Abstract
Double hydroboration of dienes is the addition of a hydrogen and a boryl group to the two double bonds of a diene molecule and represents a straightforward and effective protocol to prepare synthetically versatile bis(boryl)alkanes, provided that this reaction occurs selectively. However, this reaction can potentially yield several isomeric organoboron products, and it still remains a challenge to control the regioselectivity of this reaction, which allows the selective production of a single organoboron product, in particular, for a broad scope of dienes. By employing a readily available cobalt catalyst, here we show that this double hydroboration yields synthetically useful gem-bis(boryl)alkanes with excellent regioselectivity. In addition, the scope of dienes for this reaction is broad and encompasses a wide range of conjugated and non-conjugated dienes. Furthermore, mechanistic studies indicate that this cobalt-catalyzed double hydroboration occurs through boryl-directed chain-walking hydroboration of alkenylboronates generated from anti-Markovnikov 1,2-hydroboration of 1,n-diene.
Subject terms: Catalytic mechanisms, Homogeneous catalysis, Synthetic chemistry methodology
Control of regioselectivity in the double hydroboration of dienes to obtain a single organoboron compound is a considerable synthetic challenge. Here, the authors show a cobalt-catalyzed chain-walking double hydroboration of 1,n-dienes to access gem-bis(boryl)alkanes with regioselective control.
Introduction
Organoboronates are versatile building blocks for chemical synthesis because of their diverse reactivity in organic reactions1,2. As an important family of organoboron compounds, gem-bis(boryl)alkanes have recently gained increasing attention in organic synthesis due to their unique reactivity3–7. For example, different reactivity of the two boryl groups in gem-bis(boryl)alkanes allows stepwise functionalization of their two C–B bonds8–10. Interestingly, gem-bis(boryl)alkanes can generate two types of carboanions, monoboryl- or gem-bis(boryl)-functionalized carboanions, via deprotonation by LiTMP (lithium tetramethylpiperidide) or alkoxide-induced deborylation11,12. Over past decades, series of catalytic reactions have been developed to access these gem-bis(boryl) compounds13–17, such as double hydroboration of alkynes18–20, diborylation of alkenes21,22, hydroboration of 1-borylalkene23–25, or C–H borylation reactions26–29. Nevertheless, a general and practical approach that combines high catalytic activity, easy accessibility and handling of starting materials, and structural diversity of gem-bis(boryl) products is still lacking.
Metal-catalyzed hydroboration of 1,3-dienes has been developed into selective approaches to prepare allylic or homoallylic organoboron compounds30–35. However, double hydroboration of 1,3-dienes has been barely studied, mainly because alkenylboron products from the first hydroboration contain an unactivated internal alkene that does not readily undergo the second hydroboration. There was only one early example of rhodium-catalyzed double hydroboration of 1,3-dienes reported by Hayashi, and this reaction selectively afforded 1,3-bis(boryl)alkane products36. Catalysts for selective double hydroboration of 1,n-dienes to synthesize gem-bis(boryl)alkanes still remains unknown.
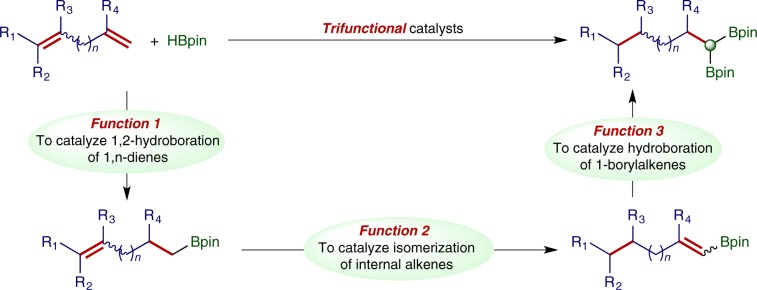
Recently, metal-mediated chain walking has been emerging as a useful tool for remote functionalization of organic molecules37–54. The directions for chain walking can be controlled by catalysts or directing groups in organic substrates. For example, aryl or boryl groups in the substrates can direct the chain walking towards them46,48,51,54. Therefore, double hydroboration of 1,n-dienes can be potentially developed into an effective and selective protocol to prepare gem-bis(boryl)alkanes, provided that a trifunctional catalyst can be identified to catalyze these three transformations: selective hydroboration of 1,n-dienes to produce alkenylboronates, the isomerization of alkenylboronates to 1-borylalkenes, and the subsequent hydroboration of 1-borylalkenes to yield gem-bis(boryl)alkanes (Fig. 1).
Fig. 1. Hydroboration of dienes.
Trifunctional catalysts for chain-walking double hydroboration of 1,n-dienes.
In recent years, cobalt compounds have been extensively studied as catalysts for hydroboration and isomerization of alkenes55–63. In 2015, Chirik and coworkers showed one example of cobalt-catalyzed hydroboration of a boryl-containing terminal alkene to give a gem-bis(boryl)alkane product by taking the advantage of boryl-directed alkene isomerization59. During our continuous efforts in developing cobalt-catalyzed hydroboration of unsaturated hydrocarbons64–66, we become interested in identifying a selective cobalt catalyst for double hydroboration of 1,n-dienes to synthesize gem-bis(boryl)alkanes. We envisioned that it would be more challenging to develop a selective double hydroboration of aryl-substituted 1,n-dienes because both aryl and boryl groups in alkenylboronate products of the first hydroboration can control the direction of subsequent alkene isomerization, which would probably decrease the selectivity for the second hydroboration. Indeed, such decreased selectivity has been encountered in a recent study on NiH-catalyzed remote hydroarylation of a phenyl-containing alkenylboronate54. Here, we show that double hydroboration of these 1,n-dienes takes place to yield synthetically versatile gem-bis(boryl)alkanes with high regioselectivity in the presence of Co(acac)2 and 1,2-bis(dicyclohexylphosphino)ethane (dcpe).
