return” for recovery in hepatic function could be reached.64 In the absence of validated predictors of liver failure recovery or transplant avoidance, decompensated patients require close monitoring after achieving SVR. Those with a decreased MELD score below 15 may be removed from the liver transplant list but will be warned about their risk of liver-related complications.
Mortality, competing risks of death and extrahepatic events
Given the positive impact of viral clearance on hepatic events, it is not surprising to observe a decrease in liver-related mortality and, as a consequence, overall mortality. The extent to which SVR also decreases extrahepatic mortality will necessitate a longer follow-up and dedicated analysis, as liver-related and non-liver-related events influence survival in a competing risk framework.5 Nevertheless, it is tempting to speculate that unlike cirrhotic patients with active HCV replication, who predominantly die from liver-related complications or the progression of disease, patients with extrahepatic conditions and an accumulation of comorbidities following SVR will constitute a growing burden over time.
SVR has been associated with improved survival in patients with bridging fibrosis/cirrhosis who achieved SVR after treatment with both interferon regimens13,65 and DAAs.14,26,66 The Veterans Affairs system recently reported a 74% reduction in the risk of death in patients who achieved SVR compared to those who did not achieve SVR after multiple adjustments.66 In addition, large prospective studies have confirmed a reduction in mortality in patients with HCV-related cirrhosis. In both the French ANRS CO12 CirVir and ANRS CO22 Hepather cohorts,14,26 a decrease in all-cause mortality (HR 0.27; 95% CI 0.18–0.42 in the CirVir cohort; adjusted HR 0.48; 95% CI 0.33–0.70 in the Hepather cohort) was confirmed after adjustment for multiple confounders (age, gender, body mass index, geographical origin, infection route, fibrosis score, HCV treatment-naïve status, HCV genotype, alcohol consumption, diabetes, arterial hypertension, biological variables, and MELD score in patients with cirrhosis). Exposure to DAAs also impacted on non-liver-related mortality in the ANRS C022 Hepather cohort (HR 0.40; 95% CI 0.19–0.83).26
Diabetes
Several studies conducted in the interferon treatment era revealed an improvement in insulin resistance following HCV eradication.67,68 As a consequence, a reduction in the risk of type 2 diabetes was also reported.67 Similarly, exposure to DAAs seems to improve glycaemic control in patients with diabetes.69 Diabetes is one of the main clinical predictors of HCC in cirrhotic patients following SVR (Fig. 2C).14 Dedicated studies are needed to accurately assess the effects of SVR on the incidence of diabetes in patients and in particular to determine if better control of this condition is able to reduce the residual risk of HCC.
Cardiovascular events
HCV infection has been linked to numerous cardiovascular diseases.70,71 Even though the underlying mechanisms are not completely understood, the positive impact of viral eradication on cardiovascular diseases has been reported in various clinical settings.[72], [73], [74] More recently, in dedicated analyses performed in cirrhotic patients with a long prospective follow-up, SVR significantly and independently reduced the occurrence of major adverse cardiovascular events (MACEs) (HR 0.42; 95% CI 0.25–0.69).75 This effect was reported in patients treated with both interferon and DAA and was associated with improved outcomes, as cirrhotic patients who had experienced MACEs during follow-up had decreased survival (Fig. 2C).
Extrahepatic cancers
Extrahepatic cancers (EHCs) are frequent in cirrhotic patients with HCV infection. EHCs can be linked to the virus (lymphomas) or to high rates of comorbidities (excessive alcohol or tobacco consumption, metabolic syndrome) usually observed in these populations.76 Numerous studies have shown a decrease in the incidence of lymphomas following HCV eradication.77,78 In a recent study focusing on patients who achieved SVR and who were mostly free of cirrhosis, EHC was the second most frequent cause of death after complications associated with injected drugs.79 Recently, an increased age-adjusted incidence of EHC was reported in French patients with HCV cirrhosis compared with that in the general population (standardised mortality ratio [SMR] 1.31; 95% CI 1.04–1.64).80 This increase was even greater in those who had previously achieved SVR (SMR 1.57; 95% CI 1.08–2.22) (Fig. 2D). A diagnosis was made in younger patients, and EHC was the leading cause of mortality in cirrhotic patients who achieved an SVR, as a consequence of the competing risks of death highlighted by the benefits of SVR on liver-related complications. In these patients, past excessive alcohol intake and ongoing tobacco consumption were associated with oral cancer, while diabetes mellitus was associated with the occurrence of all EHCs following HCV clearance.
