Abstract
Aim
This cross-sectional analysis of the New Orleans Alcohol Use in HIV (NOAH) study assesses whether current and lifetime alcohol use in people living with HIV (PLWH) are associated with greater liver disease and how hepatitis C-viral (HCV) co-infection (HIV/HCV+) modifies the association.
Methods
Alcohol use was measured by Lifetime Drinking History (LDH), a 30-day Timeline Followback calendar, the Alcohol Use Disorder Identification Test, and phosphatidylethanol. Liver disease was estimated by alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST platelet ratio-index (APRI), fibrosis-4 index (FIB-4) and nonalcoholic fatty liver disease-fibrosis score. Associations between alcohol consumption and liver disease were estimated with multivariable logistic regression. Models were adjusted for age, sex, body-mass index, hepatitis B and HIV viral load.
Results
Participants (N = 353) were majority male (69%) and black (84%) with a mean age of 48.3 ± 10 years. LDH was significantly associated with advanced liver fibrosis (FIB-4 aOR = 22.22 [1.22–403.72]) only among HIV/HCV+ participants with an LDH of 100–600 kg. HIV/HCV+ participants had a higher prevalence of intermediate and advanced liver disease markers than HIV/HCV− (P < 0.0001). Advanced markers of liver disease were most strongly associated with hazardous drinking (≥40(women)/60(men) grams/day) (APRI aOR = 15.87 (3.22–78.12); FIB-4 aOR = 6.76 (1.81–7.16)) and PEth ≥400 ng/ml (APRI aOR = 17.52 (2.55–120.54); FIB-4 aOR = 17.75 (3.30–95.630).
Conclusion
Results indicate a greater association of current alcohol use with liver disease than lifetime alcohol use, which varied by HCV status. These findings stress the importance of reducing alcohol use in PLWH to decrease risk of liver disease and fibrosis.
This cross-sectional analysis of NOAH study participants aimed to compare the association between alcohol drinking measures and liver disease markers among people living with HIV (PLWH). Current drinking was more significantly associated with liver disease than lifetime drinking, and co-infection with hepatitis C modified results.
INTRODUCTION
Increased uptake of effective combination antiretroviral therapy (cART) in recent years has allowed people living with HIV (PLWH) to extend survival approaching that of HIV-negative counterparts (Lee et al., 2001). This, in turn, has limited AIDS-related death and increased the opportunity for the development of chronic comorbidities associated with aging and lifestyle behaviors among PLWH. One of these comorbidities is chronic liver disease, which remains one of the leading causes of morbidity and mortality in PLWH (Chaudhry et al., 2009). The main contributor to this is hepatitis C virus (HCV) (Thornton et al., 2017), which affects an estimated 25% of PLWH in the USA (Centers for Disease Control, 2019). Both HIV and HCV are independently associated with liver disease progression, with coinfection of HIV and HCV further accelerating liver disease (Gaslightwala and Bini, 2006; Labarga et al., 2015).
Although there are numerous ways in which liver disease manifests, the most serious is liver fibrosis and when untreated, liver fibrosis advances to liver cirrhosis and in some cases to hepatocellular carcinoma (American Liver Foundation, 2019). The gold standard for the clinical assessment of hepatic fibrosis is liver biopsy (Gebo et al., 2002; American Association for the Study of Liver Disease, 2019). However, this procedure is invasive and subject to sampling error (Regev et al., 2002). In contrast, noninvasive markers of liver disease that utilize liver enzymes such as aspartate aminotransferase (AST) to platelet ratio index (APRI) and fibrosis-4 index score (FIB-4) correlate well with liver biopsy findings and have been validated for use in both HIV and HCV populations (Vallet-Pichard et al., 2007; Adler et al., 2008; Sebastiani et al., 2008). Studies have shown that subjects with HCV have increased risk of moderate to severe fibrosis, as indicated by these markers (Adler et al., 2008; Fuster et al., 2012). Increasing evidence suggests that HIV mono-infection can also induce fibrotic changes (Pembroke et al., 2017).
Alcohol use can further exacerbate liver injury produced by HIV and HCV alone or when co-infection exists (Rosenthal et al., 2003; Bilal et al., 2016). Both HIV+ and HCV− infected individuals demonstrate higher rates of hazardous drinking and alcohol use disorders (Hahn and Samet, 2010), which have been associated with greater susceptibility to viral disease progression (Chander et al., 2006; Baum et al., 2010) and development of chronic comorbidities. Alcohol consumption has been well documented to accelerate liver disease progression among those monoinfected with HCV (Muga et al., 2012), and a 2014 study found that heavy alcohol consumption may be more predictive of liver disease progression than HCV coinfection among patients with HIV (HIV/HCV) (Mankal et al., 2015). Indeed, higher Alcohol Use Disorder Identification Test (AUDIT-C) scores are associated with advanced hepatic fibrosis among HIV, HCV, HIV/HCV co-infected and noninfected individuals, with the greatest association being among HIV/HCV individuals (Lim et al., 2014).
Previous studies on the association between alcohol consumption and liver disease in PLWH that have focused on current drinking patterns, have failed to include biomarkers of alcohol assessment (Benhamou et al., 1999; Justice et al., 2006; Blackard et al., 2011). Furthermore, these studies reported conflicting data on the effect of alcohol consumption on liver disease among HIV/HCV patients (Benhamou et al., 1999; Blackard et al., 2011). Few studies have had sufficient data on participants’ alcohol consumption patterns to effectively compare associations of long-term and current alcohol use with markers of liver disease in PLWH or have not seen an association between alcohol use and liver fibrosis in those with HIV and HIV/HCV coinfection (Blackard et al., 2011; Fuster et al., 2012; Muga et al., 2012).