Results
Evaluation of reaction conditions
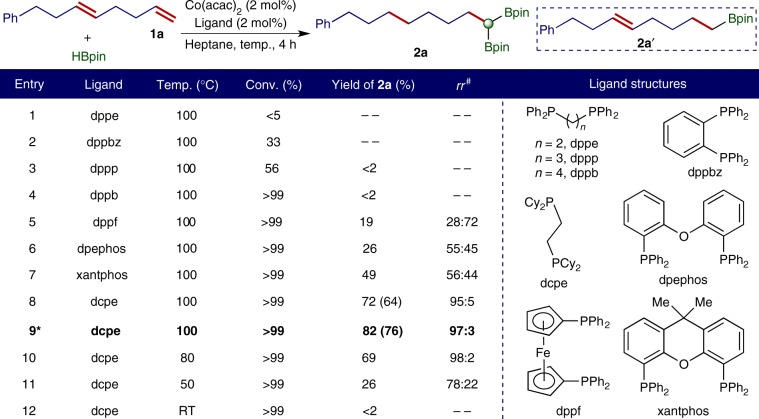
We chose the reaction of (E)-octa-3,7-dien-1-ylbenzene (1a) with HBpin to identify a cobalt catalyst and the conditions that favor the formation of the gem-bis(boryl)alkane product 2a (Fig. 2). The cobalt catalysts we intended to evaluate were generated in situ from Co(acac)2 and bisphosphine ligands and activated by the reaction with HBpin. In general, the targeted double hydroboration reactions were conducted with 2 mol% cobalt catalyst in the presence of 2.5 equivalents of HBpin for 4 h at 100 °C. The selected examples of these reactions are summarized in Fig. 2.
Fig. 2. Evaluation of conditions for cobalt-catalyzed double hydroboration of 1,5-diene 1a.
Reaction conditions: 1a (0.200 mmol), HBpin (0.500 mmol), Co(acac)2 (4.0 μmol), ligand (4.0 μmol), heptane (0.5 mL), 4 h, yield was determined by GC (gas chromatography) analysis using tridecane as internal standard and yield in parentheses was isolated yield of 2a. #rr is regioisomeric ratio and represents the ratio of the desired gem-bis(boryl)alkane product to the sum of all other bis(boryl)alkane isomers as determined by gas chromatography analysis.*3 mol % cobalt catalyst.
The reaction conducted with Co(acac)2 and dppe proceeded sluggishly to very low conversion (<5%) of 1a and the desired product 2a was not formed (Fig. 2, entry 1). The reaction catalyzed by Co(acac)2 and dppbz occurred to a low conversion (33%) of 1a and only a trace amount of 2a was detected together with several other isomeric 1,n-dienes that were resulted from the isomerization of 1a (Fig. 2, entry 2). The reactions of 1a with HBpin afforded alkenylboronate 2a′ as a major product when conducted with Co(acac)2 and dppp or dppb ligand (Fig. 2, entries 3 and 4). The reactions run with Co(acac)2 and dppf, dpephos, or xantphos afforded eight isomeric bis(boryl)alkane products (Fig. 2, entries 5−7), and the selectivity for the desired product 2a was only low to modest (28−56%). To our delight, the reaction catalyzed by 2 mol % Co(acac)2/dcpe produced 2a in good yield (72%) and high regioselectivity (95% rr, Fig. 2, entry 8). In particular, the reaction conducted with 3 mol% catalyst afforded 2a in high isolated yield (76%) with excellent regioselectivity (97% rr, Fig. 2, entry 9). We also tested various temperatures for this double hydroboration reaction catalyzed by Co(acac)2/dcpe. Similar results were obtained for the reactions run at 100 °C and 80 °C (Fig. 2, entries 8 and 10). Further lowering the temperature to 50 °C led to a lower yield of 2a with a diminished regioselectivity (Fig. 2, entry 11). Particularly, the reaction performed at room temperature afforded only the alkenylboronate product 2a′, which was formed from hydroboration of the terminal double bond of 1a (Fig. 2, entry 12).
Substrate scope of conjugated and non-conjugated 1,n-dienes
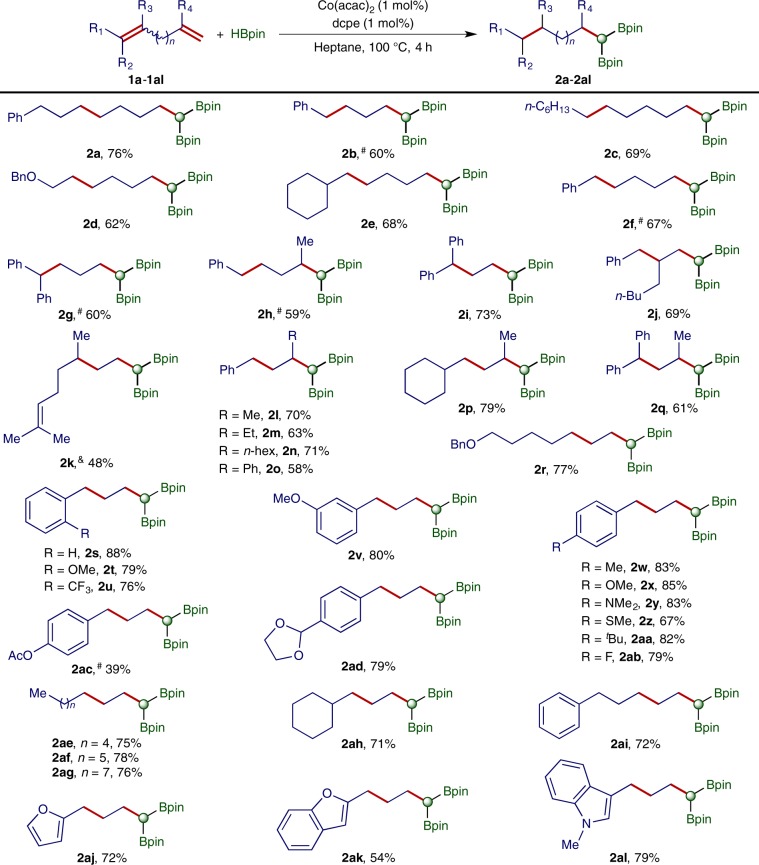
With the identified cobalt catalyst and reliable conditions in hand, we studied the scope of 1,n-dienes that undergo this cobalt-catalyzed double hydroboration for the synthesis of gem-bis(boryl)alkanes, and the results are summarized in Fig. 3. In general, a wide range of non-conjugated (Z/E)−1,n-dienes (1a−1h) and conjugated (Z/E)−1,3-dienes (1i−1al) smoothly reacted with HBpin in the presence of 1 mol% Co(acac)2 and 1 mol% dcpe, yielding the corresponding gem-bis(boryl)alkanes (2a−2al) in modest to high isolated yields (58−88%) with excellent regioselectivity (up to 99%). Noticeably, carbocyclic products resulted from cyclization of 1,6-diene 1c were not detected.
Fig. 3. Scope of 1,n-dienes.
Reaction conditions: 1,n-diene (0.300 mmol), HBpin (0.750 mmol), Co(acac)2 (3.0 μmol), dcpe (3.0 μmol), heptane (0.5 mL), 100 °C, 4 h, and isolated yields. #3 mol % catalyst; &80 °C.