Surveillance of patients with pre-therapeutic advanced liver fibrosis or cirrhosis
The available guidelines do not provide specific recommendations for the management of cirrhotic patients who achieve SVR. The adaptation of surveillance modalities will be enhanced by the growth of this population and will require a longer follow-up or complete knowledge of the course of disease in the long term. Until then, we propose a pragmatic approach based on the available data and knowledge (Fig. 3), although limited, which may guide physicians in ensuring a safe and reasonable follow-up of their patients, based on the most frequent experiences they may face in routine practice. More importantly, improving patient education through intervention by trained personnel in a dedicated setting is pivotal. The use of modern tools, including websites, educational videos, and smartphone applications, must be encouraged. The involvement of patients in the decision-making process is the cornerstone of the improvement of compliance and surveillance, both of which have been shown to increase survival.81,82 Finally, the implication of primary care providers could also strengthen compliance to surveillance programmes when simple and succinct guidelines become available.
HCC surveillance
Is HCC surveillance recommended for all patients with chronic advanced liver disease who achieve SVR?
All guidelines endorse the surveillance of patients with cirrhosis, regardless of the cause or its eventual treatment.6,9,10 The situation for patients with F3 fibrosis is more complex, as recommendations differ according to the guidelines. The AASLD HCV guidance statement recommends HCC surveillance in F3 stage patients and those who have achieved SVR,83 while the AASLD HCC guidance statement does not specifically and clearly address the issue of F3 patients.9 The EASL and ESMO HCC guidelines recommend the surveillance of all patients with F3 fibrosis,6,10 irrespective of the cause, and do not specifically address the issue of patients who have achieved HCV clearance. This issue is difficult to address because the evaluation of extensive fibrosis may not be reliable in numerous cases, leading to a high risk of misclassification or the underreporting of cirrhosis. Furthermore, despite viral clearance, patients with comorbidities are predisposed to ultimately develop cirrhosis following SVR, a fact which is highlighted by the longitudinal assessment of non-invasive tests in these patients.42 Overall, the difference between advanced fibrosis and cirrhosis is very small, and failing to recognise the transition between these 2 stages leads to the exclusion of some patients from surveillance programmes. For these reasons, a pragmatic suggestion is to maintain all F3 patients in HCC surveillance programmes until further scientific evidence is able to accurately define which subgroups of patients are unlikely to progress towards cirrhosis and/or HCC.
Should surveillance modalities differ from other causes of liver diseases?
While there is no reason to change surveillance by means of the recommended biannual ultrasound screening following SVR, the case for biological surveillance might be considered, as alpha-fetoprotein (AFP) assessment is not endorsed by non-Asian guidelines.6,9 Biochemical parameters (in particular the levels of aminotransferases and AFP in serum) are expected to rapidly decline and normalise in the majority of cirrhotic patients following SVR. However, these parameters must be monitored, as their increase might not only reflect the development of liver injury related to a comorbidity but also the emergence of an HCC.84 Indeed, it was shown via the assessment of these parameters that post-treatment alanine aminotransferase and AFP levels were fairly well correlated with the risk of developing HCC in patients who achieved SVR. Given the aforementioned poor sensitivity of ultrasound for the detection of small-sized focal lesions, the monitoring of biochemical parameters and AFP levels might be informative in cirrhotic patients who have achieved SVR, for whom the virally induced necrotic-inflammatory process in the liver is expected to be suppressed; an increase in these parameters could indeed rapidly trigger evaluation using contrast-enhanced imaging techniques to improve early HCC diagnosis outside of ultrasound examination.
Are non-invasive tests useful to decide who no longer requires surveillance?