The primary objective of this study was to assess whether lifetime drinking history (LDH) and current hazardous or harmful drinking, as defined by National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines, AUDIT and the biological marker phosphatidylethanol (PEth) were associated with liver disease as measured by APRI and FIB-4. We additionally examined the association of these alcohol use measures with indicators of liver injury such as abnormal AST and alanine aminotransferase (ALT) levels and nonalcoholic fatty liver disease fibrosis score (NAFLD-FS). Our secondary objective was to examine whether coinfection with HCV acts as an effect modifier in the association between alcohol consumption and liver disease markers in PLWH.
METHODS
Study population
This was a cross-sectional analysis of the New Orleans Alcohol Use in HIV (NOAH) study, a longitudinal study conducted by the Comprehensive Alcohol Research Center (CARC) at the Louisiana State University Health Sciences Center in New Orleans, Louisiana. The NOAH study is a translational investigation of alcohol use disorder, HIV, and ART in aging and exacerbation of comorbid conditions in an underserved cohort of PLWH. NOAH participants are adults (≥18 years old) with an HIV/AIDS diagnosis who are currently under care. Additional NOAH study methods have been previously published (Welsh et al., 2019). Of the 365 participants in the NOAH study, 353 had available information on liver disease markers and thus were included in the current analysis. There was no significant difference in demographic characteristics between those included and excluded due to missing liver disease markers (P-value ranged from 0.08 to 0.98). A medical record of a positive HCV ribonucleic acid (RNA) test was used to define diagnosis of HCV in this population, yielding a total of 56 (16%) participants who were co-infected with HIV and HCV.
Alcohol exposure
The primary exposure of interest, LDH, was defined as alcohol consumption over the life course and was measured in kilograms. This drinking measure, aimed at quantifying long-term alcohol exposure and drinking patterns, was assessed through a structured interview in which the participant was asked about alcohol consumption patterns spanning from the first year of regular drinking to the present. A standard drink was equated to 14 g of alcohol, and the total grams of alcohol consumed was then converted to kilograms of alcohol consumed. When categorizing participants’ LDH, the cut points used were <100, 100–600, and >600 kg. The reference category of <100 kg LDH was chosen using previously established evidence that alcoholic liver disease is less likely to develop below a lifetime alcohol ingestion of 100 kg, which equates to a daily alcohol intake of 30 g (or about 2 standard drinks/day) over a span of 10 years (Bellentani and Tiribelli, 2001). The second clinically relevant threshold of 600 kg was selected due to its approximate correspondence to a drinking pattern of >4 standard drinks a day for 28 years, as four standard drinks per day has been established as a threshold for advanced liver fibrosis in HCV infection (Fuster et al., 2013).
The 30-day Timeline Followback (TLFB) calendar was used to obtain estimates of daily alcohol consumption in the past 30 days by asking NOAH participants to retrospectively recount the number of drinks consumed on each day. These data were used to calculate alcohol consumption (in grams) per day and used to assess hazardous drinking as defined by NIAAA ≥40 g/day for females and ≥60 g/day for males.
The AUDIT, developed by the World Health Organization, consists of 10 questions that result in a score between 0 and 40 (Alcohol Use Disorders Identification Test, 2019). An AUDIT score <8 corresponds to a low risk for AUD, a score between 8 and 15 corresponds to a moderate risk for AUD, and a score ≥16 corresponds to a high risk of AUD.
Serum phosphatidylethanol (PEth) is a biological marker that reflects alcohol use within an approximate 3–4-week period. PEth is an abnormal phospholipid that is only formed in the presence of ethanol by the enzyme phospholipase D (Viel et al., 2012). As defined by previously established relevant thresholds (Afshar et al., 2017), participants were categorized as showing no indication of alcohol misuse (PEth <250 ng/ml), any misuse of alcohol (250–400 ng/ml) or severe misuse of alcohol.
Liver outcomes
AST to Platelet Ratio Index (APRI). Widely accepted as a noninvasive alternative to liver biopsy, APRI is a tool for the assessment of liver fibrosis. This measure is calculated using participants’ AST level, platelet count, and the upper limit of normal AST levels (Wai et al., 2003). When calculating APRI score, the upper normal limit used was 40 for all participants based on recent data (Neuschwander-Tetri et al., 2004). An APRI score >0.4 was defined as intermediate liver fibrosis and advanced liver fibrosis was defined as an APRI >1.5.
Fibrosis-4 (FIB-4). Like APRI, FIB-4 is a noninvasive tool used to measure liver fibrosis. Calculated using participants’ age, AST level, ALT level and platelet count, a FIB-4 index score of <1.45 has a negative predictive value of over 90% for advanced liver fibrosis and a score of >3.25 has a positive predictive value of 65% for advanced liver fibrosis and specificity of 97% (Sterling et al., 2006). Using these cut points, an FIB-4 index score >1.45 was classified as intermediate and a score >3.25 was classified as advanced liver fibrosis.
Additional Liver Enzymes. To further assess the association between alcohol consumption and liver dysfunction, liver enzyme levels indicative of hepatic damage were also measured. AST is also produced in smaller amounts by the heart, kidneys, brain and muscles, making it less specific for the diagnosis of liver disease. Although an upper limit of ALT 40 U/L was used in this study for determining APRI and FIB-4 associations, there is evidence that ALT levels in the historically “normal range” may suggest liver injury and that these thresholds appear to differ for females and males (Kim et al., 2004). Therefore, we performed a secondary analysis of abnormal ALT defined as >19 U/L for females and >30 U/L for males and AST >40 U/L as an indicator of liver injury for both females and males (Kim et al., 2008). Finally, because of the high prevalence of nonalcoholic fatty liver disease in HIV+ patients, we included the Non-Alcoholic Fatty Liver Disease Fibrosis Score (NAFLD-FS) (Angulo et al., 2007; Crum-Cianflone et al., 2009). This measure is derived from fasting glucose, age, AST levels, ALT levels, platelet count, body mass index (BMI) and albumin levels. We defined an NAFLD-FS of ≤−1.455 as normal, >−1.455 as intermediate and >0.676 as an indication of advanced liver fibrosis.