The scope of 1,n-dienes encompasses both alkyl- and aryl-substituted 1,n-dienes with substituents at various positions (e.g. 1g−1q). Particularly, the double hydroboration of 1,n-dienes (1h and 1l−1q) containing 1,1-disubstituted alkene units afforded β-branched gem-bis(boryl)alkanes (2h and 2l−2q), which are not accessible via double hydroboration of terminal alkynes. In addition, 1,3-dienes containing ortho-substituted aryl groups also smoothly reacted under identified conditions to afforded the corresponding gem-bis(boryl)alkanes (2t and 2u) in high isolated yields. This cobalt-catalyzed double hydroboration can tolerate a range of functional groups, such as ether (2d and 2v), tertiary amine (2y), thioether (2z), fluoride (2ab), carboxylic ester (2ac), and acetal (2ad). Nitrogen- and oxygen-containing 1,3-dienes also reacted to afford the desired products (2aj−2al) in high isolated yields.
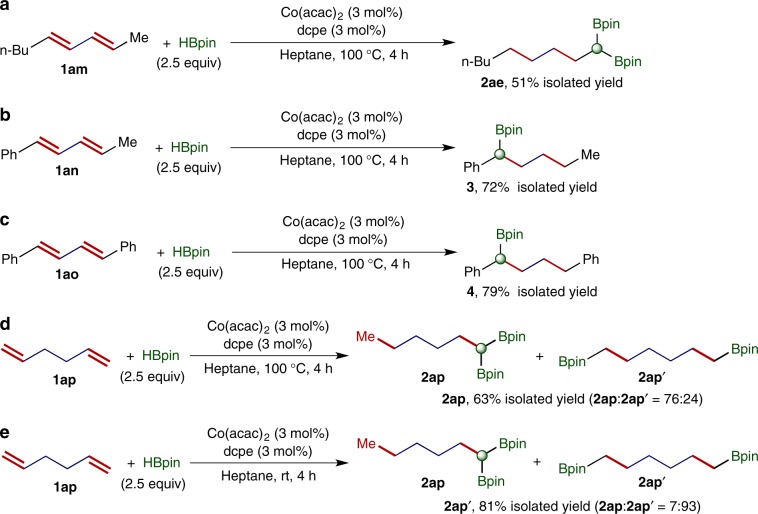
In addition, we also tested this cobalt catalyst for hydroboration reactions of dienes containing two internal or two terminal carbon-carbon double bonds, and the results are shown in Fig. 4. Under standard conditions, bis(alkyl)-substituted 1,3-diene 1am reacted smoothly with HBpin to afford gem-bis(boryl)alkane product 2ae in 51% isolated yield (Fig. 4a). Product 2ae contains two Bpin groups on the terminal sp3-carbon, indicating the isomerization of both internal double bonds to terminal double bonds. The hydroboration reactions of aryl,alkyl- or aryl,aryl-disubstituted 1,3-dienes 1an and 1ao produced benzylic boronates 3 and 4, respectively, in high yields (Fig. 4b, c). These benzylic boronate products were formed by cobalt-catalyzed sequential hydroboration and hydrogenation of 1an and 1ao. The double hydroboration of 1,5-hexadiene 1ap, a diene containing two terminal double bonds, yielded two bis(boryl)alkane products, 1,1-bis(boryl)hexane 2ap and 1,6-bis(boryl)hexane 2ap′, and the ratios of these two products were temperature dependent. For example, the reaction conducted at 100 °C afforded 1,1-bis(boryl)hexane 2ap as the major product with 2ap:2ap′ of 76:24, and gem-bis(boryl)alkane product 2ap was isolated in 63% yield (Fig. 4d). However, the corresponding reaction run at room temperature gave 1,6-bis(boryl)hexane 2ap’ in 81% isolated yield with high regioselectivity (2ap:2ap′ = 7:93, Fig. 4e).
Fig. 4. Hydroboration of dienes containing two internal double bonds or two terminal double bonds.
a Double hydroboration of diene 1am. b Sequential hydroboration/hydrogenation of diene 1an. c Sequential hydroboration/hydrogenation of diene 1ao. d Double hydroboration of diene 1ap at 100 °C. e Hydroboration of diene 1ap at room temperature.
Synthetic utilities
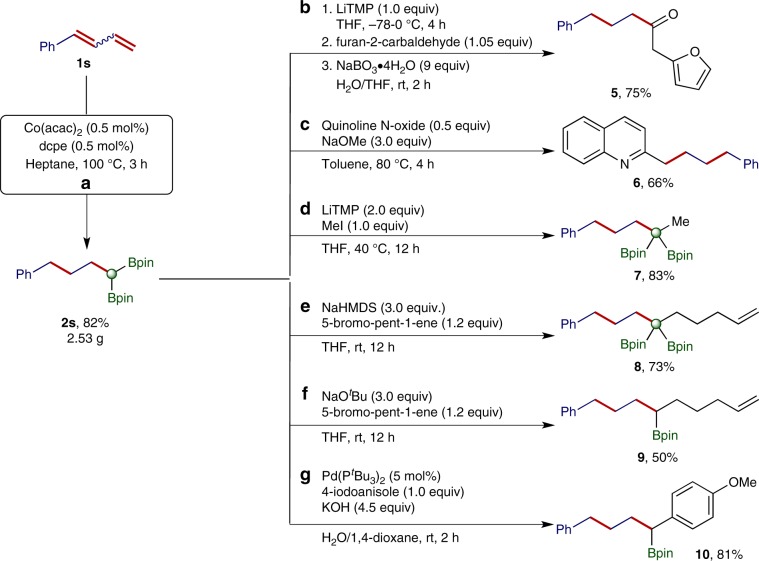
This cobalt-catalyzed chain-walking double hydroboration of 1,n-dienes can be conducted on gram-scales with a decreased catalyst loading. For example, the reaction of (Z/E)-buta-1,3-dien-1-ylbenzene 1s (Z:E = 45:55) with HBpin on a 8.0-mmol scale proceeded to full conversion of 1s in the presence of 0.5 mol% Co(acac)2/dcpe and afforded the desired product 2s (2.53 g) in 82% isolated yield (Fig. 5a). We subsequently show the utility of gem-Bis(boryl)alkane 2s as a versatile building block in organic synthesis by conducting a series of organic transformations with 2s (Fig. 5b−f). For example, 2s could undergo a Boron-Wittig reaction with an aldehyde to afford ketone 5 after oxidative workup with NaBO3•4H2O (ref.10) (Fig. 5b). 2s could also be used as an alkylating reagent for the alkylation of quinoline N-oxide to prepare 2-alkylquinoline 6 (ref.67) (Fig. 5c). In addition, the carbanion generated by the deprotonation of 2s with LiTMP, NaHMDS, or NaOtBu reacted with iodomethane or 5-bromopent-1-ene to afford boryl-functionalized alkenes 7, 8, and 9, respectively11 (Fig. 5d–f). Furthermore, gem-bis(boryl)alkane 2 s readily underwent Pd-catalyzed Suzuki-Miyaura coupling with 4-iodoanisole to give alkylboronate 10 in 81% isolated yield (Fig. 5g).