Although the regression of cirrhosis is possible, the proportion of patients who will experience such improvement is not known. In fact, it is tempting to speculate that most will not experience fibrosis regression at all, particularly those who are affected by comorbidities. Nevertheless, as has already been shown, large-scale longitudinal studies of non-invasive evaluations are currently increasing in number and suggest that even in cases of the regression of these parameters, the risk of HCC remains high enough to justify surveillance.42 In this case, unless contradictory reports emerge, the inclusion of patients with advanced fibrosis or cirrhosis in HCC surveillance programmes is expected to involve a lifelong commitment between the patient and the practitioner.
Assessment of portal hypertension
Are non-invasive tests useful to decide who no longer requires surveillance following SVR?
The Baveno VI criteria have been shown to be applicable to cirrhotic patients who have achieved SVR.56 The measurement of liver stiffness and platelet count should thus be performed once a year to avoid unnecessary endoscopic surveillance in these patients. The use of expanded Baveno VI criteria (platelet count >110,000/ml and liver stiffness measurement <25 kPa) is still under evaluation and has not yet been validated in patients who have achieved SVR.85
Which interval of surveillance should be used in the case of positive Baveno VI criteria?
The interval depends on the risk of the progression of PHT. In patients who have achieved SVR, the probability will strongly depend upon i) the baseline level of PHT and ii) the existence of comorbidities, particularly excessive alcohol consumption and metabolic syndrome. Because of this, patients with small varices, a prior history of liver decompensation or who present with the potential persistence of liver injury despite achieving SVR should be included in 1- to 2-year surveillance programmes. In all other cases, a 3-year follow-up is recommended.86
Management of comorbidities
Despite SVR achievement, some patients will have persistent liver inflammation and fibrosis progression, leading to an increased risk of critical hepatic events. The main environmental factors associated with deleterious outcomes after SVR include alcohol intake, diabetes, obesity, and viral coinfection with hepatitis B and/or hepatitis D and/or HIV due to shared routes of contamination. These comorbidities also impact on the occurrence of extrahepatic life-threatening events, such as cardiovascular disease and EHCs, in these patients, who are expected to live longer in the era of global HCV eradication. In this setting, the management of patients with SVR requires the careful evaluation of comorbidities and extensive counselling regarding alcohol minimisation, the optimisation of weight and the features of metabolic syndrome. Dedicated surveillance of cardiovascular and/or EHC risks should also be implemented as recommended and in cooperation with specialised physicians and/or general practitioners.
The impact of ongoing alcohol consumption on “all-stages of fibrosis” in HCV-related chronic hepatitis, as defined before the DAA era, is controversial.87,88 In patients with advanced liver fibrosis, alcohol increases the risk of liver events. In a prospective study conducted in 74 patients with HCV-related cirrhosis, light to moderate alcohol intake and a lack of viral eradication were associated with the risk of HCC (HR for alcohol consumption: 3.43; 95% CI 1.49–7.92; p = 0.004). The lowest risk of HCC was observed for patients without alcohol intake and with viral eradication (0%), followed by patients with alcohol intake and viral eradication (6.2%; 95% CI 0–18.4), patients without alcohol intake and no viral eradication (15.9%; 95% CI 7.1–24.7), and patients with alcohol intake and no viral eradication (29.2%; 95% CI 16.5–41.9).89 In contrast, the impact of ongoing alcohol consumption seemed to be minimal in the ANRS CO12 CirVir cohort because most patients with previous high alcohol intake stopped drinking or drank only a limited amount of alcohol during follow-up evaluation. However, past excessive alcohol intake was an independent predictive factor of HCC occurrence in patients with HCV-related cirrhosis enrolled in this cohort.90 A “safe” threshold for alcohol intake has not been determined in patients with advanced liver fibrosis, and minimal consumption should be advised.