Statistical analysis
Descriptive statistics were used to summarize patient demographics in this population, stratified by LDH to evaluate differences in potential confounding variables among those with varying long-term alcohol exposure. To assess differences in current and lifetime drinking patterns among co-infected with HIV/HCV+ and those PLWH without HCV (HIV/HCV−), chi-squared tests were conducted to test for differences in proportions for LDH, TLFB, AUDIT and PEth categories. Bivariate analyses were also conducted to examine all markers of liver disease among HIV/HCV+ and HIV/HCV−.
Multinomial logistic regression was used to determine differences in intermediate and advanced indications of liver fibrosis (APRI, FIB-4, NAFLD-FS, ALT and AST). To assess the effect of alcohol use on liver disease, multinomial logistic regression was used and all models were adjusted for age, sex, BMI, hepatitis B virus (HBV), HIV viral load and current drinking (quantified by PEth) or conversely LDH. To assess potential effect modification by HCV, an interaction term was added to the lifetime alcohol use model and current alcohol use model. Results were then stratified by HCV status. All significance testing was conducted at an alpha level of 0.05 and all analyses were conducted using SAS 9.4.
RESULTS
The average age of participants was 48.3 with a standard deviation ±10.3 years, and the study population was 69.4% male and 83.9% black (Table 1). An estimated 38.3% of participants were normal weight and 28.9% were overweight as determined by BMI. The majority were current smokers (59.8%) and 16.7% were former smokers. More than 75% had an undetectable HIV viral load of ≤50 copies/ml. The overall prevalence of HBV in the sample was 5.1%. Only 0.9% of the NOAH study participants are co-infected with HCV and HBV. Those with an LDH >600 kg were more likely to be older (P < 0.0001), male (P = 0.001) and current smokers (P = 0.002) compared with those with an LDH <100 kg. There were no statistically significant differences in the distribution of race, BMI category, HIV viral load or diagnosis of HBV by the LDH categories.
Table 1.
Demographics of included participants from the NOAH study
| All participants (n = 353) | LDH* <100 kg (n = 155) | LDH 100–600 kg (n = 142) | LDH >600 kg (n = 56) | P-value | ||
|---|---|---|---|---|---|---|
| Mean age (SD) | 48.3 (10.3) | 44.7 (11.0) | 50.4 (9.4) | 52.7 (7.2) | <0.0001 | |
| % (n) | ||||||
| Sex | 0.001 | |||||
| Female | 30.6 (108) | 40.6 (63) | 23.2 (33) | 21.4 (12) | ||
| Male | 69.4 (245) | 59.4 (92) | 76.8 (109) | 78.6 (44) | ||
| Race | 0.448 | |||||
| Black | 83.9 (296) | 85.8 (133) | 81.7 (116) | 83.9 (47) | ||
| White | 15.3 (54) | 12.3 (19) | 18.3 (26) | 16.1 (9) | ||
| Other | 0.8 (3) | 1.9 (3) | - | - | ||
| BMI category | 0.099 | |||||
| Underweight | 4.3 (15) | 3.9 (6) | 3.6 (5) | 7.3 (4) | ||
| Normal weight | 38.3 (134) | 34.2 (53) | 45.0 (63) | 32.7 (18) | ||
| Overweight | 28.9 (101) | 30.3 (47) | 25.0 (35) | 34.6 (19) | ||
| Obese | 17.7 (62) | 16.8 (26) | 16.4 (23) | 23.6 (13) | ||
| Extremely obese (>35) | 10.9 (38) | 14.8 (23) | 10.0 (14) | 1.8 (1) | ||
| Smoking status | 0.002 | |||||
| Never | 23.5 (83) | 33.6 (52) | 16.9 (24) | 12.5 (7) | ||
| Former | 16.7 (59) | 14.2 (22) | 19.7 (28) | 16.1 (9) | ||
| Current | 59.8 (211) | 52.3 (81) | 63.4 (90) | 71.4 (40) | ||
| Viral load | 0.112 | |||||
| ≤50 | 75.6 (267) | 71.0 (110) | 78.9 (112) | 80.4 (45) | ||
| 51–200 | 8.2 (29) | 7.7 (12) | 7.8 (11) | 10.7 (6) | ||
| 201–1000 | 4.8 (17) | 4.5 (7) | 6.3 (9) | 1.8 (1) | ||
| >1000 | 11.3 (40) | 16.8 (26) | 7.0 (10) | 7.1 (4) | ||
| Hepatitis B | 5.1 (18) | 5.2 (8) | 7.0 (10) | - | 0.128 | |
LDH = lifetime drinking history; SD = standard deviation; BMI = body mass index.
Table A1.