Fig. 5. Gram-scale synthesis of gem-bis(boryl)alkane 2s and its further transformations.
a Gram-scale synthesis of 2s. b Synthesis of 5. c Synthesis of 6. d Synthesis of 7. e Synthesis of 8. f Synthesis of 9. g Synthesis of 10.
Discussion
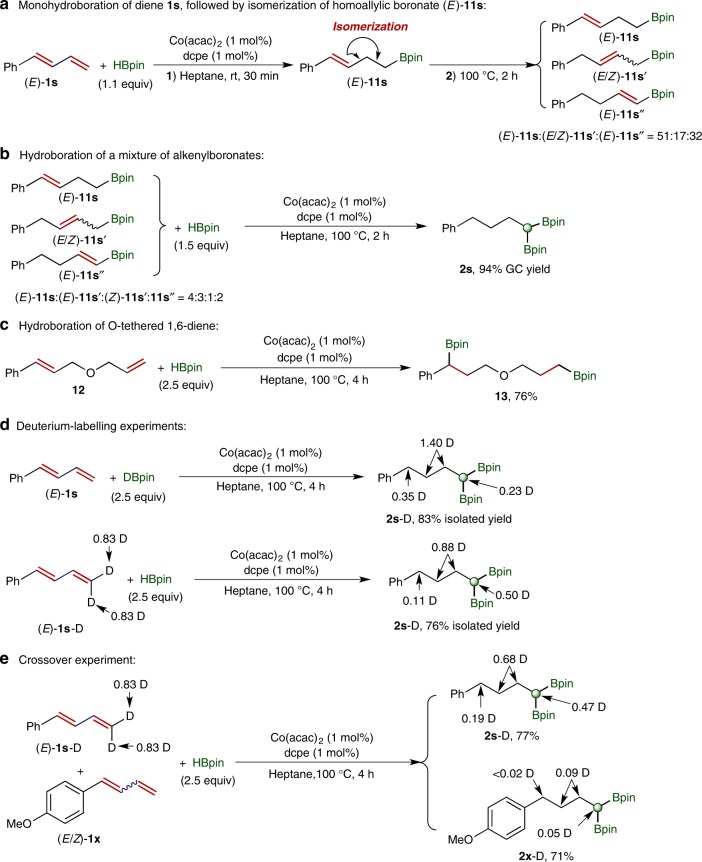
We subsequently conducted a series of experiments to gain insights into the mechanism of this cobalt-catalyzed double hydroboration reactions of 1,n-dienes, and the results of these experiments are summarized in Fig. 6. Similar to monohydroboration of 1,5-diene 1a at room temperature (Fig. 2, entry 12), monohydroboration of 1,3-diene 1s with 1.1 equiv. of HBpin occurred to completion in 30 min at room temperature and afforded alkenylboronate 11s selectively (Fig. 6a). Subsequent heating the reaction mixture at 100 °C for 2 h resulted in the isomerization of 11s to a mixture of alkenylboronates 11s, 11s′, and 11s″ with a ratio of 51:17:32 (Fig. 6a). We then tested a mixture of these alkenylboronates for hydroboration with HBpin in the presence of 1 mol % of Co(acac)2/dcpe, and all these isomeric alkenylboronates were converted to gem-bis(boryl)alkane 2s in high yield (Fig. 6b). The conversion of 11s, 11s′, and 11s″ to a single product 2s indicates that boryl group has a stronger directing ability for this cobalt-catalyzed chain-walking hydroboration than phenyl group, which may stem from the interaction of the d-electrons of the cobalt catalyst with the empty p-orbital on boron68.
Fig. 6. Deuterium-labeling experiment and control experiments. a Monohydroboration of diene 1s and isomerization of alkenylboronate (E)-11s.
b Hydroboration of a mixture of alkenylboronates. c Hydroboration of O-tethered 1,6-diene 12. d Deuterium-labeling experiments. e Crossover experiment.
A control experiment of this double hydroboration was conducted with a substrate containing an oxygen-tethered 1,6-diene 12 (Fig. 6c). This reaction afforded 1,7-bis(boryl)alkane 13 and chain-walking double hydroboration was not observed. This suggests that the chain walking takes place through reversible β-hydrogen elimination and reinsertion steps. A deuterium-labeling experiment of the double hydroboration of 1,3-diene (E)-1s was then conducted with DBpin, and deuterium incorporation at all positions of the aliphatic chain of gem-bis(boryl)alkane 2s-D was observed (Fig. 6d). When the deuterium-labeling experiment was performed with (E)-1s-D and HBpin, deuterium scrambling at all positions of the aliphatic chain of 2s-D was observed as well (Fig. 6d). In addition, when a crossover experiment of this double hydroboration was run with (E)-1s-D and (E/Z)-1×, similar deuterium incorporation and deuterium scrambling map was also observed for gem-bis(boryl)alkane products 2s-D and 2×-D (Fig. 6e). The results of this crossover experiment indicate that dissociation and re-association of Co-H/D from the Co-H/D-olefin complex occurs during the chain walking process.