Non-alcoholic steatohepatitis may be present before SVR or develop de novo after SVR, leading to a worsening of liver disease. Metabolic disease has been shown to be the main comorbidity influencing the natural history of liver disease after SVR in patients with HCV-related cirrhosis, conferring a significantly higher risk of HCC in affected patients.14 Cirrhotic patients should be advised to maintain weight as close to a normal value as possible91 and regularly engage in physical exercise as recommended.92
Conclusion
The management of patients with SVR and pre-therapeutic advanced chronic liver disease is a new challenge for hepatologists. As this population will continue to increase in size over time, our knowledge of long-term outcomes will also increase, and new challenges will emerge for our community as these patients will live longer. Future areas for research are extensive and include risk stratification and the refinement of surveillance strategies to optimise the allocation of medical resources.
Financial support
The authors received no financial support to produce this manuscript.
Conflicts of interest
Prof. Nahon has received honoraria/grants from AbbVie, AstraZeneca, Bayer, Bristol-Myers Squibb, Gilead and Ipsen. Prof. Ganne-Carrié has received honoraria from AbbVie, Bayer, Bristol-Myers Squibb, and Gilead.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors' contributions
Both authors contributed equally to the production of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2019.11.001.
Supplementary data
References
- 1.Pawlotsky J.-M., Negro F., Aghemo A., Berenguer M., Dalgard O., Dusheiko G. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Ioannou G.N., Feld J.J. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis C virus infection? Gastroenterology. 2019;156:446–460.e2. doi: 10.1053/j.gastro.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Terrault N.A., Hassanein T.I. Management of the patient with SVR. J Hepatol. 2016;65:S120–S129. doi: 10.1016/j.jhep.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Trinchet J.C., Bourcier V., Chaffaut C., Ait Ahmed M., Allam S., Marcellin P. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort) Hepatology. 2015;62:737–750. doi: 10.1002/hep.27743. [DOI] [PubMed] [Google Scholar]
- 5.Jepsen P., Vilstrup H., Andersen P.K. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology. 2015;62:292–302. doi: 10.1002/hep.27598. [DOI] [PubMed] [Google Scholar]
- 6.EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Lok A.S., Seeff L.B., Morgan T.R., di Bisceglie A.M., Sterling R.K., Curto T.M. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan T.R., Ghany M.G., Kim H.Y., Snow K.K., Shiffman M.L., De Santo J.L. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 10.Vogel A., Cervantes A., Chau I., Daniele B., Llovet J.M., Meyer T. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:871–873. doi: 10.1093/annonc/mdy510. [DOI] [PubMed] [Google Scholar]
- 11.Farhang Zangneh H., Wong W.W.L., Sander B., Bell C.M., Mumtaz K., Kowgier M. Cost effectiveness of hepatocellular carcinoma surveillance after a sustained virologic response to therapy in patients with hepatitis C virus infection and advanced fibrosis. Clin Gastroenterol Hepatol. 2019;17:1840–1849.e16. doi: 10.1016/j.cgh.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Morgan R.L., Baack B., Smith B.D., Yartel A., Pitasi M., Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Annals of internal medicine. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 13.van der Meer A.J., Veldt B.J., Feld J.J., Wedemeyer H., Dufour J.F., Lammert F. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 14.Nahon P., Bourcier V., Layese R., Audureau E., Cagnot C., Marcellin P. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017;152:142–156.e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Cheung M.C.M., Walker A.J., Hudson B.E., Verma S., McLauchlan J., Mutimer D.J. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Conti F., Buonfiglioli F., Scuteri A., Crespi C., Bolondi L., Caraceni P. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Ravi S., Axley P., Jones D., Kodali S., Simpson H., McGuire B.M. Unusually high rates of hepatocellular carcinoma after treatment with direct-acting antiviral therapy for hepatitis C related cirrhosis. Gastroenterology. 