Adjusted* odds ratios of intermediate and advanced liver fibrosis by liver disease markers by AUDIT and phosphatidylethanol (PEth), stratified by hepatitis-c viral (HCV) status, NOAH study.
| Intermediate liver damage/fibrosis | Advanced liver fibrosis | |||||||
|---|---|---|---|---|---|---|---|---|
| ALT | AST | NAFLD-FS | APRI | FIB-4 | NAFLD-FS | APRI | FIB-4 | |
| Odds ratio (95% CI) | Odds ratio (95% CI) | |||||||
| AUDIT <8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Moderate risk 8–16 | ||||||||
| All | 0.65 (0.33, 1.29) | 1.04 (0.43, 2.52) | 0.85 (0.41, 1.75) | 0.88 (0.41, 1.86) | 0.56 (0.26, 1.21) | 0.92 (0.27, 3.15) | 0.73 (0.12, 4.65) | 1.77 (0.47, 6.58) |
| HCV+ | 0.35 (0.10, 1.25) | 0.49 (0.12, 2.02) | 3.48 (0.62, 19.66) | 0.84 (0.23, 3.05) | 1.10 (0.21, 5.68) | 4.05 (0.31, 53.19) | - | 1.14 (0.11, 12.04) |
| HCV− | 0.81 (0.38, 1.75) | 1.61 (0.57, 4.57) | 0.63 (0.29, 1.39) | 0.83 (0.34, 2.05) | 0.47 (0.19, 1.14) | 0.66 (0.16, 2.66) | 1.70 (0.23, 12.45) | 3.67 (0.80, 16.93) |
| PEth <250 | Ref. | Ref. | Ref. | Ref. | ||||
| Any misuse 250–400 | ||||||||
| All | 3.83 (1.49, 9.83) | 7.70 (2.38, 24.94) | 1.45 (0.52, 4.04) | 4.86 (1.68, 14.11) | 3.33 (1.05, 10.56) | 1.99 (0.31, 12.67) | 25.39 (3.15, 205.08) | 17.52 (3.23, 95.18) |
| HCV+ | - | - | 0.39 (0.03, 5.79) | - | - | - | - | - |
| HCV− | 3.73 (1.38, 10.08) | 8.03 (2.20, 29.33) | 1.85 (0.59, 5.84) | 4.40 (4.31, 4.50) | 3.02 (0.92, 9.87) | 2.66 (0.39, 18.36) | 3.83 (3.67, 4.00) | 33.6 (4.00, 283.91) |
| PEth <250 | Ref. | Ref. | Ref. | Ref. | ||||
| ≥250 | ||||||||
| All | 2.60 (1.36, 5.00) | 5.00 (2.13, 11.69) | 3.28 (1.59, 6.79) | 4.13 (1.95, 8.75) | 3.71 (1.70, 8.14) | 4.57 (1.11, 18.87) | 20.23 (3.54, 115.48) | 17.92 (4.34, 74.01) |
| HCV+ | 0.83 (0.16, 4.21) | 1.78 (0.32, 10.02) | 4.33 (0.68, 27.69) | 1.79 (0.26, 12.10) | 14.54 (1.04, 203.45) | - | 19.98 (0.71, 559.91) | 8.52 (0.34, 215.46) |
| HCV− | 16.83 (2.46, 115.13) | 4.72 (2.14, 10.43) | 3.09 (1.56, 6.15) | 3.15 (1.45, 6.82) | 3.11 (1.37, 7.06) | 4.85 (1.13, 20.77) | 6.54 (2.53, 16.88) | 41.44 (6.48, 264.96) |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; APRI = AST to platelet ratio index; FIB-4 = fibrosis-4; NAFLD FS = non-alcoholic fatty liver disease fibrosis score.
*All models adjusted for age, sex, BMI, hepatitis-B virus status, smoking status, viral load and lifetime drinking history.
The results of the bivariate analyses conducted to compare alcohol use measures among PLWH with and without HCV (HCV+/−) are shown in Fig. 1. The only alcohol consumption measure that was significantly different between HIV/HCV+ and HIV/HCV− was LDH (P = 0.019), with more HIV/HCV+ participants having an LDH >600 kg (26.8 vs. 13.8%) (Fig. 1A). Those with HIV/HCV+ coinfection had significantly higher rates of all noninvasive markers of liver disease than their HIV/HCV−counterparts (P < 0.0001) except for advanced liver fibrosis as measured by APRI and NAFLD-FS (P > 0.05). Prevalence of intermediate liver fibrosis ranged from 41.1 to 64.3% among those with HIV/HCV+ coinfection and 8.8–45.8% among HIV/HCV−.
Fig. 1.
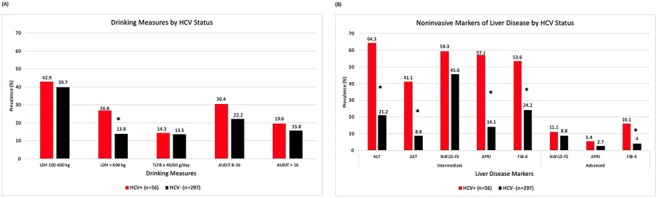
Prevalence of current and lifetime drinking measures (A) and noninvasive markers of liver disease (B) among NOAH study participants, stratified by HCV status.
Figure 2 illustrates the relationship between LDH and PEth and liver disease markers (APRI and FIB-4) by HCV status. Fig. 2A and 2B shows that HIV/HCV+ co-infected participants have higher liver disease markers than HIV monoinfected participants regardless of LDH. There is a positive association between PEth and APRI for both HIV/HCV+ co-infected and HIV/HCV−, and PEth and FIB-4 among HIV/HCV−.
Fig. 2.
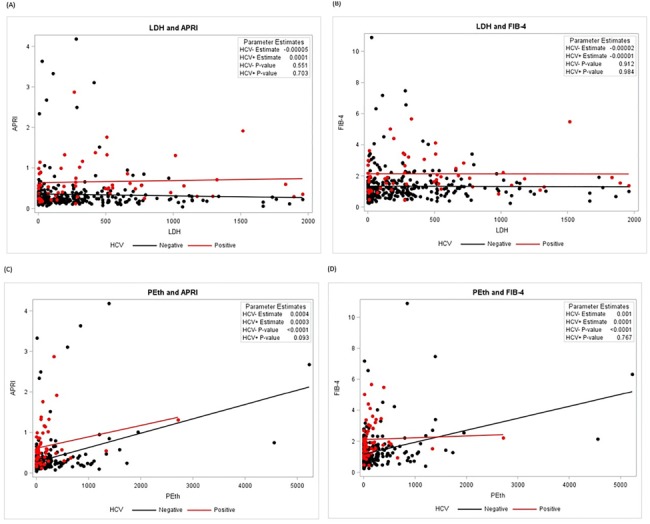
Alcohol use measures and liver disease markers by HCV status among people living with HIV, NOAH study.