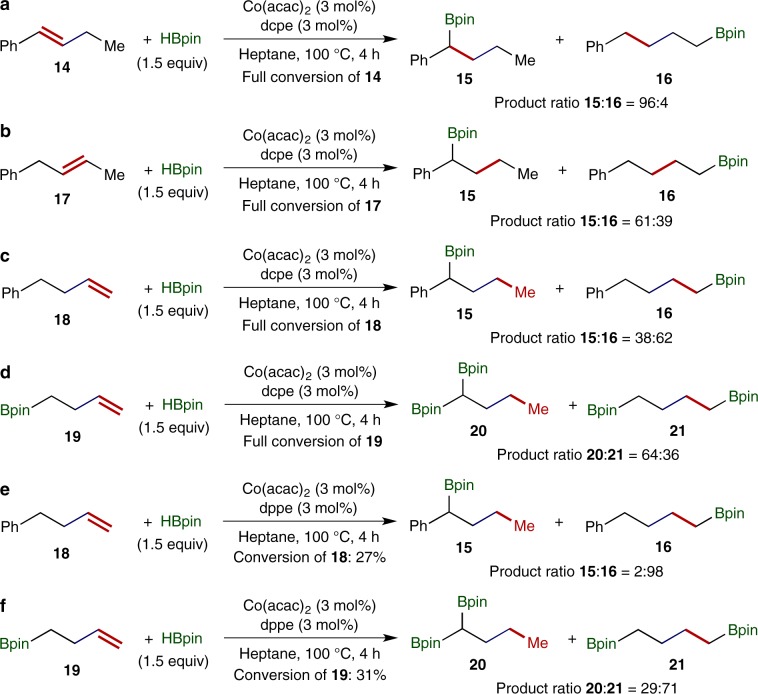
We then tested this cobalt catalyst, Co(acac)2/dcpe, for chain-walking hydroboration of phenyl- and boryl-containing alkenes with double bonds at various positions, and the results are summarized in Fig. 7. (E)-But-1-en-1-ylbenzene (14) reacted smoothly with 1.5 equivalents HBpin in the presence of 3 mol% Co(acac)2/dcpe at 100 °C to give alkylboroante 15 as a major product together with a trace amount (4%) of alkylboroante 16, a byproduct from chain-walking hydroboration (Fig. 7a). Similarly, the reaction between (E)-but-2-en-1-ylbenzene (17) and HBpin under standard conditions afforded alkylboroante products 15 and 16, albeit with a low regioselectivity (15:16 = 61:39), and both products were resulted from chain-walking hydroboration (Fig. 7b). But-3-en-1-ylbenzene (18) also underwent this cobalt-catalyzed chain-walking hydroboration, but the major product (16) of this reaction was from the hydroboration of terminal double bond of 18 (Fig. 7c). For comparison, we also conducted the reaction between a boryl-containing terminal alkene 19 and HBpin under standard conditions (Fig. 7d), and the major product, gem-bis(boryl)alkane 20, was formed by the cobalt-catalyzed chain-walking hydroboration. The results of chain-walking hydrobration reactions conducted with but-3-en-1-ylbenzene (18) and but-3-en-1-ylboronic pinacol ester (19) indicate that the Bpin group has a stronger directing ability for chain-walking hydroboration than the phenyl group.
Fig. 7. Chaining-walking hydroboration of phenyl- and boryl-containing alkenes.
a Hydroboration of alkene 14. b Hydroboration of alkene 17. c Hydroboration of alkene 18. d Hydroboration of alkene 19. e Hydroboration of alkene 18 catalyzed by Co(acac)2/dppe. f Hydroboration of alkene 19 catalyzed by Co(acac)2/dppe.
In addition, we also tested a cobalt catalyst generated from Co(acac)2 and dppe (1,2-bis(diphenylphosphino)ethane), a bisphosphine ligand having a similar steric but different electronic property, for hydroboration of terminal alkenes 18 and 19 with HBpin (Fig. 7e and f). These two reactions proceeded very sluggishly and approximately 30% of 18 and 19 were converted at 100 °C in 24 h. Interestingly, major products (16 and 21) of these two reactions were resulted from direct anti-Markovnikov hydroboration of 18 and 19, and much less byproducts (15 and 20) were formed by chain-walking hydroboration relative to the corresponding reactions catalyzed by the cobalt catalyst generated from Co(acac)2 and dcpe (Fig. 7c, d). The results of these four reactions (Fig. 7c–f) suggest that the electron-rich property of dcpe ligand facilitates the chain-walking process, thus promoting chain-walking hydroboration.
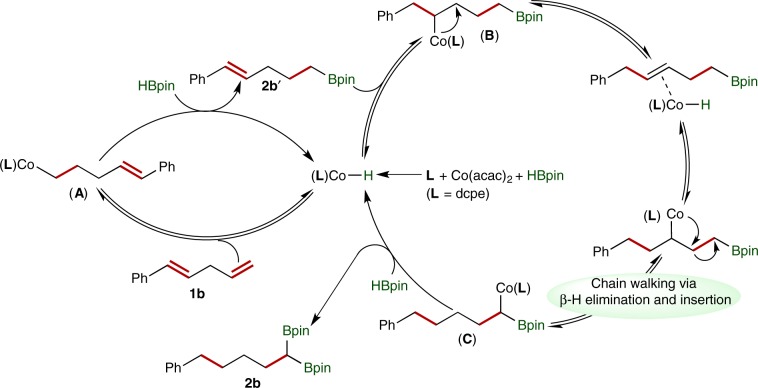
Based on the results of mechanistic studies, we proposed a catalytic pathway for this cobalt-catalyzed chain-walking double hydroboration of 1,4-diene 1b, as depicted in Fig. 8. The activation of Co(acac)2 with HBpin in the presence of dcpe (L) forms a cobalt hydride species (L)Co-H (ref. 69). Migratory insertion of the terminal alkene of 1b into (L)Co-H generates an alkenylcobalt intermediate A, which then undergoes σ-bond metathesis with HBpin to produce alkenylboronate 2b′ and regenerates (L)Co-H. The double bond in 2b′ then inserts into (L)Co-H to form an alkylcobalt species B, which undergoes isomerization to form the alkylcobalt intermediate C through reversible β-hydrogen elimination and reinsertion. In the last step, σ-bond metathesis between alkylcobalt species C and HBpin yields gem-bis(boryl)alkane 2b and regenerates the catalytically active (L)Co-H intermediate.
Fig. 8. Proposed mechanism.
The proposed catalytic cycle for this cobalt-catalyzed chain-walking double hydroboration.
In summary, we have developed an effective and convenient protocol to prepare gem-bis(boryl)alkanes via a selective cobalt-catalyzed double hydroboration of 1,n-dienes. A wide range of conjugated and non-conjugated 1,n-dienes reacted with pinacolborane to produce gem-bis(boryl)alkanes in high isolated yields with excellent regioselectivity in the presence of a catalyst generated in situ from Co(acac)2 and dcpe ligand. Mechanistic studies suggest that this cobalt-catalyzed double hydroboration occurs through an initial anti-Markovnikov monohydroboration of 1,n-dienes followed by a sequential boryl-directed chain-walking hydroboration of the resulting alkenylboronates. This cobalt-catalyzed double hydroboration provides a straightforward approach to access structurally diverse and synthetically versatile gem-bis(boryl)alkanes from readily available 1,n-dienes.