2017;152:911–912. doi: 10.1053/j.gastro.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Serti E., Park H., Keane M., O'Keefe A.C., Rivera E., Liang T.J. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα. Gut. 2017;66:724–735. doi: 10.1136/gutjnl-2015-310033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debes J.D., van Tilborg M., Groothuismink Z.M.A., Hansen B.E., Schulze Zur Wiesch J., von Felden J. Levels of cytokines in serum associate with development of hepatocellular carcinoma in patients with HCV infection treated with direct-acting antivirals. Gastroenterology. 2018;154:515–517.e13. doi: 10.1053/j.gastro.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Faillaci F., Marzi L., Critelli R., Milosa F., Schepis F., Turola E. Liver angiopoietin-2 is a key predictor of de novo or recurrent hepatocellular cancer after hepatitis C virus direct-acting antivirals. Hepatology. 2018;68:1010–1024. doi: 10.1002/hep.29911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marino Z., Darnell A., Lens S., Sapena V., Diaz A., Belmonte E. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: relevance of non-characterized nodules. J Hepatol. 2019;70:874–884. doi: 10.1016/j.jhep.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Nahon P., Layese R., Bourcier V., Cagnot C., Marcellin P., Guyader D. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155:1436–1450.e6. doi: 10.1053/j.gastro.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Innes H., Barclay S.T., Hayes P.C., Fraser A., Dillon J.F., Stanley A. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68:646–654. doi: 10.1016/j.jhep.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Ioannou G.N., Green P.K., Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanwal F., Kramer J., Asch S.M., Chayanupatkul M., Cao Y., El-Serag H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005.e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Carrat F., Fontaine H., Dorival C., Simony M., Diallo A., Hezode C. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453–1464. doi: 10.1016/S0140-6736(18)32111-1. [DOI] [PubMed] [Google Scholar]
- 27.Calvaruso V., Cabibbo G., Cacciola I., Petta S., Madonia S., Bellia A. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155:411–421.e4. doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Waziry R., Hajarizadeh B., Grebely J., Amin J., Law M., Danta M. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Li D.K., Ren Y., Fierer D.S., Rutledge S., Shaikh O.S., Lo Re V., 3rd The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: an ERCHIVES study. Hepatology. 2018;67:2244–2253. doi: 10.1002/hep.29707. [DOI] [PubMed] [Google Scholar]
- 30.van der Meer A.J., Feld J.J., Hofer H., Almasio P.L., Calvaruso V., Fernandez-Rodriguez C.M. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485–493. doi: 10.1016/j.jhep.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita N., Ohho A., Yamasaki A., Kurokawa M., Kotoh K., Kajiwara E. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: significance of lifelong periodic cancer screening for improving outcomes. J Gastroenterol. 2014;49:1504–1513. doi: 10.1007/s00535-013-0921-z. [DOI] [PubMed] [Google Scholar]
- 32.El-Serag H.B., Kanwal F., Richardson P., Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George S.L., Bacon B.R., Brunt E.M., Mihindukulasuriya K.L., Hoffmann J., Di Bisceglie A.M. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729–738. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhtar E., Manne V., Saab S. Cirrhosis regression in hepatitis C patients with sustained virological response after antiviral therapy: a meta-analysis. Liver Int. 2015;35:30–36. doi: 10.1111/liv.12576. [DOI] [PubMed] [Google Scholar]
- 35.Poynard T., McHutchison J., Manns M., Trepo C., Lindsay K., Goodman Z. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 36.Tachi Y., Hirai T., Miyata A., Ohara K., Iida T., Ishizu Y. Progressive fibrosis significantly correlates with hepatocellular carcinoma in patients with a sustained virological response. Hepatol Res. 2015;45:238–246. doi: 10.1111/hepr.12331. [DOI] [PubMed] [Google Scholar]