Multinomial logistic regression analyses of LDH categories and liver disease markers showed very few statistically significant associations (Table 2). In stratified analyses, associations between LDH and markers of liver disease had higher magnitude among HIV/HCV+ participants, but again did not reach statistical significance for any measure of liver disease except for FIB-4. Compared to LDH <100 kg, LDH 100–600 kg had an association with advanced liver fibrosis (FIB-4 aOR = 21.89 (95% confidence interval [CI]: 1.19, 402.36).
Table 2.
Adjusted* odds ratios of intermediate and advanced liver fibrosis by liver disease markers for lifetime alcohol use patterns, stratified by HCV status, the NOAH study
| Intermediate liver damage/fibrosis | Advanced liver fibrosis | |||||||
|---|---|---|---|---|---|---|---|---|
| ALT | AST | NAFLD-FS | APRI | FIB-4 | NAFLD-FS | APRI | FIB-4 | |
| LDH | Odds ratios (95% CI) | Odds ratios (95% CI) | ||||||
| <100 kg 100–600 kg | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| All | 1.09 (0.58, 2.04) | 1.76 (0.71, 4.37) | 1.73 (0.91, 3.30) | 1.04 (0.50, 2.16) | 1.09 (0.55, 2.16) | 1.49 (0.48, 4.68) | 1.93 (0.37, 10.17) | 2.67 (0.68, 10.49) |
| HCV+ | 1.36 (0.36, 5.18) | 2.43 (0.52, 11.43) | 0.89 (0.16, 4.90) | 2.28 (0.54, 9.67) | 4.65 (0.57, 38.13) | 5.88 (0.31, 113.41) | - | 21.89 (1.19, 402.36) |
| HCV− | 1.05 (0.53, 2.09) | 1.50 (0.51, 4.44) | 1.93 (0.97, 3.87) | 0.81 (0.35, 1.89) | 0.94 (0.45, 1.97) | 1.04 (0.29, 3.69) | 1.41 (0.24, 8.22) | 1.41 (0.28, 7.11) |
| >600 kg | ||||||||
| All | 1.55 (0.70, 3.44) | 2.46 (0.86, 7.06) | 1.03 (0.46, 2.33) | 0.86 (0.35, 2.15) | 0.41 (0.16, 1.00) | 0.50 (0.10, 2.58) | 0.29 (0.02, 5.04) | 0.22 (0.03, 1.75) |
| HCV+ | 3.82 (0.75, 19.53) | 3.58 (0.67, 19.13) | 0.41 (0.07, 2.42) | 1.17 (0.25, 5.38) | 0.31 (0.06, 1.75) | 0.89 (0.03, 26.63) | - | 0.19 (0.01, 5.72) |
| HCV− | 1.13 (0.44, 2.91) | 1.94 (0.50, 7.56) | 1.31 (0.52, 3.29) | 0.84 (0.28, 2.52) | 0.48 (0.17, 1.33) | 0.45 (0.07, 3.00) | - | 0.31 (0.02, 3.90) |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; NAFLD-FS = non-alcoholic fatty liver disease fibrosis score; APRI = AST to platelet ratio index; FIB-4 = fibrosis-4.
*All models adjusted for age, sex, BMI, hepatitis-B virus status, smoking status, viral load and PEth concentration.
Results for current alcohol use and liver disease markers are shown in Table 3. Among the entire sample, TLFB hazardous drinking was associated with intermediate liver disease [ALT aOR = 3.33 (1.58, 7.02); AST aOR = 5.74 (1.40, 7.85); APRI aOR = 2.44 (1.02, 5.81); NAFLD-FS aOR = 3.31 (1.40, 7.85)], and advanced liver fibrosis [APRI aOR = 15.87 (3.22, 78.12); FIB-4 aOR = 6.76 (1.81, 25.33)]. In contrast, in HIV/HCV− individuals, hazardous drinking was significantly associated with intermediate liver disease/fibrosis [ALT aOR = 2.94 (1.35, 6.39); AST aOR = 5.57 (2.15, 14.48); NAFLD-FS aOR = 4.55 (1.76, 11.73); APRI aOR = 2.90 (1.18, 7.16)] and advanced liver fibrosis [APRI aOR = 11.79 (2.17, 64.16); FIB-4 aOR = 7.68 (1.90, 31.08)]. While moderate AUD risk was not significantly associated with liver fibrosis (Appendix A), high risk of AUD (Table 3) was significantly associated with advanced fibrosis [APRI aOR = 7.07 (1.31, 38.26; FIB-4 aOR = 5.83 (1.44, 23.68)] and intermediate liver disease (AST aOR = 3.12 (1.22, 7.99)]. In stratified analysis, HIV/HCV+ participants with a high risk of AUD were significantly associated with intermediate liver disease [AST aOR = 8.81 (1.36, 57.19)]; and among HIV/HCV− participants, there was a ninefold increased odds of having an advanced liver fibrosis with APRI score (aOR = 8.91 (1.56, 50.90)) and a sevenfold increased odds with FIB-4 score (aOR = 7.08 (1.60, 31.25)). The interaction terms to assess effect modification for HCV status did not reach statistical significance.
Table 3.