Methods
General procedure for double hydroboration of 1,n-dienes
In an Argon-filled glovebox, a 4-mL screw-capped vial was charged with Co(acac)2 (0.8 mg, 3.0 µmol), dcpe (1.3 mg, 3.0 μmol), 1,n-diene (0.30 mmol), heptane (0.5 mL) and a magnetic stirring bar. The solution was stirred for 5 min and pincolborane (96.0 mg, 0.75 mmol) was added to the vial. The vial was sealed with a cap containing a PTFE septum and removed from the glovebox. The mixture was then heated at 100 °C for 4 h until complete consumption of starting material as monitored by TLC and GC-MS analysis. Subsequently, the solvent was removed under reduced pressure. The residue was purified by silica gel flash column chromatography (hexane/ethyl acetate = 40:1) to afford the desired products. See the Supplementary Information for detailed experimental procedures and the characterization data of all the products.
Supplementary information
Acknowledgements
This work was supported by the grant from the Ministry of Education of Singapore (R-143-000-A07-112 and R-143-000-A93-112).
Author contributions
M.H. planned and conducted most of the experiments. S.G. directed the projects, and S.G. and M.H. co-wrote the manuscript. Both authors contributed to the discussion.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Ilan Marek, Naohiko Yoshikai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-14543-2.
References
- 1.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 2.Brooks WLA, Sumerlin BS. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem. Rev. 2016;116:1375–1397. doi: 10.1021/acs.chemrev.5b00300. [DOI] [PubMed] [Google Scholar]
- 3.Miralles N, Maza RJ, Fernández E. Synthesis and reactivity of 1,1-diborylalkanes towards C–C bond formation and related mechanisms. Adv. Synth. Catal. 2018;360:1306–1327. doi: 10.1002/adsc.201701390. [DOI] [Google Scholar]
- 4.Nallagonda R, Padala K, Masarwa A. gem-Diborylalkanes: recent advances in their preparation, transformation and application. Org. Biomol. Chem. 2018;16:1050–1064. doi: 10.1039/C7OB02978K. [DOI] [PubMed] [Google Scholar]
- 5.Wu C, Wang J. Geminal bis(boron) compounds: their preparation and synthetic applications. Tetrahedron Lett. 2018;59:2128–2140. doi: 10.1016/j.tetlet.2018.04.061. [DOI] [Google Scholar]
- 6.Marek I, Normant J-F. Synthesis and reactivity of sp3 germinated organobismetallics. Chem. Rev. 1996;96:3241–3267. doi: 10.1021/cr9600161. [DOI] [PubMed] [Google Scholar]
- 7.Marek I. Synthesis and reactivity of sp2 germinated organobismetallics. Chem. Rev. 2000;100:2887–2900. doi: 10.1021/cr990288e. [DOI] [PubMed] [Google Scholar]
- 8.Li H, et al. Palladium(0)-catalyzed cross-coupling of 1,1-diboronates with vinyl bromides and 1,1-dibromoalkenes. Angew. Chem. Int. Ed. 2014;53:11921–11925. doi: 10.1002/anie.201407000. [DOI] [PubMed] [Google Scholar]
- 9.Potter B, Edelstein EK, Morken JP. Modular, catalytic enantioselective construction of quaternary carbon stereocenters by sequential cross-coupling reactions. Org. Lett. 2016;18:3286–3289. doi: 10.1021/acs.orglett.6b01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens TC, Pattison G. Transition-metal-free homologative cross-coupling of aldehydes and ketones with geminal bis(boron) compounds. Org. Lett. 2017;19:3498–3501. doi: 10.1021/acs.orglett.7b01474. [DOI] [PubMed] [Google Scholar]
- 11.Hong K, Liu X, Morken JP. Simple access to elusive α-boryl carbanions and their alkylation: an umpolung construction for organic synthesis. J. Am. Chem. Soc. 2014;136:10581–10584. doi: 10.1021/ja505455z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Deaton TM, Haeffner F, Morken JP. A boron alkylidene–alkene cycloaddition reaction: application to the synthesis of aphanamal. Angew. Chem. Int. Ed. 2017;56:11485–11489. doi: 10.1002/anie.201705720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masaki S, et al. Synthesis and applications of 1,1-diborylated cyclopropanes: facile route to 1,2-diboryl-3-methylenecyclopentenes. Chem. Lett. 2006;35:1222–1223. doi: 10.1246/cl.2006.1222. [DOI] [Google Scholar]
- 14.Wang L, et al. C–O functionalization of α-oxyboronates: a deoxygenative gem-diborylation and gem-silylborylation of aldehydes and ketones. J. Am. Chem. Soc. 2017;139:5257–5264. doi: 10.1021/jacs.7b02518. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Park J, Cho SH. Generation and application of (diborylmethyl)zinc(II) species: access to enantioenriched gem-diborylalkanes by an asymmetric allylic substitution. Angew. Chem. Int. Ed. 2018;57:12930–12934. doi: 10.1002/anie.201805476. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Lee Y, Cho SH. Palladium-catalyzed chemoselective negishi cross-coupling of bis[(pinacolato)boryl]methylzinc halides with aryl (pseudo)halides. Org. Lett. 2019;21:5912–5916. doi: 10.1021/acs.orglett.9b02050. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B, et al. An olefinic 1,2-boryl-migration enabled by radical addition: construction of gem-bis(boryl)alkanes. Angew. Chem. Int. Ed. 2019;58:9448–9452. doi: 10.1002/anie.201903721. [DOI] [PubMed] [Google Scholar]
- 18.Endo K, Hirokami M, Shibata T. Synthesis of 1,1-organodiboronates via Rh(I)Cl-catalyzed sequential regioselective hydroboration of 1-alkynes. Synlett. 2009;2009:1331–1335. doi: 10.1055/s-0028-1088131. [DOI] [Google Scholar]
- 19.Lee S, Li D, Yun J. Copper-catalyzed synthesis of 1,1-diborylalkanes through regioselective dihydroboration of terminal alkynes. Chem. Asian J. 2014;9:2440–2443. doi: 10.1002/asia.201402458. [DOI] [PubMed] [Google Scholar]