- 37.Mallet V., Gilgenkrantz H., Serpaggi J., Verkarre V., Vallet-Pichard A., Fontaine H. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403. doi: 10.7326/0003-4819-149-6-200809160-00006. [DOI] [PubMed] [Google Scholar]
- 38.Bachofner J.A., Valli P.V., Kroger A., Bergamin I., Kunzler P., Baserga A. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369–376. doi: 10.1111/liv.13256. [DOI] [PubMed] [Google Scholar]
- 39.D'Ambrosio R., Aghemo A., Rumi M.G., Degasperi E., Sangiovanni A., Maggioni M. Persistence of hepatocellular carcinoma risk in hepatitis C patients with a response to IFN and cirrhosis regression. Liver Int. 2018;38:1459–1467. doi: 10.1111/liv.13707. [DOI] [PubMed] [Google Scholar]
- 40.Sultanik P., Kramer L., Soudan D., Bouam S., Meritet J.F., Vallet-Pichard A. The relationship between liver stiffness measurement and outcome in patients with chronic hepatitis C and cirrhosis: a retrospective longitudinal hospital study. Aliment Pharmacol Ther. 2016;44:505–513. doi: 10.1111/apt.13722. [DOI] [PubMed] [Google Scholar]
- 41.Wang J.H., Yen Y.H., Yao C.C., Hung C.H., Chen C.H., Hu T.H. Liver stiffness-based score in hepatoma risk assessment for chronic hepatitis C patients after successful antiviral therapy. Liver Int. 2016;36:1793–1799. doi: 10.1111/liv.13179. [DOI] [PubMed] [Google Scholar]
- 42.Kanwal F., Kramer J.R., Asch S.M., Cao Y., Li L., El-Serag H.B. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology. 2019 doi: 10.1002/hep.30823. [DOI] [PubMed] [Google Scholar]
- 43.Prasad V., Lenzer J., Newman D.H. Why cancer screening has never been shown to “save lives”–and what we can do about it. BMJ. 2016;352:h6080. doi: 10.1136/bmj.h6080. [DOI] [PubMed] [Google Scholar]
- 44.Tzartzeva K., Obi J., Rich N.E., Parikh N.D., Marrero J.A., Yopp A. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S.Y., An J., Lim Y.S., Han S., Lee J.Y., Byun J.H. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA oncology. 2017;3:456–463. doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berhane S., Toyoda H., Tada T., Kumada T., Kagebayashi C., Satomura S. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 2016;14:875–886.e6. doi: 10.1016/j.cgh.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 47.Nahon P., Zucman-Rossi J. Single nucleotide polymorphisms and risk of hepatocellular carcinoma in cirrhosis. J Hepatol. 2012;57:663–674. doi: 10.1016/j.jhep.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 48.Sherman M. HCC risk scores: useful or not? Semin Liver Dis. 2017;37:287–295. doi: 10.1055/s-0037-1607452. [DOI] [PubMed] [Google Scholar]
- 49.Ioannou G.N., Green P.K., Beste L.A., Mun E.J., Kerr K.F., Berry K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018;69:1088–1098. doi: 10.1016/j.jhep.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goossens N., Singal A.G., King L.Y., Andersson K.L., Fuchs B.C., Besa C. Cost-effectiveness of risk score-stratified hepatocellular carcinoma screening in patients with cirrhosis. Clin Transl Gastroenterol. 2017;8:e101. doi: 10.1038/ctg.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandorfer M., Kozbial K., Schwabl P., Freissmuth C., Schwarzer R., Stern R. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692–699. doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Lens S., Alvarado-Tapias E., Marino Z., Londono M.C., LLop E., Martinez J. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017;153:1273–1283.e1. doi: 10.1053/j.gastro.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Bruno S., Crosignani A., Facciotto C., Rossi S., Roffi L., Redaelli A. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069–2076. doi: 10.1002/hep.23528. [DOI] [PubMed] [Google Scholar]