Adjusted* odds ratios of intermediate and advanced liver fibrosis by liver disease markers for current alcohol use patterns, stratified by HCV status, the NOAH study
| Intermediate liver damage/fibrosis | Advanced liver fibrosis | |||||||
|---|---|---|---|---|---|---|---|---|
| ALT | AST | NAFLD-FS | APRI | FIB-4 | NAFLD-FS | APRI | FIB-4 | |
| Odds ratios (95% CI) | Odds ratios (95% CI) | |||||||
| TLFB hazardous ≥40/60 | ||||||||
| All | 3.33 (1.58, 7.02) | 5.74 (2.33, 14.10) | 3.31 (1.40, 7.85) | 2.44 (1.02, 5.81) | 1.79 (0.74, 4.32) | 4.08 (0.81, 20.60) | 15.87 (3.22, 78.12) | 6.76 (1.81, 25.33) |
| HCV+ | - | 6.98 (0.64, 75.60) | 0.45 (0.05, 4.00) | 1.06 (0.14, 8.11) | 1.52 (0.17, 13.93) | 6.48 (0.28, 149.68) | 67.55 (1.09, 765.63) | 3.68 (0.23, 60.33) |
| HCV− | 2.94 (1.35, 6.39) | 5.57 (2.15, 14.48) | 4.55 (1.76, 11.73) | 2.90 (1.18, 7.16) | 1.81 (0.71, 4.62) | 3.62 (0.56, 23.32) | 11.79 (2.17, 64.16) | 7.68 (1.90, 31.08) |
| AUDIT high risk >16 | ||||||||
| All | 1.53 (0.72, 3.29) | 3.12 (1.22, 7.99) | 1.24 (0.56, 2.75) | 1.38 (0.57, 3.37) | 0.76 (0.31, 1.88) | 1.40 (0.28, 7.07) | 7.07 (1.31, 38.26) | 5.83 (1.44, 23.68) |
| HCV+ | 5.18 (0.56, 48.25) | 8.81 (1.36, 57.19) | 0.73 (0.13, 4.27) | 2.78 (0.51, 14.93) | 0.49 (0.08, 2.90) | 2.37 (0.12, 48.21) | 4.43 (0.13, 147.70) | 2.46 (0.19, 31.60) |
| HCV− | 1.29 (0.57, 2.94) | 2.30 (0.80, 6.67) | 1.36 (0.58, 3.16) | 1.49 (0.55, 4.05) | 0.82 (0.31, 2.17) | 1.14 (0.18, 7.25) | 8.91 (1.56, 50.90) | 7.08 (1.60, 31.25) |
| PEth severe misuse >400 | ||||||||
| All | 2.05 (0.93, 4.52) | 3.90 (1.45, 10.49) | 5.51 (2.23, 13.62) | 3.75 (1.55, 9.07) | 3.96 (1.56, 10.07) | 8.43 (1.32, 53.72) | 17.52 (2.55, 120.5) | 17.75 (3.30, 95.63) |
| HCV+ | 0.29 (0.04, 2.10) | 0.63 (0.08, 5.13) | 26.09 (1.97, 345.82) | 1.40 (1.33, 1.48) | 16.57 (1.12, 244.2) | - | 0.41 (0.35, 0.49) | - |
| HCV− | 2.77 (1.23, 6.25) | 5.89 (2.03, 17.07) | 4.42 (1.71, 11.42) | 3.18 (3.12, 3.24) | 3.16 (1.17, 8.51) | 7.66 (1.18, 49.85) | 26.20 (25.71, 26.70) | 46.95 (6.15, 358.70) |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; NAFLD-FS = non-alcoholic fatty liver disease fibrosis score; APRI = AST to platelet ratio index; FIB-4 = fibrosis-4.
*All models adjusted for age, sex, BMI, hepatitis-B virus status, smoking status, viral load and lifetime drinking history.
Moderate risk of AUD was not significantly associated with any liver disease markers among HIV/HCV− or HIV/HCV+ co-infected participants (Appendix A). Any misuse of alcohol identified by PEth was significantly associated with intermediate liver disease as measured by APRI, FIB-4, ALT, and AST and advanced liver disease/fibrosis with APRI and FIB-4.
DISCUSSION
This study aimed to investigate whether current and lifetime alcohol use patterns are associated with markers of liver disease in a predominantly virally suppressed majority black cohort of PLWH. We found that advanced markers of liver disease were more strongly associated with hazardous drinking in the last 30 days. LDH was only significantly associated with advanced liver fibrosis as measured by FIB-4. Our results show that HIV/HCV+ co-infected individuals had a higher prevalence of intermediate or advanced fibrosis than HIV/HCV− participants, suggesting that HCV exacerbates liver damage in PLWH. However, the effects of HCV status were not consistent and the interaction term for HCV and alcohol did not reach statistical significance.
One of the main findings of our analysis is that current drinking patterns in the NOAH study were more associated with liver disease among both HIV/HCV− and HIV/HCV+ co-infected individuals than LDH. This was consistent with previous studies that have found significant associations between both AUDIT-C (Lim et al., 2014) and current hazardous drinking (Muga et al., 2012) with fibrosis markers. While all of the drinking measures that captured current alcohol use patterns were significantly associated with at least one of the markers of liver injury, TLFB hazardous drinking and PEth concentrations were significantly associated with most markers, with PEth concentrations showing slightly greater association. Among HIV/HCV+ co-infected patients, a PEth concentration >400 was significantly associated with intermediate liver disease (APRI and FIB-4) and was significantly associated with the presence of all liver disease markers in HIV/HCV− participants. Unlike the other measures of current and lifetime alcohol use utilized in this study, PEth is a biological marker and does not rely on self-report by participants. While LDH, TLFB and AUDIT are all validated and widely used screening tools for alcohol use and abuse, they are still subject to both recall and stigma biases, both of which could have impacted our findings.