- 20.Zuo Z, Huang Z. Synthesis of 1,1-diboronate esters by cobalt-catalyzed sequential hydroboration of terminal alkynes. Org. Chem. Front. 2016;3:434–438. doi: 10.1039/C5QO00426H. [DOI] [Google Scholar]
- 21.Lee JCH, McDonald R, Hall DG. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nat. Chem. 2011;3:894. doi: 10.1038/nchem.1150. [DOI] [PubMed] [Google Scholar]
- 22.Feng X, Jeon H, Yun J. Regio- and enantioselective copper(I)-catalyzed hydroboration of borylalkenes: asymmetric synthesis of 1,1-diborylalkanes. Angew. Chem. Int. Ed. 2013;52:3989–3992. doi: 10.1002/anie.201208610. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Gong T, Lu X, Xiao B, Fu Y. Nickel-catalyzed synthesis of 1,1-diborylalkanes from terminal alkenes. Nat. Commun. 2017;8:345. doi: 10.1038/s41467-017-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teo WJ, Ge S. Cobalt-catalyzed diborylation of 1,1-disubstituted vinylarenes: a practical route to branched gem-bis(boryl)alkanes. Angew. Chem. Int. Ed. 2018;57:1654–1658. doi: 10.1002/anie.201710389. [DOI] [PubMed] [Google Scholar]
- 25.Teo WJ, Ge S. Cobalt-catalyzed enantioselective synthesis of chiral gem-bis(boryl)alkanes. Angew. Chem. Int. Ed. 2018;57:12935–12939. doi: 10.1002/anie.201805705. [DOI] [PubMed] [Google Scholar]
- 26.Cho SH, Hartwig JF. Iridium-catalyzed diborylation of benzylic C–H bonds directed by a hydrosilyl group: synthesis of 1,1-benzyldiboronate esters. Chem. Sci. 2014;5:694–698. doi: 10.1039/C3SC52824C. [DOI] [Google Scholar]
- 27.Palmer WN, Obligacion JV, Pappas I, Chirik PJ. Cobalt-catalyzed benzylic borylation: enabling polyborylation and functionalization of remote, unactivated C(sp3)–H bonds. J. Am. Chem. Soc. 2016;138:766–769. doi: 10.1021/jacs.5b12249. [DOI] [PubMed] [Google Scholar]
- 28.Palmer WN, Zarate C, Chirik PJ. Benzyltriboronates: building blocks for diastereoselective carbon–carbon bond formation. J. Am. Chem. Soc. 2017;139:2589–2592. doi: 10.1021/jacs.6b12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto T, Ishibashi A, Suginome M, Boryl-directed, Ir-catalyzed C. (sp3)–H borylation of alkylboronic acids leading to site-selective synthesis of polyborylalkanes. Org. Lett. 2019;21:6235–6240. doi: 10.1021/acs.orglett.9b02112. [DOI] [PubMed] [Google Scholar]
- 30.Wu JY, Moreau B, Ritter T. Iron-catalyzed 1,4-hydroboration of 1,3-dienes. J. Am. Chem. Soc. 2009;131:12915–12917. doi: 10.1021/ja9048493. [DOI] [PubMed] [Google Scholar]
- 31.Ely RJ, Morken JP. Regio- and stereoselective ni-catalyzed 1,4-hydroboration of 1,3-dienes: access to stereodefined (Z)-allylboron reagents and derived allylic alcohols. J. Am. Chem. Soc. 2010;132:2534–2535. doi: 10.1021/ja910750b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, et al. Selective synthesis of secondary benzylic (Z)-allylboronates by Fe-catalyzed 1,4-hydroboration of 1-aryl-substituted 1,3-dienes. Org. Chem. Front. 2014;1:1101–1106. doi: 10.1039/C4QO00206G. [DOI] [Google Scholar]
- 33.Fiorito D, Mazet C. Ir-catalyzed selective hydroboration of 2-substituted 1,3-dienes: a general method to access homoallylic boronates. ACS Catal. 2018;8:9382–9387. doi: 10.1021/acscatal.8b02334. [DOI] [Google Scholar]
- 34.Duvvuri K, et al. Cationic Co(I)-intermediates for hydrofunctionalization reactions: regio- and enantioselective cobalt-catalyzed 1,2-hydroboration of 1,3-dienes. J. Am. Chem. Soc. 2019;141:7365–7375. doi: 10.1021/jacs.8b13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng S, Yang J, Liu G, Huang Z. Ligand controlled cobalt catalyzed regiodivergent 1,2-hydroboration of 1,3-dienes. Sci. China Chem. 2019;62:336–340. doi: 10.1007/s11426-018-9418-7. [DOI] [Google Scholar]
- 36.Matsumoto Y, Hayashi T. Asymmetric double hydroboration of 1,3-dienes catalyzed by chiral phosphine-rhodium complexes. Tetrahedron Lett. 1991;32:3387–3390. doi: 10.1016/S0040-4039(00)92714-X. [DOI] [Google Scholar]
- 37.Bair JS, et al. Linear-selective hydroarylation of unactivated terminal and internal olefins with trifluoromethyl-substituted arenes. J. Am. Chem. Soc. 2014;136:13098–13101. doi: 10.1021/ja505579f. [DOI] [PubMed] [Google Scholar]
- 38.Larionov E, Li H, Mazet C. Well-defined transition metal hydrides in catalytic isomerizations. Chem. Commun. 2014;50:9816–9826. doi: 10.1039/C4CC02399D. [DOI] [PubMed] [Google Scholar]
- 39.Larionov E, Lin L, Guénée L, Mazet C. Scope and mechanism in palladium-catalyzed isomerizations of highly substituted allylic, homoallylic, and alkenyl alcohols. J. Am. Chem. Soc. 2014;136:16882–16894. doi: 10.1021/ja508736u. [DOI] [PubMed] [Google Scholar]
- 40.Mei T-S, Patel HH, Sigman MS. Enantioselective construction of remote quaternary stereocentres. Nature. 2014;508:340. doi: 10.1038/nature13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamakawa T, Yoshikai N. Alkene isomerization–hydroarylation tandem catalysis: indole C2-alkylation with aryl-substituted alkenes leading to 1,1-diarylalkanes. Chem. Asian J. 2014;9:1242–1246. doi: 10.1002/asia.201400135. [DOI] [PubMed] [Google Scholar]
- 42.Buslov I, Becouse J, Mazza S, Montandon-Clerc M, Hu X. Chemoselective alkene hydrosilylation catalyzed by nickel pincer complexes. Angew. Chem. Int. Ed. 2015;54:14523–14526. doi: 10.1002/anie.201507829. [DOI] [PubMed] [Google Scholar]
- 43.Dupuy S, Zhang K-F, Goutierre A-S, Baudoin O. Terminal-selective functionalization of alkyl chains by regioconvergent cross-coupling. Angew. Chem. Int. Ed. 2016;55:14793–14797. doi: 10.1002/anie.201608535. [DOI] [PubMed] [Google Scholar]
- 44.Lin L, Romano C, Mazet C. Palladium-catalyzed long-range deconjugative isomerization of highly substituted α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 2016;138:10344–10350. doi: 10.1021/jacs.6b06390. [DOI] [PubMed] [Google Scholar]