- 54.Di Marco V., Calvaruso V., Ferraro D., Bavetta M.G., Cabibbo G., Conte E. Effects of eradicating hepatitis C virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology. 2016;151:130–139.e2. doi: 10.1053/j.gastro.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 55.de Franchis R., Baveno V.I.F. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Thabut D., Bureau C., Layese R., Bourcier V., Hammouche M., Cagnot C. Validation of Baveno VI criteria for screening and surveillance of esophageal varices in patients with compensated cirrhosis and a sustained response to antiviral therapy. Gastroenterology. 2019;156:997–1009.e5. doi: 10.1053/j.gastro.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 57.Nahon P., Lescat M., Layese R., Bourcier V., Talmat N., Allam S. Bacterial infection in compensated viral cirrhosis impairs 5-year survival (ANRS CO12 CirVir prospective cohort) Gut. 2017;66:330–341. doi: 10.1136/gutjnl-2015-310275. [DOI] [PubMed] [Google Scholar]
- 58.Curry M.P., O'Leary J.G., Bzowej N., Muir A.J., Korenblat K.M., Fenkel J.M. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 59.Manns M., Samuel D., Gane E.J., Mutimer D., McCaughan G., Buti M. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 60.Charlton M., Everson G.T., Flamm S.L., Kumar P., Landis C., Brown R.S., Jr. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Freemantle S.J., Spinella M.J., Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- 62.Foster G.R., Irving W.L., Cheung M.C., Walker A.J., Hudson B.E., Verma S. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 63.Gentile I., Scotto R., Coppola C., Staiano L., Amoruso D.C., De Simone T. Treatment with direct-acting antivirals improves the clinical outcome in patients with HCV-related decompensated cirrhosis: results from an Italian real-life cohort (Liver Network Activity-LINA cohort) Hepatol Int. 2019;13:66–74. doi: 10.1007/s12072-018-9914-6. [DOI] [PubMed] [Google Scholar]
- 64.McCaughan G.W., Thwaites P.A., Roberts S.K., Strasser S.I., Mitchell J., Morales B. Sofosbuvir and daclatasvir therapy in patients with hepatitis C-related advanced decompensated liver disease (MELD >/= 15) Aliment Pharmacol Ther. 2018;47:401–411. doi: 10.1111/apt.14404. [DOI] [PubMed] [Google Scholar]
- 65.Backus L.I., Boothroyd D.B., Phillips B.R., Belperio P., Halloran J., Mole L.A. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516.e1. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Backus L.I., Belperio P.S., Shahoumian T.A., Mole L.A. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69:487–497. doi: 10.1002/hep.29408. [DOI] [PubMed] [Google Scholar]
- 67.Arase Y., Suzuki F., Suzuki Y., Akuta N., Kobayashi M., Kawamura Y. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 68.Delgado-Borrego A., Jordan S.H., Negre B., Healey D., Lin W., Kamegaya Y. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:458–462. doi: 10.1016/j.cgh.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hum J., Jou J.H., Green P.K., Berry K., Lundblad J., Hettinger B.D. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40:1173–1180. doi: 10.2337/dc17-0485. [DOI] [PubMed] [Google Scholar]
- 70.Vassalle C., Masini S., Bianchi F., Zucchelli G.C. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565–566. doi: 10.1136/hrt.2003.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishizaka N., Ishizaka Y., Takahashi E., Tooda E., Hashimoto H., Nagai R. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133–135. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 72.Hsu Y.C., Ho H.J., Huang Y.T., Wang H.H., Wu M.S., Lin J.T. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. doi: 10.1136/gutjnl-2014-308163. [DOI] [PubMed] [Google Scholar]
- 73.Hsu Y.C., Lin J.T., Ho H.J., Kao Y.H., Huang Y.T., Hsiao N.W. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 74.Mostafa A., Mohamed M.K., Saeed M., Hasan A., Fontanet A., Godsland I. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135–1140. doi: 10.1136/gut.2009.202317. [DOI] [PubMed] [Google Scholar]
- 75.Cacoub P., Nahon P., Layese R., Blaise L., Desbois A.C., Bourcier V. Prognostic value of viral eradication for major adverse cardiovascular events in hepatitis C cirrhotic patients. Am Heart J. 2018;198:4–17. doi: 10.1016/j.ahj.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 76.Pol S., Vallet-Pichard A., Hermine O. Extrahepatic cancers and chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2018;15:283–290. doi: 10.1038/nrgastro.2017.172. [DOI] [PubMed] [Google Scholar]
- 77.Arcaini L., Besson C., Frigeni M., Fontaine H., Goldaniga M., Casato M. Interferon-free antiviral treatment in B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood. 2016;128:2527–2532. doi: 10.1182/blood-2016-05-714667. [DOI] [PubMed] [Google Scholar]