Our results are in partial contrast to those conducted by Fuster et al. that found no statistically significant associations between lifetime alcohol exposure and liver fibrosis markers in HIV/HCV+ patients (Fuster et al., 2013). Our primary exposure of interest, LDH, was significantly positively associated with advanced liver fibrosis (FIB-4 score) among those with HIV/HCV+ for LDH of 100–600 kg. In contrast to our hypothesis, PLWH with higher LDH (>600 kg) showed a lower association with liver fibrosis. We speculate that this is not suggestive that higher lifetime alcohol exposure is associated with lower risk of liver damage. Rather, we believe this is likely due to either discontinuation of current alcohol use because of health concerns related to lifetime alcohol exposure of this caliber (Shaper et al., 1988). Conversely, continued heavy alcohol use in healthier PLWH could erroneously have resulted in an apparent protective effect of increased alcohol consumption. This pattern of alcohol abstention parallels the “sick-quitter” hypothesis. This hypothesis, first proposed by Shaper et al., states that groups of abstainers in studies include many former drinkers who quit drinking because of illness or alcohol’s interaction with prescription drugs (Shaper et al., 1988). While the hepatic injury done by alcohol can be reversed by this period of abstinence, the cumulative nature of LDH does not allow for the classification of exposure to be reduced, which could weaken the association.
Our finding could have also been affected by the standard calculation of lifetime drinking history that average alcohol consumption by decade and the heavier months or years of drinking are averaged over a larger period of time potentially decreasing the intensity of the consumption. While 600 kg of alcohol could equate to an average of two drinks a day for about 59 years, it could also be six drinks a day for roughly 20 years. These patterns of use correspond to significantly different levels of health risk for participants but would result in the same classification of LDH.
Strengths of our study include that this is the first study to our knowledge to examine four distinct drinking measures of alcohol use with noninvasive markers of liver disease in a population of PLWH both with and without HCV. In addition to the robust nature of exposure information, this study had the strength of access to a relatively virally suppressed cohort of PLWH. Approximately, 97% of participants are currently on ART and 75% had an undetectable HIV viral load. These participants were all under care in the New Orleans metropolitan area. This provision makes our findings more clinically relevant in the age of higher rates of antiretroviral treatment and suppression among PLWH. Our population also differed from previous studies in that we included men and women, mostly African Americans that are in care.
There are several limitations to consider when interpreting the findings of this study. First and foremost, the 16% prevalence of HCV in this population of HIV+ individuals is lower than anticipated. This led to extremely wide confidence intervals on parameter estimates (or an inability to obtain estimates) for HIV/HCV+ co-infected individuals. This could have also impacted the ability to detect a statistically significant interaction term in our models as we explored effect modification by HCV status. An additional limitation is that our analysis was cross sectional in nature, which allowed us to only consider alcohol use and liver disease markers at one time point. This is an important consideration because all measures utilized either AST or ALT in their calculations, and these enzyme levels can fluctuate over time, possibly leading to outcome misclassification. To reduce this potential for misclassification, outcome status was also evaluated as categorical rather than continuous. As previously mentioned, another limitation stems from the self-report method of data collection to classify exposure status of LDH, TLFB and AUDIT. While there was a possibility for misclassification of exposure status resulting from participants’ underreporting alcohol use, this misclassification would have likely diluted true associations. The final limitation of this study was that it only consists of HIV+ participants, precluding dissection of the relationship between HIV status, alcohol use and HCV. Future studies will expand recruitment to HIV− subjects.
Despite the limitations of the current study, there are several potential implications for clinical practice suggested by the findings. While HIV/HCV+ co-infected participants seem to engage less in hazardous or risky drinking than HIV/HVC- participants, they were still active alcohol consumers, despite a surplus of evidence on the detrimental effects of HIV, HCV, and alcohol use on the liver (Lim et al., 2014; Mankal et al., 2015). This suggests a need for additional counseling and information dissemination on the topic of alcohol use in this population. This study also highlights the necessity of using alcohol-related biological markers in this clinical setting. When biological markers are not feasible, clinicians should consider multiple alcohol measures in PLWH, both with and without concurrent HCV infection, when classifying disease risk. Very few of the alcohol use measures were significantly associated with all markers of liver disease in this population, so using the alcohol use measures to supplement one another has the potential to lead to more accurate identification of those at risk of developing liver disease, thus reducing the burden of advanced liver disease in this population.
Acknowledgements
This work is supported by the National Institutes of Health award P60 AA009803. We thank the NOAH study participants for their commitment to participate. The authors acknowledge the investigators and research staff affiliated with the LSUHSC Comprehensive Alcohol-HIV/AIDS Research Center for their scientific contributions, including Virginia Garrison, Rebecca Gonzales, Rhonda Martinez, Erin Meyaski, Mary Meyaski-Schluter, Ikenna Nnamani, Oluwaseun Oguntomole, Connie Porretta, Wendemi Sawadogo, Jane Schexnayder, Aneisha Simon, Curtis Vande Stouwe and Arnold Zea.
References
- Adler M, Gulbis B, Moreno C, et al. (2008) The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology 47:762–3author reply 763. [DOI] [PubMed] [Google Scholar]
- Afshar M, Burnham EL, Joyce C, et al. (2017) Cut-point levels of phosphatidylethanol to identify alcohol misuse in a mixed cohort including critically ill patients. Alcohol Clin Exp Res 41:1745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcohol Use Disorders Identification Test Alcohol Use Disorders Identification Test (AUDIT) [online] in2019. https://auditscreen.org/.
- American Association For The Study Of Liver Disease Practice Guidelines|AASLD [online] in2019. https://www.aasld.org/publications/practice-guidelines-0.
- American Liver Foundation The Stages of Liver Disease - American Liver Foundation [online] in2019. @liverUSA https://liverfoundation.org/for-patients/about-the-liver/the-progression-of-liver-disease/.