- 45.Vasseur A, Bruffaerts J, Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 2016;8:209. doi: 10.1038/nchem.2445. [DOI] [PubMed] [Google Scholar]
- 46.Chen F, et al. Remote migratory cross-electrophile coupling and olefin hydroarylation reactions enabled by in situ generation of NiH. J. Am. Chem. Soc. 2017;139:13929–13935. doi: 10.1021/jacs.7b08064. [DOI] [PubMed] [Google Scholar]
- 47.Gaydou M, Moragas T, Juliá-Hernández F, Martin R. Site-selective catalytic carboxylation of unsaturated hydrocarbons with CO2 and water. J. Am. Chem. Soc. 2017;139:12161–12164. doi: 10.1021/jacs.7b07637. [DOI] [PubMed] [Google Scholar]
- 48.He Y, Cai Y, Zhu S. Mild and regioselective benzylic C–H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 2017;139:1061–1064. doi: 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]
- 49.Juliá-Hernández F, Moragas T, Cornella J, Martin R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature. 2017;545:84. doi: 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]
- 50.Singh S, Bruffaerts J, Vasseur A, Marek I. A unique pd-catalysed heck arylation as a remote trigger for cyclopropane selective ring-opening. Nat. Commun. 2017;8:14200. doi: 10.1038/ncomms14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng L, et al. Ligand-controlled nickel-catalyzed reductive relay cross-coupling of alkyl bromides and aryl bromides. ACS Catal. 2018;8:310–313. doi: 10.1021/acscatal.7b03388. [DOI] [Google Scholar]
- 52.Sommer H, Juliá-Hernández F, Martin R, Marek I. Walking metals for remote functionalization. ACS Centr. Sci. 2018;4:153–165. doi: 10.1021/acscentsci.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Yin H, Fu GC. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature. 2018;563:379–383. doi: 10.1038/s41586-018-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Han B, Zhu S. Rapid access to highly functionalized alkyl boronates by NiH-catalyzed remote hydroarylation of boron-containing alkenes. Angew. Chem. Int. Ed. 2019;58:13860–13864. doi: 10.1002/anie.201907185. [DOI] [PubMed] [Google Scholar]
- 55.Obligacion JV, Chirik PJ. Bis(imino)pyridine cobalt-catalyzed alkene isomerization–hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 2013;135:19107–19110. doi: 10.1021/ja4108148. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Dugan TR, Brennessel WW, Weix DJ, Holland PL. Z-selective alkene isomerization by high-spin cobalt(II) complexes. J. Am. Chem. Soc. 2014;136:945–955. doi: 10.1021/ja408238n. [DOI] [PubMed] [Google Scholar]
- 57.Ruddy, A. J., Sydora, O. L., Small, B. L., Stradiotto, M. & Turculet, L. (N-phosphinoamidinate) cobalt-catalyzed hydroboration: alkene isomerization affords terminal selectivity. Chemistry20, 13918–13922 (2014). [DOI] [PubMed]
- 58.Palmer WN, Diao T, Pappas I, Chirik PJ. High-activity cobalt catalysts for alkene hydroboration with electronically responsive terpyridine and α-diimine ligands. ACS Catal. 2015;5:622–626. doi: 10.1021/cs501639r. [DOI] [Google Scholar]
- 59.Scheuermann ML, Johnson EJ, Chirik PJ. Alkene isomerization–hydroboration promoted by phosphine-ligated cobalt catalysts. Org. Lett. 2015;17:2716–2719. doi: 10.1021/acs.orglett.5b01135. [DOI] [PubMed] [Google Scholar]
- 60.Peng J, Docherty JH, Dominey AP, Thomas SP. Cobalt-catalysed Markovnikov selective hydroboration of vinylarenes. Chem. Commun. 2017;53:4726–4729. doi: 10.1039/C7CC01085K. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Cheng Z, Guo J, Lu Z. Asymmetric remote C-H borylation of internal alkenes via alkene isomerization. Nat. Commun. 2018;9:3939. doi: 10.1038/s41467-018-06240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, et al. Cobalt-catalyzed regioselective olefin isomerization under kinetic control. J. Am. Chem. Soc. 2018;140:6873–6882. doi: 10.1021/jacs.8b01815. [DOI] [PubMed] [Google Scholar]
- 63.Meng Q-Y, Schirmer TE, Katou K, König B. Controllable isomerization of alkenes by dual visible-light-cobalt catalysis. Angew. Chem. Int. Ed. 2019;58:5723–5728. doi: 10.1002/anie.201900849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu S, Wu C, Ge S. Cobalt-catalyzed asymmetric hydroboration/cyclization of 1,6-enynes with pinacolborane. J. Am. Chem. Soc. 2017;139:6526–6529. doi: 10.1021/jacs.7b01708. [DOI] [PubMed] [Google Scholar]
- 65.Wang C, Ge S. Versatile cobalt-catalyzed enantioselective entry to boryl-functionalized all-carbon quaternary stereogenic centers. J. Am. Chem. Soc. 2018;140:10687–10690. doi: 10.1021/jacs.8b06814. [DOI] [PubMed] [Google Scholar]
- 66.Wu C, Liao J, Ge S. Cobalt-catalyzed enantioselective hydroboration/cyclization of 1,7-enynes: asymmetric synthesis of chiral quinolinones containing quaternary stereogenic centers. Angew. Chem. Int. Ed. 2019;58:8882–8886. doi: 10.1002/anie.201903377. [DOI] [PubMed] [Google Scholar]
- 67.Jo W, Kim J, Choi S, Cho SH. Transition-metal-free regioselective alkylation of pyridine N-oxides using 1,1-diborylalkanes as alkylating reagents. Angew. Chem. Int. Ed. 2016;55:9690–9694. doi: 10.1002/anie.201603329. [DOI] [PubMed] [Google Scholar]
- 68.Green MLH. A new approach to the formal classification of covalent compounds of the elements. J. Organomet. Chem. 1995;500:127–148. doi: 10.1016/0022-328X(95)00508-N. [DOI] [Google Scholar]
- 69.Ai W, Zhong R, Liu X, Liu Q. Hydride transfer reactions catalyzed by cobalt complexes. Chem. Rev. 2019;119:2876–2953. doi: 10.1021/acs.chemrev.8b00404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.