- 78.Hosry J., Mahale P., Turturro F., Miranda R.N., Economides M.P., Granwehr B.P. Antiviral therapy improves overall survival in hepatitis C virus-infected patients who develop diffuse large B-cell lymphoma. Int J Cancer. 2016;139:2519–2528. doi: 10.1002/ijc.30372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Innes H., McDonald S., Hayes P., Dillon J.F., Allen S., Goldberg D. Mortality in hepatitis C patients who achieve a sustained viral response compared to the general population. J Hepatol. 2017;66:19–27. doi: 10.1016/j.jhep.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Allaire M., Nahon P., Layese R., Bourcier V., Cagnot C., Marcellin P. Extrahepatic cancers are the leading cause of death in patients achieving hepatitis B virus control or hepatitis C virus eradication. Hepatology. 2018;68:1245–1259. doi: 10.1002/hep.30034. [DOI] [PubMed] [Google Scholar]
- 81.Singal A.G., Tiro J.A., Marrero J.A., McCallister K., Mejias C., Adamson B. Mailed outreach program increases ultrasound screening of patients with cirrhosis for hepatocellular carcinoma. Gastroenterology. 2017;152:608–615.e4. doi: 10.1053/j.gastro.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costentin C.E., Layese R., Bourcier V., Cagnot C., Marcellin P., Guyader D. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead-time adjusted survival of patients with compensated viral cirrhosis: a multi-center cohort study. Gastroenterology. 2018;155:431–442.e10. doi: 10.1053/j.gastro.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 83.Chung R.T., Ghany M.G., Kim A.Y., Marks K.M., Naggie S., Vargas H.E. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018;67:1477–1492. doi: 10.1093/cid/ciy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asahina Y., Tsuchiya K., Nishimura T., Muraoka M., Suzuki Y., Tamaki N. alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253–1262. doi: 10.1002/hep.26442. [DOI] [PubMed] [Google Scholar]
- 85.Stafylidou M., Paschos P., Katsoula A., Malandris K., Ioakim K., Bekiari E. Performance of Baveno VI and Expanded Baveno VI criteria for excluding high-risk varices in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1744–1755.e1. doi: 10.1016/j.cgh.2019.04.062. [DOI] [PubMed] [Google Scholar]
- 86.Jakab S.S., Garcia-Tsao G. Screening and surveillance of varices in patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17:26–29. doi: 10.1016/j.cgh.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hezode C., Lonjon I., Roudot-Thoraval F., Pawlotsky J.M., Zafrani E.S., Dhumeaux D. Impact of moderate alcohol consumption on histological activity and fibrosis in patients with chronic hepatitis C, and specific influence of steatosis: a prospective study. Aliment Pharmacol Ther. 2003;17:1031–1037. doi: 10.1046/j.1365-2036.2003.01546.x. [DOI] [PubMed] [Google Scholar]
- 88.Cheung O., Sterling R.K., Salvatori J., Williams K., Hubbard S., Luketic V.A. Mild alcohol consumption is not associated with increased fibrosis in patients with chronic hepatitis C. J Clin Gastroenterol. 2011;45:76–82. doi: 10.1097/MCG.0b013e3181e12511. [DOI] [PubMed] [Google Scholar]
- 89.Vandenbulcke H., Moreno C., Colle I., Knebel J.F., Francque S., Serste T. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: a prospective study. J Hepatol. 2016;65:543–551. doi: 10.1016/j.jhep.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 90.Ganne-Carrie N., Layese R., Bourcier V., Cagnot C., Marcellin P., Guyader D. Nomogram for individualized prediction of hepatocellular carcinoma occurrence in hepatitis C virus cirrhosis (ANRS CO12 CirVir) Hepatology. 2016;64:1136–1147. doi: 10.1002/hep.28702. [DOI] [PubMed] [Google Scholar]
- 91.Nishikawa H., Osaki Y. Liver cirrhosis: evaluation, nutritional status, and prognosis. Mediators Inflamm. 2015;2015:872152. doi: 10.1155/2015/872152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smart N.A., King N., McFarlane J.R., Graham P.L., Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis. Br J Sports Med. 2018;52:834–843. doi: 10.1136/bjsports-2016-096197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.