- Angulo P, Hui JM, Marchesini G, et al. (2007) The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45:846–54. [DOI] [PubMed] [Google Scholar]
- Baum Mk, Rafie C, Lai S, et al. (2010) Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses 26:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Tiribelli C (2001) The spectrum of liver disease in the general population: Lesson from the Dionysos study. J Hepatol 35:531–7. [DOI] [PubMed] [Google Scholar]
- Benhamou Y, Bochet M, Di Martino V, et al. (1999) Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 30:1054–8. [DOI] [PubMed] [Google Scholar]
- Bilal U, Lau B, Lazo M, et al. (2016) Interaction between alcohol consumption patterns, antiretroviral therapy type, and liver fibrosis in persons living with HIV. AIDS Patient Care STDS 30:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard JT, Welge JA, Taylor LE, et al. (2011) HIV mono-infection is associated with FIB-4 - a noninvasive index of liver fibrosis - in women. Clin Infect Dis 52:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers For Disease Control Coinfection with HIV and Viral Hepatitis |Division of Viral Hepatitis|CDC [online] in2019. https://www.cdc.gov/hepatitis/hiv-hepatitis-coinfection.htm.
- Chander G, Lau B, Moore RD (2006) Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr 43:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry AA, Sulkowski MS, Chander G, et al. (2009) Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med 10:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone N, Dilay A, Collins G, et al. (2009) Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr 50:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster D, Tor J, Rey-Joly C, et al. (2012) Pathogenic interactions between alcohol and hepatitis C. Med Clin (Barc) 138:627–32. [DOI] [PubMed] [Google Scholar]
- Fuster D, Tsui JI, Cheng DM, et al. (2013) Impact of lifetime alcohol use on liver fibrosis in a population of HIV-infected patients with and without hepatitis C coinfection. Alcohol Clin Exp Res 37:1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaslightwala I, Bini Ej (2006) Impact of human immunodeficiency virus infection on the prevalence and severity of steatosis in patients with chronic hepatitis C virus infection. J Hepatol 44:1026–32. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Herlong HF, Torbenson MS, et al. (2002) Role of liver biopsy in management of chronic hepatitis C: A systematic review. Hepatology 36:S161–72. [DOI] [PubMed] [Google Scholar]
- Hahn JA, Samet JH (2010) Alcohol and HIV disease progression: Weighing the evidence. Curr HIV/AIDS Rep 7:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, et al. (2006) Veterans aging cohort study (VACS): Overview and description. Med Care 44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HC, Nam CM, Jee SH, et al. (2004) Normal serum aminotransferase concentration and risk of mortality from liver diseases: Prospective cohort study. BMJ 328:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WR, Flamm SL, DI Bisceglie AM, et al. (2008) Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 47:1363–70. [DOI] [PubMed] [Google Scholar]
- Labarga P, Fernandez-Montero JV, DE Mendoza C, et al. (2015) Liver fibrosis progression despite HCV cure with antiviral therapy in HIV-HCV-coinfected patients. Antivir Ther 20:329–34. [DOI] [PubMed] [Google Scholar]
- Lee LM, Karon JM, Selik R, et al. (2001) Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984-1997. JAMA 285:1308–15. [DOI] [PubMed] [Google Scholar]
- Lim JK, Tate JP, Fultz SL, et al. (2014) Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis 58:1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankal PK, Abed J, Aristy JD, et al. (2015) Relative effects of heavy alcohol use and hepatitis C in decompensated chronic liver disease in a hospital inpatient population. Am J Drug Alcohol Abuse 41:177–82. [DOI] [PubMed] [Google Scholar]
- Muga R, Sanvisens A, Fuster D, et al. (2012) Unhealthy alcohol use, HIV infection and risk of liver fibrosis in drug users with hepatitis C. PLoS ONE 7:e46810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Ünalp A, Creer MH (2004) The upper limits of normal for serum ALT levels reported by clinical laboratories depend on local reference populations. Arch Intern Med 168:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembroke T, Deschenes M, Lebouche B, et al. (2017) Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J Hepatol 67:801–8. [DOI] [PubMed] [Google Scholar]
- Regev A, Berho M, Jeffers LJ, et al. (2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 97:2614–8. [DOI] [PubMed] [Google Scholar]
- Rosenthal E, Poiree M, Pradier C, et al. (2003) Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study). Aids 17:1803–9. [DOI] [PubMed] [Google Scholar]
- Sebastiani G, Vario A, Guido M, et al. (2008) Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 15:212–8. [DOI] [PubMed] [Google Scholar]
- Shaper AG, Wannamethee G, Walker M (1988) Alcohol and mortality in British men: Explaining the U-shaped curve. Lancet 2:1267–73. [DOI] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, et al. (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43:1317–25. [DOI] [PubMed] [Google Scholar]
- Thornton AC, Jose S, Bhagani S, et al. (2017) Hepatitis B, hepatitis C, and mortality among HIV-positive individuals. Aids 31:2525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Pichard A, Mallet V, Nalpas B, et al. (2007) FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 46:32–6. [DOI] [PubMed] [Google Scholar]
- Viel G, Boscolo-Berto R, Cecchetto G, et al. (2012) Phosphatidylethanol in blood as a marker of chronic alcohol use: A systematic review and meta-analysis. Int J Mol Sci 13:14788–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, et al. (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38:518–26. [DOI] [PubMed] [Google Scholar]
- Welsh DA, Ferguson T, Theall KP, et al. (2019) The New Orleans alcohol use in HIV study: Launching a translational investigation of the interaction of alcohol use with biological and socioenvironmental risk factors for multimorbidity in people living with HIV. Alcohol Clin Exp Res 43:704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


